Abstract
NK cells develop in the bone marrow and complete their maturation in peripheral organs, but the molecular events controlling maturation are incompletely understood. The miR-15/16 family of microRNA regulates key cellular processes, and is abundantly expressed in NK cells. Here, we identify a critical role for miR-15/16 in the normal maturation of NK cells using a mouse model of NK-specific deletion, in which immature NK cells accumulate in the absence of miR-15/16. The transcription factor c-Myb (Myb) is expressed preferentially by immature NK cells, is a direct target of miR-15/16, and is increased in 15a/16-1FKO NK cells. Importantly, maturation of 15a/16-1FKO NK cells was rescued by Myb knockdown. Moreover, Myb overexpression in wild-type NK cells caused a defective NK cell maturation phenotype similar to deletion of miR-15/16, and Myb overexpression enforces an immature NK cell transcriptional profile. Thus, miR-15/16 regulation of Myb controls the NK cell maturation program.
Keywords: innate immunity, NK cell, microRNA, cellular differentiation, Myb, miR-15/16
INTRODUCTION
Natural Killer (NK) cells are innate lymphoid cells that are distinct from adaptive T and B lymphocytes in developmental and functional properties (1–3). Although all lineages develop from the common lymphocyte progenitor, NK cell development proceeds through a distinct set of intermediates in the bone marrow (BM), where they undergo an education process and become functionally competent (4). NK cells further mature in the periphery, with changes in function marked by different cell surface proteins including CD11b, CD27, CD43, and CD62L (5). In the most common murine NK cell differentiation classification, increased maturation correlates with decreasing proliferative capacity and increasing functional capacity as defined by a 4-stage sequential model in which NK cells mature from CD27−CD11b− (Stage I) to CD27+CD11b− (Stage II), CD27+CD11b+ (Stage III), and finally CD27−CD11b+ (Stage IV) (6, 7). Mouse NK cell functional capacity may be measured by the release of immunomodulatory cytokines, such as interferon gamma (IFN–γ), through stimulation of surface activating receptors such as NK1.1, Ly49H, and NKG2D, as well as cytokine signals such as those provided by IL-12, IL-15, and IL-18 (8–10). In addition, NK cell function is defined by the directed release of cytotoxic granules containing granzyme B (GzmB) and perforin (Prf1) onto the surface of target cells, thereby inducing an apoptotic-like cell death (11, 12). Thus, effective NK cell functional responses depend on appropriate maturation events in the periphery.
Despite the known functional differences between immature and mature NK cells, our understanding of the molecular networks governing NK cell maturation remains limited (13, 14). Early NK cell development is controlled by factors such as Nfil3 (15), Id2 (16), Hnf1a (Tcf-1) (17), and Tox (18). Of these, Nfil3 has been shown to be the most specific for the NK lineage, but is critical only in early NK cell development, and its requirement can be superseded by activation receptor-driven expansion (19). In later differentiation numerous factors are required, such as Gata3 (20), Prdm1 (Blimp1) (21), Ets1 (22), T-bet (23), and Eomes (24). Additional molecular mechanisms regulating NK cell maturation remain to be elucidated.
MicroRNAs (miRNAs) are small, 18-22 nucleotide non-coding RNAs that regulate protein production by binding to semi-complementary sites in the 3’UTR of target mRNAs (25). In lymphocytes, a number of miRNAs have been shown to control the development and regulation of immune responses (26, 27), and several miRNAs regulate the development and biology of NK cells (28, 29). One highly conserved miRNA family, miR-15/16 (30) is comprised of mature miR-15a, miR-15b, and miR-16 in lymphocytes, and share a high degree of sequence homology and predicted mRNA targets. These miRNAs are highly expressed in NK cells (31, 32), and have been found to inhibit B cell proliferation (33), and promote cellular apoptosis (34). miR-15/16 members are transcribed from two distinct genomic loci: the miR-15a/16-1 cluster located intronic to the DLEU2 gene and the miR-15b/16-2 cluster found intronic to the SMC4 gene. The miR-15/16 miRNA family contribution to the regulation of NK cell biology is unknown.
Myb (also known as c-Myb) is the prototypical transcription factor of the Myb family (35), which includes Myb, Mybl1 (a-Myb), and Mybl2 (b-Myb), and operates primarily as a transcriptional activator through binding of the sequence t/cAACt/gG (36). Myb is required for normal hematopoiesis, and the Myb global genetic knock-out is embryonic lethal in mice due to hematopoietic failure (37). Myb is predicted to be regulated by a number of miRNAs, including miR-15/16 in human cell lines (38). Another miRNA highly expressed in NK cells, miR-150, was shown to target Myb in B cells (39), with deletion of miR-150 leading to enhanced proliferation and defective B cell differentiation. miR-150 has also been shown to regulate NK and NK-T cell development (40), with its global deletion leading to defects in the development and maturation of NK cells, potentially through its role in regulating Myb. In this report, the impact of Myb on NK cells was examined in global Myb+/− mice, which were shown to have increased NK cell percentages, correlating with expanded NK cell percentages in miR-150-overexpressing transgenic mice (40). Thus, Myb is a transcription factor important for hematopoietic development, and the regulation of its expression in NK cells remains a relevant question in lymphocyte biology.
In this study, we hypothesized that miR-15/16 family miRNAs contribute to the regulation of NK cell development and/or function. To address this we generated a previously unreported mouse model that specifically deletes the miR-15a-16-1 loci in NK cells using Cre/Lox technology, thereby resulting in reduced expression of miR-15/16 miRNAs. This mouse manifested defective NK cell maturation with a block in terminal differentiation into stage IV CD27−CD11b+ NK cells, and an accumulation of immature stage II and III NK cells. Further, the Myb 3’UTR was biochemically confirmed as a target of miR-15/16, and Myb mRNA and protein were differentially expressed between miR-15/16-deficient and -sufficient immature NK cells in vivo. Utilizing lentiviral gene expression in immature NK cells followed by adoptive transfer, we demonstrate that miR-15/16 restoration or Myb knockdown restored defective NK cell maturation in vivo. Finally, overexpression of Myb in an NK cell line directly promoted an immature NK cell gene transcription profile. Collectively these data indicate that the regulation of Myb protein expression by miR-15/16 is critical for the normal maturation of NK cells in vivo.
MATERIALS AND METHODS
Mice
15a/16-1FKO mice were generated by crossing either Tg(Ncr1-iCre)265Sxl mice (41) or B6.Cg-Tg(CD2-cre)4Kio/J (42) with mice containing a LoxP-flanked miR-15a/16-1 allele (33) as well as a Rosa26-STOP-eYFP allele, obtained from The Jackson Laboratory as B6.129X1-Gt(ROSA)26Sortm1(EYFP)Cos/J(43). In some experiments, Nkp46iCre knock-in mice (44) were used instead of Tg(Ncr1-iCre)265Sxl, and had an equivalent phenotype. CD45.1 congenic mice were obtained from the National Cancer Institute. RAG−/−γc−/− mice were obtained from Taconic. All mice were either generated on a C57BL/6 background, or backcrossed at least 10 times to B6. All mice have been bred and maintained in specific pathogen-free housing, and all experiments were conducted in accordance with the guidelines of and with the approval of the Washington University Animal Studies Committee. Age-matched mice were used between 8 and 12 weeks of age for all experiments.
Cell Lines
293T cells were maintained in complete DMEM (DMEM with 10% FBS, 10mM HEPES, 50μM 2-mercaptoethanol, 100U/mL penicillin and streptomycin, 1mM sodium pyruvate, and 1x non-essential amino acids). KY-1 and KY-2 cell lines were maintained in complete RPMI (RPMI with the above additives) + 200 IU/mL rhIL-2.
Cell isolation and sorting
Mouse tissues were isolated as previously described (45, 46).
Immunoblots
1×106 sorted mouse YFP+CD27+/− or human CD56bright/dim NK cells were lysed in RIPA buffer, run on an 8-10% SDS-Page Gel (Bio-RAD) and transferred to Immobilon-P (Millipore) membrane. Immunostaining was performed with Myb @ 1:200, β-actin @ 1:500 overnight at 4 degrees. Densitometric quantification was performed using ImageJ (NIH).
IFN-γ assays
IFN-γ assays were performed as previously described (47).
Quantitative real-time RT-PCR
RNA from sorted cells was isolated using TRIzol (Invitrogen) according to the manufacturer’s instructions. cDNA was generated using the High-Capacity cDNA Reverse Transcription Kit (Life Technologies). Myb expression was detected in sorted NK cells using IDT PrimeTime Assay Hs.PT.56a.1442895, and normalized to 18S rRNA. miR-15a/miR-15b/miR-16/sno-135 were detected using TaqMan primer/probes, and normalized to sno-135 by the ΔΔCt method. Myb-targeted genes were validated using PrimerBank primers, the sequences of which are available upon request. All qPCR was performed on an ABI StepOne machine.
Luciferase assays
Mature miRNAs and luciferase reporter plasmids were overexpressed as previously described (32). Briefly, the Myb 3’ UTR was cloned from Myb cDNA into the psiCheck2 (Promega, Madison, WI) vector using PCR. The two predicted miR-15/16 binding sites were disrupted (cgctgcta to cgGACGta, and tgctgct to tgGACct) using the QuikChange II site-directed mutagenesis Kit (Agilent Technologies, Santa Clara, CA) following the manufacturer’s instructions. Luciferase assays were performed using the Dual-Glo Luciferase Assay (Promega, Madison, WI) as described previously (46, 47). Renilla luciferase (experimental) was normalized to Firefly luciferase (transfection control) followed by comparison of Renilla:Firefly ratios of the same psiCheck2 sensor plasmid co-transfected with an empty GFP-only control vector. For assays with the mutated Myb 3’UTR, the ratio of miR15/16 OE + empty psiCheck2 plasmid (control) was set equal to 1 and the luciferase activity of miR15/16 OE + psiCheck2-Myb or Myb-mutant are normalized relative to the control.
