Abstract
Purpose
A melanoma vaccine incorporating six peptides designed to induce helper T cell responses to melanoma antigens has induced Th1-dominant CD4+ T cell responses in most patients, and induced durable clinical responses or stable disease in 24% of evaluable patients. The present study tested whether this vaccine also induced antibody (Ab) responses to each peptide, and whether Ab responses were associated with T cell responses and with clinical outcome.
Patients and Methods
Serum samples were studied from 35 patients with stage III–IV melanomas vaccinated with 6 melanoma helper peptides (6MHP). IgG Ab responses were measured by ELISA. Associations with immune response and overall survival were assessed by log-rank test and Chi square analysis of Kaplan-Meier data.
Results
Ab responses to 6MHP were detected by week 7 in 77% of patients, and increased to peak 6 weeks after the last vaccine and persisted to 6 months. Ab responses were induced most frequently to longer peptides. Of those with T cell responses, 82% had early Ab responses. Survival was improved for patients with early Ab response (p = 0.0011) or with early T cell response (p < 0.006), and was best for those with both Ab and T cell responses (p = 0.0002).
Conclusion
Vaccination with helper peptides induced both Ab responses and T cell responses, associated with favorable clinical outcome. Such immune responses may predict favorable clinical outcome to guide combination immunotherapy. Further studies are warranted to understand mechanisms of interaction of these Abs, T cell responses, and tumor control.
Keywords: Melanoma, Immune therapy, Vaccination, Antibody, Helper T cell, peptides, Cancer Vaccines, Human, Clinical Trial, Survival
Introduction
A primary goal of cancer vaccines is to elicit immune responses to cancer antigens, and thus to mediate lysis of malignant cells. Many cancer vaccines employ peptide antigens, and are primarily designed to elicit CD8+ and/or CD4+ T-cell responses (1, 2). Few studies of peptide vaccines have addressed whether cancer vaccines also elicit humoral responses and whether this impacts clinical outcome. A melanoma vaccine incorporating six peptides designed to induce helper T cell responses to melanoma antigens has induced Th1-dominant CD4+ T cell responses in 81% of patients, and induced durable clinical responses or stable disease in 24% of evaluable patients (3, 4). We hypothesized that this 6MHP vaccine may induce Ab responses to peptides in the vaccine.
Spontaneous autoantibodies are present in patients with a variety of malignancies (5); however, whether such Abs support or inhibit immune-mediated tumor control is debated (6–8) Spontaneous Ab to the cancer testis antigen NY-ESO-1 (9) has been detected in about 50% of patients with NY-ESO-1-expressing melanomas while undetectable in patients with NY-ESO-1 negative tumors and in healthy adults (10) and have been associated with tumor progression (11). Several studies have reported clinical benefits in patients with an integrated humoral (Ab) and cellular response to cancer vaccines or to CTLA4 blockade (12–14), whereas others have not identified clinical impact of Abs induced by cancer vaccines (15, 16). Vaccination of melanoma patients with the MAGE-A3 or NY-ESO-1 recombinant protein or peptides induced T-cell responses and Ab responses but clinical impact was not reported (17–19), while another study suggested overall clinical benefit in patients vaccinated with NY-ESO-1 who developed Ab responses, but control groups were not available for comparison (20). Despite the reported humoral responses in these studies, cancer vaccine research remains focused on T-cell responses, while the effects of Ab responses remain unclear.
The capacity to produce Ab to specific antigen is the primary purpose of B cells and plasma cells, and Ab may either enhance the immune response to tumor (21, 22) or promote tumor growth (7, 23). The presence of Ab to TAA may also serve as prognostic (7, 24) or diagnostic (25) biomarkers. The role of B cells in the tumor microenvironment has received new attention as active participants in the host response to TAA (26) but they also can have regulatory function (27) in the tumor microenvironment. Changes in the B cell compartment of tumor-involved nodes have also been reported in patients with malignant disease (28, 29) indicating a role for B cells in the response to tumor. Indeed, increases in the both T and B lineage cells (including CD138+ plasma cells) in the tumor microenvironment correlated with increased survival of patients with melanoma metastases (30). A vaccine containing six MHC Class II peptides (6MHP) has elicited CD4+ T-cell responses and has shown evidence of clinical activity (4). The 6MHP vaccine induced helper T cells with a Th1 bias (3) and CD8 responses to MHC Class I peptides (31), suggesting epitope spreading. As recent studies show that some cancer vaccines are able to induce robust Ab responses, we examined whether vaccination of patients with 6MHP induced Ab responses, and whether this may be associated with T cell response and with patient survival.
Patients and Methods
Patients and Immunization protocol
Details of clinical trial (NCT00089219) design and patients have been previously described (3, 4). Briefly, 37 eligible patients with stage IIIB to IV melanoma were administered six immunizations of a vaccine containing 6 melanoma helper peptides (6MHP) at increasing doses of 200mcg (Arm A, 12 patients), 400mcg (Arm B, 12 patients), or 800mcg (Arm C, 13 patients) per vaccine. Peptides were administered in emulsions with incomplete Freund’s adjuvant (IFA, Seppic Inc, Paris, France) and GM-CSF (Berlex, Seattle WA) over a seven-week period. Seventeen of these patients had measurable disease. Blood, for lymphocytes and serum, was collected prevaccine (week 0), one week after vaccines 1, 3, 4, and 6 (weeks 1, 3, 5, and 7) and in follow-up at weeks 12 and 18 and months 6, 9, 12, 18, and 24 (4). The peptides in the vaccine are listed in Table 1.
Table 1.
