Abstract
Background
Focal treatment of Parkinson’s disease tremor by botulinum toxin type A incobotulinumtoxinA (BoNT-A) injections has been inadequately investigated and at best provides modest relief with significant muscle weakness. Complexity of multi-joint tremulous movements results in non-individualized dosing regimens. This 38-week open-label study used kinematic technology to guide muscle selection and improve efficacy of incobotulinumtoxinA (BoNT-A) injections for Parkinson’s disease tremor.
Methods
Participants (n=28) attended study visits at weeks 0, 6, 16, 22, 32, and 38, and were injected with BoNT-A at weeks 0, 16, and 32. During each visit, clinical tremor scales, the Unified Parkinson’s Disease Rating Scale (UPDRS) and the Fahn–Tolosa–Marin (FTM), and kinematic assessments were conducted. Participants performed rest and postural scripted tasks with motion sensors placed over the wrist, elbow, and shoulder joints where tremor was quantified by angular root mean square (RMS) amplitude in multiple degrees of freedom at each joint. Injection parameters were determined using the clinician’s interpretation of which muscles would contribute to the upper limb tremor biomechanics analyzed kinematically.
Results
Kinematic measures of tremor amplitude allowed detailed segmentation of tremor into directional components at each arm joint permitting a statistically significant decrease in mean UPDRS item 20 (rest tremor) at week 16 (p=0.006) and at week 32 (p=0.014), and in FTM tremor severity scores at week 6 (p=0.024). Ten participants perceived mild muscle weakness following the third treatment, which did not interfere with performing activities of daily living.
Discussion
Kinematics is a simple method for standardizing assessments and treatment of upper limb Parkinson’s disease tremor, thereby personalizing tremor therapy and optimizing the effect of BoNT-A injections for Parkinson’s disease tremor.
Keywords: Parkinson’s disease, tremor, movement disorders, botulinum toxin, kinematics, biomechanics
Introduction
Tremor is a cardinal sign of Parkinson’s disease (PD) and is one of the most challenging symptoms to treat. In PD patients, tremor is predominantly present at rest compared to posture or task-specific movement.1–3 Tremor causes difficulty in performing daily activities and significantly affects quality of life.4 Levodopa remains the most potent drug for managing PD symptoms yet it results in significant complications such as “wearing off” motor fluctuations and dyskinesia, and thus its use as a starting therapy for PD tremor is discouraged.5–7 Dopamine agonists and anticholinergic medications can be used concomitantly with levodopa to treat tremor but may be accompanied by neuropsychiatric and cognitive side effects.8,9 Deep brain stimulation is an effective treatment for treating recalcitrant PD tremor, but this is an invasive procedure and optimization of programming parameters still remains unclear. Therefore, physicians and patients are reluctant to use conventional pharmacotherapy as the first line of defense for tremor. Alternative methods for treating tremor must be considered, as an effective therapy is an enormous unmet need in tremor-dominant PD patients.
Visual assessment of upper limb tremor is restricted by the difficultly in separating multi-joint, whole-arm movements. Characteristics of tremor such as severity at the fingers, wrist, elbow, and shoulder vary per patient and voluntary tasks alter upper limb biomechanics.2 Wrist tremor is complicated by the wrist’s ability to simultaneously flex–extend, pronate–supinate, and deviate from side to side, commonly seen during rest and described as a “pill-rolling” action in the hand.10 Elbow and shoulder tremors are challenging to segment due to the size of these joints, and consequently their small amplitude movements make a significant impact at the most distal part of the arm. Similar to the wrist, the biomechanics of the shoulder simultaneously moves in two directions, abduction–adduction (Abd/Add) and flexion–extension (F/E).11 Thus, understanding tremor biomechanics is crucial for targeting specific muscle groups for effective symptomatic treatment by BoNT-A injections.
Treating upper limb tremor with BoNT-A intramuscular injections has not been widely adopted in clinical practice in PD tremor, despite some success in reducing tremor severity and improving functional rating scores.12,13 Limited improvements were attributed mainly to muscle weakness. Significant muscle weakness from rigid protocols using a fixed dose and predetermined group of muscles to inject, regardless of the patient’s tremor characteristics, may contribute to limb weakness and subsequent loss of function.14,15 Even with techniques such as electromyograph- (EMG) or ultrasound-guided needle injections that minimize toxin spread, muscle weakness can still occur.16 Another factor contributing to the low efficacy reported in previous studies may have been due to only having one or two treatment cycles with short follow-up visits.17,18 The lack of objective tremor assessments to monitor the dynamic movements at each joint may also be a factor hindering optimization capability and therapeutic outcome. Ultimately the selection of appropriate muscles to inject at each joint remains the most important issue that kinematics can solve by simplifying assessment of tremor and guide therapy.
Kinematic technology has been used to study the biomechanics of motion in many scenarios, including gait and whole-body characteristics.19,20 The use of such multi-sensor motion recordings for tremor feature extraction is well understood.21 Successful focal tremor therapy has recently been performed by using the biomechanics of tremor at each of the three arm joints for standardizing selection of injection parameters.21 Thus, efficacious use of BoNT-A, as a focal treatment, requires appropriately determined injection sites and dosage per muscle.22 To determine these parameters, a clinician can use kinematic characterization of a patient’s upper limb tremor to select muscles known to contribute to the joint movement. This was investigated in the longest open label study to date involving 28 PD participants who had received three BoNT-A injection treatments based upon kinematically guided muscle selection criteria for upper limb PD tremor every 16 weeks over a 38-week duration.
Methods
This open label, single-center, single-injector study (Health Canada CTA# 178589) recruited a convenience sampling of 28 PD participants from the London Movement Disorders Centre in London, Ontario, who provided written consent and attended six study visits at weeks 0, 6, 16, 22, 32, and 38 and were treated with BoNT-A at weeks 0, 16, and 32. Treatment-naïve participants were maintained on monotherapy of BoNT-A injections for their PD throughout the study whereas participants on treatment did not change their medications throughout the study. Participants on stable PD medication, with inadequate tremor relief, were assessed in the “ON” state during study visits, at approximately the same time of the day. Each study visit involved completion of clinical scales and kinematic tremor measurements. BoNT-A (0.5 mL of saline per 100 unit vial) was injected into the tremor dominant limb under the guidance of EMG using a Clavis portable EMG device (1′′ long 30 g injectable EMG needle).
