Abstract
Significance: Mesenchymal stem cells (MSCs) are being administered to cutaneous wounds with the goal of accelerating wound closure and promoting regeneration instead of scar formation. An ongoing challenge for cell-based therapies is achieving effective and optimal targeted delivery and engraftment at the site of injury. Contributing to this challenge is our incomplete understanding of endogenous MSC homing to sites of injury.
Recent Advances: Chemokines and their receptors are now recognized as important mediators of stem cell homing. To date, the most studied chemokine–chemokine receptor axis in MSC homing to wounds is CXCL12-CXCR4 but recent work suggests that CCL27-CCR10 and CCL21-CCR7 may also be involved.
Critical Issues: Strategies to enhance chemokine-mediated MSC homing to wounds are using a variety of approaches to amplify the chemokine signal at the wound site and/or overexpress specific chemokine receptors on the surface of the MSC.
Future Directions: Harnessing chemokine signaling may enhance the therapeutic effects of stem cell therapy by increasing the number of both exogenous and endogenous stem cells recruited to the site of injury. Alternatively, chemokine-based therapies directly targeting endogenous stem cells may circumvent the need for the time-consuming and costly isolation and expansion of autologous stem cells prior to therapeutic administration.

Anne M. Hocking, PhD
Scope and Significance
Mesenchymal stem cells (MSCs) are being administered to cutaneous wounds with the goal of accelerating wound closure and promoting regeneration instead of scar formation.1 An ongoing challenge for cell-based therapies is achieving effective and optimal targeted delivery and engraftment at the site of injury. Contributing to this challenge is our incomplete understanding of endogenous MSC homing to sites of injury.2 This review will summarize our current knowledge about the role of chemokines in MSC recruitment to wounds.
Translational Relevance
The translational relevance for research defining the chemokines responsible for stem cell homing is significant with a direct impact on stem cell therapy. Harnessing chemokine signaling may enhance the therapeutic effects of stem cell therapy by increasing the number of both exogenous and endogenous stem cells recruited to the site of injury. Alternatively, chemokine-based therapies directly targeting endogenous stem cells may circumvent the need for the time-consuming and costly isolation and expansion of autologous stem cells prior to therapeutic administration.
Clinical Relevance
Ongoing clinical trials are investigating the safety and efficacy of MSC therapy for the treatment of burns and chronic wounds including venous ulcers, pressure ulcers, and diabetic foot ulcers.1 Chronic wounds are becoming a global health problem with the aging population and the increased incidence of both diabetes mellitus and obesity.3 Despite this increased prevalence, current therapies have limited efficacy in accelerating wound closure and promoting scarless healing.
Background
Bone marrow-derived MSCs improve wound healing
MSCs are multipotent adult stem cells that were first isolated from bone marrow but have since been detected in all adult tissues.1,2,4,5 Clinical interest in MSC is based on their ability to enhance repair and regeneration of injured tissue and to modulate the immune system in inflammatory diseases. For therapeutic use, MSCs are being harvested from a variety of tissues including adipose tissue, umbilical cord, and bone marrow. This review will primarily focus on bone marrow-derived MSCs given both endogenous and systemically administered bone marrow-derived MSC home to sites of tissue injury.2
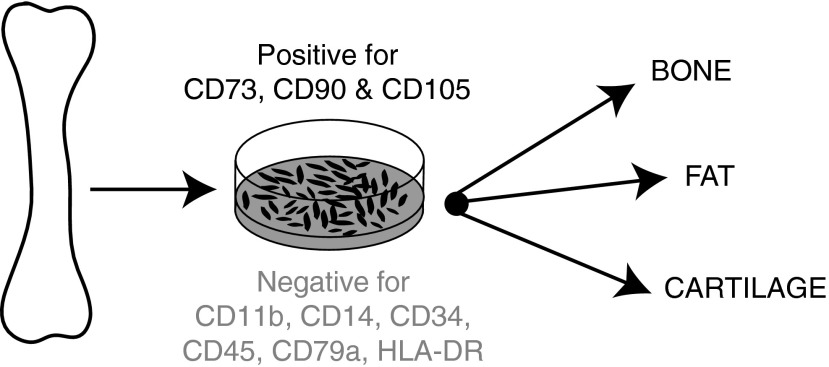
Bone marrow-derived MSCs are a heterogeneous population of plastic adherent cells that represent 0.001–0.01% of total marrow.1,2,4 In 2006, the International Society for Cellular Therapy outlined three minimal criteria for defining a bone marrow-derived MSC5 (Fig. 1). Bone marrow-derived MSC must be (i) plastic adherent; (ii) positive for the stromal cell surface markers CD73, CD90, and CD105 while negative for the hematopoietic lineage markers CD45, CD34, CD14, CD11b, CD79a, and HLA-DR; and (iii) able to differentiate into bone, fat, and cartilage in vitro. Although these criteria have helped to standardize the definition of MSC, it is important to note that they are based on in vitro properties and may not necessarily reflect the in vivo phenotype. Resolving this issue is an important goal for the field. Also important is characterization of the subpopulations of MSC. These studies are needed to clarify whether MSC populations from different tissues are the same and whether there are discrete subpopulations of true stem cells and/or specific subpopulations with enhanced therapeutic effects. It is clear that the definition of MSC phenotype will change as these studies progress.
Figure 1.