Antibody and T cell responses to each peptide in the 6MHP vaccine.
| Epitope | Sequence | # of aa | Ab Responsea | T cell responseb |
|---|---|---|---|---|
| Tyrosinase386-406 | FLLHHAFVDSIFEQWLQRHRP | 21 | 78% | 32% |
| Melan-A/MART151-73 | RNGYRALMDKSLHVGTQCALTRR | 23 | 66% | 24% |
| gp10044-59 | WNRQLYPEWTEAQRLD | 16 | 41% | 5% |
| Tyrosinase56-70 | AQNILLSNAPLGPQFP | 15 | 6% | 5% |
| MAGE-A3281-295 | TSYVKVLHHMVKISG | 15 | 0% | 49% |
| MAGE-A1,2,3,6121-134 | LLKYRAREPVTKAE | 14 | 0% | 22% |
% of patients with Ab response to each peptide, n = 32
% of patients with T cell response to each peptide, as previously reported, n = 37 (31)
Of the 37 patients eligible for the study, two were excluded from this analysis because they came off study for tumor progression before week 7, and serum specimens were not available after week 3. Serum samples were evaluable for serological responses through week 7 in 35 patients, and beyond week 7 in 31 patients. Data from one patient were inconsistent in repeat assays and were excluded from analysis. Thus, 34 patients (92%) were evaluated for early serological responses (weeks 5–7) and 30 patients (81%) were additionally evaluated for Ab responses at later time points (> 10 weeks). The study was performed with informed consent, Institution Review Board (HIC#10464) and FDA approvals (BB-IND # 10825).
ELISA Method
Patient sera were evaluated by ELISA (32) for IgG antibody to each peptide in the 6MHP vaccine, or to the pool of all 6 peptides, before (pre, week 0), 1 week after 4–6 injections (weeks 5–7), and (for 30 of the patients) at later time points (weeks 11 or later) as well. Briefly, 96-well half-area cluster plates (Corning Costar) were coated with 30 mcL of 6MHPs (individually or pooled) diluted in carbonate/bicarbonate buffer (pH 9.4; Sigma-Aldrich) at 1.67 mcg/mL of each peptide. For quantitation of specific serum levels of anti-peptide antibody, purified IgG immunoglobulin (Fitzgerald Industries International, Acton MA) was prepared in coating buffer at 1 mcg/mL, serially diluted four-fold to 0.25 ng/mL, and 30 mcL of each dilution added to duplicate wells. After incubation overnight at 4°C, plates were washed with PBS with 0.1% Tween 20 (TPBS), then blocked for 1 hour with 5% nonfat dry milk in TPBS (blocking buffer). Beginning at 1:100, four-fold serial dilutions of patient and control sera were prepared in blocking buffer and added to individual wells. After 2 hours of incubation at room temperature (RT) and subsequent washing, secondary antibody (Goat anti-human IgG AP conjugate, Southern Biotech, Birmingham, AL) was added to all wells, incubated 1 hour at RT then washed. Attophos substrate (Sigma) was added to each well for 30 minutes. After incubation, 3N NaOH was added to stop the reaction, and fluorescence recorded on a Molecular Devices (Sunnyvale, CA) SPECTRAmax Gemini EM Fluorescent plate reader, excitation 450 nm, emission 580 nm. A positive control serum was used in all assays with individual peptides, where that serum was obtained from a patient on a different trial with the 6MHP vaccine and reacted against 5 of the 6 peptides by this ELISA assay (all except MAGE-A3281-295; Supplementary Fig S1).
Titer Analysis
The FORECAST function in Microsoft Excel was used to calculate the Ab titer of patients’ sera (32). The titer is defined as the reciprocal of the serum dilution that yields a fluorescent intensity ten times greater than the cutoff value. The cutoff value is defined as the average fluorescence obtained from the first four dilutions of serially diluted normal donor serum (negative control). Antibody titers less than 100 were considered negative. A standard curve of IgG concentration and fluorescent intensity was generated from data averaged across 18 plates from 5 separate assays. Upper and lower limits were established based on the lowest and highest fluorescence of IgG standard concentrations bracketing the values used to produce a polynomial curve with a correlation coefficient greater than 0.99. Anti-peptide IgG serum concentrations were extrapolated according to the polynomial expression derived from this curve.
Reactivity to individual peptides
Patients identified as having reactivity to 6MHP were evaluated further to define reactivity to each of the 6 peptides. Sera from early (≤7 weeks after first vaccine) and at the time of peak reactivity (>10 weeks) were assayed at one dilution for reactivity to each of those peptides. Wells were coated with peptide at 30 mcL at 1 mcg/ml. The ELISA assay was performed as described above, using 1:200 dilution of patient serum. Positive responses were defined as fluorescence intensity 10-fold than the mean normal donor response to the peptides.
CD4+ T cell responses
T cell responses have been measured by 5-day proliferation assay, as described (4).
Data Analysis
Kaplan-Meier survival curves were generated with MedCalc software (Ostend, Belgium) and utilized updated patient clinical follow-up data in the Cancer Center clinical trials office database, and previously published data on the CD4+ T-cell responses to 6MHP vaccine (4); survival curves were compared with log-rank test and chi-square. Differences in Ab titer between study arms were compared using a two-tailed Student’s t-test with equal variance. Early and late serum titers were compared using the Student’s t-test for paired samples.
