Abstract
Epstein-Barr virus Latent Membrane Protein 2A (LMP2A) is expressed in EBV-infected B cells in the germinal center, a site of significant apoptosis induced by engagement of Fas on activated B cells. Signals from the B cell receptor (BCR) protect germinal center B cells from Fas-mediated apoptosis, and since LMP2A is a BCR mimic, we hypothesized that LMP2A would also protect B cells from Fas-mediated apoptosis. Surprisingly, latently-infected human and murine B cell lines expressing LMP2A were more sensitive to Fas-mediated apoptosis, as determined by increases in Annexin-V staining, and cleavage of caspase-8, −3 and PARP. Additional studies show that LMP2A-expressing B cell lines demonstrate a Lyn- and Syk-dependent increase in sensitivity to Fas-mediated apoptosis, due to an LMP2A-dependent enhancement in Fas expression. These findings demonstrate the ability for LMP2A to directly increase a pro-apoptotic molecule and have implications for EBV latency as well as the treatment of EBV-associated malignancies.
Keywords: B cells, Epstein-Barr virus, Latency Membrane Protein 2A (LMP2A), B cell receptor (BCR), Lyn, Syk, Fas (CD95), apoptosis, and PARP
Introduction
Epstein-Barr virus (EBV) is a member of the herpesvirus family that infects over 90% of the world’s population [1]. For many individuals, EBV infection manifests without symptoms. However in adolescents, the acquisition of EBV can lead to infectious mononucleosis, which is a disease that results in lymphadenopathy, fever, pharyngitis, and severe fatigue [2]. After initial lytic infection, the virus alters its gene expression profile into a state in which all latency genes are expressed, including the six different EBV nuclear antigens (EBNAs), three Latency Membrane Proteins (LMP) −1 and −2A, −2B , and EBV encoded small RNAs (EBERs) [3]. Ultimately, the immune system controls EBV production and EBV transitions into a latent state in which a more limited number of latency genes are expressed [4]. Most individuals will harbor latently-infected B cells for the rest of their life with little consequence. However, EBV can be a source of significant morbidity and mortality in people who become immunocompromised or garner genetic mutations that predispose them to tumor development [5, 6].
As mentioned above, EBV expresses few viral genes during latency in vivo [7-10]. However, one EBV transcript that is identified in both normal latency and pathogenic states is Latent Membrane Protein 2A (LMP2A) [10-13]. LMP2A is a 12 transmembrane protein that contains an amino terminal tail that is constitutively phosphorylated [14]. There are multiple sites for phosphorylation within the cytoplasmic tail, including tyrosine 112 that activates Lyn tyrosine kinase, and an immunoreceptor tyrosine activation motif (ITAM) that activates Syk. LMP2A functions as a B cell receptor (BCR) mimic [15, 16] and activates many of the same proteins induced by the BCR after activation with antigen. Both the BCR and LMP2A initially activate Lyn tyrosine kinase, followed by Syk [17, 18]. Subsequent to the activation of Syk, LMP2A activates B cell Linker protein (BLNK) [19], the Ras/PI3K/AKT pathway [20], NF-kB [21, 22] and the MAPK/ERK pathway [23]. The LMP2A-dependent activation of these pathways confers the many effects of LMP2A on B cell biology and lymphomagenesis.
LMP2A signaling influences multiple functions of B cells, but most importantly promotes cell survival [15, 20, 24-26]. The signaling of LMP2A directly prevents apoptosis by activating the Ras/PI3K/AKT pathway to increase the levels of Bcl family members [20]. Additionally, LMP2A-mediated activation of the PI3K/AKT pathway prevents TGF-β1-induced apoptosis by decreasing the cleavage of PARP and subsequent DNA fragmentation [27]. LMP2A also protects B cells from BCR-induced apoptosis, but makes them exquisitely more dependent on NF-kB to mediate this effect [21]. Alternatively, LMP2A indirectly prevents apoptosis by increasing the production of the pro-survival cytokine, IL-10, in human B cell lines [28]. Taken together, EBV uses LMP2A to hijack normal BCR signaling to protect its host cell from apoptosis and is therefore proposed to ultimately aid in prolonging EBV latent infection and promoting tumor development.
As mentioned above, EBV is associated with the development of B cell tumors including Burkitt’s lymphoma, Hodgkin’s lymphoma and lymphoproliferative disorders in the immunocompromised [4, 5, 29]. Due to the anti-apoptotic abilities of LMP2A, multiple studies have addressed the possible mechanisms by which LMP2A contributes to tumor development. In a mouse model, LMP2A accelerates the development of Burkitt’s lymphoma by decreasing apoptosis [30] and increasing the percentage of proliferating cells [31, 32], resulting in the advanced onset of tumor development [30-32]. Additionally, in human cells, LMP2A directly promotes the survival of surface immunoglobulin-negative B cells [15], which are the precursors of Hodgkin’s Reed Sternberg cells in Hodgkin’s lymphoma [5]. Thus, there is significant evidence that LMP2A contributes to tumor development by protecting cells from apoptosis that are normally induced by overexpression of c-myc and/or the loss of surface immunoglobulin.
An alternative means by which LMP2A could promote tumor development is by dampening the anti-tumor immune response that develops against cells harboring mutations that are seen as “foreign” by the immune system. LMP2A increases IL-10 production [28], which not only promotes tumor survival, but this cytokine is also immunosuppressive to anti-tumor immune responses [33, 34]. Another mechanism by which the immune system attacks tumors is via the cytotoxic T cell (CTL) response. CTLs kill tumor cells through the interaction between Fas ligand (FasL) on the CTL and Fas on the tumor cell [35]. Engagement of Fas on cells results in the formation of the death-induced active protease complex (DISC) that cleaves caspase 8, which is sufficient for apoptosis induction [36, 37]. However, the ability of LMP2A to modulate Fas expression and/or Fas-mediated apoptosis has not been investigated.
Despite the paucity of research investigating the ability of LMP2A to influence Fas-mediated apoptosis, there is evidence that signaling through the BCR blocks Fas-mediated apoptosis. Previous work indicates that engagement of the BCR on a murine B cell line, A20, protects B lymphocytes from Fas-mediated apoptosis through multiple mechanisms [38-40]. Catlett and Bishop demonstrated that BCR signaling protects A20 B cells from Fas-mediated apoptosis by directly blocking the activation of caspase 8 [39]. Additionally, another group showed that BCR signaling inhibited the activation of IL-1 β-converting enzyme to prevent Fas-mediated apoptosis [38]. Also, BCR signaling provides protection from Fas-mediated apoptosis through the up-regulation of anti-apoptotic Bcl-2 and Bcl-xL [38]. Finally, BCR stimulation activates the PI3K/Akt signaling pathway to provide a survival signal against simultaneous or ongoing Fas-mediated apoptosis by up-regulating c-FLIP expression [40].
Therefore, since LMP2A acts as a BCR mimic, we hypothesized that LMP2A would protect B cells from Fas-mediated apoptosis. In contrast to our hypothesis, LMP2A expression increased the sensitivity of both A20 B cells and EBV transformed B cell lines (lymphoblastoid cell lines (LCLs)) to Fas-mediated apoptosis. Furthermore, this increased sensitivity is due to a LMP2A-dependent increase in Fas expression, since mutations that inhibit the LMP2A-mediated activation of Lyn and Syk decreased Fas expression concomitantly with decreased sensitivity to apoptosis.