The minimal criteria for defining human bone marrow-derived MSC are: plastic adherence; positive for stromal markers and negative for hematopoietic markers; and capacity to differentiate in vitro into bone, fat, and cartilage. MSC, mesenchymal stem cell.
Preclinical human studies have investigated the effect of MSC treatment on chronic wounds,6,7 burns,8 radiation wounds,9 and blistering skin disorders.10 The results from these studies and animal models have been promising with positive outcomes on wound repair. In general, MSC treatment results in accelerated wound closure, increased angiogenesis, and increased granulation tissue formation.1 There is also evidence of increased wound tensile strength, reduced scarring, and regeneration of epidermal appendages such as sweat glands, sebaceous glands, and hair follicles.1,11,12 In addition, MSCs also have the potential to restore the structural integrity of the skin by producing extracellular matrix proteins. For example, exogenous MSCs have been shown to provide collagen VII to restore the damaged epidermal–dermal junction in the blistering disorder dystrophic epidermolysis bullosa.10,13
MSC mediate their beneficial effects on wound repair through paracrine signaling, immune modulation, and differentiation.1 MSC paracrine signaling regulates local cellular responses to injury. The MSC-derived signals include growth factors, cytokines, chemokines, and prostaglandins.14 Interestingly, exosomes (microvesicles that transfer proteins and RNA between cells) have recently been implicated in MSC paracrine signaling: in a rat model of burn injury, MSC-derived exosomes increased cell proliferation and accelerated reepithelialization in wounds.15 MSC have also been reported to modulate the inflammatory response to injury. MSCs regulate macrophage activation16 and promote the conversion of M1 proinflammatory macrophages to M2 anti-inflammatory macrophages in wounds.17 Additionally, MSC differentiation contributes to wound repair by replacing damaged tissue. In wounds, MSCs have been observed to differentiate into keratinocytes, endothelial cells, and pericytes.1 The impact of MSC paracrine signaling, immune modulation, and differentiation on wound repair is currently limited by the small number of MSCs that actually home and engraft to wound.1,2 Strategies to optimize stem cell recruitment to sites of injury are clearly needed to maximize the therapeutic benefits of MSC therapy.
Chemokines, their receptors and chemotaxis
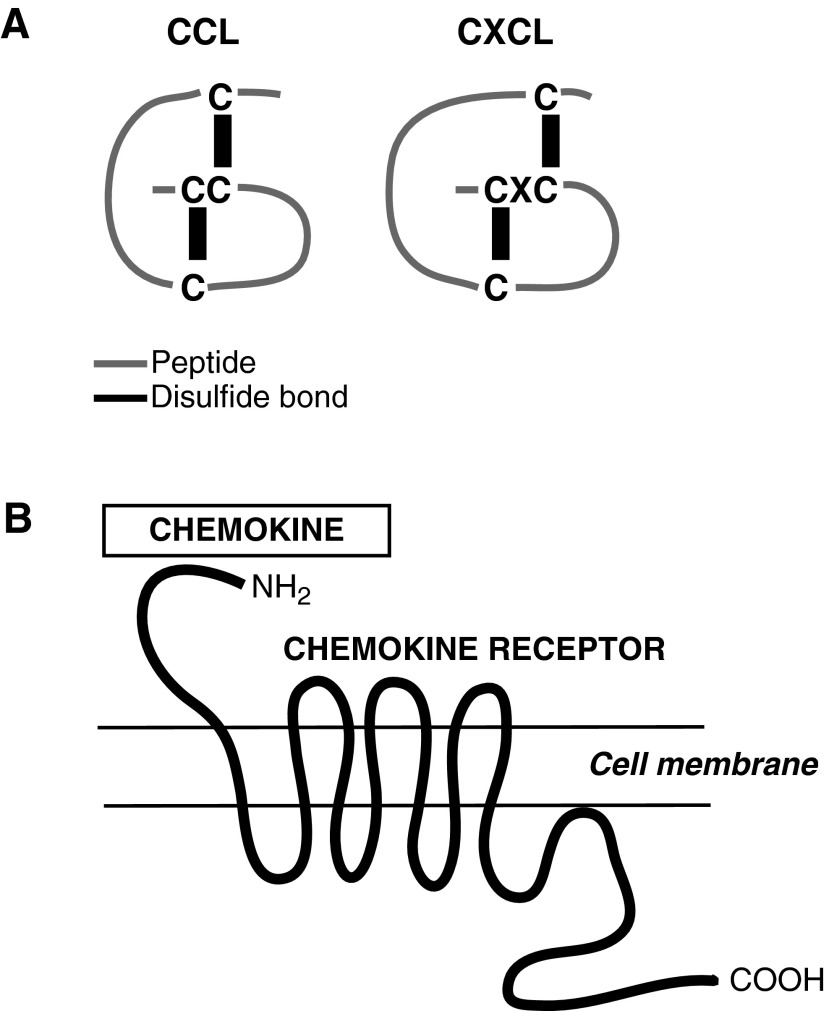
Chemokines are positively charged short peptides ranging in size from 7 to 13 kDa.18 The three-dimensional shape of the peptide is due in large part to the disulfide bonding between four amino terminal cysteines (Fig. 2). The arrangement of these four cysteines has been used to group chemokines into structurally related families. To date, four families have been identified: the CCL family with adjacent cysteine residues; the CXCL family with the cysteines separated by a single amino acid; the CL family with two instead of four cysteines; and the CX3CL family with the cysteines separated by three amino acids. The CCL and CXCL families are the largest with 26 and 17 members respectively.
