Abstract
We report the phenotype of mice with targeted disruption of the Trpv6 (Trpv6 KO) epithelial calcium channel. The mice exhibit disordered Ca2+ homeostasis, including defective intestinal Ca2+ absorption, increased urinary Ca2+ excretion, decreased BMD, deficient weight gain, and reduced fertility. Although our Trpv6 KO affects the closely adjacent EphB6 gene, the phenotype reported here is not related to EphB6 dysfunction.
Introduction
The mechanisms underlying intestinal Ca2+ absorption are crucial for overall Ca2+ homeostasis, because diet is the only source of all new Ca2+ in the body. Trpv6 encodes a Ca2+-permeable cation channel responsible for vitamin D–dependent intestinal Ca2+ absorption. Trpv6 is expressed in the intestine and also in the skin, placenta, kidney, and exocrine organs.
Materials and Methods
To determine the in vivo function of TRPV6, we generated mice with targeted disruption of the Trpv6 (Trpv6 KO) gene.
Results
Trpv6 KO mice are viable but exhibit disordered Ca2+ homeostasis, including a 60% decrease in intestinal Ca2+ absorption, deficient weight gain, decreased BMD, and reduced fertility. When kept on a regular (1% Ca2+) diet, Trpv6 KO mice have deficient intestinal Ca2+ absorption, despite elevated levels of serum PTH (3.8-fold) and 1,25-dihydroxyvitamin D (2.4-fold). They also have decreased urinary osmolality and increased Ca2+ excretion. Their serum Ca2+ is normal, but when challenged with a low (0.25%) Ca2+ diet, Trpv6 KO mice fail to further increase serum PTH and vitamin D, ultimately developing hypocalcemia. Trpv6 KO mice have normal urinary deoxypyridinoline excretion, although exhibiting a 9.3% reduction in femoral mineral density at 2 months of age, which is not restored by treatment for 1 month with a high (2%) Ca2+ “rescue” diet. In addition to their deranged Ca2+ homeostasis, the skin of Trpv6 KO mice has fewer and thinner layers of stratum corneum, decreased total Ca2+ content, and loss of the normal Ca2+ gradient. Twenty percent of all Trpv6 KO animals develop alopecia and dermatitis.
Conclusions
Trpv6 KO mice exhibit an array of abnormalities in multiple tissues/organs. At least some of these are caused by tissue-specific mechanisms. In addition, the kidneys and bones of Trpv6 KO mice do not respond to their elevated levels of PTH and 1,25-dihydroxyvitamin D. These data indicate that the TRPV6 channel plays an important role in Ca2+ homeostasis and in other tissues not directly involved in this process.
Keywords: intestinal calcium absorption, renal excretion, TRPV6, alopecia, vitamin D
INTRODUCTION
Calcium is the most abundant ion in the body. It is present in all tissues and organs and is necessary for numerous vital functions such as cardiac and skeletal muscle contraction, neurotransmission, tissue differentiation, and cell metabolism. All bodily Ca2+ is acquired through absorption from the diet. The vast majority is stored in the bones and a small percentage is recycled daily.(1)
The extracellular concentration of Ca2+ is maintained within a narrow range by the orchestrated interactions of hormones, Ca2+ channels, transporters, and sensors. When serum Ca2+ concentration decreases, the levels of PTH and 1,25-dihydroxyvitamin D increase and activate mechanisms that shunt Ca2+ into the blood. These mechanisms include intestinal absorption of Ca2+ from the diet, Ca2+ reabsorption by the kidneys, and resorption of Ca2+ from the bones. Because the diet is the sole source of new Ca2+, the mechanisms involved in intestinal Ca2+ absorption are key players in overall Ca2+ homeostasis. Alterations in intestinal absorption (and/or renal reabsorption) of ions often result in pathophysiological conditions.
Vitamin D–sensitive intestinal Ca2+ absorption is an active process whereby Ca2+ is absorbed through the transcellular pathway. This pathway is believed to be the primary mechanism for Ca2+ influx into the body under conditions of normal or low Ca2+ intake (e.g., luminal [Ca2+] in the millimolar or submillimolar range).(2) It requires at least three steps: (1) entry through the apical brush border membrane; (2) transport across the cell to the basolateral side of the epithelium; and (3) active extrusion into the bloodstream. Two epithelial Ca2+ channels, TRPV5 and TRPV6, are now considered key molecules in the active (transcellular) entry of Ca2+ across the apical epithelia of the kidney and gut. TRPV5 and TRPV6 belong to the vanilloid subfamily of the Transient Receptor Potential (TRP) superfamily, and are predominately expressed in Ca2+-transporting epithelia. They share 75% homology with one another, but are <40% homologous to other TRP family members. Both TRPV5 and TRPV6 are responsive to 1,25-dihydroxyvitamin D and have been shown to be selective for Ca2+ in electrophysiological studies.(3,4) Trpv5 expression is rather restricted to the kidney, where its levels are high, whereas Trpv6 is abundantly expressed in the duodenum and upper jejunum, as well as in the developing kidney, but only moderately expressed in the adult kidney.(5) In addition, Trpv6 is expressed in the skin, placenta, and exocrine organs.(6)
To study the roles of TRPV6 in vitamin D–regulated intestinal Ca2+ absorption, we developed a Trpv6 knockout (Trpv6 KO) mouse. This animal model is also useful for characterizing the contributions of the TRPV6 Ca2+ channel to other sites where active Ca2+ transport is important, thereby unraveling the relevance of TRPV6 to Ca2+ homeostasis and physiology.
