Abstract
Several cutaneous inflammatory diseases and their clinical phenotypes are recapitulated in animal models of skin disease. However, the identification of shared pathways for disease progression is limited by the ability to delineate the complex biochemical processes fundamental for development of the disease. Identifying common signaling pathways that contribute to cutaneous inflammation and immune function will facilitate better scientific and therapeutic strategies to span a variety of inflammatory skin diseases. Aberrant antimicrobial peptide (AMP) expression and activity is one mechanism behind the development and severity of several inflammatory skin diseases and directly influences the susceptibility of skin to microbial infections. Our studies have recently exposed a newly identified pathway for negative regulation of AMPs in the skin by the cholinergic anti-inflammatory pathway via acetylcholine (ACh). The role of ACh in AMP regulation of immune and permeability barrier function in keratinocytes is reviewed, and the importance for a better comprehension of cutaneous disease progression by cholinergic signaling is discussed.
INTRODUCTION AND SCOPE OF THE PROBLEM
Inflammatory skin diseases are a major cause of morbidity and mortality for patients, where the atypical cytokine and cellular milieu creates a reservoir for cutaneous infection and diminished barrier function. Loss of skin permeability barrier function leads to excessive water loss and penetration of irritants, allergens, and microbes. Moreover, barrier function studies demonstrate that physical cutaneous permeability barrier (e.g. electrolyte and water movement) and the chemical antimicrobial barrier (e.g. AMP) are co-dependent (Ahrens, Schunck et al. 2011). Studies have shown that a diminished permeability barrier function is associated with reduced lipid synthesis and AMP production (Aberg, Man et al. 2008). Injury and infection act as a trigger for the development of numerous skin diseases by providing a stimulus for activation of distinct inflammatory pathways. Infections caused by viral, bacterial or fungal agents can not only initiate disease directly, but further exacerbate inflammatory skin diseases. Although staphylococcal and streptococcal infections often precede the development of psoriasis, which is generally considered the most stress-responsive skin disease compared to atopic dermatitis (AD) and acne vulgaris (Griffiths and Richards 2001), psoriatic plaques are generally resistant to infection (Raychaudhuri and Raychaudhuri 1993). AD is also initiated and worsened by prolonged psychological stress and injury or infection; however lesions from these patients tend to be more susceptible to colonization and infection following development of the disease (Ong, Ohtake et al. 2002; Nomura, Goleva et al. 2003). Thus, the inflammatory pathways activated in response to primary injury or infection further exacerbate the barrier defect to facilitate disease progression. The current paradigm tends to categorize inflammatory responses as either pro- or anti-inflammatory, but fails to recognize the unifying concepts that initiate these injury and infection response patterns.
Both anecdotal and clinical evidence for the role of perceived stress in the pathophysiology of skin diseases has existed for decades (Kimyai-Asadi and Usman 2001; Picardi and Abeni 2001). This effect is twofold, where stress can initiate the onset of symptoms and, at a later time, the disease manifestations can become a major source of stress for the patients to further exacerbate the clinical manifestations of their disease. Although the influence of glucocorticoids (GCs) and catecholamines on skin barrier and innate immune function have been more thoroughly studied in the context of skin infection and disease progression, less attention has been given to the role of the cholinergic system on epidermal permeability barrier and innate immune function. Our recent investigations into how the neuroendocrine system influences epidermal AMP function has uncovered a newly recognized pathway for the negative regulation of AMPs in the skin through the activation of nicotinic acetylcholine receptors (nAChRs) by ACh. Here, we will review the current mechanisms related to cholinergic signaling believed to trigger stress-induced skin disorders by modulating keratinocyte innate immune and barrier function, with a focus on AMP regulation.
THE HPA AXIS, ADRENERGIC PATHWAY, AND THE CHOLINERGIC PATHWAY OF THE STRESS RESPONSE
Within the last few decades, a significant amount of evidence suggests that an elaborate communication network exists between the nervous, endocrine, and innate immune systems to delicately balance the response to microbial invasion and/or injury. The immediate host reaction to physical insult, such as wounding or pathogen challenge, or to psychological stress is the rapid activation of the evolutionarily conserved “fight or flight” response. This initial danger-associated response promotes inflammation and immune cell infiltration to combat an infectious threat or injury, which is then followed by immunosuppression via neuroendocrine pathways to return to homeostasis (Tracey 2002). Once the stressors are eliminated, the biochemical profile of the organism is expected to return to normal physiologic homeostasis. However, prolonged activation of neuroendocrine responses can directly promote systemic immunosuppression (reviewed in (Dhabhar 2000)).
Stress leads to activation of the parasympathetic (PSNS) and sympathetic nervous systems (SNS), as well as the hypothalamic-pituitary-adrenal axis (HPA). Activation of the PSNS results in the release of ACh from nerve fibers innervating the organs, resulting in activation of nAChR and/or muscarinic acetylcholine receptors (mAChRs). Activation of the SNS results in the release of epinephrine/norepinephrine (E/NE), activating α- or β-adrenergic receptors. PSNS and SNS activation mediates a variety of immunological and homeostatic functions, including changes in cellular proliferation, differentiation, and migration ((Maestroni 2006) (reviewed in (Slominski and Wortsman 2000)). Activation of the HPA axis stimulates the release of several neuroendocrine mediators, such as GCs (e.g. cortisol), into the bloodstream that act upon specific cell populations and tissues to generate an immunosuppressive effect and return proinflammatory mediators to normal, baseline levels (Herman and Cullinan 1997). However, prolonged or chronic stress tends to be immunosuppressive, in part, by dampening cytokine production and immune cell phagocytic capacity ((Abang, Baum et al. 2006); (Ashcraft and Bonneau 2008); (Dhabhar, Saul et al. 2010)). This feedback loop was discovered over a decade ago by Kevin Tracey and colleagues. This pathway was later coined the “Cholinergic anti-inflammatory pathway” after efferent activity in the vagus nerve was found to inhibit local pro-inflammatory production of cytokines by signaling through the innervated regions of major organs involved in the response to endotoxin, without affecting the production of anti-inflammatory cytokines (Tajima, Endo et al. 1996; Bernik, Friedman et al. 2002; Saeed, Varma et al. 2005; Pavlov, Ochani et al. 2006). Our studies recently revealed that cholinergic activation in non-neuronal keratinocytes is a major pathway involved in the suppression of keratinocyte antimicrobial activity, and is the focus of this review.
LINKING STRESS RESPONSES TO EPIDERMAL INNATE IMMUNE FUNCTION DURING INJURY OR INFECTION
Dynamic cross-talk is required between the nervous, endocrine, and immune systems to regulate inflammatory processes across a vast array of cell types and tissues (Elenkov, Wilder et al. 2000; Tracey 2002). Many of the neuroendocrine ligands and ligand-receptors derived from all three branches of the stress response pathways participate in cellular communication and are expressed by immune, nerve, and epidermal cells. For example, macrophages release TNF-α during injury or infection to amplify the inflammatory response by stimulating the release of pro-inflammatory mediators, including IL-1, reactive oxygen species (ROS), nitric oxide (NO), and eicosanoids (Wood, Jackson et al. 1992; Nickoloff and Naidu 1994). During the initial fight-or-flight response, all three pathways of the stress response are coordinately activated to modulate inflammation. For example, E/NE can block macrophage activation and dampen production of TNF-α and other proinflammatory cytokines (Chrousos 2000; Molina 2001; Molina, Bagby et al. 2001). Alternatively, E/NE can induce the release of the anti-inflammatory cytokine, interleukin-10 (IL-10) from monocytes (van der Poll, Coyle et al. 1996; Woiciechowsky, Asadullah et al. 1998). In parallel, ACh has the capacity to inhibit macrophage activation and release of pro-inflammatory cytokines, such as TNF-α, IL-1, and high mobility group protein B1 (HMBG1) (Borovikova, Ivanova et al. 2000), ultimately limiting tissue damage caused by excessive inflammation.
Psychological stress was initially found to delay wound healing and diminish epidermal barrier homeostasis (Kiecolt-Glaser, Marucha et al. 1995; Denda, Tsuchiya et al. 1998; Marucha, Kiecolt-Glaser et al. 1998; Garg, Chren et al. 2001; Rojas, Padgett et al. 2002), mediated through the activation of both GC and adrenergic signaling pathways in the skin. Mice subjected to insomnia stress for 3 days demonstrated a decrease in the protein abundance of two major skin AMPs, CAMP and mBD-3, compared to unstressed mice. Moreover, these mice were more susceptible to cutaneous Group A Streptococcus (GAS) infection, as indicated by larger skin lesions compared to unstressed mice. The adverse affect of stress on AMP expression and lesion size was reversed by systemic administration of RU-486, a GC/progesterone antagonist, suggesting GC-receptor mediated activation was involved in the suppression of AMP expression (Aberg, Radek et al. 2007). These studies were the first to establish a direct connection between stress and AMP expression in the context of cutaneous infection. Earlier studies showed that catecholamines promote the release of fully processed active AMPs from the skin surface (Benson and Hadley 1969; Zasloff 1987). More recent observations in keratinocytes demonstrated that β-adrenergic activation impairs cell motility and wound closure, indicating a pathway by which stress impairs cutaneous healing (Pullar, Rizzo et al. 2006; Sivamani, Pullar et al. 2009). Together, mediators of the stress response can directly or indirectly influence several key components of epidermal defense.
THE EVOLUTION OF THE CHOLINERGIC “INFLAMMATORY REFLEX”
During embryonic development, the skin and nervous system are both derived the neuroectoderm. Consequently, many key factors that influence neuronal CNS activities also contribute to non-neuronal cellular function, since most immune and epidermal cells express several components of the cholinergic system ((reviewed in (Radek 2010). ACh can facilitate the communication between immune and epidermal cells to mount a physiologic response to injury or infection. Langerhans cells, mast cells, neutrophils, and macrophages all express various components required for cholinergic signaling via ACh, including the enzymes mediating ACh synthesis and degradation, as well as nAChR and mAChR subunits (reviewed in (Kawashima and Fujii 2004)). Previous work by Kevin Tracey and colleagues identified that the α7 nAChR is the primary receptor involved in the cholinergic anti-inflammatory pathway in bone-marrow derived immune cells (Wang, Yu et al. 2003). During bacterial infection or following injury, TNF-α acts as an inflammatory input signal to the autonomic nervous system (ANS), which reflexively responds by stimulating the vagus nerve, resulting in activation of humoral anti-inflammatory responses (Tracey 2002). Preclinical animal studies have shown that vagus nerve stimulators designed to promote release of ACh are extremely effective in suppressing cytokine-mediated inflammation and damage through α7 nAChRs expressed on cytokine-producing cells (e.g. macrophages, dendritic cells). These observations exposed a central regulatory role for vagus nerve-mediated ACh activity in suppressing systemic cytokine release in organs and in serum, and attenuation of disease severity in response to a variety of inflammatory stimuli.
Keratinocytes comprise the epidermal non-neuronal cholinergic system, where the “non-neuronal cholinergic system” refers to cholinergic signaling pertaining to or composed of nonconducting cells of the nervous system (e.g. cells other than neurons) (Grando, Kist et al. 1993; Grando, Kawashima et al. 2007). ACh released from keratinocytes in response to pathogen challenge or wounding likely contributes to the regulation of local immune responses, and potentially that of infiltrating immune cells. Keratinocytes express different nAChR subtypes during the course of epidermal differentiation, and knock-out animals for several nAChR subtypes display diverse phenotypes (reviewed in (Grando, Pittelkow et al. 2006)). While the role of cholinergic signaling in keratinocyte proliferation, differentiation, and migration has been well described (reviewed in(Grando, Pittelkow et al. 2006)), the role in keratinocyte innate immunity remains elusive.
Our new observations identifying cholinergic activation as a mechanism for keratinocyte AMP regulation supports the paradigm upon which the inflammatory reflex is based to describe a global response involving a biochemical triad of the nervous, immune, and cutaneous systems to orchestrate anti-inflammatory pathways. The cholinergic pathway in both neuronal and non-neuronal cells is likely activated by the prolonged stress response in an attempt to restrain excessive pathological inflammation (Tracey 2007). Ultimately, this creates a detrimental negative feedback loop in the epidermis that results in immunosuppresion of AMP production and activity, and likely other pro-inflammatory cytokines, to diminish the capacity of the epidermis to resist infection (Figure 1). Therefore, it is of critical importance to identify which nAChR subtype(s) is/are primarily involved AMP expression. Our work in both keratinocytes and mouse models of nAChR activation determined that the α7 nAChR subtype is a major player involved in AMP suppression in the epidermis (Radek, Elias et al. 2010), which parallels the work by Kevin Tracey and colleagues (Borovikova, Ivanova et al. 2000; Bernik, Friedman et al. 2002; Bernik, Friedman et al. 2002; Tracey 2002; Tracey 2007). Current studies in our laboratory are further exploring the mechanism behind cholinergic regulation of AMPs in keratinocytes. The potential involvement of aberrant cholinergic activation in the development of cutaneous disease in the context of AMPs and barrier function is later discussed and is summarized in Table 1.
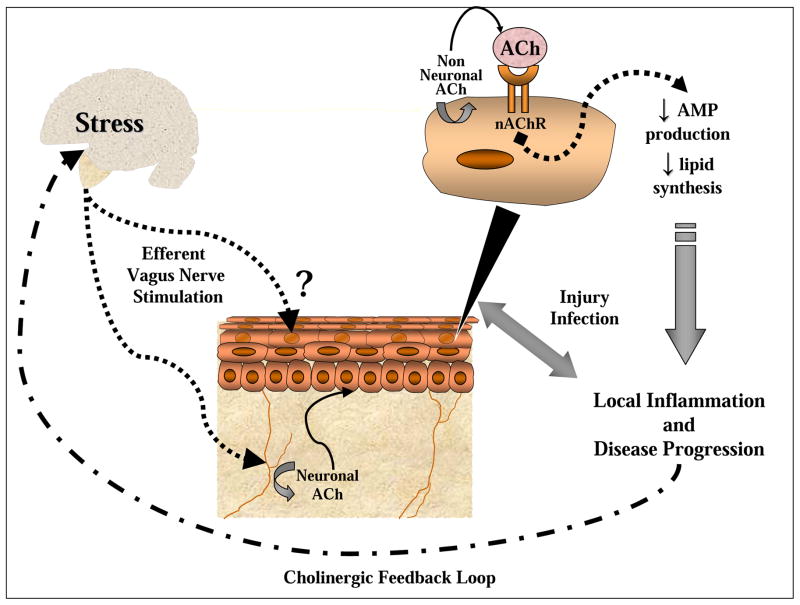
Figure 1. Elements of the systemic and local cholinergic anti-inflammatory pathway proposed to augment cutaneous disease progression.