Flow cytometry
All flow cytometry was performed on a Gallios Flow Cytometer (Beckman Coulter), and analyzed in FlowJo (TreeStar).
Cell isolation and sorting
Briefly, single-cell suspensions were generated from spleen (mechanical disruption through a 70-μM filter or glass tissue homogenizer), BM (flushing tibia with K10), liver (glass tissue homogenization followed by Percoll gradient isolation), or blood (cardiac puncture). RBC lysis was performed using ACK buffer, and viable cell numbers were determined by Nexcelom cellometer (Nexcelom) (PI-exclusion) or hematocytometer (trypan blue exclusion). Isolation of highly purified NK cells (≥98% pure) was performed by flow cytometric sorting on a BD Aria-II (Beckton Dickinson) by gating on FSC/SSC, CD45+CD3−NK1.1+YFP+, and where indicated, GFP+ cells.
Normal Human Donors
Anonymous human NK cells were obtained from platelet donors undergoing apheresis at the Barnes Jewish Hospital apheresis center. Leukocyte reduction filters are obtained immediately after apheresis, flushed, and fresh PBMC and NK cells were isolated as described (47).
Lentiviral transduction and adoptive transfer
YFP+CD27+CD11b− NK cells were sorted from Ncr1-iCre x 15a/16-1F/F (15a/16-1FKO) or Ncr1-iCre x 15a/16-1WT/WT (Ctrl) mice. Lentiviruses of MND-driven constructs were generated and used to transduce primary mouse NK cells as previously described (47), except that GFP+ NK cells were sorted after 4-5 days of culture, and then additionally expanded for 7-10 days to generate enough GFP+ cells for adoptive transfer. 5-10×104 GFP+YFP+CD45.2+ donor NK cells were injected into recipient CD45.1 mice, and allowed to mature. After ten days, spleens were harvested from recipient mice, and YFP/GFP was used to mark donor cells, which were also stained for NK1.1, CD27, and CD11b.
mRNA microarrays
YFP+CD27+NK1.1+CD3− splenic NK cells were sorted from hCD2-Cre x 15a/16-1FKO mice or the corresponding controls, and resuspended in TRIzol (Invitrogen). RNA was then isolated using the Zymo DirectZol Kit (Zymo Research). RNA was amplified using the Ovation PicoSL Kit and run on an Affymetrix Gene1.0ST Chip by the SCC Molecular and Genomic Analysis (MGA) Core. Results were analyzed in Partek, using RMA normalization. mRNA microarray data are available in the Gene Expression Omnibus (GEO) database under the accession numbers http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?token=sredgocablchhoh&acc=GSE55033 and http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?token=mfuriquyldgxjoz&acc=GSE58268 KY-1 and KY-2 cells (a kind gift of W. Yokoyama) were transduced with either GFP or GFP-P2A-FLAG-Myb. Live GFP+NK1.1+CD3− cells were sorted and resuspended in TRIzol. RNA was processed as for the splenic NK cells. Data for comparisons to primary NK cells were accessed from GSE13229 (6), and filtered for probe sets with a mean expression greater than three when analyzed using Partek with normalization as above. Myb was additionally excluded from this analysis.
Statistical Analysis
Statistical comparisons were made using the Student’s T test or ANOVA as appropriate with a p value of <0.05 considered significant.
RESULTS
miR-15/16 are highly expressed in NK cells, and is predicted to target genes involved in cell proliferation and differentiation
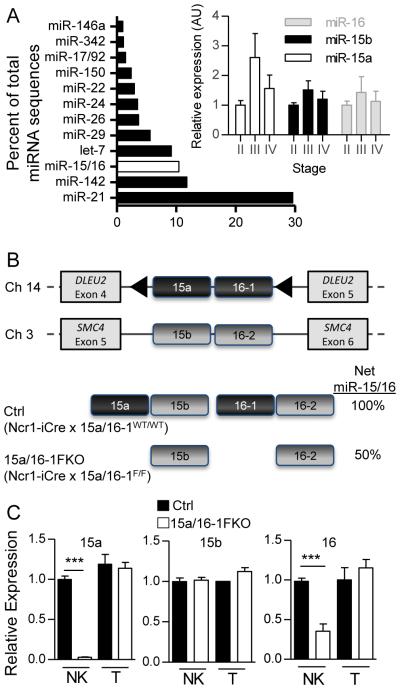
The miR-15/16 family is highly conserved, and highly expressed in both mouse and human NK cells. As a family, mature miR-15/16 miRNA sequences represent 10% of the overall miRNA sequence in mouse NK cells, and are expressed throughout NK cell differentiation (32) (Fig. 1A). miR-15/16 is bioinformatically predicted (48) to target a number of genes involved in fundamental pathways (DAVID) (49) including developmental processes, protein modification, intracellular signaling cascades, cell cycle, cell proliferation and differentiation, cell cycle control, regulated exocytosis (P-value < 3.1E-04, FDR<0.01). In order to investigate the NK cell-intrinsic molecular regulation by miR-15/16, we generated a model of NK cell-specific miR-15/16 deficiency. This model combined a conditional knockout (LoxP-flanked) mir-15a/16-1 allele (33) with an NK cell-specific Cre recombinase (Ncr1-iCre) (Fig. 1B) (41), which results in excision of mir-15a/16-1 in NK cells. As previously reported, this Cre model results in ~85% excision exclusively in NK cells as measured by a Rosa-YFP reporter (47), and except where indicated, YFP was included in all analyses to accurately report Cre+ NK cells. We assessed mature miR-15/16 levels in Ctrl (Ncr1-iCre x miR-15a/16-1WT/WT) or 15a/16-1FKO (Ncr1-iCre x miR-15a/16-1F/F) cells using qRT-PCR (Fig. 1C), and observed that in 15a/16-1FKO YFP+ NK cells, miR-15a was undetectable, miR-16 was reduced, and miR-15b levels were normal, as expected. Therefore, this model eliminates approximately 50% of the overall miR-15/16 family expression in Cre-expressing (YFP+) NK cells.
Figure 1. miR-15/16 is highly expressed in NK cells, and miR-15a/16-1 is specifically deleted upon Cre-mediated excision.
(A) Illumina miR-seq of miRNA families contributing to more than 1% of the overall miRNA sequences in resting mouse splenic NK cells (32). The mature miR-15/16 family sequences (miR-15a, miR-15b, and miR-16) are summed and highlighted (no fill). (inset) Relative mature miR-15a, 15b, and 16 expression by RT-qPCR in flow-sorted stage II, III, and IV murine splenic NK cells (N=4 mice in 2 independent experiments). (B) Diagram of NK cell specific mir-15a/16-1 floxed knockout (FKO) mice, consisting of a LoxP-flanked (triangle) 15a/16-1 allele, combined with an Ncr1-iCre. Not shown is the Rosa-LSL-YFP allele that expresses YFP after Cre-mediated excision, providing an explicit marker for Cre expression. This model eliminates miR-15a and miR-16-1 expression, but miR-15b and miR-16-2 expression remains intact. (C) RT-qPCR of the primary miR-15/16 family members in flow sorted Ctrl and 15a/16-1FKO YFP+ NK cells and YFP− T cells. Results are shown as the mean ± SEM of duplicate measurements from three independent experiments. These data demonstrate that 15a/16-1FKO NK cells express approximately 50% of the wild type miR15/16 family mature miRNA sequences. ***p<0.001 by t-test.
