Abstract
The blood-brain barrier (BBB) remains one of the most significant limitations to treatments of central nervous system (CNS) disorders including brain tumors, neurodegenerative diseases and psychiatric disorders. It is now well-established that focused ultrasound (FUS) in conjunction with contrast agent microbubbles may be used to non-invasively and temporarily disrupt the BBB, allowing localized delivery of systemically administered therapeutic agents as large as 100 nm in size to the CNS. Importantly, recent technological advances now permit FUS application through the intact human skull, obviating the need for invasive and risky surgical procedures. When used in combination with magnetic resonance imaging, FUS may be applied precisely to pre-selected CNS targets. Indeed, FUS devices capable of sub-millimeter precision are currently in several clinical trials. FUS mediated BBB disruption has the potential to fundamentally change how CNS diseases are treated, unlocking potential for combinatorial treatments with nanotechnology, markedly increasing the efficacy of existing therapeutics that otherwise do not cross the BBB effectively, and permitting safe repeated treatments. This article comprehensively reviews recent studies on the targeted delivery of therapeutics into the CNS with FUS and offers perspectives on the future of this technology.
Keywords: Focused ultrasound, blood-brain barrier, CNS drug delivery, nanoparticles
Graphical abstract
1. Introduction
Many diseases of the central nervous system (CNS) present tremendous challenges for clinicians. Both primary and metastatic brain tumors carry dismal survival rates [1,2], and the increasing age of the population in the developed world has created a dramatic increase [3] in the number of people living with age-related neurodegenerative diseases like dementia, Alzheimer’s and Parkinson’s. Additionally, nearly 20% of the adult population [4] experiences the debilitating effects of a mental illness like obsessive-compulsive disorder or clinical depression each year, generating over $44 billion in lost productivity in the US alone [5]. The commonality in this wide range of CNS disorders is the inherent difficulty of treatment. The blood-brain barrier (BBB) provides excellent protection for the body’s most privileged organ, preventing the vast majority of molecules in circulation from entering brain tissue. However, because of this, the BBB also presents a significant challenge for CNS treatments, as systemic therapies are rarely capable of crossing the BBB. Recently, the ability of focused ultrasound (FUS) in conjunction with microbubbles (MBs) to facilitate the noninvasive, localized, and reversible opening of the BBB has led to the emergence of this technology as a viable new option for delivering therapeutics to the CNS. Here, we review recent studies on FUS-mediated delivery of drugs and genes into the CNS. For convenience, we have included a table of key references (Table 1).
Table 1.
Key references demonstrating drug or gene delivery with focused ultrasound.
| Disease Application |
Author, Year |
Animal Model | Substance Delivered |
Vehicle | Ref. |
|---|---|---|---|---|---|
| Generalized | Kinoshita 2006 | Swiss-Webster mice |
Dopamine D4 receptor-targeting antibody |
Unencapsulated | [115] |
| Generalized | Burgess 2012 |
Wistar Rats | siRNA oligonucleotide |
Unencapsulated | [210] |
| Generalized | Huang 2012 | Kunming Mice | BDNF-eGFP Plasmid |
Cationic MBs | [163] |
| Generalized | Huang 2012 | Kunming Mice | CMV-EGFP Plasmid |
Cationic MBs | [162] |
| Generalized | Thevenot 2012 |
C56BL/6 Mice | CB-GFP Gene | scAAV9 | [161] |
| Generalized | Alonso 2013 | Wistar Rats | CMV-nlsLacZ Gene |
AAV2/1 | [170] |
| Generalized | Hsu 2013 | Mice | CMV-GFP Gene | AAV2 | [171] |
| Generalized | Nance 2014 | Sprague-Dawley Rats |
Polystyrene Tracer | PEGylated NPs | [211] |
| Generalized | Wang 2014 | Mice | Synapsin-eGFP Gene |
AAV1 and AAV2 | [169] |
| Generalized | Weber- Adrian 2015 |
Wistar Rats | CB-GFP Gene | scAAV9 | [168] |
| Alzheimer’s | Raymond 2008 |
APPswe:PSEN1 dE9 and PDAPP Mice |
Anti-Aβ antibody | Unencapsulated | [116] |
| Alzheimer’s | Jordão 2010 | TgCRND8 Mice | Anti-Aβ antibody | Unencapsulated | [86] |
| Alzheimer’s | Jordão 2013 | TgCRND8 Mice | Endogenous IgG | Unencapsulated | [68] |
| Alzheimer’s | Leinenga 2015 |
APP23 Mice | NA | Unencapsulated | [72] |
| Brain Metastasis |
Kinoshita 2006 |
Swiss-Webster Mice |
Herceptin | Unencapsulated | [117] |
| GBM | Ting 2012 | C6 glioma in Sprague-Dawley Rats |
BCNU | MBs | [138] |
| GBM | Treat 2012 | 9L gliosarcoma in Sprague- Dawley Rats |
Doxorubicin | PEGylated liposomes |
[132] |
| GBM | Yang 2012 | GBM8401 in NOD-scid Mice |
Doxorubicin | PEGylated liposomes, AP-1 targeted, 111In- labeled |
[133] |
| GBM | Yang 2012 | GBM8401 in NOD-scid Mice |
Doxorubicin | PEGylated liposomes, AP-1 targeted |
[90] |
| GBM | Alkins 2013 | 9L glioma in Fischer 344 Rats |
BPA-f | Unencapsulated | [91] |
| GBM | Aryal 2013 | 9L gliosarcoma in Sprague- Dawley Rats |
Doxorubicin | PEGylated liposomes |
[83] |
| GBM | Fan 2013 | C6 glioma in Sprague-Dawley Rats |
Doxorubicin | SPIO-conjugated MBs |
[139] |
| GBM | Fan 2013 | C6 glioma in Sprague-Dawley Rats |
BCNU | VEGF-targeted MBs | [89] |
| GBM | Kovacs 2014 | GL261 or SMA- 560 glioma in Mice |
Doxorubicin | Unencapsulated | [105] |
| GBM | Yang 2014 | F98 glioma in Fischer 344 Rats |
BPA-f | Unencapsulated | [111] |
| GBM | Aryal 2015 | 9L gliosarcoma in Sprague- Dawley Rats |
Doxorubicin | PEGylated liposomes |
[88] |
| GBM | Aryal 2015 | 9L gliosarcoma in Sprague- Dawley Rats |
Doxorubicin | PEGylated liposomes |
[134] |
| GBM | Chen 2015 | C6 glioma in Sprague-Dawley Rats |
IL-12 | Unencapsulated | [126] |
1.1 The Blood-Brain Barrier
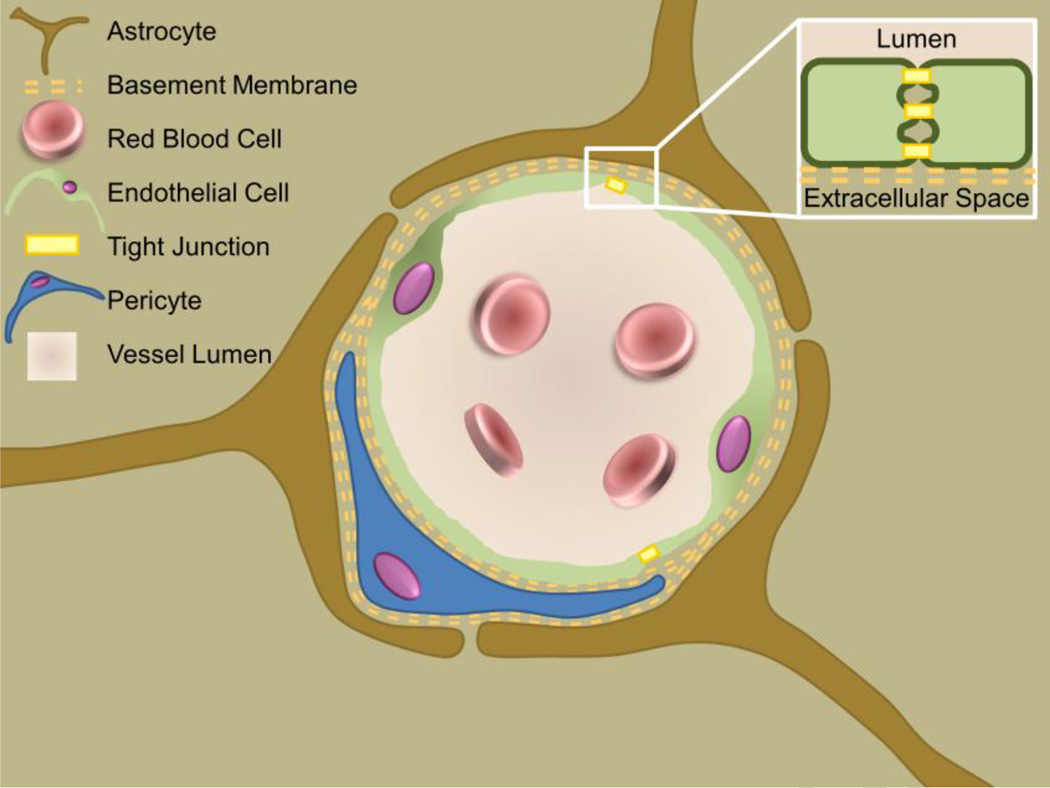
The BBB provides a formidable obstacle for drug delivery in the brain (Figure 1). Through a unique combination of transmembrane proteins and tightly regulated channels not seen elsewhere in the body, the BBB prevents nearly 100% of large molecule (>500 Da) drugs, including recombinant proteins and antibodies, and 98% of small molecule drugs, from passing into the brain [6]. Lipid soluble small molecule drugs may cross the BBB if they are capable of diffusing through the endothelial cell membrane itself [6], but few drugs fall into this category. The BBB’s remarkable exclusionary capability is attributed to tight junctions that join the endothelial cells lining the vasculature throughout the brain [7]. Tight junctions are comprised of several proteins, including various claudins, occludins, junctional adhesion molecules, and cadherins, which function to prevent molecules from passively diffusing between cells and out of the vasculature [7]. Rather, small molecules must pass through the endothelial cells themselves, either through diffusion, for lipid soluble molecules, or active transport, for most nutrients and other substances necessary for normal brain function [7]. Furthermore, if a certain molecule does manage to pass through the endothelial cell layer, the basement membrane provides an additional barrier to diffusion. Simply stated, nature’s best defense against infection significantly hinders our ability to treat diseases of the CNS by preventing drug delivery to the brain.
Figure 1.
Blood-Brain Barrier Biology. The blood-brain barrier presents a major obstacle to therapeutic delivery in the central nervous system. It is comprised of unusually abundant and structurally unique tight junctions between the vascular endothelial cells and a thick basement membrane. Regulation via astrocytes and pericytes maintain this barrier, preventing the passage of the vast majority of therapeutics.
1.2 Conventional Approaches for Bypassing the Blood-Brain Barrier
Given the central role of the BBB in limiting drug and gene delivery to the CNS, numerous methods have been developed to bypass this barrier. For example, specific viruses or nanoparticles (NPs) with BBB-targeting ligands can cross the BBB after systemic administration[8]. However, in order to achieve effective concentrations in the brain, they must be administered in doses which are associated with adverse effects in peripheral organs[9]. For this reason, the majority of preclinical and clinical studies have used direct intracranial administration as a strategy to locally increase therapeutic concentration without off-target effects. Specific brain regions can be accessed with needles or catheters and more recent strategies have utilized fluid convection to enhance distribution of therapeutics in the brain[10]. By maintaining bulk flow with hydrostatic pressure differentials, convection enhanced delivery has demonstrated marked improvement over conventional direct intracranial injection methodologies[11,12]. Unfortunately, despite promising results for direct injection in several preclinical and clinical trials[13–17], these strategies are risky and surgical complications have hindered widespread adoption. Furthermore, macromolecular agents require long dissemination times and typically cannot spread beyond a few millimeters[11]. Indeed, the invasive nature of strategies like intracranial injections is not compatible with drugs that need to be dosed repeatedly.
In order to reduce risks associated with direct injection, less-invasive strategies to enhance therapeutic delivery across the BBB have been developed. These include intranasal administration and chemical disruption of the BBB by intra-arterial infusion of the osmotic agent mannitol[18] or vasodilators[19–21]. Intranasal administration permits transport to the brain through perineural or perivascular channels[22]. While intranasal drug delivery is non-invasive and obviates peripheral side effects associated with intravenous administration, it is limited by poor absorption across the nasal epithelium, inconsistent delivery efficiency and poor localization[22,23]. Similarly, mannitol infusions lead to global BBB disruption, causing non-specific uptake and potentiating adverse off-target effects. Infusion of mannitol into the carotid artery leads to an osmotic-driven movement of fluid out of endothelial cells[24], shrinking them and leading to fenestration of cerebral vessels. While disruption of the BBB with mannitol is reasonably safe, therapeutic delivery is inconsistent with up to 10-fold variations in drug concentrations[25].
1.3 Opening the Blood-Brain Barrier with Focused Ultrasound
FUS has the advantage of being the only modality capable of achieving non-invasive, safe, repeated, and targeted BBB disruption to enhance drug or gene delivery to the CNS. With the advent of MR-compatible transducers with sub-millimeter precision, it is now possible to apply image-guided transcranial FUS to the human brain [26–29] in an extremely localized manner, greatly reducing the risk of off-target effects. FUS treatments can be performed on awake patients, eliminating the need for general anesthetic and permitting real-time patient feedback. In the future, FUS may be capable of replacing traditional surgical techniques, eliminating invasive procedures and greatly increasing the feasibility of repeated treatments. Importantly, MR and integrated passive cavitation detection (PCD) facilitate real-time intraoperative treatment monitoring, while post-treatment MR imaging allows confirmation of treatment success[30–33] and safety[34,35]. The development of transcranial FUS has been a long process. Groundbreaking research by the Fry brothers performed over 50 years ago demonstrated that ultrasound could produce bioeffects in the human brain[36]. However, it wasn’t until recent technological advances were made in both ultrasound and MRI that the field experienced a surge in interest. In the past ten years, there has been an increase in the number of papers investigating the potential applications of ultrasound in the brain.
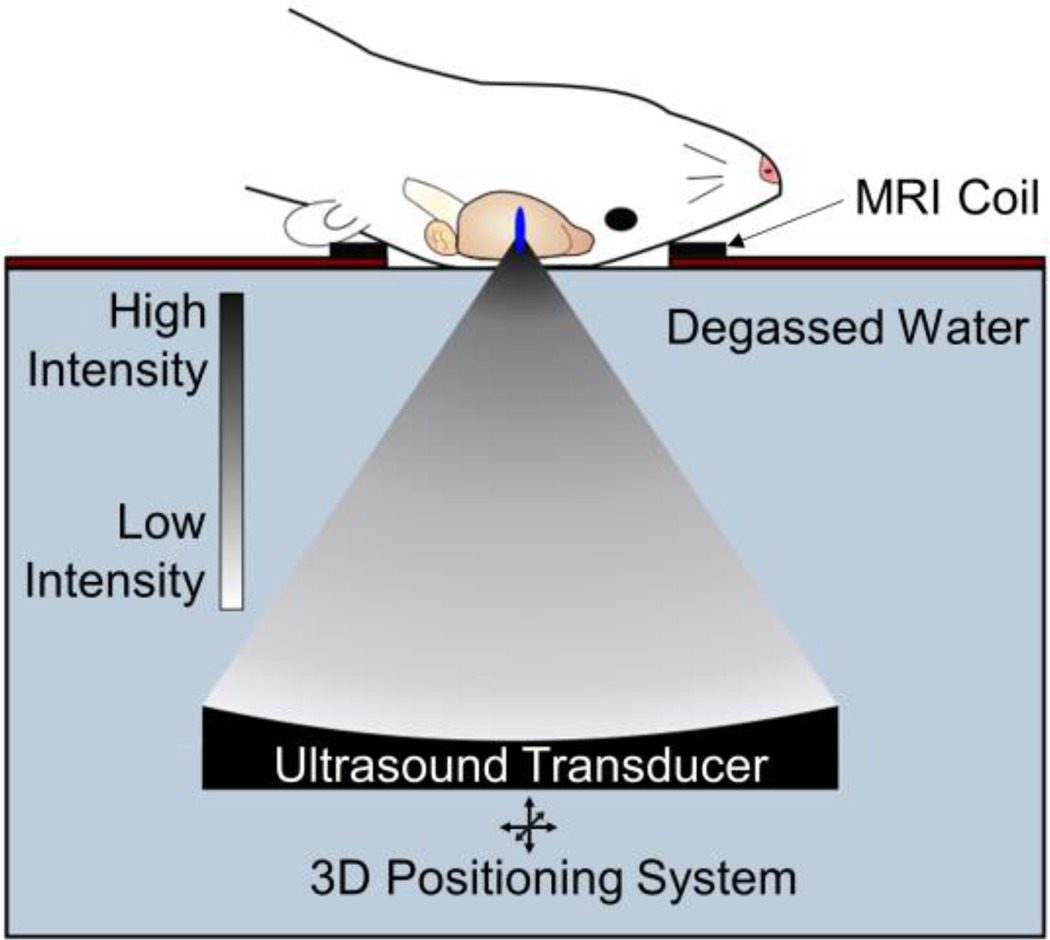
Ultrasound is, at its most basic, a pressure wave. As the wave passes through the tissue, the tissue experiences alternating periods of high pressure (compression) and low pressure (rarefaction). Ultrasound can be applied in a continuous fashion, common in treatments that require heat deposition, or in a pulsed manner, which is utilized for blood-brain barrier disruption (BBBD). Focusing the ultrasound beam (i.e. FUS) provides high spatial accuracy (less than 1 mm resolution in some cases) and localizes bioeffects. However, reflection and diffraction of the ultrasound wave at material interfaces (i.e. skull-tissue interface) can distort the focus and decrease the energy delivered at the target. While the favorable skull geometry of rats or mice allows the use of single-element transducers in pre-clinical trials (Figure 2), the far more complex topography of the human skull requires the use of a multi-element array with phase-correction software to re-focus the ultrasound beam as it passes through the skull. There are many combinations of FUS parameters (frequency, pressure, pulsing protocol) suitable for BBBD, but lower frequencies (≤1.0 MHz) experience less attenuation and distortion by the skull.
