ABSTRACT
Previous studies have demonstrated an interaction between sorting nexin 17 and the L2 capsid proteins from a variety of papillomavirus types. This interaction is required for late endosomal trafficking of the L2 protein and entry of the L2/DNA complex into the nucleus during infection. Here we show an interaction between papillomavirus L2 proteins and the related PX-FERM family member sorting nexin 27 (SNX27), which is mediated in part by a novel interaction between the PDZ domain of SNX27 and sequences in a central portion of L2. The interaction is direct and, unlike that with SNX17, is variable in strength depending on the papillomavirus type. We show that small interfering RNA (siRNA)-mediated knockdown of SNX27 alone leads to a marginal reduction in the efficiency of viral infection but that double knockdown of both sorting nexins results in a striking reduction in infection, greater than that observed for the knockdown of either sorting nexin alone. These results suggest that the HPV L2 proteins can interact through distinct mechanisms with multiple components of the cellular cargo-sorting machinery.
IMPORTANCE The trafficking of papillomaviruses to the host cell nucleus during their natural infectious life cycle is an incompletely understood process. Studies have suggested that the virus minor capsid protein L2 can interact with the endosomal recycling pathway, in part by association with sorting nexin 17, to ensure that virus DNA bound to L2 is recycled through the trans-Golgi network rather than back to the plasma membrane. In this study, we characterize the interaction between L2 and a second sorting nexin, SNX27, which is also part of the retromer complex. The study furthers our understanding of papillomavirus infection dynamics and provides potential tools for the further dissection of endosomal structure and function.
INTRODUCTION
The entry of papillomaviruses (PVs) into host cells involves a series of coordinated steps whereby virus particles attach to cell surface receptors and are internalized into endocytic vesicles. This allows the virus capsid proteins, tethered to the viral genome, to interact with cellular protein complexes within endosomes, which are exploited to allow virion disassembly, followed by the transport of L2 and the viral genome to the nucleus and their entry into the nucleus, for subsequent expression of viral transcripts. The precise details of the first step in this cascade of events remain, to a degree, controversial, although it seems that different papillomavirus types exploit different mechanisms to gain entry to the cell (1–3). Subsequent steps during viral infection may be common to all papillomavirus types and, although incompletely understood, involve virion disassembly during endosome acidification and subsequent separation of L1 and L2. While the majority of L1 is sorted into lysosomes, L2, tethered to the viral genome, is transported through the trans-Golgi network (4) and finally enters the nucleus during mitosis (5), when the nuclear membrane is dissolved, where it localizes, together with the viral genome, at promyelocytic leukemia protein (PML) nuclear bodies (6). Various interactions between L2 and cellular proteins have been shown to be important for infection; the interaction of cyclophilins with L2 has been shown to be involved in capsid disassembly and the separation of L1 from the L2/viral DNA complex in acidified endosomes (7), as has the L2 interaction with sorting nexin 17 (SNX17) (8). Additionally, the transit of vesicles to the trans-Golgi network and subsequent nuclear entry probably involve movement along microtubules by way of molecular motors, since L2 has been shown to interact with dynein (9), an association that is also essential for virus infection.
The endosomal pathway is a vesicular transport system involved in the intracellular processing of receptor-ligand complexes, lipids, membrane proteins, extracellular nutrients, and cell debris, as well as bacteria and viruses that have entered the cell by endocytosis. Mechanistically, it sorts cargos into vesicular compartments whose contents can then be stored, recycled, activated, silenced, or degraded, thereby regulating many essential pathways in the cell (for a review, see reference 10). The ultimate fate of cargos within endosomal compartments is determined by the protein complexes that are recruited into them, and the exploitation and manipulation of this system have been the evolutionary strategy by which several virus types, including papillomaviruses, gain entry into the cell nucleus. Previous studies from this laboratory have shown that human papillomavirus 16 (HPV-16) L2 binds to SNX17 and that this interaction is required for the entry of the viral genome into the nucleus (8), possibly by retention of L2 and the associated viral DNA in late endosomes in a manner analogous to the retention of P-selectin by SNX17 (11), whereas the dissociated L1 protein is sorted into a lysosomal compartment for degradation (12). The related protein sorting nexin 27 (SNX27) shares several structural features with SNX17. Both proteins belong to a subfamily of PX-FERM endosomal membrane scaffolds that play major roles in cargo fate determination (13). SNX27 has been identified in early endosomes by virtue of the interaction of its PX domain with phosphatidylinositol-3-monophosphate groups (PtdIns 3P) (14). SNX27, like SNX17, can bind cargos with an NPxY or NxxY motif, through amino acid residues in its C terminus, but SNX27 additionally has an amino-terminal PDZ domain that allows it to target a subset of other proteins that SNX17 cannot target. Such targets include the Na/H exchanger regulatory factor (NHERF) (15), the Cytohesin-associated scaffolding protein (CASP) (16), the junctional protein zonula occludens-2 (ZO-2) (17), and the β-2 adrenergic receptor (β2AR) (18–20); the last interaction has been suggested to involve the interaction of SNX27 with the SNX-BAR (sorting nexin with a Bin/Amphyphysin/Rvs domain)-retromer complex (19). Because more-recent studies have confirmed the requirement of the retromer complex for papillomavirus infection (21) and the direct interaction of HPV-16 L2 with the retromer (22), we were interested in determining whether SNX27 might also interact with papillomavirus L2 proteins and play a role in virus infection.
MATERIALS AND METHODS
Plasmid constructs.
SNX27a and SNX27b, which express the two isoforms of SNX27 (Fig. 1A), and the SNX27b ΔPDZ and SNX27b ΔRA/FERM domains, all of which express myc-tagged SNX27 and fragments thereof (see Fig. 7A), were cloned into plasmid pCI-neo. The plasmid construct expressing SNX17-Flag has been described previously (23). Plasmid pCA16L2, expressing wild-type (WT) HPV-16 L2 (16L2) with amino-terminal Flag and hemagglutinin (HA) tags, has been described previously (8), and the 16L2 truncation mutants used in this study were produced by the introduction of stop codons into the open reading frame of pCA16L2 using the GeneArt site-directed mutagenesis system (Invitrogen), as were the SNX17 and SNX27 C-terminal mutations and the SNX27 Δ67–77 and Δ96–107 mutations.
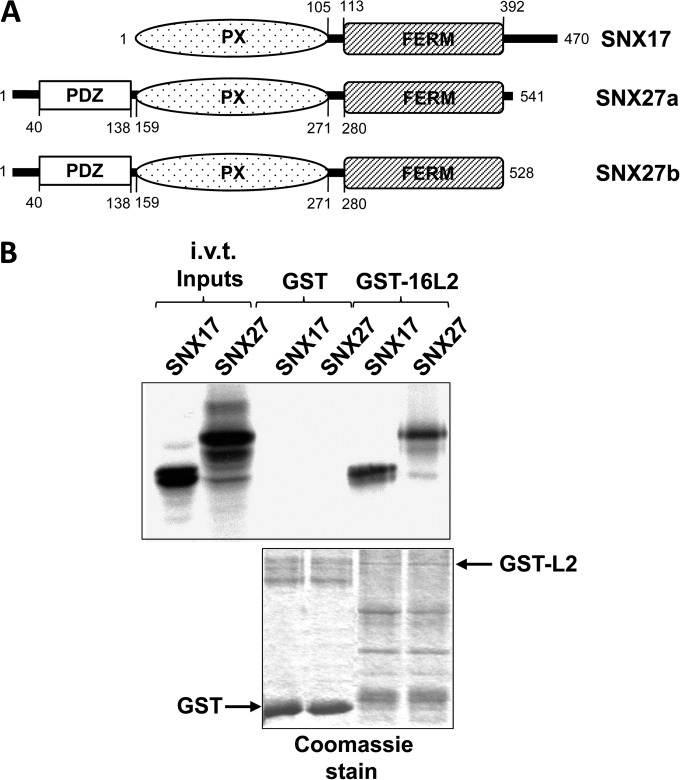
FIG 1.