miR-15a/16-1FKO mice have defective NK cell maturation
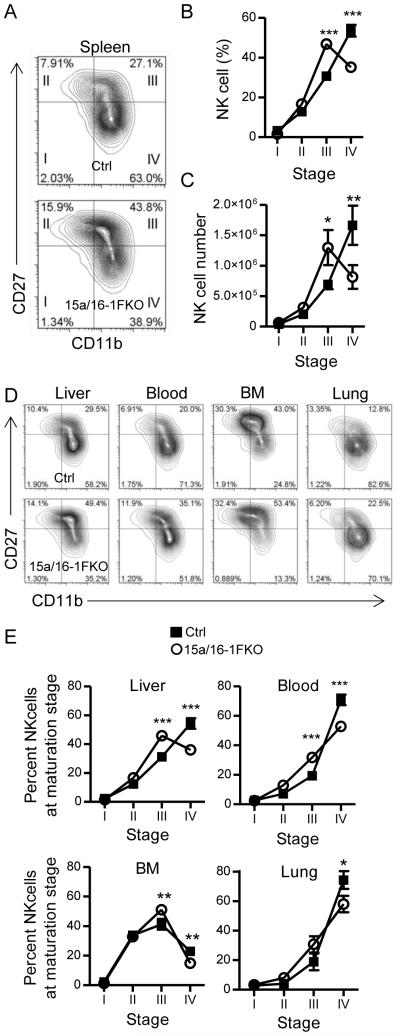
Mice with NK cell-specific miR-15a/16-1 deletion (15a/16-1FKO) had normal NK cell frequencies in the spleen, BM, liver, and blood. Additionally, in a complementary model of lymphocyte-specific mir-15a/16-1 excision (utilizing hCD2-Cre mice) (46), lymphocyte frequencies, NK cell frequencies, and NK receptor repertoires were also normal (Fig S1). We hypothesized, due to miR-15/16’s predicted targeting of cellular differentiation pathways, that 15a/16-1FKO NK cells may have developmental defects. We analyzed early NK cell development in the BM, and found no differences in early NK cell developmental precursors (Fig. S1H-I). However, peripheral NK cell maturation was markedly disrupted in 15a/16-1FKO mice (Fig. 2A,B), predominantly in the stage III to stage IV transition, when CD27 is down-regulated. The expression of CD43, a marker of terminal NK cell maturation, was also reduced in 15a/16-1FKO consistent with a less mature NK cell phenotype (Fig. S2A). In the spleen, total NK cell numbers and percentages were unchanged; however, the number of 15a/16-1FKO NK cells at stage III was increased, while stage IV NK cell numbers were reduced, suggesting a block in maturation (Fig. 2C). This phenotype was consistent in other tissues examined (Fig. 2D,E), and we observed a modest decrease in the absolute number of NK cells in the lung, fitting with their phenotype as stage IV NK cells (Fig S1). We also confirmed these phenotypes in the hCD2-Cre model (Fig. S1A-B). Moreover, we were unable to detect any changes in the death of stage IV NK cells from 15a/16-1FKO mice immediately ex vivo, suggesting that the change in maturation stages was not due to reduced survival of stage IV NK cells (Fig. S2C-D). Collectively, these findings are consistent with miR-15a/16-1FKO NK cells having a block in maturation transition from stage III to stage IV.
Figure 2. NK cell-intrinsic deficiency of miR-15/16 results in defective maturation.
(A) Representative flow cytometry contour plot demonstrating reduced stage IV (CD27−CD11b+) and increased stage III (CD27+CD11b+) NK cells from 15a/16-1FKO (bottom) compared to Ctrl (top) splenic YFP+ NK cells. (B and C) Summary data combined from two independent experiments (N=4-5 mice/group) demonstrating the mean percentage ± SEM (B) or mean absolute number ± SEM (C) of splenic YFP+ NK cells at the indicated CD27/CD11b maturation stage. (D and E) Representative flow cytometry (D) and combined summary mean ± SEM percentage data (E) demonstrating altered NK cell maturation stages of 15a/16-1FKO (bottom) compared to Ctrl (top) YFP+ NK cells from the indicated organs. Results are from three independent experiments (N=5-7 mice/group). *p<0.05; **p<0.01, ***p<0.001 by t-test.
15a/16-1FKO NK cells have an increased proliferative capacity and in vivo persistence
As the reduced number of stage IV NK cells was not apparently caused by increased cell death, we next examined the possibility that altered proliferation of 15a/16-1FKO NK cells led to skewing of NK cell maturation in these mice. We observed low levels of proliferation (detected by Ki-67 staining) in NK cells from adult mice analyzed directly ex vivo (Fig. S3A), suggesting that constitutively increased proliferation in immature NK cells does not account for the expansion of stage III NK cells. However, immature miR-15a/16-1FKO NK cells did have significantly enhanced IL-15-induced proliferation in vitro, compared to controls (Fig. S3B,C). In order to determine whether this effect also occurred during homeostatic proliferation of NK cells, we sorted immature (CD27+) YFP+ NK cells from 15a/16-1FKO or control mice, and transferred 5×104 into immunodeficient (RAG−/−γc−/−) recipient mice (Fig. S3D,E). Following transfer, 15a/16-1FKO NK cells expanded with a durable increase in NK cell numbers, compared to control YFP+ NK cells (Fig. S3D,E). Thus, 15a/16-1FKO NK cells exhibit enhanced proliferation after stimulation with IL-15, and increased expansion in vivo under conditions that result in homeostatic proliferation. Thus, enhanced proliferation may contribute to the accumulation of stage III NK cells in 15a/16-1FKO mice, but does not appear to explain the decreased number of stage IV NK cells.
15a/16-1FKO NK cells have altered IFN-γ production due to a maturation defect
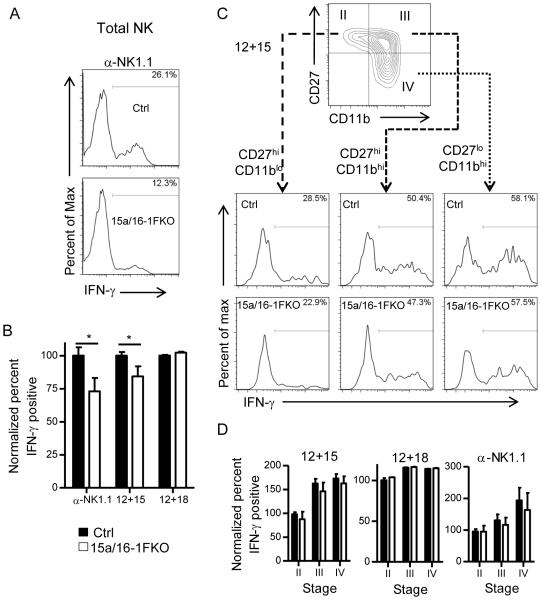
We next investigated whether miR-15a/16-1 deletion altered the function of NK cells. We found that total NK cell IFN-γ responses to varying types of stimuli that induce a spectrum of NK cell production by NK cells (activating receptor ligation and cytokine receptor stimulation with IL-12+IL-15, IL-12+IL-18) were modestly, but significantly diminished (Fig. 3A,B) in 15a/16-1FKO mice. Since mature NK cells produce IFN-γ and this NK cell stage was diminished in 15a/16-1FKO mice, we next assessed the IFN-γ responses on a per maturation stage basis using low-intensity (anti-NK1.1) or middle-intensity (IL-12+IL-15) stimuli. Indeed, following IL-12+IL-15 or α-NK1.1 stimulation the miR-15a/16-1FKO mice produced equivalent IFN-γ protein compared to controls within each maturation stage. Thus we reasoned that the decreased IFN-γ production by total NK cell within 15a/16-1FKO mice was due to reduced numbers of stage IV NK cells (Fig. 3C,D). IL-12 and IL-18-combined stimulation is a high-intensity stimulus for NK cell-IFN-γ production, and 15a/16-1FKO NK cells produced comparable amounts of IFN-γ to control NK cells with this maximal stimulation. We also examined NK cell degranulation (CD107a surface expression) in response to activating NK cell receptor ligation, and cytotoxicity against YAC-1 tumor targets. In both assays, NK cell responses were similar for 15a/16-1FKO and control mice (data not shown), consistent with the variable correlations between NK cell terminal maturation and cytotoxicity against YAC-1 targets (5, 7). Thus, miR-15a/16-1FKO NK cells as a population exhibit a diminished IFN-γ response due to an altered maturation profile, but intact degranulation and cytotoxicity.
Figure 3. 15a/16-1FKO NK cells have modestly decreased IFN–γ production due to reduced mature NK cells.
(A) Representative intracellular IFN–γ protein detected by flow cytometry by 15a/16-1FKO or Ctrl YFP+ NK cells following activating receptor (NK1.1) ligation. (B) Summary data of normalized mean ± SEM IFN–γ percentage positive NK cells after stimulation with α–NK1.1, IL-12+IL-15, or IL-12+IL-18. There was decreased IFN–γ production by 15a/16-FKO NK cells, compared to controls, by total NK cells (NK1.1+CD3−YFP+). (C) Representative flow cytometry showing the percentage of INF-γ positive YFP+ NK cells at the indicated CD27/CD11b maturation stages after stimulation with IL-12+IL-15. (D) NK cell maturation stage-specific summary data of mean ± SEM IFN–γ percentage positive NK cells after stimulation with α–NK1.1, IL-12+IL-15, and IL-12+IL-18. This analysis demonstrated no difference in IFN–γ production by maturation subsets of 15a/16-1FKO and Ctrl NK cells, indicating that the findings in (A) and (B) are due to altered maturation in 15a/16-1FKO NK cells. Data are from three independent experiments. *p<0.05
miR-15/16-deficiency alters the expression of multiple mRNAs in NK cells
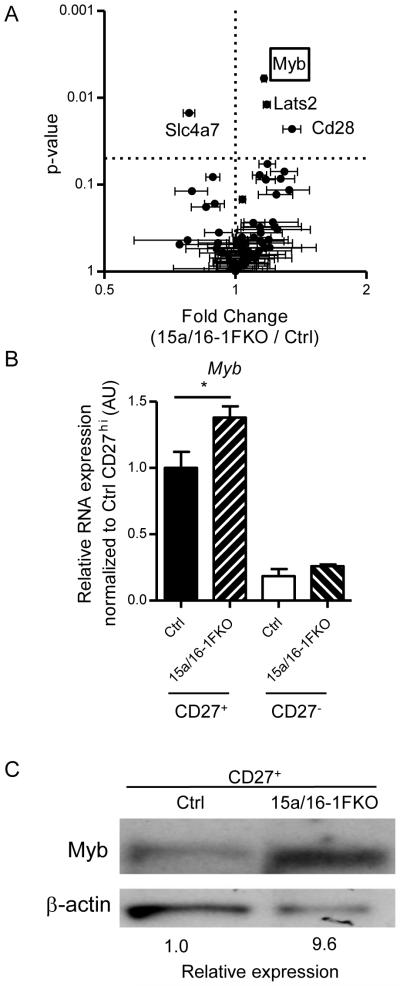
As miR-15/16 reduction led to both decreased maturation in vivo and increased proliferation, we sought to identify relevant gene targets of miR-15/16 for these processes in NK cells. Using TargetScan and DAVID functional analyses, we found that miR-15/16 was predicted to target a number of genes in pathways involved in cell proliferation and differentiation (BP00224), including those expressed in NK cells (50). To directly compare the global gene expression of 15a/16-1FKO and control NK cells, we performed mRNA microarrays on flow-sorted immature stage II/III (CD27+) NK cells, in order to control for known differences in gene expression based on maturation stage. We identified a number of modest, yet significant, mRNA differences between the two genotypes. In order to enrich for genes directly regulated by miR-15/16, we filtered the microarray dataset for genes predicted to be targeted by miR-15/16, and found two genes that were upregulated with a high degree of significance and validated by RT-qPCR: Myb (Fig. 4A) and Lats2 (fold increase of 2 in 15/16-1FKO, P<0.05, qRT-PCR). Lats2 has previously been shown to limit proliferation (51), which was inconsistent with the observed increased proliferation in 15a/16-1FKO NK cells. In contrast, Myb has been shown to promote cellular proliferation and is known to be important in hematopoietic development. We validated that RNA levels of Myb expression were higher in miR-15a/16-1FKO CD27+ NK cells by RT-qPCR (Fig. 4B). Utilizing immunoblot analysis of flow sorted CD27+ NK cells Myb protein levels were increased in 15a/16-1FKO NK cells (Fig. 4C). We therefore focused on Myb as a potential mechanistic target for the NK cell maturation defect present with miR-15/16 deficiency.
