SUMMARY
Nuclear DNA repair capacity is a critical determinant of cell fate under genotoxic stress conditions. DNA repair is a well-defined energy consuming process; however, it is unclear how DNA repair is fueled and whether mitochondrial energy production contributes to nuclear DNA repair. Here, we report a dynamic enhancement of oxygen consumption and mitochondrial ATP generation in irradiated normal cells, paralleled with increased mitochondrial relocation of cell cycle kinase CDK1 and nuclear DNA repair. The basal and radiation-induced mitochondrial ATP generation is significantly reduced in cells harboring CDK1 phosphorylation deficient mutant complex I subunits. Similarly, mitochondrial ATP generation and nuclear DNA repair are also severely compromised in cells harboring mitochondrial-targeted kinase deficient CDK1. These results demonstrate a mechanism governing the communication between mitochondria and nucleus, by which CDK1 boosts mitochondrial bioenergetics to meet the increased cellular fuel demand for DNA repair and cell survival under genotoxic stress.
Keywords: CDK1, mitochondria bioenergetics, DNA repair, radiation
INTRODUCTION
It is well-known that radiation induces cell death due to nuclear DNA damage. Attempting to survive, cells need to maintain their genomic stability via checkpoint activation and rapid DNA repair process; lack of or insufficient repair mechanism could lead to apoptosis or abnormal cell proliferation and cancer risk (Hoeijmakers, 2009; Kastan and Bartek, 2004). DNA repair is long believed to be a highly energy-demanding process consuming a large amount of cellular adenosine triphosphate (ATP) (Bakkenist and Kastan, 2004; Hopfner et al., 2000; Paull and Gellert, 1999; Ward and Chen, 2004). As the powerhouse of mammalian cells, mitochondria provide the major cellular fuel for many critical processes in cell proliferation and survival (Pagliarini and Dixon, 2006; Scheibye-Knudsen et al., 2015). Inhibition of respiration chain suppresses the spontaneous and H2O2-induced DNA damage repair in peripheral blood mononuclear cells (Gafter-Gvili et al., 2011). Mutations of mitochondrial genes encoding respiration chain subunits sensitize B-lymphoblastoid cells to radiation with decreased ATP generation and DNA repair gene expression (Kulkarni et al., 2011). However, the exact mechanism regulating mitochondrial bioenergetics to coordinate the nuclear DNA repair capacity under genotoxic stress conditions remains unknown.
Recent findings reveal that the Cyclin B1/CDK1, a well-defined cell cycle kinase governing key steps of cell cycle progression (Gautier et al., 1990; Hochegger et al., 2008), functions in the communication between mitochondrial activity and cell cycle progression. CDK1 is involved in the integration of mitochondrial fission during G2/M transition through phosphorylation of mitochondrial fission proteins (Taguchi et al., 2007; Yamano and Youle, 2011). Cyclin B1/CDK1 is also found to be able to phosphorylate and activate MnSOD and p53 in mitochondria to enhance cell survival (Candas et al., 2013; Nantajit et al., 2010). Recent data further reveal that a fraction of mitochondrial-relocated Cyclin B1/CDK1 can assist the G2/M transition by boosting mitochondrial ATP generation via phosphorylation of mitochondrial respiration chain complex I (CI) subunits (Wang et al., 2014), and that CDK1-mediated Tom6 phosphorylation enhances the mitochondrial protein influx required for mitochondrial biogenesis and activity (Harbauer et al., 2014). Here we report that Cyclin B1/CDK1 mitochondrial relocation and mitochondrial ATP generation are enhanced paralleled with nuclear DNA repair in irradiated cells. Expression of CDK1 phosphorylation-deficient CI subunits or mitochondria-targeted kinase-deficient CDK1 inhibits radiation-induced mitochondrial ATP generation and DNA repair. These results provide the evidence indicating the communication between mitochondrial bioenergetics and nuclear DNA repair, in which CDK1-mediated phosphorylation and activation of mitochondrial CI enhances mitochondrial ATP production to meet the increased energy demands for DNA damage repair and cell survival.