(A) Schematic diagram of SNX17 and the SNX27a and SNX27b isoforms for comparison. (B) Sorting nexin 27 binds to HPV-16 L2 protein. In vitro-translated (i.v.t.), radiolabeled SNX17 and SNX27a were incubated with GST alone or GST-16L2 bound to glutathione resin. After washing, bound proteins were separated by SDS-PAGE, and the gel was stained with Coomassie blue (bottom), dried, and exposed to X-ray film (top).
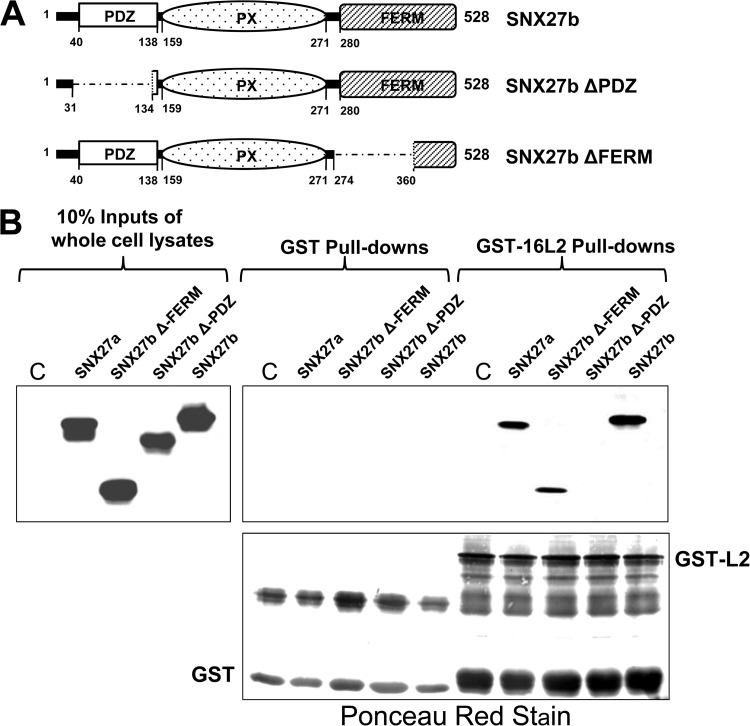
FIG 7.
A fragment of SNX27 that lacks its amino-terminal PDZ domain is unable to bind to HPV-16 L2. (A) Schematic of full-length SNX27b and the SNX27b ΔPDZ and ΔFERM mutants. (B) Full-length myc-tagged SNX27a and SNX27b isoforms and deletion mutants of SNX27b were expressed in HEK293 cells. An untransfected control cell lysate (C) and the transfected cell lysates were incubated either with GST alone or with GST-16L2 bound to glutathione resin. SNX27 mutants that bound to L2 were visualized after SDS-PAGE and Western blotting, by probing with anti-myc antibodies (top), and the GST fusion protein levels were analyzed by Ponceau red staining of the membrane (bottom).
Cell lines.
HEK293 cells (human embryonic kidney cells), H1299 cells, HaCaT cells (spontaneously immortalized human keratinocytes), and HEK293TT cells (24) were grown in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (FBS), penicillin-streptomycin (100 U/ml), and glutamine (300 μg/ml).
Pseudovirion production.
Pseudovirions with a packaged luciferase reporter were generated by transfecting HEK293TT cells with constructs expressing the various papillomavirus L1 and L2 open reading frames in codon-optimized bicistronic plasmid constructs along with the luciferase reporter pGL3Luci, as described previously (24).
Infectivity assays.
HaCaT cells were seeded in a 12-well plate at a density of 3 × 104/well. After adherence, small interfering RNAs (siRNAs) against SNX17 and SNX27, and siSTABLE nontargeting siRNA 1 (referred to below as siScr [scrambled siRNA]), as a control (Dharmacon), were added to cells in pairs of wells by using Lipofectamine RNAiMax (Invitrogen) according to the manufacturer's instructions. siRNAs were dissolved and were used at a concentration of 20 μM, and because we observed differences in knockdown efficiency, their optimal inputs were determined empirically by pilot experiments. All siRNA inputs were balanced with the control siScr to bring the total siRNA concentration to the same amount per transfection. Briefly, for each two-well transfection, for the series of control, SNX17, SNX27, and double (SNX17-plus-SNX27) knockdowns, we used 4 μl siScr, 1 μl siSNX17 plus 3 μl siScr, 3 μl siSNX27 plus 1 μl SiScr, and 3 μl siSNX27 plus 1 μl siSNX17, respectively. After 48 h, HPV-16 pseudovirions were added at a concentration of approximately 12 ng/ml. Infection was monitored 48 h later by luminometric analysis of firefly luciferase activity in the cell lysates using a luciferase assay system kit (Promega), according to the manufacturer's instructions. The efficiency of siRNA knockdown was confirmed by SDS-PAGE and Western blotting of cell lysates from the luminometry assays. Blots were serially probed with anti-SNX17 antibodies (Santa Cruz), anti-SNX27 antibodies (Abcam), and anti-α-actinin antibodies (Sigma-Aldrich) and were visualized using the Amersham ECL Western blotting detection kit (GE Healthcare).
In vivo expression for coimmunoprecipitation or GST-binding assays.
Proteins were in vitro transcribed, translated, and radiolabeled using a TnT rabbit reticulocyte lysate kit (Promega) labeling with either [35S]methionine or [35S]cysteine. The radiolabeled proteins were incubated with glutathione S-transferase (GST) or GST-fused recombinant proteins in phosphate-buffered saline (PBS) with 1% Triton X-100. After a wash in the same buffer, bound proteins were eluted and separated by SDS-PAGE, and the gel was stained with Coomassie blue, dried, and exposed to X-ray film.
For the binding of GST fusion proteins to proteins expressed in mammalian cells, HEK293 cells were first transfected with plasmids for in vivo expression by the calcium phosphate precipitation method (25) and then lysed in a buffer containing 50 mM HEPES (pH 7.0), 250 mM NaCl, 0.1% NP-40, and 20 mg ml−1 aprotinin. GST or GST-fused recombinant proteins bound to glutathione agarose (Sigma) were incubated with the cell lysates for 1 to 2 h at room temperature; then, after several washes in the lysis buffer, the bound proteins were eluted from the resin in PAGE sample buffer, separated by SDS-PAGE, transferred to nitrocellulose membranes, and probed with appropriate antibodies. Bound proteins were visualized using the Amersham ECL Western blotting detection kit (GE Healthcare).
For detection of the interaction between endogenous SNX27 and 16L2, transfection of the construct expressing Flag-tagged 16L2 (pCA16L2) was carried out as described above, but after 24 h, the cells were lysed in a buffer containing 20 mM Tris (pH 7.5), 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, and 1% Triton X-100. Immunoprecipitations were carried out using 1 μl of anti-SNX27 antibodies (Abcam) or 1 μl of rabbit anti-Flag antibodies (Sigma-Aldrich) for 2 h at 4°C. Then immune complexes were collected using protein A Sepharose that had previously been washed in lysis buffer. After SDS-PAGE separation of the immunoprecipitated proteins and transfer to nitrocellulose membranes, the Western blots were probed with either anti-SNX27 or anti-Flag antibodies to visualize the coimmunoprecipitated proteins. The precipitated proteins were detected using a SuperSignal West Femto Maximum Sensitivity Substrate kit (Pierce).
Immunofluorescence.
H1299 cells were plated out on coverslips at approximately 105 per well. After 24 h, plasmids were transfected by calcium phosphate-mediated transfer (25). After a further 24 h, cells were washed once with PBS, fixed with 3.7% paraformaldehyde for 20 min at room temperature, washed once with PBS, and then permeabilized with 0.5% Triton X-100 in PBS for 5 min at room temperature. After two washes with PBS and one with 0.1 M glycine in PBS, coverslips were incubated with primary antibodies appropriately diluted in PBS for 2 h at 37°C. After three washes with PBS, the coverslips were incubated with fluorescein-conjugated secondary antibodies (Life Technologies) diluted in PBS for 1 h at 37°C. After a further three washes in PBS, and a final wash in distilled water (dH2O), the coverslips were mounted on glass slides for UV microscopy.