Figure 4. Myb expression is elevated in 15a/16-1FKO NK cells.
(A) Gene1.0ST microarray of three independently generated groups of flow sorted CD27+ (immature) NK cells from 15a/16-1FKO or Ctrl mice, filtered based on predicted miR-15/16 targeting. Results are shown summarizing the mean ± SEM fold change (increased values indicate higher expression in 15a/16-1FKO) versus the significance (P value) using ANOVA with a Bonferroni multiple comparison correction. Dashed lines indicate no fold change (vertical) or p<0.05 (horizontal). Myb is annotated with a red box. (B) Quantitative real-time RT-qPCR of Myb expression in YFP+ Ctrl or YFP+ 15a/16-1FKO NK cells of the indicated maturation stage that validate the mRNA changes in Myb in 15a/16-1FKO NK cells. Data summarizes 3 independent experiments. *p<0.05 by t-test. (C) Western blot analysis of flow sorted CD27+ (immature) NK cells from 15a/16-1FKO or Ctrl NK cells for expression of Myb protein. B-actin is shown and used as a loading control for quantification. The immunoblot shown is representative of two independent experiments.
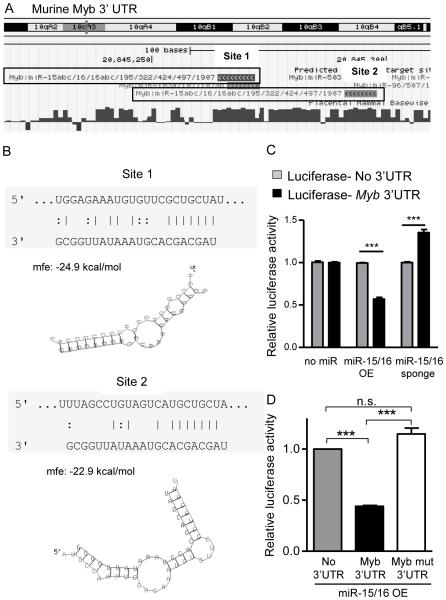
The transcription factor Myb is a direct target of miR-15/16
The two predicted miR-15/16 binding sites present in the murine Myb 3’UTR are highly conserved with humans (Fig. 5A), and were energetically favorable targets for miRNA repression (Fig. 5B). Consistent with several reports in human cell lines and primary cells (38, 52, 53), we observed that the mouse Myb 3’UTR is a target of miR-15/16 family miRNAs using an in vitro luciferase reporter assay. When miR-15/16 overexpression plasmids were co-transfected with psiCheck2-luciferase sensor plasmid controlled by the murine Myb-3’UTR we observed significant repression of the luciferase signal. Conversely, when miR-15/16 miRNAs were inhibited using co-transfection of a miR-15/16 sponge construct, the Myb-3’UTR luciferase signal was enhanced (Fig. 5C). Finally, when the predicted seed sequences in the Myb-3’UTR are disrupted to prevent binding of miR-15/16 (Myb-Mutant), miR-15/16 is no longer able suppress relative luciferase activity (Fig. 5D). Thus, murine Myb is a direct target of miR-15/16 family miRNAs, consistent with the increased mRNA and protein expression in 15a/16-1FKO NK cells.
Figure 5. Myb is a highly conserved miR-15/16 target.
(A) UCSC genome browser indication of miRNA target sites and their mammalian conservation (dark blue bars, longer bars is greater inter-species conservation). (B) Schematic representation of the two miR-15/16 target sites in the 3’UTR of Myb (top strand myb RNA, bottom strand miR-15a sequence. Below each site, RNAHybrid (53) graphical representation and analysis of the two miR-15/16 target sites in the 3’UTR of Myb. (C) miR-15/16 targets the Myb 3’UTR in vitro detected via a sensor plasmid. Luciferase sensor plasmid assay for Myb 3’UTR compared to control 3’UTR, with overexpression of GFP (no miRNA), GFP-miR-15/16 overexpression (miR-15/16 OE) or GFP-miR-15/16 sponge inhibitor (miR-15/16 sponge) that reduces available mature miR-15/16 in HEK293T cells. Data are normalized to the control 3’UTR plus no miRNA condition. (D) miR-15/16 repression of the Myb 3’ UTR relies on predicted seed sequences to suppress translation from the Myb 3’UTR. Luciferase sensor plasmid assay for miR-15/16 OE without Myb 3’UTR (control), with Myb 3’UTR, or with Myb 3’UTR-mutant (seed sites mutated to disrupt miR-15/16 binding). Data are normalized to luciferase activity of their respective 3’UTR in the absence of miR-15/16 OE. Luciferase activity is relative to the control. Transfection efficiency as measured by GFP+ cells was >80% in all experiments. Data summarize two independent experiments. ***p<0.001 by 2-way ANOVA (C) or ANOVA (D). mfe: mean free energy.
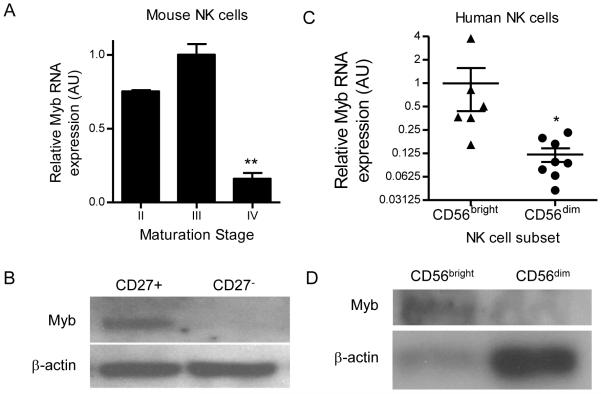
Myb expression is primarily confined to stage II/III mouse and CD56bright human NK cells
The expression of Myb mRNA has been shown to be decreased in mature stage IV CD27-CD11b+ NK cells (6, 40); however, Myb protein expression has not been examined in mouse NK cells, nor Myb expression changes investigated in human NK cells. We therefore assessed the levels of Myb mRNA and protein in the different maturation stages of NK cells, and confirmed that levels of Myb were increased in CD27+ immature mouse NK cells compared to CD27− mature NK cells by real-time RT-qPCR (Fig. 6A). Further, in wild type mice we found abundant expression of Myb protein in immature CD27+ NK cells, compared to near undetectable Myb protein levels in mature CD27− NK cells by immunoblot (Fig. 6B). We next investigated Myb mRNA (Fig. 6C) and protein (Fig. 6D) expression in human NK cell subsets, and found that Myb expression was elevated in CD56bright NK cells as compared to the more mature, CD56dim NK cells. Thus, Myb was expressed in immature NK cells and down regulated concurrent with terminal NK cell maturation at the mRNA and protein levels in both mouse and human NK cells.
Figure 6. In both mice and humans Myb is preferentially expressed in immature NK cells and down regulated at the stage of terminal maturation.