Figure 2.
Transcranial FUS with microbubbles is the only modality capable of achieving non-invasive, safe, repeated and targeted BBB disruption, leading to improved drug or gene delivery to the brain. Pre-clinical FUS studies in animals including mice and rats permit use of a single-element FUS transducer, due to favorable skull geometry. FUS can be guided with MR imaging and is capable of sub-millimeter resolution allowing precise targeting of structures in the CNS with minimal off-target effects.
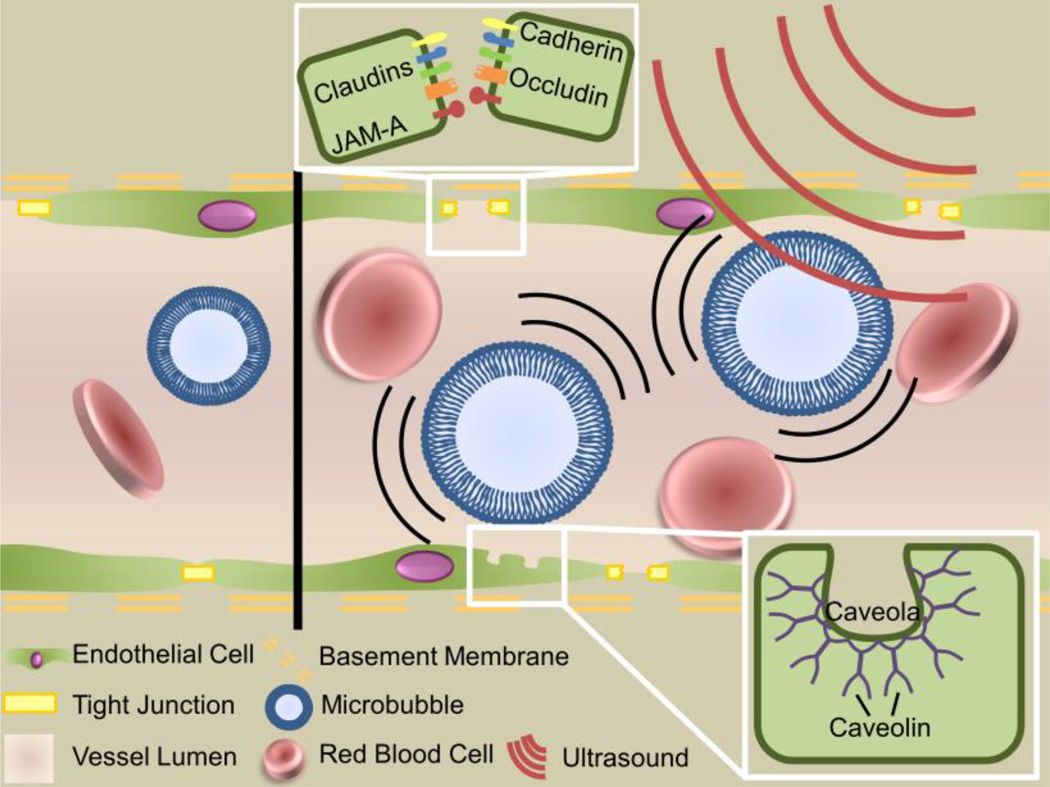
Transcranial FUS is typically applied in conjunction with intravenously administered MBs to effect BBBD. MBs are small (1–10 µm) lipid or protein shelled bubbles filled with an inert gas, most commonly a perfluorocarbon and are FDA-approved as a contrast agent during ultrasound imaging. Importantly, circulating MBs reduce the acoustic energy required to open the BBB by two orders of magnitude and confine mechanical effects to the vasculature[37]. This permits the use of low pressure FUS and virtually eliminates any concerns about skull heating during treatment. Extended off-time (low duty cycle) between FUS pulses allows MB reperfusion and thermal dissipation at the focus. At the lower ultrasonic pressures used for BBBD, MBs oscillate stably in the FUS field, expanding during rarefaction and contracting during compression, producing mechanical shear forces[38] and microstreaming[39] effects which act on the vessel wall. This behavior domain, called stable oscillation, is preferred for BBBD as its effects are more predictable. In contrast, at higher acoustic pressures, MBs experience unstable oscillations and eventually collapse inward, producing elevated local temperatures and high-pressure jet streams in a process termed inertial cavitation. While FUS-MB induced BBBD occurs in both regimes, inertial cavitation is more violent and is generally avoided for applications in healthy brain tissue. However, it may find use in diseased tissue, or for the delivery of very large (~100 nm) therapeutics, when the potential benefits outweigh the risks.
A collection of in vitro and ex vivo work[40–42] has demonstrated that the mechanical forces exerted by stably oscillating MBs cause vessel distension and invagination, as well as changes in the endothelial cells’ cytoskeletons and cell-cell interactions (Figure 3). Together, these effects produce BBBD via three mechanisms: disruption of tight junctions, induction of transcytosis, and sonoporation of the vascular endothelium. Work using transmission electron microscopy imaging[43] has demonstrated both a reduction and altered distribution of claudins 1 and 5, occludin, and ZO-1 after FUS exposure. Most notably, tight junction proteins were no longer clustered along the edges of endothelial cells, suggesting that they were no longer contributing to tight junction complexes. Furthermore, penetration of horse radish peroxidase between endothelial cells was evident, demonstrating that tight junctions were no longer sealing paracellular pathways from the vasculature to the brain parenchyma. In addition to this paracellular pathway, horseradish peroxidase was also taken up by the vascular endothelial cells after sonication. Later work[44–46] demonstrated increased expression of caveolin-1 in the vascular endothelium after sonication, identifying caveoli as the most likely transcytotic pathway. A unique approach using two-photon microscopy provided further support for both paracellular and transcellular pathways. Here, it was noted that dextrans crossed the BBB after FUS via either a fast or slow mechanism (i.e. less/greater than 10 min) and postulated that the fast and slow pathways were most likely paracellular (tight junction disruption) and transcellular (increased transcytosis and sonoporation), respectively[47]. Interestingly, 70kDa dextrans appeared to have a higher pressure threshold for BBB crossing than smaller 10kDa dextrans, in agreement with other studies indicating that the extent of BBBD (particularly the size of junctional clefts) is related to pressure[48]. Furthermore, smaller vessels (i.e. less than ~25–30 µm) were significantly more likely to be disrupted by FUS[49] than larger vessels, and fast leakage[47] (i.e. paracellular) was the dominant mode of transport in these vessels. This difference is attributed to the interactions between MBs and the vessel wall – in smaller vessels, oscillating MBs are more likely to come in close contact with the vessel wall, generating larger circumferential stresses than in larger vessels. Going forward, achieving a better understanding of the dynamics of these transport pathways will be critical for enabling more predictable BBBD, especially with the increased use of larger therapeutics such as antibodies, liposomes and polymer nanoparticles.
Figure 3.
Mechanism of focused ultrasound mediated blood-brain barrier disruption. Circulating microbubbles oscillate in the ultrasonic field, producing forces that act on the vessel wall to generate three bioeffects that permit transport across the blood-brain barrier: disruption of tight juctions, sonoporation of the vascular endothelial cells and upregulation of transcytosis.
1.4 Safety and Monitoring of Blood-Brain Barrier Disruption
While it is well known that driving MBs into inertial cavitation with high acoustic pressures can lead to irreversible capillary damage and the leakage of red blood cells across the BBB [50], thresholds have been established wherein BBBD can be achieved without toxicity or damage. BBBD is transient and, depending on acoustic pressure[51], barrier function is typically restored within 4–6 hours after treatment (Figure 4A,B) [43,52,53]. Importantly, no motor or acuity deficits were found after repeated BBBD procedures with FUS at numerous targets in monkeys[54,55]. Interestingly, some of these monkeys had T2* hypointensities in post-FUS MR imaging, indicating minor red blood cell accumulation; however, these minor capillary leakages did not lead to any changes in visual acuity or motor skills. Furthermore, it is important to emphasize that, even in the rare occurrence of erythrocyte extravasation at the lower pressures used in these studies, no apoptotic bodies were found and cognitive function of the animals was not impaired[54]. These findings are in agreement with other studies suggesting that minor capillary damage and red blood cell extravasation is not expected to lead to long term effects[32,56]. Indeed, it is possible that such damage would be acceptable in treatment of debilitating or life-threatening neurological diseases. It is important to further emphasize that FUS-related safety issues would be minor compared to those of other treatment strategies like intracranial injection, which can lead to extensive damage along the needle tract, or even non-invasive treatments like gamma knife radiosurgery[57,58].
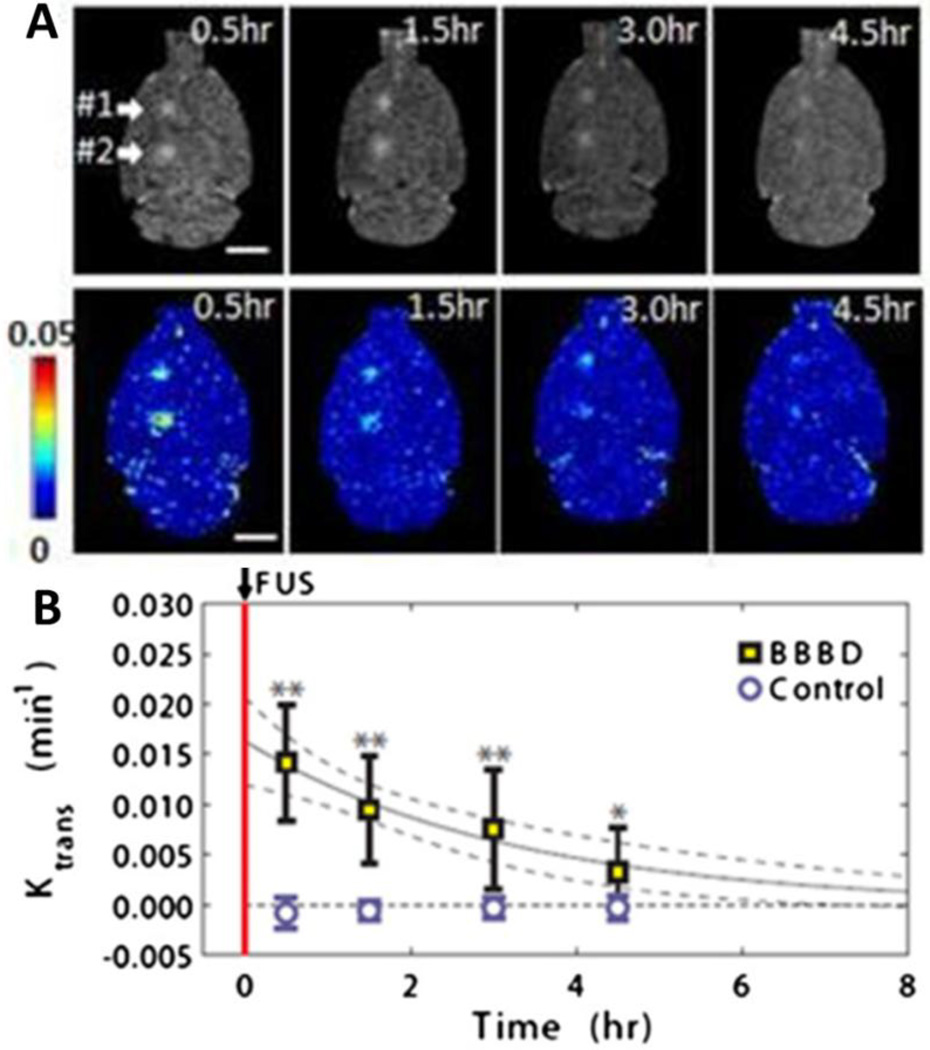
Figure 4.
Transcranial FUS leads to temporary and localized BBBD with no long term damage. A. Representative transverse contrast enhanced T1 MRI (top) and permeability maps (bottom) obtained at four time points after FUS-mediated BBBD. Arrows indicate the two FUS-treated regions. Ktrans values indicated by the color bar. B. Mean Ktrans values over time in FUS treated regions. Control indicates contralateral hemisphere at same anatomical location. Reprinted from Journal of Controlled Release, 162, J. Park, Y. Zhang, N. Vykhodtseva, F. a Jolesz, N.J. McDannold, The kinetics of blood brain barrier permeability and targeted doxorubicin delivery into brain induced by focused ultrasound, 134–42, (2012) with permission from Elsevier.
While FUS-mediated BBBD has been shown to be safe in numerous animal models, intraoperative monitoring with passive cavitation detection or MR imaging further reduces chances of aberrant FUS treatments. Passive cavitation detection (PCD) allows real-time assessment of MB cavitation[59]. While stable cavitation is most likely responsible for reversible BBBD[50,60], inertial cavitation has been linked with tissue damage[61]. Importantly, acquired PCD intensity is well correlated with BBBD[50,60]. PCD non-invasively detects the acoustic signatures resulting from MB oscillations, and can distinguish between stable oscillations and MB collapse.[62]. Stably oscillating MBs emit harmonic, subharmonic or ultraharmonic frequency acoustic emissions, whereas collapsing MBs emit broadband acoustic signals[63,64]. PCD has been used to ensure safe FUS settings in several large animal BBBD models[54,65] and systems are currently in development that will allow fully automatic feedback to control FUS sonications[66]. Once the FUS treatment is complete, MR imaging sequences including T2* and susceptibility weighted imaging (SWI) are sensitive tools that can be used to detect blood products present in tissue[34,52,67] and have been shown to be sensitive to measure even minor capillary damage[54].
1.5 Therapeutic Bioeffects of Focused Ultrasound
This review is focused on the delivery of drugs and genes across the BBB with FUS; however, it is also important to note that recent advances in our understanding of biological responses to FUS have potentiated novel approaches to treatments of brain disease, even without administration of pharmacological agents. For example, BBBD with FUS alone in a mouse model of Alzheimer’s disease led to a significant reduction of plaque burden four days after a single treatment[68]. The authors showed that plaque reduction was linked to significant enhancement of endogenous antibodies bound to the Aβ plaque as well as to activation of microglia and astrocytes in the FUS-treated region. Additionally, five successive treatments with ultrasound were shown to lead to further plaque clearance and improved subject performance on several memory tasks[69]. While these inflammatory responses bear further investigating, safety studies on repeated FUS treatments in primates have shown no deleterious effects [70] and a localized immune response may be beneficial in some applications. Non-thermal FUS has been used in other disease models to enhance the body’s anti-tumor immune response with great success [71], and it has been postulated that FUS-mediated BBBD may provide a similar benefit in brain tumors by increasing endogenous antibody delivery and reducing barriers to immune cell migration in the brain parenchyma [68,72]. In addition to alterations in immune function and cell behavior within the brain, FUS-mediated BBBD has been shown to enhance neurogenesis[73], which is attributed to the demonstrated upregulation of BDNF[74] and Akt [75] after FUS. This observation has led to suggestions that FUS could be used as a non-invasive alternative to deep brain stimulation for treatment of depression[76]. Importantly, low intensity FUS has also shown ability to transiently stimulate neurons in both animals[74,77–80] and humans[81,82], and has the ability to elicit acute sensory responses in the fingers and hands[82], potentially allowing non-invasive brain mapping. Ultimately, we believe that FUS will permit several therapeutic options in the CNS, beyond those dependent upon drug and/or gene delivery across the BBB.
2. Drug Delivery
FUS-mediated BBBD permits the delivery of a wide range of therapeutics, and improves the efficacy and safety profile of the few drugs which can cross the BBB by reducing the required systemic dose. Furthermore, in disease states with existing BBB impairment, FUS is capable of increasing delivery and improving drug distribution by producing homogeneous, targeted BBB disruption[83]. FUS has demonstrated remarkable ability to deliver a wide range of payloads, including small molecule drugs[79,85], ~150 kDa antibodies[86], recombinant proteins[87] and even ~100 nm liposomal drug vehicles[88,83]. As FUS technology has improved over the last decade, work has progressed from the delivery of free small molecule drugs [79,85] such as temozolomide to larger plaque-binding antibodies [86] and ~100 nm liposomal drug vehicles [88,83]. In addition, functionalized MBs [89], targeting moieties[90] and two-step processes like boron neutron capture therapy [91] have also been investigated in conjunction with FUS to further enhance delivery efficiency into the CNS. Here, we review work demonstrating the delivery of systemically administered small molecule-, recombinant protein- and antibody-based therapeutics in the brain using FUS-mediated BBBD.