Results
Generation of A20 B cell lines expressing LMP2A
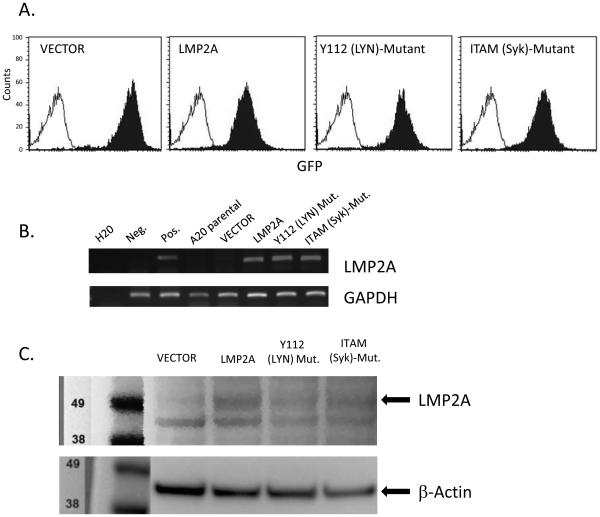
Previous reports identify that Epstein-Barr virus infection protects B cells from Fas-mediated apoptosis and that this protection is afforded through LMP1 expression [41]. However, additional data indicate that the ability of LMP1 to protect cells from Fas-mediated apoptosis may be cell-type dependent [42]. Due to the ability of the EBV latency protein, LMP2A, to protect cells from apoptosis induced by numerous pro-apoptotic triggers [15, 20, 27, 30], we tested the hypothesis that LMP2A would protect B cells from Fas-mediated apoptosis. Our rationale was further strengthened by the fact that LMP2A is a BCR mimic [15, 16] and BCR signaling protects B cells from Fas-mediated apoptosis [38-40]. The A20 B cell line is an experimental cell line that has been very useful in elucidating how signaling through the BCR protects B cells from Fas-mediated apoptosis [38-40]. Therefore, to test the ability of LMP2A to protect B cells from Fas-mediated apoptosis, we stably transduced the A20 B cell line using retroviruses containing only the vector backbone, which encodes for GFP and zeocin resistance, or the vector backbone containing wildtype LMP2A (designated LMP2A), or a mutated form of LMP2A which contains tyrosine to phenylalanine mutations at tyrosine 112 (designated Y112 (Lyn)-Mutant) or tyrosines 74 and 85 in the ITAM motif (designated ITAM (Syk)-Mutant). Stably-transduced cell lines were selected for growth in zeocin and expression of the integrated retroviral DNA was confirmed by multiple means. All cell lines (VECTOR, LMP2A, Y112 (Lyn)-Mutant and ITAM (Syk)-Mutant) were positive for GFP expression from the retroviral construct as determined by flow cytometry (Figure 1A). Additionally, we confirmed the expression of LMP2A in the LMP2A, Y112 (LYN)-Mutant and ITAM (Syk)-Mutant cell lines by RT-PCR (Figure 1B) and Western blot analysis (Figure 1C). Since phosphorylation of Y112 is a pre-requisite for LMP2A-mediated Syk activation [43], we confirmed that enhanced levels of phosphorylated Syk were not detected in Y112 (Lyn)-Mutant and ITAM (Syk)-Mutant (data not shown). Taken together, the generation of these cell lines provide an experimental system to determine if LMP2A protects B cells from Fas-mediated apoptosis by using a cell line that has been extensively studied to understand how BCR signaling protects B cells from Fas-mediated apoptosis [39, 40].
Figure 1.
LMP2A expression in retrovirally-transduced A20 B cell lines. (A) GFP expression from the retroviral backbone was assessed by flow cytometry in VECTOR, LMP2A, Y112 (Lyn)-Mutant and ITAM (Syk)-Mutant cell lines. Shaded histograms are the VECTOR, LMP2A, Y112 (Lyn)-Mutant and ITAM (Syk)-Mutant cell lines, while the open histogram is the A20 parental cell line that is GFP-negative. (B) RT-PCR analysis for LMP2A and GAPDH in A20 cell lines, with DNA from c57Bl/6 and LMP2A-Tg mice [26] as negative and positive controls for the LMP2A PCR reaction, respectively. (C) Western blot analysis for LMP2A and β-actin in A20 cell lines. Experiments in A-C are representative of at least 3 experiments with similar results.
Fas-mediated apoptosis in LMP2A-expressing cells
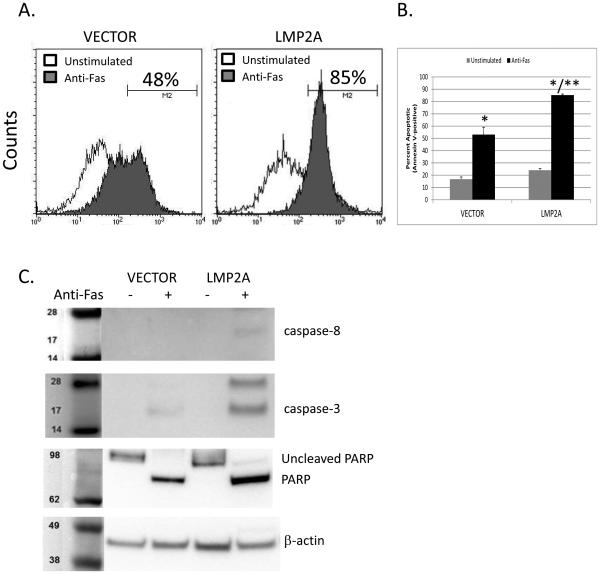
To determine if LMP2A protects the A20 B cell line from apoptosis after Fas-engagement, VECTOR and LMP2A cell lines were cultured in the absence or presence of anti-Fas (CD95) mAb for four hours and then stained for Annexin V positivity. Flow cytometric analysis of Annexin V staining, which is an indicator of apoptosis induction, demonstrates that activation of Fas on either VECTOR or LMP2A cell lines increases the percentage of cells staining positive for Annexin V (Figure 2A), confirming that these cells were sensitive to Fas-mediated apoptosis. In contrast to our hypothesis, LMP2A-expressing cells showed a dramatic increase in the percentage of Annexin V-positive cells when compared to VECTOR-containing cells after incubation with a Fas-stimulating antibody (Figure 2A-48% Annexin V+ for VECTOR cells vs 85% Annexin V+ for LMP2A cells). These initial findings were confirmed in repeated assays showing that the approximate 30% increase in Fas-mediated apoptosis in LMP2A-expressing cells was statistically higher than Fas-mediated apoptosis in VECTOR cells (Figure 2B). Since Annexin V staining is considered an “early” marker of apoptosis, we extended our findings to confirm that LMP2A enhances Fas-dependent cleavage of pro-apoptotic enzymes. Fas engagement results in the cleavage of caspase-8, caspase-3 and subsequently PARP [44, 45]. Therefore, we tested whether LMP2A-expressing cells demonstrate an increase in Caspase-8, −3, and PARP cleavage after stimulation through Fas. As shown in Figure 2C, LMP2A-expressing cells demonstrated a substantial increase in the amount of cleaved caspase—8, −3, and PARP in comparison to cells that do not express LMP2A. Finally, further experiments were performed using the human B cell line, BJAB, or an additional clone of A20 cells that express vector alone or LMP2A, which show similar results (Supplemental Figure 1A, C). These data indicate that LMP2A expression sensitizes B cells to Fas-mediated apoptosis.
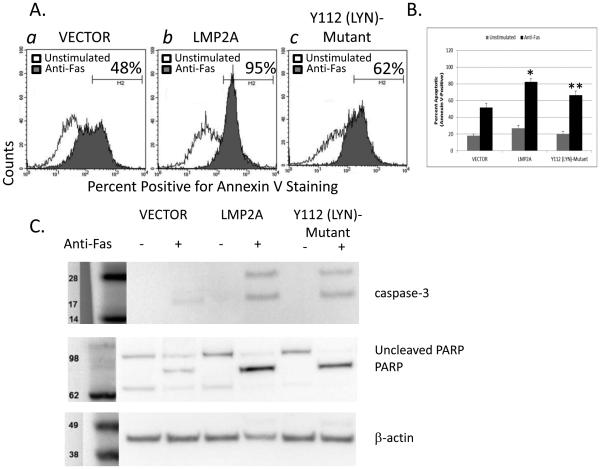
Figure 2.
Fas-mediated apoptosis in the absence or presence of LMP2A. (A) Flow cytometric analysis of Annexin V positivity in A20 VECTOR or LMP2A cell line after incubation in the absence or presence of anti-Fas (CD95) mAb. (B) Combination of 3 experiments analyzing Fas-mediated apoptosis in A20 VECTOR and LMP2A cells. Data are shown as the mean +/− standard error. (C) Analysis of caspase-8, caspase-3, and PARP cleavage by Western blot in A20 VECTOR or LMP2A cell line in the absence or presence of anti-Fas (CD95) mAb. * indicates p< 0.05 when compared to unstimulated cells. ** indicates p<0.05 when comparing Fas-mediated apoptosis between anti-Fas treated VECTOR and LMP2A cells.
Fas expression in LMP2A-expressing cells
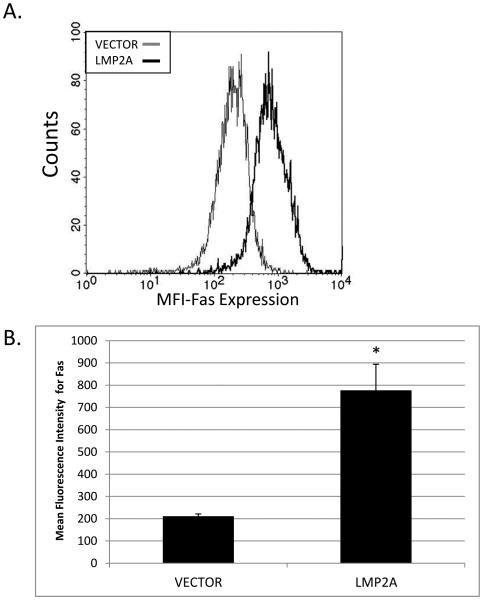
There are multiple mechanisms by which LMP2A expression could increase the sensitivity of the A20 cell line to apoptosis. However, the simplest explanation is that LMP2A may increase the level of Fas expression on A20 B cells, since previous studies indicate that the levels of Fas expression directly correlate with a cell’s sensitivity to Fas-mediated apoptosis [46-49]. To test this possibility, VECTOR and LMP2A cell lines were stained with an APC-conjugated anti-Fas mAb and the level of Fas expression was determined by flow cytometry. As shown in Figure 3A, LMP2A-expressing cells demonstrate an increase in Fas expression, as indicated by an increase in the mean fluorescence intensity (MFI) in Fas-specific staining of LMP2A cells compared to VECTOR cells (LMP2A: black line versus VECTOR: gray line). When multiple experiments are combined, a comparison of LMP2A cells and VECTOR cells demonstrate that there is an approximate 3.8 fold increase in the level of Fas expression in the LMP2A-expressing cell lines (Figure 3B). The LMP2A-dependent increase in Fas expression was also seen in an additional LMP2A-negative and -positive BJAB cell line and A20 cell clone (Supplemental Figure 1B, D). These data indicate that LMP2A-mediated increases in Fas expression could be responsible for the enhanced susceptibility of these cells to Fas-mediated apoptosis.