(A) Quantitative real-time RT-qPCR of Myb mRNA expression in YFP+ Ctrl NK cells of the indicated NK cell maturation stage. Data are normalized to stage III NK cells, and summarize 3 independent experiments. (B) Western blot of Myb expression in flow sorted CD27+ and CD27- NK cells from YFP+ Ctrl NK cells. Immunoblot is representative of two independent experiments. (C) Quantitative real-time RT-qPCR of Myb mRNA expression flow sorted CD56bright or CD56dim human NK cells. Data are normalized to CD56bright NK cells, and summarize 3 independent experiments. (D) Western blot of Myb protein expression by CD56bright and CD56dim NK cells. Immunoblot is representative of three normal donors. *p<0.05, **p<0.01 by one-way ANOVA or *p<0.05 by t-test.
miR-15/16-deficient NK cells maintain a cell-intrinsic maturation defect after adoptive transfer
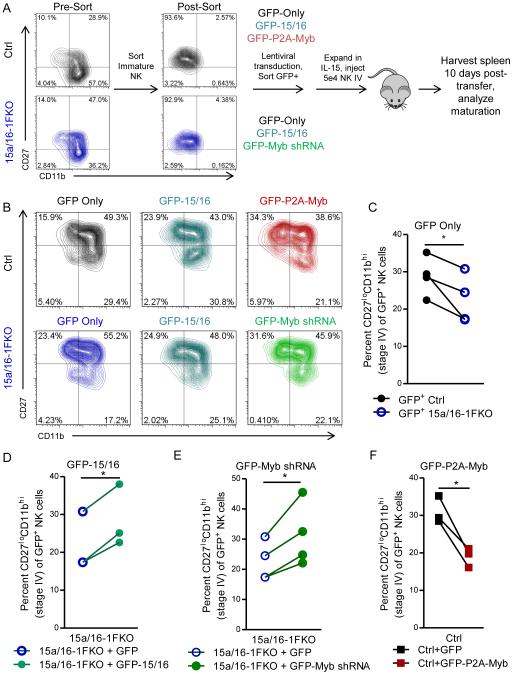
Ncr1 (NKp46), the promoter driving Cre in our model, is also expressed in lymphoid tissue inducer (LTi), NK-22, and related innate lymphoid cells, and we therefore confirmed the cell-intrinsic nature of the Ncr1-driven 15a/16-1FKO maturation defect by adoptive transfer (Fig. 6A-C). Additionally, this adoptive transfer system established an in vivo system for lentiviral manipulation and evaluation of primary murine NK cell maturation (Fig. 7A). We flow-sorted immature stage II (CD27+CD11b−) YFP+ NK cells from Ctrl or 15a/16-1FKO mice, and transduced these cells with a lentiviral construct overexpressing GFP to track the NK cells. GFP+ lentivirus-transduced NK cells were sorted, expanded briefly in IL-15, and 5×104 GFP+ NK cells were injected intravenously into recipient mice and allowed to mature in vivo for 10 days. This time point was chosen based on a balance of detecting the adoptively transferred NK cells, and the ability to observe consistent NK cell transitions from stage II to stage III and IV in vivo. The spleens from recipient mice were harvested, and the transferred GFP+ NK cells were analyzed for maturation (Fig. 7B). We found that 15a/16-1FKO NK cells had defective maturation after adoptive transfer (Fig. 7C), confirming the cell-intrinsic nature of the defect and establishing the validity of this system for the ectopic expression/repression experiments in primary NK cells.
Figure 7. miR-15/16-deficient NK cells fail to mature due to increased Myb expression.
(A) Overview of experimental system, in which immature (CD27+CD11b−) YFP+ NK cells are sorted, transduced with indicated lentiviruses (Ctrl with GFP only, GFP-15/16, or GFP-P2A-Myb; 15a/16-1FKO with GFP only, GFP-15/16, or GFP-Myb shRNA), and sorted for GFP+ cells after two days of culture. After sorting, cells were expanded for 6-10 days in IL-15, and 5×104 cells were injected into recipient mice. After 10 days, spleens from recipient mice were harvested and analyzed for donor GFP+ NK cells. (B) Representative flow cytometry plots of adoptively transferred NK cells from the indicated genotypes (Ctrl, top; 15a/16-1FKO, bottom), and lentiviral manipulation. (C-F) Summary data of Ctrl vs. 15a/16-1FKO NK cells transduced with the GFP only virus (C), 15a/16-1FKO NK cells transduced with GFP only or with the GFP-15/16 virus to restore miR-15/16 levels (D), 15a/16-1FKO NK cells transduced with GFP only or with GFP-Myb shRNA to reduce Myb levels (E), and Ctrl NK cells transduced with GFP only or GFP-P2A-Myb to mimic the Myb overexpression phenotype of the 15a/16-1FKO (F). Data summarize 3-4 experiments, using the same controls (GFP only) for multiple panels (C and E share the Ctrl GFP only control, while C, D, and E share the 15a/16-1FKO GFP only control). One experiment of 15a/16-1FKO + GFP in (D) did not have a corresponding 15a/16-1FKO + GFP-15/16, and the control in this panel is censored. *p<0.05 by paired t-test.
miR-15/16 overexpression or Myb knock-down in adoptively transferred 15a/16–1FKO NK cells rescues NK cell maturation
In order to correct the aberrant overexpression of Myb in the miR-15a/16-1FKO NK cells, we generated two vectors: 1) a lentiviral shRNA directed against Myb (GFP-Myb shRNA), which was highly effective in repressing Myb protein expression, and 2) a lentiviral overexpression construct for miR-15/16, which effectively restores miR-15a/16 and was similarly effective at repressing Myb protein expression (Fig. S4). Both lentiviruses include GFP to mark transduced cells for sorting and in vivo tracking. Ctrl and 15a/16-1FKO CD27+CD11b− cells were transduced with viruses generated from these constructs, adoptively transferred, and then evaluated for maturation after 10 days (Fig. 7A). As expected, miR-15/16 reinstatement restored miR-15a/16-1FKO NK cell maturation (Fig. 7D). Additionally, Myb knockdown was able to restore the maturation of the miR-15a/16-1FKO NK cells to comparable levels as replacement of miR-15/16 (Fig. 7E). Therefore, miR-15/16’s direct targeting of Myb mRNA appears crucial to NK cell maturation, as 15a/16-1FKO NK cell maturation can be rescued by either restoration of miR-15/16 or forced downregulation of Myb.
Myb overexpression phenocopies the miR-15a/16-1FKO maturation defect
In order to specifically address the role of Myb in NK cell maturation, we cloned the Myb del10 mRNA isoform (MybΔ10), the major form of mouse Myb expressed in NK cells. The del10 isoform is also the dominant from of Myb expressed in B and T cells (50), and shares 90% homology with human Myb. We generated an overexpression construct for MybΔ10 (GFP-P2A-Myb) (Fig. S4), and verified that Myb is overexpressed via immunoblot. We found that Ctrl stage II NK cells transduced to overexpress Myb had reduced maturation to stage IV after 10 days in vivo (Fig. 7F), similar to the 15a/16-1FKO mice (Fig. 7C). Thus, forced Myb overexpression directly prevented NK cell maturation in vivo.
Myb overexpression drives an immature NK cell mRNA program
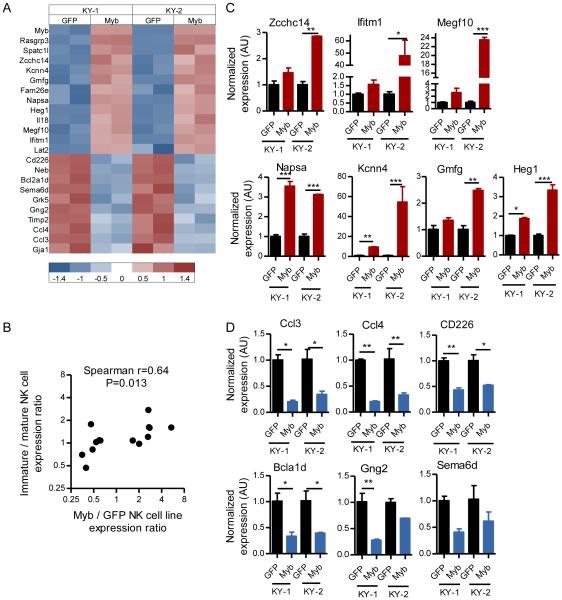
Differential expression of mRNAs in immature versus mature murine NK cells have been identified by mRNA microarray analyses (6). We hypothesized that Myb overexpression in NK cells would result in enhanced mRNA expression of genes specific for immature NK cells, and diminish expression of genes specific for mature NK cells. In order to test this hypothesis and directly assess the function of Myb as a transcription factor in NK cells, we transduced two independently generated NK cell lines (KY-1 and KY-2) (54) with either GFP or GFP-P2A-Myb expression vectors, and performed a global analysis of mRNA expression. In this experiment NK cell lines were used after multiple attempts at transducing primary immature NK cells failed to yield sufficient cell numbers to generate adequate RNA mass and quality for mRNA profiling following viral transduction. We identified 13 genes that were increased by at least 1.5-fold and 10 genes that were decreased by at least 1.5-fold consistently in both cell lines after Myb overexpression (Fig. 8A). There was a high degree of correlation between genes induced by Myb overexpression and genes known to be preferentially expressed within the immature NK cells (6). Correlation was also found between genes repressed by Myb overexpression and those preferentially expressed in mature NK cell (Fig. 8B). Bioinformatic analysis of the regulatory regions of these genes revealed the presence of canonical Myb binding sites, suggesting a direct role for Myb in these mRNA alterations. We orthogonally validated gene expression changes that occur with Myb overexpression discovered via microarray (Fig. 8A) using RT-qPCR (Fig. 8 C,D). Collectively, these results support a model in which Myb induces and/or supports an immature NK cell program, and Myb downregulation results in mRNA expression changes that facilitate terminal NK cell maturation.
Figure 8. Myb promotes an immature NK cell transcriptional profile.