2.1 Unencapsulated Drug
Currently, the most common application for FUS-mediated delivery of unencapsulated drugs is glioblastoma multiforme (GBM). While the tumor core can be resected, GBM is notorious for tumor recurrence due to the infiltrating tumor cells outside of the tumor core. Indeed, these regions are refractory to traditional systemic therapies due to the existence of an intact BBB. Thus, it has been hypothesized that the delivery of therapeutics via FUS-mediated BBBD may improve patient outcomes by providing drug delivery to these “protected” infiltrating cells [84,92]. Additionally, while the bulk of the tumor may exhibit BBB impairment, this impairment is often heterogeneous and accompanied by high interstitial pressures. FUS treatment has been shown to provide more extensive and more homogeneous drug delivery even in areas with impaired BBB function [83]. This application is particularly promising for patients with brain metastases, as many therapeutics which are efficacious against the primary tumor do not cross the BBB. FUS-mediated delivery across the BBB can significantly improve drug delivery and efficacy in the brain, and may permit a wider range of treatment options for patients with brain metastases, which are far more common than primary brain tumors.
Temozolomide (TMZ), a small molecule drug, is currently part of the recommended approach to the clinical treatment of GBM, and many clinical trials continue to test various TMZ dosing regimens as well as drug combinations [93–96]. In a rat model of GBM, BBBD in combination with orally administered medium dose TMZ significantly increased survival (ISTmedian=15% compared to controls) and controlled tumor growth as well as high dose TMZ alone [92]. A study using the U87 glioma model in mice further demonstrated that FUS treatment improves tumor growth control and survival over TMZ alone across a range of TMZ doses (ISTmedian=111% compared to control for highest dose TMZ + FUS), although the benefit is most pronounced for low dose regimens [84]. This effect appeared to be due to an increase in TMZ concentration and retention time (2.7- and 1.5-fold, respectively) in sonicated tissue [84]. These studies demonstrate that BBBD with FUS can enhance the efficacy of even “gold standard” drugs in GBM.
While able to cross the BBB, carmustine (bis-chloroethylnitrosourea, BCNU) is another small molecule chemotherapeutic drug whose effectiveness could be enhanced by improved local delivery, as it is highly toxic and degrades within 15 minutes. BCNU-loaded polifeprosan 20 Gliadel wafers were one of the first uses of biodegradable polymers for drug delivery in humans [97–100], representing a unique solution to the problems posed by BCNU. Disappointingly, Gliadel produced only mild improvements in patient survival (2.3 months compared to placebo), and is now only recommended for patients with fully resectable tumors[100]. Given these limitations, FUS-mediated delivery has been hypothesized to provide similar benefits. To this end, intravenous BCNU has been administered in conjunction with FUS-mediated BBBD, which doubled BCNU deposition in a C6 glioma model. It was shown that this combined treatment provides better tumor growth control and improved animal survival (ISTmedian=86% compared to control) [101]. This study also indicates that it may be possible to decrease the intravenous dose administered while maintaining therapeutically relevant drug concentrations in the brain, thus reducing systemic toxicity effects without the need for surgery. Ultimately, it is evident that BBBD with FUS can improve outcomes, even when used in conjunction with drugs that are able to cross the BBB, by increasing local drug concentrations and decreasing systemic toxicity.
PEGylated liposomal doxorubicin formulations are able to extravasate and collect in tumors, and they have been used in the treatment of glioma with some success (see Section 2.2). The liposomal formulation is necessary since free doxorubicin (DOX) exhibits systemic toxicity and is unable to cross the BBB [102]. FUS-mediated BBBD may facilitate the use of free DOX, generating high intratumor drug concentrations while preventing systemic toxicities associated with the liposomal formulation. While FUS was capable of delivering up to 17-fold increases in DOX concentration in healthy brain tissue, in the GL261 mouse model of GBM, treatment with FUS and free DOX increased DOX concentrations in the tumors by only 4-fold compared to contralateral controls, although this increase was significant. Animals treated with FUS + free DOX had improved survival times (ISTmedian = 68%) as well, and did not show effects of systemic drug toxicities [105]. While this work indicates that FUS can improve the delivery of free drug across the blood-tumor barrier, it is also apparent that FUS parameters may need to be optimized for tumor biology rather than healthy brain tissue.
Boron neutron capture therapy (BNCT) offers the ability to eradicate tumor cells without damaging healthy tissue, a characteristic which is particularly appealing for brain applications. It relies on the accumulation of a stable boron isotope in the tumor tissue, followed by irradiation with low-energy neutrons. The accumulated boron absorbs the neutrons and releases high energy particles, destroying the tumor cells [106]. BNCT has achieved some success in head-and-neck cancers [107], as well as GBM [108,109], but it is believed that FUS-mediated BBBD may improve BNCT efficacy by increasing the concentration of boron in the tumor tissue. Several rodent studies [91,110,111] have demonstrated that FUS significantly increases the concentration of BPA-f, a boron containing drug, in tumor tissue, as well as homogenizing distribution [91]. Nonetheless, it remains to be seen whether this increase correlates to an improvement in treatment efficacy.
Therapeutic antibodies, while currently showing promise in the treatment of numerous cancers, are too large to cross the BBB. Therefore, antibodies which have shown success against various cancers are not beneficial for patients with brain metastases [112], and antibodies designed to treat neurodegenerative diseases require a delivery system [113]. Early work indicated that FUS-mediated BBBD could be used to deliver endogenous IgG antibodies [114] as well as functionally intact D(4) receptor targeting antibody[115], opening the door for therapeutic applications. An exciting recent study in the TgCRND8 model of Alzheimer’s disease showed that FUS-mediated BBBD increased glial cell activation and the delivery of endogenous IgG and IgM antibodies, which led to a reduction in plaque load [68]. Further work in this model demonstrated the delivery of anti-Aβ across the BBB (Figure 5A), which then bound to the plaques (Figure 5B) and caused a significant 23% decrease in plaque surface area (Figure 5C). Plaque number and size were both decreased in the anti-Aβ + FUS group [86]. An earlier study in the APPswe/PSEN1dE9 Alzheimer’s model indicated that FUS-mediated BBBD produces a 3-fold increase in plaque-bound anti-Aβ compared to non-sonicated tissue [116]. These studies suggest the potential use of FUS-mediated antibody delivery for the treatment of neurodegenerative disorders. Indeed, compared to other transcranial delivery methods, FUS is particularly suited for the long term repeated treatments necessitated by the nature of these disorders due to its noninvasive application and highly localized effects.
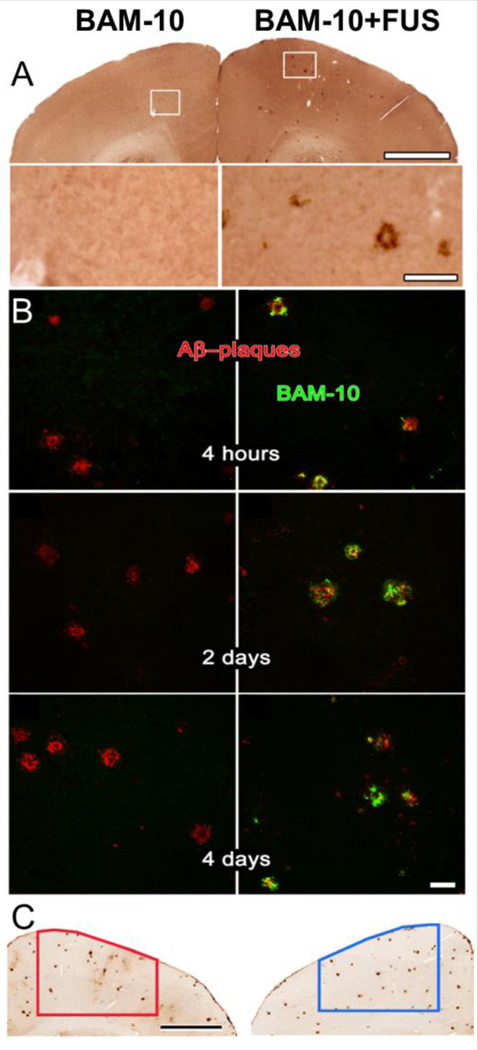
Figure 5.
FUS mediated delivery of anti-AB antibody reduces plaque load in the TgCRND8 mouse model of Alzheimer’s disease. A) Treatment with FUS increases delivery of anti-AB antibody BAM-10 in the targeted region (right) compared to the non-sonicated control (left). White boxes indicate area selected for inset. Scale bar 1mm, inset 100 µm. B) BAM-10 delivered with FUS (right column) colocalizes with plaque within 4 hrs post delivery and remains up to 4 days. Unsonicated control regions (left column) show no BAM-10 delivery. Scale bars 50 µm. C) FUS-MB enhanced delivery of BAM-10 reduces plaque load 4 days post treatment. Scale bar 1mm. Reprinted from PLoS One, 5(5), Jordão JF, Ayala-Grosso CA, Markham K, Huang Y, Chopra R, McLaurin J, Hynynen K, Aubert I, Antibodies targeted to the brain with image-guided focused ultrasound reduces amyloid-beta plaque load in the TgCRND8 mouse model of Alzheimer’s disease, e10549, (2010) with permission.
Trastuzumab (Herceptin), a monoclonal antibody which binds to the Her2 receptor, has shown promise in the treatment of breast cancer [112], which frequently metastasizes to the brain. In healthy animals, FUS-mediated BBBD significantly increased the delivery of trastuzumab in sonicated tissue with no apparent toxicity [117]. In a rat model of breast cancer brain metastasis, animals receiving FUS + trastuzumab had significantly smaller tumors (4 of 10 tumors resolved completely) with an ISTmedian of 32% compared to untreated controls. Commonly, patients with brain metastases are omitted from clinical trials, as many therapeutics which work well against the primary tumor do not cross the BBB and have no efficacy against brain metastases. FUS-mediated delivery across the BBB can significantly improve drug delivery and efficacy in the brain, and may permit a wider range of treatment options for patients with brain metastases.
Neurotrophic factor administration has been shown to ameliorate a variety of CNS disorders, including schizophrenia [118], depression [119], autism [120], and Parkinson’s [121], and FUS delivery can improve delivery and distribution of neurotrophic factors across the BBB. BDNF, which shows promise as a neuroprotective agent [121], maintains its bioactivity after FUS-mediated delivery across the BBB and generates significant downstream signaling activity[122]. Neurturin (NTN), another factor that has been identified as a potential therapy for neurodegenerative diseases [121], has also been delivered successfully [122]. FUS-mediated delivery increased NTN bioavailability by 25-fold compared to direct injection, and activation of signaling downstream of NTN indicated retention of function [87]. Nonetheless, despite the success with BDNF and NTN, glial cell-line derived neurotrophic factor (GDNF) continues to pose problems for FUS-based delivery. One study [123] demonstrated a significant increase in the delivery of GDNF in FUS-treated regions; however, another was unable to detect GDNF delivery across the BBB due to rapid breakdown in the bloodstream [122].
Immunotherapy is especially intriguing for brain tumor applications, because toxicities associated with traditional drugs pose significant problems for healthy brain tissue[124,125]. However, the presence of the BBB confounds most traditional immunotherapeutic approaches. FUS-mediated delivery of immunostimulatory interleukin-12 (IL-12) significantly increased IL-12 deposition in intracranial C6 gliomas, improved tumor growth control and increased survival (ISTmedian=43%) [126]. This effect was attributed to a significant improvement in the cytotoxic T-lymphocyte/regulatory T-cell ratio in the FUS + IL-12 group, presumably due to a combination of increased IL-12 concentration and vascular permeability, which permitted enhanced cytotoxic T-lymphocyte infiltration [126]. With the success of recent immunotherapy trials, we speculate that FUS-mediated immunotherapy delivery may permit the inclusion of patients with brain metastases who would normally be denied treatment and ultimately represent a turning point in how brain metastases are treated.
2.2 Liposomes
Liposomal drug formulations are popular due to their versatility and biocompatibility [127]. Their structure, comprised of an aqueous core and a lipid shell, permits the loading of both hydrophilic and hydrophobic drugs [128], and the formulation of the lipid shell can be easily modulated for PEGylation [129], thermosensitivity, and/or targeting [130]. Furthermore, both the size and composition of the liposome can be altered to control circulation time and degradation rate [131]. Liposomes are particularly beneficial for packaging highly toxic drugs, since encapsulated drugs are not bioavailable. Conversely, their larger size makes them more difficult to deliver and FUS may trigger release of the drug payload. The liposomal formulation of doxorubicin, a potent anthracycline, was one of the first drug delivery systems used in combination with FUS[88,83,90,132–134]. Treat et al demonstrated that a single treatment combining FUS and liposomal DOX delayed tumor growth and improved survival time (ISTmedian=24% compared to 16% for liposomal DOX alone) in a rat gliosarcoma model. Later work by the same group showed that 3 weekly FUS + liposomal DOX treatments drastically improved survival (Figure 6B) compared to the liposomal DOX-only group (ISTmedian=100% and 16%, respectively), with complete tumor resolution (Figure 6A) in several animals in the FUS + liposomal DOX group [83]. Nonetheless, several animals did suffer from side effects, including skin toxicity, neural loss and intratumoral hemorrhage [83]. To verify that the combination of FUS and liposomal DOX was not causing additional toxicity, a safety study in healthy animals was conducted that demonstrated only minor damage at the focus in animals that received both liposomal DOX and FUS, believed to be due to high local concentrations of DOX deposited by aggressive FUS settings. Of note, the authors also demonstrated that administering liposomal DOX after treatment caused a 32% decrease in DOX delivery across the BBB, a finding we have substantiated with 60 nm polymeric NPs (unpublished studies). A study with animals bearing bilateral 9L gliosarcomas indicated that even late stage tumors benefit from FUS-mediated delivery, with treated tumors showing a two-fold increase in DOX concentration compared to unsonicated controls [88]. FUS treatment also significantly increased the delivery of tumor targeted liposomal DOX formulations in an intracranial mouse xenograft model [90,133], while decreasing some elements of DOX-related toxicity [133], presumably due to lower levels of drug in circulation post sonication. While it is still unclear whether intact liposomes cross the BBB, it is clear that the combination of liposomal encapsulation and FUS-mediated delivery provide excellent therapeutic results, increasing drug concentrations at the target while minimizing systemic toxicities.
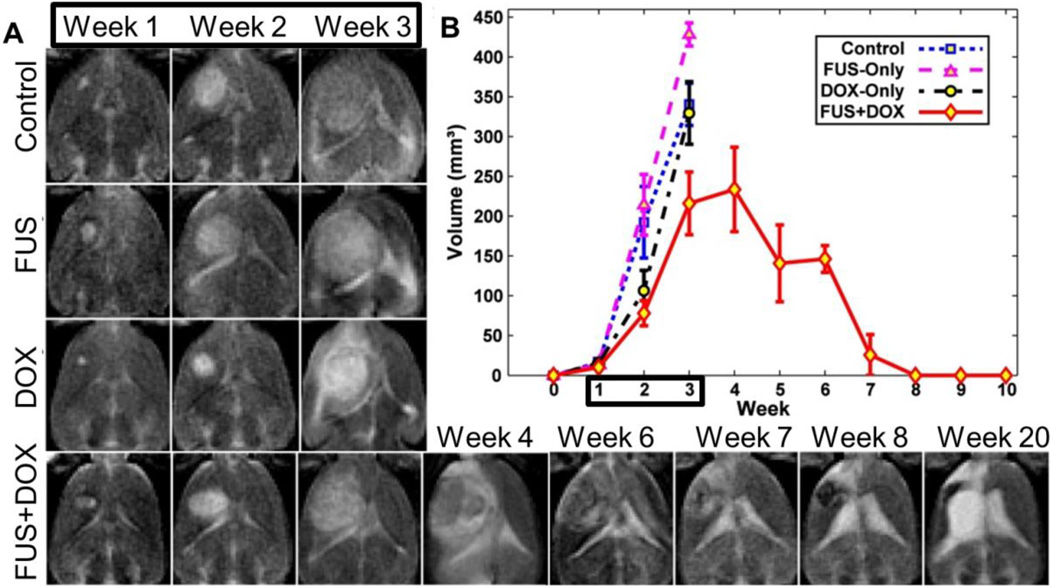
Figure 6.
