Abstract
Human Cytomegalovirus (HCMV), an ubiquitous β-herpesvirus, is a significant pathogen that causes medically severe diseases in immunocompromised individuals and in congenitally infected neonates. RhoB belongs to the family of Rho GTPases, which regulates diverse cellular processes. Rho proteins are implicated in the entry and egress from the host cell of mainly α- and γ-herpesviruses, whereas β-herpesviruses are the least studied in this regard. Here, we studied the role of RhoB GTPase during HCMV lytic infection. Microscopy analysis, both in fixed and live infected cells showed that RhoB was translocated to the assembly complex/compartment (AC) of HCMV, a cytoplasmic zone in infected cells where many viral structural proteins are known to accumulate and assembly of new virions takes place. Furthermore, RhoB was localized at the AC even when the expression of the late HCMV AC proteins was inhibited. At the very late stages of infection, cellular projections were formed containing RhoB and HCMV virions, potentially contributing to the successful viral spread. Interestingly, the knockdown of RhoB in HCMV-infected cells resulted in a significant reduction of the virus titer and could also affect the accumulation of AC viral proteins at this subcellular compartment. RhoB knockdown also affected actin fibers' structure. Actin reorganization was observed at late stages of infection originating from the viral AC and surrounding the cellular projections, implying a potential interplay between RhoB and actin during HCMV assembly and egress. In conclusion, our results demonstrate for the first time that RhoB is a constituent of the viral AC and is required for HCMV productive infection.
Keywords: HCMV, RhoB, assembly complex, assembly compartment, pUL32, pp65, pUL97, actin, cytoskeleton, cellular projections
Introduction
Human Cytomegalovirus (HCMV), a member of β-herpesvirus subfamily, is considered among the most successful human pathogens, due to its ability to establish persistent and latent infections and to inactivate, modulate or evade adaptive and innate immune responses. After primary infection, HCMV establishes lifelong latency in the host and periodically it can reactivate. Primary infection usually causes mild or subclinical diseases in otherwise healthy individuals; however, it causes severe complications in the case of immunocompromised patients, such as transplant recipients, cancer patients following chemotherapy and patients with advanced AIDS.1,2 HCMV infection in pregnant women is also the leading viral cause of congenital abnormalities and mental retardation in newborns.3,4 HCMV pathogenesis largely depends on the effect of the viral proteins on the host cell.
During lytic infection, viral genes are transcribed in a remarkably predefined temporal cascade, initiated with the transcription of the immediate-early genes (IE), followed by the transcription of the early (E) and finally the late (L) genes.5 The latter phase is characterized by the assembly and release of new virions from the host cell, a process involving the interaction of viral components with nuclear and cytoplasmic cellular proteins, structures and compartments which are often modified or relocated. Nascent nucleocapsids, upon exiting the nucleus, embark on a maturation journey that involves additional tegumantation and final envelopment and takes place in a specific cytoplasmic region called the assembly complex/compartment (AC). The AC consists of structural envelop and tegument viral proteins as well as cellular proteins. The interior of the AC contains early endosomal vesicles that are surrounded by trans-Golgi and Golgi vesicles in the form of concentric cylinders, whereas the ER forms a ring around the AC. Markers of the recycling and late endosomes also localize at the AC. The endosomal sorting complex required for transport (ESCRT) machinery and multivesicular bodies (MVB) play a role in virion maturation.6-14
RhoB belongs to the Rho GTPase family of proteins that comprises 6 subfamilies.15 The RhoA subfamily consists of RhoA, RhoB and RhoC, which exhibit approximately 85% amino acid sequence identity.15,16 RhoB, contrary to RhoA and RhoC, is generally regarded as a tumor suppressor protein.17 RhoB protein can be either geranylgeranylated (RhoB-GG) or farnesylated (RhoB-F)18 and this may affect its intracellular localization (reviewed in17). It has been reported that RhoB-GG localizes to multivesicular late endosomes and RhoB-F localizes to the plasma membrane.19 RhoB has also been reported to localize to early endosomes, to a pre-lysosomal compartment, to Golgi and motile peri-Golgi vesicles, to cytoplasmic endosomes associated with the perinuclear recycling compartment, to the nuclear membrane/margin and to the nucleoplasm.19-24 RhoB plays a role in epidermal growth factor receptor (EGFR) trafficking by recruiting protein kinase C-related protein kinase 1 (PRK1) and the PI3-kinase effector kinase PDK1 to the multivesicular body late endosomal compartment,25,26 inhibiting the sorting of EGFR to the lysosome and increasing EGFR recycling to the plasma membrane, (reviewed in17). Moreover, RhoB regulates the Akt nuclear localization in endothelial cells.23
Rho GTPases act as molecular switches regulating many aspects of cell behavior, including cell cycle progression, migration, morphogenesis, chemotaxis, apoptosis and many others.27-30 Intriguingly, the regulation of the aforementioned biological processes is mediated by the dynamic changes and the remodeling of the actin cytoskeleton in response to extracellular signals (reviewed in31,32). As regards RhoB, it has been shown that this particular Rho GTPase, in concert with actin polymerization, coordinates Src activation with endosome-mediated delivery to the membrane.24 Furthermore, RhoB regulates endosome transport by promoting actin assembly on endosomal membranes and the polymerisation of an actin coat around early endosomes through the Diaphanous-related formin, Dia1.33 Moreover, experiments using cancer cells have demonstrated that the depletion of RhoB inhibits cell spreading and stable lamellipodium extension and promotes migration, but impairs persistence and directionality.34
The role of Rho proteins has been studied in relation to herpesviruses, especially α- and gamma-herpesviruses. There is increasing evidence that herpesviruses are capable of usurping Rho GTPases during the main stages of the viral lifecycle, such as virus entry, translocation of the viral particles to the nucleus and viral egress.35,36 Depending on the endocytic uptake routes engaged during the initial interaction of the herpesviruses with the host, specific Rho GTPase signaling is triggered and significant actin cytoskeleton rearrangements are induced,37-40 creating an optimal microenvironment for viral replication, persistence and dissemination. It is of interest that apart from lytic infection, the interactions between gamma-herpeviruses and Rho GTPase signaling remain active even during non-productive infection, facilitating either the establishment of latency41 or affecting cell motility and invasion and promoting the oncogenic properties of these viruses.36,42
Less information is available for β-herpesviruses. During the initial phase of HCMV infection, total and activated-RhoA levels are decreased and this contributes to the disruption of actin stress fibers,43 a phenomenon that facilitates HCMV nuclear translocation.44-46 HCMV-encoded chemokine receptor US-28 activates RhoA via Pyk2 mediating cellular motility and also activates b-catenin involving the Rho-Rho kinase (ROCK) pathway.47,48 Moreover, the Rho GTPase CDC42 is miRNA-regulated and plays a role in HCMV AC morphogenesis along with RAB5C, RAB11A and SNAP23.49
Herein, we addressed the role of RhoB during human cytomegalovirus lytic infection. We extensively examined the subcellular distribution of RhoB both in fixed and live HCMV-infected cells and found that RhoB translocates to the cytoplasmic area that corresponds to the HCMV AC. Interestingly, depletion of RhoB results in significant reduction of the virus titer and could affect the proper accumulation of several viral proteins at the AC while the dynamic redistribution of RhoB at late stages of the infection is coupled with actin reorganization. Actin de-polymerization adversely affects the late stages of HCMV lytic infection. Overall, RhoB plays a role in the assembly of HCMV and is required for the infectious viruses production.
Results
RhoB is upregulated upon HCMV infection and localizes at the AC in HCMV-infected cells
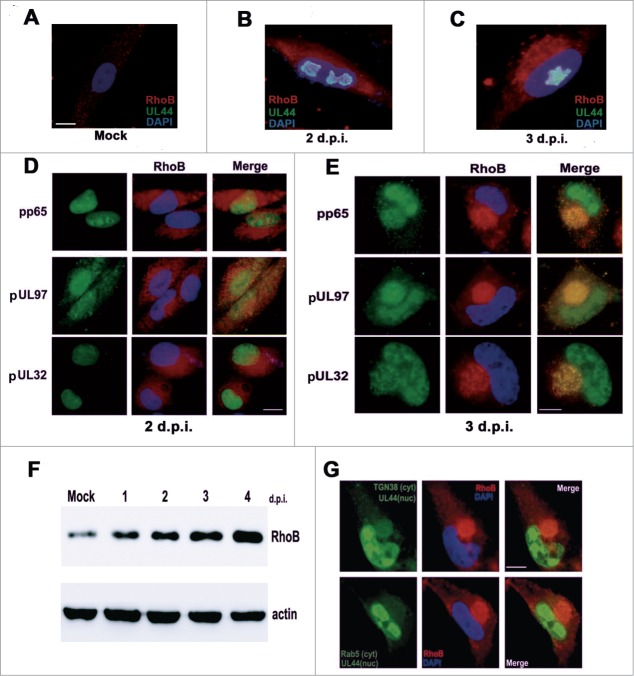
In the first series of experiments, we studied the cytoplasmic distribution of RhoB at Early and Late stages of HCMV infection in fixed cells by immunofluorescence microscopy. Infected cells were stained with the viral nuclear protein pUL44 or with the viral proteins, pp65, pUL97, and pUL32 that were used to define the cytoplasmic area that corresponded to the AC at late stages of the infection,11,50 although at earlier timepoints they localize at the nucleus.51-53 Specifically, infected fibroblasts with an MOI of 0.5 pfu/cell were fixed and stained for RhoB and each of these viral proteins 2 days post-infection (d.p.i.), prior to AC formation, as well as 3 d.p.i. when the AC was formed. RhoB underwent significant changes in the abundance and localization over the time course studied. Compared to the hardly detectable, scattered, punctuate distribution of RhoB in the cytoplasm of mock infected cells (Fig. 1A), RhoB accumulated more intensely throughout the cytoplasm in HCMV infected cells and progressively localized more strikingly close to the nucleus 2 d.p.i. (Fig. 1B and D), a phenomenon that culminated after 3 days of infection when the AC was formed. At this late stage of infection, RhoB was recruited to the assembly site and remarkably localized at the AC along with the above viral AC markers, demonstrating that RhoB is associated in this viral compartment (Fig. 1C and E). RhoB cytoplasmic translocation to the AC was accompanied by increased RhoB protein levels, as determined by western blot analysis. RhoB gradual upregulation paralleled the time course of AC formation and RhoB protein levels reached maximum levels 4 d.p.i., when the AC was well-formed (Fig. 1F). A positive and a negative cellular AC marker, the trans-Golgi Network marker, TGN-38, and the early endosomal marker, Rab5, respectively, were also employed to further study the subcellular localization of RhoB in infected cells. RhoB clearly showed the same distribution pattern with the AC marker, TGN-38, in HCMV-infected cells (Fig. 1G upper panel). On the contrary, the early endosomal protein, Rab5, exhibited limited subcellular association with the AC-recruited RhoB, as Rab5 was rather scattered in the cytoplasm than localized in the AC, exclusively (Fig. 1G lower panel). The same pattern of subcellular association has been observed among several TGN markers, Rab5 and HCMV AC proteins6,7,11,54 corroborating the specificity of RhoB localization in the assembly site of the virus.
Figure 1.