Stress can initiate a systemic response to activate the systemic cholinergic anti-inflammatory pathway through an efferent relay and stimulate acetylcholine (ACh) release from neuronal cells in the skin (e.g. nerve cells) to potentially act on keratinocytes. The non-neuronal cholinergic system in keratinocytes is capable of producing ACh that can be released into the extracellular space to activate nAChRs. Consequently, nAChR activation dampens AMP production and epidermal barrier integrity. Injury or infection during stress can either initiate or exacerbate existing skin pathologies. Episodes of more frequent or severe outbreaks of skin disease can signal a negative feedback loop back to the brain to provoke a secondary stress response to further suppress keratinocyte AMP and barrier function.
Table 1.
A select list of cutaneous diseases involving aberrant cholinergic signaling
| SKIN DISEASE | FACTORS CONTRIBUTING TO DISEASE PATHOLOGY AND COMPLEMENTARY EVIDENCE TO INDICATE INVOLVEMENT OF CHOLINERGIC SIGNALING | REFERENCES |
|---|---|---|
| Atopic Dermatitis |
|
(Ellison, Patel et al. 2000; Ong, Ohtake et al. 2002; Raap, Werfel et al. 2003; Rieg, Steffen et al. 2005; Choi, Demerjian et al. 2006; Howell, Wollenberg et al. 2006) (Raap, Werfel et al. 2003) (Kurzen and Schallreuter 2004) |
| Psoriasis |
|
(Madsen, Rasmussen et al. 1992; Nonomura, Yamanishi et al. 1994; Harder, Bartels et al. 1997; Tsuji, Okamoto et al. 2003; O’Leary, Creamer et al. 2004; Fortune, Richards et al. 2005) (Arredondo, Chernyavsky et al. 2006) (Arnetz, Fjellner et al. 1985; Richards, Ray et al. 2005; Evers, Verhoeven et al. 2010) (Lande, Gregorio et al. 2007) (Naldi, Chatenoud et al. 2005) |
| Mal de Maleda |
|
(Chimienti, Hogg et al. 2003) |
| Rosacea |
|
(Yamasaki, Schauber et al. 2006; Yamasaki, Di Nardo et al. 2007) |
| Pemphigus |
|
(Nguyen, Ndoye et al. 2000; Nguyen, Ndoye et al. 2001) |
| Palmoplantar pustulosis |
|
(Hagforsen, Edvinsson et al. 2002) |
Abbreviations: AMPs, Antimicrobial peptides, hBD, human Beta-Defensin, nAChR, nicotinic acetylcholine receptor, SLURP-1: Secreted mammalian Ly-6/uPAR-related protein-1, SLURP-2: Secreted mammalian Ly-6/uPAR-related protein-2
EVIDENCE FOR A CUTANEOUS NON-NEURONAL CHOLINERGIC SYSTEM
The non-neuronal cholinergic system in keratinocytes is primarily regulated through the binding of the neurotransmitter, ACh, to nAChRs and mAChRs classified based on their respective agonists, nicotine and muscarine (Grando, Pittelkow et al. 2006). ACh is synthesized from coenzyme A and choline by cholineacetyltransferase (ChAT) and is degraded by Acetylcholinesterase (AChE). Keratinocytes have the capacity to synthesize, process, store, and release ACh that can bind to nAChRs or mAChRs expressed on their cell surface to regulate an array of cellular processes, including migration, differentiation, proliferation, and apoptosis (Grando 1993; Denda, Tsuchiya et al. 2000) Keratinocyte cells can synthesize a average of 2 × 10−17 mol and secrete 7 × 10−19 mol of ACh per minute ((Grando, Kist et al. 1993). However, a gradient of ACh expression throughout the epidermis is observed, with highest ACh levels present in the uppermost epidermal compartment (Nguyen, Ndoye et al. 2001), likely because the highest amounts of ChAT, the ACh degrading enzyme is located predominantly in the basal layer (Johansson and Wang 1993; Klapproth, Reinheimer et al. 1997) . This intricate ACh-dependent network is referred to as the non-neuronal cholinergic system (Klapproth, Reinheimer et al. 1997; Wessler, Kirkpatrick et al. 1998; Wessler, Kirkpatrick et al. 1999).
The nAChR superfamily of ligand-gated ionotropic receptors are comprised of homo-or heterotypic combinations of nine α subunits (α2–α10) and three β subunits (β2–β4) (Steinbach 1990). During the course of keratinocyte differentiation, the expression of nAChR subunits and ACh regulatory enzymes is modified accordingly to support essential functions required for the specific microenvironment of each stratified layer of the epidermis (Kurzen, Berger et al. 2004) ( Figure 2). Keratinocyte transition from the granular cell to the flattened corneocyte is facilitated by ACh to induce apoptotic secretion, which refers to the sporadic extrusion of cytoplasmic components and the release of humectants to prevent epidermal water loss (Nguyen, Ndoye et al. 2001). Knockdown of α7 nAChR in cultured keratinocytes blocked terminal differentiation, which was coupled to the increased expression of pro-apoptotic signaling factors. Furthermore, the inhibition of α7 nAChR signaling using the pharmacologic antagonist α-Bungarotoxin promoted cell cycle progression (Arredondo, Nguyen et al. 2003). This suggests that α7 nAChR signaling facilitates normal apoptotic signals to generate the stratum corneum. Combined pharmacologic nAChR agonist and mAChR antagonist was also found to increase intracellular Ca2+ and trigger apoptotic secretion in vitro (Nguyen, Ndoye et al. 2001). Collectively, ACh signaling is mediated by distinct combinations of nAChRs as keratinocytes mature, where each subunit regulates a particular cell function at a specific point in epidermal differentiation. Our recent observations identifying a novel pathway for keratinocyte AMP regulation through cholinergic signaling further extends the functional contribution of ACh to include epidermal barrier function through elements of the innate immune system.
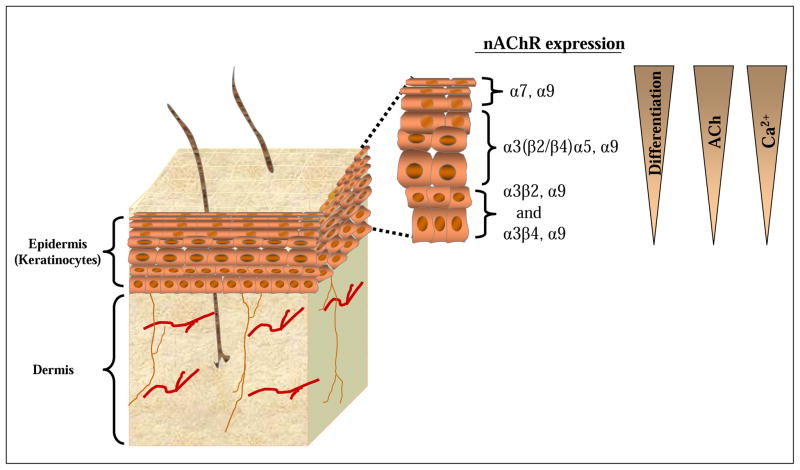
Figure 2. Expression of nicotinic acetylcholine receptor (nAChR) subtypes is dependent upon differentiation, free acetylcholine (ACh) and free Ca2+ concentration gradients.
The nAChR superfamily of ligand-gated ionotropic receptors are differentially expressed during the course of keratinocyte differentiation. Corneocyte cells comprising the top layer of the epidermis (stratum corneum) express primarily α7 nAChR. Transitional (prickle) keratinocytes located in the middle layer of the epidermis express primarily α3(β2/β4)α5 nAChR. Immature (basal) keratinocytes located within the base layer of the epidermis express primarily α3β2 or nAChRα3β4 nAChR. All keratinocyte cells appear to express α9 nAChR. Activation of each nAChR subtype is associated with a different cellular response, including apoptosis, cornification, proliferation, migration, or differentiation. Atypical expression of specific nAChR subtypes throughout the epidermis has been correlated with disease. The gradients on the right represent the state of differentiation and the concentrations of ACh and free calcium, with the greatest degree of differentiation and highest ACh and free calcium concentrations occurring at the interface with the external environment (e.g. stratum corneum).
KERATINOCYTES AS KEY MEDIATORS OF CUTANEOUS BARRIER FUNCTION
Physical maintenance of skin permeability barrier function by the stratum corneum
The fundamental purpose of the stratum corneum is to prevent external water loss and maintain a homeostatic microenvironment within the epidermis. The stratum corneum defends against pathogen invasion by creating both a physical and chemical shield comprised mainly of lipids and AMPs, respectively. Corneocytes are terminally differentiated keratinocyte cells located in the stratum corneum that comprise both the physical and chemical barrier to protect the skin against physical insult, ultraviolet light, chemical exposure, and microbial invasion, as well as prevent water and electrolyte loss into the external environment (Feingold 2009). The physical permeability barrier is created by the presence of a hydrophobic extracellular matrix composed of free fatty acids (FFAs), cholesterol, and ceramides secreted from lamellar bodies, which are secretory organelles unique to keratinocytes (Rassner, Feingold et al. 1999).
An effective permeability barrier depends on tight regulation and organization of cholesterols, ceramides, and FFAs within the stratum corneum intersticies (reviewed in (Elias 2007; Cork, Danby et al. 2009,{Borkowski, 2011 #1747; Ahrens, Schunck et al. 2011; Borkowski and Gallo 2011). Strong cell-cell physical associations are created by cellular desmosomal junctions and secretion of lipid species from lamellar bodies, which covalently bind cell membranes and forms the corneocyte lipid envelope (Behne, Uchida et al. 2000). Experimentally, transepidermal water loss (TEWL) measurements are a useful biomarker for determining the integrity and health of the stratum corneum (Kalia, Alberti et al. 2000). Barrier recovery can be determined by measuring changes in TEWL values following barrier disruption via tape-stripping or acetone treatment. Tape stripping is a type of superficial wounding that physically remove the corneocyte layer (Pinkus 1951). Contradictory to earlier reports suggesting acetone treatment extracted epidermal lipids while leaving the corneocyte layer mostly intact, it was recently demonstrated that acetone treatment also removes the corneocyte layer, but only mildly extracts lipids (Rissmann, Oudshoorn et al. 2009). Elevated TEWL values can be observed following chemical exposure, physical disruption, or several disease states, including but not limited to AD (Gupta, Grube et al. 2008) perioral dermatitis (Dirschka, Tronnier et al. 2004), and psoriasis (Motta, Monti et al. 1994).
Initial studies aimed at determining factors that negatively regulate epidermal lipid synthesis began by investigating the influence of psychological stress created by either immobilization or crowding of mice (Denda, Tsuchiya et al. 1998). Stressed animals exhibited delayed barrier recovery following barrier disruption via tape stripping indicated by higher TEWL measurements. Conversely, pretreatment with a sedative or GC receptor antagonist prevented the altered barrier recovery observed (Denda, Tsuchiya et al. 1998; Denda, Tsuchiya et al. 2000). Psychological stress in mice was also shown to decrease epidermal cell proliferation and differentiation while simultaneously reducing the production and secretion of lamellar bodies (Choi, Brown et al. 2005). This effect was reversed by topical lipid application, or systemic GC receptor antagonist administration (Choi, Demerjian et al. 2006), establishing a direct effect of neuroendocrine mediators on biochemical pathways required for normal skin barrier homeostasis. Preliminary studies in healthy humans using two validated measures, mood states and the perceived stress scale, demonstrated the first correlation between perceived psychological stress and deteriorated cutaneous barriers, providing the first evidence that stress-induced alterations in the epithelial barrier may trigger the onset of clinical manifestations of skin disease pathogenesis (Garg, Chren et al. 2001).
AMPs are multifunctional peptides that not only protect against microbial invasion and insult through direct pathogen killing, but also signal to the innate immune system and contribute to the generation and maintenance of the permeability barrier. In addition to its antimicrobial function, one AMP in particular, murine cathelicidin (CAMP) was found to have a crucial role in upholding epidermal barrier homeostasis. Mice deficient in cathelicidin (Camp−/−) have a diminished capacity to restrict barrier permeability, since they exhibit delayed barrier recovery following tape stripping (Aberg, Man et al. 2008). Camp−/− mice also display decreased lamellar body formation and secretion, and profound lipid composition defects compared to wild-type mice (Aberg, Man et al. 2008). The role of AMPs in protection against cutaneous infection is discussed below.
Chemical maintenance of the skin barrier by lipids and AMPs
Apart from the physical barrier comprised of keratinocytes and lipids, the skin contains a chemical barrier partially consisting of lipids, but largely consisting of AMPs to restrict microbial growth on the surface of the skin and help maintain barrier integrity (reviewed in (Braff, Bardan et al. 2005)). Long chain bases, such as sphingosines, and FFAs, such as lauric acid and sapienic acid, are derived from the partial hydrolysis of ceramide by ceramidases (Houben, Holleran et al. 2006), and are the most potent antimicrobial lipid species in the stratum corneum (Miller, Aly et al. 1988; Bibel, Aly et al. 1992). Sphingoid bases have broad antifungal and antimicrobial properties against a wide range of skin pathogens, including gram-positive bacteria, (e.g. Staphylococcus. aureus, Staphylococcus epidermidis, and Streptococcus pyogenes), gram-negative bacteria (e.g. Escherichia coli), and yeast, (e.g. Candida. albicans) (Drake, Brogden et al. 2008);Gell, G. 1993; Wertz, P.W. 1995). FFAs are selective against gram-positive bacteria, (e.g. S. aureus) but not gram-negative bacteria and yeast (e.g. C. albicans) (Bibel, Miller et al. 1989; Bibel, Aly et al. 1992; Aly, Bayles et al. 1994; Bibel, Aly et al. 1995). The importance of understanding the contribution of FFAs to host defense is highlighted by the fact that different combinations of FFAs may exert different antimicrobial potencies against microbial species. For example, sapienic acid is more effective for treatment of methicillin-resistant S. aureus (MRSA) infection than mupirocin, the current “gold standard” of treatment of MRSA infections (Drake, Brogden et al. 2008). Overall, the lipid component of the skin permeability barrier contributes to antimicrobial defense in parallel with AMPs.