Figure 2.
Structures of chemokines and their receptors. (A) Arrangement of four amino terminal cysteines in the CCL and CXCL families. (B) Chemokine receptors are G-protein-coupled receptors with seven transmembrane domains. Chemokines bind to the N-terminus of the receptor.
Chemokines bind G protein-coupled receptors, which are seven-transmembrane receptors signaling via heterotrimeric G proteins (Fig. 2).18 The chemokine receptors are classified according to their interaction with specific chemokine families so CXCR receptors bind CXC chemokines, whereas CCR receptors bind CC chemokines. It should be noted that there are fewer chemokine receptors than chemokines. As a result a single chemokine receptor may bind several different chemokines and a single chemokine may bind more than one receptor.
Chemokines and their receptors were first defined by their role in mediating chemotaxis, which is directed migration in response to a chemical stimulus. To date, most of our knowledge about the mechanism of mammalian chemotaxis has been from the study of neutrophil and lymphocyte trafficking.19 These circulating leukocytes sense chemokine gradients immobilized via glycosaminoglycans on the endothelium, and polarize to form leading and trailing ends. This gradient sensing and cytoskeletal reorganization is mediated by chemokines interacting with specific chemokine receptors on the leukocyte cell surface.
Discussion of Findings and Relevant Literature
Bone marrow-derived MSC home to cutaneous wounds
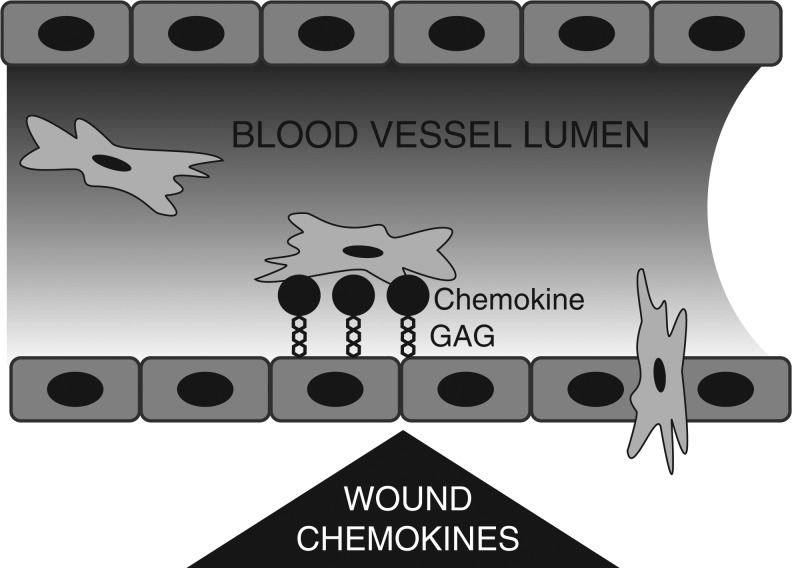
Bone marrow-derived MSCs have an inherent capacity to home to sites of injury and inflammation.1,2,20 Endogenous and systemically administered MSC have been detected in injured skin, eye, vasculature, and heart. Systemically administered MSC also home to injured brain, lung, kidney, and liver. This homing is a multi-step process involving directed migration to the site of injury where MSCs adhere to the vasculature and then transmigrate from the vasculature into the injured tissue (Fig. 3). In the case of endogenous MSCs, homing also involves mobilization from the bone marrow niche into the peripheral circulation. A growing list of factors have been identified that participate in MSC homing including cytokines, chemokines, growth factors, bioactive lipids, and adhesion molecules.20 Despite this general understanding of MSC homing, much work remains to be done to elucidate the molecular mechanisms responsible for each step.
Figure 3.
MSC homing to sites of injury involves chemotaxis. Chemokines released by wounds are immobilized on glycosaminoglycans on the endothelium. MSC migrate toward this gradient, adhere, and then transmigrate through the vessel wall into the wound.
Chemokines regulate MSC homing to cutaneous wounds
Endogenous bone marrow-derived MSCs home to cutaneous wounds. We and other investigators using bone marrow chimeras have observed MSCs localized to the dermis and blood vessels in wounds.21–24 Additional evidence that MSCs are recruited to wounds is provided by studies injecting MSCs intravenously.25–27 These systemically administered MSCs home to wounds and enhance wound healing. For bone marrow-derived MSCs to home to wounds, MSCs must be expressing a chemokine receptor that recognizes chemokines produced at the site of injury.
Gene expression analyses show that murine bone marrow-derived MSCs express the following chemokine receptors: CCR1, CCR2, CCR3, CCR4, CCR5, CCR6, CCR7, CCR9, CCR10, CXCR3, CXCR4, and CXCR7,28 whereas human bone marrow-derived MSCs express all the members of the CCR and CXCR families plus CXC3R and XCR.29,30 Interestingly, the MSC chemokine receptor profile has recently been reported to be sensitive to time in culture. For example, the expression levels for CCR1, CCR2, and CXCR4 in murine MSCs in long-term culture (>10 passages) were at least fivefold lower than MSC in short-term culture (<4 passages).28 It is possible that time in culture selects growth of a discrete MSC subpopulation with a unique chemokine receptor repertoire. Further investigation is warranted to determine whether this sensitivity to time in culture explains the divergent receptor profiles that have been previously reported for human MSCs.29 These results underscore the need to assess the effects of MSC isolation and expansion on chemokine receptor profile. Also necessary are studies to determine whether the chemokine receptor profile is different between MSCs in vivo and in vitro. Again, having specific lineage markers for MSCs would greatly facilitate characterization of the MSC chemokine receptor profile. Understanding the regulation of the chemokine receptor profile is clearly important for optimizing both endogenous and exogenous MSC homing to wounds.