RESULTS
Both SNX17 and SNX27 bind to HPV-16 L2.
To examine whether SNX27 can interact with HPV-16 L2 similarly to SNX17, we incubated in vitro-translated, radiolabeled SNX17 and SNX27 with GST alone or GST-fused HPV-16 L2, both bound to glutathione agarose. After several washes, bound proteins were separated by SDS-PAGE, and the gel was stained with Coomassie blue, dried, and exposed to X-ray film. The results (Fig. 1B) show that SNX27, like SNX17, can interact with HPV-16 L2.
HPV-16 L2 binds to endogenous SNX27 in HEK293 cells.
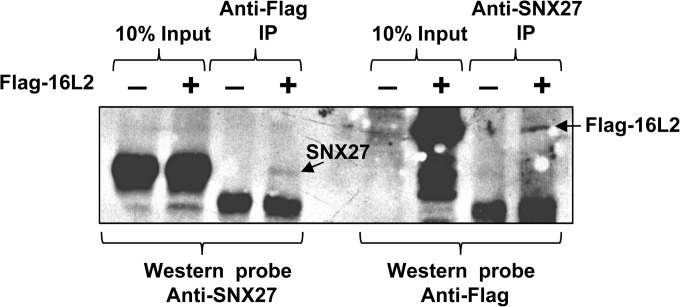
To verify that the HPV-16 L2–SNX27 interaction also occurs in vivo, we transfected HEK293 cells with either an empty plasmid or a plasmid expressing Flag-tagged HPV-16 L2. The whole-cell lysates were divided in two, retaining 10% as an input control, and were immunoprecipitated using anti-Flag or anti-SNX27 antibodies. The immunoprecipitates were analyzed by SDS-PAGE and Western blotting. The membrane from the anti-Flag immunoprecipitation was probed with anti-SNX27 antibodies to detect any SNX27 binding to HPV-16 L2, and the membrane from the anti-SNX27 immunoprecipitation was probed with anti-Flag antibodies to detect any bound HPV-16 L2. The results (Fig. 2) show that HPV-16 L2 can bind to endogenous SNX27 within HEK293 cells, although the levels of interaction between the two proteins appear to be low.
FIG 2.
The interaction between HPV-16 L2 and sorting nexin 27 occurs in vivo. HEK293 cells were transfected with Flag-tagged HPV-16 L2, and extracts were immunoprecipitated (IP) with anti-Flag or anti-SNX27 antibodies. After SDS-PAGE and transfer to membranes by Western blotting, the membranes were probed with anti-SNX27 and anti-Flag antibodies, respectively.
The SNX27-L2 interaction is stronger with HPV-16 than with the other papillomavirus types examined.
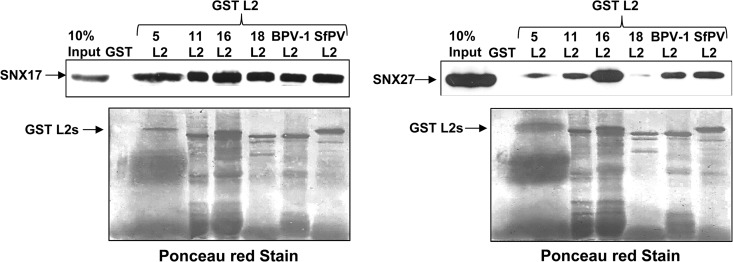
Previous studies have demonstrated that the interaction between L2 and SNX17 is highly conserved in a number of papillomavirus types other than HPV-16 (8). To examine the abilities of other papillomavirus L2 proteins to interact with SNX27, we expressed the L2 proteins from HPV-16, HPV-11, HPV-18, HPV-5, bovine papillomavirus 1 (BPV-1), and Sylvilagus floridanus papillomavirus (SfPV) as GST fusion proteins, bound to glutathione agarose, and incubated them with lysates from HEK293 cells in which either SNX17 or SNX27 was overexpressed. After washing, bound proteins were eluted in sample buffer, separated by SDS-PAGE, and analyzed by Western blotting. Bound proteins were visualized by probing with anti-Flag antibodies (SNX17) or anti-myc antibodies (SNX27). The results (Fig. 3) show that SNX27 binds most strongly to HPV-16 L2, followed by weaker interactions with HPV-11 L2, BPV-1 L2, and SfPV L2, while levels of interaction with HPV-5 L2 and HPV-18 L2 are lower still. This is in contrast to the interactions with SNX17, which are equally strong for all the papillomavirus types tested.
FIG 3.
Comparison of the binding affinities of SNX17 and SNX27 with different papillomavirus L2 proteins. Flag-tagged SNX17 or myc-tagged SNX27a was expressed in HEK293 cells. After 24 h, cells were lysed, and the extracts were incubated either with GST or with one of the indicated L2 proteins, expressed as a GST fusion protein bound to glutathione agarose. Numbers above “L2” indicate HPV types. After washing, bound proteins were eluted and were analyzed by SDS-PAGE and Western blotting. (Top) Bound SNX17 was detected by probing with anti-Flag antibodies (left), and bound SNX27 was detected by probing with anti-myc antibodies (right). (Bottom) Membranes were stained with Ponceau red to show the levels of GST-L2 fusion proteins.
The interactions between HPV-16 L2 and both SNX17 and SNX27 are direct.
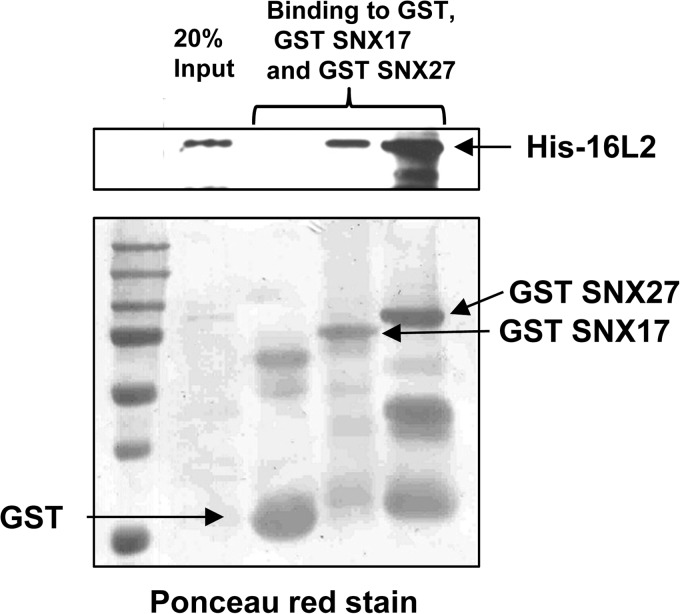
In order to investigate whether the interactions between HPV-16 L2 and the two sorting nexins are direct, we purified recombinant His-tagged HPV-16 L2 and incubated it with purified GST SNX17 and GST SNX27, bound to glutathione agarose. After several washes, we eluted the bound proteins in sample buffer, separated them by SDS-PAGE, transferred them to nitrocellulose membranes, and probed for recombinant L2 with anti-His antibodies. The results (Fig. 4) show that both SNX17 and SNX27 interact directly with HPV-16 L2.
FIG 4.
The interactions between HPV-16 L2 and both SNX17 and SNX27 are direct and are not mediated by other cellular proteins. Recombinant His-tagged HPV-16 L2 was incubated with recombinant GST alone or GST-fused SNX17 or SNX27a, bound to glutathione resin. (Top) After washing, separation by SDS-PAGE, and transfer to membranes for Western blotting, bound proteins were visualized using anti-His antibodies. (Bottom) Levels of GST fusion proteins were visualized by staining the membrane with Ponceau red.
HPV-16 L2 binds to SNX17 and SNX27 via different domains.