RESULTS
Radiation Enhances Mitochondrial Bioenergetics in Cells
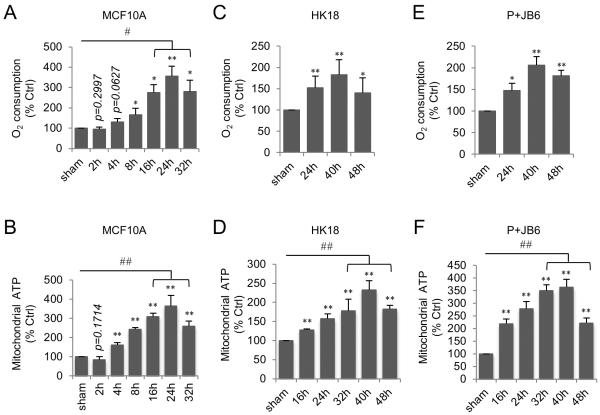
We recently reported that cell cycle kinase CDK1 is involved in the control of mitochondrial bioenergetics to fuel G2/M transition in normal cell cycle progression (Wang et al., 2014). In this study, we investigated whether CDK1-mediated mitochondrial bioenergetics contributes to the energy-supply for nuclear DNA repair under genotoxic stress conditions. To this end, we first tested how mitochondrial respiration was altered after irradiation in three immortalized normal cell lines: human mammary epithelial MCF10A cells, human skin keratinocytes (HK18) and mouse skin epithelial P+JB6 cells. We found that in all cell lines tested, mitochondrial ATP generation and oxygen consumption were significantly enhanced by radiation although the peak values and times were varied. In MCF10A cells, mitochondrial ATP generation showed a 3.63-fold increase, while oxygen consumption showed 3.46-fold increase at the peak time of 24 h post radiation compared to no radiation sham control (Figure 1A and 1B). Meanwhile, mitochondrial superoxide levels were found increased paralleled with slight increase in MnSOD activity in irradiated MCF10A cells (Figure S1A and S1B). Enhanced mitochondrial ATP generation and oxygen consumption was also observed in HK18 (Figure 1C–1D) and p+JB6 (Figure 1E–1F) cell lines with a peak time around 40 h post radiation, indicating that the induction of mitochondrial respiration by radiation is not cell-type specific (MCF10A cells were used in the subsequent experiments). Overall, these data demonstrate that mitochondrial bioenergetics is enhanced by a single dose of ionizing radiation in normal cells.
Figure 1. Radiation Enhances Mitochondrial ATP Generation and Oxygen Consumption.
Oxygen consumption (A, C, E) and mitochondrial ATP generation (B, D, F) were measured in MCF10A (A–B), HK18 (C–D), and P+JB6 (E–F) cells after 5 Gy radiation. Data represented as mean ± S.E.M.; *, P < 0.05; **, P < 0.01 irradiated cells vs. non-irradiated sham control; #, P < 0.05; ##, P < 0.01 peak time vs. non-irradiated sham control; n = 3. See also Figure S1.
Radiation-Induced DNA Damage Repair Links to Mitochondrial Bioenergetics
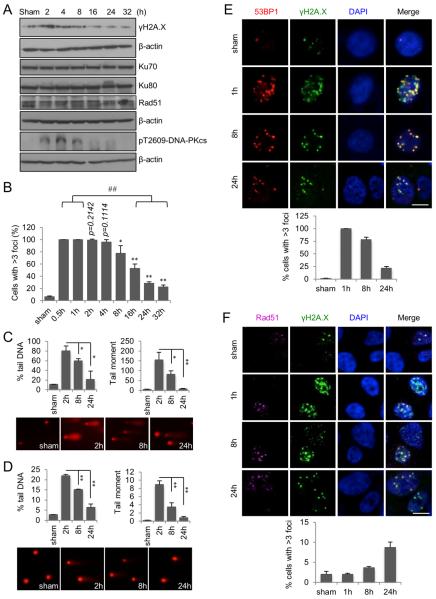
Since repair of radiation-induced DNA damage is essential for cell survival and maintenance of genomic stability, and DNA repair is an ATP-consuming process, we hypothesized that the irradiated cells enhance their mitochondrial ATP generation to meet the increased energy demand for nuclear DNA repair. We first determined the levels of γH2A.X, a marker of DNA double strain breaks (DSBs), which was increased 2 h post radiation, and then gradually decreased (Figure 2A). γH2A.X foci formation in irradiated cells can be detected as early as 15 min with 100% cells showing nuclear γH2A.X foci formation (Figure S2A), which remained constant till 2 h (Figure 2B and S2A). The number of γH2A.X foci containing cells was gradually decreased from 4 h post radiation (Figure 2B and S2A). Comet assay data displayed a similar DNA repair pattern shown by tail DNA intensity and tail moment in irradiated cells (Figure 3C–D).
Figure 2. Nuclear DNA Repair Capacity Is Paralleled with Mitochondrial ATP Generation.
(A) Western blotting analysis of radiation-induced DNA damage repair.
(B) Percentage of cells with >3 nuclear γH2A.X foci at different time intervals after 5 Gy radiation. Data represented as mean ± S.E.M.; *, P < 0.05; **, P < 0.01; n = 3. ##, P < 0.01; n = 3. See also Figure S2A.
(C) Alkaline comet assay of DNA damage. Data represented as mean ± S.E.M.; *, P < 0.05; **, P < 0.01. Lower panel, representative images of tailed DNA. Magnification, 20 ×.
(D) Neutral comet assay of DNA damage. Data represented as mean ± S.E.M.; **, P < 0.01. Lower panel, representative images of tailed DNA. Magnification, 20 ×.
(E) 53BP1 foci formation representing NHEJ repair of radiation-induced DNA DSBs. Scale bar, 10 μm. See also Figure S2B.
(F) Rad51 foci formation representing HR repair of radiation-induced DNA DSBs. Scale bar, 10 μm.
