Abstract
IL6 is a pleiotropic cytokine that is made in response to perturbations in homeostasis. IL6 becomes elevated in the acute response to host injury and can activate immune cells, direct immune cell trafficking, signal protective responses in local tissue, initial the acute phase response or initiate wound healing. In the short term this proinflammatory response is protective and limits host damage. It is when this acute response remains chronically activated that IL6 becomes pathogenic to the host. Chronically elevated IL6 levels lead to chronic inflammation and fibrotic disorders. The heart is a tissue where this temporal regulation of IL6 is very apparent. Studies from myocardial infarction show how short-term IL6 signaling can protect and preserve the heart tissue in response to acute damage, where long term IL6 signaling or an over-production of IL6R protein plays a causal role in cardiovascular disease. Thus, IL6 can be both protective and pathogenic, depending on the kinetics of the host response.
Keywords: IL6, gp130, trans-signaling, heart failure, myocarditis, CVD
1. Introduction
IL6 is a pleiotropic cytokine which bridges the innate and adaptive immune systems [1]. Perturbations or dysfunction in the transition from innate to adaptive immunity have long term consequences for inflammation and autoimmunity [2]. The acute response to IL6, which is largely protective, to chronic, long term signaling leading to pathogenic inflammation and autoimmunity is an example of the varying faces of IL6 [3].
IL6 has a wide array of biological functions and is produced by many cells of the body. Originally identified as a B-cell differentiation factor, IL6 is now recognized as a cytokine that regulates many processes such as the acute-phase response, inflammation and hematopoiesis. IL6 can be made by most tissues as well as virtually all cells of the immune system. IL6 can signal either through membrane-bound receptors or, uniquely within the IL-6 family of cytokines, can signal in trans, with a soluble form of its receptor. IL6 has been shown to participate in neurogenesis, wound healing and hepatic regeneration [4–6]. Acutely, IL6 responds to almost all perturbations of homeostasis. However, when IL6 remains elevated chronically, the protective roles IL6 have maintaining tissue integrity and signaling the immune response, are no longer required and constant signaling becomes associated with fibrosis and chronic inflammation. This dual role of IL6, from acute and beneficial to chronic and harmful, is the subject of this review.
2. Population Based Studies
Meta-analysis of human studies has demonstrated that long-term elevation of IL6 levels more than double a person’s life-time risk of coronary heart disease [7]. These studies, among many others, demonstrate an association between pathology and chronic IL6 levels. Recent studies have established a causal role of increased IL6R protein levels in coronary heart disease (CHD) [8–10]. Not understood is whether elevated IL6 was a byproduct of the cardiovascular disease (CVD) or was serving a pathogenic function. Where as association studies have suggested that long-term IL6 levels have adverse consequences for cardiac health, these 2 studies have finally given clear evidence that IL6, a proinflammatory cytokine, plays a causal role in determining CVD risk. The studies focused on a genetic variant in the population that is associated with increased IL6 levels circulating in the blood but decreased IL6R signaling. Interestingly, these groups when on to look at the effect of the variant compared to the anti-IL6R drug, tocilizumab, and found that the variant was associated with the same biological changes as the inhibiting drug. The findings of these studies suggest that targeting IL6 or, in particular IL-6R-mediated signaling, may be a possible therapeutic intervention for CVD, including a possible preventative measure in high risk individuals.
The many polymorphisms in the IL6 promoter region as well as polymorphisms in the IL6R gene locus which exist in the population are associated with inflammation and increased disease risk [11–14]. A particular polymorphism in the promoter region of IL6 was shown to lead to higher systemic levels of IL6 [11–13]. This variant is uniquely associated with susceptibility to systemic juvenile idiopathic arthritis and importantly, led to the use of anti-IL6R antibody for its treatment [14–17]. The polymorphisms associated with elevated protein levels of IL6R are also associated with inflammation and are predictive of adverse coronary outcomes such as cardiovascular disease [9] and abdominal aortic aneurism [18]. Elevated IL6 serum levels in patients may be predictive of poor outcomes, thus providing a potential prognostic tool, in a variety of heart-related diseases such as heart failure, myocardial infarction (MI), and angina[19–22]. Human studies clearly implicate IL6 signaling in the heart to be pathogenic over time, however some experimental data using animal models of acute insult to the heart, contradict these associations. The final outcome of IL6 signaling seems to be greatly dependent the duration of the signaling, as well as the downstream signaling cascades activated.
The identification and description of IL6 trans-signaling has begun to explain how IL6, uniquely in the IL6-family, has been shown to be protective in acute inflammation and disease such as septic shock but pathogenic in chronic disease [23–27]. Early in the study of IL6, chronic overproduction of the cytokine was implicated in the pathogenesis of many inflammatory conditions including rheumatoid arthritis (RA), Castleman’s disease and cardiac myxoma [28–30]. In all these disease states, a constitutively increased IL6 level explained the pathogenic inflammatory symptoms of the patients. Because of this, a therapeutic antibody targeting IL6 signaling, anti-IL6R, which targets membrane-bound as well as a soluble receptor, has been used to treat RA, Castleman’s disease and multiple myeloma in small studies [31–33]. Targeting the IL6R has been shown to be particularly effective in clinical trials for severe RA [34–36] and Crohn’s disease [37] which is important because IL6 trans-signaling is particularly pathogenic in these diseases where high levels of sIL6R have been found in patients [38–41] and associate with worse disease outcomes [41]. Trans-signaling of IL6 may be more common in chronic IL6 pathology and thus a way to target chronic signaling in the long-term, while preserving classical IL6 signaling, which is required during acute tissue insult.
3. IL6 biological functions and signaling
IL6 is a member of the IL6 family of cytokines that also includes cardiotrophin-1 (CT-1), cardiotrophin-like cytokine (CLC), cilliary neurotrophic factor (CNTF) leukemia inhibitory factor (LIF), neuropoietin (NPN), and oncostatin M [49–51].
As a multi-functional cytokine, IL6 acts on the immune system as well as other local tissues. Within the immune system, IL6 can direct the development and activation status of both innate and adaptive immune cells. IL6 signaling up-regulates anti-apoptotic molecules in T cells [52–54]. In addition, IL6 is required for Th17-lineage differentiation through STAT3 dependent mechanisms [55, 56]. This is particularly important because the Th17 lineage has been implicated as a contributor to pathogenesis in many autoimmune diseases. IL6 also has functions in the innate immune system, where it induces the differentiation of monocytes to macrophages rather than dendritic cells [57]. IL6 may also influence DC activity as it can suppress DC CCR7 expression and IL6 secretion by DCs can affect the immunosuppressive activity of Tregs [58–60], thus bridging the innate and adaptive immune responses. And importantly for the initiation of many inflammatory responses, in the tissue IL6 suppresses neutrophil infiltration while promoting the infiltration and activation of mononuclear leukocytes [61–65]. Together, these studies show how IL6 can direct a proinflammatory immune response that can trigger an auto-aggressive response through the Th17 lineage if not properly controlled.
The IL6 cytokine family signals through a cytokine-specific receptor complexed with at least one subunit of the signal-transducing protein gp130 [50]. IL6 specifically signals through a complex of the IL6R (also known as IL6R-alpha) [66] and the IL6-family common receptor gp130 [67, 68]. GP130 signaling mediates a variety of cellular processes including cell survival, apoptosis, growth, proliferation, differentiation and survival [47, 69–71]. GP130 is part of the receptor complex for CNTF in the brain, LIF, oncostatin M, NPN, cardiotrophin (CT-1) in the heart, IL11, IL27 and IL31 [72–78]. Importantly, gp130 is expressed on nearly all cells in the body. Therefore what gives IL-6 family cytokines tissue-specificity is the cellular expression of the co-receptor for each family member cytokine.
The IL6R is mainly expressed on hepatocytes and immune cells. However, IL6 is unique in the IL6-family because it has a soluble form of its receptor. Therefore, cells lacking the IL6R can still respond to IL6 because the naturally occurring soluble form of the IL6R exists and can create a complex with IL6. IL6 first binds to the IL6R and this complex of IL6 and IL6R then binds with gp130[79, 80].The soluble IL6R (sIL6R) is generated either by cleavage of the membrane-associated receptor or, independently, by translation of an alternatively spliced mRNA [81–83]. This signaling of the sIL6R and the membrane bound gp130 is referred to as IL6 trans-signaling [84]. Trans-signaling has been shown to be active in many systems where cells only become responsive to IL6 in the presence of the sIL6R, such as in hematopoietic progenitor cells [85, 86], T cells [87, 88], and endothelial cells [89].