Two murine NK cell lines, KY-1 and KY-2, were transduced with GFP or GFP-P2A-Myb lentiviruses, and mRNA expression was assessed using microarrays (A and B) or RT-qPCR (C and D). (A) Heat map showing significantly altered mRNA expression in two NK cell lines (KY-1, KY-2) with (GFP-P2A-Myb) or without (GFP) Myb overexpression. Values below graph represent gene expression values, scaled to a mean of zero and a standard deviation of one for each row. (B) Correlation between Myb-induced expression in the KY-1 and KY-2 NK cell lines and preferential expression in immature NK cells. The Y-axis shows the ratio of immature to mature NK cell expression (from ref. (6)) of the differentially expressed mRNAs identified in (A), with expression > 1 indicating increased expression in immature NK cells and < 1 indicating increased expression in mature NK cells. The X-axis is the ratio of expression between the GFP-P2A-Myb to GFP-transduced NK cell lines, with values > 1 indicating increased expression in Myb-OE cells, and values < 1 indicating decreased expression in Myb-OE cells (>1.5-fold, FDR<0.1). Genes are filtered as described in the methods section. These data show a correlation between expression of NK cell stage-specific mRNAs following Myb over-expression. (C-D) Orthogonal RT-qPCR validation of mRNA microarray findings in (A), with expression increased (C) or decreased (D) after Myb overexpression in KY-1 and KY-2 NK cell lines transduced with either GFP or GFP-P2A-Myb.
DISCUSSION
The role of specific miRNAs in the regulation of NK cell development and maturation remains largely unknown (28). This study revealed a critical role for miR-15/16, one of the most abundant miRNA families in NK cells, in the regulation of NK cell maturation. Using NK cell-lineage restricted miR-15/16 genetic deficiency models, we show that a fifty percent reduction of miR-15/16 expression resulted in decreased maturation of the CD27−CD11b+ (stage IV) NK cell compartment. Further, we identified the transcriptional regulator Myb as one key mediator of this miR-15/16 NK cell maturation effect. Myb was confirmed as a direct target of miR-15/16 in vitro using both gain- and loss-of-function approaches. Myb mRNA and protein expression were reduced in vivo in immature miR-15/16-deficient NK cells, demonstrating the relevant regulation of this predicted target in the cellular context under study – primary NK cells. Further, Myb was directly linked to the maturation defect in miR-15/16-deficient NK cells using an in vivo primary NK cell maturation assay. These experiments identified that either restoration of miR-15/16 expression or Myb knockdown reversed the maturation block in miR-15/16 deficient NK cells. Moreover, forced Myb overexpression in WT NK cells also resulted in reduced NK cell maturation. These insights lead to new questions about the impact of Myb (and Myb’s transcriptional targets) on normal NK cell maturation. Myb overexpression in NK cell lines resulted in enforcement of an immature NK cell mRNA profile, suggesting that Myb may be a key transcriptional regulator of the final steps of NK cell maturation. Thus, our study demonstrates that NK cell-intrinsic genetic alterations of a miRNA family can reveal new aspects of the NK cell molecular program.
The impact of miR-15/16 deficiency on NK cell maturation differs between tissues examined, and appears modest in the BM and lung likely due to the maturation stages present. In the BM, CD27−CD11b+ stage IV NK cells are a minor population, and the minor change in NK cell stage frequency likely reflects the normal BM NK cell skew toward immature stage II/III NK cells. In the lung, NK cells are almost exclusively stage IV, and we detected a decrease in the absolute NK cell numbers in the lung, consistent with loss of stage IV NK cells in other tissues. We expect that decreased numbers of mature NK cells in miR-15a/16-2FKO mice will lead to reduced IFN-γ production in the spleen and liver, and potentially result in diminished host control of pathogens that require IFN-γ for control, including MCMV and Listeria. Future studies will examine the effect of miR-15/16-deficincy on NK cell responses to infection and malignancy in the setting of complete miR-15/16 loss in vivo.
The transcriptional regulation of the miR-15a/16-1 and mir-15b/16-2 loci in NK cells remains unclear, with E2F1, BSAP, Myc, and Myb reported as regulators in other cell types, suggesting complex regulation of transcription in NK cells. Additional studies are required to define the mechanism of abundant miR-15/16 family expression in mouse and human NK cells. In the 15a/16-1FKO mice only one of two loci that express miR-15/16 is deleted, resulting in mature miR-15/16 sequence expression analogous to heterozygous deletion - approximately half compared to wild type NK cells. The presence of residual miR15b and miR-16 expression from the mir-15b/16-2 locus likely impacts several aspects of our study. For example, residual miR-15/16 may have limited our ability to detect robust mRNA expression differences when profiling immature NK cells from 15a/16-1FKO and control mice. This is may be compounded since miR-15/16 may predominantly exert its effect on protein translation, rather than RNA degradation, supported by the much larger increase in Myb protein expression (as compared to RNA expression) in the 15a/16-1FKO NK cells. Additionally, target prediction for miR-15/16 is imperfect, and overestimates the number of true biological targets. We acknowledge that mRNA level analysis of potential miRNA targets cannot provide comprehensive identification of miRNA-targets or protein-level alterations in miR-15/16 deficient NK cells, which highlights the importance of applying miRNA:mRNA target identification techniques in the cell of interest, for example RISC-Seq/CLIP-SEQ or proteomic approaches. In addition, we observed minimal impact of miR-15a-16-1 deletion on intrinsic NK cell functionality, despite prior evidence that this miRNA targeted the murine IFN-γ 3'UTR (46). Thus, miRNA:mRNA profiling and additional functional studies on NK cells will be most informative in the setting of complete miR-15/16 ablation to maximize the ability to clearly identify miR-15/16 targets, and current efforts include generation of miR-15b/16-2 floxed mice to define the impact of complete miR-15/16 family loss in NK cells.
When miR-15/16 is deficient, Myb mRNA and protein levels are increased, correlating with increased proliferation and disrupted stage III to IV maturation. The implication of these findings is that Myb must be down regulated in order for NK cells to complete maturation. Supporting these data, there is a large decrease in Myb mRNA and protein levels during this final phase of maturation. Further, 15a/16-1FKO NK cells that are able to mature to stage IV also have low levels of Myb. Collectively, these observations support a model in which lack of miR-15/16 boosts Myb levels in immature stage II/III NK cells, but NK cell maturation can still proceed, albeit at reduced efficiency, if a critical threshold of Myb is reached, at which time Myb gene expression is attenuated by mechanisms independent of miR-15/16. The identification of other factors that promote or inhibit the transcription and translation of Myb in NK cells, which has a number of regulatory mechanisms (35), will further enhance our understanding of NK cell maturation. Interestingly, other factors known to be required for NK cell maturation are either stably expressed (Eomes, failure at stage II to III and T-bet, failure at III to IV) or increased (Prdm1, failure at stage III to IV) throughout NK cell maturation. Since Prdm1 is a transcriptional repressor that increases during NK cell maturation (while Myb is reduced), it will be interesting to investigate whether Myb is downstream of Prdm1-regulated pathways. Additionally, Myb levels are increased in the absence of Ets1, indicating that Ets1 may promote the transcription of Myb, and this increased Myb expression may contribute to the maturation phenotype in the Ets1−/− mouse (22).
Myb has been previously shown to have a role in the differentiation of lymphocytes and other cells, and is known to be targeted by miR-150 in NK cells (40). This report analyzed haploinsufficient Myb+/− mice, and identified increased numbers of mature NK cells, consistent with findings in miR-150 transgenic mice. It will be of interest to study conditional deletion of Myb in the NK cell lineage. We investigated the combined effect of miR-150 and miR-15/16 on Myb, given that the two miRNAs target distinct sites in the 3’UTR of Myb, but found no additive or synergistic effect using an in vitro luciferase assay. However, this does not exclude an in vivo cooperation of these two miRNAs at physiologic expression levels, and it will be interesting to investigate miR-150 and miR-15/16 cooperation in vivo. Interestingly, miR-150 deficiency was shown to decrease IFN-γ production, similar to miR-15/16-deficient mice. For the miR-150-deficient mice the reduced IFN-γ was not apparently explained by the maturation change in those mice (40), while miR-15/16-deficien mice had overall IFN-γ production defects, with equivalent IFN-γ production in all stages of NK cell maturation compared to controls. Both stage III and IV NK cells are major producers of IFN-γ, and loss of stage IV NK cells in miR-15/16 deficiency resulted in modestly reduced INF-γ production, without stage-specific differences when gating on stage II, III, or IV NK cells. We also observed no major differences between miR-15/16-deficient and control NK cells in cytotoxicity responses to YAC-1 tumor targets, not unexpected based on the inconsistent correlation of CD27/CD11b maturation subsets and NK cell cytotoxicity (5, 7); However, we acknowledge the possibility that miR-15/16 may also intrinsically modulate NK cell cytotoxicity. Further, altered miR-150 dosage led to additional effects on NK cells, for example an altered Ly49A expression, an effect that was not observed in the miR-15a/16-1FKO. Moreover, while NK cells from miR-15a/16-1FKO exhibited enhanced proliferation, this effect was not reported in the global miR-150−/− model. These contrasting phenotypes suggest that while these highly expressed miRNAs have distinct pleiotropic sets of targets in NK cells, there may be targeting overlap for selected mRNAs, such as Myb.