RhoB is upregulated upon HCMV infection and localizes at the AC in HCMV-infected cells. HFFs were either mock infected (A) or infected with HCMV AD169 at MOI = 0.5 pfu/cell. (B and C) RhoB cytoplasmic expression pattern in HCMV infected cells 2 d.p.i. and 3 d.p.i., respectively. HFFs were stained for the viral nuclear protein pUL44 (green) and RhoB (red). Nuclei were stained with DAPI. (D and E) HCMV-infected cells were fixed either 2 d.p.i. or 3 d.p.i. and they were immunostained for the viral AC markers pp65, pUL97, pUL32 and for RhoB, followed by incubation with a mouse-specific Alexa Fluor 488 conjugate (green, viral proteins) or a rabbit-specific Cy3 conjugate (red, RhoB) and DAPI. (F) Kinetics of RhoB expression during the course of HCMV infection determined by western blot. (G) Subcellular localization of RhoB (red) in association with the cytoplasmic (cyt) distribution of the trans-Golgi Network marker TGN-38 (green, upper panel) and Rab5 (green, lower panel) 3 d.p.i. The cells were also co-stained for pUL44 (green) as a nuclear (nuc) marker of infection progress. (bar: 10 μm).
Live cell imaging of AC formation and RhoB AC recruitment
The results on fixed cells indicating that a considerable proportion of RhoB progressively translocates to the AC, prompted us to study the dynamics of this phenomenon in live infected cells. HFF cells were electroporated with the retroviral vector mRFP-RhoB that expresses RhoB fused to the red fluorescent molecule, mRFP and infected with the recombinant virus UL32-EGFP-HCMV-TB40 expressing the capsid-associated tegument protein pUL32 fused to EGFP. Immunogold labeling of EGFP and subsequent electron microscopy using this recombinant virus has proven that the EGFP signal represents true virion particles and does not originate from EGFP polypeptides while the kinetics and the proper localization of pUL32 by newly synthesized HCMV particles during the late stages of infection using this recombinant virus have been successfully shown in HFF infected cells.51 However, upon electroporation of HFFs, we observed a slight delay regarding the timeframe of expression of viral proteins in infected cells. Time-lapse microscopy was carried out by sequential photography of infected fibroblasts cultures in an inverted fluorescence microscope. In uninfected cells transiently expressing mRFP-RhoB, the exogenous RhoB was evenly distributed throughout the cytoplasm, slightly more intense around the nucleus (Fig. 2A, a). Images captured from live cells infected with UL32-EGFP-HCMV-TB40 (MOI=5) showed that a subset of both RhoB and pUL32 protein were recruited at the AC, at late stages of the infection (Fig. 2A, b–d). The remainder fraction of pUL32 protein was observed in the nucleus (Fig. 2A, b) while both proteins were also observed all over the cytoplasm of the infected cells (Fig. 2A, b–d).
Figure 2.
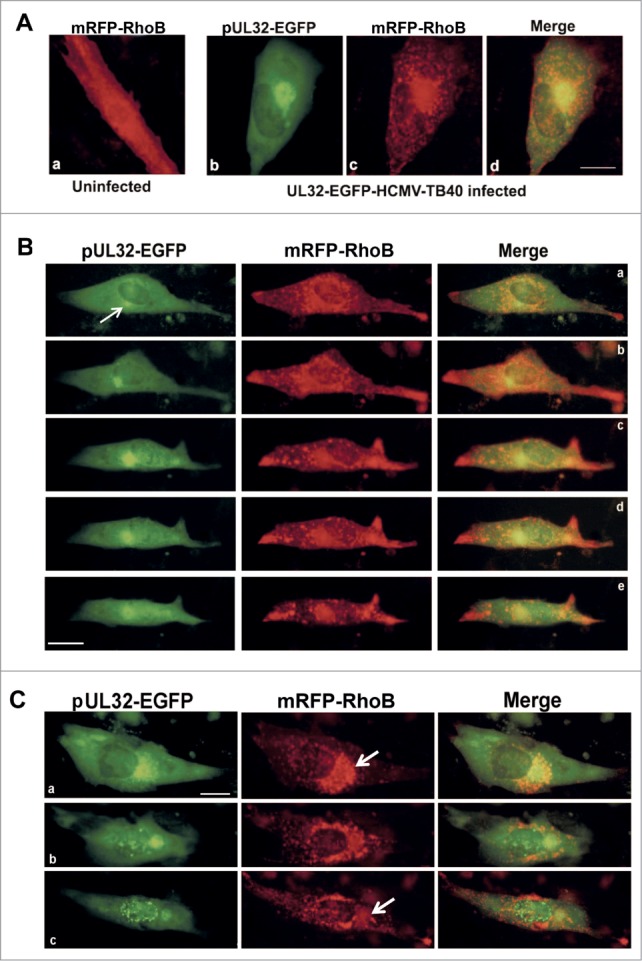
Dynamic recruitment of RhoB at the viral AC in live HCMV infected fibroblasts and cytoplasmic translocaion of RhoB and pUL32 at late stages of infection. (A) Cellular distribution of RhoB following transfection with the mRFP-RhoB plasmid in a live non-infected cell (a). Live human fibroblasts transiently expressing mRFP-RhoB were infected with the UL32-EGFP-HCMV-TB40 virus at MOI=5 pfu/cell and the image was captured 5 d.p.i. (b–d). (B) Live HFFs as above were monitored by time-lapse microscopy and images were obtained every 6 hours, initiating at 108 hours post-infection (h.p.i.). Presented are selected double-labeled images that illustrate the dynamic recruitment of RhoB at the pUL32-containing AC. Arrow indicates the position of the HCMV AC. (C) Visualization of RhoB (red) and pUL32 (green) translocation in the cytoplasm of a single live-infected cell similarly transfected and infected as in A and B, above, at 6 d.p.i. (a), 7 d.p.i (b) and 8 d.p.i. (c) Arrows indicate the HCMV AC site (bar: 10 μm).
In HCMV-infected cells, RhoB exhibited a dynamic rearrangement throughout the course of the infection compared to uninfected cells. RhoB cytoplasmic accumulation was first more striking around and adjacent to the nucleus (Fig. 2B, a). The initiation of AC formation became evident by the appearance of a small green fluorescent focus representing the pUL32 viral protein and conceivably newly synthesized pUL32-containing virions, adjacent to the nucleus that progressively increased in size (Fig. 2B, a, see arrow and b–c). Concomitantly, the nascent AC was surrounded by a cytoplasmic accumulation of RhoB that progressively became more compact and shortly, a significant proportion of RhoB was localized along with the pUL32 at the HCMV AC (Fig. 2B, c–e). RhoB distribution at the AC followed the dynamic changes regarding the shape of the AC (Fig. S1A and B). RhoB cytoplasmic localization was initially more striking at the AC (Fig. 2B, c–e), but progressively accumulated at the periphery of the cells at late stages of the infection (Fig. 2B, b–e and Fig. S2). Interestingly, detailed microscopic analysis showed that after the formation of the AC, a proportion of exogenous RhoB seemed to leave the AC and it was found in the cytoplasmic area outside the AC, whereas a fraction of RhoB remained at the AC (Fig. 2C, arrows). EGFP foci representing pUL32-containing newly synthesized virions were also observed in the cytoplasmic area between the AC and the cell membrane at late stages of infection. Viral particle movements at this stage of the infection were extensively presented in a previous study51 and it would be of interest to correlate RhoB dynamic cytoplasmic distribution and the movement of virions toward cell periphery in a future study.
RhoB localization and actin organization in cellular projections at late stages of HCMV infection
Live cell microscopy experiments revealed a dynamic redistribution of RhoB during the course of infection. Indeed, monitoring single-infected cells for prolonged times illustrated that the initial bright perinuclear accumulations of RhoB were followed by its recruitment to the AC adjacent to the nucleus and the subsequent progressive distribution of these moving puncta through the cytoplasm, reaching at the periphery and strikingly accumulating at cellular projections, at later times of the infection.
Infected fibroblasts with the recombinant HCMV expressing pUL32-EGFP and transiently overexpressing mRFP-RhoB formed remarkable cellular projections at the final stages of infection which were often branched (Fig. 3A). Overexpressed mRFP-RhoB, apart from the AC, was also strikingly localized at the edges of these projections, while pUL32-containing HCMV particles were also found in these formations (Fig. 3A), although after detailed microscopic analysis the spatial association with RhoB was not complete. Live cell analysis showed that the protrusions in the infected cells were not formed due to passive retraction of the cells, but they were actively produced during infection. On the contrary, in live uninfected cells mRFP-RhoB was distributed evenly throughout the cytoplasm and slightly more intense around the nucleus (Fig. 2A, a). This pattern remained unaltered in mock infected cells even at incubation times longer than the timeframe of HCMV infection (Fig. S3), confirming that RhoB specifically translocates to the tips of cellular protrusions due to HCMV at late stages of the infection. The fact that HCMV infection causes accumulation of mRFP-RhoB at cellular projections in live cells was further verified with the wild-type HCMV (data not shown).
Figure 3.
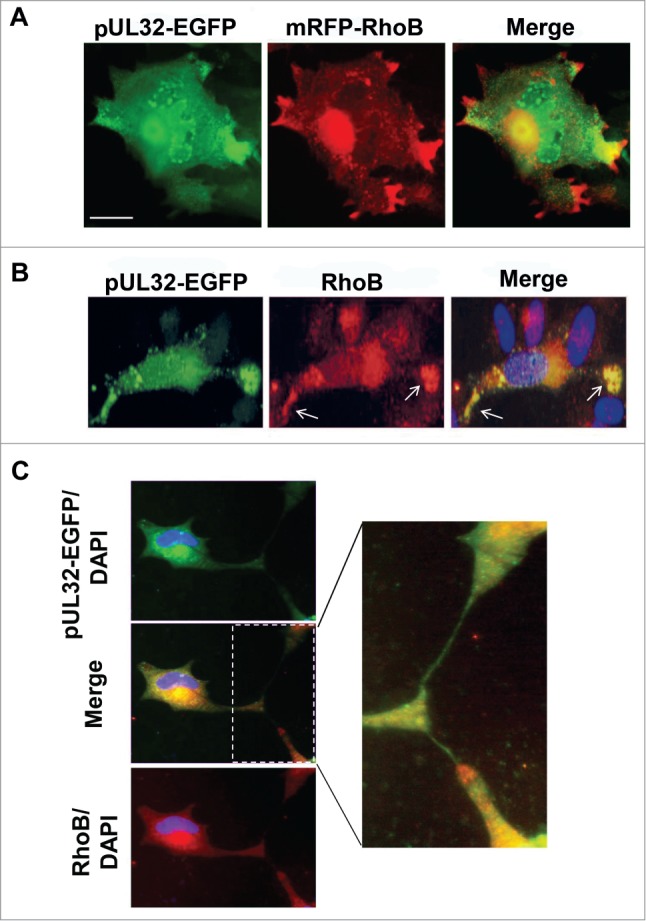
RhoB localization in cellular projections at late stages of HCMV infection. (A) Live HFFs expressing mRFP-RhoB were infected with the UL32-EGFP-HCMV-TB40 virus at MOI=5 pfu/cell. Images were obtained by timelapse microscopy 11 d.p.i. (B) Striking localization of the endogenous RhoB at the edges of cellular projections (arrows) containing pUL32-EGFP labeled virus particles at very late stages of HCMV infection. (C) The endogenous distribution of RhoB was visualized in fixed cells by indirect immunofluorescence. The enlargement illustrates the intimate contacts established between UL32-EGFP-HCMV-TB40 infected cells in non-confluent cell cultures. Nuclei were stained with DAPI. (bar: 10 μm).