Results
Vaccination with 6MHP induces IgG antibody responses
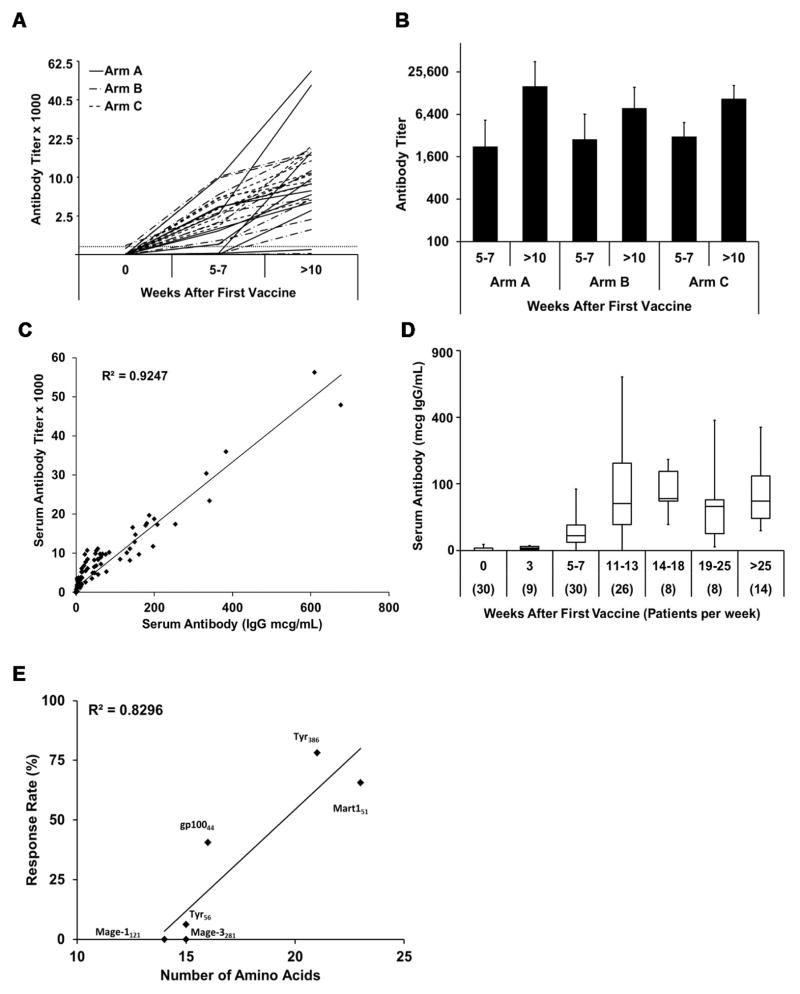
Ab responses to the 6MHP vaccine were assessed in 30 patients both early (weeks 5–7) and late (> 10 weeks). There was a 4.2-fold (mean) increase in Ab titer between early and late time points (p = 0.0001) in 26 of the 30 patients (Figure 1A). Of these patients, 77% had positive Ab titers (> 100) after 4–6 vaccines (weeks 5–7), and 87% had positive Ab titers at the later time points, more than 5 weeks after the 6th vaccine (Table 2). Ab titers increased significantly from 5–7 weeks into the vaccine schedule to more than 10 weeks (> 5 weeks after the last vaccine) at each dose level (Figure 1B, p < 0.04, paired Student’s t-test). However, Ab titers did not differ among vaccine doses at either time point (p > 0.9 at weeks 5–7; p > 0.15 at > 10 weeks; Student’s t-test with equal variance).
Figure 1. Antibody response to the 6MHP vaccine mixture.
A) The Ab response to 6MHP peptides, as the serum Ab titer prevaccine (week 0), early after vaccination (week 5–7), or at maximal titer time point (more than 10 weeks) plotted on a square root scale. B) Ab responses to 6MHP (plotted on a log to the base 4 scale) early vs late by study arm (Arm A p = 0.03, Arm B p = 0.01, Arm C p = 0.003). C) Ab responses were defined by titer and by serum concentration, and these measures were closely correlated (R2 = 0.92). D) Serum Ab concentrations measured through week 25, plotted on a square root scale, with box plots (each box 25th–75th percentiles; vertical lines define maximum and minimum; horizontal lines represent median values). E) % of patients with detectable Ab to each peptide (graphed by peptide amino acid length).
Table 2.
Antibody responses as function of study arm and time on study.
| Arm | A | B | C | Overall | ||||
|---|---|---|---|---|---|---|---|---|
| Peptide Vaccine Dose | 200 mcg | 400 mcg | 800 mcg | All Doses | ||||
| Evaluated Patients | 10 | 10 | 10 | 30 | ||||
| Weeks post 1st Vaccine | 5–7 | >10 | 5–7 | >10 | 5–7 | >10 | 5–7 | >10 |
| Number of Responders | 7 | 9 | 7 | 8 | 9 | 9 | 23 | 26 |
| Response Rate | 70% | 90% | 70% | 80% | 90% | 90% | 77% | 87% |
Association of T cell responses and Ab responses, within 7 weeks
The CD4+ T cell proliferative response (stimulation index) has been reported previously (4). Here we report also the timing of that response, as it relates to the Ab response. The overall T cell response rate in PBMC was 57% (21/37) (4). Eighty-four percent of those responses were evident by week 7 (18/37, 49%). In that study, we also assessed immune response in the vaccine-draining lymph node (sentinel immunized node, SIN) in 36 patients, which was collected at week 3. T cell responses were detected in the SIN in 28/36 patients (78%), including all 3 of the patients who developed T cell responses in the blood after week 7, plus 9 patients who did not have T cell responses detected in PBMC. Thus, 30 of 37 patients (81%) had T cell responses evident by week 7 in PBMC or SIN (data not shown). Of the 34 patients with Ab and T cell data available by week 7, 28 (82%) had T cell responses in PBMC or SIN by week 7, and 25 (74%) had Ab responses by week 5–7. This includes 23 with Ab and T cell responses, 4 with neither, 2 with Ab only, and 5 with T cell only. Thus, there was substantial concordance of Ab and T cell responses: 82% of those with T cell responses also had Ab responses by week 7, and, conversely, 92% of those with Ab responses by week 7 also had T cell response. This association approached significance (p = 0.051, chi-square, MedCalc).
Quantification of serum Ab to peptide, over time
Ab titer was closely correlated with calculated concentration of anti-peptide Ab (R2 > 0.92, Figure 1C). Ab concentration increased from weeks 5–7 to peak at weeks 11–13 and was maintained at a relatively consistent level through week 25 or later (Figure 1D). With one exception (3%), all pre-vaccine sera had titers less than 100 (< 0.7 mcg/mL). One patient had a pre-vaccine titer of 141 (0.8 mcg/mL) that increased to > 5,000 after 10 weeks. Similar to what was found with titer, peak peptide-specific IgG levels in serum were similar among dosage arms (p values 0.14, 0.83, and 0.14 for A vs B, B vs C, and A vs C, respectively; Student’s t-Test, with equal variance; Table 3).
Table 3.
