Abstract
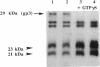
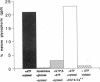
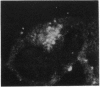
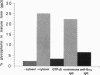
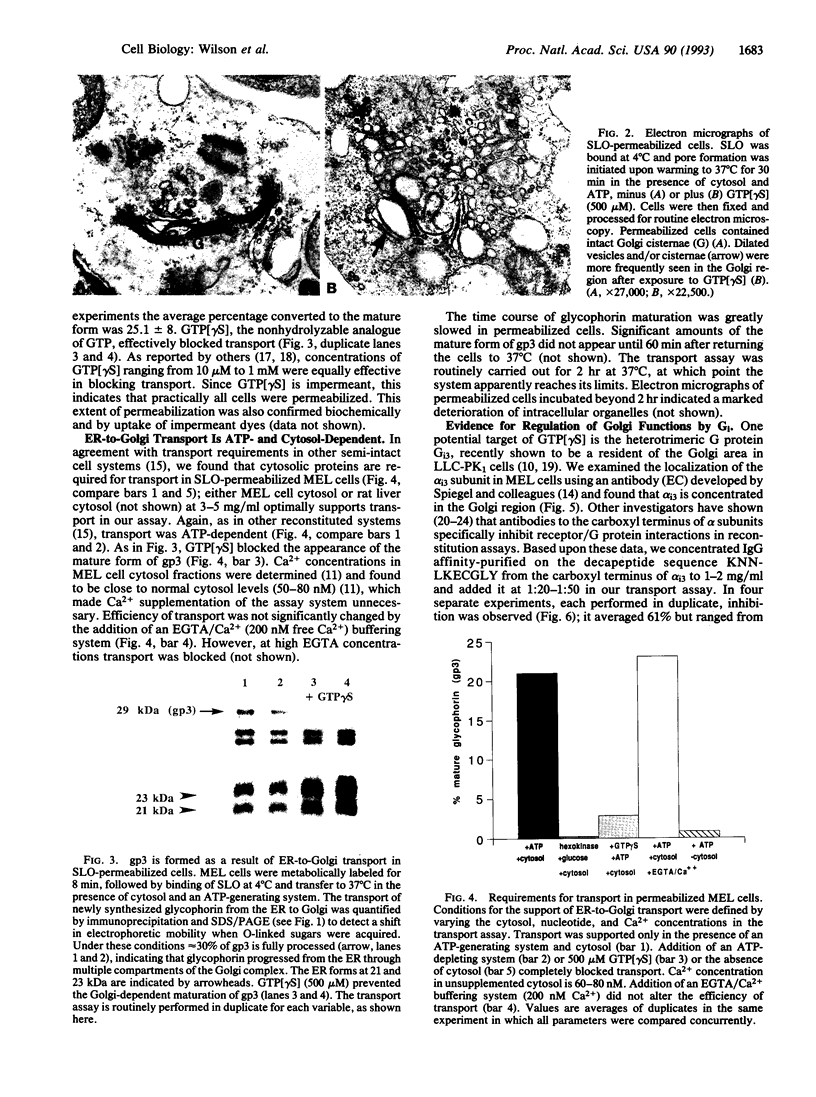
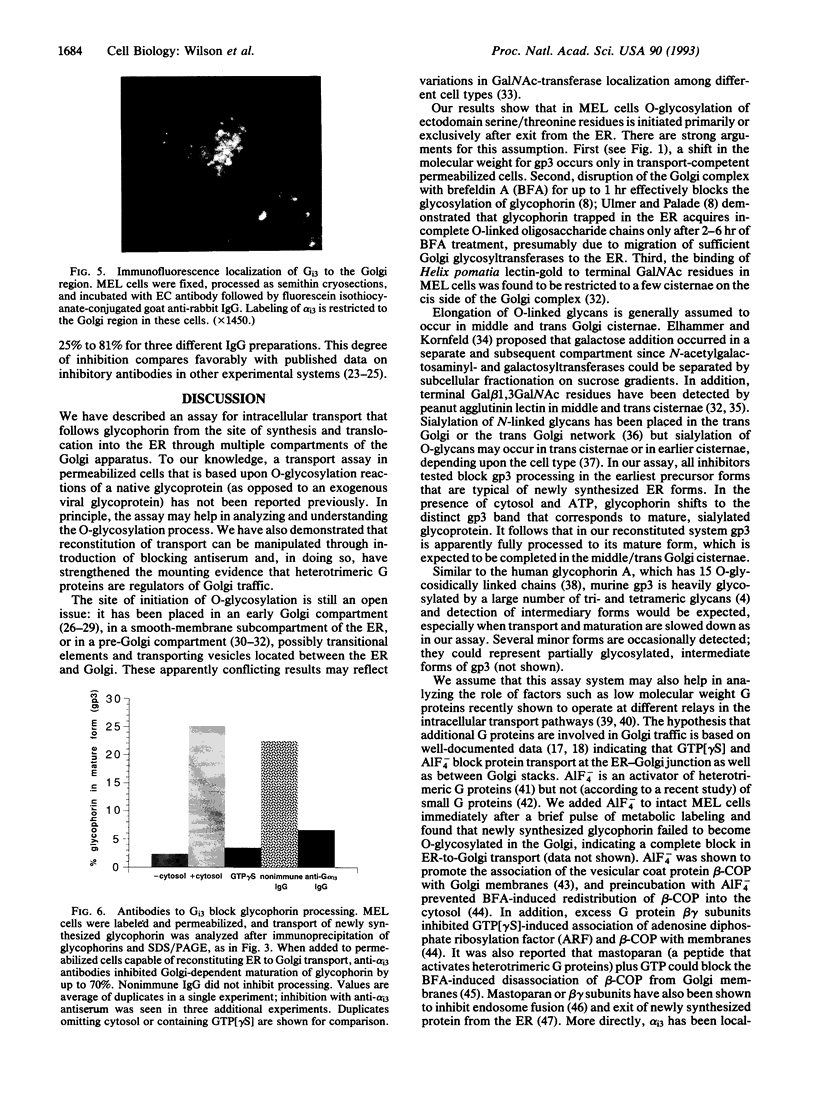
An assay for endoplasmic reticulum (ER)-through-Golgi transport has been developed in streptolysin O-permeabilized murine erythroleukemia (MEL) cells. The reporter proteins are metabolically labeled native murine glycophorins, which display a distinctive shift in electrophoretic mobility after acquisition of O-linked oligosaccharides. The O-linked sugars are acquired at a site distal to a brefeldin A block, presumably in a cis Golgi compartment, and sialylation occurs in middle and/or trans Golgi compartments. In permeabilized cells supplemented with cytosolic proteins and an ATP-generating system, 20-50% of the radiolabeled precursor glycophorins can be converted to the mature, sialylated form. This maturation process is ATP- and cytosol-dependent and is blocked by guanosine 5'-[gamma-thio]triphosphate (GTP[gamma S]). Electron microscopy of permeabilized MEL cells shows retention of ER elements, stacked Golgi cisternae, free polysomes, and other subcellular components. In the presence of GTP[gamma S], dilated vesicles accumulate around the Golgi stacks. Antisera to the carboxyl terminus of the Golgi resident alpha subunit of Gi3 inhibit maturation of glycophorin. To our knowledge, a transport assay utilizing O-glycosylation of an endogenous protein as a monitor of ER-through-Golgi traffic in permeabilized cells has not been reported previously. Furthermore, the data provide evidence for heterotrimeric GTP-binding protein involvement in Golgi function.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abeijon C., Hirschberg C. B. Subcellular site of synthesis of the N-acetylgalactosamine (alpha 1-0) serine (or threonine) linkage in rat liver. J Biol Chem. 1987 Mar 25;262(9):4153–4159. [PubMed] [Google Scholar]
- Anderson R. A., Lovrien R. E. Glycophorin is linked by band 4.1 protein to the human erythrocyte membrane skeleton. Nature. 1984 Feb 16;307(5952):655–658. doi: 10.1038/307655a0. [DOI] [PubMed] [Google Scholar]
- Balch W. E. Biochemistry of interorganelle transport. A new frontier in enzymology emerges from versatile in vitro model systems. J Biol Chem. 1989 Oct 15;264(29):16965–16968. [PubMed] [Google Scholar]
- Balch W. E., Rothman J. E. Characterization of protein transport between successive compartments of the Golgi apparatus: asymmetric properties of donor and acceptor activities in a cell-free system. Arch Biochem Biophys. 1985 Jul;240(1):413–425. doi: 10.1016/0003-9861(85)90046-3. [DOI] [PubMed] [Google Scholar]
- Balch W. E. Small GTP-binding proteins in vesicular transport. Trends Biochem Sci. 1990 Dec;15(12):473–477. doi: 10.1016/0968-0004(90)90301-q. [DOI] [PubMed] [Google Scholar]