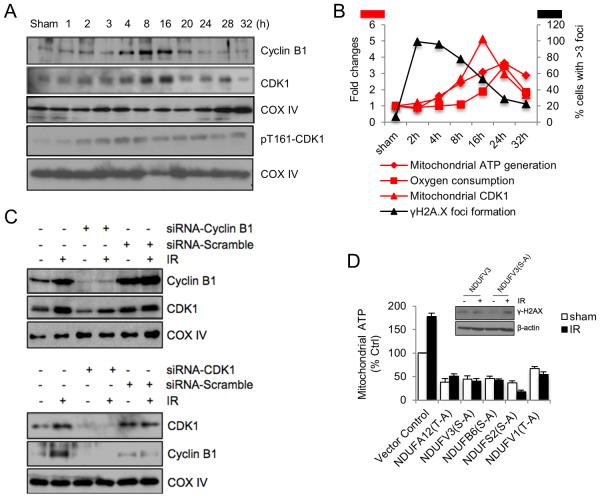
Figure 3. Mitochondrial CDK1 mediated Phosphorylation of CI Subunits Contributes to Radiation-Enhanced Mitochondrial ATP Generation.
(A) Western blotting of enhanced mitochondrial relocation of Cyclin B1 and CDK1 in irradiated cells. COXIV, mitochondria loading control. See also Figure S3.
(B) Summary of coordinative Cyclin B1/CDK1 mitochondrial relocation with enhanced mitochondrial respiration and DNA repair. Left Y-axis, fold changes in mitochondrial ATP generation, oxygen consumption and the levels of mitochondria relocated CDK1; right Y-axis, percentage of cells with >3 nuclear γH2A.X foci. See also Figure S3A.
(C) Cells were transfected with Cyclin B1 (upper panel) or CDK1 (lower panel) siRNA for 24 h and irradiated with 5 Gy followed by western blotting of mitochondria fractions 24 h after radiation. IR, ionizing radiation.
(D) Cells were transfected with each of CDK1 phosphorylation-deficient mutant CI subunits for 24 h and irradiated with 5 Gy followed by measurement of mitochondrial ATP generation 24 h post radiation (n = 3). Insert: γH2A.X expression levels measured in irradiated cells transfected with wild type or phosphorylation-deficient mutant NDUFV3 subunit.
Next, we examined the involvement of non-homologous end joining (NHEJ) and homologous recombination (HR) in the DNA DSB repair. The NHEJ pathway was found activated early post radiation indicated by 53BP1 foci formation (Figure 2E and Figure S2B) and increased DNAPKcs phosphorylation along with a constant level of Ku70/80 (Figure 2A). In contrast, HR repair was evidently increased 24 h post radiation measured by Rad51 foci formation (Figure 2F) without the apparent enhancement of Rad51 protein levels (Figure 2A). These data demonstrate a possibility that although both NHEJ and HR repair pathways are involved in irradiated cells, only HR repair coincidences with radiation-enhanced mitochondrial ATP enhancement in terms of timing, which needs to be further investigated.
Radiation induced G2/M arrest is believed to help cells for DNA damage repair, and the cells with unrepaired lesions undergo apoptosis or tumorigenesis (Hoeijmakers, 2009; Kastan and Bartek, 2004). Our data showed that the percentage of cells in G2/M phase was significantly increased 2–8 h after radiation (Figure S2C) with a relative small fraction of apoptotic cells detected during the same period of time (Figure S2D, S2E).
Radiation-Induced Mitochondrial Relocation of Cyclin B1 and CDK1
Our previous study demonstrated that radiation enhances mitochondrial relocation of Cyclin B1 and CDK1 in multiple normal and cancer cell lines (Candas et al., 2013; Nantajit et al., 2010), and that mitochondrial relocated CDK1 phosphorylates CI subunits, and consequently upregulates CI activity and mitochondrial ATP generation during G2/M transition (Wang et al., 2014). We were then wondering whether CDK1-enhanced mitochondrial ATP generation is required for nuclear DNA repair and cell survival under genotoxic conditions. We found that the first detectable increase in the mitochondrial relocation of Cyclin B1 and CDK1 appeared 4 h (Figure 3A) when mitochondria ATP production started to increase (Figure 1B) and DNA repair activity was detectable (Figure 2B), and both peaked around 16 h post radiation (Figure 3A), when mitochondrial ATP generation reached its peak time (Figure 1B) and most of damaged DNA has been repaired (Figure 2B). Taken together, as is summarized in Figure 3B and Figure S3A, the enhanced CDK1 mitochondrial relocation is coordinated with the increased mitochondria bioenergetics and DNA repair in irradiated cells. Notably, pT161-CDK1 (active phosphorylated CDK1) levels in both total cell lysates and mitochondrial fractions were increased by radiation around 2 h and remained high throughout the experiment (Figure 3A and S3B; the purity of mitochondrial preparation was shown in Figure S3C). In contrast to the enhanced mitochondrial CDK1 and Cyclin B1, total cellular CDK1 and Cyclin B1 protein remained unchanged or slightly increased within 32 h tested (Figure S3B). CDK1 mitochondrial relocation was further confirmed by electron microscopy (Figure S3D) and immunostaining (Figure S3E). Inhibition of Cyclin B1 expression using siRNA knockdown significantly decreased mitochondrial CDK1 level, and vice versa (Figure 3C), suggesting the possibility that both proteins relocate to mitochondrial together. In addition, radiation-induced mitochondrial relocation of Cyclin B1 and CDK1 appeared not to be dose-dependent (Figure S3F).