Downstream signaling of the IL6R combined with gp130, whether soluble or membrane bound, signals through either JAK-STAT, Ras-MAPK, or PI3K, pathways [90, 91]. Within the JAK-STAT pathway, IL6 specifically signals through STAT3, which dimerizes and then translocates to the nucleus [92]. Regulation and termination of downstream IL6 signaling is mediated through suppressor of cytokine signaling (SOCS) proteins [93–95]. The negative regulator of IL6-STAT3 activation, SOCS3, may in part regulate the protective versus pathogenic affects of IL6.
4. IL-6 in mouse models of inflammatory disease
IL6 is pathogenic in a variety of inflammatory mouse models. IL6KO mice are resistant to experimentally induced RA [96], colitis [87], experimental autoimmune encephalitis (EAE) [97], experimental autoimmune myocarditis (EAM) [98] and autoimmune kidney disease [99]. Additionally, antibodies that target IL6 signaling block the development of many of these same diseases. IL6R blockade ameliorates colitis [87], inhibits the onset of autoimmune kidney disease [100] and inhibits the development of collagen induced arthritis [101]. How exactly IL6 is exerting its effects in each model may show a role for signaling both to the immune system as well as the local affected tissue.
Evidence for the role of local, tissue-specific IL6 signaling in the pathogenesis of chronic inflammatory diseases comes from mouse studies that specifically target IL6 trans-signaling. The argument can be made that whole-animal knockouts of IL6 or systemic blockade of IL6 have many effects and thus the specific role that IL6 is contributing cannot be teased away from these off-target effects. However, by targeting trans-signaling, classical IL6 signaling is preserved, therefore only cells that do not express the IL6R are impacted. In many studies this translates into the local blockage of IL6 signaling in the tissue by blocking IL-6 trans-signaling as immune cells have the IL6R. In one study of renal pathology in lupus-prone mice, an inhibitor of trans-signaling, sgp130Fc was overexpressed in Lyn-deficient mice and its effect on lupus-associated pathology was measured. IL6-deletion in Lyn-deficient mice leads to decreased inflammation, decreased autoantibodies and decreased nephritis [102]. In the sgp130Fc mice, that have classical IL6 signaling but lack trans-signaling, there was no changes in immune cells, however, there was significantly attenuated glomerulonephritis and improved renal function and reduced complement fixation, showing a role for IL6 in the local kidney response [102]. Additional studies support these findings in other mouse models. It has long been known that IL6KO mice do not develop RA [103] but further studies have shown that targeting IL6 trans-signaling ameliorates RA [104]. Methods to target the local response to IL6 have been developed, such as tissue-restricted IL6 production where the whole animal is an IL6KO except for a tissue of interest. CNS-specific production of IL6 in a mouse model of EAE showed that IL6 production in the brain increases inflammatory cell infiltration impairs the blood-brain barrier and worsens disease outcome [105]. Collectively, these studies demonstrate a powerful, pathogenic role of IL6 in the local tissue that potentially can be therapeutically targeted through the sIL6R.
5. IL-6 in the Heart
The cellular response to IL6 in the heart has been well characterized. Cardiac tissue provides a revealing example where the duration of signaling, from acute to chronic, demonstrates the protective and pathogenic transition.
IL6 family signaling on cardiac myocytes is cardio protective during the acute response however, when remains elevated chronically, induces maladaptive hypertrophy and decreases contractile function [106, 107]. Myocytes themselves make IL6 in response to injury and in addition to increase Il6 signaling, increased IL6 production is associated with depressed cardiac function [108]. Acutely, IL6-family cytokines protect myocytes against oxidative stress and its signaling induces an anti-apoptotic program [107, 109, 110]. However, IL6-family signaling also depresses the basal contractility of the myocytes as well as the beta-adrenergic responsiveness of the cells leading to decreased function [111]. IL6 family signaling also induces gene expression in the myocytes that is associated with pathological hypertrophy [106]. In chronically exposed myocytes, the depressive effects on contractility of IL6 are mediated by enhancing de novo synthesis and activation of calcium-independent iNOS proteins [112]. Interestingly, the IL6-driven decrease in contractility was associated with JAK/STAT signaling but not the alternative downstream signaling, ERK pathway, suggesting that differential regulation of downstream signaling is a factor in fine-tuning the cellular response to IL6-family signaling [112].
The best characterized protective functions of IL-6 family signaling have been studied in ischemia-reperfusion injury and myocardial infarction which both induce IL6 production by cardiac myocytes [113–117]. Increased IL6 plays a role in late phase pre-conditioning that confers cardio protection [44, 45]. STAT3, the downstream signaling molecule of IL6, is also required for pre-conditioning [44]. However, chronic elevated myocardial production of IL6-family cytokines, which occurs post-MI and in HF, have been associated with worse heart outcomes [107, 109, 118]. IL6 is consistently upregulated in the infarct zone after experimental MI and is associated with left ventricle (LV) enlargement [115, 119, 120]. It is thought that the combined effects of IL6, anti-apoptosis, depressed contractility and hypertrophy, will lead to preserved myocardium in the infarct border zone [115]. Thus, the deleterious effects of IL6 chronically, serve a protective function in MI. Acutely, the combined effects of IL6 production and signaling by the myocytes leads to preserved cardiac tissue, where damage is limited by reducing the contractility of the cells and inducing an anti-apoptotic program. In the short term these experiments have led to smaller infarct zones and thus acute IL6 is protective in MI. However, in both mice and human studies showed that when this elevated IL6 continues past the initial requirement to preserve the insulted tissue, these same effects become deleterious. By inducing an anti-apoptotic program and reducing contractility in the long-term, the tissue is less effective as a muscle and begins to induce a genetic program related to hypertrophy, which can ultimately result in heart failure. Thus continued of IL6 signaling is pathogenic.
IL6 signaling has also been studied from the perspective of limiting or terminating signaling. In cultured cardiac myocytes overexpression of SOCS3 (limiting IL6-family signaling) completely suppressed the ability of the IL6-family cytokines to be anti-apoptotic as well as inducing hypertrophy[121]. This is mirrored in human data where a decrease in myocardium SOCS3 protein expression, which would lead to continuous IL6 signaling, has also been found in the LV of patients with DCM[48]. Fine-tuning the signal cascade of IL6 may solve the apparent discrepancy of high IL6 levels associating with poor heart outcomes, but experimentally evidence showing gp130 signaling to be cardio protective. In a mouse model of MI, high levels of IL6 increased adverse LV remodeling and heart failure because of impaired regulation of the downstream signaling of IL6, leading to pathogenic, sustained gp-130 mediated STAT3 activation [122]. This study was particularly clarifying because the authors identified that signaling through the tyrosine-757 residue on the gp130 receptor mediated these outcomes and lead to prolonged and enhanced JAK/STAT activation, without ERK and Akt signaling, thus pinpointing a specific cascade [122]. The identification of a specific downstream signaling cascade is an important goal because it may identify how or why acute IL6 is not properly regulated and instead shifts to a chronic signal.
In the myocardium, both chronically elevated IL6 and increased IL6R expression lead to continuous activation of gp130, which results in hypertrophy [110, 123]. To identify the role that STAT3 signaling in particular plays in pathogenic IL6 signaling, mice were created that over-express STAT3 in the heart. Mice with a cardiac-specific increase in expression of STAT3, the downstream signaling target of the IL6 cytokines, develop hypertrophy without stimuli [124]. This demonstrates that uncontrolled, continuous STAT3 signaling causes pathogenic changes in the myocardium, independent of initial tissue insult. Alternatively, complete loss of the myocyte expression of gp130 through cardiac-specific knockout results in a heart with normal structure and function, although is susceptible to cardiac myocyte apoptosis and dilation in response to pressure-overload[125]. This illustrates the need for IL6 in the acute response to injury. IL6 signaling thus plays a role in both helpful and harmful effects in the myocardium as cardiac myocyte loss, which IL6 normally protects against, contributes to the progression of compensatory LV hypertrophy to heart failure [126].