AMPs are an evolutionarily conserved component of the innate immune system that function at the external periphery as the initial line of defense against invasion by pathogens (Gallo and Nizet 2003; Ganz 2003; Nizet and Gallo 2003). Several families of AMPs, including cathelicidins (LL-37), defensins, and Chromogranins are expressed in immune cells (e.g. neutrophils, natural killer cells, mast cells, macrophages), as well as epithelial cells such as sebocytes and keratinocytes (Agerberth, Charo et al. 2000; Di Nardo, Vitiello et al. 2003; Braff and Gallo 2006; Lee, Yamasaki et al. 2008). To date, there exist over 20 epidermal AMPs (reviewed in (Schauber and Gallo 2009)). Several key epidermal AMPs, ribonuclease (RNAse) 7 and lysozyme are constitutively active and expressed at low basal levels (Harder, Glaser et al. 2007). Other AMPs, such as hBD-2 and hBD-3, and the CAMP cleavage product, LL-37, are only expressed in response to inflammation (Harder and Schroder 2002; Glaser, Harder et al. 2005); (Gudmundsson, Agerberth et al. 1996; Harder, Bartels et al. 1997; Schroder and Harder 1999; Dorschner, Pestonjamasp et al. 2001; Harder, Bartels et al. 2001; Nizet, Ohtake et al. 2001; Pazgier, Hoover et al. 2006). Other epidermal AMPs, such as dermcidin, are sweat-gland derived (Schittek, Hipfel et al. 2001), while the S100 protein psoriasin is focally present at varying levels throughout healthy skin (Glaser, Harder et al. 2005), and induced upon wounding (Lee and Eckert 2007) or skin inflammation (Madsen, Rasmussen et al. 1991).
Cathelicidin is proteolytically cleaved from the larger hCAP18 pro-protein into the catalytically active peptide (human LL-37, murine CAMP) by kallikrein proteases produced by keratinocytes, or by neutrophil-derived Proteinase-3 (Hansson, Stromqvist et al. 1994; Ekholm, Brattsand et al. 2000). In human keratinocytes, cathelicidin expression can be augmented in response to pathogen challenge, Vitamin D3 exposure, and wounding (Dorschner, Pestonjamasp et al. 2001; Butmarc, Yufit et al. 2004; Wang, Nestel et al. 2004; Schauber, Oda et al. 2008) where it functions as natural antibiotics to not only directly eradicate microbial pathogens, but also coordinate multiple components of the innate and adaptive immune responses (Brogden, Ackermann et al. 2003; Kandler, Shaykhiev et al. 2006). For example, LL-37 can directly promote the secretion of chemokines from keratinocytes (Niyonsaba, Ushio et al. 2007). Furthermore, LL-37 has the capacity to enhance Toll like receptor (TLR) signaling in immune cells and initiate a cytokine cascade responsible for bacterial recognition (Niyonsaba, Someya et al. 2001), while stimulating the release of reactive oxygen species (ROS) from activated neutrophils (Zheng, Niyonsaba et al. 2007) to enhance the overall response to infection. Conflicting reports of LL-37 immunoreactivity throughout the epidermis have been described, with some demonstrating localization in the extracellular space between the stratum granulosum and stratum corneum (Goo, Ji et al. 2010) and others to the stratum basale ((Heilborn, Nilsson et al. 2003; Mallbris, Carlen et al. 2010). Since the expression of specific nAChR and mAChR subtypes varies throughout the epidermis, understanding how ACh contributes to LL-37 expression/activity will require further investigation into its localization.
The role of AMPs in keratinocyte innate defense has been primarily described using murine model systems. However, it should be noted that while most AMPs appear to be conserved between mice and humans, several important differences between expression patterns and regulatory mechanisms have been described. For example, humans have both epidermal and neutrophil-derived defensins, whereas mice only express defensins in epithelial cells, suggesting that murine and human innate immune responses may be significantly different (Eisenhauer and Lehrer 1992; Harder, Bartels et al. 1997). The mechanisms regulating human CAMP and murine Camp also vary considerable with respect to the cathelicidin promoter. In humans, CAMP transcription requires binding of the Vitamin D Receptor (VDR) to the Vitamin D Response Element (VDRE) consensus sequence in the cathelicidin promoter (Gombart, Borregaard et al. 2005). However, the nocturnal nature of mice likely explains the absence of the VDRE consensus sequence in the murine Camp promoter (Gombart, Borregaard et al. 2005). Recent studies in mice with a keratinocyte-specific deletion of Hypoxia-Inducible Factor 1-α (HIF1α) indicates that Camp expression in mice is partially regulated by HIF1α. HIF1α-null neutrophils from these mice presented with reduced levels of cathelicidin and exhibited a decreased phagocytic capacity of skin pathogens (Peyssonnaux, Datta et al. 2005). Together, the variability in AMP regulation between species is of critical importance and should be taken into consideration when interpreting data acquired from human and mouse experiments. A more relevant model system would be the rhesus monkey, as defensin and cathelicidin AMPs are expressed in the same cell types and tissues as in humans (Bals, Lang et al. 2001), and the cathelicidin promoter contains VDREs (Gombart, Borregaard et al. 2005), suggesting that similar mechanisms of AMP transcriptional regulation occur between humans and non-human primates.
THE ROLE OF α7 nAChR IN KERATINOCYE BARRIER FUNCTION: LESSONS FROM MURINE KNOCKOUTS
Although numerous nAChR subtypes exist in the epidermis, there is evidence to suggest that similarly to the systemic, neuronal cholinergic anti-inflammatory pathway, the α7 nAChR subtype is likely to play a significant role in the regulation of the local cutaneous innate immune and barrier function. Loss of α7 nAChR expression in Chrna7 −/− mice resulted in profound decreases (34–56%) in genes related to terminal differentiation, including murine Filaggrin, Cytokeratin-1, and Cytokeratin-10. Furthermore, these mice also displayed a reduction in the production of extracellular matrix proteins Collagen 1α1 and Elastin, and Metalloproteinase-1 (Arredondo, Nguyen et al. 2002). Dramatic differences between the histology of Chrna7 −/− and Chrna7+/+ were observed in 1–3 week old mice, where Chrna7+/+ skin exhibited the normal, upper compact horny layer derived from dead corneocytes, 1–2 layers of live, nucleated keratinocytes, and a single row of suprabasal keratinocytes. In contrast. Chrna7 −/− skin had a loose, widened corneocyte layer, 1–3 layers of granulated keratinocytes, and 1–3 extra rows of suprabasilar enlarged keratinocytes, a phenotype consistent with retention hyperkeratosis (Arredondo, Nguyen et al. 2002). Interestingly, Chrna7 −/− mice also show a differential gene expression of the other nAChR subunits, suggesting that a compensatory mechanism may have developed to regulate keratinocyte cell cycle progression, apoptosis, and terminal differentiation genes in the absence of α7 nAChR signaling (Arredondo, Nguyen et al. 2002).
LINKING CHOLINERGIC SIGNALING WITH EPIDERMAL AMP ACTIVITY AND SUSCEPTIBILITY TO INFECTION
For decades, it has been well documented that stress strongly correlates with an increased incidence of infection (Locke 1982). Only recently have we begun to elucidate the neuroendrocrine contribution of epithelial immunity. Stressed individuals have increased epithelial ACh levels (Schlereth, Schonefeld et al. 2007) and are more susceptible to opportunistic infections (Wu, Zaborina et al. 2005; Ashcraft, Hunzeker et al. 2008). Overactivation of the stress-response pathways, as observed during chronic stress, results in a relative state of immunosuppression that can be partly attributed to activation of the cholinergic anti-inflammatory pathway described earlier.
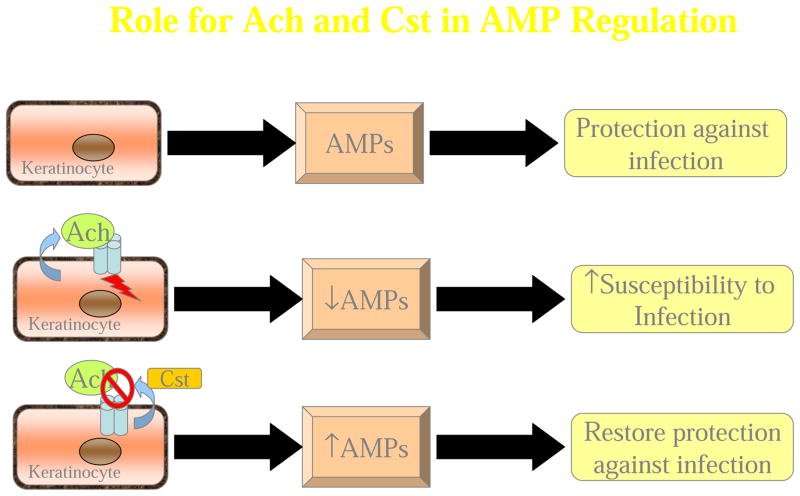
Since the cholinergic system is directly involved in systemic immunosuppression, it was likely that non-neuronal cholinergic activation could suppress the local cutaneous AMP response in keratinocytes. Normal human epidermal keratinocytes (NHEKs) stimulated with the bioactive form of vitamin D, 1,25-dihydroxy VitaminD3 (VD3), and the TLR2/6 agonist, macrophage activating lipoprotein-2 (MALP2) allows for maximal gene and protein expression of both cathelicidin and β-defensin-2 through differentiation and bacterial stimuli, respectively (Weber, Heilborn et al. 2005; Schauber, Dorschner et al. 2006). Our initial results identified that the in vitro expression of CAMP in NHEKs was induced ~100-fold over baseline levels by VD3 +MALP-2, whereas the presence of 0.01nM ACh resulted in a ~50% reduction in CAMP (Radek, Elias et al. 2010). We observed a similar suppression of hBD-2 gene and protein expression by ACh compared the MALP-2 alone. To further elucidate the specific receptor subtype mediating the dampening response by ACh, additional experiments were performed using two pharmacologic antagonists, α-Bungarotoxin, which is selective for the α7 nAChR subunit, and mecamylamine, with known selectivity for α3β4 nAChR subtypes (Grando, Pittelkow et al. 2006), or an endogenous epidermal nAChR antagonist, Cst (Radek, Lopez-Garcia et al. 2008). CAMP suppression by ACh was restored by the presence of Cst and mecamylamine, with maximal restoration observed with α-Bungarotoxin. The induction of CAMP and β-defensin expression paralleled the capacity of NHEKs to restrict the growth of the major skin pathogen S. aureus. Lysates of NHEKs treated with VD3+MALP-2 restricted staphylococcal growth by 90% as compared to lysates from untreated cells. Treatment with 0.01nM ACh suppressed the antimicrobial activity of VD3+MALP-2 lysates to that of untreated cell lysates. This effect was again restored by the presence of Cst or α-Bungarotoxin. This strongly indicates that nAChR signaling dampens the direct extractable anti-bacterial activity in NHEKs consistent with the critical role of AMPs in this response (Radek, Elias et al. 2010). Treatment of NHEKs with muscarine, a mAChR-specific agonist or propranolol, a β-adrenergic pathway agonist, were unable to suppress the induced AMP expression observed with VD3/MALP2 treatment, suggesting ACh regulation of AMP expression in keratinocytes is through direct activation of nAChRs (Radek, Elias et al. 2010). The observation that AMP suppression by ACh could be fully restored by the presence of α-Bungarotoxin, and mostly restored by the addition of mecamylamine or Cst, suggests that ACh likely facilitates AMP suppression via α7 nAChR, similarly to the neuronal cholinergic response to infection (Tracey 2007). Our results indicate that the changes in direct extractable anti-bacterial activity in these NHEKs lysates was likely due to, in part, decreased cathelicidin and hBD-2 expression However, it is possible that the levels of other inducible AMPs, (e.g. RNAse7 and hBD-3) or constitutive AMPs, (e.g. psoriasin), normally increased by the presence of bacterial membrane components were also suppressed by the presence of ACh (Harder, Bartels et al. 2001; Harder and Schroder 2002). Similar studies designed to identify changes in specific AMPs are currently in progress.
Cst was initially identified as an AMP, as it exhibited a broad spectrum killing against several pathogens including S. aureus, Group A Streptococcus (GAS), E. coli, Pseudomonas aeruginosa, and C. albicans, as well as several varieties of filamentous fungi in vitro (Briolat, Wu et al. 2005; Radek, Lopez-Garcia et al. 2008). Since the concentration of Cst required for microbicidal killing was much higher than levels observed in normal skin (Radek, Lopez-Garcia et al. 2008), we proposed that the antimicrobial properties may be secondary to its primary role as a natural, endogenous nAChR antagonist. Like several neuroendocrine-derived molecules, such as α-Melanocyte Stimulating Hormone (α-MSH), Neurokinin-1 (NK-1), and Adrenomedullin (AM), Cst may exhibit microbicidal activity due to their inherently small, cationic, amphipathic structure (Reviewed in (Radek and Gallo 2007). In our studies, we found that AMP suppression by ACh was mostly restored by the presence of Cst, indicating that Cst can block the effects of ACh by acting primarily as an endogenous nAChR antagonist (Radek, Elias et al. 2010).
