Abstract
The expression of several differentiation markers in normal human mammary gland myoepithelium and in certain stromal fibroblasts ("myofibroblasts") associated with breast carcinomas was studied by immunofluorescence microscopy of frozen sections. Several antibodies to smooth muscle-specific proteins (smooth muscle alpha-actin, smooth muscle myosin heavy chains, calponin, alpha 1-integrin, and high molecular weight caldesmon) and to epithelial-specific proteins (cytokeratins, E-cadherin, and desmoplakin) were used to show that myoepithelial cells concomitantly express epithelial and smooth muscle markers whereas adjacent luminal cells express only epithelial markers. The same antibodies were used to establish that stromal myofibroblasts exhibit smooth muscle phenotypic properties characterized by the expression of all the smooth muscle markers examined except for high molecular weight caldesmon. In addition, both myoepithelium and myofibroblasts show a significant degree of heterogeneity in smooth muscle protein expression. Thus, myoepithelial cells and stromal myofibroblasts are epithelial and mesenchymal cells, respectively, which coordinately express a set of smooth muscle markers while maintaining their specific original features. The dual nature of myoepithelial cells and the phenotypic transition of fibroblasts to myofibroblasts are examples of the plasticity of the differentiated cell phenotype.
Full text
PDF
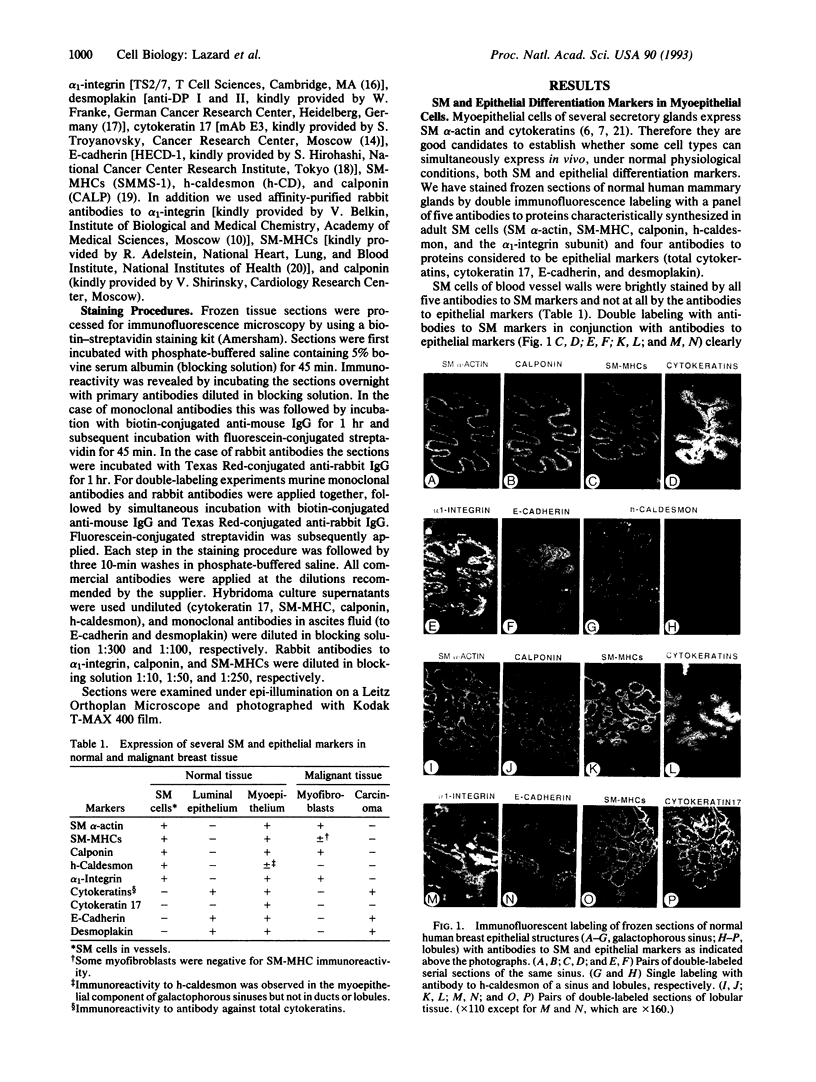
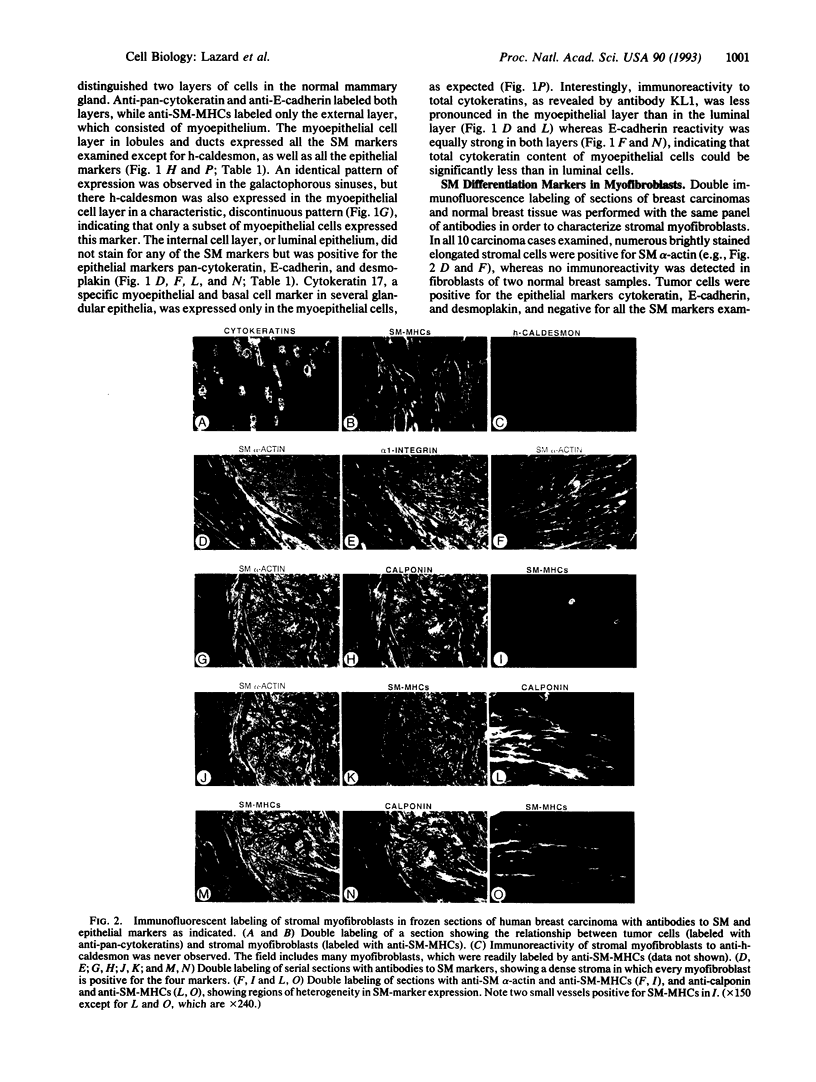


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Belkin V. M., Belkin A. M., Koteliansky V. E. Human smooth muscle VLA-1 integrin: purification, substrate specificity, localization in aorta, and expression during development. J Cell Biol. 1990 Nov;111(5 Pt 1):2159–2170. doi: 10.1083/jcb.111.5.2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blau H. M., Baltimore D. Differentiation requires continuous regulation. J Cell Biol. 1991 Mar;112(5):781–783. doi: 10.1083/jcb.112.5.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blau H. M., Pavlath G. K., Hardeman E. C., Chiu C. P., Silberstein L., Webster S. G., Miller S. C., Webster C. Plasticity of the differentiated state. Science. 1985 Nov 15;230(4727):758–766. doi: 10.1126/science.2414846. [DOI] [PubMed] [Google Scholar]
- Boyer B., Thiery J. P. Epithelial cell adhesion mechanisms. J Membr Biol. 1989 Dec;112(2):97–108. doi: 10.1007/BF01871271. [DOI] [PubMed] [Google Scholar]
- Cintorino M., Bellizzi de Marco E., Leoncini P., Tripodi S. A., Xu L. J., Sappino A. P., Schmitt-Gräff A., Gabbiani G. Expression of alpha-smooth-muscle actin in stromal cells of the uterine cervix during epithelial neoplastic changes. Int J Cancer. 1991 Apr 1;47(6):843–846. doi: 10.1002/ijc.2910470609. [DOI] [PubMed] [Google Scholar]