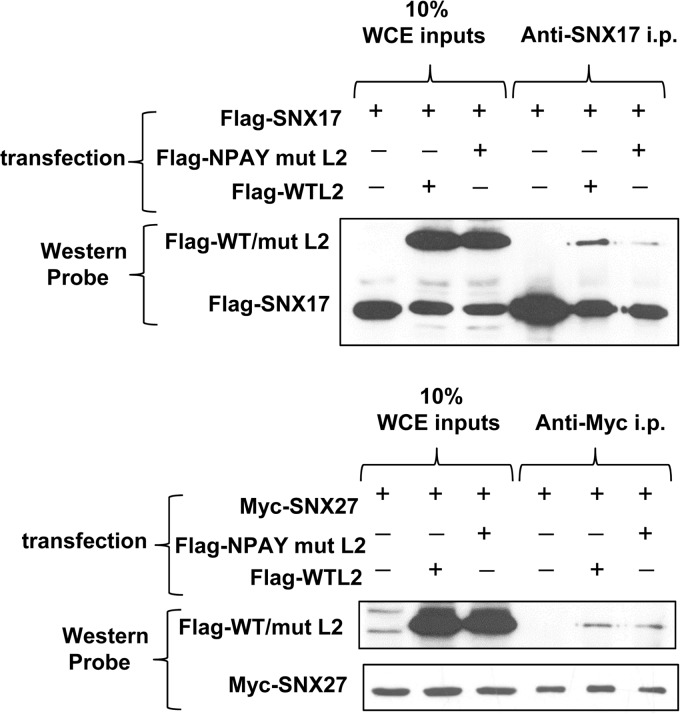
The interaction between papillomavirus L2 proteins and SNX17 has been shown to depend on highly conserved NPxY motifs in the L2 proteins (8). Sequences in the carboxy termini of both SNX17 and SNX27 have been shown to interact with conserved NPxY motifs in some substrates, such as P-selectin (11, 26), and we were interested in determining whether L2 was bound in a similar manner. First, we coexpressed Flag-tagged wild-type HPV-16 L2 or the SNX17 binding-defective mutant of HPV-16 L2 in which NPAY is replaced with AAAA (8) with either myc-tagged SNX27 or Flag-tagged SNX17, and then we immunoprecipitated the cell lysates with either anti-myc antibodies (for SNX27 immunoprecipitations) or anti-SNX17 antibodies (for SNX17 immunoprecipitations). Immunoprecipitated proteins were separated by SDS-PAGE, transferred to nitrocellulose membranes, and probed with anti-Flag antibodies to assess the levels of wild-type and mutant HPV-16 L2 binding to either sorting nexin. After stripping, the membranes were reprobed with anti-myc antibodies to visualize the levels of myc-tagged SNX27. As can be seen in Fig. 5, the NPAY-to-AAAA mutant of L2 binds to SNX27 as efficiently as wild type L2, suggesting that the NPAY binding motif on L2 is not involved in the interaction with SNX27.
FIG 5.
A mutation in the NPxY motif of HPV-16 L2 reduces its interaction with SNX17 but not with SNX27. HEK293 cells were transfected with plasmid constructs expressing Flag-tagged wild-type HPV-16 L2 or Flag-tagged NPAY mutant (mut) L2, together with Flag-tagged SNX17 (top) or myc-tagged SNX27a (bottom). After lysis, the whole-cell extracts (WCE) were immunoprecipitated (i.p.) with anti-SNX17 antibodies (top) or anti-myc antibodies (bottom). After separation by SDS-PAGE and transfer to membranes for Western blotting, bound WT or mutant L2 was detected with anti-Flag antibodies.
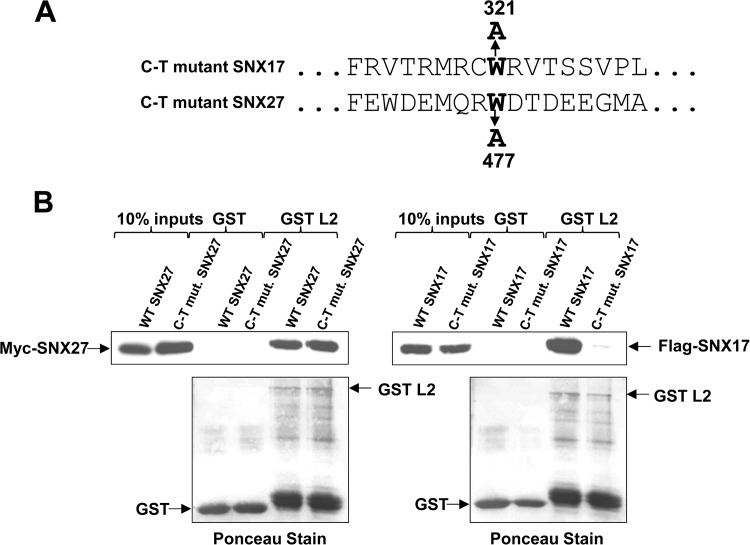
To confirm that the carboxy-terminal NPxY motif-binding region of SNX27 is not required for its interaction with L2, we made mutations in both SNX17 and SNX27 (Fig. 6A) that had been shown previously to abolish P-selectin binding (26), expressed the mutants in HEK293 cells, and incubated the cell lysates with GST or GST-L2 bound to glutathione resin. After washing, separation of bound proteins by SDS-PAGE, and transfer to membranes, bound proteins were visualized by probing with anti-myc (for SNX27) or anti-Flag (for SNX17) antibodies. The results, shown in Fig. 6B, indicate clearly that while the mutation in the C terminus of SNX17 abolishes its interaction with L2, the corresponding mutation in SNX27 does not.
FIG 6.
Different domains of SNX17 and SNX27 are involved in their interactions with HPV-16 L2. (A) Mutations in the carboxy termini of SNX17 and SNX27 that were previously shown to abolish interaction with NPxY motif-containing cargos. (B) The carboxy-terminal (C-T) mutation that abolishes the interaction of SNX17 with HPV-16 L2 (right) does not prevent the binding of L2 to SNX27a (left).
Taken together, these data show conclusively that the residues required for the L2–SNX27 interaction are completely different from those required for the interaction with SNX17.
HPV-16 L2 interacts with the PDZ domain of SNX27.