Study inclusion/exclusion criteria
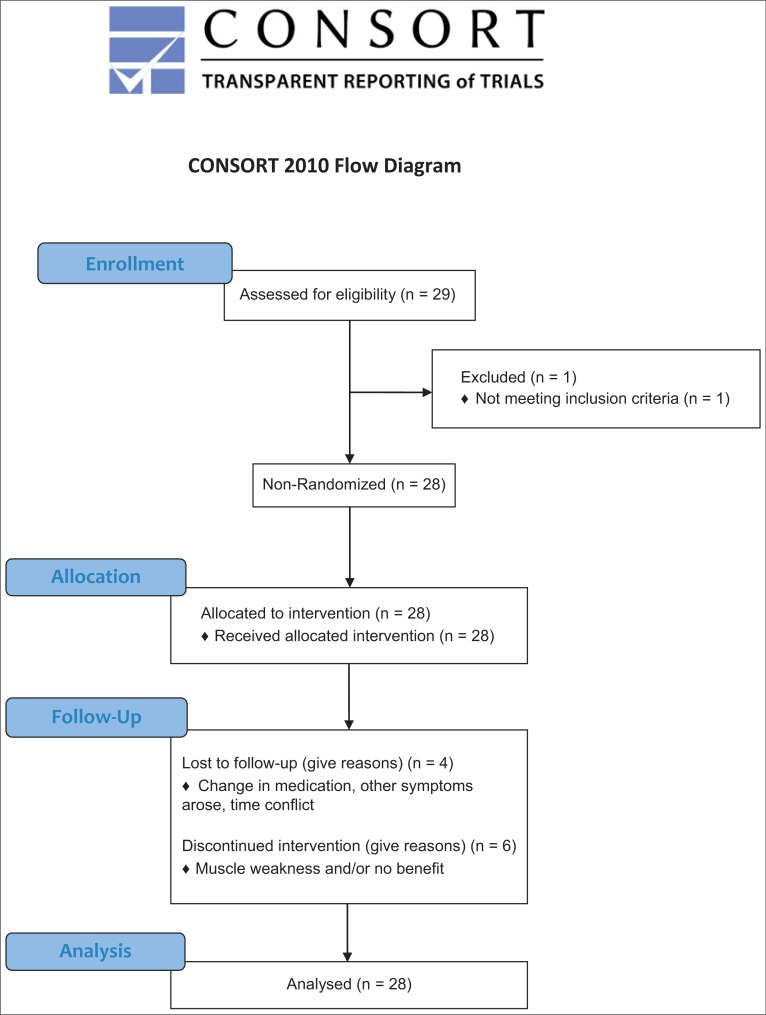
The Western University Health Sciences Research Ethics Board approved this clinical phase IIb pilot study protocol (REB#18445). The first visit of the first participant and last visit of the last participant occurred on November 2012 and August 2013, respectively. The authors confirm that all ongoing and related trials for this drug/intervention are now registered (ClinicalTrials.gov Identifier: NCT02427646). The study’s progress was outlined in the CONSORT flowchart displayed in Figure 1.
Figure 1. CONSORT Flow Diagram Displaying the Progress of the Study Design. Progress through the various stages of a trial including flow of participants, number reasoning of withdrawals and the number of participants included for analysis.
Inclusion criteria were consenting male and female participants diagnosed with PD by the UK Brain Bank Criteria with. Hoehn and Yahr stage 1–3 disease, aged 18–80 years, having tremor as their most bothersome and important symptom while on stable medication management for at least 6 months before enrolment, with no medication withheld or adjusted during the time of the study, and BoNT-A-naïve. Participant criteria excluded those who had a history of stroke, contraindications according to the BoNT-A drug monograph, pregnancy, and existing pharmacological therapy with tremor-inducing side effects (e.g. lithium, valproate, etc.).
Clinical scale assessment
Well-established tremor severity and functional rating scales were used as primary outcomes for measuring efficacy and tolerability of BoNT-A injections. Participants completed the Fahn–Tolosa–Marin (FTM) scale, consisting of parts A–C: rating tremor severity, and writing and functional disability caused by tremor. The movement disorders neurologist, blinded to prior results, assessed motor Unified Parkinson’s Disease Rating Scale (UPDRS) items 20 and 21, rest and action tremor respectively, during injection visits. The assessor monitored muscle weakness by measuring maximal grip strength using a dynamometer. Participants reported any perceived muscle weakness using a Likert scale (ranging from 0, no weakness, to 4, severe weakness in injected arm muscles).
Kinematic assessment
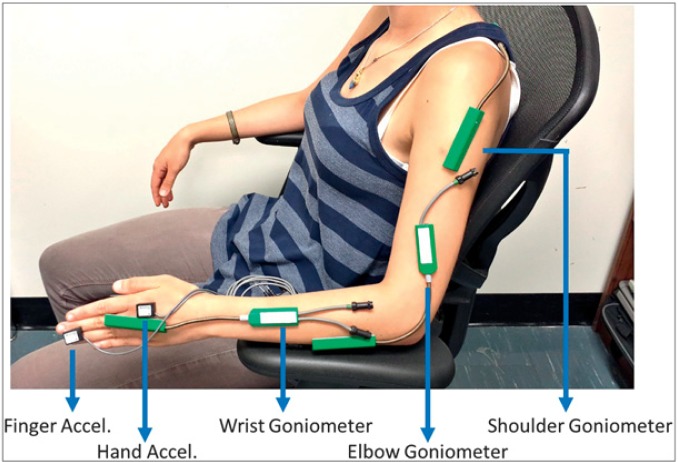
Kinematic measures of tremor were conducted while participants were in their “ON” state rather than their “OFF” state to reduce any overestimation of tremor severity. As participants were already stable on their oral medications, kinematic assessment was deemed to be best determined after taking into account the optimal medication response that was in the “ON” state. A series of scripted tasks was performed by each participant while seated with motion sensor devices placed over each arm joint (Figure 2). Wrist tremor was measured by angular position. Electrogoniometers (SG150, Biometrics Ltd) were used to measure the wrist in two degrees of freedom (DOF): flexion-extension (F/E) and radial–ulnar deviation (R/U). A torsiometer (Q150, Biometrics Ltd) was placed on the dorsal surface of the forearm to measure wrist pronation–supination (P/S). The torsiometer provided the third angular DOF of rotational motion about the wrist. The relative motion of the wrist and the forearm was recorded by the electrogoniometer. An electrogoniometer measured the elbow in one DOF: F/E. An electrogoniometer placed on the shoulder measured two DOF: F/E and Abd/Add. Thus, a total of four motion sensors were required to capture each joint movement. Motion sensor data were collected at 1500 Hz by TeleMyo 2400T G2 and PC interface (MyoResearch XP Master Edition 1.08.09, Noraxon). Sensors were attached using 3M hypoallergenic micropore medical grade tape (Ref no.: 1530–1). Recordings at each joint were mutually exclusive, with each sensor recording data only from a particular joint. Accelerometer sensor data were additionally collected as a comparison tool to prior studies and were not used for determining tremor composition.
Figure 2. Kinematic Sensor Placement for Tremor Assessment. Placement of biometric motion sensors along the arm: shoulder electrogoniometer, elbow electrogoniometer, wrist electrogoniometer. Accelerometers were placed on the forearm, hand, and third finger.
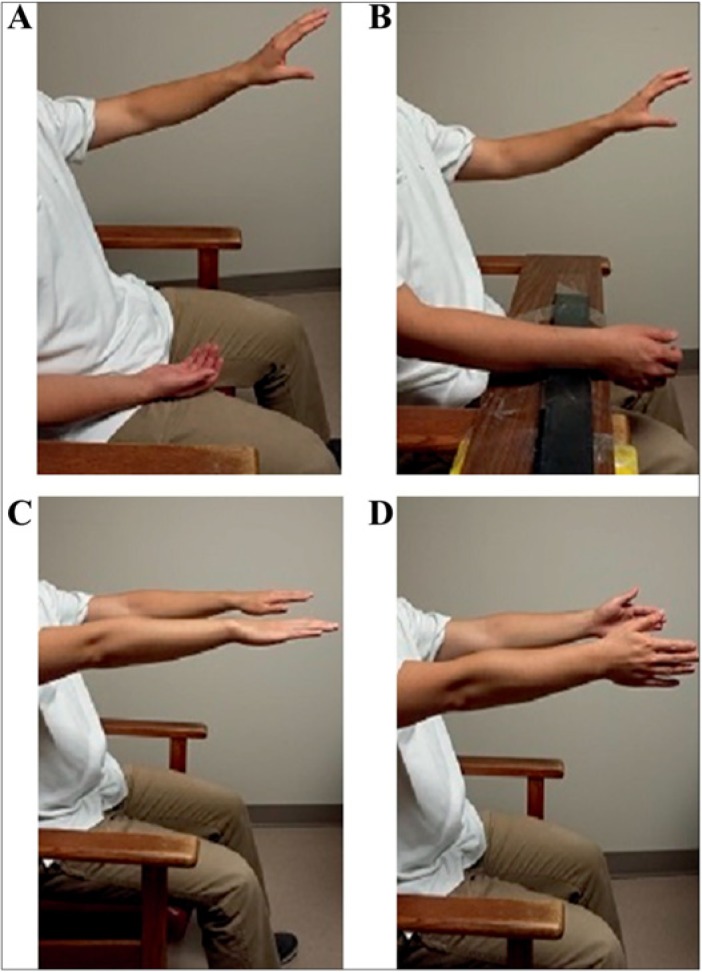
Sensors captured tremor severity in angular RMS amplitude at each arm joint producing biomechanical tremor data in multiple DOF: F/E, P/S, and R/U in the wrist, F/E in the elbow, and F/E and Abd/Add in the shoulder. Sensor calibration was completed with the forearm supported and with the hand fixed against a vertical plane in the neutral F/E, R/U, and P/S positions, and was held for 5 seconds. Additional sensor calibration was performed with the participant’s arm held straight while standing, the elbow extended with fingers pointing down for five seconds. A total of four consecutive scripted tasks were repeated three times in series (Figure 3). Twenty seconds were allotted to each task that involved two rest positions (focusing on F/E and P/S movements), and two postural positions (focusing on F/E and R/U movements). Rest tasks, denoted “Rest-1” and “Rest-2” were performed with a distraction (Figure 3A,B). Postural tasks involved the participant extending both arms outstretched in front of the body with palms facing downwards or inwards, denoted “posture-1” and “posture-2”, respectively. Tremor was captured simultaneously with the motion sensors at the middle finger and on the back of the hand using accelerometers, but accelerometry data were not utilized for determining injection parameters.