Peak concentrations of IgG-Ab to 6MHP vaccine (mcg/mL)
| Arm A | Arm B | Arm C | Overall | |
|---|---|---|---|---|
| Patients evaluated | 10 | 10 | 10 | 30 |
| Mean (mcg/mL) | 191 | 77 | 94 | 121 |
| Minimum – Maximum (mcg/mL) | 0.9–678 | 0.3–200 | 0.6–209 | 0.3 – 678 |
| Median (mcg/mL) | 54 | 15 | 60 | 56 |
Longest peptides induced Ab responses at highest frequency
Ab responses were most frequent to the longer helper peptides in the vaccine (Table 1). The two peptides with more than 20 amino acids (FLL: tyrosinase386-406,, RNG: MART-1/Melan-A51-73) induced IgG responses in 78% and 66% of the patients, respectively (Figure 1E). The third longest peptide (WNR: gp10044-59, 16 amino acids) induced IgG responses in 41%, whereas slightly shorter peptides were much less immunogenic (0–6%). Peptide length and Ab response rate were closely associated (R2 ~ 0.82, Figure 1E). Ab responses to individual peptides were assessed in paired serum from 23 patients at two time points (Supplementary Fig S2), and show increases for 3 of the 4 peptides (Tyrosinase386-406 (p<0.0001), MelanA/MART151-73 (p<0.0001), and gp10044-59 (p = 0.012). Only 2 patients responded to the Tyrosinase56-70 with one showing an increase and the other patient not showing an increase over time.
Patient survival is improved in patients with both Ab and CD4+ T-cell responses to 6MHP vaccination
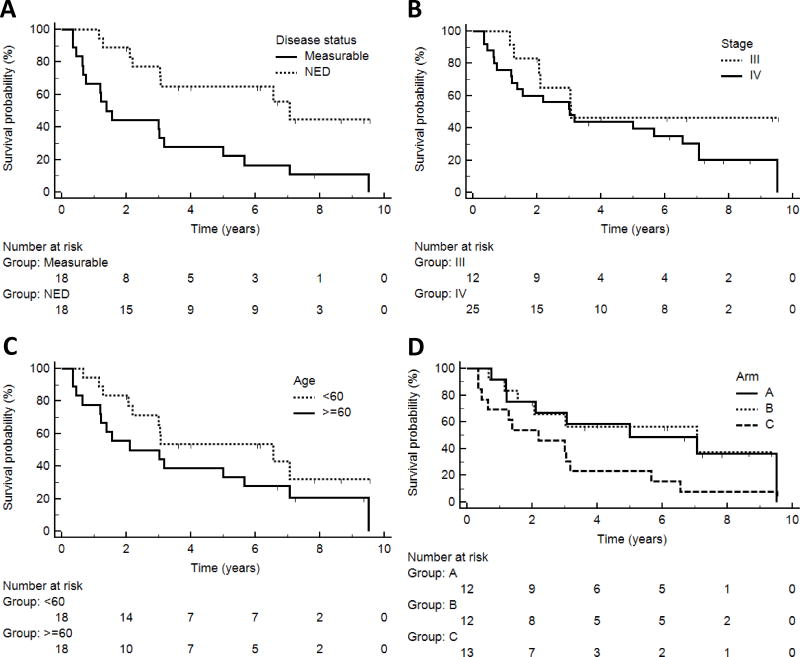
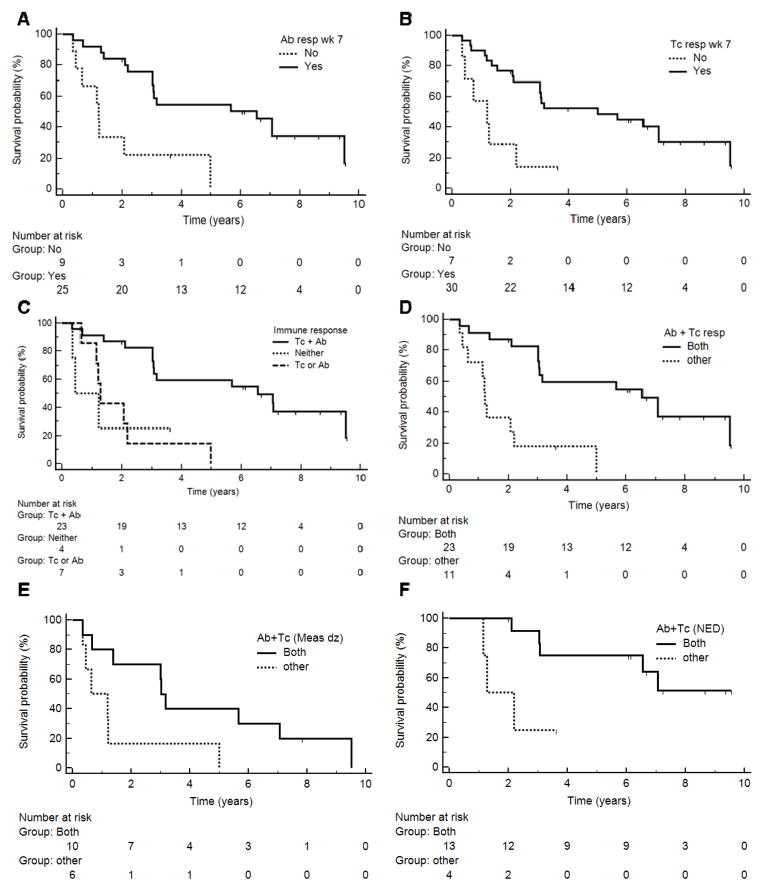
The study population included a range of patient presentations (stage III–IV, with or without measurable disease). Not surprisingly, patients without measurable disease had better survival than those with measurable disease (p = 0.003, Figure 2A). However, patient survival was not associated with stage (p = 0.21, Figure 2B), age (p = 0.16, Figure 2C), or gender (p = 0.18, not shown). The survival curve for patients in Arm C (highest dose) is lower than that for Arms A and B, but this was not significant (p = 0.10, Figure 2D): Arm C differed from Arms A+B by having more stage IV patients (77% vs 67%), more measurable disease (54% vs. 42%), and fewer patients with performance status 0 (54% vs. 71%) (4) Although Arm C may have had a lower survival curve than Arms A and B, the response rates for Ab production by week 7 were 70%, 73% and 77% for Arms A, B and C, respectively. On the other hand, immune responses to peptides in the vaccine were associated with better survival: this was true for early (by week 5–7) Ab response (p = 0.0011, Figure 3A, median survival 6.6 vs. 1.2 years) and for T cell response [(4); p < 0.006, Figure 3B, median survival 5.0 vs 1.2 years]. Survival was best for those with both Ab and T cell responses by week 7 (n = 23, median 6.6 years), less for those with only Ab or T cell responses (n = 7, median 1.3 years), and least for those with neither (n = 4, median 0.8 years; p = 0.001 across the 3 groups, Figure 3C). Similarly, survival was better for those with both Ab and T cell responses compared to all others (p = 0.0002, Figure 3D).