To verify the functional relevance of cholinergic activation and keratinocyte AMP regulation in vivo, we used a pharmacologic and genetic approach using two murine models of cholinergic activation (Radek, Elias et al. 2010). For the pharmacologic approach, we applied α-Bungarotoxin or vehicle topically to psychologically stressed mice (e.g. insomnia stress) and assessed their susceptibility to infection. We found that stressed mice treated with α-Bungarotoxin were less susceptible to GAS infection compared to vehicle treated mice, as indicated by smaller lesion size, less surviving bacteria in lesions, and lower dissemination into distal organs (Radek, Elias et al. 2010). Mice treated topically with nicotine (in the absence of stress) demonstrated a significant reduction in extractable AMP activity, indicating that direct activation of nAChRs negatively regulates AMP expression (Radek, Elias et al. 2010). We later defined the role of nAChR activation in regulating AMP activity in the skin by employing a genetic approach by utilizing CHGA−/− mice, which lack the endogenous nAChR antagonist Cst and hence, exhibit unopposed nAChR activation. CHGA−/− mice were significantly more susceptible to both S. aureus and GAS skin infection. These mice also exhibited a significant reduction in extractable AMP activity, which was restored by the presence of topical of α-Bungarotoxin. Together, these data suggested that the regulation of epidermal AMP expression and activity by ACh is highly dependent on the α7 nAChR subtype, but may also involve other subtypes to a lesser degree (Radek, Elias et al. 2010). Additionally, the increase in bacterial survival following prolonged nAChR activation was likely the result of several immunosuppressive responses, including AMP-dependent production of proinflammatory cytokines and enhancement of bacterial survival and proliferation through changes in the epidermal microenvironment (Freestone and Lyte 2008). In humans, a likely detrimental consequence of a diminished epidermal immune response following nAChR activation would lead to bacterial dissemination from initial sites of infection and colonization in distal tissues and organs.
Our previous investigations also provided a critical piece of data that nAChR-mediated cathelicidin dysregulation is a potential mechanism for increased susceptibility to infection. We found that stress did not augment the susceptibility to infection in cathelicidin-deficient (Camp−/) or CHGA−/− mice. This indicates that nAChR activation is a major contributing factor to the suppression of the AMP response to infection during prolonged nAChR activation by stress, and that cathelicidin is a key AMP influenced by cholinergic activation of nAChRs (Radek, Elias et al. 2010). If other AMPs or neuroendocrine mediators were more involved in AMP suppression, we would expect larger lesions and greater bacterial burden in both Camp −/− and CHGA−/− mice following stress. In addition, cathelicidin is an autocrine regulator of the response to purinergic agonists through modification of receptors involved in the tissue response to inflammation or injury, indicating a direct role for cathelicidin in cholinergic-mediated immunosuppression (Pochet, Tandel et al. 2006). Thus, the global suppression of AMP activity and cathelicidin seen with nAChR activation may exacerbate immunosuppression by blocking necessary stimulation of downstream immune responses that are AMP-dependent.
IMPLICATIONS FOR THE ROLE OF CHOLINERGIC ACTIVATION IN THE DEVELOPMENT OF SKIN PATHOLOGIES
Delayed wound healing
AMPs have an important role in modulating wound healing responses by increasing the expression of chemokines and chemokine receptors, stimulating epithelial proliferation, and promoting adaptive immune responses and angiogenesis (Scott, Davidson et al. 2002; Mookherjee and Hancock 2007; Lai and Gallo 2009). Therefore, a defective permeability barrier, arising from altered skin pH, lipid composition, or flawed cell-cell junctions, in addition to an insufficient AMP repertoire, can result in overactive cytokine/chemokine cascades and can foster a destructive inflammatory milieu. Since AMPs have a multi-functional role in regulating innate immune responses, inflammation, and barrier function, it is critical to maintain their homeostatic balance to allow for maximal wound repair processes.
Cholinergic signaling has been implicated in the regulation of re-epithelialization during tissue repair. Immediately following barrier disruption and wounding, pro-inflammatory cytokines, such as TNF-α, IL-1-α, and IL-1β, are released by immune and keratinocytec cells to stimulate cellular proliferation required for epidermal regeneration (e.g. re-epithelialization) (Wood, Jackson et al. 1992; Nickoloff and Naidu 1994). At the onset of re-epithelialization, keratinocyte cell-cell desmosomal and hemidesmosomal connections are dissolved, followed by the development of peripheral cytoplasmic actin filaments (Gabbiani, Chaponnier et al. 1978; Goliger and Paul 1995). Concurrently, keratinocyte integrin expression increases to allow for lateral migration across the wound bed (Clark 1990; Clark, Ashcroft et al. 1996), which ceases when keratinocytes collide to form new cell-cell junctions and ensue with terminal differentiation (Adams and Watt 1990; Martin 1997; Singer and Clark 1999). Cholinergic signaling by several nAChRs were found to be directly involved in keratinocyte chemotaxis and chemokinesis, respectively. In in vitro migration assays, reorganization of α7 nAChRs along the leading edge of keratinocytes occurred prior to the conventional crescent shape formation and directional migration of human keratinocytes in the presence of a chemoattractant, while several nAChRs were found to localize with integrin-β1 expressed on the keratinocyte cell membrane (Chernyavsky, Arredondo et al. 2004). Experimental evidence also suggests that α9 nAChR activation leads to downstream phosphorylation of cytoskelatal proteins required for cell motility (Szonyi, Csermely et al. 1999) and keratinocyte cell-cell adhesion (Nguyen, Ndoye et al. 2000). Collectively, this suggests that ACh likely contributes to both the initiation and termination of re-epilithelialization during wound repair to re-establish the epidermal permeability barrier. (Grando, Pittelkow et al. 2006).
Atopic Dermatitis (AD) and Psoriasis
A key factor in a myriad of cutaneous inflammatory diseases include AMPs. Up to 90% of AD skin lesions are typically colonized by S. aureus (Leung 2003). In the past, greater susceptibility was attributed to an overall decrease in AMP expression, since it was demonstrated that cathelicidin (LL-37), human Beta Defensin-2 (hBD-2), and human Beta Defensin-3 (hBD-3) levels were all decreased in AD skin when compared to psoriatic skin (Ong, Ohtake et al. 2002). Therefore, it was presumed that the overall decrease in AMP expression directly resulted in suppressed antimicrobial potential and a compromised barrier. More recently, a study with a large patient cohort of Atopic Excema (AE), psoriatic, and normal controls demonstrated that AE lesions have LL-37 levels similar to that of normal, non-lesional skin and healthy skin from normal donors (Gambichler, Skrygan et al. 2008). Psoriasin secretion was also profoundly increased (up to 1500-fold) when comparing AD skin to healthy skin from the same individual (Glaser, Meyer-Hoffert et al. 2009). These conflicting reports highlight the importance of understanding the factors that contribute to AMP regulation. Although the baseline levels of AMP expression in AD patients may be equal to or higher than normal, uninjured skin, the antimicrobial potential of these AMPs remains to be determined. It has been demonstrated that the increased LL-37 expression following injury observed in healthy volunteers was not observed in AD lesions (Mallbris, Carlen et al. 2010), suggesting that while the baseline level of LL-37 among normal, atopic, and psoriatic skin appears about the same, increased microbial colonization likely arises from the inability to induce LL-37 following wounding or in the presence of active infection. Furthermore, it has been shown that Th2 cytokines, IL-4 and IL-13, directly down-regulate AMP expression in cultured human keratinocytes (Ong, Ohtake et al. 2002; Nomura, Goleva et al. 2003). In parallel, AD patients have an elevated Th2 cytokine milieu, at least in comparison to psoriatic skin (Nomura, Goleva et al. 2003). This altered cytokine milieu is believed to directly suppress the AMP response to injury and infection in the skin, providing further evidence that the increased susceptibility to infection observed in AD patients is multifaceted, arising from defects in innate antimicrobial responses as well as adaptive immunity and cytokine responses. These observations emphasize the importance of identifying common mechanisms behind AMP induction and suppression that may yield unconventional therapeutic interventions that can be customized to treat cutaneous disorders with AMP dysregulation as a major contributing factor.
AD is a common skin disease where clinical onset is usually triggered by environmental or psychological stress (Raap, Werfel et al. 2003). Variable differences in expression of ACh receptor subunits has been observed in patients with AD as well as in response to minimal trauma (Kurzen and Schallreuter 2004). Patients with AD have a significant increase (14-fold) in ACh expression in the skin, as compared to normal individuals (Wessler, Reinheimer et al. 2003). Moreover, ACh contributes to the pathophysiologic characteristics of AD, such as increased pruritis, or itch, due to its ability to cause vasodilatation and the wheal and flare reaction (Heyer, Vogelgsang et al. 1997; Rukwied and Heyer 1999; Wessler, Reinheimer et al. 2003).
NC/Nga mice exhibit increased serum IgE, scaly AD-like lesions, and mast cell accumulation similar to serum and skin lesions of AD patients (Suto, Matsuda et al. 1999), and these mice are frequently used as a murine model of AD. Psychological stress was shown to trigger the development of these histopathologies in NC/Nga mice, where pretreatment with corticotropin releasing hormone (CRH), a neurogenic peptide released in response to cholinergic stimulation, completely prevented the onset of AD-lesions in these animals (Amano, Negishi et al. 2008). Epidermal cells expressing CRH receptors may play a role in the mechanism by which stress-induced cholinergic activation triggers the onset and exacerbation of AD (Amano, Negishi et al. 2008). These data strongly suggest a direct role for ACh and cholinergic signaling in the development of AD pathophysiology.
Lesions from patients with psoriasis present with epidermal hyperplasia and increased AMP expression, excess inflammation, and a diminished susceptibility to infection (Madsen, Rasmussen et al. 1991; Ong, Ohtake et al. 2002; Rieg, Steffen et al. 2005; Howell, Boguniewicz et al. 2006). Extensive studies in humans indicate a direct interaction between smoking (e.g. exposure to nicotine) and the development of psoriatic arthritis through genetic polymorphisms in the gene encoding for the Th2 cytokine, Interleukin-13 (Duffin, Freeny et al. 2009). The clinical onset and exacerbation of psoriasis is associated with environmental and psychological stress, as well as increased CRH levels (Kim, Cho et al. 2007). Since CRH release is dependent upon cholinergic activation in the hypothalamus (Karanth, Lyson et al. 1999), it is possible that the increased CRH levels are result of enhanced cholinergic regulation in the skin. Furthermore, it was recently demonstrated that Secreted mammalian Ly-6/uPAR-related protein-1 (SLURP-2) is upregulated three-fold in hyperproliferative skin from patients with psoriasis (Tsuji, Okamoto et al. 2003; Arredondo, Chernyavsky et al. 2006). SLURP-2 appears to preferentially bind the α3 nAChR subtype, which indicates a potential role for this nAChR subtype in the progression of psoriasis (Arredondo, Chernyavsky et al. 2006).
Pemphigus
Pemphigus is a rare, blistering autoimmune skin disorder that targets the skin and mucous membranes and is mediated by the production of auto-antibodies against keratinocyte antigens, including Desmogleins, proteins important for cell-cell junctions, and also several nAChRs (reviewed in (Grando 2000)). Pemphigus patients have reported that an emotional, stressful event is typically a worsening or precipitating factor of pemphigus (Morell-Dubois, Carpentier et al. 2008). In neonatal mice, nAChR antibodies can induce pemphigus-like lesions, suggesting that these receptors may contribute to the pathogenesis of Pemphigus Vulgaris (Nguyen, Ndoye et al. 2000). The role of AMPs in this disease has yet to be elucidated. However, the experimental evidence described here suggests that it is highly likely that the result of desensitized nAChR signaling in keratinocytes could potentially augment cutaneous inflammation and/or AMP expression to further aggravate this disease.
Mal de Meleda
Mal de Meleda is an autosomal recessive inflammatory and keratotic palmoplantar disorder, resulting from mutation of Secreted mammalian Ly-6/uPAR-related protein-1 (SLURP-1)(Arredondo, Chernyavsky et al. 2005), which functions as a cofactor to fine-tune cholinergic signaling in keratinocytes. SLURP-1 facilitates keratinization and apoptosis (Mastrangeli, Donini et al. 2003) and shows high amino-acid composition homology with α-Bungarotoxin to function as an allosteric agonist to potentiate acetlycholine-mediated signaling via the α7 nAChR subtype (Arredondo, Chernyavsky et al. 2005; Grando 2008).
Rosacea
Patients with Rosacea present with excessive facial skin inflammation determined to be triggered, in part, by increased expression of cutaneous serine proteases, Kallikrein 5 and 7. Overactive serine-protease activity in the stratum corneum results in shorter LL-37 peptides possessing immunostimulatory activity due to additional post-translational cleavage (Yamasaki, Di Nardo et al. 2007). Clinical observation has indicated that Rosacea symptoms improve when patients are actively smoking (Mills 1998), while symptoms appear to worsen upon cessation of nicotine exposure. This insinuates that nAChR activation, at least in some cases, may serve a protective role to prevent inflammation.
Netherton’s syndrome
Patients with Netherton’s syndrome, a genetically conferred disease, are predisposed to AD and demonstrate a higher risk for cutaneous infection (Stryk, Siegfried et al. 1999; Chao, Richard et al. 2005). Patients with Netherton’s syndrome carry a mutation in the Serine protease inhibitor Kazal-type 5 (SPINK5) gene, which codes for Lympho-epithelial Kazal-type-related inhibitor (LEKTI) (Magert, Standker et al. 1999), a serine proteinase required for processing of kallikrein proteases into active form (Schechter, Choi et al. 2005). Loss of LEKTI may contribute to AD predisposition by altering expression of both structural/cell-cell junction proteins, therefore destabilizing the epidermal barrier. In parallel, the observed increase in the expression of Kallikrein proteases in this disease likely augments the proteolytic processing of AMPs and, consequently, inflammation (as indicated for Rosacea above).
Vitiligo
Vitiligo is a chronic skin disorder where loss of pigment results in irregular white patches. The abundance of AChE, the enzyme responsible for degrading ACh, is considerably reduced in vitiliginous skin during depigmentation, resulting in elevated ACh levels and concomitant pruritis (Iyengar 1989). While cholinergic signaling has not been directly correlated with this disease, high ACh levels make it likely that excessive nAChR signaling contributes to disease pathology.