Figure 3.
Fas expression in VECTOR and LMP2A cells. (A) Flow cytometric analysis of Fas expression on VECTOR and LMP2A cells. (B) Combination of the mean fluorescence intensity in 3 experiments analyzing the levels of Fas expression in A20 VECTOR and LMP2A cells. Data are shown as the mean +/− standard error. * indicates p<0.05 when comparing Fas expression between VECTOR and LMP2A cells.
LMP2A signaling and Fas expression
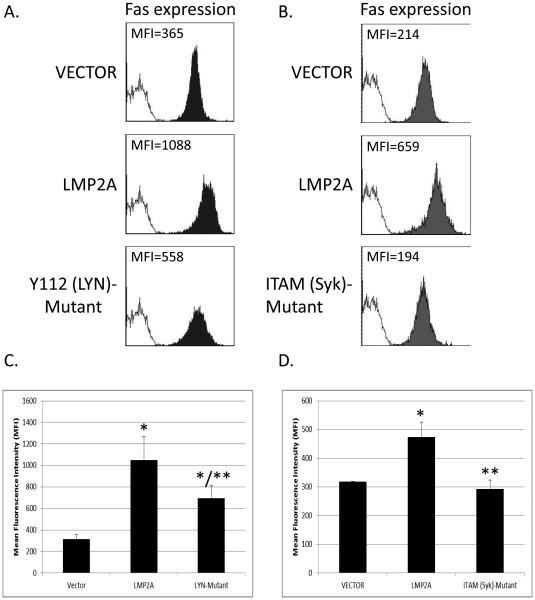
LMP2A contains an 119 amino acid cytoplasmic tail that is responsible for constitutive signaling to B cells and mimics BCR signaling by activating Lyn and Syk tyrosine kinases [14]. Within this cytoplasmic tail is tyrosine 112, which binds to Lyn, and an immunoreceptor tyrosine activation motif (ITAM) that binds to Syk [17, 18]. Since Lyn is one of the first tyrosine kinases activated in the LMP2A signaling pathway, we wanted to determine if LMP2A uses Lyn to increase Fas expression. To test this possibility, we used the stably transduced A20 cell line that expresses LMP2A containing a point mutation that results in the change of the tyrosine 112 into a phenylalanine, which results in a loss of LMP2A-mediated activation of Lyn [18]. We focused on the use of LMP2A point mutants to test our hypothesis instead of pharmacological inhibitors, since the tyrosine kinase inhibitors would also affect tonic signaling through the B cell receptor. Therefore, the use of cell lines with point mutations that are specific to LMP2A allow for the ability to directly test only the effect of LMP2A-mediated signaling on Fas expression. VECTOR, LMP2A, and Y112 (Lyn)-Mutant cell lines were stained with an APC-conjugated anti-Fas mAb and the level of Fas expression was determined by flow cytometry. As shown in Figure 4A, the cell line harboring a mutation in Y112 demonstrates a decrease in the mean fluorescence intensity of Fas expression when compared to cell lines expressing wildtype LMP2A. Furthermore, when multiple experiments are combined, the data demonstrate that Y112 (Lyn)-Mutant expresses significantly less Fas than the LMP2A cell line, but slightly more than the VECTOR control cell line (Figure 4C). These data indicate that Lyn activation is partially responsible for the LMP2A-mediated increase in Fas expression.
Figure 4.
Fas expression in cells expressing LMP2A with a mutation in the Lyn binding site (Y112 (Lyn)-Mutant cells) or the Syk Binding site (ITAM (Syk)-Mutant cells.) (A) Flow cytometric analysis of Fas expression on VECTOR, LMP2A, and Y112 (Lyn)-Mutant cells. (B) Flow cytometric analysis of Fas expression on VECTOR, LMP2A, and ITAM (Syk)-Mutant cells. In (A-B), the shaded histograms represent the cell lines stained with an APC-conjugated anti-Fas antibody, while the open histogram represents a combination of all the transduced cell lines that were unstained as a negative control. Data in (C) are a combination of the mean fluorescence intensity in 3 experiments analyzing the levels of Fas expression in VECTOR, LMP2A, and Y112 (Lyn)-Mutant cells and (D) Combination of the mean fluorescence intensity in 3 experiments analyzing the levels of Fas expression in VECTOR , LMP2A, and ITAM (Syk)-Mutant cells. Data are shown as the mean +/− standard error. * indicates p<0.05 when comparing Fas expression on VECTOR cells ** indicates p<0.05 when comparing Fas expression between cell lines expressing Wildtype LMP2A (LMP2A) and LMP2A Y112 (Lyn)-Mutant or ITAM (Syk)-Mutant
Many of the LMP2A-induced changes in B cell biology require activation of Syk [50]. Due to this fact, and since Syk activation is downstream of Lyn, we hypothesized that signaling through Syk tyrosine kinase would be required for the LMP2A-mediated increase in Fas expression. To test this hypothesis, VECTOR, LMP2A, and ITAM (Syk)-Mutant cells were analyzed for Fas expression as described above. The data demonstrate that the ITAM (Syk)-Mutant cell line expresses levels of Fas that are similar to the VECTOR control, and are much lower than the LMP2A cell line (Figure 4B). When multiple experiments are combined, the data indicate that mutations in the ITAM motif are significantly less than the cell line expressing wildtype LMP2A and equal to the VECTOR control (Figure 4D), indicating that LMP2A requires Syk activation to increase Fas expression.
Fas-mediated apoptosis in Y112 (Lyn)-Mutant and ITAM (Syk)-Mutant B cell lines
If the increase in Fas-mediated apoptosis in the LMP2A cell line is due to the increase in Fas expression, then we would expect that the cell lines harboring mutations in the Y112 and ITAM of LMP2A would be less susceptible to Fas-mediated apoptosis. We initially tested if the reduction in Fas expression in the Y112 (Lyn)-Mutant cell line would influence Fas-mediated apoptosis when compared to wildtype LMP2A and VECTOR controls. Therefore, VECTOR, LMP2A, and Y112 (Lyn)-Mutant cell lines were incubated in the absence or presence of anti-Fas mAb for four hours, and evaluated for Annexin V-positivity by flow cytometry. Analysis of the percentage of Annexin V-positive cells indicates that a lower percentage of the Y112 (Lyn)-Mutant cells are Annexin V-positive after incubation with an activating anti-Fas mAb when compared to the LMP2A cell lines (Figure 5A: panel (c) Y112 (Lyn)-Mutant 62% Annexin V+ versus panel (b) LMP2A 95% Annexin V+). When multiple experiments are combined, the Y112 (Lyn)-Mutant is statistically less sensitive to Fas-mediated apoptosis than LMP2A and is not different than VECTOR controls (Figure 5B). Additional experiments demonstrate that the level of caspase-3 and PARP cleavage is less in the Y112 (Lyn)-Mutant in comparison to the LMP2A-expressing cell lines (Figure 5C), further suggesting that Lyn is an important component used by LMP2A to increase Fas-mediated apoptosis.
Figure 5.