Figure 2. Associations of clinical factors with patient survival.
Kaplan-Meier curves represent overall survival of patients with clinical features A) disease status: measurable disease vs no evidence of disease (NED, p = 0.003); B) AJCC stage (III vs IV, p = 0.21); C) Age (< 60 vs ≥ 60, p = 0.16); and D) study arm (p = 0.10).
Figure 3. Associations of immune response with patient survival.
Kaplan-Meier curves represent overall survival of patients with immune response findings: A) Antibody response by week 7 (p = 0.0011); B) T cell response in PBMC or SIN by week 7 (p < 0.006); C,D) Combined Ab and/or T-cell responses (p = 0.001 and p = 0.0002, respectively); E) Combined Ab plus T cell response for patients with measurable disease (p = 0.033); F) Combined Ab plus T cell response for patients with no evidence of disease (NED, p = 0.015).
Even when accounting for disease status, the association of survival with combined Ab and T cell immune response was supported. The associations of immune responses and survival could not be ascribed to the immune responses to any single peptide (data not shown) but were associated with responses to the 6MHP mixture. Early Ab responses were detected in 15/17 patients without evidence of melanoma (88%) and in 10/16 patients with measurable disease (63%); this difference is not significant (p = 0.19, chi-square). Among the subset of patients with measurable disease, there was better survival with early Ab response (p = 0.03). Among the subset without measurable disease, only 2 patients failed to generate early Ab responses and they had poor outcomes, but there were too few patients in that group for a meaningful statistical comparision. However, a combined Ab + T cell immune response by week 7 was associated with improved survival for those with measurable disease (p = 0.033, Figure 3E, median 3.0 vs 0.6 years), and for those with no evidence of disease, (p = 0.015, Figure 3F, median 7.1 vs 1.3 years).
Among the patients on this trial with measurable disease, two (12%) experienced objective partial responses (PRs), and two others (12%) experienced durable stable disease (SD); all 4 of these PRs and SD were durable, for 1 to 7 years (4). Ab responses were detected by week 7 in all 4 of those patients, and CD4+ T cell responses were also observed in all 4 of these patients. Thus, combined Ab and T cell responses were associated both with improved survival and with objective clinical responses.
Discussion
Peptide vaccines have been designed to stimulate T cell responses to defined cancer antigens, either using short peptides restricted by Class I MHC molecules to stimulate CD8+ T cells, or longer peptides restricted by Class II MHC molecules to stimulate CD4+ T cells. Both approaches have induced T cell responses, but optimal vaccine regimens remain to be defined. Most peptides used in cancer vaccines, including those in the present study, are from intracellular proteins; so Ab to the peptides are not expected to bind to viable tumor cells and thus have not been the focus of immune monitoring of cancer vaccines. However, Ab induced by vaccines could have implications for vaccine immunogenicity or for tumor control. On one hand, Ab could neutralize peptides and reduce T cell responses on repeat immunization. On the other hand, Ab could opsonize peptides in immune complexes, which could increase uptake by dendritic cells, limit peptide degradation, and enhance T cell responses. Also, Ab may opsonize intracellular melanocytic proteins after tumor cell death, to support cross-presentation of proteins released by dying tumor cells. Thus, in the present study, we have assessed IgG Ab responses to each of the 6 peptides in this vaccine, and have evaluated associations with T cell responses and patient survival.
We have found that a vaccine incorporating six HLA-DR restricted peptides derived from melanocytic differentiation proteins (MDP) and cancer-testis antigens (CTA) induced IgG humoral immune responses in melanoma patients in addition to CD4+ T cell responses. Anti-peptide IgG Ab were detected 5–7 weeks after the first vaccine (in 77% of patients), peaked about 6 weeks after the last vaccine to a maximum Ab response rate of 87%, and were long-lasting, persisting to 6 months. The Ab responses were of high magnitude (median 56 mcg/ml, mean 121 mcg/ml; Table 3). There was no significant difference in Ab response rate or magnitude by vaccine dose; however, there were marked differences in immunogenicity by peptide. Tyros386-406 was immunogenic in 76% of patients. The next most immunogenic was MelanA/MART-151-73. Overall, Ab responses were greatest for the longest peptides (> 20 amino acids), and also for peptides of melanocytic proteins. Other studies have shown that long (25–30 amino acids) peptides from the cancer-testis antigen NY-ESO-1 induce strong Ab responses (19, 33). Also a shorter (14 amino acid) NY-ESO-1 peptide converted 2 seronegative patients to seropositive after 5–9 months, but Ab was not detected at early time points. We conclude that early immunogenicity may depend in part on peptide length, but that peptides from both melanocytic antigens and cancer-testis antigens can induce Ab responses.
Ab was detected as early as 5 weeks in some patients, but was not detected at 3 weeks (n = 9). In prior analyses of this trial (4), CD4+ T cell responses were detected at 3 weeks in the sentinel immunized lymph node in 78% of patients evaluated. The Ab responses persisted without apparent change to 6 months or later (Figure 1D). T cell responses peaked at week 7 then declined slightly but were still detected at 9 months (4). Thus, Ab responses have appeared later than the T cell responses but both commonly persisted, and overlapped temporally. Interestingly, the most immunodominant peptides for CD4+ T cell responses were MAGE-A3281-295 and Tyros386-406, with responses in 49% and 32% of patients, respectively, and there was no association with peptide length (31). The Tyros386-406 peptide is highly immunogenic for Ab as well as for T cells, whereas the MAGE-A3281-295 peptide is immunogenic only for T cells (Table 1), MART-1/MelanA51-73 was also immunogenic for T cells and for Ab. These data reveal that Ab and T cells may respond to the same or to different peptides. Since Ab responses occurred to two peptides that were also highly immunogenic for CD4+ T cells, it appears unlikely that the Ab interferes with induction or persistence of CD4+ T cell responses.