We then tested whether CDK1-mediated CI activation contributes to radiation-enhanced mitochondrial ATP generation and DNA repair. As is shown in Figure 3D, both basal and radiation-induced mitochondrial ATP was significantly reduced in cells harboring CDK1 phosphorylation-deficient CI subunits compared to the empty vector control cells, and moreover, these mutant CI subunit-harboring cells did not show any radiation-enhanced mitochondrial ATP. In addition, γH2A.X protein levels were significantly increased in irradiated NDUFV3(S-A)-harboring cells 24 h post radiation but not in irradiated NDUFV3wt-harboring cells (Figure 3D insert; the expression of GFP-tagged NDUFV3 was shown in Figure S3G). These data indicate that CDK1-mediated phosphorylation of mitochondria CI subunits is associated with radiation-enhanced mitochondrial ATP generation and DNA repair.
Mitochondrial Targeted Expression of Kinase-Deficient CDK1 Compromises Radiation-Enhanced Mitochondrial Bioenergetics and Inhibits DNA Repair
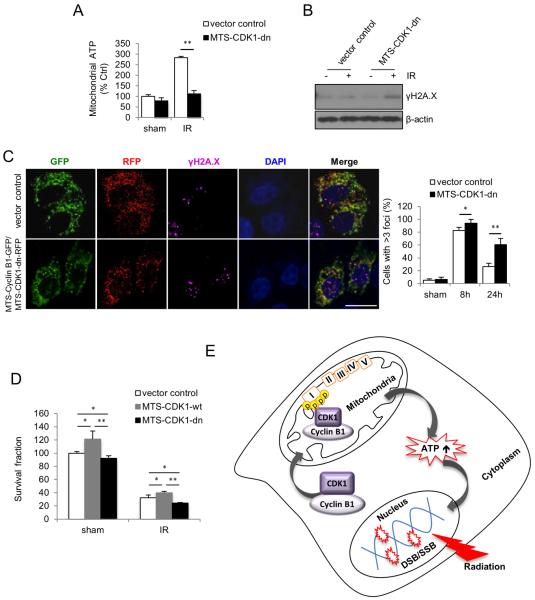
To further investigate whether mitochondrial CDK1 kinase activity is required for radiation-enhanced mitochondria bioenergetics and DNA repair, we transfected MCF10A cells with mitochondria-targeted Cyclin B1 together with kinase-deficient mutant CDK1 (MTS-CDK1-dn), and measured mitochondrial ATP generation and DNA repair. The schematics of DNA constructs for knockdown of mitochondria active CDK1, and the co-expression of fluorescence-tagged Cyclin B1 (GFP) and CDK1 (RFP) in transfected cells were shown in Figure S4A and S4B. With a mitochondrial targeting sequence (MTS) cloned in frame upstream of Cyclin B1 and CDK1 cDNA, expression of Cyclin B1 and CDK1 can be executed specifically in the mitochondria to avoid disturbing their functions in nucleus and cytosol. Radiation-induced mitochondrial ATP (Figure 4A) and superoxide (Figure S4C) were significantly compromised in MTS-CDK1-dn cells compared to empty vector control cells. Likewise, elevated γH2A.X protein level (Figure 4B) and increased γH2A.X foci containing cells (Figure 4C) were detected in the irradiated MTS-CDK1-dn cells. Further, clonogenic survival was reduced by about 25% in irradiated cells harboring MTS-CDK1-dn compared to empty vector control cells (Figure 4D). Together, these results suggest that kinase active mitochondrial CDK1 contributes to the radiation-enhanced mitochondrial ATP generation to meet the increased cellular energy demands for DNA repair in irradiated cells.
Figure 4. Mitochondria-Targeted Kinase Deficient CDK1 Compromises Enhanced Mitochondrial Bioenergetics and inhibits DNA Repair.
(A–C) Mitochondrial ATP generation (A), γH2A.X protein levels (B) and γH2A.X foci formation (Scale bar, 20 μm) (C) were measured in mitochondria-targeted kinase-deficient CDK1 harboring cells and empty vector control cells. In A, data represented as mean ± S.E.M.; **, P < 0.01; n = 3. In C right panel, data represented as mean ± S.E.M.; *, P < 0.05; **, P < 0.01; n = 3.
(D) Clonogenic survival of cells harboring mitochondria-targeted wild type or kinase-deficient mutant CDK1 and empty vector control cells under 5 Gy radiation. Data represented as mean ± S.E.M.; *, P < 0.05; **, P < 0.01; n = 3.
(E) A proposed function of CDK1 in coordinating mitochondrial bioenergetics with nuclear DNA repair in irradiated cells. Mitochondrial relocation of CDK1 is enhanced by radiation, which subsequently upregulates the mitochondrial ATP generation via phosphorylation of CI subunits to meet the increased cellular fuel demand on DNA repair.