BOTULINUM TOXIN-A AS A POTENTIAL ANTI-CHOLINERGIC THERAPEUTIC FOR TREATING SKIN DERMATOSES
Botulinum toxin-A (BoNT/A) inhibits the release of ACh from presynaptic vesicles (Hallett 2000; Huang, Foster et al. 2000), making it a potential therapy for inflammatory diseases correlated with hyperactive nAChR signaling. Several clinical trials have already reported positive outcomes. Subcutaneous injection of BoNT/A in numerous AD clinical trials improved pruritis symptoms (Swartling, Naver et al. 2002; Wollina and Karamfilov 2002). Successful treatments of acne, notalgia paresthetica, and inverse psoriasis has also been described (Diamond and Jankovic 2006). In a double-blind, placebo controlled study of human patients with normal skin, subcutaneous injection of BoNT/A decreased the itch intensity, blood flow and neurogenic inflammation in response to the histamine prick test (Gazerani, Pedersen et al. 2009). Thus, blockade of cholinergic signaling in targeted regions of the skin may help alleviate clinical manifestations of cutaneous diseases associated with ACh-mediated symptoms.
FUTURE DIRECTIONS
Based on the clinical and experimental evidence presented in this review, it is evident that the cholinergic anti-inflammatory pathway spans several macro- and microenvironments to provide a universal network to control inflammation. In the skin, it remains to be determined if neuronal ACh released from neural components in the skin is acting in parallel with or independently from non-neuronal ACh released from keratinocytes to regulation AMP expression and activity in the epidermis. Our in vitro results indicate that the epidermis is comprised of all of the necessary components to autonomously dampen the epidermal AMP response to infection. The local coordination of ACh signaling within the epidermis makes topical therapeutics a promising solution for directly targeting the cells responsible for ACh-dependent downregulation of AMP expression and eliminates the need to systemically target the vagus nerve. However, it cannot be ruled out that the immunosuppressive effects of nAChR activation are not multifaceted, where topical agonists or antagonists may activate other resident skin cells, including Langerhans and γδ-T cells, or sensory nerve cells to further inhibit inflammatory pathways in the skin. Elucidating the contribution of each particular nAChR subtype to keratinocyte AMP production and synthesis of structural elements necessary for barrier function is critical for developing unique therapeutic strategies for cutaneous diseases with AMP function as a central component. Moreover, a high degree of cross-talk likely exists between the three branches of the stress response. Dissecting the contributions of each particular stress pathway will be of vital factor in the development of therapies. A possibility exists that specific concentrations of ACh must be appropriately maintained within the stratified epidermis to maintain AMP expression and activity during periods of acute stress, which is likely skewed during periods of prolonged stress. This imbalance will consequently augment nAChR activation, resulting in immunosuppression and compromised barrier function. Collectively, a complex network of cellular and biochemical mechanisms facilitate the pathogenesis skin disease upon activation of the stress response. Activation of the HPA axis and the adrenergic system are the most extensively studied pathways to date, but a greater emphasis must be made on the role of the cholinergic system in maintaining the chemical and physical skin barrier. Ultimately, these investigations will yield new strategies in the treatment of inflammatory skin disorders.
Acknowledgments
Data from Radek et al. presented in this manuscript was supported by the following VA and NIH/NIAID funds: HHSN26620040029C, ADB contracts N01-AI-40029AI48176, AR45676, AI052453 (awarded to R. L. Gallo), and NIH F32-AR054220-01A2 (awarded to K.A. Radek). Current Loyola University Medical Center research support by NIH/NIAAA 1P30AA019373-01 and 5T32AA013527-09.
Abbreviations used
- ACh
Acetylcholine
- AChE
Acetylcholinesterase
- ACTH
Adrenocorticotropin hormone
- AM
Adrenomedullin
- AMP
Antimicrobial peptides
- AD
Atopic Dermatitis
- AE
Atopic Excema
- ANS
Autonomic nervous system
- BoNT/A
Botulinum toxin-A
- CAMP
cathelicidin antimicrobial peptide (human)
- Cst
Catestatin
- CRH
Corticotropin releasing hormone
- E/NE
Epinephrine/norepinephrine
- GAS
Group A Streptococcus
- GCs
Glucocorticoids
- HMBG1
High Mobility Group protein B1
- HPA
Hypothalamic-pituitary-adrenal axis
- hβD-2
Human Beta Defensin-2
- hβD-3
Human Beta Defensin-3
- HIF1α
Hypoxia-Inducible Factor 1-α
- IL-1
Interleukin-1
- LEKTI
Lympho-epithelial Kazal-type-related inhibitor
- MALP2
Macrophage Activating Lipoprotein-2
- mAChRs
Muscarinic acetylcholine receptors
- α-MSH
α-Melanocyte Stimulating Hormone
- NK-1
Neurokinin-1
- nAChRs
Nicotinic acetylcholine receptors
- NO
Nitric oxide
- NHEKs
Normal human epidermal keratinocytes
- PSNS
Parasympathetic nervous system
- ROS
Reactive oxygen species
- SLURP-1
Secreted mammalian Ly-6/uPAR-related protein-1
- SLURP-2
Secreted mammalian Ly-6/uPAR-related protein-2
- SNS
Sympathetic nervous system
- TLR
Toll like receptor
- TEWL
Transepidermal water loss
- TNF-α
Tumor Necrosis Factor-α
- VD3
1,25 Dihydroxy VitaminD3
- VDRE
Vitamin D Response Element
Footnotes
CONFLICT OF INTEREST:
The authors declare no conflict of interest.
Contributor Information
Brenda J. Curtis, Loyola University Medical Center, Department of Surgery, Burn and Shock Trauma Institute
Katherine A. Radek, Loyola University Medical Center, Department of Surgery, Burn and Shock Trauma Institute, Maywood, IL USA
References
- Abang MM, Baum M, et al. Differential Selection on Rhynchosporium secalis During Parasitic and Saprophytic Phases in the Barley Scald Disease Cycle. Phytopathology. 2006;96(11):1214–1222. doi: 10.1094/PHYTO-96-1214. [DOI] [PubMed] [Google Scholar]
- Aberg KM, Man MQ, et al. Co-regulation and interdependence of the mammalian epidermal permeability and antimicrobial barriers. J Invest Dermatol. 2008;128(4):917–925. doi: 10.1038/sj.jid.5701099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aberg KM, Radek KA, et al. Psychological stress downregulates epidermal antimicrobial peptide expression and increases severity of cutaneous infections in mice. J Clin Invest. 2007;117(11):3339–3349. doi: 10.1172/JCI31726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams JC, Watt FM. Changes in keratinocyte adhesion during terminal differentiation: reduction in fibronectin binding precedes alpha 5 beta 1 integrin loss from the cell surface. Cell. 1990;63(2):425–435. doi: 10.1016/0092-8674(90)90175-e. [DOI] [PubMed] [Google Scholar]
- Agerberth B, Charo J, et al. The human antimicrobial and chemotactic peptides LL-37 and alpha-defensins are expressed by specific lymphocyte and monocyte populations. Blood. 2000;96(9):3086–3093. [PubMed] [Google Scholar]
- Ahrens K, Schunck M, et al. Mechanical and Metabolic Injury to the Skin Barrier Leads to Increased Expression of Murine beta-Defensin-1, −3, and −14. J Invest Dermatol. 2011;131(2):443–452. doi: 10.1038/jid.2010.289. [DOI] [PubMed] [Google Scholar]
- Aly R, Bayles CI, et al. Topical griseofulvin in the treatment of dermatophytoses. Clin Exp Dermatol. 1994;19(1):43–46. doi: 10.1111/j.1365-2230.1994.tb01113.x. [DOI] [PubMed] [Google Scholar]
- Amano H, Negishi I, et al. Psychological stress can trigger atopic dermatitis in NC/Nga mice: an inhibitory effect of corticotropin-releasing factor. Neuropsychopharmacology. 2008;33(3):566–573. doi: 10.1038/sj.npp.1301435. [DOI] [PubMed] [Google Scholar]
- Arnetz BB, Fjellner B, et al. Stress and psoriasis: psychoendocrine and metabolic reactions in psoriatic patients during standardized stressor exposure. Psychosom Med. 1985;47(6):528–541. doi: 10.1097/00006842-198511000-00003. [DOI] [PubMed] [Google Scholar]
- Arredondo J, Chernyavsky AI, et al. SLURP-2: A novel cholinergic signaling peptide in human mucocutaneous epithelium. J Cell Physiol. 2006;208(1):238–245. doi: 10.1002/jcp.20661. [DOI] [PubMed] [Google Scholar]
- Arredondo J, Chernyavsky AI, et al. Biological effects of SLURP-1 on human keratinocytes. J Invest Dermatol. 2005;125(6):1236–1241. doi: 10.1111/j.0022-202X.2005.23973.x. [DOI] [PubMed] [Google Scholar]
- Arredondo J, V, Nguyen T, et al. Central role of alpha7 nicotinic receptor in differentiation of the stratified squamous epithelium. J Cell Biol. 2002;159(2):325–336. doi: 10.1083/jcb.200206096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arredondo J, V, Nguyen T, et al. Functional role of alpha7 nicotinic receptor in physiological control of cutaneous homeostasis. Life Sci. 2003;72(18–19):2063–2067. doi: 10.1016/s0024-3205(03)00084-5. [DOI] [PubMed] [Google Scholar]
- Ashcraft KA, Bonneau RH. Psychological stress exacerbates primary vaginal herpes simplex virus type 1 (HSV-1) infection by impairing both innate and adaptive immune responses. Brain Behav Immun. 2008;22(8):1231–1240. doi: 10.1016/j.bbi.2008.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashcraft KA, Hunzeker J, et al. Psychological stress impairs the local CD8+ T cell response to mucosal HSV-1 infection and allows for increased pathogenicity via a glucocorticoid receptor-mediated mechanism. Psychoneuroendocrinology. 2008;33(7):951–963. doi: 10.1016/j.psyneuen.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bals R, Lang C, et al. Rhesus monkey (Macaca mulatta) mucosal antimicrobial peptides are close homologues of human molecules. Clin Diagn Lab Immunol. 2001;8(2):370–375. doi: 10.1128/CDLI.8.2.370-375.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behne M, Uchida Y, et al. Omega-hydroxyceramides are required for corneocyte lipid envelope (CLE) formation and normal epidermal permeability barrier function. J Invest Dermatol. 2000;114(1):185–192. doi: 10.1046/j.1523-1747.2000.00846.x. [DOI] [PubMed] [Google Scholar]
- Benson BJ, Hadley ME. In vitro characterization of adrenergic receptors controlling skin gland secretion in two anurans Rana pipiens and Xenopus laevis. Comp Biochem Physiol. 1969;30(5):857–864. doi: 10.1016/0010-406x(69)90040-1. [DOI] [PubMed] [Google Scholar]
- Bernik TR, Friedman SG, et al. Cholinergic antiinflammatory pathway inhibition of tumor necrosis factor during ischemia reperfusion. J Vasc Surg. 2002;36(6):1231–1236. doi: 10.1067/mva.2002.129643. [DOI] [PubMed] [Google Scholar]
- Bernik TR, Friedman SG, et al. Pharmacological stimulation of the cholinergic antiinflammatory pathway. J Exp Med. 2002;195(6):781–788. doi: 10.1084/jem.20011714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bibel DJ, Aly R, et al. Antimicrobial activity of sphingosines. J Invest Dermatol. 1992;98(3):269–273. doi: 10.1111/1523-1747.ep12497842. [DOI] [PubMed] [Google Scholar]
- Bibel DJ, Aly R, et al. Topical sphingolipids in antisepsis and antifungal therapy. Clin Exp Dermatol. 1995;20(5):395–400. doi: 10.1111/j.1365-2230.1995.tb01356.x. [DOI] [PubMed] [Google Scholar]
- Bibel DJ, Miller SJ, et al. Antimicrobial activity of stratum corneum lipids from normal and essential fatty acid-deficient mice. J Invest Dermatol. 1989;92(4):632–638. doi: 10.1111/1523-1747.ep12712202. [DOI] [PubMed] [Google Scholar]
- Borkowski AW, Gallo RL. The coordinated response of the physical and antimicrobial Peptide barriers of the skin. J Invest Dermatol. 2011;131(2):285–287. doi: 10.1038/jid.2010.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borovikova LV, Ivanova S, et al. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature. 2000;405(6785):458–462. doi: 10.1038/35013070. [DOI] [PubMed] [Google Scholar]
- Braff MH, Bardan A, et al. Cutaneous defense mechanisms by antimicrobial peptides. J Invest Dermatol. 2005;125(1):9–13. doi: 10.1111/j.0022-202X.2004.23587.x. [DOI] [PubMed] [Google Scholar]
- Braff MH, Gallo RL. Antimicrobial peptides: an essential component of the skin defensive barrier. Curr Top Microbiol Immunol. 2006;306:91–110. doi: 10.1007/3-540-29916-5_4. [DOI] [PubMed] [Google Scholar]
- Briolat J, Wu SD, et al. New antimicrobial activity for the catecholamine release-inhibitory peptide from chromogranin A. Cell Mol Life Sci. 2005;62(3):377–385. doi: 10.1007/s00018-004-4461-9. [DOI] [PubMed] [Google Scholar]