MATERIALS AND METHODS
Animals
The Trpv6 KO mice used in this study were from our colony housed at the Harvard Institutes of Medicine Animal Facility and, unless otherwise stated, had free access to water and food. They were fed a high Ca2+ (2% Ca2+ and 1.25% P), low Ca2+ (0.25% Ca2+ and 0.4% P), no added Ca2+ (contaminant Ca2+ ~0.005–0.01%; 0.4% P), or regular Ca2+ (1% Ca2+, 0.4% P) diet. All special diets were from Harlan-Teklad (Madison, WI, USA). The Trpv6 KO mice were backcrossed with the C57/B6 mice for three generations. They still carried a mixed background of B6/129. Thus, we always bred heterozygous pairs and used the wildtype littermates as controls for all experiments described herein. All animal protocols used were approved by the Institutional Animal Care and Use Committee (IACUC) of the Center of Animal Resources and Comparative Medicine (ARCM) of the Harvard Medical School.
The EphB6 KO mice used in this study carried the C57/B6 background. They were backcrossed to C57/B6 for eight generations. Age-matched C57/B6 (2.5–6 months old) were used as controls.
Reagents
Unless otherwise specified, all reagents were from Sigma-Aldrich (St Louis, MO, USA).
Targeting vector and generation of Trpv6 null mice
The targeting vector (Fig. 1A) was constructed using a short and a long arm of the Trpv6 sequence flanking the Neomycin resistance gene. The short arm is a 762-bp fragment flanked by the Trpv6 primer sequences “CATSA2” (5′-CTTTCAGATGTTCCAACACCTG-3′) and “CATSA1” (5′-CATCCTTAGGGGTCACATAGG-3′). This segment includes part of the sixth intron, the seventh and eighth exons, and part of the ninth Trpv6 exon. The sequence includes the last of a series of ankyrin repeats, the first transmembrane domain, and most of the first extracellular loop. It was inserted into the 5′-end of the Neo cassette using a HindIII site. The long arm is a 9.5-kb DNA fragment from a mouse genomic library. It was inserted into the targeting vector using an AflII site. The sequence of the long arm was found to start in the 14th intron of the EphB6 gene, which is oriented tail to tail with Trpv6 in the mouse genome and is separated by 120 bp. As a result, most of the coding sequence of Trpv6 (from transmembrane domain 2 to the end of the protein) was replaced by the Neo cassette. In addition, the last four exons of the EphB6 gene were also deleted. Ten micrograms of the targeting vector was linearized with NotI and transfected by electroporation into 129 SvEv embryonic stem cells. After selection in G418, surviving colonies were expanded and screened for homologous recombination by PCR using CATSA12 and Neo1 as primers. CATSA12 is the 23-bp fragment, 5′-CAGGCTTGGAATGATTCCTGGAG-3′, outside of the short arm and 170 bp upstream of CATSA2. Neo1 (5′-TGCGAGGCCAGAGGCCACTTGTGTAGC-3′) is located in the 5′-promoter region of the neo-cassette. Positive clones, recognized by the amplification of a 1.1-bp fragment, were microinjected into C57BL/6J blastocysts to generate chimeric mice with germline transmission of the disrupted gene.
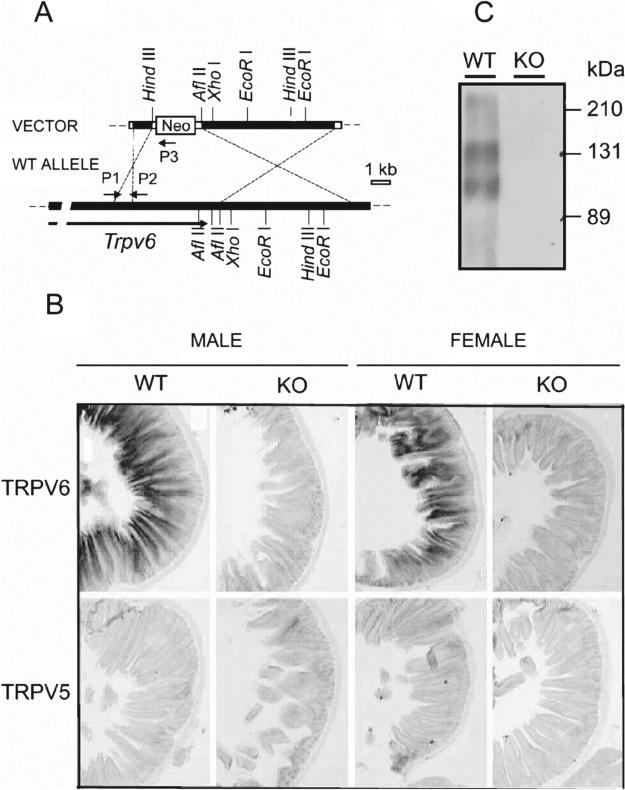
FIG. 1.
(A) Trpv6 targeting vector (top) and predicted product loci (bottom). P1, P2, and P3 indicate the positions of the primers used for genotyping. Filled boxes, exons with coding sequence; open boxes, exons for untranslated sequences (please refer to Materials and Methods section for details on the construction of the targeting vector). (B) In situ hybridization for Trpv6 and Trpv5 in the duodenum of adult wildtype (+) or Trpv6 null mice (−) male (M) or female (F) mice (−/−). (C) Western blot for Trpv6 in the intestine of adult wildtype (+/+) or Trpv6 null mice.
EphB6 is not known to be involved in Ca2+ homeostasis, and it is not expressed in mouse intestine.(7) Although no Ca2+-related abnormalities were reported in the EphB6 null mice,(8,9) we tested the EphB6 KO mice for some of the calcium abnormalities found in the Trpv6 KO mice described here.