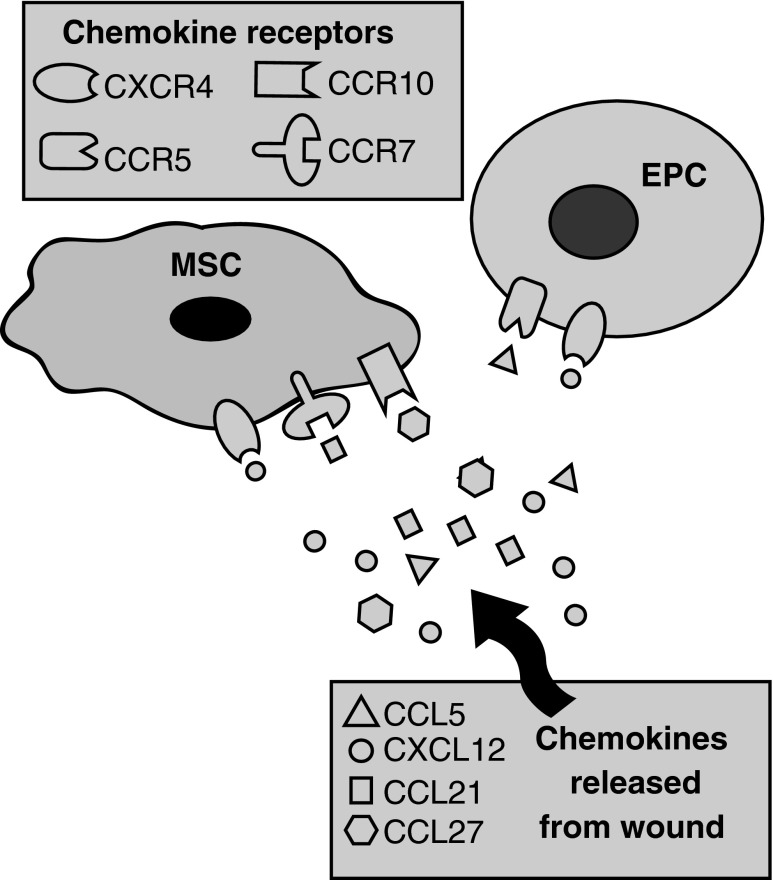
Cutaneous wounds produce a plethora of chemokines that may serve as chemoattractants for MSCs. The chemokine expression profile of a wound is regulated temporally and spatially during the repair process.18 Each chemokine has a specific expression pattern and unique role in inducing the directed migration of cells into and within the wound. The inflammatory response to injury provides an example of the sequential expression of chemokines to attract specific immune cell types.18 CXCL8 (also known as interleukin-8) produced soon after injury attracts neutrophils to the wound. CCL2 is then released by wound neutrophils to recruit monocytes, which are macrophage precursors. Lastly, expression of CCL3, CCL4, and CCL5 in the granulation tissue mediates B and T lymphocyte migration into the wound. Also pertinent to our understanding of chemokine function during wound repair is that a single chemokine may regulate the migration of multiple different cell types. CXCL8 illustrates this point as it serves as chemoattractant for neutrophils, endothelial cells, and dermal fibroblasts.18 A recent review by Martins-Green et al. summarizes the chemokines participating in each of the key cellular responses to injury.18 There have been a number of chemokine–chemokine receptor axes implicated in homing of bone marrow-derived MSCs to cutaneous wounds. These axes include CXCL12-CXCR4, CCL27-CCR10, and CCL21-CCR7 (Fig. 4).
Figure 4.
Chemokine–chemokine receptor axes mediating stem cell homing to wounds. Homing of bone marrow-derived MSCs to wounds is mediated by the following chemokine–chemokine receptor axes: CXCL12-CXCR4, CCL27-CCR10, and CCL21-CCR4. Homing of EPC to wounds is mediated by CXCL12-CXCR4 and CCL5-CCR5. EPC, endothelial progenitor cells.