Figure 3. Standard Scripted Tasks Performed by Each Participant during Kinematic Recording Sessions. (A) Rest position (rest-1) with relaxed forearm in lap measuring flexion–extension (F/E) wrist movements. (B) Rest position (rest-2) with arm supported measuring P/S movements. (C) Posture-1 position with shoulders flexed at 90° with arms extended anteriorly and pronated (palms facing downwards). (D) Posture-2 position with shoulders flexed at 90° with arms extended anteriorly, palms facing inwards.
Injection determination
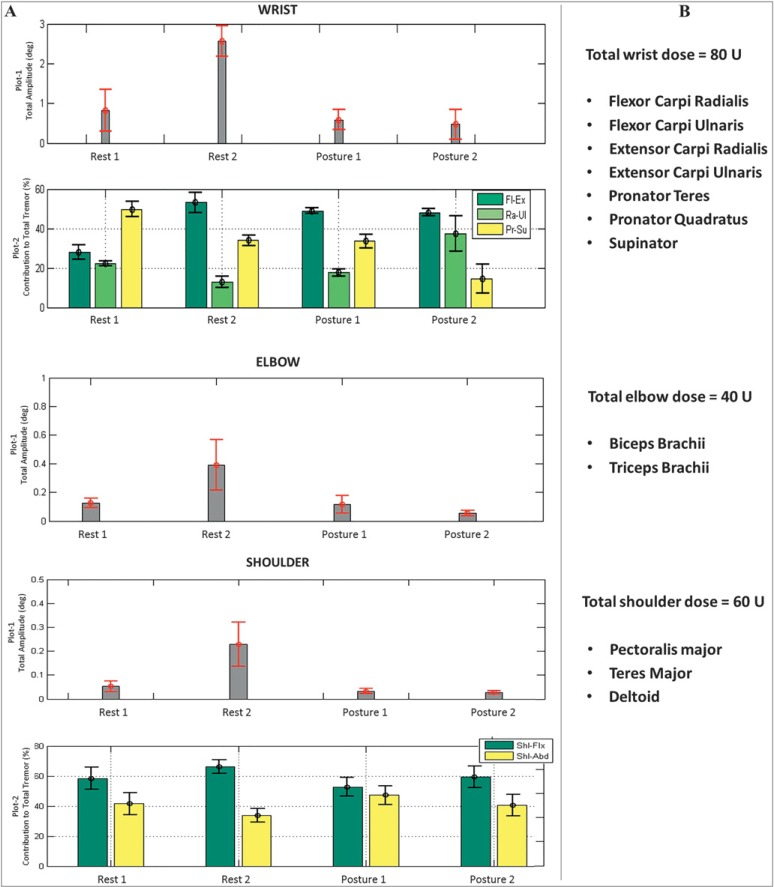
Raw angular signal data were processed using custom-written software in MatLab (R2011a).11,21 The interpreted data sets displayed total tremor severity measured in angular RMS amplitude at each arm joint for each scripted task (Figure 4A), which was reviewed by the movement disorders neurologist before injection. Kinematic analyses provided a percentage of the directional contribution of tremor, segmentation of tremor at the wrist and shoulder joints (the elbow moves in one DOF), during a specific task. Focusing on each joint individually, the movement disorders neurologist’s best clinical judgment determined a BoNT-A dosage solely using the total tremor amplitude measured kinematically. This selected BoNT-A dose was divided using the percentage contribution data. Muscles selected for injection were on the basis of the known anatomical basis of movement at each joint. The amount of BoNT-A was allocated to appropriate muscles for injection and thus injection patterns were determined solely on the basis of the kinematic tremor composition and the injector’s clinical expertise. Subsequent dosages were determined by the injector’s comparisons of kinematic data with the previous visit. This approach allowed the movement disorder neurologist to use the kinematic data to individualize BoNT-A injection parameters to the participant’s unique tremor aspects at each arm joint. This ensured that the most appropriate muscles were selected, making this approach user-friendly and generalizable in the experienced clinician’s hands.
Figure 4. Sample Participant Kinematic Data Readout of Tremor Generated from the Wrist, Elbow, and Shoulder Joints and Individualized Muscle Selection Based on the Kinematic Tremor Profile and the Injector’s Best Clinical Judgment. (A) Total tremor severity (plot 1) is displayed in angular RMS amplitude and the percentage of directional contribution of tremulous movement (plot 2) by three degrees of freedom in the wrist and by two degrees of freedom in the shoulder joint. Error bars indicate standard deviation over three trials. (B) Injector’s interpretation of the kinematic results showing selection of total dose allocated to wrist, elbow, and shoulder muscle groups based on tremor severity and the muscles selected on the basis of the amount of tremor present in each degree of freedom each arm joint moves in. Abbreviations: Fl-Ex, flexion-extension; Ra-Ul, radial-ulnar; Pr-Su, pronation-supination; Shl-Flx, shoulder flexion-extension; Shl-Abd, shoulder abduction-adduction.
Statistical analyses
The means and standard deviations of both kinematic and clinical data were analyzed using SPSS version 21 by performing one-way repeated measures analysis of variance (ANOVA) using confidence intervals of 95% (α=0.05) with post hoc Bonferroni corrections for multiple comparisons performed across all time points. Clinical scales were represented by mean and standard deviations of the population for each time point. The mean angular RMS tremor amplitude for all three trials per task at each time point was log-transformed as tremor amplitudes generated positively skewed distributions. Tremor accelerometry values captured in the x-, y-, and z-axes for each task were averaged per participant at each time point. Missing random variable analysis tests were conducted to ensure incomplete data sets for all independent variables were missing completely at random. The means from each clinical rating scale and from the kinematic tremor analyses that met criteria were tested for normality using the Shapiro–Wilk test and z-score for skewness and kurtosis. The means that met parametric analysis criteria underwent parametric ANOVA tests to determine the presence of significant changes between time points compared with week 0. Means that did not meet parametric test criteria were tested using Friedman’s test. Partial eta-squared (partial η2) was reported as an estimate of the population effect size.