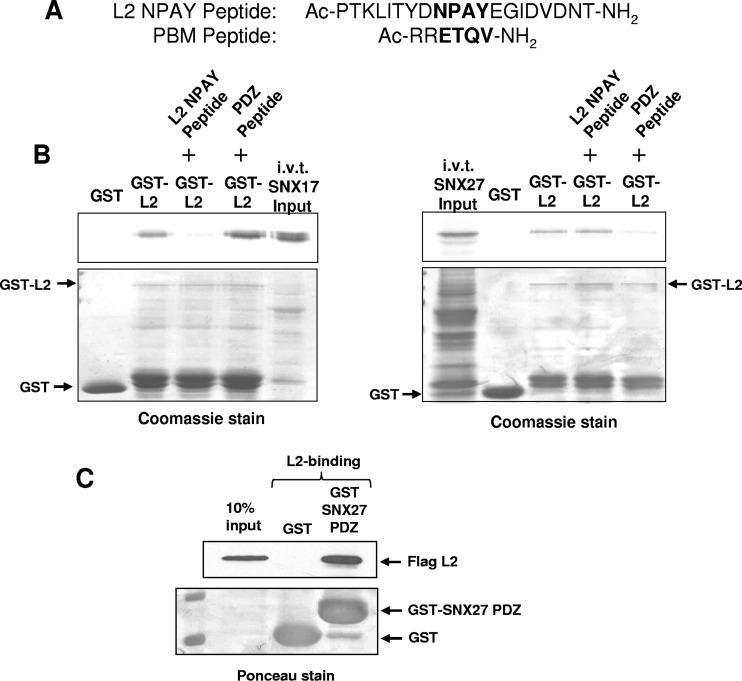
To analyze which region of SNX27 might be involved in the interaction with L2, we incubated GST or GST-L2 bound to glutathione resin with lysates from HEK293 cells overexpressing either the wild-type SNX27a or SNX27b isoform (shown schematically in Fig. 1A) (SNX27a has a C-terminal stretch of 15 amino acids that replace 2 residues in isoform b [14]) or mutants of SNX27b lacking the PDZ domain (SNX27b ΔPDZ) or the FERM domain (SNX27b ΔFERM) (shown schematically in Fig. 7A). After washing, bound proteins were eluted in sample buffer and were separated by SDS-PAGE. After transfer to nitrocellulose membranes, interacting proteins were visualized by probing with anti-myc antibodies. The results (Fig. 7B) show that the interaction between HPV-16 L2 and SNX27 requires an intact PDZ domain. We note that the ΔFERM mutant runs considerably faster than would be predicted considering the number of residues removed, but this is accordance with previous observations (Martin P. Playford, personal observations). To confirm this finding, we used peptides derived from the NPAY region of HPV-16 L2 and a PDZ-binding motif (PBM) derived from the carboxyl terminus of HPV-18 E6 (Fig. 8A) to attempt to block the interaction between HPV-16 L2 and either SNX17 or SNX27. We preincubated GST or GST-L2 bound to glutathione agarose with the peptides and then added in vitro-translated, radiolabeled SNX17 or SNX27. The results (Fig. 8B) demonstrate that the PBM-derived peptide inhibits the interaction between HPV-16 L2 and SNX27, but not that between HPV-16 L2 and SNX17. Similarly, the NPAY motif-derived peptide inhibits the interaction between L2 and SNX17, but not that between L2 and SNX27. These results strongly suggest that the interaction of L2 with SNX27 is mediated by the SNX27 PDZ domain. To confirm this, we purified the isolated PDZ domain of SNX27 as a GST fusion protein, bound to glutathione agarose, and incubated it with a cell extract in which 16L2 was expressed. The results (Fig. 8C) show that this domain alone is capable of interacting with L2.
FIG 8.
Identification of the SNX27 PDZ domain for HPV-16 L2 binding. Shown is peptide inhibition of the interaction of SNX17 or SNX27 with HPV-16 L2. (A) Peptides used in the inhibition assay. The top peptide is derived from residues that constitute the identified NPxY binding site for SNX17 on L2. The bottom peptide is derived from the HPV-18 E6 PDZ binding domain (PBM). (B) (Left) The interaction between in vitro-translated SNX17 and GST-fused HPV-16 L2 can be inhibited by the NPAY peptide but not by the PBM peptide. (Right) The interaction between in vitro-translated SNX27a and GST-fused HPV-16 L2 can be inhibited by the PBM peptide but not by the NPAY peptide. (C) The isolated PDZ domain of SNX27 expressed as a GST fusion can bind to HPV-16 L2 expressed in HEK293 cells.
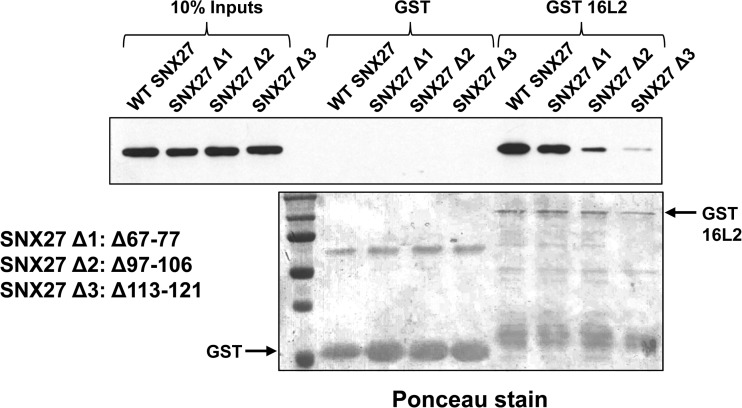
Since papillomavirus L2 proteins do not contain a typical carboxy-terminal PBM, we asked how L2 might be able to interact with the PDZ domain of SNX27. Based on X-ray crystallographic and nuclear magnetic resonance (NMR) structure predictions of the SNX27 PDZ domain (27), we made three large deletions in the SNX27 PDZ domain to investigate which residues might interact with 16L2. One unique feature of the SNX27 PDZ domain is a stretch of amino acid residues upstream of the core PDZ pocket that mediate binding between SNX27 and the VPS26 subunit of the retromer complex, thus allowing SNX27 to link to the complex while still permitting cargos with a PBM to bind to the SNX27 PDZ domain (28) and also increasing the affinity of the PBM-PDZ interaction (27). We speculated that the same stretch of amino acids might be responsible for the SNX27–L2 interaction and made a deletion mutant, Δ67–77, that lacks this region and that has been shown to be unable to interact with VPS26 (28). However, when we incubated 16L2 as a GST fusion protein with a cell extract in which this mutant protein was expressed, we observed binding at wild-type levels (Fig. 9). In contrast, two deletion mutants that remove residues deemed to be part of the PDZ domain based on structural analysis (27)—the Δ97–106 mutant, which perturbs a β-sheet, and the Δ113–121 mutant, which perturbs an α-helix—show greatly reduced ability to bind to GST L2 (Fig. 9), suggesting that it is residues within the PDZ domain itself that are involved in the interaction with L2.
FIG 9.
Dissection of the regions within the SNX27 PDZ domain that are required for its interaction with HPV-16 L2. SNX27a deletion mutant Δ1 has been shown to be unable to bind to the retromer complex subunit Vps26 while retaining the ability to bind cargos with a PBM; this mutation fails to abolish the interaction with L2. Mutants Δ2 and Δ3 have lost critical structural features within the core PDZ domain and have greatly reduced abilities to bind L2.
Identification of the HPV-16 L2 amino acid residues involved in the interaction with SNX27.
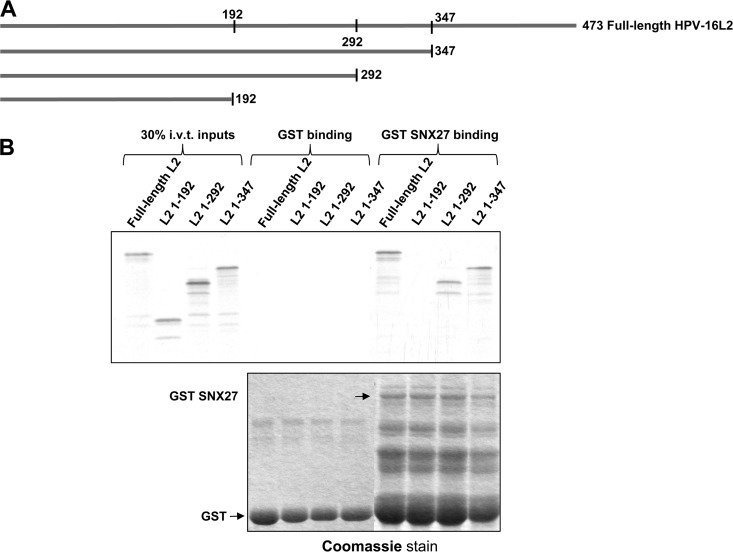
The finding that HPV-16 L2 interacts with the PDZ domain of SNX27 was unexpected, since L2 lacks a classical carboxy-terminal class 1 PBM. Although internal PBMs have been reported, and some residues within L2 might be considered to fall into this consensus, it is not clear whether such putative PBMs lie in regions of L2 where the molecular conformation would position them optimally for interaction with a PDZ domain. However, we note that sequence analysis of several papillomavirus L2 proteins by a Eukaryotic Linear Motif (ELM) predictive program suggests that the C termini of some (though not all) L2 proteins constitute class 2 PBMs. To search for the region of L2 involved in the interaction with SNX27, we made a series of L2 truncation mutants by inserting stop codons into the L2 open reading frame to generate proteins that terminate after amino acid 192, 292, or 347 (Fig. 10A). Wild-type 16L2 and each of these mutants were first in vitro translated and radiolabeled and then incubated with either GST alone or GST-SNX27, both bound to glutathione agarose. After washing, bound proteins were eluted and analyzed by SDS-PAGE and autoradiography. The results are shown in Fig. 10B, where it can be seen that the L2 truncation construct terminating at amino acid 192 was unable to bind to SNX27 but that the remaining L2 truncation mutants bound as well as full-length L2. This also shows that sequences in the carboxy terminus of L2 are not directly involved in the interaction with SNX27.