- Darby I., Skalli O., Gabbiani G. Alpha-smooth muscle actin is transiently expressed by myofibroblasts during experimental wound healing. Lab Invest. 1990 Jul;63(1):21–29. [PubMed] [Google Scholar]
- Franke W. W., Schmid E., Freudenstein C., Appelhans B., Osborn M., Weber K., Keenan T. W. Intermediate-sized filaments of the prekeratin type in myoepithelial cells. J Cell Biol. 1980 Mar;84(3):633–654. doi: 10.1083/jcb.84.3.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frid M. G., Shekhonin B. V., Koteliansky V. E., Glukhova M. A. Phenotypic changes of human smooth muscle cells during development: late expression of heavy caldesmon and calponin. Dev Biol. 1992 Oct;153(2):185–193. doi: 10.1016/0012-1606(92)90104-o. [DOI] [PubMed] [Google Scholar]
- Glukhova M. A., Kabakov A. E., Frid M. G., Ornatsky O. I., Belkin A. M., Mukhin D. N., Orekhov A. N., Koteliansky V. E., Smirnov V. N. Modulation of human aorta smooth muscle cell phenotype: a study of muscle-specific variants of vinculin, caldesmon, and actin expression. Proc Natl Acad Sci U S A. 1988 Dec;85(24):9542–9546. doi: 10.1073/pnas.85.24.9542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glukhova M. A., Kabakov A. E., Ornatsky O. I., Vasilevskaya T. D., Koteliansky V. E., Smirnov V. N. Immunoreactive forms of caldesmon in cultivated human vascular smooth muscle cells. FEBS Lett. 1987 Jun 29;218(2):292–294. doi: 10.1016/0014-5793(87)81064-5. [DOI] [PubMed] [Google Scholar]
- Glukhova M. A., Shekhonin B. V., Kruth H., Koteliansky V. E. Expression of cytokeratin 8 in human aortic smooth muscle cells. Am J Physiol. 1991 Oct;261(4 Suppl):72–77. doi: 10.1152/ajpheart.1991.261.4.72. [DOI] [PubMed] [Google Scholar]
- Gown A. M., Boyd H. C., Chang Y., Ferguson M., Reichler B., Tippens D. Smooth muscle cells can express cytokeratins of "simple" epithelium. Immunocytochemical and biochemical studies in vitro and in vivo. Am J Pathol. 1988 Aug;132(2):223–232. [PMC free article] [PubMed] [Google Scholar]
- Gown A. M. The mysteries of the myofibroblast (partially) unmasked. Lab Invest. 1990 Jul;63(1):1–3. [PubMed] [Google Scholar]
- Gown A. M., Vogel A. M., Gordon D., Lu P. L. A smooth muscle-specific monoclonal antibody recognizes smooth muscle actin isozymes. J Cell Biol. 1985 Mar;100(3):807–813. doi: 10.1083/jcb.100.3.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gugliotta P., Sapino A., Macrí L., Skalli O., Gabbiani G., Bussolati G. Specific demonstration of myoepithelial cells by anti-alpha smooth muscle actin antibody. J Histochem Cytochem. 1988 Jun;36(6):659–663. doi: 10.1177/36.6.3367051. [DOI] [PubMed] [Google Scholar]