Similar localization of endogenous RhoB in cellular projections was observed in UL32-EGFP-HCMV-TB40-infected cells (Fig. 3B). These projections were also found to contain numerous pUL32-EGFP puncta representing HCMV capsids.51 It was observed that both endogenous RhoB and pUL32-EGFP-containing virions, occasionally accumulated in cellular projections (Fig. 3B, see arrows). In non-confluent cell cultures, these cell projections established intimate contacts with neighboring cells (Fig. 3C, enlargement).
Staining of similarly infected cells with rhodamine-labeled phalloidin, showed that compared to mock infected cells (Fig. 4a), they formed wide cellular projections that contained elongated actin stress fibers along with the releasing pUL32-EGFP virions (Fig. 4b–g). Detailed microscopic analysis revealed that the intense stress fibers were exclusively located at the stripe sides of each cellular projection, whereas the actin cortex was depleted at the projection tips and actin was locally reorganized at the egress sites, forming a blind edge (Fig. 4, enlargements e–g). Thus, the formation of the aforementioned actin-containing cellular projections combined with the dynamic translocation of RhoB and the capsid-associated tegument protein, pUL32, suggests a potential role in the process of enhancing the intercellular and/or intracellular viral spread, a phenomenon that would be very interesting to further study with more detailed experiments.
Figure 4.
Cellular projections containing elongated actin stress fibers enclose accumulations of new virions at late stages of HCMV infection. HFFs were either mock infected (a) or infected with the UL32-EGFP-HCMV-TB40 virus at MOI = 5 pfu/cell and fixed 8 d.p.i. (b–d). Actin fibers were stained with rhodamine-labeled phalloidin and the nuclei were stained with DAPI. The enlargements (e–g) show in detail the stress fibers formed, surrounding pUL32-EGFP labeled virions. (bar: 10 μm).
RhoB localizes at the AC in the absence of late viral proteins from the AC and affects their recruitment to the AC
The spatial-temporal localization of the RhoB GTPase in the HCMV AC raised the question whether RhoB attracts the viral proteins to the AC or whether the viral proteins forming the AC recruit RhoB to the AC. To address this issue, HFFs were either mock infected or infected with HCMV AD169 and either treated with the antiviral drug, ganciclovir (GCV), that inhibits viral DNA synthesis or with the drug solvent. Cells were fixed 4 d.p.i. and stained for the viral AC markers, pp65 or pUL32, as well as for RhoB (Fig. 5). Treatment of mock infected cells with ganciclovir did not affect the localization of endogenous RhoB (Fig. 5b, c, k, l). The immunofluorescence analysis illustrated that despite the failure of recruitment of the above viral proteins and presumably and other viral proteins to the AC and the lack of formation of a typical HCMV AC containing viral proteins in the GCV-treated cells, (Fig. 5g and p), RhoB retained its ability to translocate to the cytoplasmic area, in a spherical structure juxtaposed to the nucleus, apparently corresponding to the AC (Fig. 5h and q). Therefore, in infected cells RhoB translocates to the AC, regardless the presence or absence of the antiviral drug GCV and regardless the presence (Fig. 5, d–f and m–o) or absence (Fig. 5, g–i and p–r) of the viral AC proteins at the AC.
Figure 5.
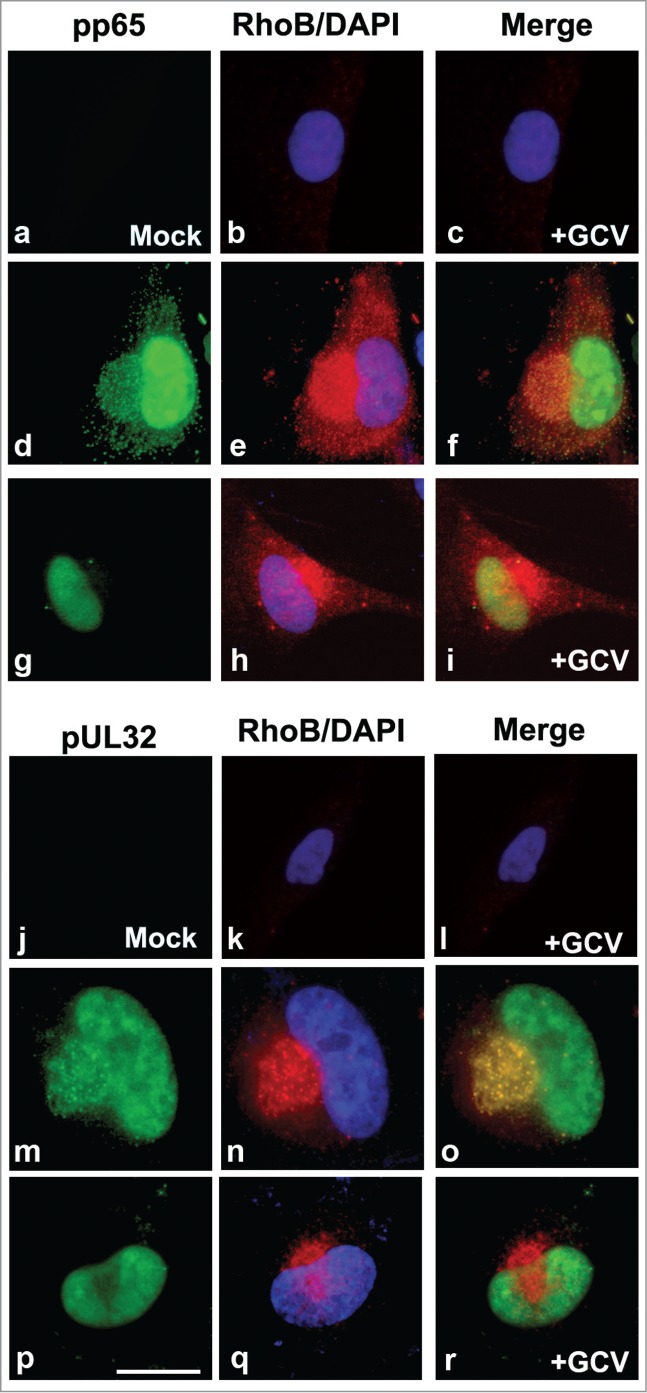
In HCMV infected cells, RhoB localizes at the AC in the absence of late viral proteins from the AC. HFFs were either mock infected and treated with ganciclovir (GCV) (a–c and j–l) or were infected with HCMV AD169 at MOI = 0.5 pfu/cell and either treated with the drug solvent (d–f and m–o) or with GCV (g–i and p–r). Cells were fixed 4 d.p.i. and immunostained for the viral AC markers, pp65 or pUL32 (green), as well as for RhoB (red). Nuclei were stained with DAPI. (bar: 25 μm).
To address the reverse scenario, HFFs were transduced with the doxycycline-inducible lentiviral vector, TRIPZshRhoB that targets RhoB and subsequently infected with the UL32-EGFP-HCMV-TB40 virus. A doxycycline-inducible TRIPZshRNA lentiviral non-targeting (scrambled) vector (named TRIPZshscr) was also used, serving as a negative shRNA control vector. Although the shRNA cassette, inducibly expressed by the lentiviral TRIPZshRhoB vector, effectively downregulated RhoB expression as detected by western blotting in total cell extracts, immunofluorescence microscopy showed that individual cells could still express residual RhoB protein which was localized at the AC in the setting of infection (data not shown). Apparently, the remaining protein after the imperfect RhoB depletion is still capable of being recruited at this viral compartment. When RhoB was knocked down upon the doxycycline-induction of the shRNA expression, we could observe cells in which pUL32-EGFP did not localize at the AC or cells in which the pUL32-EGFP accumulation at the AC was affected 5 d.p.i. (Fig. 6A, upper panel), whereas at the same timeframe, non-induced (−Dox) cells (Fig. 6A, middle panel) or cells expressing the shscr control vector (Fig. 6A, lower panel) exhibited a typical AC, confirming the specificity of the effect in RhoB-silenced cells. That was also the case when similarly lentiviral transduced cells as described above, were stained for pp65 (Fig. 6B) or pUL97 (Fig. 6C), 5 days after HCMV AD169 infection. The above observations provide evidence that RhoB is not only associated with the AC, but even more, it is also an important constituent, contributing to the formation of the viral AC.
Figure 6.
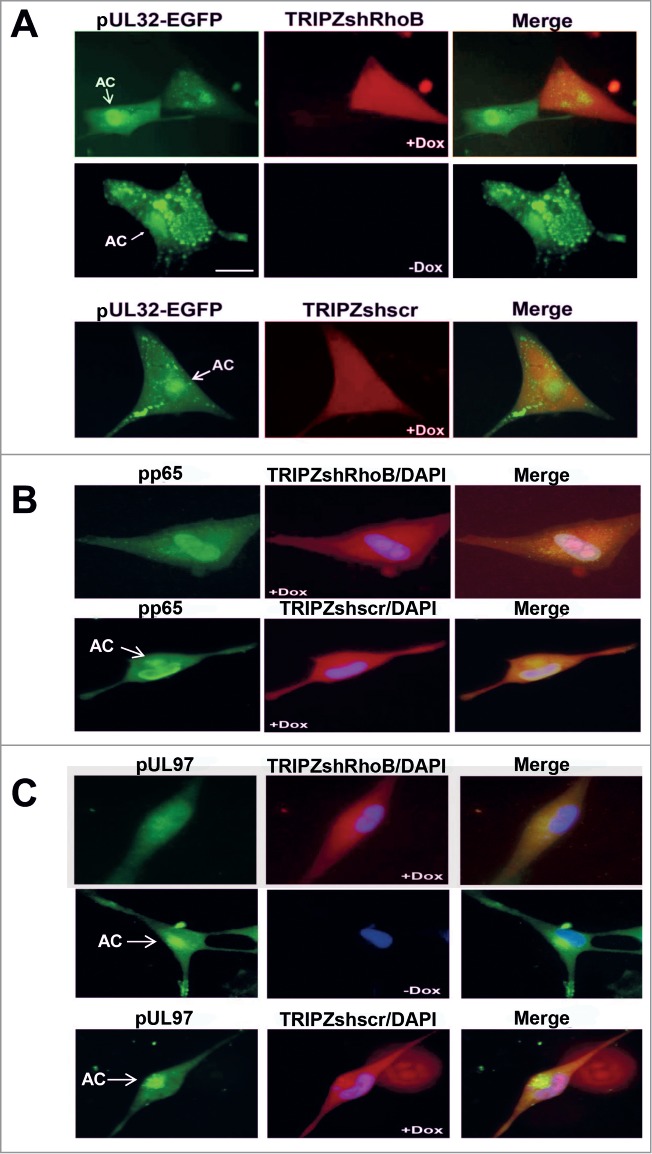
The accumulation of several viral proteins at the AC is affected in RhoB-depleted cells. HFFs were transduced with the lentiviral vector, TRIPZshRhoB, and the expression of the RhoB shRNA cassette was either induced by treatment with doxycycline (+Dox) or not (−Dox). The scrambled shRNA TRIPZ lentiviral vector (TRIPZshscr) was used as a negative control. Doxycycline-treated TRIPZshRNA-expressing cells exhibited red fluorescence. The lentiviral-transduced and doxycycline-treated cells, as well as lentiviral-transduced untreated control cells, were infected either with the UL32-EGFP-HCMV-TB40 virus at MOI = 5 pfu/cell and images were captured 5 d.p.i. (A) or with HCMV AD169 at MOI = 0.5 pfu/cell and stained for pp65 (B) or pUL97 (C) 5 d.p.i. The arrows indicate the localization of the AC. Nuclei in fixed cells were stained with DAPI. (bar: 10 μm).