- Brogden KA, Ackermann M, et al. Antimicrobial peptides in animals and their role in host defences. Int J Antimicrob Agents. 2003;22(5):465–478. doi: 10.1016/s0924-8579(03)00180-8. [DOI] [PubMed] [Google Scholar]
- Butmarc J, Yufit T, et al. Human beta-defensin-2 expression is increased in chronic wounds. Wound Repair Regen. 2004;12(4):439–443. doi: 10.1111/j.1067-1927.2004.12405.x. [DOI] [PubMed] [Google Scholar]
- Chao SC, Richard G, et al. Netherton syndrome: report of two Taiwanese siblings with staphylococcal scalded skin syndrome and mutation of SPINK5. Br J Dermatol. 2005;152(1):159–165. doi: 10.1111/j.1365-2133.2005.06337.x. [DOI] [PubMed] [Google Scholar]
- Chernyavsky AI, Arredondo J, et al. Differential regulation of keratinocyte chemokinesis and chemotaxis through distinct nicotinic receptor subtypes. J Cell Sci. 2004;117(Pt 23):5665–5679. doi: 10.1242/jcs.01492. [DOI] [PubMed] [Google Scholar]
- Chimienti F, Hogg RC, et al. Identification of SLURP-1 as an epidermal neuromodulator explains the clinical phenotype of Mal de Meleda. Hum Mol Genet. 2003;12(22):3017–3024. doi: 10.1093/hmg/ddg320. [DOI] [PubMed] [Google Scholar]
- Choi EH, Brown BE, et al. Mechanisms by which psychologic stress alters cutaneous permeability barrier homeostasis and stratum corneum integrity. J Invest Dermatol. 2005;124(3):587–595. doi: 10.1111/j.0022-202X.2005.23589.x. [DOI] [PubMed] [Google Scholar]
- Choi EH, Demerjian M, et al. Glucocorticoid blockade reverses psychological stress-induced abnormalities in epidermal structure and function. Am J Physiol Regul Integr Comp Physiol. 2006;291(6):R1657–1662. doi: 10.1152/ajpregu.00010.2006. [DOI] [PubMed] [Google Scholar]
- Chrousos GP. The stress response and immune function: clinical implications. The 1999 Novera H. Spector Lecture. Ann N Y Acad Sci. 2000;917:38–67. doi: 10.1111/j.1749-6632.2000.tb05371.x. [DOI] [PubMed] [Google Scholar]
- Clark RA. Fibronectin matrix deposition and fibronectin receptor expression in healing and normal skin. J Invest Dermatol. 1990;94(6 Suppl):128S–134S. doi: 10.1111/1523-1747.ep12876104. [DOI] [PubMed] [Google Scholar]
- Clark RA, Ashcroft GS, et al. Re-epithelialization of normal human excisional wounds is associated with a switch from alpha v beta 5 to alpha v beta 6 integrins. Br J Dermatol. 1996;135(1):46–51. [PubMed] [Google Scholar]
- Cork MJ, Danby SG, et al. Epidermal barrier dysfunction in atopic dermatitis. J Invest Dermatol. 2009;129(8):1892–1908. doi: 10.1038/jid.2009.133. [DOI] [PubMed] [Google Scholar]
- Denda M, Tsuchiya T, et al. Stress alters cutaneous permeability barrier homeostasis. Am J Physiol Regul Integr Comp Physiol. 2000;278(2):R367–372. doi: 10.1152/ajpregu.2000.278.2.R367. [DOI] [PubMed] [Google Scholar]
- Denda M, Tsuchiya T, et al. Immobilization-induced and crowded environment-induced stress delay barrier recovery in murine skin. Br J Dermatol. 1998;138(5):780–785. doi: 10.1046/j.1365-2133.1998.02213.x. [DOI] [PubMed] [Google Scholar]
- Dhabhar FS. Acute stress enhances while chronic stress suppresses skin immunity. The role of stress hormones and leukocyte trafficking. Ann N Y Acad Sci. 2000;917:876–893. doi: 10.1111/j.1749-6632.2000.tb05454.x. [DOI] [PubMed] [Google Scholar]
- Dhabhar FS, Saul AN, et al. Short-term stress enhances cellular immunity and increases early resistance to squamous cell carcinoma. Brain Behav Immun. 2010;24(1):127–137. doi: 10.1016/j.bbi.2009.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Nardo A, Vitiello A, et al. Cutting edge: mast cell antimicrobial activity is mediated by expression of cathelicidin antimicrobial peptide. J Immunol. 2003;170(5):2274–2278. doi: 10.4049/jimmunol.170.5.2274. [DOI] [PubMed] [Google Scholar]
- Diamond A, Jankovic J. Botulinum toxin in dermatology - beyond wrinkles and sweat. J Cosmet Dermatol. 2006;5(2):169. doi: 10.1111/j.1473-2165.2006.00250.x. [DOI] [PubMed] [Google Scholar]
- Dirschka T, Tronnier H, et al. Epithelial barrier function and atopic diathesis in rosacea and perioral dermatitis. Br J Dermatol. 2004;150(6):1136–1141. doi: 10.1111/j.1365-2133.2004.05985.x. [DOI] [PubMed] [Google Scholar]
- Dorschner RA, V, Pestonjamasp K, et al. Cutaneous injury induces the release of cathelicidin anti-microbial peptides active against group A Streptococcus. J Invest Dermatol. 2001;117(1):91–97. doi: 10.1046/j.1523-1747.2001.01340.x. [DOI] [PubMed] [Google Scholar]
- Drake DR, Brogden KA, et al. Thematic review series: skin lipids. Antimicrobial lipids at the skin surface. J Lipid Res. 2008;49(1):4–11. doi: 10.1194/jlr.R700016-JLR200. [DOI] [PubMed] [Google Scholar]
- Duffin KC, I, Freeny C, et al. Association between IL13 polymorphisms and psoriatic arthritis is modified by smoking. J Invest Dermatol. 2009;129(12):2777–2783. doi: 10.1038/jid.2009.169. [DOI] [PubMed] [Google Scholar]
- Eisenhauer PB, Lehrer RI. Mouse neutrophils lack defensins. Infect Immun. 1992;60(8):3446–3447. doi: 10.1128/iai.60.8.3446-3447.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekholm IE, Brattsand M, et al. Stratum corneum tryptic enzyme in normal epidermis: a missing link in the desquamation process? J Invest Dermatol. 2000;114(1):56–63. doi: 10.1046/j.1523-1747.2000.00820.x. [DOI] [PubMed] [Google Scholar]
- Elenkov IJ, Wilder RL, et al. The sympathetic nerve--an integrative interface between two supersystems: the brain and the immune system. Pharmacol Rev. 2000;52(4):595–638. [PubMed] [Google Scholar]
- Elias PM. The skin barrier as an innate immune element. Semin Immunopathol. 2007;29(1):3–14. doi: 10.1007/s00281-007-0060-9. [DOI] [PubMed] [Google Scholar]
- Ellison JA, Patel L, et al. Hypothalamic-pituitary-adrenal function and glucocorticoid sensitivity in atopic dermatitis. Pediatrics. 2000;105(4 Pt 1):794–799. doi: 10.1542/peds.105.4.794. [DOI] [PubMed] [Google Scholar]
- Evers AW, Verhoeven EW, et al. How stress gets under the skin: cortisol and stress reactivity in psoriasis. Br J Dermatol. 2010;163(5):986–991. doi: 10.1111/j.1365-2133.2010.09984.x. [DOI] [PubMed] [Google Scholar]
- Feingold KR. The outer frontier: the importance of lipid metabolism in the skin. J Lipid Res. 2009;50(Suppl):S417–422. doi: 10.1194/jlr.R800039-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortune DG, Richards HL, et al. Psychologic factors in psoriasis: consequences, mechanisms, and interventions. Dermatol Clin. 2005;23(4):681–694. doi: 10.1016/j.det.2005.05.022. [DOI] [PubMed] [Google Scholar]
- Freestone PP, Lyte M. Microbial endocrinology: experimental design issues in the study of interkingdom signalling in infectious disease. Adv Appl Microbiol. 2008;64:75–105. doi: 10.1016/S0065-2164(08)00402-4. [DOI] [PubMed] [Google Scholar]
- Gabbiani G, Chaponnier C, et al. Cytoplasmic filaments and gap junctions in epithelial cells and myofibroblasts during wound healing. J Cell Biol. 1978;76(3):561–568. doi: 10.1083/jcb.76.3.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo RL, Nizet V. Endogenous production of antimicrobial peptides in innate immunity and human disease. Curr Allergy Asthma Rep. 2003;3(5):402–409. doi: 10.1007/s11882-003-0074-x. [DOI] [PubMed] [Google Scholar]
- Gambichler T, Skrygan M, et al. Differential mRNA expression of antimicrobial peptides and proteins in atopic dermatitis as compared to psoriasis vulgaris and healthy skin. Int Arch Allergy Immunol. 2008;147(1):17–24. doi: 10.1159/000128582. [DOI] [PubMed] [Google Scholar]
- Ganz T. Defensins: antimicrobial peptides of innate immunity. Nat Rev Immunol. 2003;3(9):710–720. doi: 10.1038/nri1180. [DOI] [PubMed] [Google Scholar]
- Garg A, Chren MM, et al. Psychological stress perturbs epidermal permeability barrier homeostasis: implications for the pathogenesis of stress-associated skin disorders. Arch Dermatol. 2001;137(1):53–59. doi: 10.1001/archderm.137.1.53. [DOI] [PubMed] [Google Scholar]
- Gazerani P, Pedersen NS, et al. Botulinum toxin type A reduces histamine-induced itch and vasomotor responses in human skin. Br J Dermatol. 2009;161(4):737–745. doi: 10.1111/j.1365-2133.2009.09305.x. [DOI] [PubMed] [Google Scholar]
- Glaser R, Harder J, et al. Antimicrobial psoriasin (S100A7) protects human skin from Escherichia coli infection. Nat Immunol. 2005;6(1):57–64. doi: 10.1038/ni1142. [DOI] [PubMed] [Google Scholar]
- Glaser R, Meyer-Hoffert U, et al. The antimicrobial protein psoriasin (S100A7) is upregulated in atopic dermatitis and after experimental skin barrier disruption. J Invest Dermatol. 2009;129(3):641–649. doi: 10.1038/jid.2008.268. [DOI] [PubMed] [Google Scholar]
- Goliger JA, Paul DL. Wounding alters epidermal connexin expression and gap junction-mediated intercellular communication. Mol Biol Cell. 1995;6(11):1491–1501. doi: 10.1091/mbc.6.11.1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gombart AF, Borregaard N, et al. Human cathelicidin antimicrobial peptide (CAMP) gene is a direct target of the vitamin D receptor and is strongly up-regulated in myeloid cells by 1,25-dihydroxyvitamin D3. FASEB J. 2005;19(9):1067–1077. doi: 10.1096/fj.04-3284com. [DOI] [PubMed] [Google Scholar]
- Goo J, Ji JH, et al. Expression of antimicrobial peptides such as LL-37 and hBD-2 in nonlesional skin of atopic individuals. Pediatr Dermatol. 2010;27(4):341–348. doi: 10.1111/j.1525-1470.2010.01122.x. [DOI] [PubMed] [Google Scholar]
- Grando SA. Physiology of endocrine skin interrelations. J Am Acad Dermatol. 1993;28(6):981–992. doi: 10.1016/0190-9622(93)70141-f. [DOI] [PubMed] [Google Scholar]
- Grando SA. Autoimmunity to keratinocyte acetylcholine receptors in pemphigus. Dermatology. 2000;201(4):290–295. doi: 10.1159/000051540. [DOI] [PubMed] [Google Scholar]
- Grando SA. Basic and clinical aspects of non-neuronal acetylcholine: biological and clinical significance of non-canonical ligands of epithelial nicotinic acetylcholine receptors. J Pharmacol Sci. 2008;106(2):174–179. doi: 10.1254/jphs.fm0070087. [DOI] [PubMed] [Google Scholar]
- Grando SA, Kawashima K, et al. Recent progress in understanding the non-neuronal cholinergic system in humans. Life Sci. 2007;80(24–25):2181–2185. doi: 10.1016/j.lfs.2007.03.015. [DOI] [PubMed] [Google Scholar]
- Grando SA, Kist DA, et al. Human keratinocytes synthesize, secrete, and degrade acetylcholine. J Invest Dermatol. 1993;101(1):32–36. doi: 10.1111/1523-1747.ep12358588. [DOI] [PubMed] [Google Scholar]
- Grando SA, Pittelkow MR, et al. Adrenergic and cholinergic control in the biology of epidermis: physiological and clinical significance. J Invest Dermatol. 2006;126(9):1948–1965. doi: 10.1038/sj.jid.5700151. [DOI] [PubMed] [Google Scholar]
- Griffiths CE, Richards HL. Psychological influences in psoriasis. Clin Exp Dermatol. 2001;26(4):338–342. doi: 10.1046/j.1365-2230.2001.00834.x. [DOI] [PubMed] [Google Scholar]
- Gudmundsson GH, Agerberth B, et al. The human gene FALL39 and processing of the cathelin precursor to the antibacterial peptide LL-37 in granulocytes. Eur J Biochem. 1996;238(2):325–332. doi: 10.1111/j.1432-1033.1996.0325z.x. [DOI] [PubMed] [Google Scholar]
- Gupta J, Grube E, et al. Intrinsically defective skin barrier function in children with atopic dermatitis correlates with disease severity. J Allergy Clin Immunol. 2008;121(3):725–730 e722. doi: 10.1016/j.jaci.2007.12.1161. [DOI] [PubMed] [Google Scholar]
- Hagforsen E, Edvinsson M, et al. Expression of nicotinic receptors in the skin of patients with palmoplantar pustulosis. Br J Dermatol. 2002;146(3):383–391. doi: 10.1046/j.1365-2133.2002.04640.x. [DOI] [PubMed] [Google Scholar]
- Hallett M. How does botulinum toxin work? Ann Neurol. 2000;48(1):7–8. [PubMed] [Google Scholar]
- Hansson L, Stromqvist M, et al. Cloning, expression, and characterization of stratum corneum chymotryptic enzyme. A skin-specific human serine proteinase. J Biol Chem. 1994;269(30):19420–19426. [PubMed] [Google Scholar]
- Harder J, Bartels J, et al. A peptide antibiotic from human skin. Nature. 1997;387(6636):861. doi: 10.1038/43088. [DOI] [PubMed] [Google Scholar]
- Harder J, Bartels J, et al. Isolation and characterization of human beta -defensin-3, a novel human inducible peptide antibiotic. J Biol Chem. 2001;276(8):5707–5713. doi: 10.1074/jbc.M008557200. [DOI] [PubMed] [Google Scholar]