Early Ab response was associated with improved patient survival, as was CD4+ T cell response to the peptides. The best survival overall is for those who had both early Ab response and T cell response, with median survival of 6.6 years. Even among those with advanced measureable disease at study entry, median survival with both Ab and T cell responses exceeded 3 years. There was an association between Ab responses and CD4+ T cell responses, but some patients had only Ab responses, and others had only T cell responses. As shown in Figure 3C, the 7 patients with only Ab, or only T cell response had less favorable survival than those with both. Other studies have shown that vaccination with NY-ESO-1, MAGE-A3 peptides or protein induces integrated Ab and T cell responses (12, 15–20). In 2 of those studies, there was a suggestion of clinical activity of vaccines that induce Ab and T cell responses (12, 20). Also, Ab response to surface or secreted protein vaccines (Her2 or β-hCG) in epithelial cancer patients has been associated with prolonged survival (34). CTLA4 blockade has increased Ab responses to intracellular cancer antigens, with some evidence for association of Ab response and clinical activity (13, 14). Interestingly, Ab responses also arise to cancer antigens spontaneously, with data suggesting they may support antitumor immunity or may interfere with it (5, 7, 24). In another study with the 6MHP vaccine, we have found that CD4+ T cell responses are associated with improved survival (35). The present work is the first to show statistically better survival for patients with Ab responses, and with combined Ab and CD4+ T cell responses after vaccination with peptides from intracellular proteins.
These novel findings suggest that Ab responses to a peptide vaccine may have significant prognostic value, especially when combined with T cell response data. Thus, it may be possible to identify patients early for whom benefit of the vaccine approach is unlikely, and for whom alternate therapy may be considered. The association of Ab with improved patient survival raises questions about potential mechanisms for that finding. Melanoma antigens represented in the vaccine are intracellular proteins; thus, antibodies to the peptides would not likely be involved in direct killing of tumor cells by antibody-dependent cellular cytotoxicity or complement-mediated cytotoxicity. However, the Abs we have detected may form immune complexes with the peptides, supporting their uptake by dendritic cells. The vaccines may also bind the source proteins released from dying melanoma cells. As proteins are released into this environment and Ab is present, immune complexes (ICs) may form and be taken up by dendritic cells [DC; reviewed in (36, 37)]. ICs augment cellular immune responses by activating DC, even in the absence of CD4+ T cells (38), and cross presentation is facilitated through Fc receptors and signaling pathways (39, 40). Opsonization of antigen can also lead to increases in DC migration from peripheral tissues to the draining lymph nodes (41). Alternatively, Ab may neutralize peptide, or excess IC can interfere with antigen uptake and presentation (42). However, in light of the positive association with clinical outcome, we hypothesize that in this setting the Abs are supporting antitumor immunity. Future studies are planned to test this hypothesis more directly, to characterize the IgG subtypes of Ab induced by peptides, and to identify vaccine adjuvants that are most effective at inducing Ab that support antitumor immunity.
Supplementary Material
Statement of translational relevance.
We have examined the circulating IgG antibody (Ab) response to vaccination with a mixture of 6 melanoma “helper” peptides (6MHP). Prior studies have shown that the 6MHP vaccine has clinical activity in a subset of patients. In the present study, high Ab titers were detected in most patients. Detection of Ab to one or more peptides by week 7 (end of the vaccine regimen) was associated with significantly improved patient survival. The Ab response also was associated with a helper T cell response, and the best survival was for patients with both Ab and T cell responses. The favorable clinical outcome associated with the Ab responses, and especially the combination of Ab and T cell responses, suggests that the Ab responses may participate in the clinical benefit of these helper peptide vaccines.
Acknowledgments
Financial Support: Funding for this study has been provided by the National Cancer Institute/NIH through R01 CA057653 and U01 178846 (to C.L.S.), the Rebecca Clary Harris Fellowship (to I.S.M.), and from philanthropic support from The Commonwealth Foundation for Cancer Research and Alice and Bill Goodwin, and from George and Linda Suddock.
Footnotes
Disclosure of conflicts: Dr. Slingluff is an inventor for patents on peptides used in cancer vaccines, but not for the peptides studied in the present report, and he is a consultant for companies that develop and test cancer vaccines (Immatics, and Polynoma), but the peptides studied in this report are not owned or licensed by these companies but funds from those relationships go to the University of Virginia, not to him personally. The University of Virginia also receives funds from Glaxo Smith Kline (GSK) for an unrelated vaccine trial, but GSK has licensed some of the peptides used in this trial. C.M.R, N.D.C., I.S.M. and W.C.O. have no conflicts of interest to disclose.