Three weekly FUS BBBD treatments (weeks 1–3) mediated delivery of liposomal doxorubicin and prolonged survival in a rat glioma model. A) T2 weighted images of control, FUS-only and DOX-only groups showing rapid tumor growth during weeks 1–3. T2 weighted images of a long-term survivor in the FUS+DOX group shows rapid tumor growth in weeks 1–4 followed by tumor resolution. Hyperintensity at week 20 is due to an enlarged ventricle. Black box indicates treatment period. B) Tumor growth as measured by MRI for each experimental group. Note that no control animals (untreated tumors) survived past week 3. Black box indicates treatment period. Reprinted from J Control Release, 169, Aryal M, Vykhodtseva N, Zhang YZ, Park J, McDannold N, Multiple treatments with liposomal doxorubicin and ultrasound-induced disruption of blood-tumor and blood-brain barriers improve outcomes in a rat glioma model, 103–11, (2015) with permission from
2.3 Drug Loaded Microbubbles
Microbubbles can also be functionalized for use as drug delivery vehicles. Although drug loading is limited to the lipid or protein shell, the relatively large surface area (~50 µm2) permits conjugation for both targeting and therapeutics[135]. Because of its hydrophobicity, BCNU has been incorporated into the shell of lipid MBs with some success[89,136–138]. Encapsulation of BCNU within the MB’s lipid shell permitted simultaneous BBB opening and local drug delivery similar to that seen with unencapsulated drug, with the added benefit of increased tissue retention time at the target[138]. Treatment with BCNU-MBs and FUS showed excellent tumor control 30 days post inoculation and median survival time was increased by 12% compared to controls in a C6 glioma model [138]. The addition of VEGF-R2 to the BCNU-MBs provided antiangiogenic targeting capabilities and further improvements in tumor control and animal survival [89]. The same group also developed DOX-loaded MBs conjugated with superparamagnetic iron oxide (SPIO) NPs[139], which showed a two-fold increase in DOX deposition within a rat glioma compared to a non-sonicated control. FUS treatment followed by magnetic targeting also deposited SPIO NPs released from the MBs within the tumor tissue, permitting MR-based treatment monitoring. While drug-loaded MBs offer the benefit of highly localized delivery, they may also require higher pressure to release the drug and are limited to the circulation time of the MB itself.
3. Gene Delivery
Gene therapy in the CNS is emerging as an attractive strategy for the treatment of neurological diseases like Parkinson’s disease[140–143], Alzheimer’s disease[144,145], lysosomal storage diseases[146,147] and brain tumors [148]. Indeed, despite the ability of traditional small molecule drug regimens to treat early symptoms of diseases like Parkinson’s disease, continued disease progression ultimately leads to recurrence[149]. Furthermore, the BBB requires these drugs to be administered at high systemic doses to reach effective concentrations in the brain, ultimately causing adverse peripheral side effects [9]. Alternatively, gene therapy offers the ability to treat the underlying causes of the disease and ultimately slow progression or even reverse disease pathology. Moreover, continuous transgene expression leads to long term efficacy, reducing required treatments and overall patient costs when compared to drugs or liposomes, which need to be dosed periodically. While numerous gene therapy trials for neurological disease have yielded early positive results, limited vector distribution[11] and the risk of infection[150] after intracranial injection have slowed widespread adoption. Toward this end, it has been postulated that outcomes could be improved by enhancing therapeutic distribution within the target structures[151]. High capillary density in the brain allows multiple points of entry into the CNS after FUS application, potentiating improved distribution compared to intracranial injection. Therefore, delivery of therapeutic genes into the CNS with FUS may prove to be a powerful new method for treating neurological diseases.
Despite a high number of publications demonstrating the ability of FUS to deliver gene bearing liposomes[152,153], non-viral polyplexes[154–156], viruses[157–159] and free or MB bound plasmid DNA[160] to non-CNS tissues, there are very few studies that have shown the delivery of systemically administered gene-bearing agents to the CNS with FUS. Indeed, the first successful studies showing delivery of reporter gene-bearing agents across the BBB with FUS were completed as recently at 2012[161–163]. In these studies, it was shown that FUS could deliver either naked plasmid or adeno-associated virus (AAV) across the BBB to mediate transgene expression in the brain. Recently, however, excitement for this application has led to a flurry of new studies, which will be reviewed here.
3.1 Naked Plasmid Delivery
Anionic plasmid DNA can be electrostatically bound to cationic MBs to create MB-DNA carriers. As a result, DNA will be immediately exposed to the vasculature being disrupted by FUS-activated MBs, potentiating DNA extravasation and trans-BBB delivery. Several studies have shown that linking the plasmid DNA to the MB will enhance the transfection compared to free circulating plasmid delivered with FUS[135,164–166]. Interestingly, MRIgFUS exposure to MB-DNA carriers bearing a gene for eGFP led to a significant enhancement of transgene expression in neurons in a young mouse model[162]. Using a similar system, it was shown a MB-DNA carrier bearing a gene for BDNF led to a ~20-fold increase in BDNF protein content[163]. Unfortunately, very high doses of plasmid DNA were required due to susceptibility to degradation from nucleases in the blood and the cell, which reduce the efficiency of this vector system.
3.2 Adeno-Associated Virus
Adeno-associated virus (AAV), with its small ~20 nm size, transduction efficiency, and limited immunogenicity, is a well suited vector for delivery applications across the BBB. Indeed, some AAV vectors, like the self-complementary AAV9 (scAAV9) vector, are able to cross the BBB and mediate global transgene expression in the brain after intravenous injection. However, very high doses of scAAV9 are required, with up to 1×1011 vg/g found to only transduce 19% of motor neurons in adult mice[167]. In contrast, FUS-mediated BBBD can yield transduction efficiencies of 80% in the brain [161] and 87% in the spinal cord (Figure 7) [168] at doses as low as 2.5×109 or 2×109 vg/g, respectively. This marks a robust enhancement of transgene expression in the CNS after intravenous administration of scAAV9. In each case, transgene expression was localized to the anatomical location targeted with FUS. In addition to scAAV9, other studies have shown the delivery of AAV1[169,170] or AAV2[171] across the BBB with FUS. Importantly, these studies showed that transgene expression can be limited to neurons through the use of the synapsin promoter[169]. Moreover, it was found that a transgene driven by the CMV promoter and packaged into the AAV2 capsid led to predominantly astrocytic expression after delivery with FUS[171], in contrast to intracranial injection of the same vector, which led to mostly neuronal expression[172,173]. To this end, it has been suggested that FUS could alter cellular receptor concentrations, including heparan sulfate proteoglycans. This receptor, in addition to being the cellular receptor for AAV2[174] is also known to have roles in the CNS injury response[175].
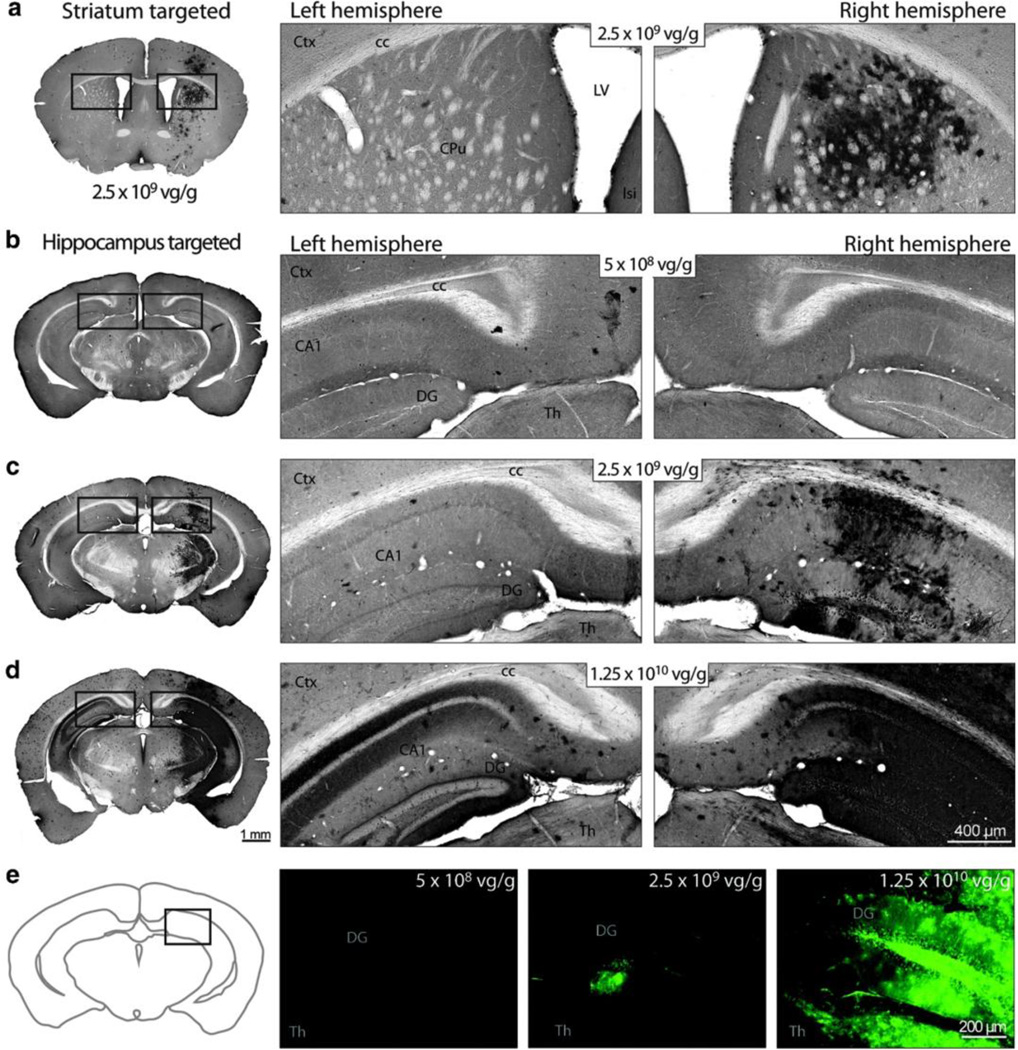
Figure 7.
FUS mediated delivery of scAAV9 leads to a dose-dependent transgene expression in mouse brain. Mice were treated with MRI guided FUS in the right striatum (a) or hippocampus (b–e) immediately prior to intravenous injection of scAAV9 bearing a gene for GFP under the ubiquitously active chicken β-actin promoter at doses of 5×108 (a,c,e left), 2.5×109 (b,e middle) or 1.25×1010 (d,e right) vg/g. Twelve days after treatment, GFP expression was assessed in coronal brain sections with immunohistrochemistry (a–d) or fluorescence microscopy (e). GFP expression was higher on the FUS-treated region (a,c,d,e, right) than the corresponding anatomical location on the contralateral hemisphere (a,c,d,e, left) at the two higher doses, but not the lowest dose. The publisher for this copyrighted material is Mary Ann Liebert, Inc. publishers.
Despite its efficiency, AAV has shown significant limitations when considered in the context of CNS gene delivery applications. Concerns about safety, limited packaging capacity, difficulties in scale-up and high production costs limit its scope as a long-term solution to CNS gene delivery. Furthermore, repeated administration of AAV leads to production of neutralizing antibody immune responses that may ultimately reduce the efficiency of the vector[176,177]. Finally, scAAV vectors and AAV vectors have packaging capacities of just 2.4 kb or 4.8 kb [178], respectively, which hampers the versatility of this vector.
4. Polymer-Based Delivery Systems
Polymer based NP delivery systems offer several advantages over non-encapsulated drugs or viral delivery systems. These include tailorability, ease of manufacture, improved drug-release profiles and protection from degradation or clearance[179,180]. Combined, these properties can reduce drug doses and drug-associated toxicity while improving therapeutic efficacy [181–186]. Polymer NPs can be loaded with a variety of payloads including soluble or non-soluble drugs[166,183,187,188], imaging or theranostic agents[189–191], or nucleic acids[154,180,192,193].
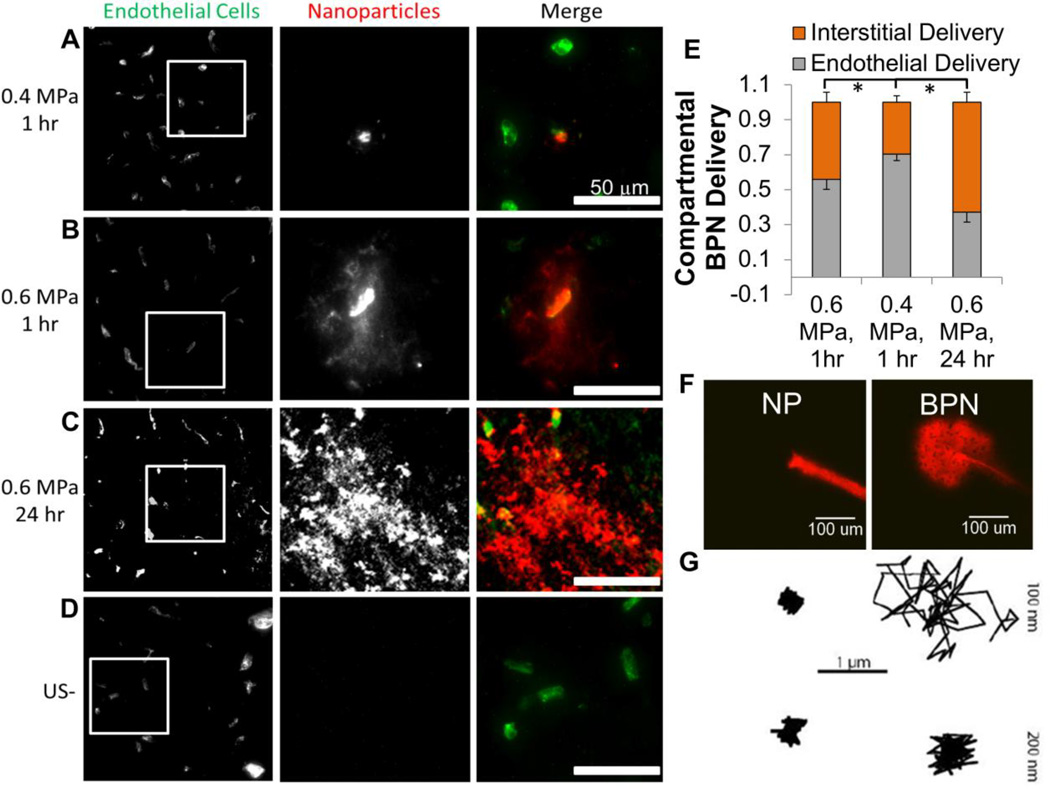
It is well known that enhancing therapeutic distribution in the brain parenchyma will improve efficacy[151]. Indeed, while the limitations imposed by the BBB are widely known, the brain parenchyma itself presents a further barrier to delivery in the brain. The brain tissue barrier consists of a dense nanoporous mesh of electrostatically charged macromolecules, including chondroitin sulfate proteoglycans, hyaluronan, and tenascins [194,195]. These charged molecules form a microstructure that hampers diffusion of macromolecules and vectors, including NPs [196,197] and viruses [12], via steric or adhesive interactions. In addition, tumors like GBM contain dense and heterogeneous networks of collagen[198] and high interstitial pressures[199] that further limit macromolecule diffusion [200–203]. As a result, until recently, it was thought that the upper size limit to diffusion in healthy brain was as small as 64 nm [204]. However, it has been shown that an extremely dense (> 9 PEG/100 nm2) coat of the bioinert and neutrally charged polymer polyethylene glycol (PEG) shields NP surface charge and reduces ECM interactions in brain tissue, permitting the diffusion of particles up to 114 nm in size [197,205,206] and improving circulation time[207]. This ultimately permits greater accumulation in tumors through the enhanced permeability and retention effect [208,209]. Dense PEG coatings have demonstrated remarkable improvements in diffusivity and efficacy with multiple types of polymer[154,180,192,205]. For example, highly-PEGylated “brain-penetrating” NPs (BPNs) continue to diffuse up to 24 hours after delivery, leading to a more homogeneous distribution within the parenchyma (Figure 8A–E) [206]. In contrast, all sizes of un-PEGylated controls were rapidly immobilized within the ECM (Figure 8F,G). Unsurprisingly, drug-loaded BPNs are more effective than their un-PEGylated counterparts in limiting tumor growth after intracranial administration. Additionally, BPNs are also an effective vehicle for gene delivery in the brain, and have demonstrated remarkable efficiency after intracranial administration[192]. Gene bearing BPN are an easily adaptable and versatile option for applications in the brain, devoid of the limitations of viral vectors. FUS is capable of delivering 60 nm BPNs across the BBB (Figure 8A–E) [205]. These BPN represent an important advance in polymeric delivery systems, as evading the BBB is only the first major obstacle to drug and gene delivery in the brain – a point eloquently demonstrated by the lack of success with the Gliadel wafers. Therapeutics must be delivered well beyond the vasculature, and particles that are able to diffuse well beyond the cerebral vasculature after FUS-mediated BBBD may greatly increase treatment homogeneity and efficacy.
Figure 8.