Results
Participant demographics
Demographics and baseline clinical scores of the 28 PD study participants are shown in Table 1. Following the first treatment at week 16, 11% of participants (3/28) withdrew due to experiencing both inadequate functional benefit and bothersome muscle weakness. Following the second treatment at week 32 and focusing on the remaining 89% of participants (25/28), one participant withdrew because of unwanted weakness, and two participants failed to maintain inclusion criteria such as lack of study attendance and medication change. Of the remaining participants (22/28), four did not continue past week 32 because of unwanted weakness (9%, 2/22), failed study attendance (4%, 1/22), and change in other PD symptoms (4%, 1/22). Thus, only a total of six PD participants (21%) experienced unwanted weakness warranting study withdrawal following three treatments. However, this implies that 79% of patients did not have enough weakness to discontinue participation in the study.
Table 1. PD Participant Demographics and Baseline UPDRS Scores.
| Baseline Scores | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Patient ID | Gender | Age | Years with Tremor | Injected Limb | Dominant Limb | Weight (lbs) | Item 20 Non-treated Arm (/4) | Item 20 Treated Arm (/4) | Item 21 Non-treated Arm (/4) | Item 21 Treated Arm (/4) |
| 1 | F | 71 | 11 | L | R | 170 | 0 | 2 | 1 | 2 |
| 2 | M | 35 | 7 | R | R | 350 | 0 | 2 | 0 | 3 |
| 3 | M | 62 | 7 | R | R | 175 | 0 | 3 | 0 | 0 |
| 4 | M | 79 | 7 | R | R | 165 | 2 | 3.5 | 1 | 1 |
| 5 | M | 53 | 10 | L | R | 2 | 3 | 1 | 2.5 | |
| 6 | M | 43 | 5 | L | R | 1 | 2 | 1 | 2 | |
| 7 | M | 60 | 7 | R | R | 225 | 1 | 3 | 1 | 2.5 |
| 8 | M | 79 | 14 | R | R | 4 | 4 | 2 | 2 | |
| 9 | M | 59 | 11 | R | R | 275 | 2 | 2 | 1 | 1 |
| 10 | F | 77 | 9 | L | R | 185 | 1 | 3 | 1 | 2 |
| 11 | M | 62 | 5 | R | R | 203 | 2 | 3 | 0 | 0 |
| 12 | M | 66 | 7 | R | R | 185 | 0 | 2.5 | 1 | 1 |
| 13 | M | 76 | 6 | R | R | 152 | 1 | 2 | 0 | 1 |
| 14 | F | 54 | 6 | R | R | 140 | 0 | 2 | 1 | 0 |
| 15 | F | 50 | R | R | 0 | 3 | 0 | 2 | ||
| 16 | F | 75 | R | R | 0 | 3 | 2 | 2 | ||
| 17 | F | 62 | 8 | L | R | 152 | 2 | 3.5 | 1 | 2.5 |
| 18 | F | 47 | 14 | R | R | 193 | 1 | 2 | 1 | 2 |
| 19 | F | 71 | R | R | 0 | 2.5 | 1 | 2 | ||
| 20 | M | 80 | 9 | R | R | 150 | 0 | 3.5 | 0 | 0 |
| 21 | M | 59 | 7 | L | R | 170 | 0 | 3 | 0 | 2 |
| 22 | M | 69 | 6 | R | R | 234 | 0 | 3.5 | 0 | 2.5 |
| 23 | F | 70 | 6 | R | R | 165 | 2 | 2 | 2.5 | 2.5 |
| 24 | M | 68 | 14 | R | R | 160 | 3 | 3 | 1 | 1 |
| 25 | M | 70 | 7 | R | R | 165 | 0 | 3.5 | 0 | 3.5 |
| 26 | M | 69 | L | R | 215 | 0 | 2 | 0 | 1 | |
| 27 | F | 80 | 5 | R | R | 150 | 1 | 2.5 | 1 | 1 |
| 28 | F | 66 | L | R | 168 | 1 | 2.5 | 0 | 1 | |
| Mean | 7F | 65.5 | 7.5 | 8L | 1L | 188.2 | 0.9 | 2.7 | 0.7 | 1.6 |
| ± SD | ± 11.5 | ± 3.1 | ± 47.5 | ± 1.0 | ± 0.6 | ± 0.7 | ± 0.9 | |||
Abbreviations: F, Female; L, Left; M, Male; PD, Parkinson’s Disease; R, Right; SD, Standard Deviation; UPDRS, Unified Parkinson’s Disease Rating Scale.
Selecting Kinematically-based BoNT-A injection parameters
Kinematics was utilized to quantify two key characteristics of tremor for optimizing BoNT-A therapy: severity of total tremor (angular RMS amplitude) and directional contribution of the tremor at each arm joint. Figure 4A displays a sample participant’s kinematic tremor measures during each of the four tasks by plotting the total tremor severity (plot 1) in the wrist, elbow, and shoulder joints and segmenting total tremor in the wrist and shoulder joints by directional movements of the total tremor (plot 2). The task with the largest tremor amplitude served as a biomechanical basis for determining BoNT-A injection parameters. The movement disorders neurologist interpreted the kinematics by basing the total dose on total tremor severity (plots 1). This total dose was ultimately divided among select muscles that generated these fundamental tremulous movements, focusing on the distribution of the total tremor in each DOF (plots 2) at each arm joint (Figure 4B). In the example in Figure 4A, the “rest-2” task, with forearm supported, generated the largest tremor severity at the wrist, elbow, and shoulder joints. Thus, allocation of the total dose was distributed according to the division of the total tremor, illustrated in plot 2 for wrist and shoulder joints in Figure 4A.
Injection parameters were optimized solely using kinematics by comparing the change in tremor at baseline to kinematic measures of tremor at 6 weeks and 16 weeks after treatment. A reduction in total tremor during a task at the 6-week follow-up visit indicated the appropriate muscles were targeted. An increase in BoNT-A dose was required if the tremor could be reduced further, as quantified by kinematics at post-treatment assessments, and there were no side effects present, as perceived by the participant (reported on a Likert scale). A reduction in dose was indicated by the participant when prolonged muscle weakness was experienced in injected muscles lasting more than 1 month, as rated by the Likert scale for muscle weakness, and weakness was reported as functionally bothersome.
The mean total dose per arm did not significantly change between the first and third treatments; however, the mean number of injected muscles gradually increased (Table 2). The total dose for the second treatment was increased for 47.6% of participants (10/21) and was reduced for 14.2% (3/21). Of those who required an increased BoNT-A dosing at the second treatment, at the third treatment, 10% of participants (1/10) required a reversal of the increased BoNT-A reverting to the original parameters and 20% of participants (2/10) required an additional increase in the total dose. One participant whose total dose increased at the second treatment was not injected at the third treatment due to prolonged moderate muscle weakness. The total dose was reduced for 13.3% of participants (2/15) whose parameters were not altered during the second treatment though required a reduced total dose at the third treatment.