Myocarditis provides a striking example of the dysregulation of protective IL6 responses leading to pathogenic IL6 outcomes. Acutely, IL6 is protective in the heart as it limits viral replication and thus cardiac damage[42]. However, once the virus has been cleared a subset of patients will eventually develop an autoimmune response to their heart and present with autoimmune myocarditis, independent of viral presence [127]. In these cases, continuous IL6 signaling is no longer protective but contributes to heart failure. Over time, patients with autoimmune myocarditis may develop dilated cardiomyopathy, which at its end-stage can only be treated with a heart transplant [127]. Circulating levels of IL6 increase with the severity of heart failure[128, 129] and upon autopsy, IL6 has been found to be increased in the heart tissue of patients with DCM [43, 130] and end-stage heart failure [129, 131, 132]. Myocarditis is an extreme example of how limited acute IL6 signaling is protective for viral clearance but the chronic, long term exposure of the heart to IL6 signaling contributes to pathology and loss of cardiac function and remodeling.
6. Conclusions
Classically IL6 is considered to be a proinflammatory cytokine. When homeostasis is disturbed within a host IL6 is elevated and induces protective responses determined by the nature of the insult. IL6 can activate immune cells, direct immune cell trafficking, signal protective responses in local tissue, initiate the acute phase response or contribute to wound healing. In the acute response, these are all vital functions. However, beyond this temporally limited role, the proinflammatory nature of IL6 can become pathogenic. In the short term, what are protective responses, increased cell infiltration, increased wound repair, can turn deleterious in the long term leading to inflammatory and fibrotic disorders. The heart is a tissue where this duality is very apparent. Studies from MI show how short-term IL6 signaling can protect and preserve the heart tissue in response to acute damage, where long term IL6 signaling or an over-production of IL6R protein plays a causal role in cardiovascular disease.
The identification of the unique nature of IL6 signaling, which occurs through both classical, membrane-bound signaling and through signaling in trans, with a soluble form of the IL6R, has created the opportunity for therapeutic intervention. Blocking all IL6 signaling has severe consequences as IL6 serves many vital functions, although is only currently used for severe cases of RA. Having a method to only block the particularly pathogenic signals of IL6 is an exciting avenue of research. The current use of the available IL6R antibody, which targets both classical and trans signaling, is limited as tocilizumab is given monthly by intravenous (IV) infusion. However, many animal studies are aimed to design more specific inhibitors, through the use of a soluble gp130 decoy, sgp130Fc, which inhibits only trans-signaling.
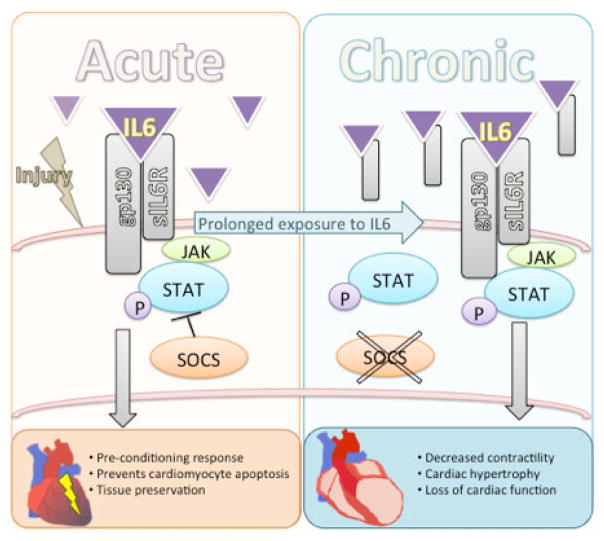
Figure 1.
The transition from acute, protective IL6 signaling, to chronic pathogenic IL6 signaling on the cardiomyocyte. In the acute phase, IL6 preserves cardiac tissue by inducing an anti-apoptotic program in the myocyte and triggers the pre-conditioning response [44,45]. When IL6 signaling continues chronically, these protective responses become pathogenic and induce depressed myocyte function. There is decreased contractility, hypertrophic genes are turned and LV enlargement occurs [115, 119, 120].
Table 1.
The dual role of IL-6 in human studies
| Beneficial | Harmful | |
|---|---|---|
| Acute signaling | ||
| Elevated IL6 serum levels in patients with myocarditis | Limits viral infection [42] | Associated with DCM [43] |
| Elevated IL6 serum levels following myocardial infarction | Limits infarct size [44, 45] | Associated with HF and LVF[46] |
| Chronic signaling | ||
| Long-term elevated IL6 serum levels | Two fold Increased life-time risk of CHD [4] | |
| Long-term elevated sIL6R serum levels | Associated with severe RA [34, 35] | |
| Polymorphism in IL6R leading to elevated IL6R protein expression in sera | Increased susceptibility to systemic juvenile idiopathic arthritis (SJIA) [14] Predictive of adverse outcomes in CVD [47] |
|
| Decreased myocardial SOCS3 protein expression | Found in myocardium of patients with DCM [48] |
Table 2.
The dual role of IL6 in mouse model studies
| Beneficial | Harmful | |
|---|---|---|
| Acute signaling | ||
| Increased myocyte production of IL6 | Anti-apoptotic Protective against oxidative stress Pre-conditioning response [44, 45] |
Depressed basal contractility Hypertrophic genes turned on Depressed b-adrenergic response LV enlargement [115, 119, 120] |
| IL6KO mice in experimental myocarditis | Resistant to experimental autoimmune myocarditis [98] | Increased susceptibility to viral-induced myocarditis mouse models [42] |
| Chronic signaling | ||
| Myocyte-specific gp130 loss | No IL-6 signaling [125] | Susceptible to myocyte apoptosis in pressure-overload models [125] |
| Cardiac-specific increase in STAT3 expression | Increased IL6-family responsiveness [124] | Mice develop hypertrophy without stimuli [124] |
| IL6KO mice | Resistant to experimentally induced RA [96], colitis [87], EAE [97], EAM [98], autoimmune kidney disease [99] | Susceptible to infections [133, 134] |
Highlights.