- Beckers C. J., Balch W. E. Calcium and GTP: essential components in vesicular trafficking between the endoplasmic reticulum and Golgi apparatus. J Cell Biol. 1989 Apr;108(4):1245–1256. doi: 10.1083/jcb.108.4.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckers C. J., Keller D. S., Balch W. E. Semi-intact cells permeable to macromolecules: use in reconstitution of protein transport from the endoplasmic reticulum to the Golgi complex. Cell. 1987 Aug 14;50(4):523–534. doi: 10.1016/0092-8674(87)90025-0. [DOI] [PubMed] [Google Scholar]
- Bourne H. R. Do GTPases direct membrane traffic in secretion? Cell. 1988 Jun 3;53(5):669–671. doi: 10.1016/0092-8674(88)90081-5. [DOI] [PubMed] [Google Scholar]
- Carraway K. L., Hull S. R. O-glycosylation pathway for mucin-type glycoproteins. Bioessays. 1989 Apr;10(4):117–121. doi: 10.1002/bies.950100406. [DOI] [PubMed] [Google Scholar]
- Colombo M. I., Mayorga L. S., Casey P. J., Stahl P. D. Evidence of a role for heterotrimeric GTP-binding proteins in endosome fusion. Science. 1992 Mar 27;255(5052):1695–1697. doi: 10.1126/science.1348148. [DOI] [PubMed] [Google Scholar]
- Cummings R. D., Kornfeld S., Schneider W. J., Hobgood K. K., Tolleshaug H., Brown M. S., Goldstein J. L. Biosynthesis of N- and O-linked oligosaccharides of the low density lipoprotein receptor. J Biol Chem. 1983 Dec 25;258(24):15261–15273. [PubMed] [Google Scholar]
- Dolci E. D., Palade G. E. The biosynthesis and fatty acid acylation of the murine erythrocyte sialoglycoproteins. J Biol Chem. 1985 Sep 5;260(19):10728–10735. [PubMed] [Google Scholar]
- Donaldson J. G., Kahn R. A., Lippincott-Schwartz J., Klausner R. D. Binding of ARF and beta-COP to Golgi membranes: possible regulation by a trimeric G protein. Science. 1991 Nov 22;254(5035):1197–1199. doi: 10.1126/science.1957170. [DOI] [PubMed] [Google Scholar]
- Donaldson J. G., Lippincott-Schwartz J., Klausner R. D. Guanine nucleotides modulate the effects of brefeldin A in semipermeable cells: regulation of the association of a 110-kD peripheral membrane protein with the Golgi apparatus. J Cell Biol. 1991 Feb;112(4):579–588. doi: 10.1083/jcb.112.4.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elhammer A., Kornfeld S. Two enzymes involved in the synthesis of O-linked oligosaccharides are localized on membranes of different densities in mouse lymphoma BW5147 cells. J Cell Biol. 1984 Jul;99(1 Pt 1):327–331. doi: 10.1083/jcb.99.1.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ercolani L., Stow J. L., Boyle J. F., Holtzman E. J., Lin H., Grove J. R., Ausiello D. A. Membrane localization of the pertussis toxin-sensitive G-protein subunits alpha i-2 and alpha i-3 and expression of a metallothionein-alpha i-2 fusion gene in LLC-PK1 cells. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4635–4639. doi: 10.1073/pnas.87.12.4635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman A. G. G proteins: transducers of receptor-generated signals. Annu Rev Biochem. 1987;56:615–649. doi: 10.1146/annurev.bi.56.070187.003151. [DOI] [PubMed] [Google Scholar]