FIG 10.
Identification of the region of L2 required for the interaction with SNX27. (A) Deletion mutants of HPV-16 used in the experiment. (B) Binding of in vitro-translated, radiolabeled full-length 16L2 and truncation mutants to GST-fused SNX27a indicates that residues between 192 and 292 may be involved in binding.
Both siRNA knockdown of SNX17 and siRNA knockdown of SNX27 affect papillomavirus pseudovirion infection.
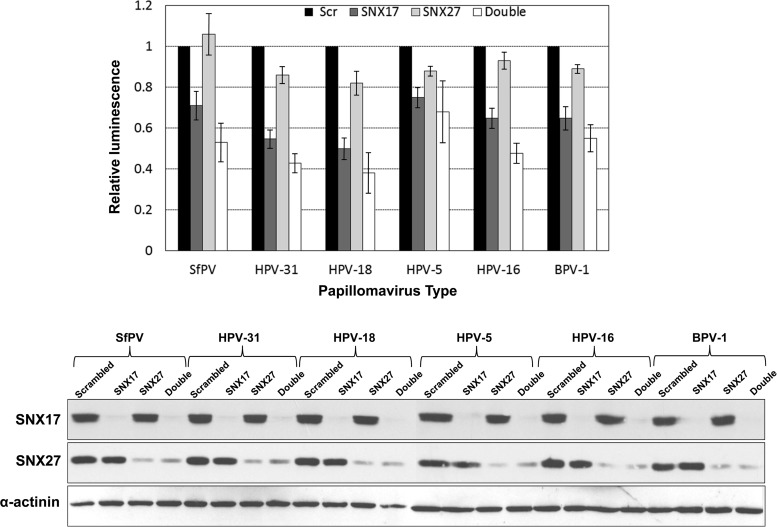
To study the role played by SNX27 in papillomavirus infection, we used a well-established pseudovirion infection system (29). We selectively downregulated SNX27 or SNX17 expression in HaCaT cells by siRNA treatment. Forty-eight hours later, we infected the cells with pseudovirions in which a luciferase reporter gene had been packaged and then monitored the infection efficiency by detection of luciferase activity in cell lysates 48 h postinfection. We used SfPV, HPV-31, HPV-18, HPV-16, HPV-5, and BPV-1 pseudovirions, with siSNX17 as a positive control and a scrambled siRNA as a negative control. The results are shown in Fig. 11, where it can be seen clearly that siRNA knockdown of SNX17 reduces infection efficiency, as demonstrated previously (8). In contrast, siRNA knockdown of SNX27 leads to only a marginal reduction in infection efficiency for most of the papillomavirus types studied, but siRNA knockdown of both SNX17 and SNX27 results in a further reduction of infection efficiency, greater than that observed for SNX17 knockdown alone.
FIG 11.
Effects of siRNA knockdown of SNX17, SNX27, and both sorting nexins together on the infectivities of luciferase-packaged pseudovirions from a range of papillomaviruses. (Top) As reported previously, siRNA knockdown of SNX17 has a marked effect on the efficiency of papillomavirus infection (8). While knockdown of SNX27 by itself has little effect on infectivity, double knockdown of both sorting nexins has an additive effect, greater than that observed for SNX17 knockdown alone. The data shown are mean infection efficiencies relative to that of the control and are derived from at least 3 separate experiments. Error bars indicate standard deviations. (Bottom) The efficiencies of SNX17 and SNX27 knockdowns were assayed by Western blot analysis of the cell lysates used for a typical luciferase assay, by probing serially with antibodies against SNX27, SNX17, and α-actinin.
To examine the statistical significance of this finding, we performed two-way analysis of variance (ANOVA) evaluating the effects of different siSNX treatments on the various PV types. The analysis shows that infection efficiency, as reflected by luciferase expression, is highly dependent on the siSNX treatment (P < 0.0001), while the differences observed between different PV types are not significant (P, 0.327 by two-way ANOVA). When we further compared different siRNA treatments, we observed significantly lower infectivity with siSNX17 (P < 0.0001) or the double knockdown (siSNX27 and siSNX17) (P < 0.0001)—but, as expected, not with siSNX27—than with siScr. Also as expected, comparisons between different siSNX treatments confirmed significantly lower infectivity for siSNX17-treated cells than for siSNX27-treated cells (P < 0.0001). More interestingly, double silencing of SNX27 and SNX17 is significantly more effective at eliminating general PV infection than the single siSNX27 (P < 0.0001) or even siSNX17 (P, <0.001 by two-way ANOVA and Tukey's multiple-comparison test) silencing. Although we typically observe an increase in infection efficiency when using the control scrambled siRNA over that obtained using no siRNA, this increase is considered to be nonspecific, and we observed no further changes in infection efficiency when excess amounts of siScr were added to the siRNA against either of the sorting nexins (data not shown). Consequently, for all siRNA transfections, the control siRNA, siScr, was used to balance inputs, as described in Materials and Methods. siRNA knockdown efficiency was verified by Western blot analysis of the cell lysates, probing sequentially for SNX27, SNX17, and α-actinin (used as a control). These results suggest the involvement of both SNX17 and SNX27 in controlling the entry of L2 and associated viral DNA into the nucleus.
16L2, when expressed ectopically together with SNX17, has a different cellular localization than when it is expressed by itself or together with SNX27.
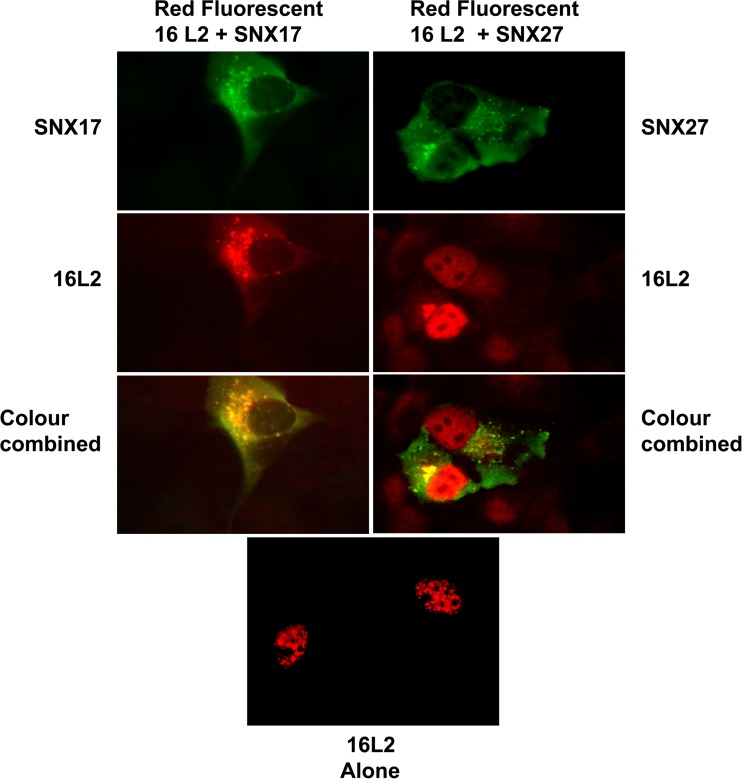
Although the result presented above suggests a level of functional redundancy between SNX17 and SNX27 with regard to pseudovirion infection, we wanted to determine any effects of ectopic expression of 16L2 with either SNX17 or SNX27 on its cellular localization. Red fluorescent 16L2 was transfected into H1299 cells on coverslips together with either myc-tagged SNX27 or Flag-tagged SNX17. After 24 h, cells were fixed and permeabilized, and the relevant proteins were detected by incubation with either mouse anti-SNX27 antibodies or rabbit anti-Flag antibodies (for SNX17), followed by the relevant fluorescein-labeled secondary antibodies. The results (Fig. 12) show that after 24 h, L2 expressed alone is localized to the nucleus. Ectopic expression of SNX27 together with L2 does not change the localization of L2 at this time point, although some colocalization of L2 and SNX27 can be observed adjacent to the nucleus in regions reminiscent of the trans-Golgi network. In contrast, L2 is localized mostly to the cytoplasm in the presence of ectopically expressed SNX17.