The CXCL12 aka stromal-derived factor-1–CXCR4 axis mediates MSC homing
To date, the most stud-ied chemokine–chemokine receptor axis in MSC homing to wounds is CXCL12-CXCR4. Indeed the CXCL12-CXCR4 axis may be a general mechanism for homing to sites of injury as CXCL12 acts as a chemoattractant for a variety of adult stem cells including MSCs, hematopoietic stem cells, neural progenitor cells, and endothelial progenitor cells.31,32 Furthermore, the CXC12-CXCR4 homing mechanism is not specific to a single type of uninjured tissue, instead it has been shown to mediate stem cell homing to diverse tissues ranging from heart, brain, skeletal muscle, kidney, liver, and skin.31
In the current literature, CXCL12 is more commonly referred to as stromal-derived factor-1 (SDF-1), which is often interchangeably used with stromal-derived factor-1 alpha (SDF-1α). In uninjured skin, SDF-1 is constitutively expressed and localized to endothelial cells, pericytes, and dermal fibroblasts.33 In response to injury, SDF-1 expression is upregulated in acute wounds and burns.33–36 The cognate receptor for SDF-1 is CXCR4, which binds SDF-1 exclusively. The CXCR7 receptor also binds SDF-1, although it is important to note that this interaction does not mediate cell migration.20 Regardless, both chemokine receptors have been detected on the cell surface of bone marrow-derived MSCs.13,28
There has been much work to investigate the role of CXCL12-CXCR4 in MSC homing to sites of injury. The majority of studies have been performed in tissues other than skin. In the limited number of studies in cutaneous wounds, almost of all these are investigating MSC homing to sites of radiation or burn injury. Despite intense interest in CXCL12-CXCR4 regulation of stem cell homing to diabetic wounds, this research has primarily focused on endothelial progenitor cells rather than MSCs.32 Regardless of the flavor of stem cells, similar strategies have been used to determine the function of the CXCL12-CXCR4 in stem cell homing to wounds. Gain of function and loss of function experiments have been performed to either modulate SDF-1 levels at the wound or modulate CXCR4 expression on the stem cells. Overexpression of SDF-1 in diabetic wounds enhances healing35,37 and increases endothelial progenitor cell homing to wounds,32 whereas knockdown of SDF-1α levels in nondiabetic wounds reduces MSC recruitment to wounds.38,39 Studies targeting the CXCR4 receptor have shown CXCR4 overexpression in MSCs increased homing to wounds, and shRNA-mediated knockdown of CXCR4 resulted in significantly less MSCs in the wound.40 The knockdown result has been recently confirmed in a study that preincubated MSCs with a CXCR4 antagonist prior to intravenous injection.41 This resulted in diminished numbers of MSCs in a burn wound. Collectively, these studies demonstrate that the CXCL12-CXCR4 axis regulates stem cell homing to wounds.
Other chemokine axes implicated in stem cell homing to wounds
Although not as well documented as the CXCL12-CXCR4 axis, three other chemokines axes have been implicated in stem cell homing to injured skin. These axes are CCL27-CCR10, CCL21-CCR7, and CCL5-CCR5. CCL27 (also known as CTACK) is a skin-specific chemokine expressed in the epidermis of uninjured skin.30 It is upregulated in wounded skin42 and has also been detected in burn wound exudates.43 To date, there have been two studies reporting that bone marrow-derived stem cells migrate toward CCL27 in vivo. The first report in 2006 demonstrated that intradermal injections of CCL27 at the wound edge increased recruitment of CD34+ bone marrow-derived cells.42 A drawback of this study is that the identity of these CD34+ bone marrow-derived cells remains unclear given CD34 is considered a negative marker for MSC.5 The second study also reported that CCL27 was a chemoattractant for stem cells in vivo.30 These investigators determined that a subpopulation of bone marrow-derived MSC express CCR10, the receptor for CCL27. Furthermore, MSC overexpressing CCR10 migrated toward an ectopic gradient of CCL27 in skin. This chemotaxis occurred for MSCs administered locally or systemically. These results demonstrate the utility of the CCL27-CCR10 axis for enhancing MSC homing to skin and wounds.
The CCL21-CCR7 axis also appears to play a role in MSC homing to wounds. Intradermal injection of CCL21 (also known as SLC) at the wound periphery increased the number of bone marrow-derived MSCs in the wound and enhanced the accelerated closure of MSC-treated wounds.26 There is also evidence that the CCL5-CCR5 axis is important in endothelial progenitor cell recruitment to wounds.44 Endothelial progenitor cells migrate toward CCR5 (also known as RANTES) in vitro. There are reduced numbers of endothelial progenitor cells in wounds of the CCR5 null mouse. Transplantation of CCR5-positive bone marrow restores the number of endothelial progenitor cells to levels comparable with wild-type animals and rescues the neovascularization defect. Despite in vitro experiments demonstrating that CCL5 mediates adipose stem cells chemotaxis,45 it remains to be determined whether the CCL5-CCR5 axis is important for MSC homing to wounds in vivo.