Fas-mediated apoptosis in LMP2A and Y112 (Lyn)-Mutant cell lines. (A) Flow cytometric analysis of Annexin V positivity in VECTOR, LMP2A, and Y112 (Lyn)-Mutant cells after incubation in the absence or presence of anti-Fas (CD95) mAb. (B) Combination of 3 experiments analyzing Fas-mediated apoptosis in VECTOR, LMP2A, and Y112 (Lyn)-Mutant cells. Data are shown as the mean +/− standard error. (C) Analysis of caspase-3 and PARP cleavage by Western blot analysis in VECTOR, LMP2A, and Y112 (Lyn)-Mutant cells after incubation in the absence or presence of anti-Fas (CD95) mAb. * indicates p<0.05 when compared to Fas-mediated apoptosis in VECTOR cells ** indicates p<0.05 when comparing Fas-mediated apoptosis between LMP2A and Y112 (Lyn)-Mutant cell lines
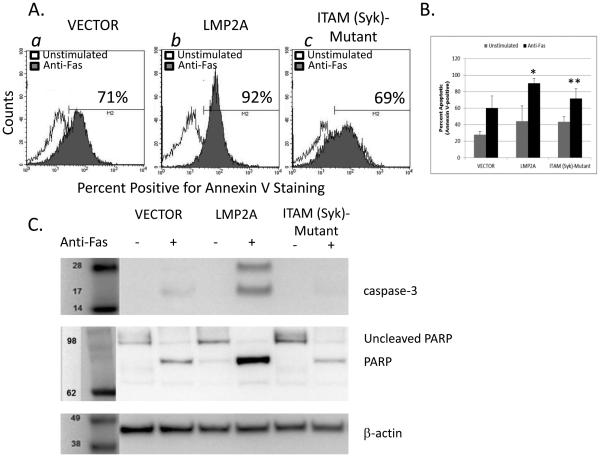
Since the ITAM (Syk)-Mutant cell lines demonstrate a lack of LMP2A-mediated increases in Fas expression, we expected that this cell line would also be deficient in the LMP2A-mediated increase in Fas-dependent apoptosis. Therefore, we tested the requirement for the LMP2A-ITAM in enhancing Fas-mediated apoptosis as described above. The data show that ITAM-(Syk) Mutant cell lines are less susceptible to Fas-mediated apoptosis when compared to the LMP2A cell line and equally sensitive to Fas-mediated apoptosis when compared to VECTOR cells (Figure 6A: (a) VECTOR-71% Annexin V+, (b) LMP2A-92% Annexin V+, and (c) ITAM-Mutant-69% Annexin V+). When multiple experiments are combined, the data demonstrate that the level of Fas-dependent apoptosis in the ITAM (Syk)-Mutant cell line is statistically decreased when compared to the LMP2A cell line and equivalent to the VECTOR control cell line (Figure 6B). Once again, these data were confirmed as determined by caspase-3 and PARP cleavage, since ITAM (Syk)-Mutant cell lines had significantly less cleaved caspase-3 and PARP when compared to cell lines expressing wildtype LMP2A (Figure 6C). Taken together, these data indicate that LMP2A requires signaling through the ITAM motif in the cytoplasmic tail to increase the sensitivity of the A20 cell line to Fas-mediated signaling and apoptosis by increasing Fas expression.
Figure 6.
Fas-mediated apoptosis in LMP2A and ITAM (Syk)-Mutant cell lines. (A) Flow cytometric analysis of Annexin V positivity in VECTOR, LMP2A, and ITAM (Syk)-Mutant cells after incubation in the absence or presence of anti-Fas (CD95) mAb. (B) Combination of 3 experiments analyzing Fas-mediated apoptosis in VECTOR, LMP2A, and ITAM (Syk)-Mutant cells. Data are shown as the mean +/− standard error. (C) Analysis of caspase-3 and PARP cleavage by Western blot analysis in VECTOR, LMP2A, and ITAM (Syk)-Mutant cells after incubation in the absence or presence of anti-Fas (CD95) mAb. * indicates p<0.05 when compared to Fas-mediated apoptosis in VECTOR cells ** indicates p<0.05 when comparing Fas-mediated apoptosis between LMP2A and ITAM (Syk)-Mutant cell lines
Fas expression and Fas-mediated apoptosis in EBV-infected B cells
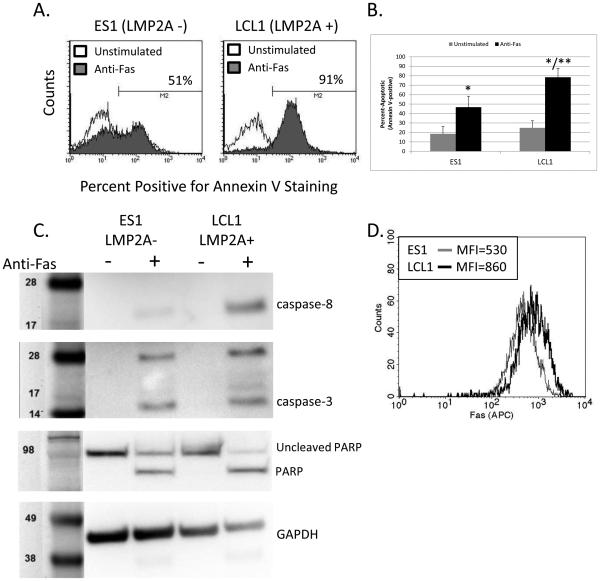
Even though the A20 B cell line has been extensively used to understand Fas-mediated apoptosis in B cells [38-40], the possibility exists that LMP2A may not act in a similar manner among other EBV latency proteins. Therefore, to confirm that LMP2A increases B cell sensitivity to Fas-mediated apoptosis, we analyzed Fas-mediated apoptosis in EBV-infected lymphoblastoid cell lines (LCLs) that either express LMP2A (LCL1) or are deficient in LMP2A (ES1). LCLs express all of the latency genes (EBNAs, LMPs, and EBERs), so by using LCLs for these studies, we can assess whether LMP2A increases B cell sensitivity to Fas-mediated apoptosis in the presence of other EBV latency proteins. As shown in Figure 7, fewer LMP2A-deficient LCLs are Annexin V-positive after Fas engagement when compared to LCLs that express LMP2A (Figure 7A-B). Analysis of caspase-8, −3, and PARP cleavage also confirms that LMP2A-deficient LCLs are less susceptible to Fas-mediated apoptosis, when compared to LCLs expressing LMP2A (Figure 7C), indicating that LMP2A increases B cell sensitivity to Fas-mediated apoptosis in the natural context of the virus. To confirm that the mechanism by which LMP2A increases the susceptibility of human B cells to apoptosis is similar to that seen in the A20 cell line, we tested whether LMP2A also increases Fas expression in LCLs. As shown in Figure 7D, LCLs that express LMP2A show a higher level of Fas expression when compared to LCLs that do not express LMP2A. Taken together, these data indicate that LMP2A increases Fas expression and sensitivity to Fas-mediated apoptosis in EBV-infected B cell lines.
Figure 7.
Fas-mediated apoptosis in LMP2A-expressing (LCL1) or LMP2A-deficient (ES1) lymphoblastoid cell lines. (A) Flow cytometric analysis of Annexin V positivity in LCL1 (LMP2A+) or ES1 (LMP2A−) lymphoblastoid cells after incubation in the absence or presence of anti-human Fas (CD95) mAb and protein G. (B) Combination of 3 experiments analyzing Fas-mediated apoptosis in LCL1 and ES1 lymphoblastoid cell lines. Data are shown as the mean +/− standard error. (C) Analysis of caspase-8, caspase-3, and PARP cleavage by Western blot analysis in LCL1 and ES1 lymphoblastoid cell lines after incubation in the absence or presence of anti-human Fas (CD95) mAb and protein G. (D) Flow cytometric analysis of Fas expression on LCL1 and ES1 lymphoblastoid cell lines. * indicates p<0.05 when compared to Fas-mediated apoptosis in non-stimulated cells ** indicates p<0.05 when comparing Fas-mediated apoptosis between ES1 and LCL1 cell lines
Discussion
Numerous studies using the A20 cell line demonstrate that BCR signaling inhibits Fas-mediated apoptosis [38-40]. Due to the ability of LMP2A to mimic BCR signaling by activating Lyn and Syk tyrosine kinases, we hypothesized that LMP2A would protect B cells from Fas-mediated apoptosis. Our findings demonstrate that LMP2A actually potentiates Fas-mediated apoptosis by increasing the levels of Fas on the surface of B cells.
Fas-mediated apoptosis can be regulated at many levels: the number of Fas receptors on the surface of the cell [46-49], the formation of the DISC complex [39], the activation of caspase 8 directly [39] or indirectly by increasing the levels of c-FLIP [40] and the levels of anti-apoptotic molecules, such as Bcl-2 and Bcl-xL [38]. Our findings demonstrate that LMP2A regulates Fas-mediated apoptosis by increasing the levels of Fas on the surface of B cells. It is possible that LMP2A directly regulates additional components of the Fas-mediated pathway. However, any inherent anti-apoptotic abilities of LMP2A are over-ridden by the increase in Fas on the B cell surface and strong Fas-induced signal. For example, while LMP2A can increase Bcl-xL levels and the phosphorylation of Bcl-2 in transgenic mice [20], the data presented here suggest that the ability of LMP2A to modulate these molecules is not sufficient to protect B cells from Fas-mediated apoptosis. Taken together, even though LMP2A is known to promote survival of B cells, the dramatic LMP2A-dependent increase in Fas expression increases the susceptibility of these cells to Fas-mediated apoptosis.