DISCUSSION
Here we report a mechanism by which CDK1 coordinates mitochondrial ATP generation with radiation-induced nuclear DNA damage repair (Figure 4E). We show that mitochondrial ATP generation is increased in irradiated cells in a time-dependent manner paralleled with radiation-induced nuclear DNA damage and mitochondrial accumulation of Cyclin B1/CDK1. Mitochondria-targeted knockdown of CDK1 as well as expression of CDK1 phosphorylation-deficient subunits of CI severely compromises enhanced mitochondrial ATP generation and inhibits DNA repair in irradiated cells.
Timely and efficient DNA repair is a critical step for cells to survive genotoxic stress such as ionizing radiation (Hoeijmakers, 2009), which requires consumption of cellular energy (Bakkenist and Kastan, 2004; Kulkarni et al., 2011; Ward and Chen, 2004). As the major contributor of cellular energy production by generating ATP (Pagliarini and Dixon, 2006; Scheibye-Knudsen et al., 2015), mitochondria-derived ATP is enhanced under oxidative stress conditions such as radiation (Jin et al., 2015; Lu et al., 2015). Besides these, whether or how mitochondrial bioenergetics affects DNA damage repair capacity of cells under radiation stress has not been defined. Single nucleotide mutations in mitochondrial genes involved in ATP synthesis result in increased number of chromosomal aberrations and decreased mitotic indices following acute radiation exposure in human lymphoblastoid cells (Kulkarni et al., 2009), suggesting that mitochondrial ATP generation is linked with the DNA repair. In irradiated Jurkat cells, cellular ATP levels continuously decrease under oligomycin containing glucose-free medium culture condition but not glucose-free only medium or oligomycin containing regular medium culture conditions (Eguchi et al., 1997), indicating that in normal cells, mitochondrial oxidative phosphorylation and glycolysis might complement each other in order to maintain the efficient energy supply. Similar to normal cells, tumor cells also show radiation-enhanced mitochondrial respiration (Gong et al., 1998; Lu et al., 2015; Meike et al., 2011; Yamamori et al., 2012), and increased cellular ATP generation accompanied by reduced DNA damage leads to radio-resistance (Artesi et al., 2015). Our results, together with these reported findings, indicate a previously unknown communication between mitochondrial bioenergetics and nuclear DNA repair, through which cells enhance the mitochondrial energy metabolism to meet the increased fuel demand to recover from DNA damages.
It should be noticed that although both of NHEJ and HR pathways are shown to be involved in DNA repair following irradiation, the HR repair seems to be more dependent on the mitochondrial ATP supplies, indicating a possibility that HR pathway counts for decreased cell survival observed in cells harboring kinase-deficient CDK1. Further investigations are in need to elucidate whether a specific DNA repair pathway requires mitochondrial bioenergetics, and whether such a dynamic communication between mitochondrial bioenergetics and nuclear DNA repair is also activated in cancer cells.
Ionizing radiation is a potent inducer of apoptosis in many different types of cell (Shao et al., 2014), which is also an ATP/energy-demanding process, and the pertinent deployment of energy between DNA repair and apoptosis will potentially determine the cell fate (Bernstein et al., 2002). Study in Jurkat cells suggested that both glycolysis and mitochondrial oxidative phosphorylation could be the energy support of apoptosis (Eguchi et al., 1997). Although in the current study, apoptosis of irradiated cells was found increased gradually in the phase of mitochondrial energy enhancement, radiation-induced apoptosis in MCF10A cells was not affected by expression of mitochondria-targeted kinase-deficient CDK1 (Candas et al., 2013), suggesting that the CDK1/CI-mediated mitochondrial bioenergetics is highly possibly not required for radiation induced apoptosis in normal cells.
Under genotoxic conditions, a specific group of stress responsive proteins including MnSOD, p53, Cyclin B1/CDK1, Cyclin D1/CDK4 and survivin (Horbinski and Chu, 2005; Jin et al., 2015; Lu et al., 2015; Nantajit et al., 2010; Pagliarini and Dixon, 2006) are inflexed to mitochondria to induce cellular adaptive protection. As a well-defined cell cycle G2/M transition regulator, the precise subcellular relocation of Cyclin B1/CDK1 is required for the regulation of cell cycle progression (Gavet and Pines, 2010). Recently, CDK1 is found to relocate to mitochondrial to coordinate energy metabolism with increased fuel demand for cell cycle G2/M transition (Wang et al., 2014). Interestingly, as the major entry point of electrons into the respiration chain, CI was identified be substrate of CDK1 during cell cycle progression (Dephoure et al., 2008; Wang et al., 2014). These findings, together with our data showing that kinase deficient CDK1 and CDK1 phosphorylation deficient CI subunits can inhibit radiation-enhanced mitochondrial ATP generation and DNA repair, support the concept that CDK1-mediated activation of mitochondrial respiration chain coordinates the nuclear DNA repair with the increased cellular energy supply.