FUS delivers large 60-nm brain-penetrating nanoparticles across the BBB. At one hour post sonication, low pressure sonication primarily delivers nanoparticles to the endothelium (A) while higher pressure delivers particles to the interstitium (B). After 24 hours, brain-penetrating particles have diffused away from the vessel, significantly increasing nanoparticle coverage in the interstitial space compared to both low and high pressure 1 hr timepoints (C, G). Control regions show no nanoparticle delivery (D). 60 nm brain-penetrating nanoparticles (BPN) diffuse in ex vivo brain tissue after injection, while uncoated particles (NP) are immobilized (E). 100 nm BPNs also exhibit diffusive behavior in brain tissue, as demonstrated by traces taken by particle tracking software, while 200 nm BPNs and both 100 and 200 nm uncoated NPs are immobilized (F). * indicates p<0.05. A,B,D,G,E reprinted from J Control Release, 189, Nance E, Timbie K, Miller GW, Song J, Louttit C, Klibanov AL, Shih TY, Swaminathan G, Tamargo RJ, Woodworth GF, Hanes J, Price RJ, Non-invasive delivery of stealth, brain-penetrating nanoparticles across the blood-brain barrier using MRI-guided focused, 123–32, (2014) with permission from Elsevier. F reprinted from ACS Nano, 8(10), Nance E, Zhang C, Shih TY, Xu Q, Schuster BS, Hanes J, Brain-penetrating nanoparticles improve paclitaxel efficacy in malignant glioma following local administration, 10655–64, (2013) with permission from AAAS.
Polymer-based NP delivery systems are well suited for brain therapies after FUS mediated BBBD. Ideal drug delivery systems for applications in the CNS would include (i) ability to homogeneously distribute within the target volume, (ii) sustained drug release and (iii) long circulation times by avoiding rapid clearance. In combination with FUS as a non-invasive strategy to bypass the BBB, polymeric brain-penetrating NPs have potential to overcome many of the hurdles associated with drug and gene delivery in the brain.
5. Conclusions and Future Perspectives
FUS is currently the only modality which allows repeated, non-invasive, and temporary BBBD to deliver drugs or genes to the CNS[30]. As FUS technology improves, it may be capable of replacing invasive surgical techniques and offers an exciting alternative to traditional approaches. Numerous studies have demonstrated the ability of FUS to deliver a wide range of payloads across the BBB including imaging agents, small molecule drugs, ~150 kDa antibodies, recombinant proteins, ~20 nm viruses, ~60 nm NPs, 100 nm liposomes and even 10 µm stem cells. As a result, FUS has opened doors to novel treatments for CNS disorders like neurodegenerative disease, GBM, and psychiatric disorders. Particularly, despite its advantages and immense potential, nanotechnology has largely been excluded from applications in the brain owing to difficulties in delivery, which can be overcome with FUS. While the BBB has long been considered the greatest bottleneck in the development of treatments for CNS disease, FUS may fundamentally revolutionize how such diseases are approached.
Acknowledgements
The authors of this work have been supported by NIH R01 CA164789, R01 CA197111, R03 EB016784, R01 EB020147, the Focused Ultrasound Foundation and the, NHLBI-sponsored Basic Cardiovascular Research Training Grant (5 T32 HL007284).
Abbreviations
- AAV
adeno-associated virus
- BBB
blood-brain barrier
- BBBD
blood-brain barrier disruption
- BCNU
bis-chloroethylnitrosourea, or carmustine
- BNCT
boron neutron capture therapy
- BPN
brain-penetrating nanoparticle
- CNS
central nervous system
- DOX
doxorubicin
- FUS
focused ultrasound
- GBM
glioblastoma multiforme
- GDNF
glial-cell derived neurotrophic factor
- MB
microbubble
- MR
magnetic resonance
- NTN
neuturin
- NP
nanoparticle
- PCD
passive cavitation detection
- PEG
polyethylene glycol
- SPIO
superparamagnetic iron oxide
- SWI
susceptibility weighted imaging
- TMZ
temozolomide
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Puhalla S, Elmquist W, Freyer D, Kleinberg L, Adkins C, Lockman P, et al. Unsanctifying the sanctuary: challenges and opportunities with brain metastases. Neuro. Oncol. 2015;17:639–651. doi: 10.1093/neuonc/nov023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Prados MD, Byron SA, Tran NL, Phillips JJ, Molinaro AM, Ligon KL, et al. Toward precision medicine in glioblastoma: the promise and the challenges. Neuro. Oncol. 2015 doi: 10.1093/neuonc/nov031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Neurological Disorders: public health challenges [Google Scholar]
- 4.Substance Abuse and Mental Health Services Administration, Results from the 2012 National Survey on Drug Use and Health : Summary of National Findings. 2013 http://store.samhsa.gov/home.
- 5.Stewart WF, Ricci JA, Chee E, Hahn SR, Morganstein D. Cost of lost productive work time among US workers with depression. JAMA. 2003;289:3135–3144. doi: 10.1001/jama.289.23.3135. [DOI] [PubMed] [Google Scholar]
- 6.Pardridge WM. The blood-brain barrier: bottleneck in brain drug development. NeuroRx. 2005;2:3–14. doi: 10.1602/neurorx.2.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bauer H-C, Krizbai IA, Bauer H, Traweger A. “You Shall Not Pass”-tight junctions of the blood brain barrier. Front. Neurosci. 2014;8:392. doi: 10.3389/fnins.2014.00392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pardridge WM, Boado RJ. Reengineering biopharmaceuticals for targeted delivery across the blood-brain barrier. 1st ed. Elsevier Inc: 2012. [DOI] [PubMed] [Google Scholar]
- 9.Deleu D, Northway MG, Hanssens Y. Clinical pharmacokinetic and pharmacodynamic properties of drugs used in the treatment of Parkinson’s disease. Clin. Pharmacokinet. 2002;41:261–309. doi: 10.2165/00003088-200241040-00003. [DOI] [PubMed] [Google Scholar]
- 10.Lonser RR, Sarntinoranont M, Morrison PF, Oldfield EH. Convection-enhanced delivery to the central nervous system. J Neurosurg. 2015;122:697–706. doi: 10.3171/2014.10.JNS14229. [DOI] [PubMed] [Google Scholar]
- 11.Morrison PF. Distributed Models of drug Kinetics. Princ. Clin. Pharmacol. 2007:107–128. [Google Scholar]
- 12.Bobo RH, Laske DW, Akbasak A, Morrisont PF, Dedrickt RL, Oldfield EH. Convection-enhanced delivery of macromolecules in the brain. 1994;91:2076–2080. doi: 10.1073/pnas.91.6.2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marks WJ, Bartus RT, Siffert J, Davis CS, Lozano A, Boulis N, et al. Gene delivery of AAV2-neurturin for Parkinson’s disease: a double-blind, randomised, controlled trial. Lancet. Neurol. 2010;9:1164–1172. doi: 10.1016/S1474-4422(10)70254-4. [DOI] [PubMed] [Google Scholar]
- 14.Marks WJ, Ostrem JL, Verhagen L, Starr Pa, Larson PS, Bakay RA. et al. Safety and tolerability of intraputaminal delivery of CERE-120 (adeno-associated virus serotype 2-neurturin) to patients with idiopathic Parkinson’s disease: an open-label, phase I trial, Lancet Neurol. 2008;7:400–408. doi: 10.1016/S1474-4422(08)70065-6. [DOI] [PubMed] [Google Scholar]
- 15.Ramaswamy S, McBride JL, Han I, Berry-Kravis EM, Zhou L, Herzog CD, et al. Intrastriatal CERE-120 (AAV-Neurturin) protects striatal and cortical neurons and delays motor deficits in a transgenic mouse model of Huntington’s disease. Neurobiol. Dis. 2009;34:40–50. doi: 10.1016/j.nbd.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 16.Gasmi M, Brandon EP, Herzog CD, Wilson A, Bishop KM, Hofer EK, et al. AAV2-mediated delivery of human neurturin to the rat nigrostriatal system: long-term efficacy and tolerability of CERE-120 for Parkinson’s disease. Neurobiol. Dis. 2007;27:67–76. doi: 10.1016/j.nbd.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 17.Ren X, Zhang T, Gong X, Hu G, Ding W, Wang X. AAV2-mediated striatum delivery of human CDNF prevents the deterioration of midbrain dopamine neurons in a 6-hydroxydopamine induced parkinsonian rat model. Exp. Neurol. 2013;248:148–156. doi: 10.1016/j.expneurol.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 18.Gonzales-Portillo GS, Sanberg PR, Franzblau M, Gonzales-Portillo C, Diamandis T, Staples M. et al. Mannitol-enhanced delivery of stem cells and their growth factors across the blood-brain barrier, Cell Transplant. 2014;23:531–539. doi: 10.3727/096368914X678337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee HJ, Zhang Y, Pardridge WM. Blood-brain barrier disruption following the internal carotid arterial perfusion of alkyl glycerols. J. Drug Target. 2002;10:463–467. doi: 10.1080/1061186021000038337. [DOI] [PubMed] [Google Scholar]
- 20.Bhattacharjee AK, Kondoh T, Nagashima T, Ikeda M, Ehara K, Tamaki N. Quantitative analysis of papaverine-mediated blood-brain barrier disruption in rats. Biochem. Biophys. Res. Commun. 2001;289:548–552. doi: 10.1006/bbrc.2001.6029. [DOI] [PubMed] [Google Scholar]
- 21.Black KL, Cloughesy T, Huang SC, Gobin YP, Zhou Y, Grous J, et al. Intracarotid infusion of RMP-7, a bradykinin analog, and transport of gallium-68 ethylenediamine tetraacetic acid into human gliomas. 1997 doi: 10.3171/jns.1997.86.4.0603. [DOI] [PubMed] [Google Scholar]
- 22.Lochhead JJ, Thorne RG. Intranasal delivery of biologics to the central nervous system. Adv. Drug Deliv. Rev. 2012;64:614–628. doi: 10.1016/j.addr.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 23.Costantino HR, Illum L, Brandt G, Johnson PH, Quay SC. Intranasal delivery: Physicochemical and therapeutic aspects. Int. J. Pharm. 2007;337:1–24. doi: 10.1016/j.ijpharm.2007.03.025. [DOI] [PubMed] [Google Scholar]
- 24.Ikeda M, Bhattacharjee AK, Kondoh T, Nagashima T, Tamaki N. Synergistic effect of cold mannitol and Na(+)/Ca(2+) exchange blocker on blood-brain barrier opening. Biochem. Biophys. Res. Commun. 2002;291:669–674. doi: 10.1006/bbrc.2002.6495. [DOI] [PubMed] [Google Scholar]
- 25.Zylber-Katz E, Gomori JM, Schwartz A, Lossos A, Bokstein F, Siegal T. Pharmacokinetics of methotrexate in cerebrospinal fluid and serum after osmotic blood-brain barrier disruption in patients with brain lymphoma. Clin. Pharmacol. Ther. 2000;67:631–641. doi: 10.1067/mcp.2000.106932. [DOI] [PubMed] [Google Scholar]
- 26.Lipsman N, Schwartz ML, Huang Y, Lee L, Sankar T, Chapman M. et al. MR-guided focused ultrasound thalamotomy for essential tremor: a proof-of-concept study, Lancet Neurol. 2013;12:462–468. doi: 10.1016/S1474-4422(13)70048-6. [DOI] [PubMed] [Google Scholar]
- 27.Elias WJ, Huss D, Voss T, Loomba J, Khaled M, Zadicario E, et al. A pilot study of focused ultrasound thalamotomy for essential tremor. N. Engl. J. Med. 2013;369:640–648. doi: 10.1056/NEJMoa1300962. [DOI] [PubMed] [Google Scholar]
- 28.Dallapiazza R, McKisic MS, Shah B, Elias WJ. Neuromodulation for movement disorders. Neurosurg. Clin. N. Am. 2014;25:47–58. doi: 10.1016/j.nec.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 29.Dobrakowski PP, Machowska-Majchrzak AK, Labuz-Roszak B, Majchrzak KG, Kluczewska E, Pierzchała KB. MR-guided focused ultrasound: a new generation treatment of Parkinson’s disease, essential tremor and neuropathic pain. [accessed May 18, 2015];Interv. Neuroradiol. 20:275–282. doi: 10.15274/INR-2014-10033. http://www.ncbi.nlm.nih.gov/pubmed/24976088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hynynen K, Mcdannold N. Imaging – guided Focal Opening of the Blood-Brain Barrier in Rabbits. 2001;1 doi: 10.1148/radiol.2202001804. [DOI] [PubMed] [Google Scholar]
- 31.Hynynen K, McDannold N, Vykhodtseva N, Jolesz FA. Non-invasive opening of BBB by focused ultrasound. Acta Neurochir. 2003;(Suppl. 86):555–558. doi: 10.1007/978-3-7091-0651-8_113. [DOI] [PubMed] [Google Scholar]
- 32.McDannold N, Vykhodtseva N, Raymond S, Jolesz Fa, Hynynen K. MRI-guided targeted blood-brain barrier disruption with focused ultrasound: Histological findings in rabbits. Ultrasound Med. Biol. 2005;31:1527–1537. doi: 10.1016/j.ultrasmedbio.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 33.Runge VM, Clanton JA, Price AC, Wehr CJ, Herzer WA, Partain CL, et al. The use of Gd DTPA as a perfusion agent and marker of blood-brain barrier disruption. Magn. Reson. Imaging. 1985;3:43–55. doi: 10.1016/0730-725x(85)90008-6. [DOI] [PubMed] [Google Scholar]
- 34.Liu HL, Wai YY, Chen WS, Chen JC, Hsu PH, Wu XY. et al. Hemorrhage Detection During Focused-Ultrasound Induced Blood-Brain-Barrier Opening by Using Susceptibility-Weighted Magnetic Resonance Imaging, Ultrasound Med. Biol. 2008;34:598–606. doi: 10.1016/j.ultrasmedbio.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 35.Cheng AL, Batool S, McCreary CR, Lauzon ML, Frayne R, Goyal M. et al. Susceptibility-weighted imaging is more reliable than T2*-weighted gradient-recalled echo mri for detecting microbleeds, Stroke. 2013;44:2782–2786. doi: 10.1161/STROKEAHA.113.002267. [DOI] [PubMed] [Google Scholar]
- 36.Fry W, Fry F, Meyers R, Eggleton RC. The Use of Ultrasound In Neurosurgery. [accessed May 12, 2015];Third Int. Conf. Med. Electron. 1960 http://www.brl.uiuc.edu/Publications/1960/Fry-TICME-453-1960.pdf.