Designing a chemokine-based therapeutic to enhance MSC homing to wounds
To date, strategies to enhance chemokine-mediated MSC homing to wounds are primarily targeting the CXCL12 (SDF-1)–CXCR4 axis. A variety of approaches are being used to amplify the SDF-1 signal at the wound site and/or overexpress the CXCR4 on the surface of the MSC. Increased SDF-1 levels in wounds has been achieved by localized intradermal injection of either SDF-1 recombinant protein or viral vectors expressing SDF-1 mRNA.31 Furthermore, both a hydrogel and an alginate scaffold have also been successfully used to deliver SDF-1 to wounds.37,46 In all cases, administration of exogenous SDF-1 resulted in increased MSC recruitment to the wound.31 However, there are likely additional benefits given that diabetes and age significantly reduce SDF-1 levels in wounds.35,47 An alternative approach to SDF-1 administration is the use of the CXCR4 antagonist, AMD3100 (Plerixafor). This antagonist is already being used in the clinic to stimulate mobilization of hematopoietic stem cells from the bone marrow into the blood in cancer patients.48 A recent study demonstrates that local injection of AMD3100 at the site of injury increases recruitment of endothelial progenitor cells to wounds in rodent models.48
An important advantage of administering either SDF-1 or AMD3100 to the wound is that it directly targets endogenous stem cells and circumvents the challenges with isolating, expanding, and then delivering stem cells to the wound. However, if the goal is to also recruit exogenous MSC then recognized challenges associated with MSC delivery to the wound need to be overcome. These challenges include entrapment of systemically administered MSCs in the lung4 and sensitivity of the MSC chemokine receptor profile to in vitro culture conditions.28 There are also safety concerns as elevated levels of SDF-1 have been correlated with cancer and inflammatory diseases.49
Direct targeting of CXCR4 levels on MSC is also being used to maximize homing to sites of injury. MSC are being genetically engineered to overexpress CXCR4 via viral vectors or cationic liposomes20 and these cells are then topically delivered to the wound. An alternative to genetic engineering is to induce the MSCs to upregulate CXCR4 expression prior to delivery to the wound. This approach is referred to as preconditioning or priming. A number of preconditioning factors have been identified including hypoxia, insulin-like growth factor 1, inhibitors of glycogen synthase kinase 3-β, and valproic acid20. All of these factors have been reported to increase CXCR4 levels by MSCs. Interestingly, preconditioning with SDF-1 may also induce MSCs to upregulate CXCR4 expression given it has been reported to elicit this effect on endothelial progenitor cells.49 There are added benefits to SDF-1 preconditioning as a number of studies demonstrate that SDF-1 increases MSC engraftment and survival at the site of injury.50 Collectively, these studies modulating the CXCL12 (SDF-1)–CXCR4 axis demonstrate feasibility and the promise of chemokine-based therapeutics for enhancing stem cell recruitment to wounds.
Summary
An understanding of MSC homing to sites of injury is critical for optimizing the therapeutic effects of these stem cells. Current research is focused on elucidating the role of chemokines in MSC recruitment to wounds. In vitro and in vivo experiments have demonstrated that MSCs migrate toward chemokine gradients. Strategies aimed at maximizing homing are amplifying MSC-specific chemokine signals in the wound and engineering chemokine receptor levels on the cell surface of the MSCs. Further work is needed to address the safety and efficacy of these approaches. Overall, these studies show promise and suggest the potential of chemokine therapies to target endogenous MSCs circumventing the need for isolation, expansion, and administration of exogenous MSCs.
Take-Home Messages.
• MSCs are adult stem cells with therapeutic benefits for both chronic wounds and burn wounds.
• MSCs home to sites of injury. However, our limited understanding of this process is an ongoing challenge for development of stem cell-based therapies for cutaneous wounds.
• Chemokines and their receptors are key participants in MSC homing to sites of injury. MSCs expressing specific chemokine receptors on their cell surface migrate toward and bind chemokines at the wound.
• Strategies to enhance chemokine-mediated MSC homing to wounds are primarily targeting the CXCL12 (SDF-1)–CXCR4 axis. A variety of approaches are being used to amplify the CXCL12 (SDF-1) signal at the wound site and/or overexpress the CXCR4 on the surface of the MSC. More work is needed to improve efficacy and to ensure safety of chemokine-based therapies targeting MSC homing.
Abbreviations and Acronyms
- EPC
endothelial progenitor cells
- MSC
mesenchymal stem cell
- SDF-1
stromal-derived factor 1
- SDF-1α
stromal-derived factor-1 alpha
Acknowledgments and Funding Sources
The author would like to acknowledge Lara Muffley for excellent assistance with the preparation of the figures and Jeffrey Bradley for critical comments on the article.
Author Disclosure and Ghostwriting
The author has no commercial associations that could lead to a conflict of interest. The author is solely responsible for writing the article and did not use ghostwriters.
About the Author
Anne M. Hocking, PhD, is a Research Associate Professor in the Department of Surgery at the University of Washington. Her research interests include wound metabolism and mesenchymal stem cell therapy for chronic wounds.