IL6 is cardio protective during acute insult to the myocardium
IL6 transitions to a pathogenic factor when it remains elevated chronically
The transition is associated with specific downstream signaling unique to IL6
Chronic IL6 signaling is associated with heart failure
In myocarditis chronic IL6 signaling contributes to progression to dilated cardiomyopathy
Acknowledgments
This work was supported by the National Institutes of Health/National Heart, Lung and Blood Institute (NIH/NHLBI) Grants R01 HL113008 (to N.R.Rose) and R01HL118183 (D. Čiháková).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jones SA. Directing transition from innate to acquired immunity: defining a role for IL-6. Journal of immunology. 2005;175:3463–8. doi: 10.4049/jimmunol.175.6.3463. [DOI] [PubMed] [Google Scholar]
- 2.Hoebe K, Janssen E, Beutler B. The interface between innate and adaptive immunity. Nature immunology. 2004;5:971–4. doi: 10.1038/ni1004-971. [DOI] [PubMed] [Google Scholar]
- 3.Kaplanski G, Marin V, Montero-Julian F, Mantovani A, Farnarier C. IL-6: a regulator of the transition from neutrophil to monocyte recruitment during inflammation. Trends in immunology. 2003;24:25–9. doi: 10.1016/s1471-4906(02)00013-3. [DOI] [PubMed] [Google Scholar]
- 4.Streetz KL, Luedde T, Manns MP, Trautwein C. Interleukin 6 and liver regeneration. Gut. 2000;47:309–12. doi: 10.1136/gut.47.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lin ZQ, Kondo T, Ishida Y, Takayasu T, Mukaida N. Essential involvement of IL-6 in the skin wound-healing process as evidenced by delayed wound healing in IL-6-deficient mice. Journal of leukocyte biology. 2003;73:713–21. doi: 10.1189/jlb.0802397. [DOI] [PubMed] [Google Scholar]
- 6.Erta M, Quintana A, Hidalgo J. Interleukin-6, a major cytokine in the central nervous system. International journal of biological sciences. 2012;8:1254–66. doi: 10.7150/ijbs.4679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Danesh J, Kaptoge S, Mann AG, Sarwar N, Wood A, Angleman SB, et al. Long-term interleukin-6 levels and subsequent risk of coronary heart disease: two new prospective studies and a systematic review. PLoS medicine. 2008;5:e78. doi: 10.1371/journal.pmed.0050078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Collaboration IRGCERF. Sarwar N, Butterworth AS, Freitag DF, Gregson J, Willeit P, et al. Interleukin-6 receptor pathways in coronary heart disease: a collaborative meta-analysis of 82 studies. Lancet. 2012;379:1205–13. doi: 10.1016/S0140-6736(11)61931-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Interleukin-6 Receptor Mendelian Randomisation Analysis C. Hingorani AD, Casas JP. The interleukin-6 receptor as a target for prevention of coronary heart disease: a mendelian randomisation analysis. Lancet. 2012;379:1214–24. doi: 10.1016/S0140-6736(12)60110-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boekholdt SM, Stroes ES. The interleukin-6 pathway and atherosclerosis. Lancet. 2012;379:1176–8. doi: 10.1016/S0140-6736(12)60361-4. [DOI] [PubMed] [Google Scholar]
- 11.Smith AJ, Zheng D, Palmen J, Pang DX, Woo P, Humphries SE. Effects of genetic variation on chromatin structure and the transcriptional machinery: analysis of the IL6 gene locus. Genes and immunity. 2012;13:583–6. doi: 10.1038/gene.2012.32. [DOI] [PubMed] [Google Scholar]
- 12.Smith AJ, D’Aiuto F, Palmen J, Cooper JA, Samuel J, Thompson S, et al. Association of serum interleukin-6 concentration with a functional IL6 -6331T>C polymorphism. Clinical chemistry. 2008;54:841–50. doi: 10.1373/clinchem.2007.098608. [DOI] [PubMed] [Google Scholar]
- 13.Samuel JM, Kelberman D, Smith AJ, Humphries SE, Woo P. Identification of a novel regulatory region in the interleukin-6 gene promoter. Cytokine. 2008;42:256–64. doi: 10.1016/j.cyto.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 14.Ogilvie EM, Fife MS, Thompson SD, Twine N, Tsoras M, Moroldo M, et al. The -174G allele of the interleukin-6 gene confers susceptibility to systemic arthritis in children: a multicenter study using simplex and multiplex juvenile idiopathic arthritis families. Arthritis and rheumatism. 2003;48:3202–6. doi: 10.1002/art.11300. [DOI] [PubMed] [Google Scholar]
- 15.Yokota S, Miyamae T, Imagawa T, Iwata N, Katakura S, Mori M, et al. Therapeutic efficacy of humanized recombinant anti-interleukin-6 receptor antibody in children with systemic-onset juvenile idiopathic arthritis. Arthritis and rheumatism. 2005;52:818–25. doi: 10.1002/art.20944. [DOI] [PubMed] [Google Scholar]
- 16.Woo P, Wilkinson N, Prieur AM, Southwood T, Leone V, Livermore P, et al. Open label phase II trial of single, ascending doses of MRA in Caucasian children with severe systemic juvenile idiopathic arthritis: proof of principle of the efficacy of IL-6 receptor blockade in this type of arthritis and demonstration of prolonged clinical improvement. Arthritis research & therapy. 2005;7:R1281–8. doi: 10.1186/ar1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.De Benedetti F, Brunner HI, Ruperto N, Kenwright A, Wright S, Calvo I, et al. Randomized trial of tocilizumab in systemic juvenile idiopathic arthritis. The New England journal of medicine. 2012;367:2385–95. doi: 10.1056/NEJMoa1112802. [DOI] [PubMed] [Google Scholar]
- 18.Harrison SC, Smith AJ, Jones GT, Swerdlow DI, Rampuri R, Bown MJ, et al. Interleukin-6 receptor pathways in abdominal aortic aneurysm. European heart journal. 2013;34:3707–16. doi: 10.1093/eurheartj/ehs354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lindmark E, Diderholm E, Wallentin L, Siegbahn A. Relationship between interleukin 6 and mortality in patients with unstable coronary artery disease: effects of an early invasive or noninvasive strategy. JAMA : the journal of the American Medical Association. 2001;286:2107–13. doi: 10.1001/jama.286.17.2107. [DOI] [PubMed] [Google Scholar]
- 20.Ikeda U, Ohkawa F, Seino Y, Yamamoto K, Hidaka Y, Kasahara T, et al. Serum interleukin 6 levels become elevated in acute myocardial infarction. Journal of molecular and cellular cardiology. 1992;24:579–84. doi: 10.1016/0022-2828(92)91042-4. [DOI] [PubMed] [Google Scholar]
- 21.Torre-Amione G, Kapadia S, Benedict C, Oral H, Young JB, Mann DL. Proinflammatory cytokine levels in patients with depressed left ventricular ejection fraction: a report from the Studies of Left Ventricular Dysfunction (SOLVD) Journal of the American College of Cardiology. 1996;27:1201–6. doi: 10.1016/0735-1097(95)00589-7. [DOI] [PubMed] [Google Scholar]
- 22.Tsutamoto T, Hisanaga T, Wada A, Maeda K, Ohnishi M, Fukai D, et al. Interleukin-6 spillover in the peripheral circulation increases with the severity of heart failure, and the high plasma level of interleukin-6 is an important prognostic predictor in patients with congestive heart failure. Journal of the American College of Cardiology. 1998;31:391–8. doi: 10.1016/s0735-1097(97)00494-4. [DOI] [PubMed] [Google Scholar]
- 23.Kallen KJ. The role of transsignalling via the agonistic soluble IL-6 receptor in human diseases. Biochimica et biophysica acta. 2002;1592:323–43. doi: 10.1016/s0167-4889(02)00325-7. [DOI] [PubMed] [Google Scholar]
- 24.Ulich TR, Yin S, Guo K, Yi ES, Remick D, del Castillo J. Intratracheal injection of endotoxin and cytokines. II. Interleukin-6 and transforming growth factor beta inhibit acute inflammation. The American journal of pathology. 1991;138:1097–101. [PMC free article] [PubMed] [Google Scholar]