Genotyping
The wildtype Trpv6 allele was amplified by PCR using P1 as forward primer (5′-AACCTGAAAGAGCCAGGGACTTTGGAAAC-3′) and P2 as reverse primer (5′-CGAGAATGGTCTGTCCAAAGAA TCGAGTG-3′). P1 is located in the 8th intron and P2 is located in the 10th exon of Trpv6. The expected product is 314 bp. The allele that was knocked out was amplified using P1 and P3 as primers, where P3 is identical to the Neomycin resistance cassette. The amplified product was 220 bp. Because the 10th Trpv6 exon is deleted in the Trpv6 KO mice, and the Neo cassette is absent in the WT mice, all primers were included in the same reaction mixture for routine genotyping. DNA amplified from heterozygous carriers yielded both fragments (314 and 220 bp).
Quantitative detection of Trpv6 mRNA by real-time PCR
Total RNA was isolated using Trizol (Invitrogen), according to the manufacturer's instructions. After checking integrity by electrophoresis, RNA samples were reverse-transcribed using the Superscript II Reverse Transcriptase (Invitrogen) according to the manufacturer's protocol. The amount of Trpv5, Trpv6, or GAPDH cDNA in each sample was determined by quantitative PCR using the LightCycler system (Roche) in 20 μl containing 2 μl of LightCycler PCR mix DNA master SYBRGreen I (Roche), 0.25 μM of primers [Trpv5: 5′-ATTGACGGACCTGCCAATTACAGAG-3′ (forward) and 5′-GTGTTCAACCCGTAAGAACCAACGTC-3′ (reverse); Trpv6: 5′-ATCGATGGCCCTGCGAACT-3′ (forward) and 5′-CAGAGTAGAGGCCATCTTGTTGCTG-3′ (reverse); and GAPDH: 5′-TCACCATCTTCCAGGAGCG-3′ (forward) and 5′-CTGCTTCACCACCTTCTTGA-3′ (reverse)], 3 mM MgCl2, and 2 μl of cDNA. After the initial denaturation at 95°C for 20 s, the samples were amplified as follows: denaturation at 95°C for 0 s (with a temperature transition rate of 20°C/s from last extension cycle), annealing at 62°C (Trpv5 and Trpv6) or 60°C (GAPDH) with a temperature transition rate of 8°C/s, extension at 72°C for 20 s (25 s for GAPDH) with a temperature transition rate of 4°C/s, followed by the fluorescence detection at 86°C (Trpv5 and Trpv6) or 85°C (GAPDH) for 3 s.
Trpv6 expression by in situ hybridization and Western blotting
Sense and antisense probes were created from plasmid constructs of Trpv6 cDNA inserted into pGEMTeasy (Promega) and synthesized using the DIG RNA labeling kit (Roche). The Trpv6 cDNA probe was linearized with SalI (for sense) or NotI (for antisense). The Trpv5 cDNA probe was linearized with SalI (for sense) or ApaI (for antisense). cRNA probes were transcribed from a fragment containing 670 kb (1934–2603) of the Trpv6 cDNA and 943 kb (1963–2905) of the Trpv5 cDNA, both flanked by promoter sequences of the T7 RNA polymerase for sense and SP6 RNA polymerase for antisense. Sense and antisense DIG-labeled transcripts were alkali-hydrolyzed to an average length of 500 nucleotides. In situ hybridization was performed on 10-μm cryosections of fresh-frozen mouse tissues. Sections were immersed in slide mailers in a hybridization solution composed of 50% formamide, 5% SSC, 2% blocking reagent (Roche), 0.02% SDS, and 0.1% N-lauroylsarcosine and were hybridized at 70°C for 60 minutes with sense or antisense probes at a concentration of 200 ng/ml. Sections were washed three times in 2% SSC and twice in 0.2% SSC for 30 minutes at 70°C. After washing, the hybridized probes were visualized by alkaline phosphatase histochemistry using alkaline phosphatase–conjugated, anti-digoxigenin Fab fragments (Roche) and 5-bromo-4-chloro-3-indolyl phosphate/nitro blue tetrazolium.
Protein expression was detected in enterocytes isolated from 6-month-old females by Western blot. Briefly, the first 15 cm of the small intestine was removed and rinsed. The epithelial layer was isolated and digested at 25°C × 200 rpm for 20 minutes in 0.1 M PBS with 1% NP-40, 0.25% sodium deoxycholate, 0.5% Triton X-100, and protease inhibitors (Roche). Digested layers were centrifuged at 15,000 rpm for 30 minutes at 4°C. Thirty micrograms of supernatant protein (Bradford) was submitted to 0.1% SDS/7.5% PAGE and transferred to a 0.45-μm nitrocellulose membrane (Pierce). Nonspecific binding was blocked with 0.5% Tween-20 and 2% skim milk in 0.1 M PBS, pH 7.4, for 30 minutes. Immunoreactive Trpv6 was detected using a 1:5000 dilution of a polyclonal chicken anti-Trpv6 anti-serum in PBS(6) and 0.5% Tween-20 overnight at 4°C. The primary antibody was detected with a 1:5000 dilution of a horseradish peroxidase (HRP)-labeled goat anti-chicken antibody (Aves Laboratories) for 2 h at room temperature. The secondary antibody was washed and the labeled Trpv6 was visualized by enhanced chemiluminescence (ECL detection system by Amersham Biosciences) according to the manufacturer's instructions.
