Abstract
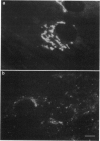
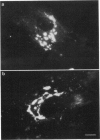
Previous studies have established that a fluorescent analog of ceramide, N-[7-(4-nitrobenzo-2-oxa-1,3-diazole)] -6-aminohexanoyl-D-erythro-sphingosine (C6-NBD-Cer), is a vital stain for the Golgi apparatus and a useful tool for studying the sorting and transport of sphingolipids along the secretory pathway in animal cells. Here, we examine the effects of various culture conditions on labeling of the Golgi apparatus of human skin fibroblasts by C6-NBD-Cer and demonstrate that cholesterol deprivation affects the fluorescence properties of the probe at this organelle. Labeling of the Golgi apparatus by C6-NBD-Cer was dramatically reduced in cells grown in medium containing lipoprotein-deficient serum compared to cells grown in medium containing normal serum. Quantitative fluorescence microscopy showed that this apparent reduction in labeling resulted from accelerated photo-bleaching of the fluorescent analog. C6-NBD-Cer labeling of the Golgi apparatus was restored in cholesterol-deprived cells by stimulating endogenous cholesterol biosynthesis with mevalonic acid or by adding exogenous nonlipoprotein cholesterol or low density lipoprotein to the culture medium. In addition, when cells grown in medium containing normal serum were perforated and treated with cholesterol oxidase, an apparent reduction in labeling resulted, further implicating an intracellular pool of cholesterol in the potentiation of C6-NBD-Cer fluorescence. These results demonstrate that cytological studies using C6-NBD-Cer are affected by cholesterol deprivation and suggest that this fluorescent lipid may be used to monitor cholesterol at the Golgi apparatus of living cells.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Blanchette-Mackie E. J., Dwyer N. K., Amende L. M., Kruth H. S., Butler J. D., Sokol J., Comly M. E., Vanier M. T., August J. T., Brady R. O. Type-C Niemann-Pick disease: low density lipoprotein uptake is associated with premature cholesterol accumulation in the Golgi complex and excessive cholesterol storage in lysosomes. Proc Natl Acad Sci U S A. 1988 Nov;85(21):8022–8026. doi: 10.1073/pnas.85.21.8022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M. S., Goldstein J. L. Multivalent feedback regulation of HMG CoA reductase, a control mechanism coordinating isoprenoid synthesis and cell growth. J Lipid Res. 1980 Jul;21(5):505–517. [PubMed] [Google Scholar]
- Cooper M. S., Cornell-Bell A. H., Chernjavsky A., Dani J. W., Smith S. J. Tubulovesicular processes emerge from trans-Golgi cisternae, extend along microtubules, and interlink adjacent trans-golgi elements into a reticulum. Cell. 1990 Apr 6;61(1):135–145. doi: 10.1016/0092-8674(90)90221-y. [DOI] [PubMed] [Google Scholar]
- Coste H., Martel M. B., Azzar G., Got R. UDPglucose-ceramide glucosyltransferase from porcine submaxillary glands is associated with the Golgi apparatus. Biochim Biophys Acta. 1985 Mar 28;814(1):1–7. doi: 10.1016/0005-2736(85)90412-2. [DOI] [PubMed] [Google Scholar]
- Coste H., Martel M. B., Got R. Topology of glucosylceramide synthesis in Golgi membranes from porcine submaxillary glands. Biochim Biophys Acta. 1986 Jun 13;858(1):6–12. doi: 10.1016/0005-2736(86)90285-3. [DOI] [PubMed] [Google Scholar]
- FORD D. K., YERGANIAN G. Observations on the chromosomes of Chinese hamster cells in tissue culture. J Natl Cancer Inst. 1958 Aug;21(2):393–425. [PubMed] [Google Scholar]
- Futerman A. H., Pagano R. E. Determination of the intracellular sites and topology of glucosylceramide synthesis in rat liver. Biochem J. 1991 Dec 1;280(Pt 2):295–302. doi: 10.1042/bj2800295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Futerman A. H., Stieger B., Hubbard A. L., Pagano R. E. Sphingomyelin synthesis in rat liver occurs predominantly at the cis and medial cisternae of the Golgi apparatus. J Biol Chem. 1990 May 25;265(15):8650–8657. [PubMed] [Google Scholar]
- Gamble W., Vaughan M., Kruth H. S., Avigan J. Procedure for determination of free and total cholesterol in micro- or nanogram amounts suitable for studies with cultured cells. J Lipid Res. 1978 Nov;19(8):1068–1070. [PubMed] [Google Scholar]
- Gravotta D., Adesnik M., Sabatini D. D. Transport of influenza HA from the trans-Golgi network to the apical surface of MDCK cells permeabilized in their basolateral plasma membranes: energy dependence and involvement of GTP-binding proteins. J Cell Biol. 1990 Dec;111(6 Pt 2):2893–2908. doi: 10.1083/jcb.111.6.2893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeckel D., Karrenbauer A., Birk R., Schmidt R. R., Wieland F. Sphingomyelin is synthesized in the cis Golgi. FEBS Lett. 1990 Feb 12;261(1):155–157. doi: 10.1016/0014-5793(90)80659-7. [DOI] [PubMed] [Google Scholar]