- Gravotta D., Adesnik M., Sabatini D. D. Transport of influenza HA from the trans-Golgi network to the apical surface of MDCK cells permeabilized in their basolateral plasma membranes: energy dependence and involvement of GTP-binding proteins. J Cell Biol. 1990 Dec;111(6 Pt 2):2893–2908. doi: 10.1083/jcb.111.6.2893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutowski S., Smrcka A., Nowak L., Wu D. G., Simon M., Sternweis P. C. Antibodies to the alpha q subfamily of guanine nucleotide-binding regulatory protein alpha subunits attenuate activation of phosphatidylinositol 4,5-bisphosphate hydrolysis by hormones. J Biol Chem. 1991 Oct 25;266(30):20519–20524. [PubMed] [Google Scholar]
- Hamm H. E., Deretic D., Hofmann K. P., Schleicher A., Kohl B. Mechanism of action of monoclonal antibodies that block the light activation of the guanyl nucleotide-binding protein, transducin. J Biol Chem. 1987 Aug 5;262(22):10831–10838. [PubMed] [Google Scholar]
- Johnson D. C., Spear P. G. O-linked oligosaccharides are acquired by herpes simplex virus glycoproteins in the Golgi apparatus. Cell. 1983 Mar;32(3):987–997. doi: 10.1016/0092-8674(83)90083-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn R. A., Kern F. G., Clark J., Gelmann E. P., Rulka C. Human ADP-ribosylation factors. A functionally conserved family of GTP-binding proteins. J Biol Chem. 1991 Feb 5;266(4):2606–2614. [PubMed] [Google Scholar]
- Krotkiewski H., Lisowska E., Angel A. S., Nilsson B. Structural analysis by fast-atom-bombardment mass spectrometry of the mixture of alditols derived from the O-linked oligosaccharides of murine glycophorins. Carbohydr Res. 1988 Dec 31;184:27–38. doi: 10.1016/0008-6215(88)80003-x. [DOI] [PubMed] [Google Scholar]
- Ktistakis N. T., Linder M. E., Roth M. G. Action of brefeldin A blocked by activation of a pertussis-toxin-sensitive G protein. Nature. 1992 Mar 26;356(6367):344–346. doi: 10.1038/356344a0. [DOI] [PubMed] [Google Scholar]
- LaMorte V. J., Goldsmith P. K., Spiegel A. M., Meinkoth J. L., Feramisco J. R. Inhibition of DNA synthesis in living cells by microinjection of Gi2 antibodies. J Biol Chem. 1992 Jan 15;267(2):691–694. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Locker J. K., Griffiths G., Horzinek M. C., Rottier P. J. O-glycosylation of the coronavirus M protein. Differential localization of sialyltransferases in N- and O-linked glycosylation. J Biol Chem. 1992 Jul 15;267(20):14094–14101. doi: 10.1016/S0021-9258(19)49683-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotan R., Skutelsky E., Danon D., Sharon N. The purification, composition, and specificity of the anti-T lectin from peanut (Arachis hypogaea). J Biol Chem. 1975 Nov 10;250(21):8518–8523. [PubMed] [Google Scholar]