Ca2+ gradient in the layers of stratum corneum
Skin samples were removed and processed for ion capture cytochemistry, as previously described.(10) Samples were minced and immediately immersed in an ice-cold fixative containing 2% glutaraldehyde, 2% formaldehyde, 90 mM potassium oxalate, and 1.4% sucrose, pH 7.4. After overnight fixation at 4°C in the dark, samples were postfixed in 1% osmium tetroxide containing 2% potassium pyroantimonate and routinely processed and embedded in an epon–epoxy resin mixture. Ultra-thin sections were double-stained with uranyl acetate/lead citrate and examined with a Zeiss electron microscope operating at 60 kV.
Trpv6 immunostaining
Human Normal-Grid multitissue slides obtained from Biomeda (Foster City, CA, USA) were deparaffinized in xylene and rehydrated in gradient alcohol and water. The slides were blocked in 5% BSA at room temperature and incubated overnight with chicken anti-Trpv6 IgY CH2747 (1:300) at 4°C. After three washings, slides were incubated with a 1:1000 dilution of the HRP-conjugated donkey anti-chicken IgG (Aves Laboratories) for 1 h at room temperature. Immunoreactivity was detected with the DAB (3,3′-diaminobenzidine tetrahydrochloride)-Chromagen system (ABC Elite from Vector Laboratories). Controls for nonspecific immunoreactivity were performed with preimmune chicken IgY.
Serum and urine Ca2+
Total serum or urinary Ca2+ was determined by the Stanbio (Liquicolor, cat no. 0150) colorimetric assay. Briefly, the Ca2+ was dissociated from binding proteins in an acidic solution and combined with ortho-cresolphthalein complexone to form a stable purple color. The absorbance at 550 nm is linear and proportional to the amount of Ca2+ upto 15 mg/dl. The Ca2+ concentrations in serum and urine were calculated from a standard curve and are presented as milligrams per deciliter.
Intestinal Ca2+ absorption after oral gavage
All mice were starved overnight before the gavage experiments. Serum 45Ca2+ was determined after oral administration of 0.15 ml/ g BW of 15 mM Tris-HCl, pH 7.4, containing 100 μM CaCl2, 125 mM NaCl2, 1.8 g/liter fructose, and 20 μCi/ ml 45Ca2+ to hand-restrained mice using a feeding needle. Blood samples were collected at 5, 10, and 15 minutes (tail bleeding) and at 30 minutes by cardiac puncture in IsoFlo-anesthetized mice. Ten microliters of serum or urine collected at the same time-points was counted by liquid scintillation (Packard Instruments), and the results are presented as nanograms 45Ca2+per milliliter serum or urine. Mouse carcasses were discarded according to the Harvard Radiation Protection Office Guidelines. In terms of the total calcium concentration used in the gavage studies (100 μM), our strategy was to minimize the undesired calcium entry at higher calcium levels (>100 μM) through the paracellular pathway, but also to keep it not too far below the apparent Trpv6 Km for calcium (440 μM for rats, 250 μM for humans).
Metabolic studies, osmolality, and deoxypyridinoline determination in the urine
The mice were housed overnight in individual metabolic cages (Nalgene) with free access to water and food. The amount of water and food consumed and the volume of urine per animal were recorded daily for 2–3 consecutive days. Urine samples from different animals were used to determine total Ca2+, osmolality, creatinine, and deoxypyridinoline (DPD). Osmolality in 10 μl of urine was determined using a vapor pressure osmometer (Wescor). The osmometer was calibrated against standard solutions just before use. The results are the mean ± SE of 10–20 samples (mmol/kg). Urinary creatinine levels in the Trpv6 KO mice were determined by the Stanbio colorimetric assay and used to normalize the urine DPD. Creatinine levels in EphB6 KO mice were determined by the Synchron LX20 Clinical System (Beckman/Couter). The urine DPD was determined by the Metra DPD EIA kit (Quidel) according to the manufacturer's instructions. Samples were transferred to a DPD monoclonal antibody-coated strip. DPD in the sample competes for the antibody with conjugated DPD-alkaline phosphatase, and the reaction is detected with a pNPP substrate. DPD levels were normalized to creatinine. Results are expressed as nmol DPD/mmol creatinine and represent the mean ± SD of 10–20 samples.
Intact PTH assay
PTH was determined using the two-site ELISA for quantitative determination of intact PTH (Immutopics International, cat no. 60–2300). Two goat polyclonal antibodies were used. One is biotinylated and recognizes the C-terminal portion (39–84) of the peptide. The other is conjugated with HRP and recognizes the N-terminal region (1-34). Twenty-five microliters of serum was incubated for 3 h at room temperature with both antibodies in a streptavidin-coated microtiter plate. The wells were washed five times and incubated with the HRP substrate at room temperature for 30 minutes. PTH was calculated by plotting the absorbencies at 450 nm against the standard curve. Results are expressed as picograms per milliliter serum.
1,25-dihydroxyvitamin D assay
1,25-dihydroxyvitamin D was determined by radioimmunoassay (RIA; Nichols Institute Diagnostics, cat no. 40–6090). 1,25-dihydroxyvitamin D was separated from other vitamin D metabolites and potential cross-reactants before the assay by immunoextraction of 200 μl of nonhemolyzed serum using a monoclonal 1,25-dihydroxyvitamin D antibody (depilation agent). The purified 1,25-dihydroxyvitamin D in the eluate was diluted 1:2 and quantitated by RIA using a polyclonal antibody and 125I-labeled 1,25 dihydroxyvitamin D as the tracer. 1,25-dihydroxyvitamin D in unknown samples was calculated against the standards, and the results are presented as picograms of 1,25-dihydroxyvitamin D per milliliter serum.