References
- 1.Hocking AM. and Gibran NS: Mesenchymal stem cells: paracrine signaling and differentiation during cutaneous wound repair. Exp Cell Res 2010;316:2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Karp JM. and Leng Teo GS: Mesenchymal stem cell homing: the devil is in the details. Cell Stem Cell 2009;4:206. [DOI] [PubMed] [Google Scholar]
- 3.Sen CK, Gordillo GM, Roy S, et al. : Human skin wounds: a major and snowballing threat to public health and the economy. Wound Repair Regen 2009;17:763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rustad KC. and Gurtner GC: Mesenchymal stem cells home to sites of injury and inflammation. Adv Wound Care 2012;1:147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dominici M, Le Blanc K, Mueller I, et al. : Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006;8:315. [DOI] [PubMed] [Google Scholar]
- 6.Falanga V, Iwamoto S, Chartier M, et al. : Autologous bone marrow-derived cultured mesenchymal stem cells delivered in a fibrin spray accelerate healing in murine and human cutaneous wounds. Tissue Eng 2007;13:1299. [DOI] [PubMed] [Google Scholar]
- 7.Vojtassak J, Danisovic L, Kubes M, et al. : Autologous biograft and mesenchymal stem cells in treatment of the diabetic foot. Neuro Endocrinol Lett 2006;27 Suppl 2:134. [PubMed] [Google Scholar]
- 8.Rasulov MF, Vasilchenkov AV, Onishchenko NA, et al. : First experience of the use bone marrow mesenchymal stem cells for the treatment of a patient with deep skin burns. Bull Exp Biol Med 2005;139:141. [DOI] [PubMed] [Google Scholar]
- 9.Bey E, Prat M, Duhamel P, et al. : Emerging therapy for improving wound repair of severe radiation burns using local bone marrow-derived stem cell administrations. Wound Repair Regen 2010;18:50. [DOI] [PubMed] [Google Scholar]
- 10.Conget P, Rodriguez F, Kramer S, et al. : Replenishment of type VII collagen and re-epithelialization of chronically ulcerated skin after intradermal administration of allogeneic mesenchymal stromal cells in two patients with recessive dystrophic epidermolysis bullosa. Cytotherapy 2010;12:429. [DOI] [PubMed] [Google Scholar]
- 11.Huang S, Lu G, Wu Y, et al. : Mesenchymal stem cells delivered in a microsphere-based engineered skin contribute to cutaneous wound healing and sweat gland repair. J Dermatol Sci 2012;66:29. [DOI] [PubMed] [Google Scholar]
- 12.Xie MW, Gorodetsky R, Micewicz ED, et al. : Marrow-derived stromal cell delivery on fibrin microbeads can correct radiation-induced wound-healing deficits. J Invest Dermatol 2013;133:553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alexeev V, Uitto J, and Igoucheva O: Gene expression signatures of mouse bone marrow-derived mesenchymal stem cells in the cutaneous environment and therapeutic implications for blistering skin disorder. Cytotherapy 2011;13:30. [DOI] [PubMed] [Google Scholar]
- 14.Ranganath SH, Levy O, Inamdar MS, and Karp JM: Harnessing the mesenchymal stem cell secretome for the treatment of cardiovascular disease. Cell Stem Cell 2012;10:244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang B, Wang M, Gong A, et al. : HucMSC-exosome mediated-Wnt4 signaling is required for cutaneous wound healing. Stem Cells 2014. June 25 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 16.Qi Y, Jiang D, Sindrilaru A, et al. : TSG-6 released from intradermally injected mesenchymal stem cells accelerates wound healing and reduces tissue fibrosis in murine full-thickness skin wounds. J Invest Dermatol 2014;134:526. [DOI] [PubMed] [Google Scholar]
- 17.Zhang QZ, Su WR, Shi SH, et al. : Human gingiva-derived mesenchymal stem cells elicit polarization of m2 macrophages and enhance cutaneous wound healing. Stem Cells 2010;28:1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martins-Green M, Petreaca M, and Wang L: Chemokines and their receptors are key players in the orchestra that regulates wound healing. Adv Wound Care 2013;2:327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bear JE. and Haugh JM: Directed migration of mesenchymal cells: where signaling and the cytoskeleton meet. Curr Opin Cell Biol 2014;30c:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marquez-Curtis LA. and Janowska-Wieczorek A: Enhancing the migration ability of mesenchymal stromal cells by targeting the SDF-1/CXCR4 axis. Biomed Res Int 2013;2013:561098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Badiavas EV, Abedi M, Butmarc J, Falanga V, and Quesenberry P: Participation of bone marrow derived cells in cutaneous wound healing. J Cell Physiol 2003;196:245. [DOI] [PubMed] [Google Scholar]
- 22.Fathke C, Wilson L, Hutter J, et al. : Contribution of bone marrow-derived cells to skin: collagen deposition and wound repair. Stem Cells 2004;22:812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Opalenik SR. and Davidson JM: Fibroblast differentiation of bone marrow-derived cells during wound repair. FASEB J 2005;19:1561. [DOI] [PubMed] [Google Scholar]
- 24.Seppanen E, Roy E, Ellis R, Bou-Gharios G, Fisk NM, and Khosrotehrani K. Distant mesenchymal progenitors contribute to skin wound healing and produce collagen: evidence from a murine fetal microchimerism model. PloS One 2013;8:e62662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McFarlin K, Gao X, Liu YB, et al. : Bone marrow-derived mesenchymal stromal cells accelerate wound healing in the rat. Wound Repair Regen 2006;14:471. [DOI] [PubMed] [Google Scholar]
- 26.Sasaki M, Abe R, Fujita Y, Ando S, Inokuma D, and Shimizu H: Mesenchymal stem cells are recruited into wounded skin and contribute to wound repair by transdifferentiation into multiple skin cell type. J Immunol 2008;180:2581. [DOI] [PubMed] [Google Scholar]