- 37.Hynynen K, Jolesz Fa. Demonstration of potential noninvasive ultrasound brain therapy through an intact skull. Ultrasound Med. Biol. 1998;24:275–283. doi: 10.1016/s0301-5629(97)00269-x. [DOI] [PubMed] [Google Scholar]
- 38.a Rooney J. Shear as a mechanism for sonically induced biological effects. J. Acoust. Soc. Am. 1972;52:1718–1724. doi: 10.1121/1.1913306. http://www.ncbi.nlm.nih.gov/pubmed/4641374. [DOI] [PubMed] [Google Scholar]
- 39.Davidson BJ, Riley N. MICROSTREAMING. East. 1971;15:217–233. [Google Scholar]
- 40.Chen H, Kreider W, Brayman AA, Bailey MR, Matula TJ. Blood vessel deformations on microsecond time scales by ultrasonic cavitation. [accessed May 21, 2015];Phys. Rev. Lett. 2011 106:034301. doi: 10.1103/PhysRevLett.106.034301. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3087441&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wu J, Ross JP, Chiu J-F. Reparable sonoporation generated by microstreaming. J. Acoust. Soc. Am. 2002;111:1460. doi: 10.1121/1.1420389. [DOI] [PubMed] [Google Scholar]
- 42.Juffermans LJM, van Dijk A, Jongenelen CAM, Drukarch B, Reijerkerk A, de Vries HE, et al. Ultrasound and microbubble-induced intra- and intercellular bioeffects in primary endothelial cells. Ultrasound Med. Biol. 2009;35:1917–1927. doi: 10.1016/j.ultrasmedbio.2009.06.1091. [DOI] [PubMed] [Google Scholar]
- 43.Sheikov N, McDannold N, Sharma S, Hynynen K. Effect of focused ultrasound applied with an ultrasound contrast agent on the tight junctional integrity of the brain microvascular endothelium. Ultrasound Med. Biol. 2008;34:1093–1104. doi: 10.1016/j.ultrasmedbio.2007.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Deng J, Huang Q, Wang F, Liu Y, Wang Z, Wang Z, et al. The role of caveolin-1 in blood-brain barrier disruption induced by focused ultrasound combined with microbubbles. J. Mol. Neurosci. 2012;46:677–687. doi: 10.1007/s12031-011-9629-9. [DOI] [PubMed] [Google Scholar]
- 45.Xia C, Zhang Z, Xue Y, Wang P, Liu Y. Mechanisms of the increase in the permeability of the blood-tumor barrier obtained by combining low-frequency ultrasound irradiation with small-dose bradykinin. J. Neurooncol. 2009;94:41–50. doi: 10.1007/s11060-009-9812-9. [DOI] [PubMed] [Google Scholar]
- 46.Meijering BDM, Juffermans LJM, van Wamel A, Henning RH, Zuhorn IS, Emmer M, et al. Ultrasound and microbubble-targeted delivery of macromolecules is regulated by induction of endocytosis and pore formation. Circ. Res. 2009;104:679–687. doi: 10.1161/CIRCRESAHA.108.183806. [DOI] [PubMed] [Google Scholar]
- 47.Nhan T, Burgess A, Cho EE, Stefanovic B, Lilge L, Hynynen K. Drug delivery to the brain by focused ultrasound induced blood–brain barrier disruption: Quantitative evaluation of enhanced permeability of cerebral vasculature using two-photon microscopy. [accessed October 28, 2013];J. Control. Release. 2013 172:274–280. doi: 10.1016/j.jconrel.2013.08.029. http://www.sciencedirect.com/science/article/pii/S0168365913004987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen H, Konofagou EE. The size of blood-brain barrier opening induced by focused ultrasound is dictated by the acoustic pressure. J. Cereb. Blood Flow Metab. 2014;34:1197–1204. doi: 10.1038/jcbfm.2014.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cho EE, Drazic J, Ganguly M, Stefanovic B, Hynynen K. Two-photon fluorescence microscopy study of cerebrovascular dynamics in ultrasound-induced blood-brain barrier opening. J. Cereb. Blood Flow Metab. 2011;31:1852–1862. doi: 10.1038/jcbfm.2011.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McDannold N, Vykhodtseva N, Hynynen K. Targeted disruption of the blood-brain barrier with focused ultrasound: association with cavitation activity. Phys. Med. Biol. 2006;51:793–807. doi: 10.1088/0031-9155/51/4/003. [DOI] [PubMed] [Google Scholar]
- 51.Samiotaki G, Vlachos F, Tung YS, Feshitan J, Borden M, Konofagou EE. Pressure and microbubble size dependence study of focused ultrasound-induced blood-brain barrier opening reversibility in vivo. AIP Conf. Proc. 2012;1481:300–306. doi: 10.1002/mrm.23063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Park J, Zhang Y, Vykhodtseva N, a Jolesz F, McDannold NJ. The kinetics of blood brain barrier permeability and targeted doxorubicin delivery into brain induced by focused ultrasound. J. Control. Release. 2012;162:134–142. doi: 10.1016/j.jconrel.2012.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Howles GP, Bing KF, Qi Y, Rosenzweig SJ, Nightingale KR, Johnson GA. Contrast-enhanced in vivo magnetic resonance microscopy of the mouse brain enabled by noninvasive opening of the blood-brain barrier with ultrasound. Magn. Reson. Med. 2010;64:995–1004. doi: 10.1002/mrm.22411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McDannold N, Arvanitis CD, Vykhodtseva N, Livingstone MS. Temporary disruption of the blood-brain barrier by use of ultrasound and microbubbles: safety and efficacy evaluation in rhesus macaques. Cancer Res. 2012;72:3652–3663. doi: 10.1158/0008-5472.CAN-12-0128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Downs ME, Buch A, Sierra C, Karakatsani ME, Chen S, Konofagou EE, et al. Long-Term Safety of Repeated Blood-Brain Barrier Opening via Focused Ultrasound with Microbubbles in Non-Human Primates Performing a Cognitive Task. PLoS One. 2015;10:e0125911. doi: 10.1371/journal.pone.0125911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hynynen K, McDannold N, a Sheikov N, a Jolesz F, Vykhodtseva N. Local and reversible blood-brain barrier disruption by noninvasive focused ultrasound at frequencies suitable for trans-skull sonications. Neuroimage. 2005;24:12–20. doi: 10.1016/j.neuroimage.2004.06.046. [DOI] [PubMed] [Google Scholar]
- 57.Rauch PJ, Park HS, Knisely JPS, Chiang VL, Vortmeyer AO. Delayed radiation-induced vasculitic leukoencephalopathy. Int. J. Radiat. Oncol. Biol. Phys. 2012;83:369–375. doi: 10.1016/j.ijrobp.2011.06.1982. [DOI] [PubMed] [Google Scholar]
- 58.Oh BC, Pagnini PG, Wang MY, Liu CY, Kim PE, Yu C. et al. Stereotactic radiosurgery: Adjacent tissue injury and response after high-dose single fraction radiation: Part I - Histology, imaging, and molecular events, Neurosurgery. 2007;60:31–44. doi: 10.1227/01.NEU.0000249191.23162.D2. [DOI] [PubMed] [Google Scholar]
- 59.O’Reilly Ma, Hynynen K. Blood-Brain Barrier: Real-time Feedback-controlled Focused Ultrasound Disruption by Using an Acoustic Emissions-based Controller. Radiology. 2012;263:96–106. doi: 10.1148/radiol.11111417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tung Y-S, Vlachos F, Choi JJ, Deffieux T, Selert K, Konofagou EE. In vivo transcranial cavitation threshold detection during ultrasound-induced blood-brain barrier opening in mice. Phys. Med. Biol. 2010;55:6141–6155. doi: 10.1088/0031-9155/55/20/007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Samuel S, a Cooper M, Bull JL, Fowlkes JB, Miller DL. An ex vivo study of the correlation between acoustic emission and microvascular damage. Ultrasound Med. Biol. 2009;35:1574–1586. doi: 10.1016/j.ultrasmedbio.2009.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Madanshetty SI, Roy RA, Apfel RE. Acoustic microcavitation: its active and passive acoustic detection. J. Acoust. Soc. Am. 1991;90:1515–1526. doi: 10.1121/1.401891. [DOI] [PubMed] [Google Scholar]
- 63.Ilyichev VI, Koretz VL, Melnikov NP. Spectral characteristics of acoustic cavitation. Ultrasonics. 1989;27:357–361. doi: 10.1016/0041-624x(89)90034-6. [DOI] [PubMed] [Google Scholar]
- 64.Leighton TG. The Acoustic Bubble. J. Acoust. Soc. Am. 1994;96:2616. [Google Scholar]
- 65.Arvanitis CD, Livingstone MS, Vykhodtseva N, McDannold N. Controlled Ultrasound-Induced Blood-Brain Barrier Disruption Using Passive Acoustic Emissions Monitoring. PLoS One. 2012;7 doi: 10.1371/journal.pone.0045783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wu SY, Tung YS, Marquet F, Downs M, Sanchez C, Chen C. et al. Transcranial cavitation detection in primates during blood-brain barrier opening-a performance assessment study, IEEE Trans. Ultrason. Ferroelectr. Freq. Control. 2014;61:966–978. doi: 10.1109/TUFFC.2014.2992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chai WY, Chu PC, Tsai MY, Lin YC, Wang JJ, Wei KC. et al. Magnetic-resonance imaging for kinetic analysis of permeability changes during focused ultrasound-induced blood-brain barrier opening and brain drug delivery, J. Control. Release. 2014;192:1–9. doi: 10.1016/j.jconrel.2014.06.023. [DOI] [PubMed] [Google Scholar]
- 68.Jordão JF, Thévenot E, Markham-Coultes K, Scarcelli T, Weng Y-Q, Xhima K, et al. Amyloid-β plaque reduction, endogenous antibody delivery and glial activation by brain-targeted, transcranial focused ultrasound. Exp. Neurol. 2013;248:16–29. doi: 10.1016/j.expneurol.2013.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Leinenga G, Götz J. Scanning ultrasound removes amyloid-b and restores memory in an Alzheimer’ s disease mouse model. Sci. Transl. Med. 2015;7 doi: 10.1126/scitranslmed.aaa2512. [DOI] [PubMed] [Google Scholar]
- 70.McDannold N, Arvanitis CD, Vykhodtseva N, Livingstone MS. Temporary disruption of the blood-brain barrier by use of ultrasound and microbubbles: safety and efficacy evaluation in rhesus macaques. Cancer Res. 2012 doi: 10.1158/0008-5472.CAN-12-0128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Burke CW, Klibanov AL, Sheehan JP, Price RJ. Inhibition of glioma growth by microbubble activation in a subcutaneous model using low duty cycle ultrasound without significant heating. J. Neurosurg. 2011;114:1654–1661. doi: 10.3171/2010.11.JNS101201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Leinenga G, Gotz J. Scanning ultrasound removes amyloid- and restores memory in an Alzheimer’s disease mouse model. Sci. Transl. Med. 2015;7:278ra33–278ra33. doi: 10.1126/scitranslmed.aaa2512. [DOI] [PubMed] [Google Scholar]
- 73.Scarcelli T, Jordão JF, a O’Reilly M, Ellens N, Hynynen K, Aubert I. Stimulation of hippocampal neurogenesis by transcranial focused ultrasound and microbubbles in adult mice. Brain Stimul. 2014;7:304–307. doi: 10.1016/j.brs.2013.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tufail Y, Matyushov A, Baldwin N, Tauchmann ML, Georges J, Yoshihiro A, et al. Transcranial pulsed ultrasound stimulates intact brain circuits. Neuron. 2010;66:681–694. doi: 10.1016/j.neuron.2010.05.008. [DOI] [PubMed] [Google Scholar]
- 75.Jalali S, Huang Y, Dumont DJ, Hynynen K. Focused ultrasound-mediated bbb disruption is associated with an increase in activation of AKT: experimental study in rats. BMC Neurol. 2010;10:114. doi: 10.1186/1471-2377-10-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tsai S-J. Transcranial focused ultrasound as a possible treatment for major depression. Med. Hypotheses. 2015;84:381–383. doi: 10.1016/j.mehy.2015.01.030. [DOI] [PubMed] [Google Scholar]
- 77.Tyler WJ, Tufail Y, Finsterwald M, Tauchmann ML, Olson EJ, Majestic C. Remote excitation of neuronal circuits using low-intensity, low-frequency ultrasound. PLoS One. 2008;3:e3511. doi: 10.1371/journal.pone.0003511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Deffieux T, Younan Y, Wattiez N, Tanter M, Pouget P, Aubry J-F. Low-intensity focused ultrasound modulates monkey visuomotor behavior. Curr. Biol. 2013;23:2430–2433. doi: 10.1016/j.cub.2013.10.029. [DOI] [PubMed] [Google Scholar]
- 79.Kim H, Taghados SJ, Fischer K, Maeng LS, Park S, Yoo SS. Noninvasive Transcranial Stimulation of Rat Abducens Nerve by Focused Ultrasound. Ultrasound Med. Biol. 2012;38:1568–1575. doi: 10.1016/j.ultrasmedbio.2012.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yoo SS, Bystritsky A, Lee JH, Zhang Y, Fischer K, Min BK. et al. Focused ultrasound modulates region-specific brain activity, Neuroimage. 2011;56:1267–1275. doi: 10.1016/j.neuroimage.2011.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Legon W, Rowlands A, Opitz A, Sato TF, Tyler WJ. Pulsed Ultrasound Differentially Stimulates Somatosensory Circuits in Humans as Indicated by EEG and fMRI. PLoS One. 2012;7 doi: 10.1371/journal.pone.0051177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lee W, Kim H, Jung Y, Song I-U, Chung YA, Yoo S-S. Image-Guided Transcranial Focused Ultrasound Stimulates Human Primary Somatosensory Cortex. Sci. Rep. 2015;5:8743. doi: 10.1038/srep08743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Aryal M, Vykhodtseva N, Zhang Y-Z, Park J, McDannold N. Multiple treatments with liposomal doxorubicin and ultrasound-induced disruption of blood-tumor and blood-brain barriers improve outcomes in a rat glioma model. J. Control. Release. 2013;169:103–111. doi: 10.1016/j.jconrel.2013.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Liu H-L, Huang C-Y, Chen J-Y, Wang H-YJ, Chen P-Y, Wei K-C. Pharmacodynamic and therapeutic investigation of focused ultrasound-induced blood-brain barrier opening for enhanced temozolomide delivery in glioma treatment. PLoS One. 2014;9:e114311. doi: 10.1371/journal.pone.0114311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Treat LH, McDannold N, Vykhodtseva N, Zhang Y, Tam K, Hynynen K. Targeted delivery of doxorubicin to the rat brain at therapeutic levels using MRI-guided focused ultrasound. Int. J. Cancer. 2007;121:901–907. doi: 10.1002/ijc.22732. [DOI] [PubMed] [Google Scholar]
- 86.Jordão JF, a Ayala-Grosso C, Markham K, Huang Y, Chopra R, McLaurin J, et al. Antibodies targeted to the brain with image-guided focused ultrasound reduces amyloid-beta plaque load in the TgCRND8 mouse model of Alzheimer’s disease. PLoS One. 2010;5:e10549. doi: 10.1371/journal.pone.0010549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Samiotaki G, Acosta C, Wang S, Konofagou EE. Enhanced delivery and bioactivity of the neurturin neurotrophic factor through focused ultrasound-mediated blood-brain barrier opening in vivo. J. Cereb. Blood Flow Metab. 2015;35:611–622. doi: 10.1038/jcbfm.2014.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Aryal M, Park J, Vykhodtseva N, Zhang Y-Z, McDannold N. Enhancement in blood-tumor barrier permeability and delivery of liposomal doxorubicin using focused ultrasound and microbubbles: evaluation during tumor progression in a rat glioma model. Phys. Med. Biol. 2015;60:2511–2527. doi: 10.1088/0031-9155/60/6/2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Fan C-H, Ting C-Y, Liu H-L, Huang C-Y, Hsieh H-Y, Yen T-C, et al. Antiangiogenic-targeting drug-loaded microbubbles combined with focused ultrasound for glioma treatment. Biomaterials. 2013;34:2142–2155. doi: 10.1016/j.biomaterials.2012.11.048. [DOI] [PubMed] [Google Scholar]
- 90.Yang F-Y, Wong T-T, Teng M-C, Liu R-S, Lu M, Liang H-F, et al. Focused ultrasound and interleukin-4 receptor-targeted liposomal doxorubicin for enhanced targeted drug delivery and antitumor effect in glioblastoma multiforme. J. Control. Release. 2012;160:652–658. doi: 10.1016/j.jconrel.2012.02.023. [DOI] [PubMed] [Google Scholar]
- 91.Alkins RD, Brodersen PM, Sodhi RNS, Hynynen K. Enhancing drug delivery for boron neutron capture therapy of brain tumors with focused ultrasound. Neuro. Oncol. 2013;15:1225–1235. doi: 10.1093/neuonc/not052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wei K-C, Chu P-C, Wang H-YJ, Huang C-Y, Chen P-Y, Tsai H-C, et al. Focused ultrasound-induced blood-brain barrier opening to enhance temozolomide delivery for glioblastoma treatment: a preclinical study. PLoS One. 2013;8:e58995. doi: 10.1371/journal.pone.0058995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Malmström A, Grønberg BH, Marosi C, Stupp R, Frappaz D, Schultz H, et al. Temozolomide versus standard 6-week radiotherapy versus hypofractionated radiotherapy in patients older than 60 years with glioblastoma: the Nordic randomised, phase 3 trial. Lancet. Oncol. 2012;13:916–926. doi: 10.1016/S1470-2045(12)70265-6. [DOI] [PubMed] [Google Scholar]