- Harder J, Glaser R, et al. Human antimicrobial proteins effectors of innate immunity. J Endotoxin Res. 2007;13(6):317–338. doi: 10.1177/0968051907088275. [DOI] [PubMed] [Google Scholar]
- Harder J, Schroder JM. RNase 7, a novel innate immune defense antimicrobial protein of healthy human skin. J Biol Chem. 2002;277(48):46779–46784. doi: 10.1074/jbc.M207587200. [DOI] [PubMed] [Google Scholar]
- Heilborn JD, Nilsson MF, et al. The cathelicidin anti-microbial peptide LL-37 is involved in re-epithelialization of human skin wounds and is lacking in chronic ulcer epithelium. J Invest Dermatol. 2003;120(3):379–389. doi: 10.1046/j.1523-1747.2003.12069.x. [DOI] [PubMed] [Google Scholar]
- Herman JP, Cullinan WE. Neurocircuitry of stress: central control of the hypothalamo-pituitary-adrenocortical axis. Trends Neurosci. 1997;20(2):78–84. doi: 10.1016/s0166-2236(96)10069-2. [DOI] [PubMed] [Google Scholar]
- Heyer G, Vogelgsang M, et al. Acetylcholine is an inducer of itching in patients with atopic eczema. J Dermatol. 1997;24(10):621–625. doi: 10.1111/j.1346-8138.1997.tb02305.x. [DOI] [PubMed] [Google Scholar]
- Houben E, Holleran WM, et al. Differentiation-associated expression of ceramidase isoforms in cultured keratinocytes and epidermis. J Lipid Res. 2006;47(5):1063–1070. doi: 10.1194/jlr.M600001-JLR200. [DOI] [PubMed] [Google Scholar]
- Howell MD, Boguniewicz M, et al. Mechanism of HBD-3 deficiency in atopic dermatitis. Clin Immunol. 2006;121(3):332–338. doi: 10.1016/j.clim.2006.08.008. [DOI] [PubMed] [Google Scholar]
- Howell MD, Wollenberg A, et al. Cathelicidin deficiency predisposes to eczema herpeticum. J Allergy Clin Immunol. 2006;117(4):836–841. doi: 10.1016/j.jaci.2005.12.1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W, Foster JA, et al. Pharmacology of botulinum toxin. J Am Acad Dermatol. 2000;43(2 Pt 1):249–259. doi: 10.1067/mjd.2000.105567. [DOI] [PubMed] [Google Scholar]
- Iyengar B. Modulation of melanocytic activity by acetylcholine. Acta Anat (Basel) 1989;136(2):139–141. doi: 10.1159/000146813. [DOI] [PubMed] [Google Scholar]
- Johansson O, Wang L. Choline acetyltransferase-like immunofluorescence in epidermis of human skin. Neurobiology (Bp) 1993;1(3):201–206. [PubMed] [Google Scholar]
- Kalia YN, Alberti I, et al. Normalization of stratum corneum barrier function and transepidermal water loss in vivo. Pharm Res. 2000;17(9):1148–1150. doi: 10.1023/a:1026474200575. [DOI] [PubMed] [Google Scholar]
- Kandler K, Shaykhiev R, et al. The anti-microbial peptide LL-37 inhibits the activation of dendritic cells by TLR ligands. Int Immunol. 2006;18(12):1729–1736. doi: 10.1093/intimm/dxl107. [DOI] [PubMed] [Google Scholar]
- Karanth S, Lyson K, et al. Effects of cholinergic agonists and antagonists on interleukin-2-induced corticotropin-releasing hormone release from the mediobasal hypothalamus. Neuroimmunomodulation. 1999;6(3):168–174. doi: 10.1159/000026378. [DOI] [PubMed] [Google Scholar]
- Kawashima K, Fujii T. Expression of non-neuronal acetylcholine in lymphocytes and its contribution to the regulation of immune function. Front Biosci. 2004;9:2063–2085. doi: 10.2741/1390. [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Marucha PT, et al. Slowing of wound healing by psychological stress. Lancet. 1995;346(8984):1194–1196. doi: 10.1016/s0140-6736(95)92899-5. [DOI] [PubMed] [Google Scholar]
- Kim JE, Cho DH, et al. Expression of the corticotropin-releasing hormone-proopiomelanocortin axis in the various clinical types of psoriasis. Exp Dermatol. 2007;16(2):104–109. doi: 10.1111/j.1600-0625.2006.00509.x. [DOI] [PubMed] [Google Scholar]
- Kimyai-Asadi A, Usman A. The role of psychological stress in skin disease. J Cutan Med Surg. 2001;5(2):140–145. doi: 10.1007/BF02737869. [DOI] [PubMed] [Google Scholar]
- Klapproth H, Reinheimer T, et al. Non-neuronal acetylcholine, a signalling molecule synthezised by surface cells of rat and man. Naunyn Schmiedebergs Arch Pharmacol. 1997;355(4):515–523. doi: 10.1007/pl00004977. [DOI] [PubMed] [Google Scholar]
- Kurzen H, Berger H, et al. Phenotypical and molecular profiling of the extraneuronal cholinergic system of the skin. J Invest Dermatol. 2004;123(5):937–949. doi: 10.1111/j.0022-202X.2004.23425.x. [DOI] [PubMed] [Google Scholar]
- Kurzen H, Schallreuter KU. Novel aspects in cutaneous biology of acetylcholine synthesis and acetylcholine receptors. Exp Dermatol. 2004;13(Suppl 4):27–30. doi: 10.1111/j.1600-0625.2004.00258.x. [DOI] [PubMed] [Google Scholar]
- Lai Y, Gallo RL. AMPed up immunity: how antimicrobial peptides have multiple roles in immune defense. Trends Immunol. 2009;30(3):131–141. doi: 10.1016/j.it.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lande R, Gregorio J, et al. Plasmacytoid dendritic cells sense self-DNA coupled with antimicrobial peptide. Nature. 2007;449(7162):564–569. doi: 10.1038/nature06116. [DOI] [PubMed] [Google Scholar]
- Lee DY, Yamasaki K, et al. Sebocytes express functional cathelicidin antimicrobial peptides and can act to kill propionibacterium acnes. J Invest Dermatol. 2008;128(7):1863–1866. doi: 10.1038/sj.jid.5701235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KC, Eckert RL. S100A7 (Psoriasin)--mechanism of antibacterial action in wounds. J Invest Dermatol. 2007;127(4):945–957. doi: 10.1038/sj.jid.5700663. [DOI] [PubMed] [Google Scholar]
- Leung DY. Infection in atopic dermatitis. Curr Opin Pediatr. 2003;15(4):399–404. doi: 10.1097/00008480-200308000-00008. [DOI] [PubMed] [Google Scholar]
- Locke SE. Stress, adaptation, and immunity: studies in humans. Gen Hosp Psychiatry. 1982;4(1):49–58. doi: 10.1016/0163-8343(82)90027-5. [DOI] [PubMed] [Google Scholar]
- Madsen P, Rasmussen HH, et al. Molecular cloning and expression of a novel keratinocyte protein (psoriasis-associated fatty acid-binding protein [PA-FABP]) that is highly up-regulated in psoriatic skin and that shares similarity to fatty acid-binding proteins. J Invest Dermatol. 1992;99(3):299–305. doi: 10.1111/1523-1747.ep12616641. [DOI] [PubMed] [Google Scholar]
- Madsen P, Rasmussen HH, et al. Molecular cloning, occurrence, and expression of a novel partially secreted protein “p” soriasin” that is highly up-regulated in psoriatic skin. J Invest Dermatol. 1991;97(4):701–712. doi: 10.1111/1523-1747.ep12484041. [DOI] [PubMed] [Google Scholar]
- Maestroni GJ. Sympathetic nervous system influence on the innate immune response. Ann N Y Acad Sci. 2006;1069:195–207. doi: 10.1196/annals.1351.017. [DOI] [PubMed] [Google Scholar]
- Magert HJ, Standker L, et al. LEKTI, a novel 15-domain type of human serine proteinase inhibitor. J Biol Chem. 1999;274(31):21499–21502. doi: 10.1074/jbc.274.31.21499. [DOI] [PubMed] [Google Scholar]
- Mallbris L, Carlen L, et al. Injury downregulates the expression of the human cathelicidin protein hCAP18/LL-37 in atopic dermatitis. Exp Dermatol. 2010;19(5):442–449. doi: 10.1111/j.1600-0625.2009.00918.x. [DOI] [PubMed] [Google Scholar]
- Martin P. Wound healing--aiming for perfect skin regeneration. Science. 1997;276(5309):75–81. doi: 10.1126/science.276.5309.75. [DOI] [PubMed] [Google Scholar]
- Marucha PT, Kiecolt-Glaser JK, et al. Mucosal wound healing is impaired by examination stress. Psychosom Med. 1998;60(3):362–365. doi: 10.1097/00006842-199805000-00025. [DOI] [PubMed] [Google Scholar]
- Mastrangeli R, Donini S, et al. ARS Component B: structural characterization, tissue expression and regulation of the gene and protein (SLURP-1) associated with Mal de Meleda. Eur J Dermatol. 2003;13(6):560–570. [PubMed] [Google Scholar]
- Miller SJ, Aly R, et al. In vitro and in vivo antistaphylococcal activity of human stratum corneum lipids. Arch Dermatol. 1988;124(2):209–215. [PubMed] [Google Scholar]
- Mills CM. Cigarette smoking, cutaneous immunity, and inflammatory response. Clin Dermatol. 1998;16(5):589–594. doi: 10.1016/s0738-081x(98)00044-3. [DOI] [PubMed] [Google Scholar]
- Molina PE. Noradrenergic inhibition of TNF upregulation in hemorrhagic shock. Neuroimmunomodulation. 2001;9(3):125–133. doi: 10.1159/000049016. [DOI] [PubMed] [Google Scholar]
- Molina PE, Bagby GJ, et al. Hemorrhage alters neuroendocrine, hemodynamic, and compartment-specific TNF responses to LPS. Shock. 2001;16(6):459–465. doi: 10.1097/00024382-200116060-00010. [DOI] [PubMed] [Google Scholar]
- Mookherjee N, Hancock RE. Cationic host defence peptides: innate immune regulatory peptides as a novel approach for treating infections. Cell Mol Life Sci. 2007;64(7–8):922–933. doi: 10.1007/s00018-007-6475-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morell-Dubois S, Carpentier O, et al. Stressful life events and pemphigus. Dermatology. 2008;216(2):104–108. doi: 10.1159/000111506. [DOI] [PubMed] [Google Scholar]
- Motta S, Monti M, et al. Abnormality of water barrier function in psoriasis. Role of ceramide fractions. Arch Dermatol. 1994;130(4):452–456. [PubMed] [Google Scholar]
- Naldi L, Chatenoud L, et al. Cigarette smoking, body mass index, and stressful life events as risk factors for psoriasis: results from an Italian case-control study. J Invest Dermatol. 2005;125(1):61–67. doi: 10.1111/j.0022-202X.2005.23681.x. [DOI] [PubMed] [Google Scholar]
- Nguyen VT, Ndoye A, et al. Novel human alpha9 acetylcholine receptor regulating keratinocyte adhesion is targeted by Pemphigus vulgaris autoimmunity. Am J Pathol. 2000;157(4):1377–1391. doi: 10.1016/s0002-9440(10)64651-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen VT, Ndoye A, et al. Programmed cell death of keratinocytes culminates in apoptotic secretion of a humectant upon secretagogue action of acetylcholine. J Cell Sci. 2001;114(Pt 6):1189–1204. doi: 10.1242/jcs.114.6.1189. [DOI] [PubMed] [Google Scholar]
- Nguyen VT, Ndoye A, et al. Antibodies against keratinocyte antigens other than desmogleins 1 and 3 can induce pemphigus vulgaris-like lesions. J Clin Invest. 2000;106(12):1467–1479. doi: 10.1172/JCI10305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickoloff BJ, Naidu Y. Perturbation of epidermal barrier function correlates with initiation of cytokine cascade in human skin. J Am Acad Dermatol. 1994;30(4):535–546. doi: 10.1016/s0190-9622(94)70059-1. [DOI] [PubMed] [Google Scholar]
- Niyonsaba F, Someya A, et al. Evaluation of the effects of peptide antibiotics human beta-defensins-1/−2 and LL-37 on histamine release and prostaglandin D(2) production from mast cells. Eur J Immunol. 2001;31(4):1066–1075. doi: 10.1002/1521-4141(200104)31:4<1066::aid-immu1066>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Niyonsaba F, Ushio H, et al. Antimicrobial peptides human beta-defensins stimulate epidermal keratinocyte migration, proliferation and production of proinflammatory cytokines and chemokines. J Invest Dermatol. 2007;127(3):594–604. doi: 10.1038/sj.jid.5700599. [DOI] [PubMed] [Google Scholar]
- Nizet V, Gallo RL. Cathelicidins and innate defense against invasive bacterial infection. Scand J Infect Dis. 2003;35(9):670–676. doi: 10.1080/00365540310015629. [DOI] [PubMed] [Google Scholar]
- Nizet V, Ohtake T, et al. Innate antimicrobial peptide protects the skin from invasive bacterial infection. Nature. 2001;414(6862):454–457. doi: 10.1038/35106587. [DOI] [PubMed] [Google Scholar]
- Nomura I, Goleva E, et al. Cytokine milieu of atopic dermatitis, as compared to psoriasis, skin prevents induction of innate immune response genes. J Immunol. 2003;171(6):3262–3269. doi: 10.4049/jimmunol.171.6.3262. [DOI] [PubMed] [Google Scholar]
- Nonomura K, Yamanishi K, et al. Up-regulation of elafin/SKALP gene expression in psoriatic epidermis. J Invest Dermatol. 1994;103(1):88–91. doi: 10.1111/1523-1747.ep12391802. [DOI] [PubMed] [Google Scholar]
- O’Leary CJ, Creamer D, et al. Perceived stress, stress attributions and psychological distress in psoriasis. J Psychosom Res. 2004;57(5):465–471. doi: 10.1016/j.jpsychores.2004.03.012. [DOI] [PubMed] [Google Scholar]
- Ong PY, Ohtake T, et al. Endogenous antimicrobial peptides and skin infections in atopic dermatitis. N Engl J Med. 2002;347(15):1151–1160. doi: 10.1056/NEJMoa021481. [DOI] [PubMed] [Google Scholar]