Myb promotes the transcription of a number of genes associated with an immature NK cell phenotype, while repressing genes associated with a mature NK cell phenotype. We identified a number of putative Myb-targeted genes in two mouse NK cell lines following forced over-expression of Myb. These Myb-promoted genes have a high degree of correlation to the expression of genes associated with immature NK cells, strongly suggesting that Myb promotes an immature NK cell phenotype. However, it will be important to validate these findings in primary NK cells using chromatin immunoprecipitation (ChIP) as robust Myb antibodies for ChIP become available. Further, bioinformatic algorithms predict that canonical Myb target sites are present and overrepresented in genes affected by Myb over-expression. These data support that Myb promotes an immature NK cell phenotype. Our findings that Myb expression is also differentially regulated in human CD56bright and CD56dim subsets are consistent with the current model that CD56bright are less mature than CD56dim NK cells. The miR-15/16 binding sites in the Myb 3’UTR are highly conserved, resulting in parallel regulation of Myb by miR-15/16 in both species. Thus, our data in mice, and species conservation between miR-15/16 and Myb in mouse and humans, suggest that miR-15/16 control of Myb may regulate human NK cell differentiation. However, this is challenging to experimentally test, since there are differences in Myb family expression between mice and humans, with the additional Myb family member Mybl1 being significantly expressed in human, but not mouse, NK cells (50). Our future studies will seek to test the hypothesis that miR-15/16 similarly targets Myb in human NK cells, and thereby regulates human NK cell maturation. Additionally, we acknowledge that miR-15/16 family miRNAs have many targets in addition to Myb that may be contributing to the differentiation phenotype.
In summary, the regulation of Myb by miR-15/16 is essential for complete physiologic NK cell maturation in vivo, revealing a previously unreported mechanism regulating NK cell development. In support of these findings, immature mouse NK cells transduced to overexpress Myb failed to mature in vivo, and 15a/16-1FKO NK cells failed to mature due to increased levels of Myb, which can be corrected either by reintroducing miR-15a/16 or siRNA-mediated reduction of Myb. Myb promotes a number of transcripts directly associated with immature NK cells, and represses those associated with mature NK cells. These data reveal a previously unreported miRNA – transcription factor regulatory pathway that controls NK cell maturation in vivo.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank the Siteman Cancer Center Flow Cytometry Core (NIH P30CA091842), the Washington University Facility of Rheumatic Diseases Core Center (NIH P30AR048335), and the Washington University Genome Technology Access Center (NIH P30CA91842 and ICTS/CTSA UL1TR000448). We thank Drs. Megan Cooper, Anthony French, Marco Colonna, and Wayne Yokoyama for insightful discussion. We thank Riccardo Dalla-Favera for providing the mir-15a/16-1 floxed mice.
This work was supported by NIH T32 HL708836 (RPS), NIH F32 CA200253 (MBE) R01AI102924 from the NIH, ASH Scholar Award from the ASH foundation, Physician-Scientist Early Career Award from HHMI (TAF).
Abbreviations
- NK
Natural killer
- BM
bone marrow
- IFN–γ
interferon gamma
- GzmB
granzyme B
- Prf1
and perforin
- miRNAs
MicroRNAs
Footnotes
AUTHOR CONTRIBUTIONS
Author contributions: R.P.S., T.A.F. designed research; R.P.S., J.W.L., S.E.S., A.R.I., M.M.B-E., A.S., T.S., B.A.J. performed research; V.S., contributed new reagents/analytic tools; R.P.S., J.W.L., S.E.S., M.M.B-E, T.A.F. analyzed data; and R.P.S. and T.A.F. wrote the paper. All authors discussed the results and approved the final version of the manuscript.
Disclosure of conflicts of interest: The authors have no financial conflicts of interest to disclose.
REFERENCES
- 1.Yokoyama WM, Kim S, French AR. The dynamic life of natural killer cells. Ann Rev Immunol. 2004;22:405–29. doi: 10.1146/annurev.immunol.22.012703.104711. [DOI] [PubMed] [Google Scholar]
- 2.Di Santo JP. Natural killer cell developmental pathways: a question of balance. Ann Rev Immunol. 2006;24:257–86. doi: 10.1146/annurev.immunol.24.021605.090700. [DOI] [PubMed] [Google Scholar]
- 3.Hesslein DGT, Lanier LL. Transcriptional control of natural killer cell development and function. Adv Immunol. 2011;109:45–85. doi: 10.1016/B978-0-12-387664-5.00002-9. [DOI] [PubMed] [Google Scholar]
- 4.Elliott JM, Yokoyama WM. Unifying concepts of MHC-dependent natural killer cell education. Trends Immunol. 2011;32:364–72. doi: 10.1016/j.it.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim S, Iizuka K, Kang H-SP, Dokun A, French AR, Greco S, Yokoyama WM. In vivo developmental stages in murine natural killer cell maturation. Nat. Immunol. 2002;3:523–8. doi: 10.1038/ni796. [DOI] [PubMed] [Google Scholar]
- 6.Chiossone L, Chaix J, Fuseri N, Roth C, Vivier E, Walzer T. Maturation of mouse NK cells is a 4-stage developmental program. Blood. 2009;113:5488–96. doi: 10.1182/blood-2008-10-187179. [DOI] [PubMed] [Google Scholar]
- 7.Hayakawa Y, Smyth MJ. CD27 Dissects Mature NK Cells into Two Subsets with Distinct Responsiveness and Migratory Capacity. J Immunol. 2006;176:1517–24. doi: 10.4049/jimmunol.176.3.1517. [DOI] [PubMed] [Google Scholar]
- 8.Lanier LL. Up on the tightrope: natural killer cell activation and inhibition. Nat Immunol. 2008;9:495–502. doi: 10.1038/ni1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Newman KC, Riley EM. Whatever turns you on: accessory-cell-dependent activation of NK cells by pathogens. Nat Rev Immunol. 2007;7:279–91. doi: 10.1038/nri2057. [DOI] [PubMed] [Google Scholar]
- 10.Vivier E, Tomasello E, Baratin M, Walzer T, Ugolini S. Functions of natural killer cells. Nat Immunol. 2008;9:503–10. doi: 10.1038/ni1582. [DOI] [PubMed] [Google Scholar]
- 11.Orange JS. Formation and function of the lytic NK-cell immunological synapse. Nat Rev Immunol. 2008;8:713–25. doi: 10.1038/nri2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Russell JH, Ley TJ. Lymphocyte-mediated cytotoxicity. Annu Rev Immunol. 2002;20:323–370. doi: 10.1146/annurev.immunol.20.100201.131730. [DOI] [PubMed] [Google Scholar]
- 13.Luevano M, Madrigal A, Saudemont A. Transcription factors involved in the regulation of natural killer cell development and function: an update. Front. Immunol. 2012;3:319. doi: 10.3389/fimmu.2012.00319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ramirez K, Kee BL. Transcriptional regulation of natural killer cell development. Curr Opin Immunol. 2010;22:193–8. doi: 10.1016/j.coi.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gascoyne DM, Long E, Veiga-Fernandes H, de Boer J, Williams O, Seddon B, Coles M, Kioussis D, Brady HJM. The basic leucine zipper transcription factor E4BP4 is essential for natural killer cell development. Nat. Immunol. 2009;10:1118–24. doi: 10.1038/ni.1787. [DOI] [PubMed] [Google Scholar]
- 16.Boos MD, Yokota Y, Eberl G, Kee BL. Mature natural killer cell and lymphoid tissue-inducing cell development requires Id2-mediated suppression of E protein activity. J. Exp. Med. 2007;204:1119–30. doi: 10.1084/jem.20061959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Held W, Kunz B, Lowin-Kropf B, van de Wetering M, Clevers H. Clonal acquisition of the Ly49A NK cell receptor is dependent on the trans-acting factor TCF-1. Immunity. 1999;11:433–42. doi: 10.1016/s1074-7613(00)80118-1. [DOI] [PubMed] [Google Scholar]
- 18.Aliahmad P, de la Torre B, Kaye J. Shared dependence on the DNA-binding factor TOX for the development of lymphoid tissue-inducer cell and NK cell lineages. Nat. Immunol. 2010;11:945–52. doi: 10.1038/ni.1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Firth M. a, Madera S, Beaulieu AM, Gasteiger G, Castillo EF, Schluns KS, Kubo M, Rothman PB, Vivier E, Sun JC. Nfil3-independent lineage maintenance and antiviral response of natural killer cells. J. Exp. Med. 2013;210:2981–90. doi: 10.1084/jem.20130417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Samson SI, Richard O, Tavian M, Ranson T, Vosshenrich C. a J., Colucci F, Buer J, Grosveld F, Godin I, Di Santo JP. GATA-3 promotes maturation, IFN-gamma production, and liver-specific homing of NK cells. Immunity. 2003;19:701–11. doi: 10.1016/s1074-7613(03)00294-2. [DOI] [PubMed] [Google Scholar]
- 21.Kallies A, Carotta S, Huntington ND, Bernard NJ, Tarlinton DM, Smyth MJ, Nutt SL. A role for Blimp1 in the transcriptional network controlling natural killer cell maturation. Blood. 2011;117:1869–1879. doi: 10.1182/blood-2010-08-303123. [DOI] [PubMed] [Google Scholar]