BMD
Adult males fed the regular, high, or no added Ca2+ diet for 1 month were weighed and killed by Isoflorane (IsoFlo) exposure at exactly 95 days of age. The right femur was dissected, and BMD was assessed by DXA using the pDEXA Sabre Bone Densitometer and the pDEXA Sabre Software version 3.9.4 (Norland Medical Systems, Fort Atkinson, WI, USA), both specially designed for small animals. The research mode scan option was used in the measurements, and the pixel spacing was set to 0.5 × 0.5 mm and scan speed to 4 mm/s. BMD values are the average of measurements from three mice.
Statistical analysis
Differences were considered significant when p ≤ 0.05 by two-way ANOVA (three or more groups) followed by the Newman-Keuls post-test for comparison. When comparing two groups, significance was tested with the Student's t-test. All regression curves were calculated and traced by the GraphPad Prism.
RESULTS
Confirmation of the knockout of the Trpv6 gene
We used the duodenum to document the absence of Trpv6 expression, because it is the most important intestinal segment for Trpv6-dependent Ca2+ absorption and has the highest levels of Trpv6 expression along the intestine. In situ hybridization of WT duodenum showed a strong signal in the epithelial cells, whereas no signal was detected in Trpv6 KO male or female mice (Fig. 1B, first row). In addition, no Trpv5 RNA could be detected in the same sections (Fig. 1B, second row). The Western blot of WT intestine detected 2 Trpv6-positive bands at 110 and 145 kDa, whereas no bands could be detected in the membranes isolated from Trpv6 KO mice (Fig. 1C). The two bands are caused by complex glycosylation of Trpv6. When expressed in Xenopus oocytes or mammalian cells, Trpv6 migrates as two major bands, which represents the core-glycosylated and complex glycosylated forms of Trpv6 channel proteins, respectively (unpublished observations). The native Trpv6 from mouse small intestine exhibited higher molecular weight bands than those expressed in transfected cells. This is likely because of the fact that proteins in native tissues can undergo more complex glycosylation. A similar phenomenon has been reported for other transport proteins, such as the thiazide sensitive Na-Cl co-transporter (NCC) in mouse kidney.(11) The absence of Trpv6 expression was corroborated by real-time PCR using the intestine (Fig. 2A) and kidneys (Fig. 2B) of Trpv6 KO mice.
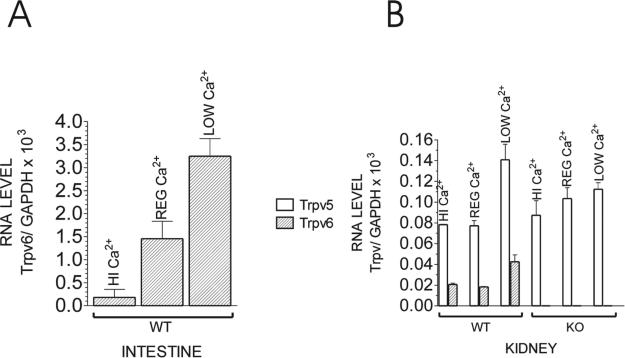
FIG. 2.
Quantitative RT-PCR for (A) Trpv6 in the intestine and (B) Trpv5 and Trpv6 in the kidney of adult WT or Trpv6 KO mice fed the low (0.25%), regular (1%), or high (2%) Ca2+ diet. Each bar is the average of two different mouse samples ± SE and is represented as the ratio of [Trpv/GAPDH] RNA × 103.
Figure 2B shows that Trpv5 mRNA in the kidney was 34% increased in Trpv6 KO mice fed a regular (1%) diet and 20% decreased in Trpv6 KO mice fed a low (0.25%) Ca2+ diet compared with WT littermates kept on the same diets. The high (2%) Ca2+ treatment had no significant effect on the level of Trpv5 or Trpv6 mRNA in the kidney compared with regular Ca2+ diet, confirming our previous observations.(5) In contrast, compared with a regular diet, Trpv6 mRNA levels in the intestine of WT mice were 87.5% reduced in response to a high Ca2+ diet and 124% increased in response to a low Ca2+ diet (Fig. 2A).
Characterization of the Trpv6 KO phenotype
Mice with targeted disruption of the Trpv6 gene have a normal life span, although they show signs of abnormal development early in life. They weigh less than their WT littermates throughout life and have impaired fertility. In contrast, EphB6 KO mice up to 24 months of age have no obvious signs of abnormal development or fertility.(8,9)
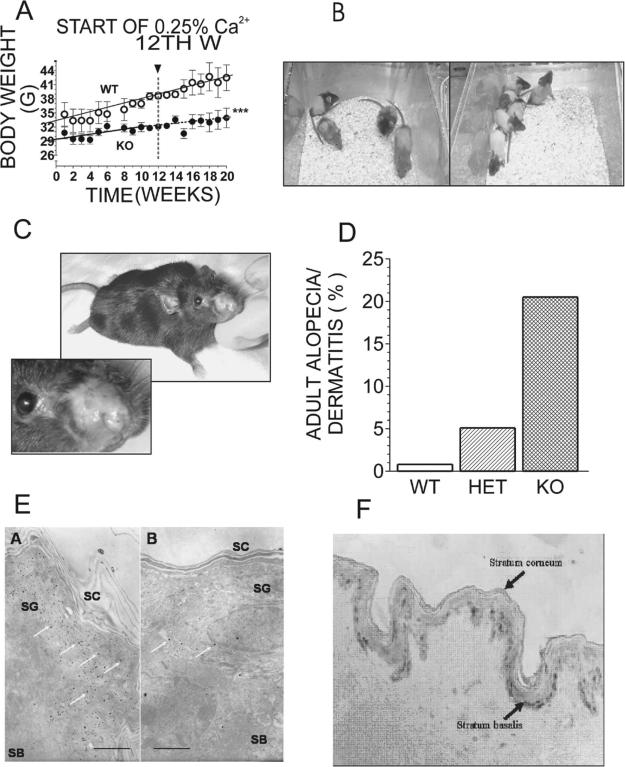
The difference in weight gain between KOs and controls slowly increased with time (Fig. 3A). The switch to the low Ca2+ diet at the 12th week of the experiment did not significantly affect the weight gain in control or Trpv6 KO mice (Fig. 3A). During the first week of the experiment, the KO mice weighed 11.3% less than the age-matched controls, and by the 20th week, they weighed 20.1% less. No difference in weight gain was observed between Trpv6 KO and heterozygous mice.