Table 2. Total Injected Dosage and Number of Muscles Injected as Determined by Injector Across all Participants.
| Week 0 (First Injection) | Week 16 (Second Injection) | Week 32 (Third Injection) | ||||
|---|---|---|---|---|---|---|
| Patient | BoNT-A Dose (U) | No. of Muscles Injected | BoNT-A Dose (U) | No. of Muscles Injected | BoNT-A Dose (U) | No. of Muscles Injected |
| 1 | 100 | 6 | No injection1 | 75 | 8 | |
| 2 | 200 | 7 | 200 | 7 | No Injection1 | |
| 3 | 100 | 6 | 100 | 6 | No Injection1 | |
| 4 | 100 | 8 | 200 | 8 | No Injection1 | |
| 5 | 100 | 8 | 100 | 8 | No Injection1 | |
| 6 | 100 | 6 | Withdrawn4 | Withdrawn4 | ||
| 7 | 200 | 8 | No Injection1 | 200 | 8 | |
| 8 | 275 | 8 | Withdrawn4 | Withdrawn4 | ||
| 9 | 260 | 9 | 390 | 11 | No Injection1 | |
| 10 | 125 | 7 | No injection2 | 125 | 7 | |
| 11 | 140 | 8 | 175 | 9 | No injection3 | |
| 12 | 100 | 8 | 170 | 8 | 100 | 8 |
| 13 | 175 | 8 | 175 | 8 | 135 | 8 |
| 14 | 95 | 7 | 95 | 7 | 95 | 7 |
| 15 | 320 | 11 | 350 | 11 | Withdrawn4 | |
| 16 | 200 | 11 | Withdrawn5 | Withdrawn5 | ||
| 17 | 200 | 11 | 280 | 9 | 300 | 8 |
| 18 | 200 | 10 | 200 | 10 | 200 | 10 |
| 19 | 200 | 6 | Withdrawn4 | Withdrawn4 | ||
| 20 | 265 | 13 | 300 | 13 | 300 | 13 |
| 21 | 200 | 8 | 280 | 12 | 300 | 13 |
| 22 | 200 | 8 | 100 | 8 | 100 | 8 |
| 23 | 190 | 11 | 170 | 11 | 170 | 11 |
| 24 | 200 | 8 | 200 | 8 | 200 | 11 |
| 25 | 300 | 12 | 300 | 12 | 300 | 12 |
| 26 | 100 | 7 | 200 | 9 | Withdrawn5 | |
| 27 | 130 | 9 | 200 | 11 | 200 | 11 |
| 28 | 100 | 6 | 80 | 6 | Withdrawn6 | |
| Mean±SD | 174.1±66.8 | 8.4±1.9 | 203.1±84.4 | 9.1±2.0 | 186.7±79.5 | 9.5±2.1 |
Abbreviations: BoNT-A, IncobotulinumtoxinA; SD, Standard Deviation.
Note: Dosing was in BoNT-A units.
Participant presented with minimal tremor at visit and injector made a clinical judgment against injection.
Participants subjectively reported prolonged mild unwanted weakness in non-injected muscles in treated arm, but had functional benefit.
Participant perceived prolonged moderate wrist extensor weakness with limited functional benefit.
Participant withdrew from study due to wrist extensor weakness.
Participant withdrew from study due to lack of time commitment.
Participant withdrew from study due to changes in Parkinson’s disease symptoms and met exclusion criteria.
The muscles selected and the mean administered dose per muscle are summarized in Table 3. For the first treatment, all participants were injected in the flexor carpi ulnaris and extensor carpi ulnaris. The most frequently injected muscles during the second injection cycle (20/21) were the extensor carpi ulnaris, pronator teres, and pronator quadratus, and flexor carpi radialis, extensor carpi radialis, pronator teres, pronator quadratus, and supinator during the third treatment (14/15).
Table 3. Mean Injection Dosage by Arm Muscle Treated at Each Treatment Time Point.
| Muscles Injected | First Injection (Week 0) | Second Injection (Week 16) | Third Injection (Week 32) | |||
|---|---|---|---|---|---|---|
| Mean±SD | No. of Patients (n=28) | Mean±SD | No. of Patients (n=21) | Mean±SD | No. of Patients (n=15) | |
| Flexor carpi radialis | 16.3±7.0 | 24 | 15.6±5.7 | 17 | 13.6±5.8 | 14 |
| Flexor carpi ulnaris | 16.8±6.7 | 28 | 16.1±5.8 | 19 | 14.2±5.5 | 13 |
| Brachioradialis | 20.0±0.0 | 1 | 20.0±0.0 | 1 | 20.0±0.0 | 1 |
| Extensor carpi radialis | 18.5±8.2 | 24 | 17.5±5.8 | 18 | 16.1±5.4 | 14 |
| Extensor carpi ulnaris | 18.6±7.9 | 28 | 17.5±5.8 | 20 | 16.5±5.3 | 13 |
| Pronator teres | 17.4±5.1 | 25 | 17.8±4.9 | 20 | 15.7±4.2 | 14 |
| Pronator quadratus | 16.0±4.9 | 25 | 17.3±5.1 | 20 | 15.4±4.8 | 14 |
| Supinator | 17.3±4.5 | 22 | 18.1±4.8 | 18 | 16.8±7.0 | 14 |
| Biceps brachii | 33.9±10.3 | 23 | 36.3±10.4 | 19 | 30.4±9.7 | 12 |
| Triceps | 29.5±10.1 | 10 | 32.7±9.6 | 11 | 28.1±9.3 | 8 |
| Pectoralis major | 33.3±8.8 | 9 | 34.5±13.0 | 11 | 28.1±9.3 | 6 |
| Teres major | 25.8±6.7 | 6 | 30.0±12.2 | 8 | 29.2±10.6 | 8 |
| Deltoid | 30.0±9.4 | 4 | 32.0±9.3 | 5 | 30.0±5.5 | 5 |
| Supraspinatus | 28.0±2.4 | 5 | 30.0±5.5 | 5 | 27.5±2.5 | 4 |
Abbreviation: SD, Standard Deviation.
Note: All the dosages are in units of BoNT-A. The mean values represent the average dose administered in the mean number of participants injected in that particular muscle.
Clinical and kinematic efficacy results
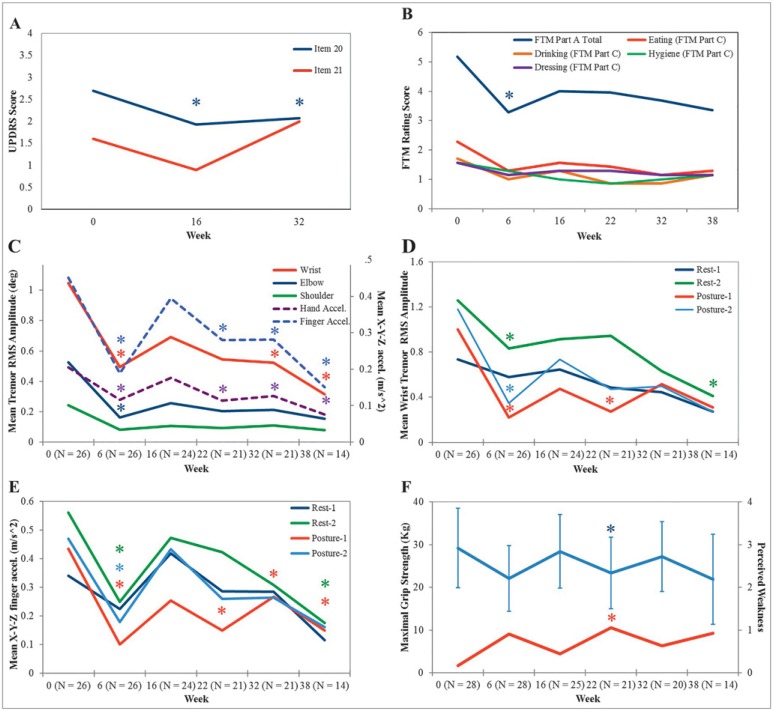
Severity of rest tremor (UPDRS item 20) in the treated arm was significantly reduced (F[2,40]=8.378, p=0.001, partial η2=0.295) from 2.7±0.6 at week 0 to 2.0±0.8 at week 16 (p=0.006) and to 2.1±0.7 at week 32 (p=0.014). Action tremor (UPDRS item 21) was reduced in the treated arm from 1.6±0.9 at week 0 to 0.9±1.0 (p=0.09) at week 16 and to 1.0±0.8 at week 32, although this was not statistically significant (F[2,40]=2.832, p=0.071, partial η2=0.124) (Figure 5A).