In conclusion, our data demonstrate a pathway through which mitochondria-relocated CDK1 phosphorylates multiple CI subunits, boosting mitochondrial ATP generation to meet the increased energy demand for DNA repair and cell survival; a unique cross-talk between mitochondrial energy metabolism and nuclear DNA repair, which may contribute to further understanding of how cell fate was decided under genotoxic stress conditions.
EXPERIMENTAL PROCEDURES
Cell Culture and Treatment
MCF-10A cells were maintained in as descripted previously (Soule et al., 1990). P+JB6 cells were maintained in MEM medium supplemented with 5% FBS and HK18 cells were maintained in DMEM medium supplemented with 10% FBS. Exponentially grown cells were irradiated with a cabinet x-ray System Faxitron Series at a dose rate of 1.382 Gy/min. See Supplemental Experimental Procedures for details.
Western Blotting
For each assay, 20 μg of protein were separated on SDS-PAGE gel and transferred to the PVDF membrane, followed by blocking of the membrane in 5% fat-free milk for 1 h. The membrane was then incubated with primary antibodies (refer to Supplementary Information for details) at 4°C overnight, followed by incubation with secondary antibodies for 1 h at room temperature. Signals were then detected with ECL detection kit.
Measurement of Mitochondrial ATP Production
Mitochondrial ATP production was measured following published method (Vives-Bauza et al., 2007) with modifications. See Supplemental Experimental Procedures for details.
Measurement of Oxygen Consumption
Exponentially growing cells (1~2 × 106) were harvested and resuspended in 300 μl of oxygen consumption buffer (25 mM sucrose, 15 mM KCl, 1 mM EGTA, 0.5 mM MgCl2 and 30 mM K2HPO4). Oxygen consumption was monitored at 30°C with succinate as complex II substrate using Clarke-type oxygen electrode (Rank Brothers Ltd) following the manufacturer's instructions.
Comet Assay
Alkaline and neutral comet assay were performed following the manufacturer's instructions (Trevigen Laboratory Inc.). DNA was stained with Propidium Iodide and images of the comets were captured under a fluorescence microscope. For each sample, a minimum of 50 cells was analyzed using software CASPlab, and the DNA damage was represented as percent tail DNA and tail moment. The experiments were repeated twice, and the control (untreated cells) was used to determine the characteristics of data for a healthy cell.
Foci Formation Assay
Cells growing on coverslips were fixed in 4% paraformaldehyde for 10 min at room temperature, permeabilized in 0.2% Triton X-100 for 10 min and blocked in 1% BSA for 1 h at room temperature. Cells were then incubated with indicated primary antibodies overnight at 4°C followed by incubation with the fluorescence-conjugated secondary antibody for 1 h in dark at room temperature. Nuclei were counterstained with DAPI contained in mounting solution. Images were acquired using a Zeiss LSM710 confocal microscope system and analyzed with ImageJ software. The experiment was repeated 3 times and at least 100 cells were scored for each sample and data represent the percentage of cells containing more than 3 foci.
Preparation of Mitochondrial Fractions from Cultured Cells
Mitochondrial and cytosolic fractions were prepared as described previously (Frezza et al., 2007) with modifications. Briefly, cells were homogenized using a Teflon-pestle in ice-cold IBc buffer. The homogenates were centrifuged at 600g for 10 min at 4°C and the supernatants were collected and further centrifuged at 7,000g for 10 min at 4°C. The supernatants were transferred to new tubes as the cytosolic fractions and the pellets were washed with ice-cold IBc buffer twice and re-suspended in cell lysis buffer as mitochondrial fractions.
Plasmid Constructs and Cell Transfection
Cyclin B1, CDK1 and CI subunits DNA constructs were established by our lab previously (Wang et al., 2014). Exponentially growing cells were transfected using METAFECTENE-PRO following the manufacturer's instructions.
Gene Silencing by siRNA
SiRNAs targeting Cyclin B1 and CDK1 mRNA were synthesized using the Silencer siRNA construction kit (Ambion) and transfected to cells using LipofectamineTM RNAi MAX (Invitrogen). See Supplemental Experimental Procedures for details.
Clonogenic Survival Assay
Cells transfected with mitochondrial targeting wild type or kinase-deficient mutant CDK1 were irradiated at 24 h after transfection, and seeded in 60 mm plates with three different cell numbers in triplicate. Colonies were stained at 7th day with Coomassie Blue dye and counted. The clonogenic survival ability of irradiated cells was represented as survival fraction normalized with that of no radiation control cells transfected with empty vector.
Statistical Analysis
Data are presented as mean ± S.E.M. from at least three independent experiments. Statistical analysis was performed with unpaired two-tailed Student's t test. P < 0.05 was considered statistically significant.
Supplementary Material
ACKNOWLEDGEMENTS
The authors are thankful to Dr. Zhenkun Lou at Mayo Clinic for providing DNA-PKcs-pT2609 antibody. L.Q. and J.J.L. designed the study. L.Q., M.F. and D.C. performed the experiments. S.P. and L.T. provided technical assistance. L.Q., D.C., G.J and J.J.L. wrote the manuscript. This work was partially supported by the grants from National Institutes of Health (CA152313 to J.J.L.) and the Department of Energy Office of Science (DE-SC0001271 to G.W., J.J.L., D.J.G.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: The authors declare no conflict of interest.