One potential difference in the outcomes between BCR-mediated protection and LMP2A-mediated sensitization to Fas-dependent apoptosis could be due to the fact that despite overwhelming similarities, there are slight differences in the quantity and/or quality in signaling [51, 52]. BCR-dependent signaling by antigen induces a short burst of high levels of tyrosine phosphorylation, while LMP2A signaling is constitutive and more similar to “tonic” signaling through the BCR [14, 16, 53]. However, the tonic signal from LMP2A must be distinct in some respects from the tonic signaling from the BCR, since resting B cells which only are receiving tonic BCR signals express low levels of Fas [46]. Future studies that analyze the signals generated downstream of Lyn and Syk that are used by LMP2A to increase Fas expression may shed light on the differences between BCR and LMP2A signaling.
Even though Fas levels are increased on activated B cells [54] and germinal center B cells [55-57], the increase is attributed to signaling through CD40 [46, 48]. The role of BCR-dependent signaling in regards to Fas expression is proposed to be limited to augmenting CD40-induced Fas expression, since BCR-signaling alone is not sufficient to increase Fas levels [46]. Therefore, the finding that LMP2A specifically increases Fas expression is somewhat unexpected. Surprisingly, the regulation of Fas expression is understudied. To date, the ability of p53, STAT1 and NF-kB have been shown to specifically induce Fas expression [42]. Previous studies using LMP2A-transgenic murine B cells demonstrate that LMP2A constitutively activates NF-kB [21, 22], and therefore it is plausible that LMP2A is using NF-kB to increase Fas expression in the B cells. Experiments using the NF-kB inhibitor (Bay 11-1075) were attempted to address whether this hypothesis was correct. However, the A20 cell line is highly sensitive to NF-kB inhibition and underwent substantial apoptosis in the absence of anti-Fas antibody (data not shown). Nevertheless, our data demonstrate that Lyn and Syk are required by LMP2A to upregulate Fas expression, and highlight a potentially new area of investigation.
Both LMP1 and LMP2A can protect B cells from apoptosis [20, 28, 58, 59], but both have now been shown to promote Fas-mediated apoptosis ([42] and current findings). During normal EBV infection, LMP2A is commonly expressed with LMP1 [60]. LMP2A mimics the BCR [14], while LMP1 mimics CD40 signaling [58, 61]. It has been proposed that co-expression of LMP2A and LMP1 allows infected cells to transit through the germinal center reaction to promote the evolution of the infected cells to become latently-infected memory cells [6]. If this is true, then one would expect that EBV-infected B cells would demonstrate numerous properties of germinal center B cells. A report by Roughan and Thorley-Lawson demonstrate that EBV-infected germinal center B cells express both LMP1 and LMP2A and numerous germinal center markers, with the appropriate down-regulation of non-germinal center markers [62]. Based on our current findings, the data suggest that LMP1 would not rescue LMP2A-expressing B cells from Fas-mediated apoptosis during the germinal center reaction induced by EBV. Future experiments that address the ability of LMP1 and LMP2A to influence the susceptibility of B cells to Fas-mediated apoptosis could provide significant insight into the interplay of these proteins in promoting B cell survival during the germinal center reaction.
The question remains as to why EBV would evolve to increase the susceptibility of EBV-infected cells to Fas-mediated apoptosis. EBV is a highly successful human pathogen, in that it is carried by over 90% of the human population. It is possible that during the co-evolution of the virus with its human host, EBV has evolved to require a level of Fas-mediated susceptibility to apoptosis in order to limit the overwhelming expansion of EBV-infected cells and promote the establishment of human/EBV equilibrium. The inability to control EBV infections can be fatal [63, 64], so therefore it makes sense for the virus to limit its expansion. EBV is controlled by a strong cytotoxic T cell response [65-67], and therefore, using the Fas/FasL pathway as a mechanism to control overwhelming infection postures the virus to establish latency in B lymphocytes.
In support of this idea, individuals with Autoimmune Lymphoproliferative Syndrome (ALPS) who have mutations that inhibit Fas-mediated apoptosis have presented with exacerbated EBV infections and a higher incidence of EBV-associated lymphoproliferative diseases [68-70]. For example, individuals with ALPS have a 14 to 51 fold higher risk of developing non-Hodgkin’s and Hodgkin’s lymphoma, respectively [70]. Additionally, In the United States, ALPS is associated with the development of chronic active EBV disorder [69], which is associated with fever, lymphadenopathy and splenomegaly due to the expansion of EBV-infected B lymphocytes [69, 71]. Therefore, while EBV needs B cells to survive in order to prolong EBV infection, there appears to be a balance that needs to be maintained between B cell life and death via Fas-mediated apoptosis.
Conclusions
This study tested the ability of the Epstein-Barr virus latency protein, LMP2A, to protect B cells from Fas-mediated apoptosis. Our findings indicate that LMP2A signals through Lyn and Syk to increase the sensitivity of B cells to Fas-mediated apoptosis by increasing the levels of Fas expression on B cells. These findings have important implications for the survival of both normal, latently-infected B cells and tumor cells that express LMP2A.
Methods
Culture of A20 cell line and Lymphoblastoid cell lines
The parental A20 cell line was a kind gift of William J. Karpus of Northwestern University. All cell lines were tested and shown to be mycoplasma free. A20 cells were cultured in complete RPMI containing 10% FCS, L-glutamine, and 1% penicillin/streptomycin at 37° C/5% CO2. Retrovirally-transduced A20 cells were cultured under the same environmental conditions in complete RPMI as above with the addition of zeocin (250 μg/ml) to select for the retention of cells containing the retroviral DNA. Lymphoblastoid cell lines (LCLs), ES1 (LMP2A-negative) and LCL1 (LMP2A-positive) [72] were cultured in cRPMI, while BJAB cell lines [73] were cultured in cRPMI with hygromycin (400 ug/ml) and gentamycin (2 ug/ml) at 37° C/5% CO2.
Generation of cell lines containing Wildtype LMP2A, Y112 mutant, or ITAM mutant
In order to generate A20 murine B cell lines harboring wildtype LMP2A or LMP2A with a mutation in the Lyn binding site (Y112) or the Syk binding site (ITAM motif), cDNA encoding wildtype LMP2A [17], Y112 (Lyn)-Mutant [18] or ITAM (Syk)-Mutant [17] was cloned into the pIEGZ retroviral vector (generous gift of Dirk Lindemann, Germany). The pIEGZ vector also encodes for GFP fluorescence and resistance to zeocin, under the control of the CMV promoter [74]. Retroviral DNA containing VECTOR alone, wildtype LMP2A, Y112 (Lyn)-Mutant, or ITAM (Syk)-Mutant was then transfected into the packaging cell line GP2-293 for 48 hours before viral supernatants were harvested and incubated at 37°C with A20 parental B cell lines in 6-well plates. Stably-expressing cell lines were gradually selected for their growth in complete RPMI1640 plus zeocin (250 μg/ml) and GFP expression by flow cytometry.
RT-PCR
A20 parental, VECTOR, and LMP2A-expressing cell lines (2×106 cells) were used to isolate RNA using the E.Z.N.A™ HP Total RNA Kit (OMEGA biotek). RNA was then DNase treated for 30 minutes at 37°C, followed by heat inactivation for 10 minutes at 65°C (Promega). The DNase treated RNA was then reverse transcribed using qScript Supermix (Quanta Biosciences) at 25°C for 5 minutes, 42°C for 30 minutes, and 85°C for 5 minutes. PCR amplification for LMP2A and GAPDH was performed as described previously [26].
Measuring Fas Expression
VECTOR, LMP2A, Y112 (Lyn)-Mutant, and ITAM (Syk)-Mutant cell lines (0.5×106 cells) were washed twice (PBS+2%FCS) and stained using APC-conjugated anti-mouse CD95 (Fas) antibody (eBiosciences), at a final concentration of 1μg/ml for 30 minutes in the dark on ice. Alternatively, LCL1 (LMP2A+) and ES1 (LMP2A-negative) LCLs were incubated with anti-human CD95 (Fas) antibody (BD Biosciences) as described above. Cells were subsequently washed twice before analysis using a FACS Calibur flow cytometer (Becton Dickinson) and analyzed using Cellquest Software.
Measuring Fas-mediated apoptosis
VECTOR, LMP2A, Y112 (Lyn)-Mutant, and ITAM (Syk)-Mutant cell lines (1×106 cells) were incubated in the absence or presence of 0.1 μg/ml rat anti-mouse Fas/CD95 mAb (BD Biosciences) in cRPMI/Zeocin at 37° C. Alternatively, human lymphoblastoid cell lines LCL1 and ES1 were incubated in the presence of 0.1 μg/ml mouse anti-human Fas/CD95 (BD Biosciences) plus 2 μg of protein G in cRPMI at 37° C. After 4 hours, the cells were washed in PBS and tested for the induction of Fas-mediated apoptosis. Cells were stained using Annexin V Apoptosis Kit APC (BioLegend) according to manufacturer’s instructions. GFP-positive cells were analyzed for Annexin V staining by flow cytometry using a Becton Dickinson FACS Calibur and analyzed using Cellquest Software.