Figure 5. IncobotulinumtoxinA Treatments Significantly Reduced Tremor Severity and Improved Arm Function in the Treated Arm of Parkinson’s Disease Participants Reported Qualitatively and Quantitatively. (A) Unified Parkinson’s Disease Rating Scale item 20 and 21 mean scores (max: four per arm) for rest and action tremor, respectively. (B) Fahn–Tolosa–Marin (FTM) part A score (max: 12 per arm), sum of tremor severity during rest, posture, and action tasks, significantly decreased. FTM part C score (max: four per category), functional disability, was significantly reduced for eating tasks (N=7). (C) Angular RMS tremor amplitude (primary y-axis) and hand and finger accelerometer values (secondary y-axis) for each arm joint were averaged over two rest and two postural tasks per time point. Significant reductions in wrist amplitude and finger accelerometry were observed. (D) Angular wrist tremor RMS amplitude and (E) mean x-y-z tremor acceleration captured from the third finger displayed for each rest and postural task showed significant reduction. (F) Maximal grip strength (blue line) and perceived muscle weakness (red line) yielded significant change at week 22. Abbreviations: UPDRS, Unified Parkinson's Disease Rating Scale; FTM, Fahn-Tolosa-Marin; RMS, root mean square.
The FTM part A score, indicating tremor severity, was significantly reduced (F[5,65]=2.043, p=0.024, partial η2=0.136) at week 6 compared with week 0 (Figure 5B), although the mean total FTM part B score assessing handwriting and pouring function did not produce a significant reduction (F[5,60]=1.820, p=0.123, partial η2=0.132). Twenty-five percent of participants (7/28) indicated their arm tremor was the root source of functional disability, as opposed to other PD symptoms interfering with activities of daily living, including eating, drinking, and working tasks (Figure 5B). For these participants, eating (solid food) FTM subcategory score was significantly reduced (F[5,30]=2.558, p=0.048, partial η2=0.299) and produced strong evidence of functional improvement from 2.3±0.4 at week 0 to 1.3±0.7 (p=0.056) at week 38, although this was not significant as demonstrated by Bonferroni pairwise comparisons.
Kinematics displayed a significant reduction in tremor severity at each arm joint during rest and postural states over the treatment course (Figure 5C). By analyzing tremor severity over all four tasks, a statistically significant reduction in RMS tremor amplitude (F[5,65]=7.096, p<0.0005, partial η2=0.353), captured by motion-sensor devices, was displayed in the wrist alone (Figure 3C); this was observed following the initial treatment at week 6 (p=0.004), at week 32 (p=0.032), and following the third treatment at week 38 (p=0.003). Although tremor acceleration, represented by averaged x-, y- and z-axes values, and joint RMS amplitudes measure tremor severity, they indicate different characteristics. Mean finger acceleration over the four tasks resembled a similar change in tremor severity to wrist joint angles and significantly decreased (F[5,65]=9.057, p<0.0005, partial η2=0.411) following the first injection at week 6 (p=0.001), following the second treatment at week 22 (p=0.028), at week 32 (p=0.03), and following the third treatment at week 38 (p=0.003) (Figure 3C). Likewise, tremor accelerometry captured at the hand demonstrated significant reduction (F[5,65]=7.786, p<0.0005, partial η2=0.375) at week 6 (p=0.003), week 22 (p=0.006), week 32 (p=0.003), and at week 38 (p=0.003) (Figure 5C). The severity of elbow tremor amplitude significantly decreased (F[5,65]=3.962, p=0.003, partial η2=0.234) from 0.46±1.240 at week 0 to 0.08±0.272 RMS at week 6 (p=0.029) (Figure 5C). Shoulder RMS tremor amplitude did not significantly change over the study course.
By analyzing the severity of tremor during each scripted task, the mean wrist RMS tremor amplitude during “rest-1”. task did not significantly change (F[5,65]=1.422, p=0.228, partial η2=0.099) (Figure 5D). Although the RMS tremor measured during “rest-2”, forearm partly pronated while supported, was significantly reduced (F[5,65]=3.740, p=0.005, partial η2=0.223) from 1.2±1.2 at baseline to 0.7±1.1 at week 6 (p=0.045) and to 0.6±0.7 at week 32 (p=0.004). The mean wrist RMS amplitude during “posture-1” was significantly reduced (F[5,65]=7.410, p<0.0005, partial η2=0.363) at week 6 (p=0.003), week 22 (p=0.026), and at week 32 (p=0.05). The wrist tremor amplitude captured during “posture-2” was significantly reduced (F[5,65]=4.205, p=0.002, partial η2=0.244) at week 6 (p=0.013). Finger acceleration significantly decreased (F[5,65]=8.538, p<0.0005, partial η2=0.396) during “posture-1” at week 6 (p=0.005), week 22 (p=0.009), week 32 (p=0.23), and at week 38 (p=0.027) (Figure 5E). Likewise, finger acceleration during “posture-2” decreased (F[3.025,40.112]=4.589, p=0.007, partial η2=0.261) at week 6 (p=0.025). During “rest-2”, finger tremor acceleration significantly reduced (F[5,65]=3.876, p=0.004, partial η2=0.230) at week 6 (p=0.023) and at week 38 (p=0.005).
Side effects
The maximal grip strength was significantly reduced (F[5,60]=6.350, p<0.0005, partial η2=0.346) from 29.2±9.5 kg at week 0 to 21.8±9.4 kg at week 22 (p=0.05) and returned to baseline strength of 24.4±8.8 kg at week 32 (Figure 5F). A significant change in maximal grip strength was perceived as mild weakness in injected muscles by participants, a mean rating of 1 out of 4 on the Likert scale of muscle weakness. Significant change in perceived weakness occurred following the second treatment: an increase from 0.2±0.4 at week 0 to 1.1±0.6 at week 22 (p=0.03). This coincided with the peak effect of BoNT-A. The mean maximal grip strength of the untreated arm was 32.3±11.1 kg at week 0 and remained unchanged over the treatment course. Severity and frequency of perceived weakness reported in the Likert scale for each time point is summarized below in Table 4.
Table 4. Number of Participants Who Perceived Weakness Using a Likert Scale Over the Treatment Course.
| Time | Number of Participants Per Likert Score | ||||
|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | |
| Week 0 (n=18) | 15 | 3 | – | – | – |
| Week 6 (n=21) | 9 | 6 | 5 | 1 | – |
| Week 16 (n=18) | 11 | 6 | 1 | – | – |
| Week 22 (n=17) | 4 | 8 | 5 | – | – |
| Week 32 (n=19) | 9 | 8 | 2 | – | – |
| Week 38 (n=14) | 4 | 8 | 1 | 1 | – |
Note: Likert scale scores ranged from 0 = no weakness, 1 = mild weakness in non-injected muscles, 2 = mild weakness in injected muscles, 3 = moderate weakness in injected muscles and 4 = severe weakness in injected muscles.