- 25.Onogawa T. Local delivery of soluble interleukin-6 receptors to improve the outcome of alpha-toxin producing Staphylococcus aureus infection in mice. Immunobiology. 2005;209:651–60. doi: 10.1016/j.imbio.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 26.Diao H, Kohanawa M. Endogenous interleukin-6 plays a crucial protective role in streptococcal toxic shock syndrome via suppression of tumor necrosis factor alpha production. Infection and immunity. 2005;73:3745–8. doi: 10.1128/IAI.73.6.3745-3748.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jones SA, Horiuchi S, Topley N, Yamamoto N, Fuller GM. The soluble interleukin 6 receptor: mechanisms of production and implications in disease. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2001;15:43–58. doi: 10.1096/fj.99-1003rev. [DOI] [PubMed] [Google Scholar]
- 28.Jourdan M, Bataille R, Seguin J, Zhang XG, Chaptal PA, Klein B. Constitutive production of interleukin-6 and immunologic features in cardiac myxomas. Arthritis and rheumatism. 1990;33:398–402. doi: 10.1002/art.1780330313. [DOI] [PubMed] [Google Scholar]
- 29.Hirano T, Matsuda T, Turner M, Miyasaka N, Buchan G, Tang B, et al. Excessive production of interleukin 6/B cell stimulatory factor-2 in rheumatoid arthritis. European journal of immunology. 1988;18:1797–801. doi: 10.1002/eji.1830181122. [DOI] [PubMed] [Google Scholar]
- 30.Yoshizaki K, Matsuda T, Nishimoto N, Kuritani T, Taeho L, Aozasa K, et al. Pathogenic significance of interleukin-6 (IL-6/BSF-2) in Castleman’s disease. Blood. 1989;74:1360–7. [PubMed] [Google Scholar]
- 31.Nishimoto N, Sasai M, Shima Y, Nakagawa M, Matsumoto T, Shirai T, et al. Improvement in Castleman’s disease by humanized anti-interleukin-6 receptor antibody therapy. Blood. 2000;95:56–61. [PubMed] [Google Scholar]
- 32.Nishimoto N, Yoshizaki K, Miyasaka N, Yamamoto K, Kawai S, Takeuchi T, et al. Treatment of rheumatoid arthritis with humanized anti-interleukin-6 receptor antibody: a multicenter, double-blind, placebo-controlled trial. Arthritis and rheumatism. 2004;50:1761–9. doi: 10.1002/art.20303. [DOI] [PubMed] [Google Scholar]
- 33.Yokota S, Imagawa T, Mori M, Miyamae T, Aihara Y, Takei S, et al. Efficacy and safety of tocilizumab in patients with systemic-onset juvenile idiopathic arthritis: a randomised, double-blind, placebo-controlled, withdrawal phase III trial. Lancet. 2008;371:998–1006. doi: 10.1016/S0140-6736(08)60454-7. [DOI] [PubMed] [Google Scholar]
- 34.Choy EH, Isenberg DA, Garrood T, Farrow S, Ioannou Y, Bird H, et al. Therapeutic benefit of blocking interleukin-6 activity with an anti-interleukin-6 receptor monoclonal antibody in rheumatoid arthritis: a randomized, double-blind, placebo-controlled, dose-escalation trial. Arthritis and rheumatism. 2002;46:3143–50. doi: 10.1002/art.10623. [DOI] [PubMed] [Google Scholar]
- 35.Nakahara H, Song J, Sugimoto M, Hagihara K, Kishimoto T, Yoshizaki K, et al. Anti-interleukin-6 receptor antibody therapy reduces vascular endothelial growth factor production in rheumatoid arthritis. Arthritis and rheumatism. 2003;48:1521–9. doi: 10.1002/art.11143. [DOI] [PubMed] [Google Scholar]
- 36.Yoshizaki K, Nishimoto N, Mihara M, Kishimoto T. Therapy of rheumatoid arthritis by blocking IL-6 signal transduction with a humanized anti-IL-6 receptor antibody. Springer seminars in immunopathology. 1998;20:247–59. doi: 10.1007/BF00832010. [DOI] [PubMed] [Google Scholar]
- 37.Ito H, Takazoe M, Fukuda Y, Hibi T, Kusugami K, Andoh A, et al. A pilot randomized trial of a human anti-interleukin-6 receptor monoclonal antibody in active Crohn’s disease. Gastroenterology. 2004;126:989–96. doi: 10.1053/j.gastro.2004.01.012. discussion 47. [DOI] [PubMed] [Google Scholar]
- 38.Desgeorges A, Gabay C, Silacci P, Novick D, Roux-Lombard P, Grau G, et al. Concentrations and origins of soluble interleukin 6 receptor-alpha in serum and synovial fluid. The Journal of rheumatology. 1997;24:1510–6. [PubMed] [Google Scholar]
- 39.Kotake S, Sato K, Kim KJ, Takahashi N, Udagawa N, Nakamura I, et al. Interleukin-6 and soluble interleukin-6 receptors in the synovial fluids from rheumatoid arthritis patients are responsible for osteoclast-like cell formation. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 1996;11:88–95. doi: 10.1002/jbmr.5650110113. [DOI] [PubMed] [Google Scholar]
- 40.Keul R, Heinrich PC, Muller-newen G, Muller K, Woo P. A possible role for soluble IL-6 receptor in the pathogenesis of systemic onset juvenile chronic arthritis. Cytokine. 1998;10:729–34. doi: 10.1006/cyto.1997.0343. [DOI] [PubMed] [Google Scholar]
- 41.Robak T, Gladalska A, Stepien H, Robak E. Serum levels of interleukin-6 type cytokines and soluble interleukin-6 receptor in patients with rheumatoid arthritis. Mediators of inflammation. 1998;7:347–53. doi: 10.1080/09629359890875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kanda T, McManus JE, Nagai R, Imai S, Suzuki T, Yang D, et al. Modification of viral myocarditis in mice by interleukin-6. Circulation research. 1996;78:848–56. doi: 10.1161/01.res.78.5.848. [DOI] [PubMed] [Google Scholar]
- 43.Buzas K, Megyeri K, Hogye M, Csanady M, Bogats G, Mandi Y. Comparative study of the roles of cytokines and apoptosis in dilated and hypertrophic cardiomyopathies. European cytokine network. 2004;15:53–9. [PubMed] [Google Scholar]
- 44.Dawn B, Xuan YT, Guo Y, Rezazadeh A, Stein AB, Hunt G, et al. IL-6 plays an obligatory role in late preconditioning via JAK-STAT signaling and upregulation of iNOS and COX-2. Cardiovascular research. 2004;64:61–71. doi: 10.1016/j.cardiores.2004.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Smart N, Mojet MH, Latchman DS, Marber MS, Duchen MR, Heads RJ. IL-6 induces PI 3-kinase and nitric oxide-dependent protection and preserves mitochondrial function in cardiomyocytes. Cardiovascular research. 2006;69:164–77. doi: 10.1016/j.cardiores.2005.08.017. [DOI] [PubMed] [Google Scholar]
- 46.Gabriel AS, Martinsson A, Wretlind B, Ahnve S. IL-6 levels in acute and post myocardial infarction: their relation to CRP levels, infarction size, left ventricular systolic function, and heart failure. European journal of internal medicine. 2004;15:523–8. doi: 10.1016/j.ejim.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 47.Fischer P, Hilfiker-Kleiner D. Role of gp130-mediated signalling pathways in the heart and its impact on potential therapeutic aspects. British journal of pharmacology. 2008;153 (Suppl 1):S414–27. doi: 10.1038/bjp.2008.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Podewski EK, Hilfiker-Kleiner D, Hilfiker A, Morawietz H, Lichtenberg A, Wollert KC, et al. Alterations in Janus kinase (JAK)-signal transducers and activators of transcription (STAT) signaling in patients with end-stage dilated cardiomyopathy. Circulation. 2003;107:798–802. doi: 10.1161/01.cir.0000057545.82749.ff. [DOI] [PubMed] [Google Scholar]
- 49.Kishimoto T. Interleukin-6: from basic science to medicine--40 years in immunology. Annual review of immunology. 2005;23:1–21. doi: 10.1146/annurev.immunol.23.021704.115806. [DOI] [PubMed] [Google Scholar]
- 50.Taga T, Kishimoto T. Gp130 and the interleukin-6 family of cytokines. Annual review of immunology. 1997;15:797–819. doi: 10.1146/annurev.immunol.15.1.797. [DOI] [PubMed] [Google Scholar]