- Jeckel D., Karrenbauer A., Burger K. N., van Meer G., Wieland F. Glucosylceramide is synthesized at the cytosolic surface of various Golgi subfractions. J Cell Biol. 1992 Apr;117(2):259–267. doi: 10.1083/jcb.117.2.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson I. D., Kang H. C., Haugland R. P. Fluorescent membrane probes incorporating dipyrrometheneboron difluoride fluorophores. Anal Biochem. 1991 Nov 1;198(2):228–237. doi: 10.1016/0003-2697(91)90418-s. [DOI] [PubMed] [Google Scholar]
- Kobayashi T., Pagano R. E. Lipid transport during mitosis. Alternative pathways for delivery of newly synthesized lipids to the cell surface. J Biol Chem. 1989 Apr 5;264(10):5966–5973. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lipsky N. G., Pagano R. E. A vital stain for the Golgi apparatus. Science. 1985 May 10;228(4700):745–747. doi: 10.1126/science.2581316. [DOI] [PubMed] [Google Scholar]
- Lipsky N. G., Pagano R. E. Intracellular translocation of fluorescent sphingolipids in cultured fibroblasts: endogenously synthesized sphingomyelin and glucocerebroside analogues pass through the Golgi apparatus en route to the plasma membrane. J Cell Biol. 1985 Jan;100(1):27–34. doi: 10.1083/jcb.100.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipsky N. G., Pagano R. E. Sphingolipid metabolism in cultured fibroblasts: microscopic and biochemical studies employing a fluorescent ceramide analogue. Proc Natl Acad Sci U S A. 1983 May;80(9):2608–2612. doi: 10.1073/pnas.80.9.2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller R. G. The use and abuse of filipin to localize cholesterol in membranes. Cell Biol Int Rep. 1984 Jul;8(7):519–535. doi: 10.1016/0309-1651(84)90050-x. [DOI] [PubMed] [Google Scholar]
- Orci L., Montesano R., Meda P., Malaisse-Lagae F., Brown D., Perrelet A., Vassalli P. Heterogeneous distribution of filipin--cholesterol complexes across the cisternae of the Golgi apparatus. Proc Natl Acad Sci U S A. 1981 Jan;78(1):293–297. doi: 10.1073/pnas.78.1.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagano R. E., Martin O. C. A series of fluorescent N-acylsphingosines: synthesis, physical properties, and studies in cultured cells. Biochemistry. 1988 Jun 14;27(12):4439–4445. doi: 10.1021/bi00412a034. [DOI] [PubMed] [Google Scholar]
- Pagano R. E., Martin O. C., Kang H. C., Haugland R. P. A novel fluorescent ceramide analogue for studying membrane traffic in animal cells: accumulation at the Golgi apparatus results in altered spectral properties of the sphingolipid precursor. J Cell Biol. 1991 Jun;113(6):1267–1279. doi: 10.1083/jcb.113.6.1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagano R. E., Sepanski M. A., Martin O. C. Molecular trapping of a fluorescent ceramide analogue at the Golgi apparatus of fixed cells: interaction with endogenous lipids provides a trans-Golgi marker for both light and electron microscopy. J Cell Biol. 1989 Nov;109(5):2067–2079. doi: 10.1083/jcb.109.5.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagano R. E. The Golgi apparatus: insights from lipid biochemistry. Biochem Soc Trans. 1990 Jun;18(3):361–366. doi: 10.1042/bst0180361. [DOI] [PubMed] [Google Scholar]
- Rothman J. E., Orci L. Molecular dissection of the secretory pathway. Nature. 1992 Jan 30;355(6359):409–415. doi: 10.1038/355409a0. [DOI] [PubMed] [Google Scholar]
- Simons K., Virta H. Perforated MDCK cells support intracellular transport. EMBO J. 1987 Aug;6(8):2241–2247. doi: 10.1002/j.1460-2075.1987.tb02496.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sleight R. G., Pagano R. E. Transport of a fluorescent phosphatidylcholine analog from the plasma membrane to the Golgi apparatus. J Cell Biol. 1984 Aug;99(2):742–751. doi: 10.1083/jcb.99.2.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virtanen I., Ekblom P., Laurila P. Subcellular compartmentalization of saccharide moieties in cultured normal and malignant cells. J Cell Biol. 1980 May;85(2):429–434. doi: 10.1083/jcb.85.2.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeagle P. L. Cholesterol and the cell membrane. Biochim Biophys Acta. 1985 Dec 9;822(3-4):267–287. doi: 10.1016/0304-4157(85)90011-5. [DOI] [PubMed] [Google Scholar]
- van 't Hof W., van Meer G. Generation of lipid polarity in intestinal epithelial (Caco-2) cells: sphingolipid synthesis in the Golgi complex and sorting before vesicular traffic to the plasma membrane. J Cell Biol. 1990 Sep;111(3):977–986. doi: 10.1083/jcb.111.3.977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Meer G., Stelzer E. H., Wijnaendts-van-Resandt R. W., Simons K. Sorting of sphingolipids in epithelial (Madin-Darby canine kidney) cells. J Cell Biol. 1987 Oct;105(4):1623–1635. doi: 10.1083/jcb.105.4.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]