FIG. 3.
(A) Weight gain in adult WT or Trpv6 KO mice fed the regular (1% Ca2+) diet. At the 12th week of the experiment, the diet was switched to the low (0.25%) Ca2+ diet. Weight gain was recorded once a week, and the results are the average of 4 mice ± SE. ***p < 0.0001 vs. WT. (B) 25-day-old pups born to Trpv6 +/− mothers bred on the high (2%) Ca2+ diet. (C) Adult (8 months old) Trpv6 KO female with severe dermatitis of the nose. (D) Incidence of alopecia/dermatitis among adult Trpv6 KO mice. Approximately 800 mice born to different combinations of breeding pairs were observed for 1 year. White bar, WT mice; striped bar, heterozygous (Het) mice; black bar, Trpv6 KO (KO) mice. (E) Electron dense deposits of Ca2+ precipitates in normal-appearing skin of adult (a) WT and (b) Trpv6 KO mice. White arrows, electron dense deposits; SG, density of Ca2+ precipitates in the stratum granulosum of the outer epidermis; SB, density of Ca2+ precipitates in the stratum basalis of the inner epidermis; SC, density of Ca2+ precipitates in the stratum corneum. Bars are 2 rm radium. (F) TRPV6 immunostaining in normal skin: Normal-Grid slides of skin tissue were incubated with anti-TRPV6, washed, and incubated with HRP-conjugated second IgG. Immunoreactivity was detected with the DAB-Chromagen system (ABC Elite). Controls for nonspecific immunoreactivity were performed with preimmune serum.
When kept on a regular diet, Trpv6 KO males rarely impregnated females: Only once was a KO male able to impregnate a heterozygous female, resulting in the birth of only one pup. KO females took longer to get pregnant than control females, and when they eventually became pregnant, they had a relatively small number of pups (usually four to six pups). About 20% of the KO mothers showed abnormal maternal behavior with at least part of the litter. Transferring KO mothers with abnormal maternal behavior to a new cage did not trigger pup retrieval or nest-building activities.
Eighty percent of all KO and 35% of all heterozygous mice exhibited alopecia as soon as their hair started to grow (Fig. 3B). These percentages decreased with age, and by adulthood, 20% of all Trpv6 KO and 5% of all heterozygous mice had persistent alopecia and an eczematous dermatitis (Figs. 3C and 3D). However, only heterozygous mice born to KO mothers developed alopecia (e.g., no cases of alopecia in heterozygotes born to WT mothers were observed). Although we cannot exclude the possibility that there was increased maternal grooming, it has been shown that the mammary gland expresses high amounts of Trpv6,(6) suggesting that Ca2+ deficiencies in the milk may have been involved.
Analysis of skin samples from WT mice revealed the expected Ca2+ gradient in which the levels of intracellular and extracellular Ca2+ progressively increase from the basal layer to the uppermost viable epidermal layer, the stratum granulosum (Fig. 3Ea). In contrast, epidermis isolated from Trpv6 KO mice exhibited a visible reduction in total Ca2+ with losses of intracellular as well as extracellular Ca2+, particularly in the uppermost stratum granulosum where the normal Ca2+ concentration is well above the serum levels. These alterations were present not only in epidermal regions exhibiting alopecia, but also in Trpv6 KO epidermis with normal appearance (Fig. 3Eb). Accordingly, epidermis from normal skin tissue show noticeable levels of Trpv6 expression (Fig. 3F). The amount of lipid secretion and the degree of lipid processing, also required for normal epidermal permeability barrier function, appeared normal in both WT and Trpv6 KO animals. Even though the EphB6 KO mice show no signs of alopecia or dermatitis, skin samples of the EphB6 KO mice are being analyzed and show no signs of abnormal Ca2+ gradient (data not shown). Although we cannot rule out the contribution of EphB6 at this point, the striking similarities in the skin (as well as the overall Ca2+ homeostasis) phenotype of the Trpv6 and VDR KO mice suggest that these epidermal defects may be related to a tissue-specific Ca2+ signaling deficiency.
Intestinal Ca2+ absorption
Intestinal 45Ca2+ absorption was deficient in Trpv6 KO mice under all conditions tested. The concentration of total calcium used (0.1 mM) is below the TRPV6 Km and was chosen to ensure that the TRPV6-mediated uptake would not be saturated. Therefore, the results would reflect the uptake through TRPV6 calcium channels. In addition, this strategy minimizes the undesired entry of calcium through the paracellular pathway.