Western Blot Analysis
A20 VECTOR, LMP2A, Y112 (Lyn)-Mutant, and ITAM (Syk)-Mutant cell lines or LCL1 (LMP2A+) and ES1 (LMP2A-negative) LCLs (5 × 106) were lysed in RIPA buffer (Pierce, Rockford IL) containing 25mM Tris-HCl (pH 7.6), 150mM NaCl, 1% NP-40, 1% sodium deoxycholate and 0.1% SDS, along with Halt™ Protease and Phosphatase inhibitors (Pierce, Rockford IL). Protein concentrations from cell lysates were quantified using a BCA assay (Pierce, Rockford IL) and equal amounts of protein were analyzed by Western blot. Antibodies against LMP2A (Clone 14B71-1) (Abcam, Eugene OR), PARP (Monoclonal, clone C.384.8) (Pierce, Rockford IL), Caspase-3 (Cell Signaling Technology, Beverly MA), Caspase-8 (Cell Signaling Technology, Beverly MA), β-actin (Pierce, Rockford IL), and anti-GAPDH primary Ab (BioLegend, San Diego CA) were diluted 1:1000 in blocking buffer containing milk, followed by washes in TBST. A secondary anti-rabbit IgG-HRP conjugated Ab (Pierce, Rockford IL) diluted in blocking buffer (1:5000) was added to the blot followed by ECL imaging (GE Healthcare, Buckinghamshire UK) using a Bio-Rad imager. Image analysis and quantification was done using ImageJ software (National Institutes of Health
Statistics
Experiments which compared two groups were analyzed using a Student’s T-test, while experiments with three or more groups were initially analyzed using a one-way analysis of variance (ANOVA) to determine if there were statistically significant differences within the experiment. Subsequently, a Bonferroni post-hoc test was used to compare individual groups. A finding is determined to be significant when the p value was equal to or less than 0.05.
Supplementary Material
Supplemental Figure 1: Fas-mediated apoptosis in the absence or presence of LMP2A with human BJAB or an additional A20 clones. (A,C) Flow cytometric analysis of Annexin V positivity in LMP2A-negative or –positive BJAB (BJAB-Vector or BJAB-LMP2A) or A20.1 (A20.1 VECTOR or A20.1 LMP2A) cell lines after incubation in the absence or presence of anti-Fas (CD95) mAb. (B,D) Fas expression on LMP2A-negative and –positive BJAB and A20 cell lines. The bar graph is a combination of at least 3 experiments analyzing Fas-mediated apoptosis in A20 VECTOR and LMP2A cells. Data are shown as the mean +/− standard error.* indicates p< 0.05 when comparing the MFI for Fas expression between LMP2A-negative and LMP2A-positive cell lines
Highlights.
The EBV protein LMP2A enhances Fas levels and Fas-induced apoptosis in B cells.
LMP2A uses both Lyn and Syk kinase activation to enhance Fas expression.
LMP2A enhances Fas-mediated apoptosis in the context of the entire virus.
Acknowledgements
The pIEGZ retroviral vector was a generous gift from Dr. Dirk Lindemann, Institut fur Virologie Technischen Universitat Dresden. This work is funded by NIH grant 1R15CA149690-01, the Biomedical Sciences Program in the College of Health Sciences, and Midwestern University Intramural Funds.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing Interests
The authors declare that they have no competing interests.
Author Contributions
RI generated the VECTOR, LMP2A, Y112 (Lyn)-Mutant and ITAM (Syk)-Mutant cell lines, performed experiments, analyzed data, and helped with the editing of the manuscript. SH performed experiments and analyzed data. KB produced the retroviral constructs used in the experiments. LACA and MJF provided reagents. MSM contributed to the design of the experiments and wrote the manuscript. All authors read and approved the final manuscript.
References
- [1].Thompson MP, Kurzrock R. Epstein-Barr virus and cancer. Clin Cancer Res. 2004;10:803–821. doi: 10.1158/1078-0432.ccr-0670-3. [DOI] [PubMed] [Google Scholar]
- [2].Clute SC, Watkin LB, Cornberg M, Naumov YN, Sullivan JL, Luzuriaga K, Welsh RM, Selin LK. Cross-reactive influenza virus-specific CD8+ T cells contribute to lymphoproliferation in Epstein-Barr virus-associated infectious mononucleosis. J Clin Invest. 2005;115:3602–3612. doi: 10.1172/JCI25078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Thorley-Lawson DA, Allday MJ. The curious case of the tumour virus: 50 years of Burkitt's lymphoma. Nat Rev Microbiol. 2008;6:913–924. doi: 10.1038/nrmicro2015. [DOI] [PubMed] [Google Scholar]
- [4].Thorley-Lawson DA, Hawkins JB, Tracy SI, Shapiro M. The pathogenesis of Epstein-Barr virus persistent infection. Curr Opin Virol. 2013;3:227–232. doi: 10.1016/j.coviro.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Seifert M, Scholtysik R, Kuppers R. Origin and pathogenesis of B cell lymphomas. Methods Mol Biol. 2013;971:1–25. doi: 10.1007/978-1-62703-269-8_1. [DOI] [PubMed] [Google Scholar]
- [6].Thorley-Lawson DA, Gross A. Persistence of the Epstein-Barr virus and the origins of associated lymphomas. N Engl J Med. 2004;350:1328–1337. doi: 10.1056/NEJMra032015. [DOI] [PubMed] [Google Scholar]
- [7].Babcock GJ, Decker LL, Volk M, Thorley-Lawson DA. EBV persistence in memory B cells in vivo. Immunity. 1998;9:395–404. doi: 10.1016/s1074-7613(00)80622-6. [DOI] [PubMed] [Google Scholar]
- [8].Babcock GJ, Hochberg D, Thorley-Lawson AD. The expression pattern of Epstein-Barr virus latent genes in vivo is dependent upon the differentiation stage of the infected B cell. Immunity. 2000;13:497–506. doi: 10.1016/s1074-7613(00)00049-2. [DOI] [PubMed] [Google Scholar]
- [9].Babcock GJ, Miyashita-Lin EM, Thorley-Lawson DA. Detection of EBV infection at the single-cell level. Precise quantitation of virus-infected cells in vivo. Methods Mol Biol. 2001;174:103–110. doi: 10.1385/1-59259-227-9:103. [DOI] [PubMed] [Google Scholar]
- [10].Decker LL, Klaman LD, Thorley-Lawson DA. Detection of the latent form of Epstein-Barr virus DNA in the peripheral blood of healthy individuals. J Virol. 1996;70:3286–3289. doi: 10.1128/jvi.70.5.3286-3289.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Babcock GJ, Thorley-Lawson DA. Tonsillar memory B cells, latently infected with Epstein-Barr virus, express the restricted pattern of latent genes previously found only in Epstein-Barr virus-associated tumors. Proc Natl Acad Sci U S A. 2000;97:12250–12255. doi: 10.1073/pnas.200366597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Bell AI, Groves K, Kelly GL, Croom-Carter D, Hui E, Chan AT, Rickinson AB. Analysis of Epstein-Barr virus latent gene expression in endemic Burkitt's lymphoma and nasopharyngeal carcinoma tumour cells by using quantitative real-time PCR assays. J Gen Virol. 2006;87:2885–2890. doi: 10.1099/vir.0.81906-0. [DOI] [PubMed] [Google Scholar]
- [13].Niedobitek G, Agathanggelou A, Herbst H, Whitehead L, Wright DH, Young LS. Epstein-Barr virus (EBV) infection in infectious mononucleosis: virus latency, replication and phenotype of EBV-infected cells. J Pathol. 1997;182:151–159. doi: 10.1002/(SICI)1096-9896(199706)182:2<151::AID-PATH824>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- [14].Pang MF, Lin KW, Peh SC. The signaling pathways of Epstein-Barr virus-encoded latent membrane protein 2A (LMP2A) in latency and cancer. Cell Mol Biol Lett. 2009;14:222–247. doi: 10.2478/s11658-008-0045-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Mancao C, Hammerschmidt W. Epstein-Barr virus latent membrane protein 2A is a B-cell receptor mimic and essential for B-cell survival. Blood. 2007;110:3715–3721. doi: 10.1182/blood-2007-05-090142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Portis T, Cooper L, Dennis P, Longnecker R. The LMP2A signalosome--a therapeutic target for Epstein-Barr virus latency and associated disease. Front Biosci. 2002;7:d414–426. doi: 10.2741/portis. [DOI] [PubMed] [Google Scholar]
- [17].Fruehling S, Longnecker R. The immunoreceptor tyrosine-based activation motif of Epstein-Barr virus LMP2A is essential for blocking BCR-mediated signal transduction. Virology. 1997;235:241–251. doi: 10.1006/viro.1997.8690. [DOI] [PubMed] [Google Scholar]