Discussion
Although tremor is not the most disabling symptom in PD, patients perceive tremor as an important symptom that requires treatment.4 Benefits from recommended treatments for PD tremor are often unsatisfactory and result in side effects of these medications.23 In addition, for PD patients with tremor as their only troublesome symptom, treatment with current oral medications becomes a therapeutic dilemma as these drugs may contribute to motor fluctuations and dyskinesia later in life. Hence, levodopa sparing becomes an important variable to consider in treatment of tremor. Previous studies have shown BoNT-A injections as a possible focal treatment for tremor, although finger and wrist extensor muscle weakness and dose-dependent limb weakness frequently occurred.14,15,18,24,25 Brin et al.14 and Pullman et al.15 applied a fixed-dosing regimen and subjectively determined injection sites that resulted in the occurrence of dose-dependent hand weakness, thereby reducing any functional efficacy of BoNT-A. Trosch and Pullman18 demonstrated in an open label study that five of the 10 PD patients moderately improved in clinical tremor scores, although accelerometry measures for rest tremor did not significantly change. The limitations of these studies that reduced the effectiveness of BoNT-A therapy were attributed to single injection studies, visually selecting muscles to inject, or using fixed-dosing parameters regardless of the patient’s tremor severity. As such, BoNT-A for PD tremor is not widely adopted in clinical practice on the basis of these past results.
The present study demonstrates that by individualizing BoNT-A injection parameters on the basis of the biomechanical pattern of tremor at the wrist, elbow, and shoulder joints, targeted focal therapy greatly improved efficacy without impairing arm function. As accelerometers placed on the hand/fingers cannot distinguish and segment tremor originating from wrist, elbow, or shoulder joints,26 this study simplified the complexity of tremor by utilizing sensor-based recordings in conjunction with custom-written software to characterize each patient’s tremor profile.27 Kinematics allows independent and separate characterization of joint motion along the arm for every patient, which is not possible with visual assessments. Furthermore, injection patterns can be tailored to each patient’s kinematics (Figure 4) instead of depending on visual methods or using a standard set of injections, as employed in previous studies.14
The significant, palliative effect of BoNT-A on whole-arm tremor severity was clearly demonstrated both clinically and kinematically (Figure 5). Kinematically determined BoNT-A parameters showed efficacious results by observing a significant decrease (UPDRS item 20) for all study time points following the first treatment. Action tremor (UPDRS item 21) severity demonstrated a trending decline in rating, although this was not significant (Figure 5A). Likewise, the FTM tremor severity score displayed significant improvement in rest, postural, and action tremor at week 6 that continued to week 38. Those seven participants who found tremor to be functionally bothersome at baseline demonstrated significantly improved eating and function of daily tasks, a significant enhancement in quality of life (Figure 5B). These functionally beneficial improvements in fine and gross motor skills continued to occur following the peak effect of BoNT-A. Reduced maximal grip strength during peak activity of BoNT-A (Figure 5F) was not perceived to be functionally bothersome as participants rated such weakness as a 1 out of 4 on the Likert scale, indicating mild weakness in injected muscles. Although maximal grip strength decreased by 25% following the first treatment and 57% of participants (12/21) experienced third finger extensor weakness, this was perceived as slight to mild weakness and these effects were reported as not troublesome. This demonstrated that kinematically based BoNT-A injection patterns minimize the likelihood of adverse functional impairments.14 For future studies, as weakness in non-injected muscles (e.g. finger extensors) and in injected wrist muscle groups did occasionally occur, a need for further refinement of injection techniques, such as incorporating ultrasound-guided injections could be considered.16
Dosages per muscle, in particular elbow and shoulder muscle groups (Table 3), were substantially lower than previous studies involving treatment of PD, and cerebellar and essential tremor.15 As dosing was calculated on the basis of the quantified tremor amplitude, the best medicated, that is “ON”, state was chosen. Thus, tremor treatment with BoNT-A was provided concomitantly over and above the best-treated oral medication state. Eight muscles were injected on average, which was more than reported in previous studies.24,25 It is possible that kinematic determination of the joint dynamics of tremor would allow better optimization of injections, thereby reducing muscle weakness.15
The study limitations were non-blinded injections and having no treatment comparator, and as this was an open-label study the results are subject to bias. As outcomes in this longitudinal study are both qualitative and quantitative, a persistent placebo response is unlikely. Since weakness is obvious to perceive by both the clinician and the participant, long-term blinded studies with BoNT-A are challenging to conduct. Validated clinical rating scales were used as primary endpoints of this study, but a need for better functional assessment scales, such as a patient global impression of change, could be incorporated for future studies. Comparative studies investigating the use of surface EMG alone versus kinematics for tremor localization and assessment may also be useful in confirming this study’s results. In addition, since tremor is variable, fluctuations in severity during each visit introduced error. However, participants were assessed around the same time of day and in the “ON” state. Visually-based versus kinematically-based treatments were not compared as prior studies have already shown the lack of reproducibility and tolerability using visually guided, fixed schedule injections.11–15,17,18 The sample size is similar to other reported studies in the literature.2,11–15,18,21,24,25,27
This study shows that individual, objective measurement of tremor at each joint in the upper limb affected by tremor allows for proper characterization and treatment of PD patients. When achieved, such characterization can be used to guide the clinician’s muscle selection for treatment of tremor. In PD tremor, individualized and optimized dosages of BoNT-A can be used successfully and without significant severe weakness over a series of injections.
Acknowledgment
We would like to acknowledge the contribution by the participants and by the post-doctoral engineering and volunteer research staff at the National Parkinson Foundation Centre of Excellence, London Movement Disorder Centre located within the London Health Sciences Centre, London, Ontario, Canada.
Footnotes
Funding: The present study was partially funded by a research grant from Merz Pharma Canada and a government-industry matched grant from MITACS.
Financial Disclosures: M.J. reports a pilot research grant from Merz Pharma while conducting the study, and non-financial support from Allergan Inc., Teva Pharmaceuticals and AbbVie. M.J., who is commercializing this medical device, has a patent PCT/CA2013/000804 pending to MDDT Inc., and a patent PCT/CA2014/050893 pending to MDDT Inc. F.R. has a patent PCT/CA2013/000804 pending to MDDT Inc., and a patent PCT/CA2014/050893 pending to MDDT Inc. O.S. reports a government industrial MITACS grant in collaboration with Merz Pharma while conducting the study.
Conflict of Interest: J.L. is a MDDT Inc. employee. F.R. now performs contract work with MDDT Inc. to continue commercial development.
Ethics Statement: This study was performed in accordance with the ethical standards detailed in the Declaration of Helsinki. The authors’ institutional ethics committee has approved this study and all patients have provided written informed consent.