FIG 12.
Effects of SNX17 and SNX27 overexpression on the localization of HPV-16 L2. H1299 cells were transfected with a plasmid expressing red fluorescent HPV-16 L2 with or without plasmid expression of SNX17 or SNX27a. Then the cells were fixed at 24 h posttransfection. HPV-16 L2 expressed alone or in the presence of overexpressed SNX27 localizes to the nucleus, whereas in the presence of overexpressed SNX17, L2 is retained in the cytoplasm.
DISCUSSION
Previous studies showed that sorting nexin 17 plays an important role in papillomavirus infection, since its siRNA-mediated knockdown reduces the levels of detectable L2 in late endosomes and impairs pseudovirion infection (8). In the present study, we have analyzed the role played by the related PX FERM-like domain protein sorting nexin 27. We show that its siRNA-mediated knockdown has a more limited effect on pseudovirion infection efficiency than SNX17 knockdown but that when both sorting nexins are knocked down, there is a further reduction in infection efficiency. This finding might argue that the two nexins play similar roles during L2 trafficking, but overexpression of these nexins has differing consequences in terms of L2 cellular localization. SNX27 overexpression does not alter the nuclear localization that is observed when HPV-16 L2 is expressed alone, whereas overexpression of SNX17 leads to cytoplasmic sequestration of L2. This result suggests that the consequences of the interaction between SNX17 and L2 may be different from those of interaction between L2 and SNX27.
Several lines of evidence suggest that SNX27 binds to HPV-16 L2 in a manner different from that of SNX17. An important common mechanism for cargo selection by both SNX17 and SNX27 involves residues in their carboxy termini. The mutations T321A within SNX17 and T447A within SNX27 both abolish binding to the cargo P-selectin through its NPAY motif (11, 26) but have different effects on binding to HPV-16 L2; the mutation in SNX17 abolishes its interaction with HPV-16 L2, while the mutation in SNX27 has no effect. In support of this observation, mutation of the NPAY motif within HPV-16 L2 markedly reduces its interaction with SNX17 but has no effect on its interaction with SNX27. However, because SNX27 has an additional amino-terminal PDZ domain that can also bind to cargos, we investigated this as a possible HPV-16 L2-interactive domain. Indeed, deletion analysis of SNX27 indicates that an intact PDZ domain is required for its interaction with HPV-16 L2, and the isolated PDZ domain of SNX27 is sufficient for its interaction with HPV-16 L2. In support of this, we have shown that the interaction between SNX17 and GST-L2 can be blocked by using an L2-derived peptide spanning the NPAY motif that was previously shown to be required for this interaction. The same peptide fails to block the interaction between SNX27 and GST-L2, whereas that interaction is blocked by a peptide derived from the C terminus of HPV-18 E6 that has been shown to block the interaction of E6 with a PDZ domain.
The three deletion mutations made within the SNX27 PDZ domain show that the PDZ binding region itself (rather than the external VPS26 binding loop, with which the PDZ domain binds the retromer complex) interacts with L2. The deletions made in SNX27 mutants Δ2 and Δ3 are large, and while their L2 binding is strongly reduced, it is not completely abolished, an observation that argues against any kind of classical PDZ-PBM interaction, given that the mutations greatly perturb previously identified structural elements within the PDZ domain (27).
Although HPV-16 L2 does not have a classical PDZ-binding motif at its carboxy terminus, analysis of the Eukaryotic Linear Motif (ELM) database suggested that at least some of the papillomavirus L2 proteins used in this study might have a carboxy-terminal class 2 PDZ binding motif. However, expression of L2 mutants truncated at the C terminus identifies a region of L2 between amino acid residues 192 and 292 (rather than the C terminus) that is clearly involved in the interaction with SNX27. Further studies are required to identify the residues within this region that are responsible for the SNX27 interaction.
Recent studies have shown an interaction between L2 and the retromer complex (22); however, the regions of L2 that are involved in this interaction are not involved in the interaction with SNX27, indicating that the interaction of L2 with the retromer is probably not mediated through its interaction with SNX27. This might also explain why SNX27 knockdown may by itself be insufficient to inhibit retromer-dependent trafficking.
While we can show an interaction between 16L2 and endogenous SNX27 in HEK293 cells, the level of this interaction is clearly extremely low. There may be several reasons for this: possibly only a specific and limited pool of SNX27 interacts with 16L2, or the interaction may be extremely transient during the trafficking process. A limited pool of L2-interacting SNX27 might also explain why we observed very little effect on HPV-16 pseudovirion infection when SNX27 alone was knocked down by siRNA treatment. We do, however, see, as a general trend, an additive effect in the reduction of infection efficiency when both SNX17 and SNX27 are knocked down, and this effect seems to have a degree of statistical significance. These results demonstrate a degree of functional redundancy between SNX17 and SNX27 in terms of the roles they play during papillomavirus infection. Knockdown of either does not completely abolish infection, although the role played by SNX17 appears to be more essential, whereas knockdown of both sorting nexins seems to have an additive, deleterious effect on papillomavirus entry. While SNX17 has been shown to be a component of recycling endosomes, its relationships to the Wiskott-Aldrich syndrome homologue (WASH) complex and the retromer complex are not well understood. The WASH complex is required in recycling endosomes for the recruitment of retromer complexes to the tail of the WASH subunit FAM21 (30). Like FAM21, SNX27 also provides a link between the two complexes and is able to transport endosomal cargos through either its C-terminal binding region or its PDZ domain. The binding of L2 to the PDZ domain, but not the C terminus, of SNX27 is likely to be significant, and we are currently investigating whether this interaction blocks the ability of SNX27 to simultaneously bind the retromer complex via its interaction with the VPS26 subunit. We predict that this would not be the case, since recruitment of the retromer to the WASH complex through binding of the FAM21 tail appears to be required for endosomal tubulation and trans-Golgi transport of bound cargos, as opposed to recycling to the plasma membrane (31). If SNX17 should prove to link to the WASH complex, this might explain why siRNA knockdown of proteins from both complexes would have a greater effect on L2 trafficking into the nucleus via the trans-Golgi network than the knockdown of proteins from either complex alone.
Finally, it is worth bearing in mind that the L2 protein is required at two time points in the viral life cycle. While we have investigated the consequences of the L2–SNX27 interaction during viral entry, this interaction could also have relevance at the end of the viral life cycle, when capsid proteins are synthesized prior to encapsidation of the viral DNA.