- [18].Fruehling S, Swart R, Dolwick KM, Kremmer E, Longnecker R. Tyrosine 112 of latent membrane protein 2A is essential for protein tyrosine kinase loading and regulation of Epstein-Barr virus latency. J Virol. 1998;72:7796–7806. doi: 10.1128/jvi.72.10.7796-7806.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Engels N, Merchant M, Pappu R, Chan AC, Longnecker R, Wienands J. Epstein-Barr virus latent membrane protein 2A (LMP2A) employs the SLP-65 signaling module. J Exp Med. 2001;194:255–264. doi: 10.1084/jem.194.3.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Portis T, Longnecker R. Epstein-Barr virus (EBV) LMP2A mediates B-lymphocyte survival through constitutive activation of the Ras/PI3K/Akt pathway. Oncogene. 2004;23:8619–8628. doi: 10.1038/sj.onc.1207905. [DOI] [PubMed] [Google Scholar]
- [21].Swanson-Mungerson M, Bultema R, Longnecker R. Epstein-Barr virus LMP2A imposes sensitivity to apoptosis. J Gen Virol. 2010;91:2197–2202. doi: 10.1099/vir.0.021444-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Swanson-Mungerson MA, Caldwell RG, Bultema R, Longnecker R. Epstein-Barr virus LMP2A alters in vivo and in vitro models of B-cell anergy, but not deletion, in response to autoantigen. J Virol. 2005;79:7355–7362. doi: 10.1128/JVI.79.12.7355-7362.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Anderson LJ, Longnecker R. EBV LMP2A provides a surrogate pre-B cell receptor signal through constitutive activation of the ERK/MAPK pathway. J Gen Virol. 2008;89:1563–1568. doi: 10.1099/vir.0.2008/001461-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Hatton O, Lambert SL, Phillips LK, Vaysberg M, Natkunam Y, Esquivel CO, Krams SM, Martinez OM. Syk-induced phosphatidylinositol-3-kinase activation in Epstein-Barr virus posttransplant lymphoproliferative disorder. Am J Transplant. 2013;13:883–890. doi: 10.1111/ajt.12137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Wasil LR, Tomaszewski MJ, Hoji A, Rowe DT. The effect of Epstein-Barr virus Latent Membrane Protein 2 expression on the kinetics of early B cell infection. PLoS One. 2013;8:e54010. doi: 10.1371/journal.pone.0054010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Caldwell RG, Wilson JB, Anderson SJ, Longnecker R. Epstein-Barr virus LMP2A drives B cell development and survival in the absence of normal B cell receptor signals. Immunity. 1998;9:405–411. doi: 10.1016/s1074-7613(00)80623-8. [DOI] [PubMed] [Google Scholar]
- [27].Fukuda M, Longnecker R. Latent membrane protein 2A inhibits transforming growth factor-beta 1-induced apoptosis through the phosphatidylinositol 3-kinase/Akt pathway. J Virol. 2004;78:1697–1705. doi: 10.1128/JVI.78.4.1697-1705.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Incrocci R, McCormack M, Swanson-Mungerson M. Epstein-Barr virus LMP2A increases IL-10 production in mitogen-stimulated primary B-cells and B-cell lymphomas. J Gen Virol. 2013;94:1127–1133. doi: 10.1099/vir.0.049221-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Bieging KT, Swanson-Mungerson M, Amick AC, Longnecker R. Epstein-Barr virus in Burkitt's lymphoma: a role for latent membrane protein 2A. Cell Cycle. 2010;9:901–908. doi: 10.4161/cc.9.5.10840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Bultema R, Longnecker R, Swanson-Mungerson M. Epstein-Barr virus LMP2A accelerates MYC-induced lymphomagenesis. Oncogene. 2009;28:1471–1476. doi: 10.1038/onc.2008.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Bieging KT, Amick AC, Longnecker R. Epstein-Barr virus LMP2A bypasses p53 inactivation in a MYC model of lymphomagenesis. Proc Natl Acad Sci U S A. 2009;106:17945–17950. doi: 10.1073/pnas.0907994106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Fish K, Chen J, Longnecker R. Epstein-Barr virus latent membrane protein 2A enhances MYC-driven cell cycle progression in a mouse model of B lymphoma. Blood. 2014;123:530–540. doi: 10.1182/blood-2013-07-517649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Bost KL, Bieligk SC, Jaffe BM. Lymphokine mRNA expression by transplantable murine B lymphocytic malignancies. Tumor-derived IL-10 as a possible mechanism for modulating the anti-tumor response. J Immunol. 1995;154:718–729. [PubMed] [Google Scholar]
- [34].Jarnicki AG, Lysaght J, Todryk S, Mills KH. Suppression of antitumor immunity by IL-10 and TGF-beta-producing T cells infiltrating the growing tumor: influence of tumor environment on the induction of CD4+ and CD8+ regulatory T cells. J Immunol. 2006;177:896–904. doi: 10.4049/jimmunol.177.2.896. [DOI] [PubMed] [Google Scholar]
- [35].Nagata S. Fas ligand-induced apoptosis. Annu Rev Genet. 1999;33:29–55. doi: 10.1146/annurev.genet.33.1.29. [DOI] [PubMed] [Google Scholar]
- [36].Brint E, O'Callaghan G, Houston A. Life in the Fas lane: differential outcomes of Fas signaling. Cell Mol Life Sci. 2013;70:4085–4099. doi: 10.1007/s00018-013-1327-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Yurchenko M, Shlapatska LM, Sidorenko SP. The multilevel regulation of CD95 signaling outcome. Exp Oncol. 2012;34:153–159. [PubMed] [Google Scholar]
- [38].Bras A, Martinez AC, Baixeras E. B cell receptor cross-linking prevents Fas-induced cell death by inactivating the IL-1 beta-converting enzyme protease and regulating Bcl-2/Bcl-x expression. J Immunol. 1997;159:3168–3177. [PubMed] [Google Scholar]
- [39].Catlett IM, Bishop GA. Cutting edge: a novel mechanism for rescue of B cells from CD95/Fas-mediated apoptosis. J Immunol. 1999;163:2378–2381. [PubMed] [Google Scholar]
- [40].Moriyama H, Yonehara S. Rapid up-regulation of c-FLIP expression by BCR signaling through the PI3K/Akt pathway inhibits simultaneously induced Fas-mediated apoptosis in murine B lymphocytes. Immunol Lett. 2007;109:36–46. doi: 10.1016/j.imlet.2006.12.009. [DOI] [PubMed] [Google Scholar]
- [41].Snow AL, Lambert SL, Natkunam Y, Esquivel CO, Krams SM, Martinez OM. EBV can protect latently infected B cell lymphomas from death receptor-induced apoptosis. J Immunol. 2006;177:3283–3293. doi: 10.4049/jimmunol.177.5.3283. [DOI] [PubMed] [Google Scholar]
- [42].Le Clorennec C, Youlyouz-Marfak I, Adriaenssens E, Coll J, Bornkamm GW, Feuillard J. EBV latency III immortalization program sensitizes B cells to induction of CD95-mediated apoptosis via LMP1: role of NF-kappaB, STAT1, and p53. Blood. 2006;107:2070–2078. doi: 10.1182/blood-2005-05-2053. [DOI] [PubMed] [Google Scholar]
- [43].Ikeda M, Longnecker R. The c-Cbl proto-oncoprotein downregulates EBV LMP2A signaling. Virology. 2009;385:183–191. doi: 10.1016/j.virol.2008.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Aredia F, Scovassi AI. Poly(ADP-ribose): A signaling molecule in different paradigms of cell death. Biochem Pharmacol. 2014;92:157–163. doi: 10.1016/j.bcp.2014.06.021. [DOI] [PubMed] [Google Scholar]
- [45].Thorburn A. Death receptor-induced cell killing. Cell Signal. 2004;16:139–144. doi: 10.1016/j.cellsig.2003.08.007. [DOI] [PubMed] [Google Scholar]
- [46].Garrone P, Neidhardt EM, Garcia E, Galibert L, van Kooten C, Banchereau J. Fas ligation induces apoptosis of CD40-activated human B lymphocytes. J Exp Med. 1995;182:1265–1273. doi: 10.1084/jem.182.5.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Park SM, Kim S, Choi JS, Hur DY, Lee WJ, Lee MS, Choe J, Lee TH. TGF-beta inhibits Fas-mediated apoptosis of a follicular dendritic cell line by down-regulating the expression of Fas and caspase-8: counteracting role of TGF-beta on TNF sensitization of Fas-mediated apoptosis. J Immunol. 2005;174:6169–6175. doi: 10.4049/jimmunol.174.10.6169. [DOI] [PubMed] [Google Scholar]