- Hemler M. E., Sanchez-Madrid F., Flotte T. J., Krensky A. M., Burakoff S. J., Bhan A. K., Springer T. A., Strominger J. L. Glycoproteins of 210,000 and 130,000 m.w. on activated T cells: cell distribution and antigenic relation to components on resting cells and T cell lines. J Immunol. 1984 Jun;132(6):3011–3018. [PubMed] [Google Scholar]
- Jahn L., Franke W. W. High frequency of cytokeratin-producing smooth muscle cells in human atherosclerotic plaques. Differentiation. 1989 Mar;40(1):55–62. doi: 10.1111/j.1432-0436.1989.tb00813.x. [DOI] [PubMed] [Google Scholar]
- Kawamoto S., Adelstein R. S. Characterization of myosin heavy chains in cultured aorta smooth muscle cells. A comparative study. J Biol Chem. 1987 May 25;262(15):7282–7288. [PubMed] [Google Scholar]
- Longtine J. A., Pinkus G. S., Fujiwara K., Corson J. M. Immunohistochemical localization of smooth muscle myosin in normal human tissues. J Histochem Cytochem. 1985 Mar;33(3):179–184. doi: 10.1177/33.3.3882826. [DOI] [PubMed] [Google Scholar]
- Moll R., Franke W. W., Schiller D. L., Geiger B., Krepler R. The catalog of human cytokeratins: patterns of expression in normal epithelia, tumors and cultured cells. Cell. 1982 Nov;31(1):11–24. doi: 10.1016/0092-8674(82)90400-7. [DOI] [PubMed] [Google Scholar]
- Sappino A. P., Schürch W., Gabbiani G. Differentiation repertoire of fibroblastic cells: expression of cytoskeletal proteins as marker of phenotypic modulations. Lab Invest. 1990 Aug;63(2):144–161. [PubMed] [Google Scholar]
- Sappino A. P., Skalli O., Jackson B., Schürch W., Gabbiani G. Smooth-muscle differentiation in stromal cells of malignant and non-malignant breast tissues. Int J Cancer. 1988 May 15;41(5):707–712. doi: 10.1002/ijc.2910410512. [DOI] [PubMed] [Google Scholar]
- Shimoyama Y., Hirohashi S., Hirano S., Noguchi M., Shimosato Y., Takeichi M., Abe O. Cadherin cell-adhesion molecules in human epithelial tissues and carcinomas. Cancer Res. 1989 Apr 15;49(8):2128–2133. [PubMed] [Google Scholar]
- Skalli O., Ropraz P., Trzeciak A., Benzonana G., Gillessen D., Gabbiani G. A monoclonal antibody against alpha-smooth muscle actin: a new probe for smooth muscle differentiation. J Cell Biol. 1986 Dec;103(6 Pt 2):2787–2796. doi: 10.1083/jcb.103.6.2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troyanovsky S. M., Guelstein V. I., Tchipysheva T. A., Krutovskikh V. A., Bannikov G. A. Patterns of expression of keratin 17 in human epithelia: dependency on cell position. J Cell Sci. 1989 Jul;93(Pt 3):419–426. doi: 10.1242/jcs.93.3.419. [DOI] [PubMed] [Google Scholar]
- Weintraub H., Davis R., Tapscott S., Thayer M., Krause M., Benezra R., Blackwell T. K., Turner D., Rupp R., Hollenberg S. The myoD gene family: nodal point during specification of the muscle cell lineage. Science. 1991 Feb 15;251(4995):761–766. doi: 10.1126/science.1846704. [DOI] [PubMed] [Google Scholar]