- McKenzie F. R., Kelly E. C., Unson C. G., Spiegel A. M., Milligan G. Antibodies which recognize the C-terminus of the inhibitory guanine-nucleotide-binding protein (Gi) demonstrate that opioid peptides and foetal-calf serum stimulate the high-affinity GTPase activity of two separate pertussis-toxin substrates. Biochem J. 1988 Feb 1;249(3):653–659. doi: 10.1042/bj2490653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melançon P., Glick B. S., Malhotra V., Weidman P. J., Serafini T., Gleason M. L., Orci L., Rothman J. E. Involvement of GTP-binding "G" proteins in transport through the Golgi stack. Cell. 1987 Dec 24;51(6):1053–1062. doi: 10.1016/0092-8674(87)90591-5. [DOI] [PubMed] [Google Scholar]
- Murray-Whelan R., Schlegel W. Brain somatostatin receptor-G protein interaction. G alpha C-terminal antibodies demonstrate coupling of the soluble receptor with Gi(1-3) but not with Go. J Biol Chem. 1992 Feb 15;267(5):2960–2965. [PubMed] [Google Scholar]
- Nair B. G., Parikh B., Milligan G., Patel T. B. Gs alpha mediates epidermal growth factor-elicited stimulation of rat cardiac adenylate cyclase. J Biol Chem. 1990 Dec 5;265(34):21317–21322. [PubMed] [Google Scholar]
- Pathak R. K., Merkle R. K., Cummings R. D., Goldstein J. L., Brown M. S., Anderson R. G. Immunocytochemical localization of mutant low density lipoprotein receptors that fail to reach the Golgi complex. J Cell Biol. 1988 Jun;106(6):1831–1841. doi: 10.1083/jcb.106.6.1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Vilar J., Hidalgo J., Velasco A. Presence of terminal N-acetylgalactosamine residues in subregions of the endoplasmic reticulum is influenced by cell differentiation in culture. J Biol Chem. 1991 Dec 15;266(35):23967–23976. [PubMed] [Google Scholar]
- Plutner H., Cox A. D., Pind S., Khosravi-Far R., Bourne J. R., Schwaninger R., Der C. J., Balch W. E. Rab1b regulates vesicular transport between the endoplasmic reticulum and successive Golgi compartments. J Cell Biol. 1991 Oct;115(1):31–43. doi: 10.1083/jcb.115.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth J. Cytochemical localization of terminal N-acetyl-D-galactosamine residues in cellular compartments of intestinal goblet cells: implications for the topology of O-glycosylation. J Cell Biol. 1984 Feb;98(2):399–406. doi: 10.1083/jcb.98.2.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth J., Taatjes D. J., Weinstein J., Paulson J. C., Greenwell P., Watkins W. M. Differential subcompartmentation of terminal glycosylation in the Golgi apparatus of intestinal absorptive and goblet cells. J Biol Chem. 1986 Oct 25;261(30):14307–14312. [PubMed] [Google Scholar]