Depletion of RhoB results in reduction of progeny virion production
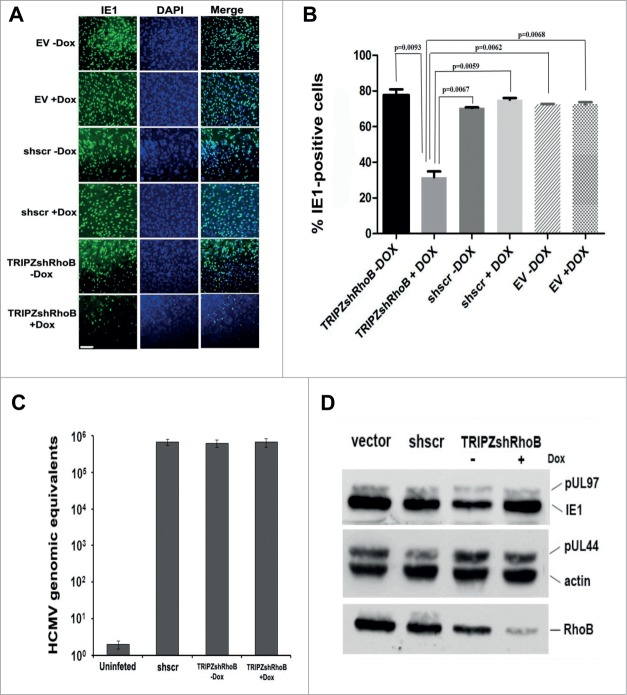
To explore the overall effect of RhoB on the viral yield, we used the above described shRNA approach. HFFs were first transduced either with the doxycycline-inducible lentiviral vector, TRIPZshRhoB, in order to knockdown RhoB or control vectors [empty vector (EV) or shRNA scramble vector (shscr) in the presence or absence of Dox] followed by HCMV AD169 infection. Supernatants from all HCMV-infected cells were harvested 5 days after infection and titrated on fresh HFFs by IE1 fluorescence, combining 2 previously described approaches.55,56 The percentage of the IE1-positive cells was statistically significantly reduced in the fresh HFFs infected with supernatants of the RhoB-depleted cells, compared to the ones infected with the supernatants of the control non-induced TRIPZshRhoB fibroblasts (Fig. 7A and B). A similar statistically significant decrease in the number of IE1-expressing cells derived from the RhoB-silenced cells was evident when they were compared to the IE1-positive cells that corresponded to the EV or shscr-transduced HCMV-infected cells, either in the presence or absence of doxycycline (Fig. 7B). Therefore, the knockdown of RhoB results in a profound decrease in the viral yield, suggesting a key role of RhoB in HCMV production.
Figure 7.
Depletion of RhoB results in the reduction of HCMV progeny virion production. (A) HFFs were first transduced with either the doxycycline-inducible lentiviral vector, TRIPZshRhoB, or control vectors [empty vector (EV) or shRNA scramble vector (shscr)], in the presence or absence of doxycycline. The transduced cells were subsequently infected with HCMV AD169 at MOI = 0.5 pfu/cell. Supernatants from all lentiviral transduced HCMV infected cells were harvested 5 days after infection, fresh HFFs were subsequently superinfected with these supernatants, and fixed and stained for viral IE1 expression 24 hours later. Cell nuclei were counterstained using DAPI. (bar: 1 μm). (B) IE1 gene expression of fresh superinfected HFFs described in (A) was analyzed by counting representative microscopy fields. A minimum of 500 cells were counted for each vector from each of 3 independent experiments. The percentage of IE1-positive cells was quantified and statistical analysis was performed by One-Way Anova. Erros bars, SD. Genomic and viral DNA and total protein extracts were examined by qPCR (C) and western blotting using the indicated antibodies (D) in similarly transduced and HCMV infected cells as in (A)
Reduction of the viral yield may be due to a block in virus entry provoked by the knockdown of RhoB. To address this issue, HFFs that had been transduced with the shRNA scramble or with the TRIPZshRhoB vector in the presence or absence of doxycycline, were infected with wild-type HCMV and the cell-associated virus was assessed 6 h.p.i. Real-time PCR analysis revealed that the number of the viral genomes that had infected the cells was comparable, irrespective of RhoB expression levels (Fig. 7C), confirming the efficient viral entry in the RhoB-depleted cells.
Decreased progeny virus was not due to an inhibition in viral protein synthesis either, as indicated by a western blot analysis of the viral proteins, IE1, pUL44 and pUL97. Regardless of the expression levels of endogenous RhoB (normal or knockdown), the expression of the above viral proteins, representing the immediate-early, early and late viral gene expression, respectively, was not affected (Fig. 7D).
Actin reorganization at the AC in HCMV-infected cells
RhoB has been reported to regulate actin fiber structure.57,58 Normal fibroblasts have a rather impressive actin filament network. Exploring the effect of RhoB depletion on the cytoskeleton by transducing HFFs with the lentiviral vector, TRIPZshRhoB, we observed a robust modification in the alignment of stress fibers, from the parallel-running actin fibers in the normal fibroblasts (Fig. 4a) to a random orientation of the fibers where a subset of bundles acquired a vertical direction to the main axis in the RhoB-depleted cells (Fig. S4A). The organization of actin cytoskeleton remained unaffected in cells transduced with the TRIPZshscr control vector (Fig. S4B), confirming that the pronounced change in the organization of actin filaments is specifically due to the genetic silencing of RhoB.
In contrast to the fine structure of actin cytoskeleton in mock infected human fibroblasts (Fig. 4a), shortly upon HCMV infection, actin fibers are disrupted and this has been reported to enhance the translocation of the incoming viral particles to the host nucleus and hence it is to the benefit of the virus.44,46,59,60 However, the fate of actin cytoskeleton at late stages of the infection has not been thoroughly studied so far. To this end, HFFs were infected with wild-type HCMV and co-stained for the cytoplasmic protein, RhoB, as well as for actin using rhodamine-phalloidin. Additionally, the large replication compartments formed at the nucleus containing pUL44, served as markers of advanced viral infection. The destructive effect of the virus on the actin cytoskeleton was still apparent 2 days after infection, when the infected cells showed less stress fiber formation compared to their mock-infected counterparts (Fig. 8). However, a progressive reconstitution of actin filaments initiating 3 d.p.i. and sustaining for several days was evident, predominantly at the site of the AC. In particular, newly synthesized actin filaments were formed, initially relatively short and progressively longer and branched, remarkably originating from a juxtanuclear location corresponding to the core of the viral AC. Thus, the formation of the AC seems to induce the reorganization of the actin network within the host cell probably facilitating HCMV assembly and likely the subsequent steps of virion migration and/or egress.
Figure 8.

Actin reorganization at the AC in HCMV infected cells. Human fibroblasts were infected with HCMV AD169 at MOI = 0.5 pfu/cell. Cells were fixed at the indicated time points after infection and stained for pUL44 (nuclear) and RhoB (cytoplasmic) proteins. Actin fibers were visualized by direct immunofluorescence using rhodamine-labeled phalloidin. Nuclei were stained with DAPI (bar: 10 μm).
Disassembly of actin fibers does not disperse the AC but reduces the viral yield
Taking into account that RhoB can affect actin structure in fibroblasts and also that actin filaments are reorganized at the AC of infected cells and at cellular projections at late times of infection, we tested whether the reduction in the viral yield upon RhoB knock down may be associated with alterations in actin fibers organization induced by RhoB depletion. HFFs were infected with HCMV AD169 and on day 5 p.i. the cell culture medium was removed, fresh medium was added and the cells were treated for 1 hour with Cytochalasin B, a potent disruptor of actin filaments. Immunofluorescence analysis showed that despite the compromised actin cytoskeleton after the Cytochalasin B treatment, the viral AC was not dispersed, as indicated by the recruitment of either RhoB or pp65 in this characteristic compartment (Fig. 9A, b, c and e). In contrast, the destruction of the microtubule network by nocodazol for 1 hour severely affected the RhoB localization at the AC (Fig. 9A, f), confirming that the integrity of the microtubule network is critical for the maintenance of the AC50 and that RhoB is a component of this viral compartment.
Figure 9.
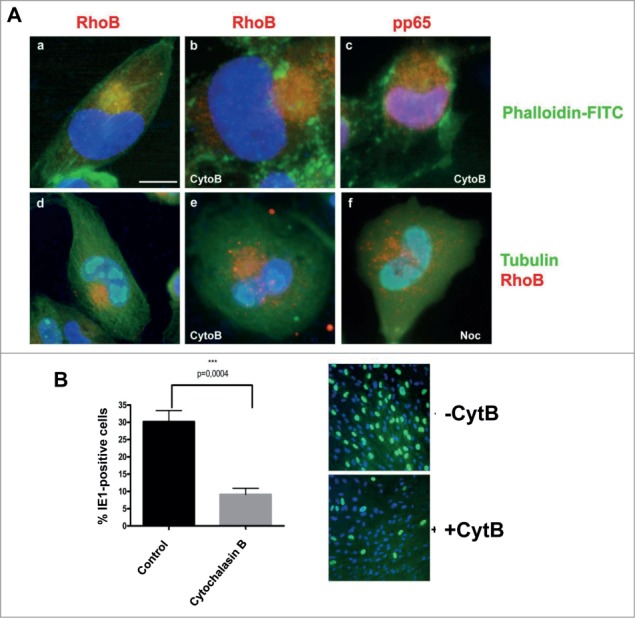
Disassembly of actin fibers does not dramatically disperse the viral AC but reduces the viral yield. (A) HCMV AD169-infected fibroblasts, 5 d.p.i., were treated either with Cytochalasin B (CytoB, b, c, e) or with nocodazol (Noc, f) or the corresponding organic solvent (a, d) for 1 hour. The cells were then fixed and stained for RhoB (a, b, d–f), pp65 (c) and α-tubulin (d–f). Actin filaments were stained with FITC-labeled phalloidin (a–c) while nuclei were stained with DAPI. (bar: 10 μm). (B) Supernatants harvested from the above Cytochalasin B-treated and control cells were quantified on fresh HFFs by IE1 fluoresence, counting representative microscopy fields, examining more than 500 cells from each of 3 independent experiments. The percentage of cells expressing IE1 was quantified and statistical analysis was carried out by One-Way Anova. Error bars, SD.
Titration of supernatants harvested from the above Cytochalasin B-treated cells was performed by IE1 fluorescence, as descibed above and showed a statistically significant reduction of virus titer compared to the non-treated cells (Fig. 9B), suggesting that although the gross structural integrity of the AC was likely retained, in terms of several of its components, its functionality might have been affected. Reduction of viral titer upon Cytochalasin B treatment implies that actin cytoskeleton could play a role during viral assembly and/or egress. Other mechanisms that remain to be elucidated in future studies may also contribute.