REFERENCES
- Artesi M, Kroonen J, Bredel M, Nguyen-Khac M, Deprez M, Schoysman L, Poulet C, Chakravarti A, Kim H, Scholtens D, et al. Connexin 30 expression inhibits growth of human malignant gliomas but protects them against radiation therapy. Neuro-oncology. 2015;17:392–406. doi: 10.1093/neuonc/nou215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakkenist CJ, Kastan MB. Initiating cellular stress responses. Cell. 2004;118:9–17. doi: 10.1016/j.cell.2004.06.023. [DOI] [PubMed] [Google Scholar]
- Bernstein C, Bernstein H, Payne CM, Garewal H. DNA repair/pro-apoptotic dual-role proteins in five major DNA repair pathways: fail-safe protection against carcinogenesis. Mutat. Res. 2002;511:145–178. doi: 10.1016/s1383-5742(02)00009-1. [DOI] [PubMed] [Google Scholar]
- Candas D, Fan M, Nantajit D, Vaughan AT, Murley JS, Woloschak GE, Grdina DJ, Li JJ. CyclinB1/Cdk1 phosphorylates mitochondrial antioxidant MnSOD in cell adaptive response to radiation stress. J. Mol. Cell Biol. 2013;5:166–175. doi: 10.1093/jmcb/mjs062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dephoure N, Zhou C, Villen J, Beausoleil SA, Bakalarski CE, Elledge SJ, Gygi SP. A quantitative atlas of mitotic phosphorylation. Proc. Natl. Acad. Sci. USA. 2008;105:10762–10767. doi: 10.1073/pnas.0805139105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eguchi Y, Shimizu S, Tsujimoto Y. Intracellular ATP levels determine cell death fate by apoptosis or necrosis. Cancer Res. 1997;57:1835–1840. [PubMed] [Google Scholar]
- Frezza C, Cipolat S, Scorrano L. Organelle isolation: functional mitochondria from mouse liver, muscle and cultured fibroblasts. Nat. Protoc. 2007;2:287–295. doi: 10.1038/nprot.2006.478. [DOI] [PubMed] [Google Scholar]
- Gafter-Gvili A, Herman M, Ori Y, Korzets A, Chagnac A, Zingerman B, Rozen-Zvi B, Gafter U, Malachi T. Inhibition of mitochondrial function reduces DNA repair in human mononuclear cells. Leuk. Res. 2011;35:219–225. doi: 10.1016/j.leukres.2010.06.009. [DOI] [PubMed] [Google Scholar]
- Gautier J, Minshull J, Lohka M, Glotzer M, Hunt T, Maller JL. Cyclin is a component of maturation-promoting factor from Xenopus. Cell. 1990;60:487–494. doi: 10.1016/0092-8674(90)90599-a. [DOI] [PubMed] [Google Scholar]
- Gavet O, Pines J. Activation of cyclin B1-Cdk1 synchronizes events in the nucleus and the cytoplasm at mitosis. J. Cell Biol. 2010;189:247–259. doi: 10.1083/jcb.200909144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong B, Chen Q, Almasan A. Ionizing radiation stimulates mitochondrial gene expression and activity. Radiat. Res. 1998;150:505–512. [PubMed] [Google Scholar]
- Harbauer AB, Opalinska M, Gerbeth C, Herman JS, Rao S, Schonfisch B, Guiard B, Schmidt O, Pfanner N, Meisinger C. Mitochondria. Cell cycle-dependent regulation of mitochondrial preprotein translocase. Science. 2014;346:1109–1113. doi: 10.1126/science.1261253. [DOI] [PubMed] [Google Scholar]
- Hochegger H, Takeda S, Hunt T. Cyclin-dependent kinases and cell-cycle transitions: does one fit all? Nat. Rev. Mol. Cell Biol. 2008;9:910–916. doi: 10.1038/nrm2510. [DOI] [PubMed] [Google Scholar]
- Hoeijmakers JH. DNA damage, aging, and cancer. New Engl. J. Med. 2009;361:1475–1485. doi: 10.1056/NEJMra0804615. [DOI] [PubMed] [Google Scholar]
- Hopfner KP, Karcher A, Shin DS, Craig L, Arthur LM, Carney JP, Tainer JA. Structural biology of Rad50 ATPase: ATP-driven conformational control in DNA double-strand break repair and the ABC-ATPase superfamily. Cell. 2000;101:789–800. doi: 10.1016/s0092-8674(00)80890-9. [DOI] [PubMed] [Google Scholar]
- Horbinski C, Chu CT. Kinase signaling cascades in the mitochondrion: a matter of life or death. Free Radical Biol. Med. 2005;38:2–11. doi: 10.1016/j.freeradbiomed.2004.09.030. [DOI] [PubMed] [Google Scholar]
- Jin C, Qin L, Shi Y, Candas D, Fan M, Lu CL, Vaughan AT, Shen R, Wu LS, Liu R, et al. CDK4-mediated MnSOD activation and mitochondrial homeostasis in radioadaptive protection. Free Radical Biol. Med. 2015;81:77–87. doi: 10.1016/j.freeradbiomed.2014.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kastan MB, Bartek J. Cell-cycle checkpoints and cancer. Nature. 2004;432:316–323. doi: 10.1038/nature03097. [DOI] [PubMed] [Google Scholar]