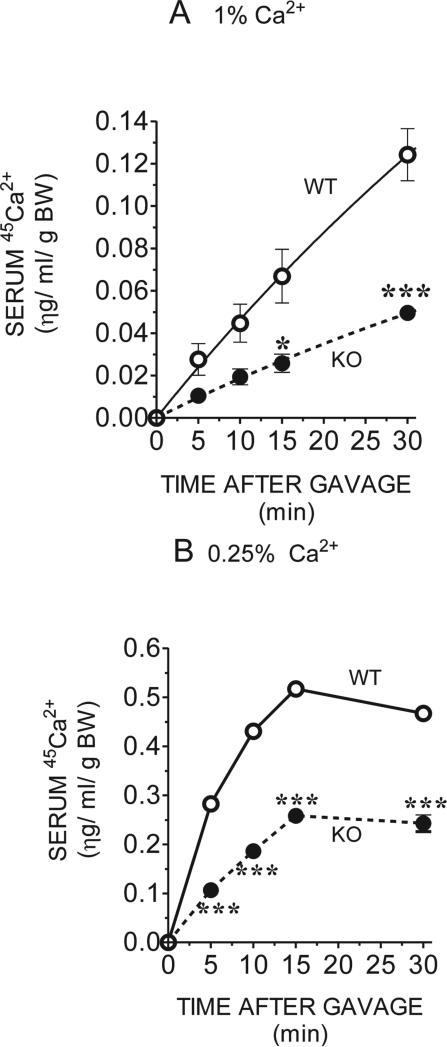
After oral administration of 45Ca2+, Trpv6 KO mice on the regular diet accumulated ~60% less 45Ca2+ in the serum from 5 to 30 minutes than did their controls (Fig. 4A). When kept on a low (0.25%) Ca2+ diet, 45Ca2+ accumulation in the KO mice was reduced by ~40% at 5 minutes, and it decreased further to 50% at 15 and 30 minutes (Fig. 4B). In addition, when kept on the regular (1%) diet, maximal serum 45Ca2+ in all mice had not been reached by 30 minutes (Fig. 4A), although all kept on the low Ca2+ diet reached a peak of serum 45Ca2+ on or around 15 minutes (Fig. 4B). In addition, feeding the low (0.25%) Ca2+ diet caused an increase in total Ca2+ absorbed at all time-points, regardless of the mouse genotype. In WT mice, this increase was ~7.5-fold, whereas in the Trpv6 KO mice it was 9.6-fold, suggesting that a TRPV6-independent pathway may be stimulated by Ca2+ restriction and that the persistent ~60% deficit in Ca2+ absorption may represent the contribution of TRPV6 to the total intestinal Ca2+ absorption capacity.
FIG. 4.
Concentration of 45Ca2+ in the serum after oral gavage of adult WT or Trpv6 KO mice fed the (A) regular (1%) or (B) low (0.25%) Ca2+ diet. Blood samples were collected at 0, 5, 10, 15, and 30 minutes after gavaging the mice with 15 μl/g BW of 15 mM Tris, 100 μM CaCl2, 125 mM NaCl2, 1.8 g/liter fructose, and 20 μCi/ml 45Ca. Ten microliters of serum was counted, and 45Ca2+ concentration was calculated by plotting the counts against a 45Ca2+ standard curve. Each point of the 1% Ca2+ curves is the average of five WT and three Trpv6 KO mice; for the 0.25% Ca2+ curves, each point is the average of three WT and two Trpv6 KO mice ± SE. *p < 0.05; ***p < 0.0001 vs. WT controls in the same diet.
Serum parameters
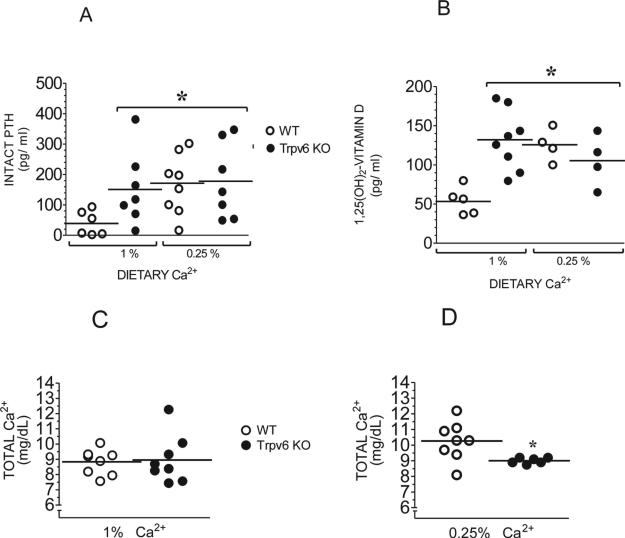
The serum intact PTH of Trpv6 KO mice fed the regular diet was significantly (3.8-fold) elevated compared with their WT counterparts maintained on the same diet (Fig. 5A). When switched to the low (0.25%) Ca2+ diet, WT mice increased their serum PTH levels 4.2-fold, reaching levels not significantly different from those of Trpv6 KO mice on the regular diet. This suggests that Trpv6 KO mice on a regular diet (i.e., not challenged with low environmental Ca2+) have maximally elevated serum PTH. When challenged with a low Ca2+ diet, Trpv6 KO mice showed only a slight, nonsignificant increase in their intact PTH (Fig. 5A).
FIG. 5.
(A) Intact PTH and (B) 1,25 dihydroxyvitamin D in the serum of adult mice fed the regular (1%) or low (0.25%) Ca2+ diet. Total Ca2+ in the serum of adult WT or Trpv6 KO mice fed a (C) regular (1%) or (D) low (0.25%) Ca2+ diet was assessed by colorimetric assay. Absorbencies were plotted against a standard Ca2+ concentration curve. Results from each group represent the mean ± SE of at least 10 serum samples from different mice. *p < 0.05 vs. WT in regular diet.