Discussion
The assembly of new HCMV virions is a complex multi-step process that initiates in the nucleus of the infected cell and progresses at the juxtanuclear cytoplasmic assembly compartment, which is an interface of many cellular and viral proteins, important for mature virion formation. RhoB was recruited at the HCMV AC along with the viral proteins, pp65, pUL32, pUL97 and pUL99 (Figs. 1E, 2A and B, and Fig. S1B). As previously described,51,52,61 we detected the pUL32 protein both at the nucleus and the cytoplasm of the infected cells at late stages of the infection. Nuclear signal was present prior to the AC formation in agreement with previous studies,51,52 however in our study, a more intense fluorescence nuclear signal was observed in fixed than in live infected cells (Fig. 1 and Fig. 2), probably due to different reagents used. Moreover, viral protein pp65 has been reported to initially localize at the nucleus of the infected cells and at later times of infection both at the nucleus and the cytoplasm, at the viral AC compartment.11,50,52 Upon GCV treatment, residual pUL32 and pp65 proteins that are expressed,62,63 are restricted to their nuclear localization (Fig. 5, g,i and p,r). The failure of viral proteins to localize at the AC after inhibition of viral DNA synthesis by GCV, is in concert with previous studies using anti-HCMV molecules.13,64 Interestingly, RhoB was still able to be recruited at the HCMV AC, even when the Late phase of the viral life-cycle was inhibited by GCV and proper AC was not formed (Fig. 5, h–i and q–r). The active translocation of RhoB at a cellular compartment which normally hosts the viral AC, even if the AC is finally not configured, further supports an important structural role of RhoB in the formation of this viral zone. The signaling pathways dictating the dynamic localization of RhoB toward the viral AC remains to be elucidated in future studies.
The striking difference regarding RhoB cytoplasmic distribution among uninfected or mock infected cells (Figs. 1A, 2A, a and Fig. S3) and HCMV-infected cells (Figs. 1B–E, 2, 3, and Figs. S1 and S2) is accompanied by a progressive upregulation of RhoB protein levels over the course of the infection (Fig. 1F). This is of interest, as it has been reported that the cellular proteins, CD63, TGN46 and CD-M6PR, that also localize at the AC are downregulated in infected cells and are incorporated in new virions.7
During the formation of the AC, extensive cytoplasmic remodeling occurs, resulting in dramatic reorientation of the secretory machinery, including the early endosomes.6,7,10,14 Although RhoB has been reported to localize to early endosomes,20 it is not fully spatially associated with the early endosome protein Rab565,66 in HCMV-infected cells (Fig. 1G lower panel). This pattern of partial subcellular association between RhoB and Rab5 is in agreement with the fact that Rab5 only partially co-localizes with the viral AC protein gB11 and does not precisely designate the viral AC.6 Moreover, limited colocalization between Rab5 and the early endosome protein EEA1 has also been observed in HCMV-infected fibroblasts, whereas EEA1 localizes at the AC.6 On the contrary, in HCMV infected cells RhoB shows the same distribution pattern as the TGN marker, TGN-38 (Fig. 1G upper panel), an observation in line with the fact that other TGN markers, such as TGN-46 and p230, also localize at the AC.6,7,11,50,54
Live cell imaging using an autofluorescent RhoB construct, mRFP-RhoB, and the recombinant UL32-EGFP-HCMV-TB40 virus, not only confirmed the observations from the experiments in fixed cells but additionally shed light into the dynamics of RhoB spatial localization in association with the pUL32 tegument protein at the late stages of HCMV infection. Apart from its marked upregulation, RhoB is also relocated during viral infection, changing from an initial cytoplasmic, relatively scattered distribution to a striking recruitment to sites where new virions are assembled, along with structural HCMV proteins, such as pUL32 (Fig. 2A and B). The RhoB spatial relocalization during HCMV infection is reminiscent of another small GTPase, the Rab27a redistribution, a regulator of lysosome-related organelle transport,67 suggesting that HCMV advantageously manipulates the secretory apparatus, resulting in its extensive but coordinated reconfiguration to facilitate viral assembly and maturation. Interestingly, RhoB has been reported to also localize to a pre-lysosomal compartment.20 Moreover, using the same experimental system, it became evident that RhoB migrates within the cytoplasm (Fig. 2C), from the HCMV AC toward the tips of cell projections (Fig. 3A and Fig. S2). This finding provides the first evidence of a regulatory role of RhoB in the cytoplasmic viral maturation or trafficking pathway.
The critical role of RhoB in HCMV life cycle was further illustrated by the compromised accumulation of viral proteins at the AC in a proportion of HCMV infected RhoB-knockdown cells (Fig. 6), followed by the substantial reduction of the progeny virus in RhoB-depleted cells (Fig. 7). Previous studies have either tested the role of Rho GTPase, RhoA43 or the role of Rho-associated protein kinases (ROCK) using specific inhibitors48,68,69 however, focusing mainly at the very early stages of HCMV infection. Rab genes, another family of small Ras-like GTPases whose products control membrane traffic, are differentially regulated during HCMV infection70 and Rab members, such as Rab3, Rab6, Rab11 and Rab27a have been shown to be essential for viral assembly and successful viral production.11,67,71,72 Immunofluorescence analysis in normal HCMV infected fibroblasts showed that pp65 was not present in all viral ACs (data not shown), a fact which is in agreement with previous studies reporting that pp65 serves as an optional scaffold protein for virion formation.73 The same was also true for pUL97 (data not shown). Moreover, in RhoB-knockdown HCMV infected cells, residual RhoB could be detected at the AC by immunofluorescence (data not shown). When staining HCMV infected RhoB-knockdown cells with the above viral AC-markers or monitoring live cells infected with the UL32-EGFP-HCMV-TB40 virus and after excluding the cells without any apparent viral protein cytoplasmic accumulation indicative of AC formation, we observed a proportion of cells with less and improper accumulation of viral AC-markers at this subcellular compartment in comparison to either non-transduced cells or to control TRIPZshscr transduced cells (Fig. 6). Our microscopy analysis provides good arguments for a role of RhoB as an important component of the viral AC, contributing to the architecture of this structure. This finding is further supported by the dynamic adjustment of RhoB cytoplasmic distribution to changes in the shape of the AC (Fig. S1) and by the fact that nocodazole, which is known to disperse the viral proteins from the AC, exerts the same effect on RhoB (Fig. 9A, f).
Our functional experiments silencing RhoB demonstrated a statistically significant reduction in the number of secreted infectious viral particles in RhoB-deficient cells (Fig. 7A and B). RhoB is mainly localized to the cytoplasmic face of the endosomal membranes20,74 and shows unique functions in the control of endocytic traffic.25 Several studies have provided a better understanding of the role that endosomal compartments play during HCMV maturation, both at the structural and the functional level. There is current evidence supporting that secondary viral envelopment takes place within early endosomal compartments by budding into endosomal vesicles, which fuse with the plasma membrane to release virions to the extracellular medium.6,8,9,75,76 It has also been suggested that HCMV acquires its final envelop by budding into Golgi-derived vacuole compartments positive for TGN-46, Rab3, gB and Mannosidase II11 and that TGN perhaps contributes to final envelopment.6,50 Considering the reduced progeny production after the depletion of RhoB, we propose that HCMV exploits the cellular machinery involved in endosomal function for its assembly. This result identifies RhoB as a player in HCMV production, further supporting a relationship between HCMV and endosomes. RhoB, among other Rho GTPases, is a major determinant of vesicular trafficking in the cytoplasm and thus, it is appealing to speculate that HCMV viral secretion may be regulated by RhoB.
Shortly upon HCMV infection, host actin filaments are disrupted43,45,46,59,68,77 and it has been described that there are 3 transient phases of actin depolymerization that occur at 20 min, 5 to 10 h and 48 to 72 h.p.i.78 This phenomenon extends throughout the cytoplasm and facilitates HCMV nuclear translocation and infectivity as indicated by the fact that Cytochalasin B treatment at early stages of HCMV infection resulted in increased viral yield.46 On the contrary, in our study, a short Cytochalasin B treatment at late stages of the infection resulted in a statistically significant reduction in the virus titer during treatment (Fig. 9B), indicating a necessity for polymerized actin cytoskeleton at this phase. The phenomena that predominate at this stage of the viral lifecycle are the assembly of new virions and their egress from the host cell. Interestingly, viperin, a protein causing actin disruption upon HCMV infection, has been reported to colocalize with vMIA to the mitochondria 1 d.p.i. and is redistributed to the AC at 3 d.p.i.,44 a timeframe coinciding with actin reorganization detected in our study. The observed reorganization of actin filaments at the area of the AC (Fig. 8) and at the cellular projections of the infected cells (Fig. 4b–g), in combination with the above viral yield result upon Cytochalasin B treatment, is suggestive of an opposite role of actin in virus assembly and/or egress compared to viral entry in the host cell.
Interestingly, actin has been shown to be present in purified herpesviruses, possibly playing a structural role, although the incorporation process is not known.39 The fact that Cytochalasin B treatment does not dramatically disperse pp65 and RhoB from the AC (Fig. 9A, b, c and e) does not downgrade the importance of actin at this stage of the infection, taking into account the contribution of actin in the structure and function of cellular organelles such as the Golgi and the TGN79-81 that are manipulated by the virus during assembly and perhaps egress. Moreover, actin pattern is remarkably different in cells in which RhoB has been knocked down compared to control cells (Fig. S4), an observation leading to a similar hypothesis. Actin remodeling is also important during endocytosis, exocytosis and membrane trafficking. Taking into account the important role of endosomes during the late stages of HCMV infection, it is of particular interest that RhoB promotes actin assembly on endosomal membranes through Dia1 and regulates endosome transport.33 The progressive overexpression of RhoB observed in our experiments and particularly at the later stages of HCMV infection, could be combined with a regulatory role of this small GTPase, either directly or indirectly through Rho effectors, such as mDia or ROCK, in the formation of actin stress fibers and in a potential mechanism involving actin and endosomes during HCMV assembly and/or egress. Further experiments are now in progress in our laboratory to address this important issue.
RhoB overexpression enhances the formation of cellular projections of HCMV-infected fibroblasts (Fig. 3A). Newly synthesized virions preferably accumulate at the projections while moving from the AC toward the cellular membrane to exit the cell (Fig. 3 and Fig. 4). Interestingly, RhoB and pUL32-containing virions occasionally accumulated simultaneously at the tips of these cellular projections. Therefore, the overexpression of RhoB at late stages of HCMV infection might provide more exit routes to new virions and may facilitate viral egress. In spite of the disassembly of the actin cytoskeleton in HCMV-infected cells, the outlines of the cell projections formed during viral egress contain newly assembled actin stress fibers (Fig. 4e–g). These projections extend to neighboring cells (Fig. 3C) and similar cell projections at late stages of infection have been detected in several α-herpesviruses, such as HSV-1, VZV and PRV enhancing the intercellular viral spread (for review35,36,39,40).
In conclusion, our study demonstrates for the first time a structural and functional role of RhoB GTPase in the formation of the HCMV AC and probably in viral egress, further supporting the role of endosomal compartments in these processes. Identifying the interactions of RhoB with other viral and host AC-partners, may provide useful insight in understanding the molecular mechanisms ruling the late stages of HCMV life-cycle and for the development of novel anti-HCMV therapeutic strategies.
Materials and Methods
Cells, viruses and small molecules
Human foreskin fibroblasts (HFFs) and HEK 293T cells were cultured in Dulbecco's modified Eagle's medium with high glucose (Biosera, UK) supplemented with 10% fetal bovine serum, 100 U/ml penicillin and 100 μg/ml streptomycin. The wild-type HCMV laboratory strain, AD169, as well as the recombinant TB40 strain, UL32-EGFP-HCMV-TB4051 (kindly provided by C. Sinzger) were employed. Both viruses were propagated in HFFs and isolated by centrifugation from the supernatants when fibroblasts exhibited excessive cytopathic effects according to standard protocols. Viral pellet was resuspended in cell culture medium and virus aliquots were stored at −80°C until use. Virus titer of viral stocks was assessed by a standard plaque assay method performed on the HFFs. For viral infections, the cells were infected with HCMV at the indicated MOI, were incubated for 2 hours and then the inoculum was removed and replaced by fresh medium. Moreover, in order to compare the viral yield in different experimental conditions, supernatants from HCMV-infected cells were harvested when indicated and titrated on fresh HFFs by IE1 fluorescence, combining 2 previously described approaches.55,56 Briefly, equal volumes of each supernatant were used to infect fresh HFFs grown on coverslips, and after 24 hours incubation, they were stained for IE1. Cell nuclei were counterstained using DAPI. The percentage of infected cells (IE1-positive cells) was calculated and statistical analysis was performed to compare the viral titers.