- Kulkarni R, Reither A, Thomas RA, Tucker JD. Mitochondrial mutant cells are hypersensitive to ionizing radiation, phleomycin and mitomycin C. Mutat. Res. 2009;663:46–51. doi: 10.1016/j.mrfmmm.2009.01.004. [DOI] [PubMed] [Google Scholar]
- Kulkarni R, Thomas RA, Tucker JD. Expression of DNA repair and apoptosis genes in mitochondrial mutant and normal cells following exposure to ionizing radiation. Environ. Mol. Mutag. 2011;52:229–237. doi: 10.1002/em.20605. [DOI] [PubMed] [Google Scholar]
- Lu CL, Qin L, Liu HC, Candas D, Fan M, Li JJ. Tumor cells switch to mitochondrial oxidative phosphorylation under radiation via mTOR-mediated hexokinase II inhibition--a Warburg-reversing effect. PLoS One. 2015;10:e0121046. doi: 10.1371/journal.pone.0121046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meike S, Yamamori T, Yasui H, Eitaki M, Matsuda A, Inanami O. 8-Aminoadenosine enhances radiation-induced cell death in human lung carcinoma A549 cells. J. Radiat. Res. 2011;52:456–463. doi: 10.1269/jrr.10188. [DOI] [PubMed] [Google Scholar]
- Nantajit D, Fan M, Duru N, Wen Y, Reed JC, Li JJ. Cyclin B1/Cdk1 phosphorylation of mitochondrial p53 induces anti-apoptotic response. PLoS ONE. 2010;5:e12341. doi: 10.1371/journal.pone.0012341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagliarini DJ, Dixon JE. Mitochondrial modulation: reversible phosphorylation takes center stage? Trends Biochem. Sci. 2006;31:26–34. doi: 10.1016/j.tibs.2005.11.005. [DOI] [PubMed] [Google Scholar]
- Paull TT, Gellert M. Nbs1 potentiates ATP-driven DNA unwinding and endonuclease cleavage by the Mre11/Rad50 complex. Genes Dev. 1999;13:1276–1288. doi: 10.1101/gad.13.10.1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheibye-Knudsen M, Fang EF, Croteau DL, Wilson DM, 3rd, Bohr VA. Protecting the mitochondrial powerhouse. Trends Cell Biol. 2015;25:158–170. doi: 10.1016/j.tcb.2014.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao L, Luo Y, Zhou D. Hematopoietic stem cell injury induced by ionizing radiation. Antioxid. Redox Signal. 2014;20:1447–1462. doi: 10.1089/ars.2013.5635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soule HD, Maloney TM, Wolman SR, Peterson WD, Jr., Brenz R, McGrath CM, Russo J, Pauley RJ, Jones RF, Brooks SC. Isolation and characterization of a spontaneously immortalized human breast epithelial cell line, MCF-10. Cancer Res. 1990;50:6075–6086. [PubMed] [Google Scholar]
- Taguchi N, Ishihara N, Jofuku A, Oka T, Mihara K. Mitotic phosphorylation of dynamin-related GTPase Drp1 participates in mitochondrial fission. J. Biol. Chem. 2007;282:11521–11529. doi: 10.1074/jbc.M607279200. [DOI] [PubMed] [Google Scholar]
- Vives-Bauza C, Yang L, Manfredi G. Assay of mitochondrial ATP synthesis in animal cells and tissues. Methods Cell Biol. 2007;80:155–171. doi: 10.1016/S0091-679X(06)80007-5. [DOI] [PubMed] [Google Scholar]
- Wang Z, Fan M, Candas D, Zhang TQ, Qin L, Eldridge A, Wachsmann-Hogiu S, Ahmed KM, Chromy BA, Nantajit D, et al. Cyclin B1/Cdk1 coordinates mitochondrial respiration for cell-cycle G2/M progression. Dev. Cell. 2014;29:217–232. doi: 10.1016/j.devcel.2014.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward I, Chen J. Early events in the DNA damage response. Curr. Top. Dev. Biol. 2004;63:1–35. doi: 10.1016/S0070-2153(04)63001-8. [DOI] [PubMed] [Google Scholar]
- Yamamori T, Yasui H, Yamazumi M, Wada Y, Nakamura Y, Nakamura H, Inanami O. Ionizing radiation induces mitochondrial reactive oxygen species production accompanied by upregulation of mitochondrial electron transport chain function and mitochondrial content under control of the cell cycle checkpoint. Free Radical Biol. Med. 2012;53:260–270. doi: 10.1016/j.freeradbiomed.2012.04.033. [DOI] [PubMed] [Google Scholar]
- Yamano K, Youle RJ. Coupling mitochondrial and cell division. Nat. Cell Biol. 2011;13:1026–1027. doi: 10.1038/ncb2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.