- Rothman J. E., Orci L. Molecular dissection of the secretory pathway. Nature. 1992 Jan 30;355(6359):409–415. doi: 10.1038/355409a0. [DOI] [PubMed] [Google Scholar]
- Saraste J., Palade G. E., Farquhar M. G. Temperature-sensitive steps in the transport of secretory proteins through the Golgi complex in exocrine pancreatic cells. Proc Natl Acad Sci U S A. 1986 Sep;83(17):6425–6429. doi: 10.1073/pnas.83.17.6425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwaninger R., Plutner H., Bokoch G. M., Balch W. E. Multiple GTP-binding proteins regulate vesicular transport from the ER to Golgi membranes. J Cell Biol. 1992 Dec;119(5):1077–1096. doi: 10.1083/jcb.119.5.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonds W. F., Goldsmith P. K., Codina J., Unson C. G., Spiegel A. M. Gi2 mediates alpha 2-adrenergic inhibition of adenylyl cyclase in platelet membranes: in situ identification with G alpha C-terminal antibodies. Proc Natl Acad Sci U S A. 1989 Oct;86(20):7809–7813. doi: 10.1073/pnas.86.20.7809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stow J. L., de Almeida J. B., Narula N., Holtzman E. J., Ercolani L., Ausiello D. A. A heterotrimeric G protein, G alpha i-3, on Golgi membranes regulates the secretion of a heparan sulfate proteoglycan in LLC-PK1 epithelial cells. J Cell Biol. 1991 Sep;114(6):1113–1124. doi: 10.1083/jcb.114.6.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tooze S. A., Tooze J., Warren G. Site of addition of N-acetyl-galactosamine to the E1 glycoprotein of mouse hepatitis virus-A59. J Cell Biol. 1988 May;106(5):1475–1487. doi: 10.1083/jcb.106.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsien R. Y., Pozzan T., Rink T. J. Calcium homeostasis in intact lymphocytes: cytoplasmic free calcium monitored with a new, intracellularly trapped fluorescent indicator. J Cell Biol. 1982 Aug;94(2):325–334. doi: 10.1083/jcb.94.2.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulmer J. B., Dolci E. D., Palade G. E. Glycophorin expression in murine erythroleukaemia cells. J Cell Sci. 1989 Feb;92(Pt 2):163–171. doi: 10.1242/jcs.92.2.163. [DOI] [PubMed] [Google Scholar]
- Ulmer J. B., Palade G. E. Anomalies in the translocation and processing of glycophorin precursors in murine erythroleukemia cells. J Biol Chem. 1989 Jan 15;264(2):1084–1091. [PubMed] [Google Scholar]
- Ulmer J. B., Palade G. E. Targeting and processing of glycophorins in murine erythroleukemia cells: use of brefeldin A as a perturbant of intracellular traffic. Proc Natl Acad Sci U S A. 1989 Sep;86(18):6992–6996. doi: 10.1073/pnas.86.18.6992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshima H., Furthmayr H., Kobata A. Structures of the asparagine-linked sugar chains of glycophorin A. J Biol Chem. 1980 Oct 25;255(20):9713–9718. [PubMed] [Google Scholar]