Ganciclovir (GCV), purchased from Sigma Chemicals (St. Louis, MO), was dissolved in distilled water. Stock solutions (10mM) were stored at −80°C, while HCMV-infected HFFs were treated with GCV at a final concentration of 30 μM. Cytochalasin B was purchased from Sigma Aldrich (Deisenhofen, Germany) and dissolved in dimethyl sulfoxide at 2 mg/ml. Prior to use, it was added to the culture medium to yield a final concentration of 2 μg/ml. The microtubule-depolymerizing agent, nocodazole (Sigma Aldrich, Deisenhofen, Germany), was also diluted in DMSO and used at a final concentration of 2 μM. HCMV-infected cells were treated with either Cytochalasin B or nocodazole for 1 hour in fresh medium, 5 d.p.i. and subsequently the cells were fixed and stained.
Transient transfection
The retroviral vector, mRFP-ENDO (mRFP-RhoB), expressesing RhoB fused to the red fluorescent molecule, mRFP, was kindly provided by L. Opresco (Pacific Northwest National Lab) and was used for transfection experiments. For live cell imaging, the HFFs were transfected with the mRFP-RhoB vector using the Amaxa Nucleofector kit for primary mammalian fibroblasts according to the manufacturer's instructions (Amaxa Biosystems, Cologne, Germany). Cells were electroporated in suspension, plated in chambers with coverslip quality glass bottoms (Lab-Tek; Nunc) and infected with the recombinant virus, UL32-EGFP-HCMV-ΤΒ40, the following day.
Live cell microscopy
Time-lapse microscopy was performed as previously described.82 Briefly, the cells were seeded into 2-well, chambered coverglass units with coverslip quality glass bottoms (Lab-Tek; Nunc) at a density of 2 × 105 cells per well. Live cells were observed under a Leica DMIRE2 inverted fluorescence microscope, equipped with a Leica DFC300 FX digital camera, using a ×63 dry objective lens. To maintain cell viability for prolonged times of the experiments, the microscope stage was enclosed within a controlled environment of constant temperature, CO2 and humidity. The excitation wavelength was controlled by mercury lamp illumination and a manual filter wheel equipped with filters suitable for EGFP, the monomeric red fluorescent protein and DAPI. The camera image acquisition was controlled by IM50 software (Leica). Particular care was taken in order to avoid overlap between channels by collecting the data from each channel sequentially and routing the emission signals through appropriate band pass filters. Single images or timed image sequences were exported as TIFF files from the IM50 software.
Lentiviral production and transduction
The doxycycline-inducible TRIPZ lentiviral shRNA vector targeting RhoB, TRIPZshRhoB, (Clone ID: V2THS_172671) as well as the TRIPZshRNA lentiviral non-targeting (scrambled) vector, TRIPZshscr, (Clone ID: RHS4743) were purchased from Thermo Scientific-Dharmacon. In these vectors, turboRFP and shRNA are part of a single transcript, allowing the visual marking of shRNA-expressing cells. HEK 293T cells were co-transfected with the vectors TRIPZshRNA (expressing either shRhoB or scrambled shRNA), pCMV/VSV-G (where VSV-G is vesicular stomatitis virus protein G) and pCMV-dR8.2 dvpr. Transfection was carried out using Turbofect transfection reagent (Thermo Scientific). The pLKO.1 lentiviral empty vector (EV) or the pLKO.1scr vector, expressing a scrambled shRNA served as a negative controls. To transduce the HFFs with the packaged viruses, early passage cells were incubated with the lentiviral supernatants in the presence of 5 µg/ml polybrene (Sigma-Aldrich, Deisenhofen, Germany) for 24 hours. Forty-eight hours later, cells were selected with puromycin (2μg/ml). Transduced cells were treated with doxycycline (500ng/ml), when required, to induce shRNA expression.
Antibodies/immunofluorescence/Western Blot analysis
The HCMV immediate-early IE1 and early pUL44 gene products were detected using monoclonal antibodies against IE1 (BS500) and pUL44 (BS510), respectively, as previously described.83 The viral tegument protein pUL32 was detected using the mouse monoclonal antibody (MAb) XP1,51 the UL97 gene product was detected using the mouse polyclonal antibody #1343,84 while the UL99-encoded pp28 mouse monoclonal antibody (10B4-29) was also used (kind gift of T. Shenk, Princeton University).85 Antibodies against HCMV pp65 (sc-71229), RhoB (sc-180), TGN38 (sc-271624), Rab5 (sc-46692) and β-actin (sc-8432) serving as a loading control in western blot analysis, were purchased from Santa Cruz (Santa Cruz, CA). Rhodamine (phalloidin R415) and fluorescein phalloidin (F432) were purchased from Molecular Probes (Eugene, OR); a monoclonal antibody against tubulin (DM1A) was purchased from Sigma.
Anti-mouse, as well as anti-rabbit horseradish peroxidase-conjugated secondary antibodies were obtained from Sigma while Alexa 488 and Cy3-conjugated secondary antibodies were purchased from Molecular Probes.
For indirect immunofluorescence experiments, the HFFs were seeded on 13-mm diameter glass coverslips and infected with either HCMV AD169 or with UL32-EGFP-HCMV-TB40 at a confluency of at least 70%. Cells were fixed and stained on the day indicated for each experiment. The conditions for fixation and immunodetection of viral and cellular proteins, were as previously described.86 For microtubule staining, cells were fixed in 4% paraformaldehyde in cytoskeleton buffer (1.1 M Na2HPO4, 0.4 M KH2PO4, 137 mM NaCl, 5 mM KCl, 2 mM MgCl2, 2 mM EGTA, 5mM PIPES, 5 mM Glucose, pH 6.1) for 5 min at room temperature.
For Western blotting, extracts from infected or lentiviral transduced cells were prepared in sodium dodecyl sulfate loading buffer, separated on sodium dodecyl sulfate-containing 8–10% polyacrylamide gels, and transferred to nitrocellulose membranes. Western blotting and chemiluminescence detection were performed as previously described.87
Real-time PCR
The Nanogen Q-CMV Real-Time PCR kit (Nanogen Advanced Diagnostics,Italy) was used to quantify the HCMV genomes in combination with the ABI 7500 Fast system (ABI). The primers for HCMV are specific for the exon 4 region of the HCMV MIEA gene (major immediate early antigen, HCMV UL123) while in parallel, a region of the human β globin gene is also amplified. The limit of detection of the assay is approximately 0.25log10 copies/ml. All reactions were performed in triplicate, following the manufacturer's instructions.
Disclosure of Potential Conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank C. Sinzger (Ulm, Germany) and B. Plachter (Mainz, Germany), M. Marschall (Erlangen, Germany) and D.J. Bigelow (Richland, WA) for kindly providing reagents.
Funding
This project was funded by The National Plan for Science, Technology, and Innovation (MAARIFAH), King Abdulaziz City for Science and Technology, Kingdom of Saudi Arabia (Award number 12-MED2652-02).
Supplemental Material
Supplemental data for this article can be accessed on the publisher's website.
References
- 1.Boeckh M, Geballe AP. Cytomegalovirus: pathogen, paradigm, and puzzle. J Clin Invest 2011; 121:1673-80; PMID:21659716; http://dx.doi.org/ 10.1172/JCI45449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bate SL, Dollard SC, Cannon MJ. Cytomegalovirus seroprevalence in the United States: the national health and nutrition examination surveys, 1988–2004. Clin Infect Dis 2010; 50:1439-47; PMID:20426575; http://dx.doi.org/ 10.1086/652438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fowler KB, Stagno S, Pass RF, Britt WJ, Boll TJ, Alford CA. The outcome of congenital cytomegalovirus infection in relation to maternal antibody status. N Engl J Med 1992; 326:663-7; PMID:1310525; http://dx.doi.org/ 10.1056/NEJM199203053261003 [DOI] [PubMed] [Google Scholar]
- 4.Hyde TB, Schmid DS, Cannon MJ. Cytomegalovirus seroconversion rates and risk factors: implications for congenital CMV. Rev Med Virol 2010; 20:311-26; PMID:20645278; http://dx.doi.org/ 10.1002/rmv.659 [DOI] [PubMed] [Google Scholar]
- 5.Landolfo S, Gariglio M, Gribaudo G, Lembo D. The human cytomegalovirus. Pharmacol Ther 2003; 98:269-97; PMID:12782241; http://dx.doi.org/ 10.1016/S0163-7258(03)00034-2 [DOI] [PubMed] [Google Scholar]
- 6.Das S, Vasanji A, Pellett PE. Three-dimensional structure of the human cytomegalovirus cytoplasmic virion assembly complex includes a reoriented secretory apparatus. J Virol 2007; 81:11861-9; PMID:17715239; http://dx.doi.org/ 10.1128/JVI.01077-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cepeda V, Esteban M, Fraile-Ramos A. Human cytomegalovirus final envelopment on membranes containing both trans-Golgi network and endosomal markers. Cell Microbiol 2010; 12:386-404; PMID:19888988; http://dx.doi.org/ 10.1111/j.1462-5822.2009.01405.x [DOI] [PubMed] [Google Scholar]
- 8.Das S, Pellett PE. Spatial relationships between markers for secretory and endosomal machinery in human cytomegalovirus-infected cells versus those in uninfected cells. J Virol 2011; 85:5864-79; PMID:21471245; http://dx.doi.org/ 10.1128/JVI.00155-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tandon R, AuCoin DP, Mocarski ES. Human cytomegalovirus exploits ESCRT machinery in the process of virion maturation. J Virol 2009; 83:10797-807; PMID:19640981; http://dx.doi.org/ 10.1128/JVI.01093-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tandon R, Mocarski ES. Viral and host control of cytomegalovirus maturation. Trends Microbiol 2012; 20:392-401; PMID:22633075; http://dx.doi.org/ 10.1016/j.tim.2012.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Homman-Loudiyi M, Hultenby K, Britt W, Soderberg-Naucler C. Envelopment of human cytomegalovirus occurs by budding into Golgi-derived vacuole compartments positive for gB, Rab 3, trans-golgi network 46, and mannosidase II. J Virol 2003; 77:3191-203; PMID:12584343; http://dx.doi.org/ 10.1128/JVI.77.5.3191-3203.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sanchez V, Sztul E, Britt WJ. Human cytomegalovirus pp28 (UL99) localizes to a cytoplasmic compartment which overlaps the endoplasmic reticulum-golgi-intermediate compartment. J Virol 2000; 74:3842-51; PMID:10729158; http://dx.doi.org/ 10.1128/JVI.74.8.3842-3851.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Das S, Ortiz DA, Gurczynski SJ, Khan F, Pellett PE. Identification of human cytomegalovirus genes important for biogenesis of the cytoplasmic virion assembly complex. J Virol 2014; 88:9086-99; PMID:24899189; http://dx.doi.org/ 10.1128/JVI.01141-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alwine JC. The human cytomegalovirus assembly compartment: a masterpiece of viral manipulation of cellular processes that facilitates assembly and egress. PLoS Pathog 2012; 8:e1002878; PMID:23028305; http://dx.doi.org/ 10.1371/journal.ppat.1002878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wheeler AP, Ridley AJ. Why three Rho proteins? RhoA, RhoB, RhoC, and cell motility. Exp Cell Res 2004; 301:43-9; PMID:15501444; http://dx.doi.org/ 10.1016/j.yexcr.2004.08.012 [DOI] [PubMed] [Google Scholar]