- 51.Derouet D, Rousseau F, Alfonsi F, Froger J, Hermann J, Barbier F, et al. Neuropoietin, a new IL-6-related cytokine signaling through the ciliary neurotrophic factor receptor. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:4827–32. doi: 10.1073/pnas.0306178101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Narimatsu M, Maeda H, Itoh S, Atsumi T, Ohtani T, Nishida K, et al. Tissue-specific autoregulation of the stat3 gene and its role in interleukin-6-induced survival signals in T cells. Molecular and cellular biology. 2001;21:6615–25. doi: 10.1128/MCB.21.19.6615-6625.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Teague TK, Schaefer BC, Hildeman D, Bender J, Mitchell T, Kappler JW, et al. Activation-induced inhibition of interleukin 6-mediated T cell survival and signal transducer and activator of transcription 1 signaling. The Journal of experimental medicine. 2000;191:915–26. doi: 10.1084/jem.191.6.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Curnow SJ, Scheel-Toellner D, Jenkinson W, Raza K, Durrani OM, Faint JM, et al. Inhibition of T cell apoptosis in the aqueous humor of patients with uveitis by IL-6/soluble IL-6 receptor trans-signaling. Journal of immunology. 2004;173:5290–7. doi: 10.4049/jimmunol.173.8.5290. [DOI] [PubMed] [Google Scholar]
- 55.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–8. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 56.Mangan PR, Harrington LE, O’Quinn DB, Helms WS, Bullard DC, Elson CO, et al. Transforming growth factor-beta induces development of the T(H)17 lineage. Nature. 2006;441:231–4. doi: 10.1038/nature04754. [DOI] [PubMed] [Google Scholar]
- 57.Chomarat P, Banchereau J, Davoust J, Palucka AK. IL-6 switches the differentiation of monocytes from dendritic cells to macrophages. Nature immunology. 2000;1:510–4. doi: 10.1038/82763. [DOI] [PubMed] [Google Scholar]
- 58.Hegde S, Pahne J, Smola-Hess S. Novel immunosuppressive properties of interleukin-6 in dendritic cells: inhibition of NF-kappaB binding activity and CCR7 expression. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2004;18:1439–41. doi: 10.1096/fj.03-0969fje. [DOI] [PubMed] [Google Scholar]
- 59.Bleier JI, Pillarisetty VG, Shah AB, DeMatteo RP. Increased and long-term generation of dendritic cells with reduced function from IL-6-deficient bone marrow. Journal of immunology. 2004;172:7408–16. doi: 10.4049/jimmunol.172.12.7408. [DOI] [PubMed] [Google Scholar]
- 60.Pasare C, Medzhitov R. Toll pathway-dependent blockade of CD4+CD25+ T cell-mediated suppression by dendritic cells. Science. 2003;299:1033–6. doi: 10.1126/science.1078231. [DOI] [PubMed] [Google Scholar]
- 61.Hurst SM, Wilkinson TS, McLoughlin RM, Jones S, Horiuchi S, Yamamoto N, et al. Il-6 and its soluble receptor orchestrate a temporal switch in the pattern of leukocyte recruitment seen during acute inflammation. Immunity. 2001;14:705–14. doi: 10.1016/s1074-7613(01)00151-0. [DOI] [PubMed] [Google Scholar]
- 62.Chen Q, Wang WC, Bruce R, Li H, Schleider DM, Mulbury MJ, et al. Central role of IL-6 receptor signal-transducing chain gp130 in activation of L-selectin adhesion by fever-range thermal stress. Immunity. 2004;20:59–70. doi: 10.1016/s1074-7613(03)00358-3. [DOI] [PubMed] [Google Scholar]
- 63.McLoughlin RM, Jenkins BJ, Grail D, Williams AS, Fielding CA, Parker CR, et al. IL-6 trans-signaling via STAT3 directs T cell infiltration in acute inflammation. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:9589–94. doi: 10.1073/pnas.0501794102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Modur V, Li Y, Zimmerman GA, Prescott SM, McIntyre TM. Retrograde inflammatory signaling from neutrophils to endothelial cells by soluble interleukin-6 receptor alpha. The Journal of clinical investigation. 1997;100:2752–6. doi: 10.1172/JCI119821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.McLoughlin RM, Hurst SM, Nowell MA, Harris DA, Horiuchi S, Morgan LW, et al. Differential regulation of neutrophil-activating chemokines by IL-6 and its soluble receptor isoforms. Journal of immunology. 2004;172:5676–83. doi: 10.4049/jimmunol.172.9.5676. [DOI] [PubMed] [Google Scholar]
- 66.Yamasaki K, Taga T, Hirata Y, Yawata H, Kawanishi Y, Seed B, et al. Cloning and expression of the human interleukin-6 (BSF-2/IFN beta 2) receptor. Science. 1988;241:825–8. doi: 10.1126/science.3136546. [DOI] [PubMed] [Google Scholar]
- 67.Hibi M, Murakami M, Saito M, Hirano T, Taga T, Kishimoto T. Molecular cloning and expression of an IL-6 signal transducer, gp130. Cell. 1990;63:1149–57. doi: 10.1016/0092-8674(90)90411-7. [DOI] [PubMed] [Google Scholar]
- 68.Taga T, Hibi M, Hirata Y, Yamasaki K, Yasukawa K, Matsuda T, et al. Interleukin-6 triggers the association of its receptor with a possible signal transducer, gp130. Cell. 1989;58:573–81. doi: 10.1016/0092-8674(89)90438-8. [DOI] [PubMed] [Google Scholar]
- 69.Heinrich PC, Behrmann I, Muller-Newen G, Schaper F, Graeve L. Interleukin-6-type cytokine signalling through the gp130/Jak/STAT pathway. Biochemical Journal. 1998;334:297–314. doi: 10.1042/bj3340297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fischer P, Hilfiker-Kleiner D. Survival pathways in hypertrophy and heart failure: the gp130-STAT3 axis. Basic research in cardiology. 2007;102:279–97. doi: 10.1007/s00395-007-0658-z. [DOI] [PubMed] [Google Scholar]
- 71.Naka T, Nishimoto N, Kishimoto T. The paradigm of IL-6: from basic science to medicine. Arthritis research. 2002;4 (Suppl 3):S233–42. doi: 10.1186/ar565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gearing DP, Comeau MR, Friend DJ, Gimpel SD, Thut CJ, McGourty J, et al. The IL-6 signal transducer, gp130: an oncostatin M receptor and affinity converter for the LIF receptor. Science. 1992;255:1434–7. doi: 10.1126/science.1542794. [DOI] [PubMed] [Google Scholar]
- 73.Ip NY, Nye SH, Boulton TG, Davis S, Taga T, Li Y, et al. CNTF and LIF act on neuronal cells via shared signaling pathways that involve the IL-6 signal transducing receptor component gp130. Cell. 1992;69:1121–32. doi: 10.1016/0092-8674(92)90634-o. [DOI] [PubMed] [Google Scholar]
- 74.Liu J, Modrell B, Aruffo A, Marken JS, Taga T, Yasukawa K, et al. Interleukin-6 signal transducer gp130 mediates oncostatin M signaling. The Journal of biological chemistry. 1992;267:16763–6. [PubMed] [Google Scholar]
- 75.Pennica D, Shaw KJ, Swanson TA, Moore MW, Shelton DL, Zioncheck KA, et al. Cardiotrophin-1. Biological activities and binding to the leukemia inhibitory factor receptor/gp130 signaling complex. The Journal of biological chemistry. 1995;270:10915–22. doi: 10.1074/jbc.270.18.10915. [DOI] [PubMed] [Google Scholar]
- 76.Yin T, Taga T, Tsang ML, Yasukawa K, Kishimoto T, Yang YC. Involvement of IL-6 signal transducer gp130 in IL-11-mediated signal transduction. Journal of immunology. 1993;151:2555–61. [PubMed] [Google Scholar]
- 77.Pflanz S, Hibbert L, Mattson J, Rosales R, Vaisberg E, Bazan JF, et al. WSX-1 and glycoprotein 130 constitute a signal-transducing receptor for IL-27. Journal of immunology. 2004;172:2225–31. doi: 10.4049/jimmunol.172.4.2225. [DOI] [PubMed] [Google Scholar]
- 78.Dillon SR, Sprecher C, Hammond A, Bilsborough J, Rosenfeld-Franklin M, Presnell SR, et al. Interleukin 31, a cytokine produced by activated T cells, induces dermatitis in mice. Nature immunology. 2004;5:752–60. doi: 10.1038/ni1084. [DOI] [PubMed] [Google Scholar]
- 79.Rose-John S. Coordination of interleukin-6 biology by membrane bound and soluble receptors. Advances in experimental medicine and biology. 2001;495:145–51. doi: 10.1007/978-1-4615-0685-0_19. [DOI] [PubMed] [Google Scholar]
- 80.Mackiewicz A, Schooltink H, Heinrich PC, Rose-John S. Complex of soluble human IL-6-receptor/IL-6 up-regulates expression of acute-phase proteins. Journal of immunology. 1992;149:2021–7. [PubMed] [Google Scholar]
- 81.Lust JA, Donovan KA, Kline MP, Greipp PR, Kyle RA, Maihle NJ. Isolation of an mRNA encoding a soluble form of the human interleukin-6 receptor. Cytokine. 1992;4:96–100. doi: 10.1016/1043-4666(92)90043-q. [DOI] [PubMed] [Google Scholar]