- 22.Ramirez K, Chandler KJ, Spaulding C, Zandi S, Sigvardsson M, Graves BJ, Kee BL. Gene deregulation and chronic activation in natural killer cells deficient in the transcription factor ETS1. Immunity. 2012;36:921–32. doi: 10.1016/j.immuni.2012.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Townsend MJ, Weinmann AS, Matsuda JL, Salomon R, Farnham PJ, a Biron C, Gapin L, Glimcher LH. T-bet regulates the terminal maturation and homeostasis of NK and Valpha14i NKT cells. Immunity. 2004;20:477–94. doi: 10.1016/s1074-7613(04)00076-7. [DOI] [PubMed] [Google Scholar]
- 24.Intlekofer AM, Takemoto N, Wherry EJ, a Longworth S, Northrup JT, Palanivel VR, Mullen AC, Gasink CR, Kaech SM, Miller JD, Gapin L, Ryan K, Russ AP, Lindsten T, Orange JS, Goldrath AW, Ahmed R, Reiner SL. Effector and memory CD8+ T cell fate coupled by T-bet and eomesodermin. Nat. Immunol. 2005;6:1236–44. doi: 10.1038/ni1268. [DOI] [PubMed] [Google Scholar]
- 25.Lewis BP, Burge CB, Bartel DP. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell. 2005;120:15–20. doi: 10.1016/j.cell.2004.12.035. [DOI] [PubMed] [Google Scholar]
- 26.Belver L, Papavasiliou FN, Ramiro AR. MicroRNA control of lymphocyte differentiation and function. Curr. Opin. Immunol. 2011;23:368–73. doi: 10.1016/j.coi.2011.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baltimore D, Boldin MP, O’Connell RM, Rao DS, Taganov KD. MicroRNAs: new regulators of immune cell development and function. Nat Immunol. 2008;9:839–845. doi: 10.1038/ni.f.209. [DOI] [PubMed] [Google Scholar]
- 28.Leong JW, Sullivan RP, Fehniger TA. microRNA management of NK-cell developmental and functional programs. Eur J Immunol. 2014;44:2862–2868. doi: 10.1002/eji.201444798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Beaulieu AM, a Bezman N, Lee JE, Matloubian M, Sun JC, Lanier LL. MicroRNA function in NK-cell biology. Immunol Rev. 2013;253:40–52. doi: 10.1111/imr.12045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yue J, Tigyi G. Conservation of miR-15a/16-1 and miR-15b/16-2 clusters. Mamm. Genome. 2010;21:88–94. doi: 10.1007/s00335-009-9240-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Basso K, Sumazin P, Morozov P, Schneider C, Maute RL, Kitagawa Y, Mandelbaum J, Haddad J, Chen C-Z, Califano A, Dalla-Favera R. Identification of the human mature B cell miRNome. Immunity. 2009;30:744–52. doi: 10.1016/j.immuni.2009.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fehniger TA, Wylie T, Germino E, Leong JW, Magrini VJ, Koul S, Keppel CR, Schneider SE, Koboldt DC, Sullivan RP, Heinz ME, Crosby SD, Nagarajan R, Ramsingh G, Link DC, Ley TJ, Mardis ER. Next-generation sequencing identifies the natural killer cell microRNA transcriptome. Genome Res. 2010;20:1590–1604. doi: 10.1101/gr.107995.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Klein U, Lia M, Crespo M, Siegel R, Shen Q, Mo T, Ambesi-Impiombato A, Califano A, Migliazza A, Bhagat G, Dalla-Favera R. The DLEU2/miR-15a/16-1 cluster controls B cell proliferation and its deletion leads to chronic lymphocytic leukemia. Cancer Cell. 2010;17:28–40. doi: 10.1016/j.ccr.2009.11.019. [DOI] [PubMed] [Google Scholar]
- 34.Cimmino A, Calin GA, Fabbri M, Ferracin M. V Iorio, M., Shimizu M, Wojcik SE, Aqeilan RI, Zupo S, Dono M, Rassenti L, Alder H, Volinia S, Liu C-G, Kipps TJ, Negrini M, Croce CM. miR-15 and miR-16 induce apoptosis by targeting BCL2. Proc Natl Acad Sci USA. 2005;102:13944–9. doi: 10.1073/pnas.0506654102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ramsay RG, Gonda TJ. MYB function in normal and cancer cells. Nat. Rev. Cancer. 2008;8:523–34. doi: 10.1038/nrc2439. [DOI] [PubMed] [Google Scholar]
- 36.Biedenkapp H, Borgmeyer U. Viral myb oncogene encodes a sequence-specific DNA-binding activity. Nature. 1988;335:835–837. doi: 10.1038/335835a0. [DOI] [PubMed] [Google Scholar]
- 37.Mucenski ML, McLain K, Kier a B., Swerdlow SH, Schreiner CM, a Miller T, Pietryga DW, Scott WJ, Potter SS. A functional c-myb gene is required for normal murine fetal hepatic hematopoiesis. Cell. 1991;65:677–89. doi: 10.1016/0092-8674(91)90099-k. [DOI] [PubMed] [Google Scholar]
- 38.Chung E, Dews M, Cozma D. c-Myb oncoprotein is an essential target of the dleu2 tumor suppressor microRNA cluster. Cancer Biol. Ther. 2008;7:1758–1764. doi: 10.4161/cbt.7.11.6722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xiao C, Calado DP, Galler G, Thai TH, Patterson HC, Wang J, Rajewsky N, Bender TP, Rajewsky K. MiR-150 controls B cell differentiation by targeting the transcription factor c-Myb. Cell. 2007;131:146–159. doi: 10.1016/j.cell.2007.07.021. [DOI] [PubMed] [Google Scholar]
- 40.Bezman NA, Chakraborty T, Bender T, Lanier LL. miR-150 regulates the development of NK and iNKT cells. J Exp Med. 2011:1–15. doi: 10.1084/jem.20111386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Eckelhart E, Warsch W, Zebedin E, Simma O, Stoiber D, Kolbe T, Rülicke T, Mueller M, Casanova E, Sexl V. A novel Ncr1-Cre mouse reveals the essential role of STAT5 for NK-cell survival and development. Blood. 2011;117:1565–73. doi: 10.1182/blood-2010-06-291633. [DOI] [PubMed] [Google Scholar]
- 42.De Boer J, Williams A, Skavdis G, Harker N, Coles M, Tolaini M, Norton T, Williams K, Roderick K, Potocnik AJ, Kioussis D. Transgenic mice with hematopoietic and lymphoid specific expression of Cre. Eur. J. Immunol. 2003;33:314–25. doi: 10.1002/immu.200310005. [DOI] [PubMed] [Google Scholar]
- 43.Srinivas S, Watanabe T, Lin CS, William CM, Tanabe Y, Jessell TM, Costantini F. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev Biol. 2001:1–4. doi: 10.1186/1471-213X-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Narni-mancinelli E, Chaix J, Fenis A, Kerdiles YM, Yessaad N, Reynders A, Gregoire C, Luche H, Ugolini S, Tomasello E, Walzer T, Vivier E. Fate mapping analysis of lymphoid cells expressing the NKp46 cell surface receptor. Proc Natl Acad Sci USA. 2011;108:18324–9. doi: 10.1073/pnas.1112064108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fehniger TA, Cai SF, Cao X, Bredemeyer AJ, Presti RM, French AR, Ley TJ. Acquisition of murine NK cell cytotoxicity requires the translation of a pre-existing pool of granzyme B and perforin mRNAs. Immunity. 2007;26:798–811. doi: 10.1016/j.immuni.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 46.Sullivan RP, Leong JW, Schneider SE, Keppel CKR, Germino E, French AR, Fehniger TA. MicroRNA Deficient NK Cells Exhibit Decreased Survival but Enhanced Function. J Immunol. 2012;188:3019–30. doi: 10.4049/jimmunol.1102294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sullivan RP, Fogel LA, Leong JW, Schneider SE, Wong R, Romee R, Thai T-H, Sexl V, Matkovich SJ, Dorn GW, French AR, a Fehniger T. miR-155 tunes both the threshold and extent of NK cell activation via targeting of multiple signaling pathways. J Immunol. 2013;191:5904–13. doi: 10.4049/jimmunol.1301950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Garcia DM, Baek D, Shin C, Bell GW, Grimson A, Bartel DP. Weak seed-pairing stability and high target-site abundance decrease the proficiency of lsy-6 and other microRNAs. Nat Struct Mol Cell Biol. 2011;18:1139–1146. doi: 10.1038/nsmb.2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Huang DW, Sherman BT, a Lempicki R. Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res. 2009;37:1–13. doi: 10.1093/nar/gkn923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shay T, Jojic V, Zuk O, Rothamel K, Puyraimond-Zemmour D, Feng T, Wakamatsu E, Benoist C, Koller D, Regev A. Conservation and divergence in the transcriptional programs of the human and mouse immune systems. Proc. Natl. Acad. Sci. U. S. A. 2013;110:2946–51. doi: 10.1073/pnas.1222738110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li Y, Pei J, Xia H, Ke H, Wang H, Tao W. Lats2, a putative tumor suppressor, inhibits G1/S transition. Oncogene. 2003;22:4398–405. doi: 10.1038/sj.onc.1206603. [DOI] [PubMed] [Google Scholar]
- 52.Zhao H, Kalota A, Jin S, Gewirtz AM. The c-myb proto-oncogene and microRNA-15a comprise an active autoregulatory feedback loop in human hematopoietic cells. Blood. 2009;113:505–16. doi: 10.1182/blood-2008-01-136218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sankaran VG, Menne TF, Šćepanović D, Vergilio J-A, Ji P, Kim J, Thiru P, Orkin SH, Lander ES, Lodish HF. MicroRNA-15a and -16-1 act via MYB to elevate fetal hemoglobin expression in human trisomy 13. Proc. Natl. Acad. Sci. U. S. A. 2011;108:1519–24. doi: 10.1073/pnas.1018384108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Karlhofer BFM, Orihuela MM, Yokoyama WM. Ly-49-independent Natural Killer (NK) Cell Specificity Revealed by NK Cell Clones Derived from p53-deficient Mice. J Exp Med. 1995:181. doi: 10.1084/jem.181.5.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.