- 16.Wennerberg K, Der CJ. Rho-family GTPases: it's not only Rac and Rho (and I like it). J Cell Sci 2004; 117:1301-12; PMID:15020670; http://dx.doi.org/ 10.1242/jcs.01118 [DOI] [PubMed] [Google Scholar]
- 17.Huang M, Prendergast GC. RhoB in cancer suppression. Histol Histopathol 2006; 21:213-8; PMID:16329046 [DOI] [PubMed] [Google Scholar]
- 18.Adamson P, Marshall CJ, Hall A, Tilbrook PA. Post-translational modifications of p21rho proteins. J Biol Chem 1992; 267:20033-8; PMID:1400319 [PubMed] [Google Scholar]
- 19.Wherlock M, Gampel A, Futter C, Mellor H. Farnesyltransferase inhibitors disrupt EGF receptor traffic through modulation of the RhoB GTPase. J Cell Sci 2004; 117:3221-31; PMID:15226397; http://dx.doi.org/ 10.1242/jcs.01193 [DOI] [PubMed] [Google Scholar]
- 20.Adamson P, Paterson HF, Hall A. Intracellular localization of the P21rho proteins. J Cell Biol 1992; 119:617-27; PMID:1383236; http://dx.doi.org/ 10.1083/jcb.119.3.617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zalcman G, Closson V, Linares-Cruz G, Lerebours F, Honore N, Tavitian A, Olofsson B. Regulation of Ras-related RhoB protein expression during the cell cycle. Oncogene 1995; 10:1935-45; PMID:7539118 [PubMed] [Google Scholar]
- 22.Michaelson D, Silletti J, Murphy G, D'Eustachio P, Rush M, Philips MR. Differential localization of Rho GTPases in live cells: regulation by hypervariable regions and RhoGDI binding. J Cell Biol 2001; 152:111-26; PMID:11149925; http://dx.doi.org/ 10.1083/jcb.152.1.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Adini I, Rabinovitz I, Sun JF, Prendergast GC, Benjamin LE. RhoB controls Akt trafficking and stage-specific survival of endothelial cells during vascular development. Genes Dev 2003; 17:2721-32; PMID:14597666; http://dx.doi.org/ 10.1101/gad.1134603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sandilands E, Cans C, Fincham VJ, Brunton VG, Mellor H, Prendergast GC, Norman JC, Superti-Furga G, Frame MC. RhoB and actin polymerization coordinate Src activation with endosome-mediated delivery to the membrane. Dev Cell 2004; 7:855-69; PMID:15572128; http://dx.doi.org/ 10.1016/j.devcel.2004.09.019 [DOI] [PubMed] [Google Scholar]
- 25.Mellor H, Flynn P, Nobes CD, Hall A, Parker PJ. PRK1 is targeted to endosomes by the small GTPase, RhoB. J Biol Chem 1998; 273:4811-4; PMID:9478917; http://dx.doi.org/ 10.1074/jbc.273.9.4811 [DOI] [PubMed] [Google Scholar]
- 26.Flynn P, Mellor H, Casamassima A, Parker PJ. Rho GTPase control of protein kinase C-related protein kinase activation by 3-phosphoinositide-dependent protein kinase. J Biol Chem 2000; 275:11064-70; PMID:10753910; http://dx.doi.org/ 10.1074/jbc.275.15.11064 [DOI] [PubMed] [Google Scholar]
- 27.Hall A. Rho GTPases and the actin cytoskeleton. Science 1998; 279:509-14; PMID:9438836; http://dx.doi.org/ 10.1126/science.279.5350.509 [DOI] [PubMed] [Google Scholar]
- 28.Ridley AJ. Rho family proteins: coordinating cell responses. Trends Cell Biol 2001; 11:471-7; PMID:11719051; http://dx.doi.org/ 10.1016/S0962-8924(01)02153-5 [DOI] [PubMed] [Google Scholar]
- 29.Ridley AJ. Rho GTPases and cell migration. J Cell Sci 2001; 114:2713-22; PMID:11683406 [DOI] [PubMed] [Google Scholar]
- 30.Papakonstanti EA, Stournaras C. Cell responses regulated by early reorganization of actin cytoskeleton. FEBS Lett 2008; 582:2120-7; PMID:18325339; http://dx.doi.org/ 10.1016/j.febslet.2008.02.064 [DOI] [PubMed] [Google Scholar]
- 31.Ridley AJ. Rho proteins: linking signaling with membrane trafficking. Traffic 2001; 2:303-10; PMID:11350626; http://dx.doi.org/ 10.1034/j.1600-0854.2001.002005303.x [DOI] [PubMed] [Google Scholar]
- 32.Kardassis D, Murphy C, Fotsis T, Moustakas A, Stournaras C. Control of transforming growth factor beta signal transduction by small GTPases. FEBS J 2009; 276:2947-65; PMID:19490100; http://dx.doi.org/ 10.1111/j.1742-4658.2009.07031.x [DOI] [PubMed] [Google Scholar]
- 33.Fernandez-Borja M, Janssen L, Verwoerd D, Hordijk P, Neefjes J. RhoB regulates endosome transport by promoting actin assembly on endosomal membranes through Dia1. J Cell Sci 2005; 118:2661-70; PMID:15944396; http://dx.doi.org/ 10.1242/jcs.02384 [DOI] [PubMed] [Google Scholar]
- 34.Vega FM, Colomba A, Reymond N, Thomas M, Ridley AJ. RhoB regulates cell migration through altered focal adhesion dynamics. Open Biol 2012; 2:120076; PMID:22724071; http://dx.doi.org/ 10.1098/rsob.120076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Favoreel HW, Enquist LW, Feierbach B. Actin and Rho GTPases in herpesvirus biology. Trends Microbiol 2007; 15:426-33; PMID:17764949; http://dx.doi.org/ 10.1016/j.tim.2007.08.003 [DOI] [PubMed] [Google Scholar]
- 36.Van den Broeke C, Favoreel HW. Actin' up: herpesvirus interactions with Rho GTPase signaling. Viruses 2011; 3:278-92; PMID:21994732; http://dx.doi.org/ 10.3390/v3040278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Swanson JA. Shaping cups into phagosomes and macropinosomes. Nat Rev Mol Cell Biol 2008; 9:639-49; PMID:18612320; http://dx.doi.org/ 10.1038/nrm2447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Doherty GJ, McMahon HT. Mechanisms of endocytosis. Annu Rev Biochem 2009; 78:857-902; PMID:19317650; http://dx.doi.org/ 10.1146/annurev.biochem.78.081307.110540 [DOI] [PubMed] [Google Scholar]
- 39.Roberts KL, Baines JD. Actin in herpesvirus infection. Viruses 2011; 3:336-46; PMID:21994736; http://dx.doi.org/ 10.3390/v3040336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lyman MG, Enquist LW. Herpesvirus interactions with the host cytoskeleton. J Virol 2009; 83:2058-66; PMID:18842724; http://dx.doi.org/ 10.1128/JVI.01718-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rodrigues L, Pires de Miranda M, Caloca MJ, Bustelo XR, Simas JP. Activation of Vav by the gammaherpesvirus M2 protein contributes to the establishment of viral latency in B lymphocytes. J Virol 2006; 80:6123-35; PMID:16731951; http://dx.doi.org/ 10.1128/JVI.02700-05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Raghu H, Sharma-Walia N, Veettil MV, Sadagopan S, Caballero A, Sivakumar R, Varga L, Bottero V, Chandran B. Lipid rafts of primary endothelial cells are essential for Kaposi's sarcoma-associated herpesvirus/human herpesvirus 8-induced phosphatidylinositol 3-kinase and RhoA-GTPases critical for microtubule dynamics and nuclear delivery of viral DNA but dispensable for binding and entry. J Virol 2007; 81:7941-59; PMID:17507466; http://dx.doi.org/ 10.1128/JVI.02848-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang X, Huang DY, Huong SM, Huang ES. Integrin alphavbeta3 is a coreceptor for human cytomegalovirus. Nat Med 2005; 11:515-21; PMID:15834425; http://dx.doi.org/ 10.1038/nm1236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Seo JY, Yaneva R, Hinson ER, Cresswell P. Human cytomegalovirus directly induces the antiviral protein viperin to enhance infectivity. Science 2011; 332:1093-7; PMID:21527675; http://dx.doi.org/ 10.1126/science.1202007 [DOI] [PubMed] [Google Scholar]
- 45.Poncet D, Pauleau AL, Szabadkai G, Vozza A, Scholz SR, Le Bras M, Briere JJ, Jalil A, Le Moigne R, Brenner C, et al.. Cytopathic effects of the cytomegalovirus-encoded apoptosis inhibitory protein vMIA. J Cell Biol 2006; 174:985-96; PMID:16982800; http://dx.doi.org/ 10.1083/jcb.200604069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jones NL, Lewis JC, Kilpatrick BA. Cytoskeletal disruption during human cytomegalovirus infection of human lung fibroblasts. Eur J Cell Biol 1986; 41:304-12; PMID:3019700 [PubMed] [Google Scholar]
- 47.Vomaske J, Varnum S, Melnychuk R, Smith P, Pasa-Tolic L, Shutthanandan JI, Streblow DN. HCMV pUS28 initiates pro-migratory signaling via activation of Pyk2 kinase. Herpesviridae 2010; 1:2; PMID:21429240; http://dx.doi.org/ 10.1186/2042-4280-1-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Langemeijer EV, Slinger E, de Munnik S, Schreiber A, Maussang D, Vischer H, Verkaar F, Leurs R, Siderius M, Smit MJ. Constitutive beta-catenin signaling by the viral chemokine receptor US28. PLoS One 2012; 7:e48935; PMID:23145028; http://dx.doi.org/ 10.1371/journal.pone.0048935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hook LM, Grey F, Grabski R, Tirabassi R, Doyle T, Hancock M, Landais I, Jeng S, McWeeney S, Britt W, et al.. Cytomegalovirus miRNAs target secretory pathway genes to facilitate formation of the virion assembly compartment and reduce cytokine secretion. Cell Host Microbe 2014; 15:363-73; PMID:24629342; http://dx.doi.org/ 10.1016/j.chom.2014.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sanchez V, Greis KD, Sztul E, Britt WJ. Accumulation of virion tegument and envelope proteins in a stable cytoplasmic compartment during human cytomegalovirus replication: characterization of a potential site of virus assembly. J Virol 2000; 74:975-86; PMID:10623760; http://dx.doi.org/ 10.1128/JVI.74.2.975-986.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sampaio KL, Cavignac Y, Stierhof YD, Sinzger C. Human cytomegalovirus labeled with green fluorescent protein for live analysis of intracellular particle movements. J Virol 2005; 79:2754-67; PMID:15708994; http://dx.doi.org/ 10.1128/JVI.79.5.2754-2767.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hensel G, Meyer H, Gartner S, Brand G, Kern HF. Nuclear localization of the human cytomegalovirus tegument protein pp150 (ppUL32). J Gen Virol 1995; 76 (Pt 7):1591-601; PMID:9049366; http://dx.doi.org/ 10.1099/0022-1317-76-7-1591 [DOI] [PubMed] [Google Scholar]