- 82.Rose-John S, Heinrich PC. Soluble receptors for cytokines and growth factors: generation and biological function. The Biochemical journal. 1994;300 ( Pt 2):281–90. doi: 10.1042/bj3000281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Matthews V, Schuster B, Schutze S, Bussmeyer I, Ludwig A, Hundhausen C, et al. Cellular cholesterol depletion triggers shedding of the human interleukin-6 receptor by ADAM10 and ADAM17 (TACE) The Journal of biological chemistry. 2003;278:38829–39. doi: 10.1074/jbc.M210584200. [DOI] [PubMed] [Google Scholar]
- 84.Jones SA, Richards PJ, Scheller J, Rose-John S. IL-6 transsignaling: the in vivo consequences. Journal of interferon & cytokine research : the official journal of the International Society for Interferon and Cytokine Research. 2005;25:241–53. doi: 10.1089/jir.2005.25.241. [DOI] [PubMed] [Google Scholar]
- 85.Audet J, Miller CL, Rose-John S, Piret JM, Eaves CJ. Distinct role of gp130 activation in promoting self-renewal divisions by mitogenically stimulated murine hematopoietic stem cells. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:1757–62. doi: 10.1073/pnas.98.4.1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hacker C, Kirsch RD, Ju XS, Hieronymus T, Gust TC, Kuhl C, et al. Transcriptional profiling identifies Id2 function in dendritic cell development. Nature immunology. 2003;4:380–6. doi: 10.1038/ni903. [DOI] [PubMed] [Google Scholar]
- 87.Atreya R, Mudter J, Finotto S, Mullberg J, Jostock T, Wirtz S, et al. Blockade of interleukin 6 trans signaling suppresses T-cell resistance against apoptosis in chronic intestinal inflammation: evidence in crohn disease and experimental colitis in vivo. Nature medicine. 2000;6:583–8. doi: 10.1038/75068. [DOI] [PubMed] [Google Scholar]
- 88.Becker C, Fantini MC, Schramm C, Lehr HA, Wirtz S, Nikolaev A, et al. TGF-beta suppresses tumor progression in colon cancer by inhibition of IL-6 trans-signaling. Immunity. 2004;21:491–501. doi: 10.1016/j.immuni.2004.07.020. [DOI] [PubMed] [Google Scholar]
- 89.Romano M, Sironi M, Toniatti C, Polentarutti N, Fruscella P, Ghezzi P, et al. Role of IL-6 and its soluble receptor in induction of chemokines and leukocyte recruitment. Immunity. 1997;6:315–25. doi: 10.1016/s1074-7613(00)80334-9. [DOI] [PubMed] [Google Scholar]
- 90.Akira S, Isshiki H, Sugita T, Tanabe O, Kinoshita S, Nishio Y, et al. A nuclear factor for IL-6 expression (NF-IL6) is a member of a C/EBP family. The EMBO journal. 1990;9:1897–906. doi: 10.1002/j.1460-2075.1990.tb08316.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Akira S, Nishio Y, Inoue M, Wang XJ, Wei S, Matsusaka T, et al. Molecular cloning of APRF, a novel IFN-stimulated gene factor 3 p91-related transcription factor involved in the gp130-mediated signaling pathway. Cell. 1994;77:63–71. doi: 10.1016/0092-8674(94)90235-6. [DOI] [PubMed] [Google Scholar]
- 92.Zhong Z, Wen Z, Darnell JE., Jr Stat3: a STAT family member activated by tyrosine phosphorylation in response to epidermal growth factor and interleukin-6. Science. 1994;264:95–8. doi: 10.1126/science.8140422. [DOI] [PubMed] [Google Scholar]
- 93.Endo TA, Masuhara M, Yokouchi M, Suzuki R, Sakamoto H, Mitsui K, et al. A new protein containing an SH2 domain that inhibits JAK kinases. Nature. 1997;387:921–4. doi: 10.1038/43213. [DOI] [PubMed] [Google Scholar]
- 94.Naka T, Narazaki M, Hirata M, Matsumoto T, Minamoto S, Aono A, et al. Structure and function of a new STAT-induced STAT inhibitor. Nature. 1997;387:924–9. doi: 10.1038/43219. [DOI] [PubMed] [Google Scholar]
- 95.Starr R, Willson TA, Viney EM, Murray LJ, Rayner JR, Jenkins BJ, et al. A family of cytokine-inducible inhibitors of signalling. Nature. 1997;387:917–21. doi: 10.1038/43206. [DOI] [PubMed] [Google Scholar]
- 96.Boe A, Baiocchi M, Carbonatto M, Papoian R, Serlupi-Crescenzi O. Interleukin 6 knock-out mice are resistant to antigen-induced experimental arthritis. Cytokine. 1999;11:1057–64. doi: 10.1006/cyto.1999.0502. [DOI] [PubMed] [Google Scholar]
- 97.Samoilova EB, Horton JL, Hilliard B, Liu TS, Chen Y. IL-6-deficient mice are resistant to experimental autoimmune encephalomyelitis: roles of IL-6 in the activation and differentiation of autoreactive T cells. Journal of immunology. 1998;161:6480–6. [PubMed] [Google Scholar]
- 98.Eriksson U, Kurrer MO, Schmitz N, Marsch SC, Fontana A, Eugster H-P, et al. Interleukin-6–Deficient Mice Resist Development of Autoimmune Myocarditis Associated With Impaired Upregulation of Complement C3. Circulation. 2003;107:320–5. doi: 10.1161/01.cir.0000043802.38699.66. [DOI] [PubMed] [Google Scholar]
- 99.Cash H, Relle M, Menke J, Brochhausen C, Jones SA, Topley N, et al. Interleukin 6 (IL-6) deficiency delays lupus nephritis in MRL-Faslpr mice: the IL-6 pathway as a new therapeutic target in treatment of autoimmune kidney disease in systemic lupus erythematosus. The Journal of rheumatology. 2010;37:60–70. doi: 10.3899/jrheum.090194. [DOI] [PubMed] [Google Scholar]
- 100.Mihara M, Takagi N, Takeda Y, Ohsugi Y. IL-6 receptor blockage inhibits the onset of autoimmune kidney disease in NZB/W F1 mice. Clinical and experimental immunology. 1998;112:397–402. doi: 10.1046/j.1365-2249.1998.00612.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Alonzi T, Fattori E, Lazzaro D, Costa P, Probert L, Kollias G, et al. Interleukin 6 is required for the development of collagen-induced arthritis. The Journal of experimental medicine. 1998;187:461–8. doi: 10.1084/jem.187.4.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Tsantikos E, Maxwell MJ, Putoczki T, Ernst M, Rose-John S, Tarlinton DM, et al. Interleukin-6 trans-signaling exacerbates inflammation and renal pathology in lupus-prone mice. Arthritis and rheumatism. 2013;65:2691–702. doi: 10.1002/art.38061. [DOI] [PubMed] [Google Scholar]
- 103.Ohshima S, Saeki Y, Mima T, Sasai M, Nishioka K, Nomura S, et al. Interleukin 6 plays a key role in the development of antigen-induced arthritis. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:8222–6. doi: 10.1073/pnas.95.14.8222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Nowell MA, Richards PJ, Horiuchi S, Yamamoto N, Rose-John S, Topley N, et al. Soluble IL-6 receptor governs IL-6 activity in experimental arthritis: blockade of arthritis severity by soluble glycoprotein 130. Journal of immunology. 2003;171:3202–9. doi: 10.4049/jimmunol.171.6.3202. [DOI] [PubMed] [Google Scholar]
- 105.Quintana A, Muller M, Frausto RF, Ramos R, Getts DR, Sanz E, et al. Site-specific production of IL-6 in the central nervous system retargets and enhances the inflammatory response in experimental autoimmune encephalomyelitis. Journal of immunology. 2009;183:2079–88. doi: 10.4049/jimmunol.0900242. [DOI] [PubMed] [Google Scholar]
- 106.Wollert KC, Taga T, Saito M, Narazaki M, Kishimoto T, Glembotski CC, et al. Cardiotrophin-1 activates a distinct form of cardiac muscle cell hypertrophy. Assembly of sarcomeric units in series VIA gp130/leukemia inhibitory factor receptor-dependent pathways. The Journal of biological chemistry. 1996;271:9535–45. doi: 10.1074/jbc.271.16.9535. [DOI] [PubMed] [Google Scholar]
- 107.Terrell AM, Crisostomo PR, Wairiuko GM, Wang M, Morrell ED, Meldrum DR. Jak/STAT/SOCS signaling circuits and associated cytokine-mediated inflammation and hypertrophy in the heart. Shock. 2006;26:226–34. doi: 10.1097/01.shk.0000226341.32786.b9. [DOI] [PubMed] [Google Scholar]
- 108.Yang S, Zheng R, Hu S, Ma Y, Choudhry MA, Messina JL, et al. Mechanism of cardiac depression after trauma-hemorrhage: increased cardiomyocyte IL-6 and effect of sex steroids on IL-6 regulation and cardiac function. American journal of physiology Heart and circulatory physiology. 2004;287:H2183–91. doi: 10.1152/ajpheart.00624.2003. [DOI] [PubMed] [Google Scholar]