References
- 1.Jankovic J. Parkinson’s disease: Clinical features and diagnosis. J Neurol Neurosurg Psychiatry. 2008;79:368–376. doi: 10.1136/jnnp.2007.131045. doi: http://dx.doi.org/10.1136/jnnp.2007.131045. [DOI] [PubMed] [Google Scholar]
- 2.Milanov I. A cross-over clinical and electromyographic assessment of treatment for parkinsonian tremor. Parkinsonism Relat Disord. 2001;8:67–73. doi: 10.1016/s1353-8020(00)00077-8. doi: http://dx.doi.org/10.1016/S1353-8020(00)00077-8. [DOI] [PubMed] [Google Scholar]
- 3.Kraus PH, Lemke MR, Reichmann H. Kinetic tremor in Parkinson’s disease – an underrated symptom. J Neural Transm. 2006;113:845–853. doi: 10.1007/s00702-005-0354-9. doi: http://dx.doi.org/10.1007/s00702-005-0354-9. [DOI] [PubMed] [Google Scholar]
- 4.Fleischman DA, Wilson RS, Schneider JA, Bienias JL, Bennett DA. Parkinsonian signs and functional disability in old age. Exp Aging Res. 2007;33:59–76. doi: 10.1080/03610730601006370. doi: http://dx.doi.org/10.1080/03610730601006370. [DOI] [PubMed] [Google Scholar]
- 5.Miyasaki JM, Martin W, Suchowersky O, Weiner WJ, Lang AE. Practice parameter: initiation of treatment for Parkinson’s disease: An evidence-based review: report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2002;58:11–17. doi: 10.1212/wnl.58.1.11. doi: http://dx.doi.org/10.1212/WNL.58.1.11. [DOI] [PubMed] [Google Scholar]
- 6.Nagatsua T, Sawadab M. L-dopa therapy for Parkinson’s disease: past, present, and future. Parkinsonism Relat Disord. 2009;15:S3–S8. doi: 10.1016/S1353-8020(09)70004-5. doi: http://dx.doi.org/10.1016/S1353-8020(09)70004-5. [DOI] [PubMed] [Google Scholar]
- 7.Stathis P, Konitsiotis S, Antonini A. Dopamine agonists early monotherapy for the delay of development of levodopa-induced dyskinesias. Expert Rev Neurother. 2015;15:1–7. doi: 10.1586/14737175.2015.1001747. doi: http://dx.doi.org/10.1586/14737175.2015.1001747. [DOI] [PubMed] [Google Scholar]
- 8.Katzenschlager R, Sampaio C, Costa J, Lees A. Anticholinergics for symptomatic management of Parkinson’s disease. Cochrane Database Syst Rev. 2003;2:CD003735. doi: 10.1002/14651858.CD003735. doi: http://dx.doi.org/10.1586/14737175.2015.1001747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schadt CR, Duffis EI, Charles PD. Pharmacological treatment of disabling tremor. Expert Opin Pharmacother. 2005;6:419–428. doi: 10.1517/14656566.6.3.419. doi: http://dx.doi.org/10.1517/14656566.6.3.419. [DOI] [PubMed] [Google Scholar]
- 10.de Lau LM, Breteler MM. Epidemiology of Parkinson’s disease. Lancet Neurol. 2006;5:525–535. doi: 10.1016/S1474-4422(06)70471-9. doi: http://dx.doi.org/10.1016/S1474-4422(06)70471-9. [DOI] [PubMed] [Google Scholar]
- 11.Rahimi F, Debicki D, Roberts-South AC, Bee C, Bapat P, Jog S. Dynamic decomposition of motion in essential and parkinsonian tremor. Can J Neurol Sci. 2015;42:116–124. doi: 10.1017/cjn.2015.12. doi: http://dx.doi.org/10.1017/cjn.2015.12. [DOI] [PubMed] [Google Scholar]
- 12.Kim S, Yiannikas C, Mahant N, Vucic S, Fung V. Treatment of proximal upper limb tremor with botulinum toxin therapy. Mov Disord. 2013;29:835–838. doi: 10.1002/mds.25739. doi: http://dx.doi.org/10.1002/mds.25739. [DOI] [PubMed] [Google Scholar]
- 13.Pacchetti C, Mancini F, Bulgheroni M, et al. Botulinum toxin treatment for functional disability induced by essential tremor. Neurol Sci. 2000;21:349–353. doi: 10.1007/s100720070049. [DOI] [PubMed] [Google Scholar]
- 14.Brin MF, Lyons KE, Doucette J, et al. A randomized, double masked, controlled trial of botulinum toxin type A in essential hand tremor. Neurology. 2001;56:1523–1528. doi: 10.1212/wnl.56.11.1523. [DOI] [PubMed] [Google Scholar]
- 15.Pullman SL, Greene P, Fahn S, Pedersen SF. Approach to the treatment of limb disorders with botulinum toxin A. Experience with 187 patients. Arch Neurol. 1996;53:617–624. doi: 10.1001/archneur.1996.00550070055012. [DOI] [PubMed] [Google Scholar]
- 16.Lim E, Quek A, Seet R. Accurate targeting of botulinum toxin injections: how to and why. Parkinsonism Relat Disord. 2011;17:S34–S39. doi: 10.1016/j.parkreldis.2011.06.016. doi: http://dx.doi.org/10.1016/j.parkreldis.2011.06.016. [DOI] [PubMed] [Google Scholar]
- 17.Jankovic J, Schwartz K. Botulinum toxin treatment of tremors. Neurology. 1991;41:1185–1188. doi: 10.1212/wnl.41.8.1185. [DOI] [PubMed] [Google Scholar]
- 18.Trosch RM, Pullman SL. Botulinum toxin a injections for the treatment of hand tremors. Mov Disord. 1994;9:601–609. doi: 10.1002/mds.870090604. [DOI] [PubMed] [Google Scholar]
- 19.Supuk T, Bajd T, Jurillo G. Assessment of reach-to-grasp trajectories toward stationary objects. Clin Biomech. 2011;26:811–818. doi: 10.1016/j.clinbiomech.2011.04.007. doi: http://dx.doi.org/10.1016/j.clinbiomech.2011.04.007. [DOI] [PubMed] [Google Scholar]
- 20.Casellato C, Zorzi G, Pedrocchi A, Ferrigno G, Nardocci N. Reaching and writing movements: sensitive and reliable tools to measure genetic dystonia in children. J Child Neurol. 2011;26:822–829. doi: 10.1177/0883073810392997. doi: http://dx.doi.org/10.1177/0883073810392997. [DOI] [PubMed] [Google Scholar]
- 21.Rahimi F, Bee C, Debicki D, Roberts AC, Bapat P, Jog M. Effectiveness of BoNT A in Parkinson’s disease upper limb tremor management. Can J Neurol Sci. 2013;40:663–669. doi: 10.1017/s031716710001489x. [DOI] [PubMed] [Google Scholar]
- 22.Rozman J, Bartolic A, Ribaric S. A new method for selective measurement of joint movement in hand tremor in Parkinson’s disease patients. J Med Eng Technol. 2007;31:305–311. doi: 10.1080/03091900600992064. [DOI] [PubMed] [Google Scholar]
- 23.Connolly BS, Lang AE. Pharmacological treatment of Parkinson disease: a review. JAMA. 2014;311:1670–1683. doi: 10.1001/jama.2014.3654. doi: http://dx.doi.org/10.1001/jama.2014.3654. [DOI] [PubMed] [Google Scholar]
- 24.Jankovic J, Schwartz K, Clemence W, Aswad A, Mordaunt J. A randomized, double-blind, placebo-controlled study to evaluate botulinum toxin type A in essential hand tremor. Mov Disord. 1996;11:250–256. doi: 10.1002/mds.870110306. [DOI] [PubMed] [Google Scholar]
- 25.Henderson JM, Ghika JA, Van Melle G, Haller E, Einstein R. Botulinum toxin A in non-dystonic tremors. Eur Neurol. 1996;36:29–35. doi: 10.1159/000117196. [DOI] [PubMed] [Google Scholar]
- 26.Machowska-Majchrzak A, Pierzchala K, Pietraszek S, Labuz-Roszak B. Essential tremor – assessment of tremor accelerometric parameter’s symmetry and the relationship between hand dominance and severity of tremor. Neurol Neurochir Pol. 2011;45:121–127. doi: 10.1016/s0028-3843(14)60022-0. [DOI] [PubMed] [Google Scholar]
- 27.Rahimi F, Bee C, South A, Debicki D, Jog M. Variability of hand tremor in rest and in posture – a pilot study. Conf Proc IEEE Eng Med Biol Soc. 2011;2011:470–473. doi: 10.1109/IEMBS.2011.6090067. doi: http://dx.doi.org/10.1109/IEMBS.2011.6090067. [DOI] [PubMed] [Google Scholar]
- 28.Van Der Walt A, Sung S, Spelman T, et al. A double-blind, randomized, controlled study of botulinum toxin type A in MS-related tremor. Neurology. 2012;79:92–99. doi: 10.1212/WNL.0b013e31825dcdd9. doi: http://dx.doi.org/10.1212/WNL.0b013e31825dcdd9. [DOI] [PubMed] [Google Scholar]