- 94.Darlix A, Baumann C, Lorgis V, Ghiringhelli F, Blonski M, Chauffert B, et al. Prolonged administration of adjuvant temozolomide improves survival in adult patients with glioblastoma. [accessed April 11, 2015];Anticancer Res. 2013 33:3467–3474. http://www.ncbi.nlm.nih.gov/pubmed/23898121. [PubMed] [Google Scholar]
- 95.Gilbert MR, Wang M, Aldape KD, Stupp R, Hegi ME, Jaeckle KA, et al. Dose-dense temozolomide for newly diagnosed glioblastoma: a randomized phase III clinical trial. J. Clin. Oncol. 2013;31:4085–4091. doi: 10.1200/JCO.2013.49.6968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Norden AD, Lesser GJ, Drappatz J, Ligon KL, Hammond SN, Lee EQ, et al. Phase 2 study of dose-intense temozolomide in recurrent glioblastoma. Neuro. Oncol. 2013;15:930–935. doi: 10.1093/neuonc/not040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Westphal M, Hilt DC, Bortey E, Delavault P, Olivares R, Warnke PC, et al. A phase 3 trial of local chemotherapy with biodegradable carmustine (BCNU) wafers (Gliadel wafers) in patients with primary malignant glioma. Neuro. Oncol. 2003;5:79–88. doi: 10.1215/S1522-8517-02-00023-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gururangan S, Cokgor L, Rich JN, Edwards S, Affronti ML, Quinn JA, et al. Phase I study of Gliadel wafers plus temozolomide in adults with recurrent supratentorial high-grade gliomas. [accessed April 11, 2015];Neuro. Oncol. 2001 3:246–2450. doi: 10.1093/neuonc/3.4.246. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1920622&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Westphal M, Ram Z, Riddle V, Hilt D, Bortey E. Gliadel wafer in initial surgery for malignant glioma: long-term follow-up of a multicenter controlled trial. Acta Neurochir. (Wien) 2006;148:269–275. doi: 10.1007/s00701-005-0707-z. discussion 275. [DOI] [PubMed] [Google Scholar]
- 100.Attenello FJ, Mukherjee D, Datoo G, McGirt MJ, Bohan E, Weingart JD, et al. Use of Gliadel (BCNU) wafer in the surgical treatment of malignant glioma: a 10-year institutional experience. Ann. Surg. Oncol. 2008;15:2887–2893. doi: 10.1245/s10434-008-0048-2. [DOI] [PubMed] [Google Scholar]
- 101.Liu H-L, Hua M-Y, Chen P-Y, Chu P-C, Pan C-H, Yang H-W, et al. Blood-brain barrier disruption with focused ultrasound enhances delivery of chemotherapeutic drugs for glioblastoma treatment. Radiology. 2010;255:415–425. doi: 10.1148/radiol.10090699. [DOI] [PubMed] [Google Scholar]
- 102.Hau P, Fabel K, Baumgart U, Rümmele P, Grauer O, Bock A. et al. Pegylated liposomal doxorubicin-efficacy in patients with recurrent high-grade glioma, Cancer. 2004;100:1199–1207. doi: 10.1002/cncr.20073. [DOI] [PubMed] [Google Scholar]
- 103.Fabel K, Dietrich J, Hau P, Wismeth C, Winner B, Przywara S, et al. Long-term stabilization in patients with malignant glioma after treatment with liposomal doxorubicin. [accessed April 12, 2015];Cancer. 2001 92:1936–1942. doi: 10.1002/1097-0142(20011001)92:7<1936::aid-cncr1712>3.0.co;2-h. http://www.ncbi.nlm.nih.gov/pubmed/11745268. [DOI] [PubMed] [Google Scholar]
- 104.Ananda S, Nowak AK, Cher L, Dowling A, Brown C, Simes J, et al. Phase 2 trial of temozolomide and pegylated liposomal doxorubicin in the treatment of patients with glioblastoma multiforme following concurrent radiotherapy and chemotherapy. J. Clin. Neurosci. 2011;18:1444–1448. doi: 10.1016/j.jocn.2011.02.026. [DOI] [PubMed] [Google Scholar]
- 105.Kovacs Z, Werner B, Rassi A, Sass JO, Martin-Fiori E, Bernasconi M. Prolonged survival upon ultrasound-enhanced doxorubicin delivery in two syngenic glioblastoma mouse models. J. Control. Release. 2014 doi: 10.1016/j.jconrel.2014.05.033. [DOI] [PubMed] [Google Scholar]
- 106.Savolainen S, Kortesniemi M, Timonen M, Reijonen V, Kuusela L, Uusi-Simola J, et al. Boron neutron capture therapy (BNCT) in Finland: technological and physical prospects after 20 years of experiences. Phys. Med. 2013;29:233–248. doi: 10.1016/j.ejmp.2012.04.008. [DOI] [PubMed] [Google Scholar]
- 107.Kankaanranta L, Seppälä T, Koivunoro H, Saarilahti K, Atula T, Collan J, et al. Boron neutron capture therapy in the treatment of locally recurred head-and-neck cancer: final analysis of a phase I/II trial. Int. J. Radiat. Oncol. Biol. Phys. 2012;82:e67–e75. doi: 10.1016/j.ijrobp.2010.09.057. [DOI] [PubMed] [Google Scholar]
- 108.Miyatake S-I, Kawabata S, Yokoyama K, Kuroiwa T, Michiue H, Sakurai Y, et al. Survival benefit of boron neutron capture therapy for recurrent malignant gliomas. Appl. Radiat. Isot. 2009;67:S22–S24. doi: 10.1016/j.apradiso.2009.03.032. [DOI] [PubMed] [Google Scholar]
- 109.Hopewell JW, Gorlia T, Pellettieri L, Giusti V, H-Stenstam B, Sköld K. Boron neutron capture therapy for newly diagnosed glioblastoma multiforme: an assessment of clinical potential. Appl. Radiat. Isot. 2011;69:1737–1740. doi: 10.1016/j.apradiso.2011.03.022. [DOI] [PubMed] [Google Scholar]
- 110.Yang F-Y, Chen Y-W, Chou F-I, Yen S-H, Lin Y-L, Wong T-T. Boron neutron capture therapy for glioblastoma multiforme: enhanced drug delivery and antitumor effect following blood-brain barrier disruption induced by focused ultrasound. Future Oncol. 2012;8:1361–1369. doi: 10.2217/fon.12.118. [DOI] [PubMed] [Google Scholar]
- 111.Yang F-Y, Lin Y-L, Chou F-I, Lin Y-C, Hsueh Liu Y-W, Chang L-W, et al. Pharmacokinetics of BPA in Gliomas with Ultrasound Induced Blood-Brain Barrier Disruption as Measured by Microdialysis. PLoS One. 2014;9:e100104. doi: 10.1371/journal.pone.0100104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kawalec P, Łopuch S, Mikrut A. Effectiveness of Targeted Therapy in Patients With Previously Untreated Metastatic Breast Cancer: A Systematic Review and Meta-Analysis. Clin. Breast Cancer. 2015;15:90–100.e1. doi: 10.1016/j.clbc.2014.10.006. [DOI] [PubMed] [Google Scholar]
- 113.Spencer B, Masliah E. Immunotherapy for Alzheimer’s disease: past, present and future. Front. Aging Neurosci. 2014;6:114. doi: 10.3389/fnagi.2014.00114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Sheikov N, McDannold N, Vykhodtseva N, Jolesz F, Hynynen K. Cellular mechanisms of the blood-brain barrier opening induced by ultrasound in presence of microbubbles. Ultrasound Med. Biol. 2004;30:979–989. doi: 10.1016/j.ultrasmedbio.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 115.Kinoshita M, McDannold N, Jolesz FA, Hynynen K. Targeted delivery of antibodies through the blood-brain barrier by MRI-guided focused ultrasound. Biochem. Biophys. Res. Commun. 2006;340:1085–1090. doi: 10.1016/j.bbrc.2005.12.112. [DOI] [PubMed] [Google Scholar]
- 116.Raymond SB, Treat LH, Dewey JD, McDannold NJ, Hynynen K, Bacskai BJ. Ultrasound enhanced delivery of molecular imaging and therapeutic agents in Alzheimer’s disease mouse models. PLoS One. 2008;3:e2175. doi: 10.1371/journal.pone.0002175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kinoshita M, McDannold N, a Jolesz F, Hynynen K. Noninvasive localized delivery of Herceptin to the mouse brain by MRI-guided focused ultrasound-induced blood-brain barrier disruption. Proc. Natl. Acad. Sci. U. S. A. 2006;103:11719–11723. doi: 10.1073/pnas.0604318103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ahmed AO, Mantini AM, Fridberg DJ, Buckley PF. Brain-derived neurotrophic factor (BDNF) and neurocognitive deficits in people with schizophrenia: A meta-analysis. Psychiatry Res. 2015;226:1–13. doi: 10.1016/j.psychres.2014.12.069. [DOI] [PubMed] [Google Scholar]
- 119.Kalia M, Costa E, Silva J. Biomarkers of psychiatric diseases: current status and future prospects. Metabolism. 2015;64:S11–S15. doi: 10.1016/j.metabol.2014.10.026. [DOI] [PubMed] [Google Scholar]
- 120.Theoharides TC, Athanassiou M, Panagiotidou S, Doyle R. Dysregulated brain immunity and neurotrophin signaling in Rett syndrome and autism spectrum disorders. J. Neuroimmunol. 2015;279:33–38. doi: 10.1016/j.jneuroim.2014.12.003. [DOI] [PubMed] [Google Scholar]
- 121.V Hegarty S, O’Keeffe GW, Sullivan AM. Neurotrophic factors: from neurodevelopmental regulators to novel therapies for Parkinson’s disease. Neural Regen. Res. 2014;9:1708–1711. doi: 10.4103/1673-5374.143410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Baseri B, Choi JJ, Deffieux T, Samiotaki G, Tung Y-S, Olumolade O, et al. Activation of signaling pathways following localized delivery of systemically administered neurotrophic factors across the blood-brain barrier using focused ultrasound and microbubbles. Phys. Med. Biol. 2012;57:N65–N81. doi: 10.1088/0031-9155/57/7/N65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Wang F, Shi Y, Lu L, Liu L, Cai Y, Zheng H, et al. Targeted delivery of GDNF through the blood-brain barrier by MRI-guided focused ultrasound. PLoS One. 2012;7:e52925. doi: 10.1371/journal.pone.0052925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Thomas AA, Brennan CW, DeAngelis LM, Omuro AM. Emerging therapies for glioblastoma. JAMA Neurol. 2014;71:1437–1444. doi: 10.1001/jamaneurol.2014.1701. [DOI] [PubMed] [Google Scholar]
- 125.Ampie L, Woolf EC, Dardis C. Immunotherapeutic advancements for glioblastoma. Front. Oncol. 2015;5:12. doi: 10.3389/fonc.2015.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Chen P-Y, Hsieh H-Y, Huang C-Y, Lin C-Y, Wei K-C, Liu H-L. Focused ultrasound-induced blood-brain barrier opening to enhance interleukin-12 delivery for brain tumor immunotherapy: a preclinical feasibility study. J. Transl. Med. 2015;13:451. doi: 10.1186/s12967-015-0451-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Bozzuto G, Molinari A. Liposomes as nanomedical devices. Int. J. Nanomedicine. 2015;10:975–999. doi: 10.2147/IJN.S68861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Mohan A, Narayanan S, Sethuraman S, Krishnan UM. Novel resveratrol and 5-fluorouracil coencapsulated in PEGylated nanoliposomes improve chemotherapeutic efficacy of combination against head and neck squamous cell carcinoma. Biomed Res. Int. 2014;2014:424239. doi: 10.1155/2014/424239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Immordino ML, Dosio F, Cattel L. Stealth liposomes: review of the basic science rationale, clinical applications, existing and potential. [accessed February 12, 2015];Int. J. Nanomedicine. 2006 1:297–315. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2426795&tool=pmcentrez&rendertype=abstract. [PMC free article] [PubMed] [Google Scholar]
- 130.Dicheva BM, Koning GA. Targeted thermosensitive liposomes: an attractive novel approach for increased drug delivery to solid tumors. Expert Opin. Drug Deliv. 2014;11:83–100. doi: 10.1517/17425247.2014.866650. [DOI] [PubMed] [Google Scholar]
- 131.Storm G, Roerdink FH, Steerenberg PA, de Jong WH, Crommelin DJ. Influence of lipid composition on the antitumor activity exerted by doxorubicin-containing liposomes in a rat solid tumor model. [accessed April 10, 2015];Cancer Res. 1987 47:3366–3372. http://www.ncbi.nlm.nih.gov/pubmed/3581073. [PubMed] [Google Scholar]
- 132.Treat LH, McDannold N, Zhang Y, Vykhodtseva N, Hynynen K. Improved anti-tumor effect of liposomal doxorubicin after targeted blood-brain barrier disruption by MRI-guided focused ultrasound in rat glioma. Ultrasound Med. Biol. 2012;38:1716–1725. doi: 10.1016/j.ultrasmedbio.2012.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Yang F-Y, Wang H-E, Liu R-S, Teng M-C, Li J-J, Lu M. et al. Pharmacokinetic Analysis of 111In-Labeled Liposomal Doxorubicin in Murine Glioblastoma after Blood-Brain Barrier Disruption by Focused Ultrasound, PLoS One. 2012;7:e45468. doi: 10.1371/journal.pone.0045468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Aryal M, Vykhodtseva N, Zhang Y-Z, McDannold N. Multiple sessions of liposomal doxorubicin delivery via focused ultrasound mediated blood-brain barrier disruption: A safety study. J. Control. Release. 2015;204:60–69. doi: 10.1016/j.jconrel.2015.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Nomikou N, Tiwari P, Trehan T, Gulati K, McHale AP, Studies on neutral. cationic and biotinylated cationic microbubbles in enhancing ultrasound-mediated gene delivery in vitro and in vivo. Acta Biomater. 2012;8:1273–1280. doi: 10.1016/j.actbio.2011.09.010. [DOI] [PubMed] [Google Scholar]
- 136.Fan C-H, Liu H-L, Ting C-Y, Lee Y-H, Huang C-Y, Ma Y-J, et al. Submicron-bubble-enhanced focused ultrasound for blood-brain barrier disruption and improved CNS drug delivery. PLoS One. 2014;9:e96327. doi: 10.1371/journal.pone.0096327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Fan C-H, Ting C-Y, Chang Y-C, Wei K-C, Liu H-L, Yeh C-K. Drug-loaded bubbles with matched focused ultrasound excitation for concurrent blood-brain barrier opening and brain-tumor drug delivery. Acta Biomater. 2015;15:89–101. doi: 10.1016/j.actbio.2014.12.026. [DOI] [PubMed] [Google Scholar]
- 138.Ting C-Y, Fan C-H, Liu H-L, Huang C-Y, Hsieh H-Y, Yen T-C, et al. Concurrent blood-brain barrier opening and local drug delivery using drug-carrying microbubbles and focused ultrasound for brain glioma treatment. Biomaterials. 2012;33:704–712. doi: 10.1016/j.biomaterials.2011.09.096. [DOI] [PubMed] [Google Scholar]
- 139.Fan C-H, Ting C-Y, Lin H-J, Wang C-H, Liu H-L, Yen T-C, et al. SPIO-conjugated, doxorubicin-loaded microbubbles for concurrent MRI and focused-ultrasound enhanced brain-tumor drug delivery. Biomaterials. 2013;34:3706–3715. doi: 10.1016/j.biomaterials.2013.01.099. [DOI] [PubMed] [Google Scholar]
- 140.Tereshchenko J, Maddalena A, Bähr M, Kügler S. Pharmacologically controlled, discontinuous GDNF gene therapy restores motor function in a rat model of Parkinson’s disease. Neurobiol. Dis. 2014;65:35–42. doi: 10.1016/j.nbd.2014.01.009. [DOI] [PubMed] [Google Scholar]
- 141.Bäck S, Peränen J, Galli E, Pulkkila P, Lonka-Nevalaita L, Tamminen T, et al. Gene therapy with AAV2-CDNF provides functional benefits in a rat model of Parkinson’s disease. Brain Behav. 2013;3:75–88. doi: 10.1002/brb3.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Bjorklund T, Kordower JH. Gene therapy for Parkinson’s disease. Mov. Disord. 2010;25(Suppl 1):S161–S173. doi: 10.1002/mds.22785. [DOI] [PubMed] [Google Scholar]
- 143.a LeWitt P, Rezai AR, a Leehey M, Ojemann SG, Flaherty AW, Eskandar EN, et al. AAV2-GAD gene therapy for advanced Parkinson’s disease: a double-blind, sham-surgery controlled, randomised trial. Lancet Neurol. 2011;10:309–319. doi: 10.1016/S1474-4422(11)70039-4. [DOI] [PubMed] [Google Scholar]
- 144.Murphy SR, Chang CCY, Dogbevia G, Bryleva EY, Bowen Z, Hasan MT, et al. Acat1 Knockdown Gene Therapy Decreases Amyloid- β in a Mouse Model of Alzheimer ‘ s Disease. 2013;21:1497–1506. doi: 10.1038/mt.2013.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Mandel RJ. CERE-110, an adeno-associated virus-based gene delivery vector expressing human nerve growth factor for the treatment of Alzheimer’s disease. Curr. Opin. Mol. Ther. 2010;12:240–247. [PubMed] [Google Scholar]
- 146.Mccurdy VJ, Rockwell HE, Arthur JR, Bradbury AM, Johnson AK, Randle AN. et al. Widespread correction of central nervous system disease after intracranial gene therapy in a feline model of Sandhoff disease, Gene Ther. 2014;22:181–189. doi: 10.1038/gt.2014.108. [DOI] [PubMed] [Google Scholar]
- 147.Berry M, Barrett L, Seymour L, Baird A, Logan A. Gene therapy for central nervous system repair. Curr. Opin. Mol. Ther. 2001;3:338–349. [PubMed] [Google Scholar]
- 148.Okura H, Smith CA, Rutka JT. Gene therapy for malignant glioma. Expert Rev. Neurother. 2006;6:479–488. doi: 10.1586/14737175.6.4.479. [DOI] [PubMed] [Google Scholar]
- 149.Bankiewicz KS, Eberling JL, Kohutnicka M, Jagust W, Pivirotto P, Bringas J, et al. Convection-enhanced delivery of AAV vector in parkinsonian monkeys; in vivo detection of gene expression and restoration of dopaminergic function using pro-drug approach. Exp. Neurol. 2000;164:2–14. doi: 10.1006/exnr.2000.7408. [DOI] [PubMed] [Google Scholar]
- 150.Shahar T, Ram Z, Kanner Aa. Convection-enhanced delivery catheter placements for high-grade gliomas: Complications and pitfalls. J. Neurooncol. 2012;107:373–378. doi: 10.1007/s11060-011-0751-x. [DOI] [PubMed] [Google Scholar]