REFERENCES
- 1.Bousarghin L, Touze A, Sizaret PY, Coursaget P. 2003. Human papillomavirus types 16, 31, and 58 use different endocytosis pathways to enter cells. J Virol 77:3846–3850. doi: 10.1128/JVI.77.6.3846-3850.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schiller JT, Day PM, Kines RC. 2010. Current understanding of the mechanism of HPV infection. Gynecol Oncol 118(1 Suppl):S12–S17. doi: 10.1016/j.ygyno.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Raff AB, Woodham AW, Raff LM, Skeate JG, Yan L, Da Silva DM, Schelhaas M, Kast WM. 2013. The evolving field of human papillomavirus receptor research: a review of binding and entry. J Virol 87:6062–6072. doi: 10.1128/JVI.00330-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Day PM, Thompson CD, Schowalter RM, Lowy DR, Schiller JT. 2013. Identification of a role for the trans-Golgi network in human papillomavirus 16 pseudovirus infection. J Virol 87:3862–3870. doi: 10.1128/JVI.03222-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aydin I, Weber S, Snijder B, Samperio Ventayol P, Kühbacher A, Becker M, Day PM, Schiller JT, Kann M, Pelkmans L, Helenius A, Schelhaas M. 2014. Large scale RNAi reveals the requirement of nuclear envelope breakdown for nuclear import of human papillomaviruses. PLoS Pathog 10:e1004162. doi: 10.1371/journal.ppat.1004162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Day PM, Baker CC, Lowy DR, Schiller JT. 2004. Establishment of papillomavirus infection is enhanced by promyelocytic leukemia protein (PML) expression. Proc Natl Acad Sci U S A 101:14252–14257. doi: 10.1073/pnas.0404229101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bienkowska-Haba M, Williams C, Kim SM, Garcea RL, Sapp M. 2012. Cyclophilins facilitate dissociation of the human papillomavirus type 16 capsid protein L1 from the L2/DNA complex following virus entry. J Virol 86:9875–9887. doi: 10.1128/JVI.00980-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bergant M, Banks L. 2013. SNX17 facilitates infection by diverse papillomavirus types. J Virol 87:1270–1273. doi: 10.1128/JVI.01991-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Florin L, Becker KA, Lambert C, Nowak T, Sapp C, Strand D, Streeck RE, Sapp M. 2006. Identification of a dynein interacting domain in the papillomavirus minor capsid protein L2. J Virol 80:6691–6696. doi: 10.1128/JVI.00057-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huotari J, Helenius A. 2011. Endosome maturation. EMBO J 30:3481–3500. doi: 10.1038/emboj.2011.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Knauth P, Schlüter T, Czubayko M, Kirsch C, Florian V, Schreckenberger S, Hahn H, Bohnensack R. 2005. Functions of sorting nexin 17 domains and recognition motif for P-selectin trafficking. J Mol Biol 347:813–825. doi: 10.1016/j.jmb.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 12.Bergant Marušič M, Ozbun MA, Campos SK, Myers MP, Banks L. 2012. Human papillomavirus L2 facilitates viral escape from late endosomes via sorting nexin 17. Traffic 13:455–467. doi: 10.1111/j.1600-0854.2011.01320.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cullen PJ. 2008. Endosomal sorting and signalling: an emerging role for sorting nexins. Nat Rev Mol Cell Biol 9:574–582. doi: 10.1038/nrm2427. [DOI] [PubMed] [Google Scholar]
- 14.Cai L, Loo LS, Atlashkin V, Hanson BJ, Hong W. 2011. Deficiency of sorting nexin 27 (SNX27) leads to growth retardation and elevated levels of N-methyl-d-aspartate receptor 2C (NR2C). Mol Cell Biol 31:1734–1747. doi: 10.1128/MCB.01044-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Joubert L, Hanson B, Barthet G, Sebben M, Claeysen S, Hong W, Dumuis A, Bockaert J. 2004. New sorting nexin (SNX27) and NHERF specifically interact with the 5-HT4a receptor splice variant: roles in receptor targeting. J Cell Sci 117:5367–5379. doi: 10.1242/jcs.01379. [DOI] [PubMed] [Google Scholar]
- 16.MacNeil AJ, Mansour M, Pohajdak B. 2007. Sorting nexin 27 interacts with the Cytohesin associated scaffolding protein (CASP) in lymphocytes. Biochem Biophys Res Commun 359:848–853. doi: 10.1016/j.bbrc.2007.05.162. [DOI] [PubMed] [Google Scholar]
- 17.Zimmerman SP, Hueschen CL, Malide D, Milgram SL, Playford MP. 2013. Sorting nexin 27 (SNX27) associates with zonula occludens-2 (ZO-2) and modulates the epithelial tight junction. Biochem J 455:95–106. doi: 10.1042/BJ20121755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lauffer BE, Melero C, Temkin P, Lei C, Hong W, Kortemme T, von Zastrow M. 2010. SNX27 mediates PDZ-directed sorting from endosomes to the plasma membrane. J Cell Biol 190:565–574. doi: 10.1083/jcb.201004060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Temkin P, Lauffer B, Jäger S, Cimermancic P, Krogan NJ, von Zastrow M. 2011. SNX27 mediates retromer tubule entry and endosome-to-plasma membrane trafficking of signalling receptors. Nat Cell Biol 13:715–721. doi: 10.1038/ncb2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nakagawa T, Asahi M. 2013. β1-Adrenergic receptor cycles via a membranous organelle, recycling endosome, by binding with sorting nexin 27. J Membr Biol 246:571–579. doi: 10.1007/s00232-013-9571-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lipovsky A, Popa A, Pimienta G, Wyler M, Bhan A, Kuruvilla L, Guie MA, Poffenberger AC, Nelson CD, Atwood WJ, DiMaio D. 2013. Genome-wide siRNA screen identifies the retromer as a cellular entry factor for human papillomavirus. Proc Natl Acad Sci U S A 110:7452–7457. doi: 10.1073/pnas.1302164110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Popa A, Zhang W, Harrison MS, Goodner K, Kazakov T, Goodwin EC, Lipovsky A, Burd CG, DiMaio D. 2015. Direct binding of retromer to human papillomavirus type 16 minor capsid protein L2 mediates endosome exit during viral infection. PLoS Pathog 11:e1004699. doi: 10.1371/journal.ppat.1004699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Burden JJ, Sun XM, Garcia AB, Soutar AK. 2004. Sorting motifs in the intracellular domain of the low density lipoprotein receptor interact with a novel domain of sorting nexin-17. J Biol Chem 279:16237–16245. doi: 10.1074/jbc.M313689200. [DOI] [PubMed] [Google Scholar]
- 24.Buck CB, Pastrana DV, Lowy DR, Schiller JT. 2005. Generation of HPV pseudovirions using transfection and their use in neutralization assays. Methods Mol Med 119:445–462. [DOI] [PubMed] [Google Scholar]
- 25.Graham FL, van der Eb AJ. 1973. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology 52:456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- 26.Ghai R, Bugarcic A, Liu H, Norwood SJ, Skeldal S, Coulson EJ, Li SS, Teasdale RD, Collins BM. 2013. Structural basis for endosomal trafficking of diverse transmembrane cargos by PX-FERM proteins. Proc Natl Acad Sci U S A 110:643–652. doi: 10.1073/pnas.1216229110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gallon M, Clairfeuille T, Steinberg F, Mas C, Ghai R, Sessions RB, Teasdale RD, Collins BM, Cullen PJ. 2014. A unique PDZ domain and arrestin-like fold interaction reveals mechanistic details of endocytic recycling bySNX27-retromer. Proc Natl Acad Sci U S A 111:E3604–E3613. doi: 10.1073/pnas.1410552111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Steinberg F, Gallon M, Winfield M, Thomas EC, Bell AJ, Heesom KJ, Tavaré JM, Cullen P. 2013. A global analysis of SNX27-retromer assembly and cargo specificity reveals a function in glucose and metal ion transport. Nat Cell Biol 15:461–471. doi: 10.1038/ncb2721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Buck CB, Thompson CD. 2007. Production of papillomavirus-based gene transfer vectors. Curr Protoc Cell Biol Chapter 26:Unit 26.1. doi: 10.1002/0471143030.cb2601s37. [DOI] [PubMed] [Google Scholar]
- 30.Harbour ME, Breusegem SY, Seaman MN. 2012. Recruitment of the endosomal WASH complex is mediated by the extended ‘tail’ of Fam21 binding to the retromer protein Vps35. Biochem J 442:209–220. doi: 10.1042/BJ20111761. [DOI] [PubMed] [Google Scholar]
- 31.McGough IJ, Steinberg F, Jia D, Barbuti PA, McMillan KJ, Heesom KJ, Whone AL, Caldwell MA, Billadeau DD, Rosen MK, Cullen PJ. 2014. Retromer binding to FAM21 and the WASH complex is perturbed by the Parkinson disease-linked VPS35 (D620N) mutation. Curr Biol 24:1670–1676. doi: 10.1016/j.cub.2014.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]