Reference List
- 1.Swartz A, Batich K, Fecci P, Sampson J. Peptide vaccines for the treatment of glioblastoma. J Neurooncol. 2014:1–8. doi: 10.1007/s11060-014-1676-y. [DOI] [PubMed] [Google Scholar]
- 2.Slingluff CL., Jr The present and future of peptide vaccines for cancer: single or multiple, long or short, alone or in combination? The Cancer Journal. 2011:17. doi: 10.1097/PPO.0b013e318233e5b2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dillon P, Olson W, Czarkowski A, Petroni G, Smolkin M, Grosh W, et al. A melanoma helper peptide vaccine increases Th1 cytokine production by leukocytes in peripheral blood and immunized lymph nodes. Journal for ImmunoTherapy of Cancer. 2014;2:23. doi: 10.1186/2051-1426-2-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Slingluff CL, Jr, Petroni GR, Olson W, Czarkowski A, Grosh WW, Smolkin M, et al. Helper T-cell responses and clinical activity of a melanoma vaccine with multiple peptides from MAGE and melanocytic differentiation antigens. J Clin Oncol. 2008;26:4973–80. doi: 10.1200/JCO.2008.17.3161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kobold S, Lütkens T, Cao Y, Bokemeyer C, Atanackovic D. Autoantibodies against tumor-related antigens: Incidence and biologic significance. Human Immunology. 2010;71:643–51. doi: 10.1016/j.humimm.2010.03.015. [DOI] [PubMed] [Google Scholar]
- 6.Jensen-Jarolim E, Singer J. Cancer vaccines inducing antibody production: more pros than cons. Expert Review of Vaccines. 2011;10:1281–9. doi: 10.1586/erv.11.105. [DOI] [PubMed] [Google Scholar]
- 7.Zörnig I, Halama N, Lorenzo Bermejo J, Ziegelmeier C, Dickes E, Migdoll A, et al. Prognostic significance of spontaneous antibody responses against tumor-associated antigens in malignant melanoma patients. Int J Cancer. 2015;136:138–51. doi: 10.1002/ijc.28980. [DOI] [PubMed] [Google Scholar]
- 8.Komatsu N, Jackson HM, Chan K, Oveissi S, Cebon J, Itoh K, et al. Fine-mapping naturally occurring NY-ESO-1 antibody epitopes in melanoma patients’ sera using short overlapping peptides and full-length recombinant protein. Molecular Immunology. 2013;54:465–71. doi: 10.1016/j.molimm.2013.01.014. [DOI] [PubMed] [Google Scholar]
- 9.Gnjatic S, Nishikawa H, Jungbluth AA, Güre AO, Ritter G, Jäger E, et al. NY-ESO-1: Review of an immunogenic tumor antigen. In: George FV, editor. Advances in Cancer Research. 95. Academic Press; 2006. pp. 1–30. [DOI] [PubMed] [Google Scholar]
- 10.Stockert E, Jäger E, Chen YT, Scanlan MJ, Gout I, Karbach J, et al. A survey of the humoral immune response of cancer patients to a panel of human tumor antigens. The Journal of Experimental Medicine. 1998;187:1349–54. doi: 10.1084/jem.187.8.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jäger E, Stockert E, Zidianakis Z, Chen YT, Karbach J, Jäger D, et al. Humoral immune responses of cancer patients against “Cancer-Testis” antigen NY-ESO-1: Correlation with clinical events. Int J Cancer. 1999;84:506–10. doi: 10.1002/(sici)1097-0215(19991022)84:5<506::aid-ijc10>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 12.Davis ID, Chen W, Jackson H, Parente P, Shackleton M, Hopkins W, et al. Recombinant NY-ESO-1 protein with ISCOMATRIX adjuvant induces broad integrated antibody and CD4+ and CD8+ T cell responses in humans. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:10697–702. doi: 10.1073/pnas.0403572101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weber JS, Hamid O, Chasalow SD, Wu DY, Parker SM, Galbraith S, et al. Ipilimumab increases activated T cells and enhances humoral immunity in patients with advanced melanoma. Journal of Immunotherapy. 2012:35. doi: 10.1097/CJI.0b013e31823aa41c. [DOI] [PubMed] [Google Scholar]
- 14.Yuan J, Adamow M, Ginsberg BA, Rasalan TS, Ritter E, Gallardo HF, et al. Integrated NY-ESO-1 antibody and CD8+ T-cell responses correlate with clinical benefit in advanced melanoma patients treated with ipilimumab. Proceedings of the National Academy of Sciences. 2011;108:16723–8. doi: 10.1073/pnas.1110814108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wada H, Isobe M, Kakimi K, Mizote Y, Eikawa S, Sato E, et al. Vaccination with NY-ESO-1 overlapping peptides mixed with Picibanil OK-432 and montanide ISA-51 in patients with cancers expressing the NY-ESO-1 antigen. Journal of Immunotherapy. 2014;37:84–92. doi: 10.1097/CJI.0000000000000017. [DOI] [PubMed] [Google Scholar]
- 16.Rapoport AP, Aqui NA, Stadtmauer EA, Vogl DT, Xu YY, Kalos M, et al. Combination immunotherapy after ASCT for multiple myeloma Using MAGE-A3/poly-ICLC immunizations followed by adoptive transfer of vaccine-primed and costimulated autologous T cells. Clinical Cancer Research. 2014;20:1355–65. doi: 10.1158/1078-0432.CCR-13-2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vantomme V, Dantinne C, Amrani N, Permanne P, Gheysen D, Bruck C, et al. Immunologic analysis of a phase I/II study of vaccination with MAGE-3 protein combined with the AS02B adjuvant in patients with MAGE-3-positive tumors. Journal of Immunotherapy. 2004:27. doi: 10.1097/00002371-200403000-00006. [DOI] [PubMed] [Google Scholar]
- 18.Adams S, O’Neill DW, Nonaka D, Hardin E, Chiriboga L, Siu K, et al. Immunization of malignant melanoma patients with full-length NY-ESO-1 protein using TLR7 agonist imiquimod as vaccine adjuvant. The Journal of Immunology. 2008;181:776–84. doi: 10.4049/jimmunol.181.1.776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sabbatini P, Tsuji T, Ferran L, Ritter E, Sedrak C, Tuballes K, et al. Phase I trial of overlapping long peptides from a tumor self-antigen and poly-ICLC shows rapid induction of integrated immune response in ovarian cancer patients. Clinical Cancer Research. 2012;18:6497–508. doi: 10.1158/1078-0432.CCR-12-2189. [DOI] [PubMed] [Google Scholar]