- [48].Schattner EJ, Elkon KB, Yoo DH, Tumang J, Krammer PH, Crow MK, Friedman SM. CD40 ligation induces Apo-1/Fas expression on human B lymphocytes and facilitates apoptosis through the Apo-1/Fas pathway. J Exp Med. 1995;182:1557–1565. doi: 10.1084/jem.182.5.1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Yasuda M, Tanaka Y, Fujii K, Yasumoto K. CD44 stimulation down-regulates Fas expression and Fas-mediated apoptosis of lung cancer cells. Int Immunol. 2001;13:1309–1319. doi: 10.1093/intimm/13.10.1309. [DOI] [PubMed] [Google Scholar]
- [50].Merchant M, Caldwell RG, Longnecker R. The LMP2A ITAM is essential for providing B cells with development and survival signals in vivo. J Virol. 2000;74:9115–9124. doi: 10.1128/jvi.74.19.9115-9124.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Portis T, Dyck P, Longnecker R. Epstein-Barr Virus (EBV) LMP2A induces alterations in gene transcription similar to those observed in Reed-Sternberg cells of Hodgkin lymphoma. Blood. 2003;102:4166–4178. doi: 10.1182/blood-2003-04-1018. [DOI] [PubMed] [Google Scholar]
- [52].Portis T, Longnecker R. Epstein-Barr virus (EBV) LMP2A alters normal transcriptional regulation following B-cell receptor activation. Virology. 2004;318:524–533. doi: 10.1016/j.virol.2003.09.017. [DOI] [PubMed] [Google Scholar]
- [53].Rickert RC. New insights into pre-BCR and BCR signalling with relevance to B cell malignancies. Nat Rev Immunol. 2013;13:578–591. doi: 10.1038/nri3487. [DOI] [PubMed] [Google Scholar]
- [54].Yoshino T, Kondo E, Cao L, Takahashi K, Hayashi K, Nomura S, Akagi T. Inverse expression of bcl-2 protein and Fas antigen in lymphoblasts in peripheral lymph nodes and activated peripheral blood T and B lymphocytes. Blood. 1994;83:1856–1861. [PubMed] [Google Scholar]
- [55].Kondo E, Yoshino T. Expression of apoptosis regulators in germinal centers and germinal center-derived B-cell lymphomas: insight into B-cell lymphomagenesis. Pathol Int. 2007;57:391–397. doi: 10.1111/j.1440-1827.2007.02115.x. [DOI] [PubMed] [Google Scholar]
- [56].Martinez-Valdez H, Guret C, de Bouteiller O, Fugier I, Banchereau J, Liu YJ. Human germinal center B cells express the apoptosis-inducing genes Fas, c-myc, P53, and Bax but not the survival gene bcl-2. J Exp Med. 1996;183:971–977. doi: 10.1084/jem.183.3.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Takahashi Y, Ohta H, Takemori T. Fas is required for clonal selection in germinal centers and the subsequent establishment of the memory B cell repertoire. Immunity. 2001;14:181–192. doi: 10.1016/s1074-7613(01)00100-5. [DOI] [PubMed] [Google Scholar]
- [58].Graham JP, Arcipowski KM, Bishop GA. Differential B-lymphocyte regulation by CD40 and its viral mimic, latent membrane protein 1. Immunol Rev. 2010;237:226–248. doi: 10.1111/j.1600-065X.2010.00932.x. [DOI] [PubMed] [Google Scholar]
- [59].Hatton O, Martinez OM, Esquivel CO. Emerging therapeutic strategies for Epstein-Barr virus+ post-transplant lymphoproliferative disorder. Pediatr Transplant. 2012;16:220–229. doi: 10.1111/j.1399-3046.2012.01656.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Thorley-Lawson DA. Epstein-Barr virus: exploiting the immune system. Nat Rev Immunol. 2001;1:75–82. doi: 10.1038/35095584. [DOI] [PubMed] [Google Scholar]
- [61].Lam N, Sugden B. CD40 and its viral mimic, LMP1: similar means to different ends. Cell Signal. 2003;15:9–16. doi: 10.1016/s0898-6568(02)00083-9. [DOI] [PubMed] [Google Scholar]
- [62].Roughan JE, Thorley-Lawson DA. The intersection of Epstein-Barr virus with the germinal center. J Virol. 2009;83:3968–3976. doi: 10.1128/JVI.02609-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Purtilo DT, Cassel CK, Yang JP, Harper R. X-linked recessive progressive combined variable immunodeficiency (Duncan's disease) Lancet. 1975;1:935–940. doi: 10.1016/s0140-6736(75)92004-8. [DOI] [PubMed] [Google Scholar]
- [64].Veillette A, Perez-Quintero LA, Latour S. X-linked lymphoproliferative syndromes and related autosomal recessive disorders. Curr Opin Allergy Clin Immunol. 2013;13:614–622. doi: 10.1097/ACI.0000000000000008. [DOI] [PubMed] [Google Scholar]
- [65].Lacerda JF, Ladanyi M, Louie DC, Fernandez JM, Papadopoulos EB, O'Reilly RJ. Human Epstein-Barr virus (EBV)-specific cytotoxic T lymphocytes home preferentially to and induce selective regressions of autologous EBV-induced B cell lymphoproliferations in xenografted C.B-17 scid/scid mice. J Exp Med. 1996;183:1215–1228. doi: 10.1084/jem.183.3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Royston I, Sullivan JL, Periman PO, Perlin E. Cell-mediated immunity to Epstein-Barr-virus-transformed lymphoblastoid cells in acute infectious mononucleosis. N Engl J Med. 1975;293:1159–1163. doi: 10.1056/NEJM197512042932301. [DOI] [PubMed] [Google Scholar]
- [67].Strang G, Rickinson AB. In vitro expansion of Epstein-Barr virus-specific HLA-restricted cytotoxic T cells direct from the blood of infectious mononucleosis patients. Immunology. 1987;62:647–654. [PMC free article] [PubMed] [Google Scholar]
- [68].Miyawaki T. Primary Immunodeficiencies Inducing EBV-Associated Severe Illnesses. Iran J Allergy Asthma Immunol. 2004;3:51–57. [PubMed] [Google Scholar]
- [69].Cohen JI, Jaffe ES, Dale JK, Pittaluga S, Heslop HE, Rooney CM, Gottschalk S, Bollard CM, Rao VK, Marques A, Burbelo PD, Turk SP, Fulton R, Wayne AS, Little RF, Cairo MS, El-Mallawany NK, Fowler D, Sportes C, Bishop MR, Wilson W, Straus SE. Characterization and treatment of chronic active Epstein-Barr virus disease: a 28-year experience in the United States. Blood. 2011;117:5835–5849. doi: 10.1182/blood-2010-11-316745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Straus SE, Jaffe ES, Puck JM, Dale JK, Elkon KB, Rosen-Wolff A, Peters AM, Sneller MC, Hallahan CW, Wang J, Fischer RE, Jackson CM, Lin AY, Baumler C, Siegert E, Marx A, Vaishnaw AK, Grodzicky T, Fleisher TA, Lenardo MJ. The development of lymphomas in families with autoimmune lymphoproliferative syndrome with germline Fas mutations and defective lymphocyte apoptosis. Blood. 2001;98:194–200. doi: 10.1182/blood.v98.1.194. [DOI] [PubMed] [Google Scholar]
- [71].Okano M, Kawa K, Kimura H, Yachie A, Wakiguchi H, Maeda A, Imai S, Ohga S, Kanegane H, Tsuchiya S, Morio T, Mori M, Yokota S, Imashuku S. Proposed guidelines for diagnosing chronic active Epstein-Barr virus infection. Am J Hematol. 2005;80:64–69. doi: 10.1002/ajh.20398. [DOI] [PubMed] [Google Scholar]
- [72].Ikeda M, Longnecker R. Cholesterol is critical for Epstein-Barr virus latent membrane protein 2A trafficking and protein stability. Virology. 2007;360:461–468. doi: 10.1016/j.virol.2006.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Miller CL, Longnecker R, Kieff E. Epstein-Barr virus latent membrane protein 2A blocks calcium mobilization in B lymphocytes. J Virol. 1993;67:3087–3094. doi: 10.1128/jvi.67.6.3087-3094.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Temme A, Morgenroth A, Schmitz M, Weigle B, Rohayem J, Lindemann D, Fussel M, Ehninger G, Rieber EP. Efficient transduction and long-term retroviral expression of the melanoma-associated tumor antigen tyrosinase in CD34(+) cord blood-derived dendritic cells. Gene Ther. 2002;9:1551–1560. doi: 10.1038/sj.gt.3301821. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1: Fas-mediated apoptosis in the absence or presence of LMP2A with human BJAB or an additional A20 clones. (A,C) Flow cytometric analysis of Annexin V positivity in LMP2A-negative or –positive BJAB (BJAB-Vector or BJAB-LMP2A) or A20.1 (A20.1 VECTOR or A20.1 LMP2A) cell lines after incubation in the absence or presence of anti-Fas (CD95) mAb. (B,D) Fas expression on LMP2A-negative and –positive BJAB and A20 cell lines. The bar graph is a combination of at least 3 experiments analyzing Fas-mediated apoptosis in A20 VECTOR and LMP2A cells. Data are shown as the mean +/− standard error.* indicates p< 0.05 when comparing the MFI for Fas expression between LMP2A-negative and LMP2A-positive cell lines