- Pavlov VA, Ochani M, et al. Central muscarinic cholinergic regulation of the systemic inflammatory response during endotoxemia. Proc Natl Acad Sci U S A. 2006;103(13):5219–5223. doi: 10.1073/pnas.0600506103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pazgier M, Hoover DM, et al. Human beta-defensins. Cell Mol Life Sci. 2006;63(11):1294–1313. doi: 10.1007/s00018-005-5540-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyssonnaux C, Datta V, et al. HIF-1alpha expression regulates the bactericidal capacity of phagocytes. J Clin Invest. 2005;115(7):1806–1815. doi: 10.1172/JCI23865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picardi A, Abeni D. Stressful life events and skin diseases: disentangling evidence from myth. Psychother Psychosom. 2001;70(3):118–136. doi: 10.1159/000056237. [DOI] [PubMed] [Google Scholar]
- Pinkus H. Examination of the epidermis by the strip method of removing horny layers. I. Observations on thickness of the horny layer, and on mitotic activity after stripping. J Invest Dermatol. 1951;16(6):383–386. doi: 10.1038/jid.1951.45. [DOI] [PubMed] [Google Scholar]
- Pochet S, Tandel S, et al. Modulation by LL-37 of the responses of salivary glands to purinergic agonists. Mol Pharmacol. 2006;69(6):2037–2046. doi: 10.1124/mol.105.021444. [DOI] [PubMed] [Google Scholar]
- Pullar CE, Rizzo A, et al. beta-Adrenergic receptor antagonists accelerate skin wound healing: evidence for a catecholamine synthesis network in the epidermis. J Biol Chem. 2006;281(30):21225–21235. doi: 10.1074/jbc.M601007200. [DOI] [PubMed] [Google Scholar]
- Raap U, Werfel T, et al. Atopic dermatitis and psychological stress. Hautarzt. 2003;54(10):925–929. doi: 10.1007/s00105-003-0609-z. [DOI] [PubMed] [Google Scholar]
- Radek K, Gallo R. Antimicrobial peptides: natural effectors of the innate immune system. Semin Immunopathol. 2007;29(1):27–43. doi: 10.1007/s00281-007-0064-5. [DOI] [PubMed] [Google Scholar]
- Radek KA. Antimicrobial anxiety: the impact of stress on antimicrobial immunity. J Leukoc Biol. 2010;88(2):263–277. doi: 10.1189/jlb.1109740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radek KA, Elias PM, et al. Neuroendocrine nicotinic receptor activation increases susceptibility to bacterial infections by suppressing antimicrobial peptide production. Cell Host Microbe. 2010;7(4):277–289. doi: 10.1016/j.chom.2010.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radek KA, Lopez-Garcia B, et al. The neuroendocrine peptide catestatin is a cutaneous antimicrobial and induced in the skin after injury. J Invest Dermatol. 2008;128(6):1525–1534. doi: 10.1038/sj.jid.5701225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rassner U, Feingold KR, et al. Coordinate assembly of lipids and enzyme proteins into epidermal lamellar bodies. Tissue Cell. 1999;31(5):489–498. doi: 10.1054/tice.1999.0050. [DOI] [PubMed] [Google Scholar]
- Raychaudhuri SP, Raychaudhuri SK. Relationship between kinetics of lesional cytokines and secondary infection in inflammatory skin disorders: a hypothesis. Int J Dermatol. 1993;32(6):409–412. doi: 10.1111/j.1365-4362.1993.tb02809.x. [DOI] [PubMed] [Google Scholar]
- Richards HL, Ray DW, et al. Response of the hypothalamic-pituitary-adrenal axis to psychological stress in patients with psoriasis. Br J Dermatol. 2005;153(6):1114–1120. doi: 10.1111/j.1365-2133.2005.06817.x. [DOI] [PubMed] [Google Scholar]
- Rieg S, Steffen H, et al. Deficiency of dermcidin-derived antimicrobial peptides in sweat of patients with atopic dermatitis correlates with an impaired innate defense of human skin in vivo. J Immunol. 2005;174(12):8003–8010. doi: 10.4049/jimmunol.174.12.8003. [DOI] [PubMed] [Google Scholar]
- Rissmann R, Oudshoorn MH, et al. Skin barrier disruption by acetone: observations in a hairless mouse skin model. Arch Dermatol Res. 2009;301(8):609–613. doi: 10.1007/s00403-009-0946-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas IG, Padgett DA, et al. Stress-induced susceptibility to bacterial infection during cutaneous wound healing. Brain Behav Immun. 2002;16(1):74–84. doi: 10.1006/brbi.2000.0619. [DOI] [PubMed] [Google Scholar]
- Rukwied R, Heyer G. Administration of acetylcholine and vasoactive intestinal polypeptide to atopic eczema patients. Exp Dermatol. 1999;8(1):39–45. doi: 10.1111/j.1600-0625.1999.tb00346.x. [DOI] [PubMed] [Google Scholar]
- Saeed RW, Varma S, et al. Cholinergic stimulation blocks endothelial cell activation and leukocyte recruitment during inflammation. J Exp Med. 2005;201(7):1113–1123. doi: 10.1084/jem.20040463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schauber J, Dorschner RA, et al. Control of the innate epithelial antimicrobial response is cell-type specific and dependent on relevant microenvironmental stimuli. Immunology. 2006;118(4):509–519. doi: 10.1111/j.1365-2567.2006.02399.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schauber J, Gallo RL. Antimicrobial peptides and the skin immune defense system. J Allergy Clin Immunol. 2009;124(3 Suppl 2):R13–18. doi: 10.1016/j.jaci.2009.07.014. [DOI] [PubMed] [Google Scholar]
- Schauber J, Oda Y, et al. Histone acetylation in keratinocytes enables control of the expression of cathelicidin and CD14 by 1,25-dihydroxyvitamin D3. J Invest Dermatol. 2008;128(4):816–824. doi: 10.1038/sj.jid.5701102. [DOI] [PubMed] [Google Scholar]
- Schechter NM, Choi EJ, et al. Inhibition of human kallikreins 5 and 7 by the serine protease inhibitor lympho-epithelial Kazal-type inhibitor (LEKTI) Biol Chem. 2005;386(11):1173–1184. doi: 10.1515/BC.2005.134. [DOI] [PubMed] [Google Scholar]
- Schittek B, Hipfel R, et al. Dermcidin: a novel human antibiotic peptide secreted by sweat glands. Nat Immunol. 2001;2(12):1133–1137. doi: 10.1038/ni732. [DOI] [PubMed] [Google Scholar]
- Schlereth T, Schonefeld S, et al. In vivo release of non-neuronal acetylcholine from human skin by dermal microdialysis: effects of sunlight, UV-A and tactile stimulus. Life Sci. 2007;80(24–25):2239–2242. doi: 10.1016/j.lfs.2007.01.008. [DOI] [PubMed] [Google Scholar]
- Schroder JM, Harder J. Human beta-defensin-2. Int J Biochem Cell Biol. 1999;31(6):645–651. doi: 10.1016/s1357-2725(99)00013-8. [DOI] [PubMed] [Google Scholar]
- Scott MG, Davidson DJ, et al. The human antimicrobial peptide LL-37 is a multifunctional modulator of innate immune responses. J Immunol. 2002;169(7):3883–3891. doi: 10.4049/jimmunol.169.7.3883. [DOI] [PubMed] [Google Scholar]
- Singer AJ, Clark RA. Cutaneous wound healing. N Engl J Med. 1999;341(10):738–746. doi: 10.1056/NEJM199909023411006. [DOI] [PubMed] [Google Scholar]
- Sivamani RK, Pullar CE, et al. Stress-mediated increases in systemic and local epinephrine impair skin wound healing: potential new indication for beta blockers. PLoS Med. 2009;6(1):e12. doi: 10.1371/journal.pmed.1000012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slominski A, Wortsman J. Neuroendocrinology of the skin. Endocr Rev. 2000;21(5):457–487. doi: 10.1210/edrv.21.5.0410. [DOI] [PubMed] [Google Scholar]
- Steinbach JH. Mechanism of action of the nicotinic acetylcholine receptor. Ciba Found Symp. 1990;152:53–61. doi: 10.1002/9780470513965.ch4. discussion 61–57. [DOI] [PubMed] [Google Scholar]
- Stryk S, Siegfried EC, et al. Selective antibody deficiency to bacterial polysaccharide antigens in patients with Netherton syndrome. Pediatr Dermatol. 1999;16(1):19–22. doi: 10.1046/j.1525-1470.1999.99005.x. [DOI] [PubMed] [Google Scholar]
- Suto H, Matsuda H, et al. NC/Nga mice: a mouse model for atopic dermatitis. Int Arch Allergy Immunol. 1999;120(Suppl 1):70–75. doi: 10.1159/000053599. [DOI] [PubMed] [Google Scholar]
- Swartling C, Naver H, et al. Treatment of dyshidrotic hand dermatitis with intradermal botulinum toxin. J Am Acad Dermatol. 2002;47(5):667–671. doi: 10.1067/mjd.2002.124605. [DOI] [PubMed] [Google Scholar]
- Szonyi M, Csermely P, et al. Acetylcholine-induced phosphorylation in isolated outer hair cells. Acta Otolaryngol. 1999;119(2):185–188. doi: 10.1080/00016489950181639. [DOI] [PubMed] [Google Scholar]
- Tajima T, Endo H, et al. Immobilization stress-induced increase of hippocampal acetylcholine and of plasma epinephrine, norepinephrine and glucose in rats. Brain Res. 1996;720(1–2):155–158. doi: 10.1016/0006-8993(96)00046-7. [DOI] [PubMed] [Google Scholar]
- Tracey KJ. The inflammatory reflex. Nature. 2002;420(6917):853–859. doi: 10.1038/nature01321. [DOI] [PubMed] [Google Scholar]
- Tracey KJ. Physiology and immunology of the cholinergic antiinflammatory pathway. J Clin Invest. 2007;117(2):289–296. doi: 10.1172/JCI30555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuji H, Okamoto K, et al. SLURP-2, a novel member of the human Ly-6 superfamily that is up-regulated in psoriasis vulgaris. Genomics. 2003;81(1):26–33. doi: 10.1016/s0888-7543(02)00025-3. [DOI] [PubMed] [Google Scholar]
- van der Poll T, Coyle SM, et al. Epinephrine inhibits tumor necrosis factor-alpha and potentiates interleukin 10 production during human endotoxemia. J Clin Invest. 1996;97(3):713–719. doi: 10.1172/JCI118469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Yu M, et al. Nicotinic acetylcholine receptor alpha7 subunit is an essential regulator of inflammation. Nature. 2003;421(6921):384–388. doi: 10.1038/nature01339. [DOI] [PubMed] [Google Scholar]
- Wang TT, Nestel FP, et al. Cutting edge: 1,25-dihydroxyvitamin D3 is a direct inducer of antimicrobial peptide gene expression. J Immunol. 2004;173(5):2909–2912. doi: 10.4049/jimmunol.173.5.2909. [DOI] [PubMed] [Google Scholar]
- Weber G, Heilborn JD, et al. Vitamin D induces the antimicrobial protein hCAP18 in human skin. J Invest Dermatol. 2005;124(5):1080–1082. doi: 10.1111/j.0022-202X.2005.23687.x. [DOI] [PubMed] [Google Scholar]
- Wessler I, Kirkpatrick CJ, et al. Non-neuronal acetylcholine, a locally acting molecule, widely distributed in biological systems: expression and function in humans. Pharmacol Ther. 1998;77(1):59–79. doi: 10.1016/s0163-7258(97)00085-5. [DOI] [PubMed] [Google Scholar]
- Wessler I, Kirkpatrick CJ, et al. The cholinergic ‘pitfall’: acetylcholine, a universal cell molecule in biological systems, including humans. Clin Exp Pharmacol Physiol. 1999;26(3):198–205. doi: 10.1046/j.1440-1681.1999.03016.x. [DOI] [PubMed] [Google Scholar]
- Wessler I, Reinheimer T, et al. Increased acetylcholine levels in skin biopsies of patients with atopic dermatitis. Life Sci. 2003;72(18–19):2169–2172. doi: 10.1016/s0024-3205(03)00079-1. [DOI] [PubMed] [Google Scholar]
- Woiciechowsky C, Asadullah K, et al. Sympathetic activation triggers systemic interleukin-10 release in immunodepression induced by brain injury. Nat Med. 1998;4(7):808–813. doi: 10.1038/nm0798-808. [DOI] [PubMed] [Google Scholar]
- Wollina U, Karamfilov T. Adjuvant botulinum toxin A in dyshidrotic hand eczema: a controlled prospective pilot study with left-right comparison. J Eur Acad Dermatol Venereol. 2002;16(1):40–42. doi: 10.1046/j.1468-3083.2002.00361.x. [DOI] [PubMed] [Google Scholar]
- Wood LC, Jackson SM, et al. Cutaneous barrier perturbation stimulates cytokine production in the epidermis of mice. J Clin Invest. 1992;90(2):482–487. doi: 10.1172/JCI115884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu LR, Zaborina O, et al. Surgical injury and metabolic stress enhance the virulence of the human opportunistic pathogen Pseudomonas aeruginosa. Surg Infect (Larchmt) 2005;6(2):185–195. doi: 10.1089/sur.2005.6.185. [DOI] [PubMed] [Google Scholar]
- Yamasaki K, Di Nardo A, et al. Increased serine protease activity and cathelicidin promotes skin inflammation in rosacea. Nat Med. 2007;13(8):975–980. doi: 10.1038/nm1616. [DOI] [PubMed] [Google Scholar]
- Yamasaki K, Schauber J, et al. Kallikrein-mediated proteolysis regulates the antimicrobial effects of cathelicidins in skin. FASEB J. 2006;20(12):2068–2080. doi: 10.1096/fj.06-6075com. [DOI] [PubMed] [Google Scholar]
- Zasloff M. Magainins, a class of antimicrobial peptides from Xenopus skin: isolation, characterization of two active forms, and partial cDNA sequence of a precursor. Proc Natl Acad Sci U S A. 1987;84(15):5449–5453. doi: 10.1073/pnas.84.15.5449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y, Niyonsaba F, et al. Cathelicidin LL-37 induces the generation of reactive oxygen species and release of human alpha-defensins from neutrophils. Br J Dermatol. 2007;157(6):1124–1131. doi: 10.1111/j.1365-2133.2007.08196.x. [DOI] [PubMed] [Google Scholar]