- 20.Odunsi K, Matsuzaki J, Karbach J, Neumann A, Mhawech-Fauceglia P, Miller A, et al. Efficacy of vaccination with recombinant vaccinia and fowlpox vectors expressing NY-ESO-1 antigen in ovarian cancer and melanoma patients. Proc Natl Acad Sci U S A. 2012;109:5797–802. doi: 10.1073/pnas.1117208109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.von Mensdorff-Pouilly S. Vaccine-induced antibody responses in patients with carcinoma. Expert Review of Vaccines. 2010;9:579–94. doi: 10.1586/erv.10.51. [DOI] [PubMed] [Google Scholar]
- 22.Namm JP, Li Q, Lao X, Lubman DM, He J, Liu Y, et al. B lymphocytes as effector cells in the immunotherapy of cancer. J Surg Oncol. 2012;105:431–5. doi: 10.1002/jso.22093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pandey JP, Kistner-Griffin E, Black L, Namboodiri AM, Iwasaki M, Kasuga Y, et al. IGKC and FcγR genotypes and humoral immunity to HER2 in breast cancer. Immunobiology. 2014;219:113–7. doi: 10.1016/j.imbio.2013.08.005. [DOI] [PubMed] [Google Scholar]
- 24.Matsueda S, Komatsu N, Kusumoto K, Koga S, Yamada A, Kuromatsu R, et al. Humoral immune responses to CTL epitope peptides from tumor-associated antigens are widely detectable in humans: A new biomarker for overall survival of patients with malignant diseases. Developmental & Comparative Immunology. 2013;41:68–76. doi: 10.1016/j.dci.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 25.Lu H, Ladd J, Feng Z, Wu M, Goodell V, Pitteri SJ, et al. Evaluation of known oncoantibodies, HER2, p53, and cyclin B1, in prediagnostic breast cancer sera. Cancer Prevention Research. 2012;5:1036–43. doi: 10.1158/1940-6207.CAPR-11-0558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nelson BH. CD20+ B cells: The other tumor-infiltrating lymphocytes. The Journal of Immunology. 2010;185:4977–82. doi: 10.4049/jimmunol.1001323. [DOI] [PubMed] [Google Scholar]
- 27.Balkwill F, Montfort A, Capasso M. B regulatory cells in cancer. Trends in Immunology. 2013;34:169–73. doi: 10.1016/j.it.2012.10.007. [DOI] [PubMed] [Google Scholar]
- 28.Fang L, Lowther DE, Meizlish ML, Anderson RCE, Bruce JN, Devine L, et al. The immune cell infiltrate populating meningiomas is composed of mature, antigen-experienced T and B cells. Neuro-Oncology. 2013;15:1479–90. doi: 10.1093/neuonc/not110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zirakzadeh AA, Marits P, Sherif A, Winqvist O. Multiplex B cell characterization in blood, lymph nodes, and tumors from patients with malignancies. The Journal of Immunology. 2013;190:5847–55. doi: 10.4049/jimmunol.1203279. [DOI] [PubMed] [Google Scholar]
- 30.Erdag G, Schaefer JT, Smolkin ME, Deacon DH, Shea SM, Dengel LT, et al. Immunotype and immunohistologic characteristics of tumor-infiltrating immune cells are associated with clinical outcome in metastatic melanoma. Cancer Research. 2012;72:1070–80. doi: 10.1158/0008-5472.CAN-11-3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hu Y, Petroni G, Olson W, Czarkowski A, Smolkin M, Grosh W, et al. Immunologic hierarchy, class II MHC promiscuity, and epitope spreading of a melanoma helper peptide vaccine. Cancer Immunol Immunother. 2014;63:779–86. doi: 10.1007/s00262-014-1551-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gnjatic S, Old LJ, Chen YT. Autoantibodies against cancer antigens. In: Tainsky MA, editor. Tumor Biomarker Discovery. 520. Humana Press; 2009. pp. 11–9. [DOI] [PubMed] [Google Scholar]
- 33.Ayyoub M, Pignon P, Dojcinovic D, Raimbaud I, Old LJ, Luescher I, et al. Assessment of vaccine-induced CD4 T cell responses to the 119–143 immunodominant region of the tumor-specific antigen NY-ESO-1 using DRB1*0101 tetramers. Clinical Cancer Research. 2010;16:4607–15. doi: 10.1158/1078-0432.CCR-10-1485. [DOI] [PubMed] [Google Scholar]
- 34.Moulton HM, Yoshihara PH, Mason DH, Iversen PL, Triozzi PL. Active specific immunotherapy with a β-human chorionic gonadotropin peptide vaccine in patients withmetastatic colorectal cancer: antibody response is associated with improved survival. Clinical Cancer Research. 2002;8:2044–51. [PubMed] [Google Scholar]
- 35.Slingluff CL, Lee S, Zhao F, Chianese-Bullock KA, Olson WC, Butterfield LH, et al. A randomized phase II trial of multiepitope vaccination with melanoma peptides for cytotoxic T cells and helper T cells for patients with metastatic melanoma (E1602) Clinical Cancer Research. 2013;19:4228–38. doi: 10.1158/1078-0432.CCR-13-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Platzer B, Stout MM, Fiebiger E. Antigen cross-presentation of immune complexes. Frontiers in Immunology. 2014:5. doi: 10.3389/fimmu.2014.00140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Baker K, Rath T, Pyzik M, Blumberg RS. The role of FcRn in antigen presentation. Frontiers in Immunology. 2014:5. doi: 10.3389/fimmu.2014.00408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schuurhuis DH, Ioan-Facsinay A, Nagelkerken B, van Schip JJ, Sedlik C, Melief CJM, et al. Antigen-Antibody Immune Complexes Empower Dendritic Cells to Efficiently Prime Specific CD8+ CTL Responses In Vivo. The Journal of Immunology. 2002;168:2240–6. doi: 10.4049/jimmunol.168.5.2240. [DOI] [PubMed] [Google Scholar]
- 39.Boross P, van Montfoort N, Stapels DAC, van der Poel CE, Bertens C, Meeldijk J, et al. FcRg-Chain ITAM Signaling Is Critically Required for Cross-Presentation of Soluble Antibody-Antigen Complexes by Dendritic Cells. The Journal of Immunology. 2014;193:5506–14. doi: 10.4049/jimmunol.1302012. [DOI] [PubMed] [Google Scholar]
- 40.Rafiq K, Bergtold A, Clynes R. Immune complex-mediated antigen presentation induces tumor immunity. J Clin Invest. 2002;110:71–9. doi: 10.1172/JCI15640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Clatworthy MR, Aronin CEP, Mathews RJ, Morgan NY, Smith KGC, Germain RN. Immune complexes stimulate CCR7-dependent dendritic cell migration to lymph nodes. Nat Med. 2014;20:1458–63. doi: 10.1038/nm.3709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yamada DH, Elsaesser H, Lux A, Timmerman JM, Morrison SL, de la Torre JC, et al. Suppression of Fcγ-Receptor-Mediated Antibody Effector Function during Persistent Viral Infection. Immunity. 2015;42:379–90. doi: 10.1016/j.immuni.2015.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.