- 109.Wollert KC, Drexler H. The role of interleukin-6 in the failing heart. Heart failure reviews. 2001;6:95–103. doi: 10.1023/a:1011401825680. [DOI] [PubMed] [Google Scholar]
- 110.Yamauchi-Takihara K, Kishimoto T. Cytokines and their receptors in cardiovascular diseases--role of gp130 signalling pathway in cardiac myocyte growth and maintenance. International journal of experimental pathology. 2000;81:1–16. doi: 10.1046/j.1365-2613.2000.00139.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Prabhu SD. Cytokine-induced modulation of cardiac function. Circulation research. 2004;95:1140–53. doi: 10.1161/01.RES.0000150734.79804.92. [DOI] [PubMed] [Google Scholar]
- 112.Yu X, Kennedy RH, Liu SJ. JAK2/STAT3, not ERK1/2, mediates interleukin-6-induced activation of inducible nitric-oxide synthase and decrease in contractility of adult ventricular myocytes. The Journal of biological chemistry. 2003;278:16304–9. doi: 10.1074/jbc.M212321200. [DOI] [PubMed] [Google Scholar]
- 113.Florholmen G, Thoresen GH, Rustan AC, Jensen J, Christensen G, Aas V. Leukaemia inhibitory factor stimulates glucose transport in isolated cardiomyocytes and induces insulin resistance after chronic exposure. Diabetologia. 2006;49:724–31. doi: 10.1007/s00125-006-0150-6. [DOI] [PubMed] [Google Scholar]
- 114.Kukielka GL, Smith CW, Manning AM, Youker KA, Michael LH, Entman ML. Induction of interleukin-6 synthesis in the myocardium. Potential role in postreperfusion inflammatory injury. Circulation. 1995;92:1866–75. doi: 10.1161/01.cir.92.7.1866. [DOI] [PubMed] [Google Scholar]
- 115.Gwechenberger M, Mendoza LH, Youker KA, Frangogiannis NG, Smith CW, Michael LH, et al. Cardiac myocytes produce interleukin-6 in culture and in viable border zone of reperfused infarctions. Circulation. 1999;99:546–51. doi: 10.1161/01.cir.99.4.546. [DOI] [PubMed] [Google Scholar]
- 116.Chandrasekar B, Mitchell DH, Colston JT, Freeman GL. Regulation of CCAAT/Enhancer binding protein, interleukin-6, interleukin-6 receptor, and gp130 expression during myocardial ischemia/reperfusion. Circulation. 1999;99:427–33. doi: 10.1161/01.cir.99.3.427. [DOI] [PubMed] [Google Scholar]
- 117.Yamauchi-Takihara K, Ihara Y, Ogata A, Yoshizaki K, Azuma J, Kishimoto T. Hypoxic stress induces cardiac myocyte-derived interleukin-6. Circulation. 1995;91:1520–4. doi: 10.1161/01.cir.91.5.1520. [DOI] [PubMed] [Google Scholar]
- 118.Frangogiannis NG, Smith CW, Entman ML. The inflammatory response in myocardial infarction. Cardiovascular research. 2002;53:31–47. doi: 10.1016/s0008-6363(01)00434-5. [DOI] [PubMed] [Google Scholar]
- 119.Frangogiannis NG. Targeting the inflammatory response in healing myocardial infarcts. Current medicinal chemistry. 2006;13:1877–93. doi: 10.2174/092986706777585086. [DOI] [PubMed] [Google Scholar]
- 120.Ono K, Matsumori A, Shioi T, Furukawa Y, Sasayama S. Cytokine gene expression after myocardial infarction in rat hearts: possible implication in left ventricular remodeling. Circulation. 1998;98:149–56. doi: 10.1161/01.cir.98.2.149. [DOI] [PubMed] [Google Scholar]
- 121.Yasukawa H, Hoshijima M, Gu Y, Nakamura T, Pradervand S, Hanada T, et al. Suppressor of cytokine signaling-3 is a biomechanical stress-inducible gene that suppresses gp130-mediated cardiac myocyte hypertrophy and survival pathways. The Journal of clinical investigation. 2001;108:1459–67. doi: 10.1172/JCI13939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hilfiker-Kleiner D, Shukla P, Klein G, Schaefer A, Stapel B, Hoch M, et al. Continuous glycoprotein-130-mediated signal transducer and activator of transcription-3 activation promotes inflammation, left ventricular rupture, and adverse outcome in subacute myocardial infarction. Circulation. 2010;122:145–55. doi: 10.1161/CIRCULATIONAHA.109.933127. [DOI] [PubMed] [Google Scholar]
- 123.Hirota H, Yoshida K, Kishimoto T, Taga T. Continuous activation of gp130, a signal-transducing receptor component for interleukin 6-related cytokines, causes myocardial hypertrophy in mice. Proceedings of the National Academy of Sciences of the United States of America. 1995;92:4862–6. doi: 10.1073/pnas.92.11.4862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kunisada K, Negoro S, Tone E, Funamoto M, Osugi T, Yamada S, et al. Signal transducer and activator of transcription 3 in the heart transduces not only a hypertrophic signal but a protective signal against doxorubicin-induced cardiomyopathy. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:315–9. doi: 10.1073/pnas.97.1.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Hirota H, Chen J, Betz UA, Rajewsky K, Gu Y, Ross J, Jr, et al. Loss of a gp130 cardiac muscle cell survival pathway is a critical event in the onset of heart failure during biomechanical stress. Cell. 1999;97:189–98. doi: 10.1016/s0092-8674(00)80729-1. [DOI] [PubMed] [Google Scholar]
- 126.Lopez N, Varo N, Diez J, Fortuno MA. Loss of myocardial LIF receptor in experimental heart failure reduces cardiotrophin-1 cytoprotection. A role for neurohumoral agonists? Cardiovascular research. 2007;75:536–45. doi: 10.1016/j.cardiores.2007.04.025. [DOI] [PubMed] [Google Scholar]
- 127.Cihakova D, Rose NR. Pathogenesis of myocarditis and dilated cardiomyopathy. Advances in immunology. 2008;99:95–114. doi: 10.1016/S0065-2776(08)00604-4. [DOI] [PubMed] [Google Scholar]
- 128.Hirota H, Izumi M, Hamaguchi T, Sugiyama S, Murakami E, Kunisada K, et al. Circulating interleukin-6 family cytokines and their receptors in patients with congestive heart failure. Heart and vessels. 2004;19:237–41. doi: 10.1007/s00380-004-0770-z. [DOI] [PubMed] [Google Scholar]
- 129.Kubota T, Miyagishima M, Alvarez RJ, Kormos R, Rosenblum WD, Demetris AJ, et al. Expression of proinflammatory cytokines in the failing human heart: comparison of recent-onset and end-stage congestive heart failure. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2000;19:819–24. doi: 10.1016/s1053-2498(00)00173-x. [DOI] [PubMed] [Google Scholar]
- 130.Plenz G, Song ZF, Reichenberg S, Tjan TD, Robenek H, Deng MC. Left-ventricular expression of interleukin-6 messenger-RNA higher in idiopathic dilated than in ischemic cardiomyopathy. The Thoracic and cardiovascular surgeon. 1998;46:213–6. doi: 10.1055/s-2007-1010227. [DOI] [PubMed] [Google Scholar]
- 131.Plenz G, Song ZF, Tjan TD, Koenig C, Baba HA, Erren M, et al. Activation of the cardiac interleukin-6 system in advanced heart failure. European journal of heart failure. 2001;3:415–21. doi: 10.1016/s1388-9842(01)00137-4. [DOI] [PubMed] [Google Scholar]
- 132.Birks EJ, Latif N, Owen V, Bowles C, Felkin LE, Mullen AJ, et al. Quantitative myocardial cytokine expression and activation of the apoptotic pathway in patients who require left ventricular assist devices. Circulation. 2001;104:I233–40. doi: 10.1161/hc37t1.094872. [DOI] [PubMed] [Google Scholar]
- 133.LeBlanc RA, Pesnicak L, Cabral ES, Godleski M, Straus SE. Lack of interleukin-6 (IL-6) enhances susceptibility to infection but does not alter latency or reactivation of herpes simplex virus type 1 in IL-6 knockout mice. Journal of virology. 1999;73:8145–51. doi: 10.1128/jvi.73.10.8145-8151.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Estrada-Villasenor E, Morales-Montor J, Rodriguez-Dorantes M, Ramos-Martinez E, Nequiz-Avendano M, Ostoa-Saloma P. IL-6 KO mice develop experimental amoebic liver infection with eosinophilia. The Journal of parasitology. 2007;93:1424–8. doi: 10.1645/GE-1223.1. [DOI] [PubMed] [Google Scholar]