- 151.Allard E, Passirani C, Benoit J-P. Convection-enhanced delivery of nanocarriers for the treatment of brain tumors. Biomaterials. 2009;30:2302–2318. doi: 10.1016/j.biomaterials.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 152.Omata D, Negishi Y, Hagiwara S, Yamamura S, Endo-Takahashi Y, Suzuki R, et al. Bubble liposomes and ultrasound promoted endosomal escape of TAT-PEG liposomes as gene delivery carriers. Mol. Pharm. 2011;8:2416–2423. doi: 10.1021/mp200353m. [DOI] [PubMed] [Google Scholar]
- 153.Omata D, Negishi Y, Suzuki R, Oda Y. Nonviral Gene Delivery Systems by the Combination of Bubble Liposomes and Ultrasound. Elsevier Ltd. 2015 doi: 10.1016/bs.adgen.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 154.Burke CW, Suk JS, Kim AJ, Hsiang Y-HJ, Klibanov AL, Hanes J. et al. Markedly Enhanced Skeletal Muscle Transfection Achieved by the Ultrasound-Targeted Delivery of Non-Viral Gene Nanocarriers with Microbubbles, J. Control. Release. 2012;162:414–421. doi: 10.1016/j.jconrel.2012.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Xenariou S, Liang HD, Griesenbach U, Zhu J, Farley R, Somerton L. et al. Low-frequency ultrasound increases non-viral gene transfer to the mouse lung, Acta Biochim. Biophys. Sin. (Shanghai) 2010;42:45–51. doi: 10.1093/abbs/gmp100. [DOI] [PubMed] [Google Scholar]
- 156.Park DH, Jung BK, Lee YS, Jang JY, Kim MK, Lee JK. et al. Evaluation of in vivo antitumor effects of ANT2 shRNA delivered using PEI and ultrasound with microbubbles, Gene Ther. 2015;22:325–332. doi: 10.1038/gt.2014.120. [DOI] [PubMed] [Google Scholar]
- 157.Xie W, Liu S, Su H, Wang Z, Zheng Y, Fu Y. Ultrasound microbubbles enhance recombinant adeno-associated virus vector delivery to retinal ganglion cells in vivo. Acad. Radiol. 2010;17:1242–1248. doi: 10.1016/j.acra.2010.05.008. [DOI] [PubMed] [Google Scholar]
- 158.Sorace AG, Warram JM, Mahoney M, Zinn KR, Hoyt K. Enhancement of Adenovirus Delivery after Ultrasound-Stimulated Therapy in a Cancer Model. Ultrasound Med. Biol. 2013;39:2374–2381. doi: 10.1016/j.ultrasmedbio.2013.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Konishi M, Kawamoto K, Izumikawa M, Kuriyama H, Yamashita T. Gene transfer into guinea pig cochlea using adeno-associated virus vectors. J. Gene Med. 2008;10:610–618. doi: 10.1002/jgm.1189. [DOI] [PubMed] [Google Scholar]
- 160.Christiansen JP, French Ba, Klibanov AL, Kaul S, Lindner JR. Targeted tissue transfection with ultrasound destruction of plasmid-bearing cationic microbubbles. Ultrasound Med. Biol. 2003;29:1759–1767. doi: 10.1016/s0301-5629(03)00976-1. [DOI] [PubMed] [Google Scholar]
- 161.Thévenot E, Jordão JF, a O’Reilly M, Markham K, Weng Y-Q, Foust KD, et al. Targeted delivery of self-complementary adeno-associated virus serotype 9 to the brain, using magnetic resonance imaging-guided focused ultrasound. Hum. Gene Ther. 2012;23:1144–1155. doi: 10.1089/hum.2012.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Huang Q, Deng J, Xie Z, Wang F, Chen S, Lei B. et al. Effective Gene Transfer into Central Nervous System Following Ultrasound-Microbubbles-Induced Opening of the Blood-Brain Barrier, Ultrasound Med. Biol. 2012;38:1234–1243. doi: 10.1016/j.ultrasmedbio.2012.02.019. [DOI] [PubMed] [Google Scholar]
- 163.Huang Q, Deng J, Wang F, Chen S, Liu Y, Wang Z, et al. Targeted gene delivery to the mouse brain by MRI-guided focused ultrasound-induced blood-brain barrier disruption. Exp. Neurol. 2012;233:350–356. doi: 10.1016/j.expneurol.2011.10.027. [DOI] [PubMed] [Google Scholar]
- 164.Wang DS, Panje C, Pysz MA, Paulmurugan R, Rosenberg J, Gambhir SS. et al. Cationic versus Neutral Microbubbles for Ultrasound-mediated Gene Delivery in Cancer, Radiology. 2012;264:721–732. doi: 10.1148/radiol.12112368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Burke CW, Hsiang Y-HJ, Alexander E, Kilbanov AL, Price RJ. Covalently linking poly(lactic-co-glycolic acid) nanoparticles to microbubbles before intravenous injection improves their ultrasound-targeted delivery to skeletal muscle. Small. 2011;7:1227–1235. doi: 10.1002/smll.201001934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Burke CW, Alexander E, Timbie K, Kilbanov AL, Price RJ. Ultrasound-activated agents comprised of 5FU-bearing nanoparticles bonded to microbubbles inhibit solid tumor growth and improve survival. Mol. Ther. 2014;22:321–328. doi: 10.1038/mt.2013.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Duque S, Joussemet B, Riviere C, Marais T, Dubreil L, Douar A-M, et al. Intravenous administration of self-complementary AAV9 enables transgene delivery to adult motor neurons. Mol. Ther. 2009;17:1187–1196. doi: 10.1038/mt.2009.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Weber-Adrian D, Thévenot E, a O’Reilly M, Oakden W, Akens MK, Ellens N, et al. Gene delivery to the spinal cord using MRI-guided focused ultrasound. Gene Ther. 2015 doi: 10.1038/gt.2015.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Wang S, Olumolade OO, Sun T, Samiotaki G, Konofagou EE. Noninvasive, neuron-specific gene therapy can be facilitated by focused ultrasound and recombinant adeno-associated virus. Gene Ther. 2014 doi: 10.1038/gt.2014.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Alonso A, Reinz E, Leuchs B, Kleinschmidt J, Fatar M, Geers B, et al. Focal Delivery of AAV2/1-transgenes Into the Rat Brain by Localized Ultrasound-induced BBB Opening. Mol. Ther. Nucleic Acids. 2013;2:e73. doi: 10.1038/mtna.2012.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Hsu P-H, Wei K-C, Huang C-Y, Wen C-J, Yen T-C, Liu C-L, et al. Noninvasive and targeted gene delivery into the brain using microbubble-facilitated focused ultrasound. PLoS One. 2013;8:e57682. doi: 10.1371/journal.pone.0057682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Kaplitt MG, Leone P, Samulski RJ, Xiao X, Pfaff DW, O’Malley KL, et al. Long-term gene expression and phenotypic correction using adeno-associated virus vectors in the mammalian brain. Nat. Genet. 1994;8:148–154. doi: 10.1038/ng1094-148. [DOI] [PubMed] [Google Scholar]
- 173.McCown TJ, Xiao X, Li J, Breese GR, Samulski RJ. Differential and persistent expression patterns of CNS gene transfer by an adeno-associated virus (AAV) vector. Brain Res. 1996;713:99–107. doi: 10.1016/0006-8993(95)01488-8. [DOI] [PubMed] [Google Scholar]
- 174.Summerford C, Samulski RJ. Membrane-associated heparan sulfate proteoglycan is a receptor for adeno-associated virus type 2 virions. J. Virol. 1998;72:1438–1445. doi: 10.1128/jvi.72.2.1438-1445.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Properzi F, Lin R, Kwok J, Naidu M, Van Kuppevelt TH, Ten Dam GB. et al. Heparan sulphate proteoglycans in glia and in the normal and injured CNS: Expression of sulphotransferases and changes in sulphation, Eur. J. Neurosci. 2008;27:593–604. doi: 10.1111/j.1460-9568.2008.06042.x. [DOI] [PubMed] [Google Scholar]
- 176.Peden CS, Burger C, Muzyczka N, Mandel RJ. Circulating Anti-Wild-Type Antibodies Inhibit Recombinant AAV2 Gene Transfer in the Brain Circulating Anti-Wild-Type Adeno-Associated Virus Type 2 (AAV2) Antibodies Inhibit Recombinant AAV2 (rAAV2) -Mediated, but Not rAAV5-Mediated. Gene Transfer in, J. Virol. 2004;78:6344–6359. doi: 10.1128/JVI.78.12.6344-6359.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Calcedo R, Wilson JM. Humoral Immune Response to AAV. Front. Immunol. 2013;4:341. doi: 10.3389/fimmu.2013.00341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Wu J, Zhao W, Zhong L, Han Z, Li B, Ma W, et al. Self-complementary recombinant adeno-associated viral vectors: packaging capacity and the role of rep proteins in vector purity. Hum. Gene Ther. 2007;18:171–182. doi: 10.1089/hum.2006.088. [DOI] [PubMed] [Google Scholar]
- 179.Wilczewska AZ, Niemirowicz K, Markiewicz KH, Car H. Nanoparticles as drug delivery systems. Pharmacol. Reports. 2012;64:1020–1037. doi: 10.1016/s1734-1140(12)70901-5. [DOI] [PubMed] [Google Scholar]
- 180.Boylan NJ, Suk JS, Lai SK, Jelinek R, Boyle MP, Cooper MJ, et al. Highly compacted DNA nanoparticles with low MW PEG coatings: in vitro, ex vivo and in vivo evaluation. J. Control. Release. 2012;157:72–79. doi: 10.1016/j.jconrel.2011.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Wong HL, Wu XY, Bendayan R. Nanotechnological advances for the delivery of CNS therapeutics. Adv. Drug Deliv. Rev. 2011 doi: 10.1016/j.addr.2011.10.007. [DOI] [PubMed] [Google Scholar]
- 182.Suh J, Choy K-L, Lai SK, Suk JS, Tang BC, Prabhu S, et al. PEGylation of nanoparticles improves their cytoplasmic transport. [accessed April 4, 2012];Int. J. Nanomedicine. 2007 2:735–741. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2676827/ [PMC free article] [PubMed] [Google Scholar]
- 183.Danhier F, Ansorena E, Silva JM, Coco R, Le Breton A, Préat V. PLGA-based nanoparticles: An overview of biomedical applications. J. Control. Release. 2012 doi: 10.1016/j.jconrel.2012.01.043. [DOI] [PubMed] [Google Scholar]
- 184.Tosi G, Bortot B, Ruozi B, Dolcetta D, Vandelli MA, Forni F, et al. Potential use of polymeric nanoparticles for drug delivery across the blood-brain barrier. [accessed November 19, 2013];Curr. Med. Chem. 2013 20:2212–2225. doi: 10.2174/0929867311320170006. http://www.ncbi.nlm.nih.gov/pubmed/23458620. [DOI] [PubMed] [Google Scholar]
- 185.Giteau A, Venier-Julienne MC, Aubert-Pouëssel A, Benoit JP. How to achieve sustained and complete protein release from PLGA-based microparticles? Int. J. Pharm. 2008;350:14–26. doi: 10.1016/j.ijpharm.2007.11.012. [DOI] [PubMed] [Google Scholar]
- 186.Dufort S, Sancey L, Coll J-L. Physico-chemical parameters that govern nanoparticles fate also dictate rules for their molecular evolution. Adv. Drug Deliv. Rev. 2012;64:179–189. doi: 10.1016/j.addr.2011.09.009. [DOI] [PubMed] [Google Scholar]
- 187.Kumari A, Yadav SK, Yadav SC. Biodegradable polymeric nanoparticles based drug delivery systems. Colloids Surfaces B Biointerfaces. 2010;75:1–18. doi: 10.1016/j.colsurfb.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 188.Chappell JC, Song J, Burke CW, Klibanov AL, Price RJ. Targeted delivery of nanoparticles bearing fibroblast growth factor-2 by ultrasonic microbubble destruction for therapeutic arteriogenesis. Small. 2008;4:1769–1777. doi: 10.1002/smll.200800806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.V Naumova A, Modo M, Moore A, Murry CE, a Frank J. Clinical imaging in regenerative medicine. Nat. Biotechnol. 2014;32:804–818. doi: 10.1038/nbt.2993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Chen F, Ehlerding EB, Cai W. Theranostic Nanoparticles. J. Nucl. Med. 2014;55:1919–1922. doi: 10.2967/jnumed.114.146019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Walia S, Acharya A. Silica micro/nanospheres for theranostics: from bimodal MRI and fluorescent imaging probes to cancer therapy. Beilstein J. Nanotechnol. 2015;6:546–558. doi: 10.3762/bjnano.6.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Mastorakos P, Zhang C, Berry S, Oh Y, Lee S, Eberhart CG, et al. Highly PEGylated DNA Nanoparticles Provide Uniform and Widespread Gene Transfer in the Brain. Adv. Healthc. Mater. 2015 doi: 10.1002/adhm.201400800. n/a–n/a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Grigsby CL, Leong KW. Balancing protection and release of DNA: tools to address a bottleneck of non-viral gene delivery. J. R. Soc. Interface. 2010;7(Suppl 1):S67–S82. doi: 10.1098/rsif.2009.0260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Zimmermann DR, Dours-Zimmermann MT. Extracellular matrix of the central nervous system: From neglect to challenge. Histochem. Cell Biol. 2008;130:635–653. doi: 10.1007/s00418-008-0485-9. [DOI] [PubMed] [Google Scholar]
- 195.Syková E, Nicholson C. Diffusion in brain extracellular space. Physiol. Rev. 2008;88:1277–1340. doi: 10.1152/physrev.00027.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Kenny GD, Bienemann AS, Tagalakis AD, Pugh Ja, Welser K, Campbell F. et al. Multifunctional receptor-targeted nanocomplexes for the delivery of therapeutic nucleic acids to the Brain, Biomaterials. 2013;34:9190–9200. doi: 10.1016/j.biomaterials.2013.07.081. [DOI] [PubMed] [Google Scholar]
- 197.Nance EA, Woodworth GF, Sailor KA, Shih T-Y, Xu Q, Swaminathan G, et al. A dense poly(ethylene glycol) coating improves penetration of large polymeric nanoparticles within brain tissue. Sci. Transl. Med. 2012;4:149ra119. doi: 10.1126/scitranslmed.3003594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.a Netti P, a Berk D, a Swartz M, Grodzinsky aJ, Jain RK. Role of extracellular matrix assembly in interstitial transport in solid tumors. Cancer Res. 2000;60:2497–2503. [PubMed] [Google Scholar]
- 199.Heldin C-H, Rubin K, Pietras K, Ostman A. High interstitial fluid pressure - an obstacle in cancer therapy. Nat. Rev. Cancer. 2004;4:806–813. doi: 10.1038/nrc1456. [DOI] [PubMed] [Google Scholar]
- 200.a Pluen, Boucher Y, Ramanujan S, McKee TD, Gohongi T, di Tomaso E, et al. Role of tumor-host interactions in interstitial diffusion of macromolecules: cranial vs. subcutaneous tumors. Proc. Natl. Acad. Sci. U. S. A. 2001;98:4628–4633. doi: 10.1073/pnas.081626898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Jain RK, Stylianopoulos T. Delivering nanomedicine to solid tumors. Nat. Rev. Clin. Oncol. 2010;7:653–664. doi: 10.1038/nrclinonc.2010.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Beyer I, Li Z, Persson J, Liu Y, van Rensburg R, Yumul R, et al. Controlled extracellular matrix degradation in breast cancer tumors improves therapy by trastuzumab. Mol. Ther. 2011;19:479–489. doi: 10.1038/mt.2010.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Stylianopoulos T, Poh MZ, Insin N, Bawendi MG, Fukumura D, Munn LL. et al. Diffusion of particles in the extracellular matrix: The effect of repulsive electrostatic interactions, Biophys. J. 2010;99:1342–1349. doi: 10.1016/j.bpj.2010.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Thorne RG, Nicholson C. In vivo diffusion analysis with quantum dots and dextrans predicts the width of brain extracellular space. Proc. Natl. Acad. Sci. U. S. A. 2006;103:5567–5572. doi: 10.1073/pnas.0509425103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Nance E, Timbie K, Miller GW, Song J, Louttit C, Klibanov AL, et al. Non-invasive delivery of stealth, brain-penetrating nanoparticles across the blood-brain barrier using MRI-guided focused ultrasound. J. Control Release. 2014;189:123–132. doi: 10.1016/j.jconrel.2014.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Nance E, Zhang C, Shih T-Y, Xu Q, Schuster BS, Hanes J. Brain-penetrating nanoparticles improve paclitaxel efficacy in malignant glioma following local administration. ACS Nano. 2014;8:10655–10664. doi: 10.1021/nn504210g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Gref R, Minamitake Y, Peracchia MT, Trubetskoy V, Torchilin V, Langer R. Biodegradable long-circulating polymeric nanospheres. Science. 1994;263:1600–1603. doi: 10.1126/science.8128245. [DOI] [PubMed] [Google Scholar]
- 208.Calvo P, Gouritin B, Chacun H, Desmaile D, D’Angelo J, Noel JP. et al. Long-circulating pegylated polycyanoacrylate nanoparticles as new drug carrier for brain delivery, Pharm. Res. 2001;18:1157–1166. doi: 10.1023/a:1010931127745. [DOI] [PubMed] [Google Scholar]
- 209.Morizet J, Eve G, El H. Poly (ethylene glycol) -Coated Hexadecylcyanoacrylate Nanospheres Display a Combined Effect for Brain Tumor Targeting. J. Pharmacol. Exp. Ther. 2002;303:928–936. doi: 10.1124/jpet.102.039669. [DOI] [PubMed] [Google Scholar]
- 210.Burgess A, Huang Y, Querbes W, Sah DW, Hynynen K. Focused ultrasound for targeted delivery of siRNA and efficient knockdown of Htt expression. J. Control. Release. 2012;163:125–129. doi: 10.1016/j.jconrel.2012.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Timbie K, Burke C, Nance E, Woodworth G, Miller GW, Hanes J, et al. Ultrasound-targeted delivery of systemically administered therapeutic nanoparticles. J. Acoust. Soc. Am. 2013;134:4047. [Google Scholar]