- 53.Michel D, Pavic I, Zimmermann A, Haupt E, Wunderlich K, Heuschmid M, Mertens T. The UL97 gene product of human cytomegalovirus is an early-late protein with a nuclear localization but is not a nucleoside kinase. J Virol 1996; 70:6340-6; PMID:8709262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Das S, Pellett PE. Members of the HCMV US12 family of predicted heptaspanning membrane proteins have unique intracellular distributions, including association with the cytoplasmic virion assembly complex. Virology 2007; 361:263-73; PMID:17188320; http://dx.doi.org/ 10.1016/j.virol.2006.11.019 [DOI] [PubMed] [Google Scholar]
- 55.Woodhall DL, Groves IJ, Reeves MB, Wilkinson G, Sinclair JH. Human Daxx-mediated repression of human cytomegalovirus gene expression correlates with a repressive chromatin structure around the major immediate early promoter. J Biol Chem 2006; 281:37652-60; PMID:17035242; http://dx.doi.org/ 10.1074/jbc.M604273200 [DOI] [PubMed] [Google Scholar]
- 56.Lorz K, Hofmann H, Berndt A, Tavalai N, Mueller R, Schlotzer-Schrehardt U, Stamminger T. Deletion of open reading frame UL26 from the human cytomegalovirus genome results in reduced viral growth, which involves impaired stability of viral particles. J Virol 2006; 80:5423-34; PMID:16699023; http://dx.doi.org/ 10.1128/JVI.02585-05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hanna S, El-Sibai M. Signaling networks of Rho GTPases in cell motility. Cell Signal 2013; 25(10):1955-61; PMID:23669310; http://dx.doi.org/ 10.1016/j.cellsig.2013.04.009 [DOI] [PubMed] [Google Scholar]
- 58.Heasman SJ, Ridley AJ. Mammalian Rho GTPases: new insights into their functions from in vivo studies. Nat Rev Mol Cell Biol 2008; 9:690-701; PMID:18719708; http://dx.doi.org/ 10.1038/nrm2476 [DOI] [PubMed] [Google Scholar]
- 59.Arcangeletti MC, Pinardi F, Medici MC, Pilotti E, De Conto F, Ferraglia F, Landini MP, Chezzi C, Dettori G. Cytoskeleton involvement during human cytomegalovirus replicative cycle in human embryo fibroblasts. New Microbiol 2000; 23:241-56; PMID:10939039 [PubMed] [Google Scholar]
- 60.Lam V, Bigley T, Terhune SS, Wakatsuki T. A method for quantifying mechanical properties of tissue following viral infection. PLoS One 2012; 7:e42197; PMID:22870300; http://dx.doi.org/ 10.1371/journal.pone.0042197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Moorman NJ, Sharon-Friling R, Shenk T, Cristea IM. A targeted spatial-temporal proteomics approach implicates multiple cellular trafficking pathways in human cytomegalovirus virion maturation. Mol Cell Proteomics 2010; 9:851-60; PMID:20023299; http://dx.doi.org/ 10.1074/mcp.M900485-MCP200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shi W, Xu B, Tian P, Li X, Sun H. A trapping ligand antagonist peptide H22-LP inhibition of human cytomegalovirus infection. J Microbiol Immunol Infect 2014; PMID:25081984; http://dx.doi.org/21752907 10.1016/j.jmii.2014.06.010 [DOI] [PubMed] [Google Scholar]
- 63.Goldner T, Hewlett G, Ettischer N, Ruebsamen-Schaeff H, Zimmermann H, Lischka P. The novel anticytomegalovirus compound AIC246 (Letermovir) inhibits human cytomegalovirus replication through a specific antiviral mechanism that involves the viral terminase. J Virol 2011; 85:10884-93; PMID:21752907; http://dx.doi.org/ 10.1128/JVI.05265-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Prichard MN, Britt WJ, Daily SL, Hartline CB, Kern ER. Human cytomegalovirus UL97 Kinase is required for the normal intranuclear distribution of pp65 and virion morphogenesis. J Virol 2005; 79:15494-502; PMID:16306620; http://dx.doi.org/ 10.1128/JVI.79.24.15494-15502.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schimmoller F, Simon I, Pfeffer SR. Rab GTPases, directors of vesicle docking. J Biol Chem 1998; 273:22161-4; PMID:9712825; http://dx.doi.org/ 10.1074/jbc.273.35.22161 [DOI] [PubMed] [Google Scholar]
- 66.Miaczynska M, Zerial M. Mosaic organization of the endocytic pathway. Exp Cell Res 2002; 272:8-14; PMID:11740860; http://dx.doi.org/ 10.1006/excr.2001.5401 [DOI] [PubMed] [Google Scholar]
- 67.Fraile-Ramos A, Cepeda V, Elstak E, van der Sluijs P. Rab27a is required for human cytomegalovirus assembly. PLoS One 2010; 5:e15318; PMID:21170347; http://dx.doi.org/ 10.1371/journal.pone.0015318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sharon-Friling R, Shenk T. Human cytomegalovirus pUL37x1-induced calcium flux activates PKCalpha, inducing altered cell shape and accumulation of cytoplasmic vesicles. Proc Natl Acad Sci U S A 2014; 111:E1140-8; PMID:24616524; http://dx.doi.org/ 10.1073/pnas.1402515111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yi HA, Kim MS, Jang SY, Lee YM, Ahn JH, Lee CH. Cellular signals involved in cyclooxygenase-2 expression induced by human cytomegalovirus. Virus Res 2009; 146:89-96; PMID:19748535; http://dx.doi.org/ 10.1016/j.virusres.2009.09.004 [DOI] [PubMed] [Google Scholar]
- 70.Hertel L, Mocarski ES. Global analysis of host cell gene expression late during cytomegalovirus infection reveals extensive dysregulation of cell cycle gene expression and induction of Pseudomitosis independent of US28 function. J Virol 2004; 78:11988-2011; PMID:15479839; http://dx.doi.org/ 10.1128/JVI.78.21.11988-12011.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Indran SV, Britt WJ. A role for the small GTPase Rab6 in assembly of human cytomegalovirus. J Virol 2011; 85:5213-9; PMID:21411515; http://dx.doi.org/ 10.1128/JVI.02605-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Krzyzaniak MA, Mach M, Britt WJ. HCMV-encoded glycoprotein M (UL100) interacts with Rab11 effector protein FIP4. Traffic 2009; 10:1439-57; PMID:19761540; http://dx.doi.org/ 10.1111/j.1600-0854.2009.00967.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Reyda S, Tenzer S, Navarro P, Gebauer W, Saur M, Krauter S, Buscher N, Plachter B. The tegument protein pp65 of human cytomegalovirus acts as an optional scaffold protein that optimizes protein uploading into viral particles. J Virol 2014; 88(17):9633-46; PMID:24920816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Robertson D, Paterson HF, Adamson P, Hall A, Monaghan P. Ultrastructural localization of ras-related proteins using epitope-tagged plasmids. J Histochem Cytochem 1995; 43:471-80; PMID:7537292; http://dx.doi.org/ 10.1177/43.5.7537292 [DOI] [PubMed] [Google Scholar]
- 75.Cepeda V, Fraile-Ramos A. A role for the SNARE protein syntaxin 3 in human cytomegalovirus morphogenesis. Cell Microbiol 2011; 13:846-58; PMID:21371234; http://dx.doi.org/ 10.1111/j.1462-5822.2011.01583.x [DOI] [PubMed] [Google Scholar]
- 76.Tooze J, Hollinshead M, Reis B, Radsak K, Kern H. Progeny vaccinia and human cytomegalovirus particles utilize early endosomal cisternae for their envelopes. Eur J Cell Biol 1993; 60:163-78; PMID:8385018 [PubMed] [Google Scholar]
- 77.Cudmore S, Reckmann I, Way M. Viral manipulations of the actin cytoskeleton. Trends Microbiol 1997; 5:142-8; PMID:9141188; http://dx.doi.org/ 10.1016/S0966-842X(97)01011-1 [DOI] [PubMed] [Google Scholar]
- 78.Jones NL, Kilpatrick BA. The effects of human cytomegalovirus infection on cytoskeleton-associated polysomes. Eur J Cell Biol 1988; 46:31-8; PMID:2456216 [PubMed] [Google Scholar]
- 79.Egea G, Lazaro-Dieguez F, Vilella M. Actin dynamics at the Golgi complex in mammalian cells. Curr Opin Cell Biol 2006; 18:168-78; PMID:16488588; http://dx.doi.org/ 10.1016/j.ceb.2006.02.007 [DOI] [PubMed] [Google Scholar]
- 80.Egea G, Serra-Peinado C. Golgi apparatus: finally mechanics comes to play in the secretory pathway. Curr Biol 2014; 24:R741-3; PMID:25137584; http://dx.doi.org/ 10.1016/j.cub.2014.07.002 [DOI] [PubMed] [Google Scholar]
- 81.von Blume J, Duran JM, Forlanelli E, Alleaume AM, Egorov M, Polishchuk R, Molina H, Malhotra V. Actin remodeling by ADF/cofilin is required for cargo sorting at the trans-Golgi network. J Cell Biol 2009; 187:1055-69; PMID:20026655; http://dx.doi.org/ 10.1083/jcb.200908040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Dimitropoulou P, Caswell R, McSharry BP, Greaves RF, Spandidos DA, Wilkinson GW, Sourvinos G. Differential relocation and stability of PML-body components during productive human cytomegalovirus infection: detailed characterization by live-cell imaging. Eur J Cell Biol 2010; 89:757-68; PMID:20599291; http://dx.doi.org/ 10.1016/j.ejcb.2010.05.006 [DOI] [PubMed] [Google Scholar]
- 83.Plachter B, Britt W, Vornhagen R, Stamminger T, Jahn G. Analysis of proteins encoded by IE regions 1 and 2 of human cytomegalovirus using monoclonal antibodies generated against recombinant antigens. Virology 1993; 193:642-52; PMID:7681609; http://dx.doi.org/ 10.1006/viro.1993.1172 [DOI] [PubMed] [Google Scholar]
- 84.Marschall M, Freitag M, Suchy P, Romaker D, Kupfer R, Hanke M, Stamminger T. The protein kinase pUL97 of human cytomegalovirus interacts with and phosphorylates the DNA polymerase processivity factor pUL44. Virology 2003; 311:60-71; PMID:12832203; http://dx.doi.org/ 10.1016/S0042-6822(03)00147-8 [DOI] [PubMed] [Google Scholar]
- 85.Silva MC, Yu QC, Enquist L, Shenk T. Human cytomegalovirus UL99-encoded pp28 is required for the cytoplasmic envelopment of tegument-associated capsids. J Virol 2003; 77:10594-605; PMID:12970444; http://dx.doi.org/ 10.1128/JVI.77.19.10594-10605.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Filippakis H, Dimitropoulou P, Eliopoulos AG, Spandidos DA, Sourvinos G. The enhanced host-cell permissiveness of human cytomegalovirus is mediated by the Ras signaling pathway. Biochim Biophys Acta 2011; 1813:1872-82; PMID:21782855; http://dx.doi.org/ 10.1016/j.bbamcr.2011.07.003 [DOI] [PubMed] [Google Scholar]
- 87.Everett RD, Sourvinos G, Orr A. Recruitment of herpes simplex virus type 1 transcriptional regulatory protein ICP4 into foci juxtaposed to ND10 in live, infected cells. J Virol 2003; 77:3680-9; PMID:12610143; http://dx.doi.org/ 10.1128/JVI.77.6.3680-3689.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.