- 27.Kidd S, Spaeth E, Dembinski JL, et al. : Direct evidence of mesenchymal stem cell tropism for tumor and wounding microenvironments using in vivo bioluminescent imaging. Stem Cells 2009;27:2614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alexeev V, Donahue A, Uitto J, Igoucheva O: Analysis of chemotactic molecules in bone marrow-derived mesenchymal stem cells and the skin: Ccl27-Ccr10 axis as a basis for targeting to cutaneous tissues. Cytotherapy 2013;15:171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ringe J, Strassburg S, Neumann K, et al. : Towards in situ tissue repair: human mesenchymal stem cells express chemokine receptors CXCR1, CXCR2 and CCR2, and migrate upon stimulation with CXCL8 but not CCL2. J Cell Biochem 2007;101:135. [DOI] [PubMed] [Google Scholar]
- 30.Fox JM, Chamberlain G, Ashton BA, and Middleton J: Recent advances into the understanding of mesenchymal stem cell trafficking. Br J Haematol 2007;137:491. [DOI] [PubMed] [Google Scholar]
- 31.Lau TT. and Wang DA: Stromal cell-derived factor-1 (SDF-1): homing factor for engineered regenerative medicine. Expert Opin Biol Ther 2011;11:189. [DOI] [PubMed] [Google Scholar]
- 32.Nishimura Y, Ii M, Qin G, et al. : CXCR4 antagonist AMD3100 accelerates impaired wound healing in diabetic mice. J Invest Dermatol 2012;132:711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Avniel S, Arik Z, Maly A, et al. : Involvement of the CXCL12/CXCR4 pathway in the recovery of skin following burns. J Invest Dermatol 2006;126:468. [DOI] [PubMed] [Google Scholar]
- 34.Toksoy A, Muller V, Gillitzer R, and Goebeler M: Biphasic expression of stromal cell-derived factor-1 during human wound healing. Br J Dermatol 2007;157:1148. [DOI] [PubMed] [Google Scholar]
- 35.Badillo AT, Chung S, Zhang L, Zoltick P, and Liechty KW: Lentiviral gene transfer of SDF-1alpha to wounds improves diabetic wound healing. J Surg Res 2007;143:35. [DOI] [PubMed] [Google Scholar]
- 36.Ding J, Hori K, Zhang R, et al. : Stromal cell-derived factor 1 (SDF-1) and its receptor CXCR4 in the formation of postburn hypertrophic scar (HTS). Wound Repair Regen 2011;19:568. [DOI] [PubMed] [Google Scholar]
- 37.Henderson PW, Singh SP, Krijgh DD, et al. : Stromal-derived factor-1 delivered via hydrogel drug-delivery vehicle accelerates wound healing in vivo. Wound Repair Regen 2011;19:420. [DOI] [PubMed] [Google Scholar]
- 38.Xu X, Zhu F, Zhang M, et al. : Stromal cell-derived factor-1 enhances wound healing through recruiting bone marrow-derived mesenchymal stem cells to the wound area and promoting neovascularization. Cells Tissues Organs 2013;197:103. [DOI] [PubMed] [Google Scholar]
- 39.Lu MH, Hu CJ, Chen L, et al. : miR-27b represses migration of mouse MSCs to burned margins and prolongs wound repair through silencing SDF-1a. PloS One 2013;8:e68972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang D, Sun S, Wang Z, Zhu P, Yang Z, and Zhang B: Stromal cell-derived factor-1 receptor CXCR4-overexpressing bone marrow mesenchymal stem cells accelerate wound healing by migrating into skin injury areas. Cell Reprogram 2013;15:206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hu C, Yong X, Li C, et al. : CXCL12/CXCR4 axis promotes mesenchymal stem cell mobilization to burn wounds and contributes to wound repair. J Surg Res 2013;183:427. [DOI] [PubMed] [Google Scholar]
- 42.Inokuma D, Abe R, Fujita Y, et al. : CTACK/CCL27 accelerates skin regeneration via accumulation of bone marrow-derived keratinocytes. Stem Cells 2006;24:2810. [DOI] [PubMed] [Google Scholar]
- 43.van den Broek LJ, Kroeze KL, Waaijman T, et al. : Differential response of human adipose tissue-derived mesenchymal stem cells, dermal fibroblasts, and keratinocytes to burn wound exudates: potential role of skin-specific chemokine CCL27. Tissue Eng Part A 2014;20:197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ishida Y, Kimura A, Kuninaka Y, et al. : Pivotal role of the CCL5/CCR5 interaction for recruitment of endothelial progenitor cells in mouse wound healing. J Clin Invest 2012;122:711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kroeze KL, Jurgens WJ, Doulabi BZ, van Milligen FJ, Scheper RJ, and Gibbs S. Chemokine-mediated migration of skin-derived stem cells: predominant role for CCL5/RANTES. J Invest Dermatol 2009;129:1569. [DOI] [PubMed] [Google Scholar]
- 46.Rabbany SY, Pastore J, Yamamoto M, et al. : Continuous delivery of stromal cell-derived factor-1 from alginate scaffolds accelerates wound healing. Cell Transplant 2010;19:399. [DOI] [PubMed] [Google Scholar]
- 47.Loh SA, Chang EI, Galvez MG, et al. : SDF-1 alpha expression during wound healing in the aged is HIF dependent. Plast Reconstr Surg 2009;123:65s. [DOI] [PubMed] [Google Scholar]
- 48.Lin Q, Wesson RN, Maeda H, et al. : Pharmacological mobilization of endogenous stem cells significantly promotes skin regeneration after full-thickness excision: the synergistic activity of AMD3100 and tacrolimus. J Invest Dermatol 2014;134:2358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Castilla DM, Liu ZJ, Tian R, Li Y, Livingstone AS, and Velazquez OC: A novel autologous cell-based therapy to promote diabetic wound healing. Ann Surg 2012;256:560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nakamura Y, Ishikawa H, Kawai K, Tabata Y, and Suzuki S: Enhanced wound healing by topical administration of mesenchymal stem cells transfected with stromal cell-derived factor-1. Biomaterials 2013;34:9393. [DOI] [PubMed] [Google Scholar]