The serum 1,25-dihydroxyvitamin D followed a similar pattern: When on regular diet, Trpv6 KO mice exhibited a 2.4-fold elevated serum 1,25-dihydroxyvitamin D. Switching WT mice to low Ca2+ diet caused a 2.3-fold increase in their serum concentration, whereas Trpv6 KO were unable to generate any further increase (Fig. 5B). Trpv6 KO mice on regular diet are normocalcemic (Fig. 5C). However, when challenged with the low Ca2+, they fail to respond in the manner exhibited by their control littermates, ending up with significantly lower levels of serum Ca2+ (Fig. 5D). The average total serum Ca2+ in all groups fed the regular diet was ~9 mg Ca2+/dl (Fig. 5C). When fed the low Ca2+diet, WT mice increased their serum Ca2+ to 10.3 mg/dl, whereas Trpv6 KO mice did not (Fig. 5D). The elevated serum Ca2+ in WT mice on this diet was observed in every male and female based on five independent experiments. As internal controls, we ran serum samples of WT mice on a no added Ca2+ diet, because these mice did not exhibit any increase in serum Ca2+ levels.
Urinary parameters
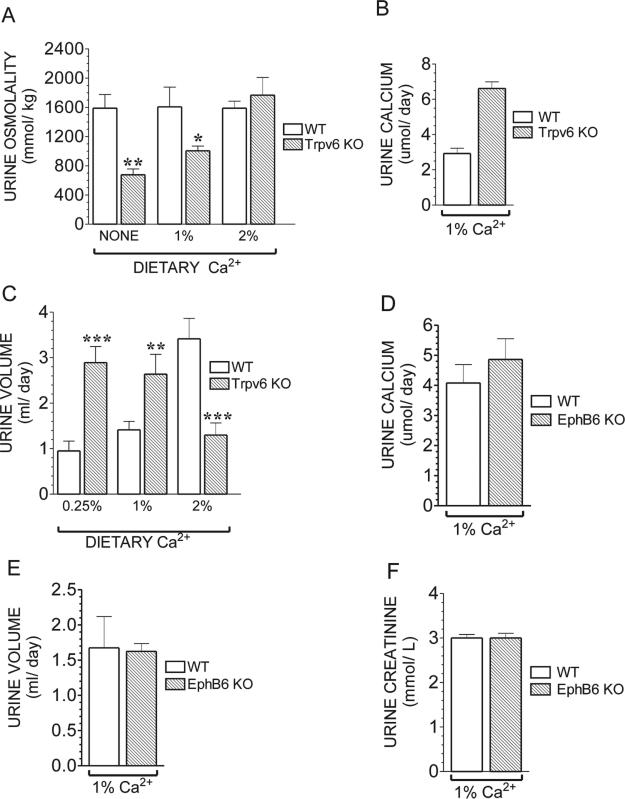
Trpv6 KO mice show several signs of renal abnormalities. The urinary osmolality in WT mice remained nearly constant under all experimental conditions (1587, 1606, and 1688 mmol/kg for low [0.25%], regular [%], and high [2%] Ca2+ diet, respectively; Fig. 6A), whereas in Trpv6 KO mice, the osmolality varied in direct proportion to the amount of Ca2+ in their diet (e.g., 677, 1005, and 1767 mmol/kg for mice maintained on the low, regular, and high Ca2+, respectively; Fig. 6A). Only Trpv6 KO mice fed the high Ca2+ diet had normal osmolality, suggesting that these mice are unable to properly concentrate their urine. In addition, Trpv6 KO mice had a significantly elevated urinary Ca2+ (6.61 ± 0.38 versus 2.93 ± 0.29 μmol/day for the KO and WT, respectively; Fig. 6B). The changes in urinary volume in Trpv6 KO mice were in the opposite direction of those observed in their WT controls under the same conditions (Fig. 6C), again suggesting abnormal urine concentration.
FIG. 6.
(A) Urine osmolality in adult WT or Trpv6 KO mice fed a no added Ca2+, regular (1%), or high (2%) Ca2+ diet. Osmolality was measured in a vapor pressure osmometer. Values are the mean ± SE of urine samples from six WT and six Trpv6 KO mice. (B) Urine Ca2+ in adult Trpv6 KO fed a regular Ca2+ diet. Ca2+ concentration was determined by a colorimetric assay. Values are the mean ± SE of urine samples from three WT and three Trpv6 KO mice. (C) Daily urine volume in adult WT or Trpv6 KO mice fed a low (0.25%), regular (1%), or high (2%) Ca2+ diet. Values are the mean ± SE of urine samples from seven WT and seven Trpv6 KO mice. All samples were collected after 3 months of diet treatment. Open bars, WT mice; black bars, Trpv6 KO mice. (D) Daily urine Ca2+ excretion in adult EphB6 KO mice fed a regular diet. (E) Daily urine volume in adult EphB6 KO mice fed a regular Ca2+ diet. (F) Urine creatinine levels in adult EphB6 KO mice fed a regular Ca2+ diet. The urine results from EphB6 mice represent the mean ± SE of urine samples from seven WT (14 samples collected in 2 different days) and four EphB6 KO mice (8 samples collected in 2 different days). *p < 0.05 vs. WT in the same diet; *p < 0.05 or **p < 0.001 vs. WT in the same diet.
EphB6 has recently been shown to be expressed in adult mouse kidney and, whereas lacking the tyrosine kinase activity common to other members of its family,(12) was postulated to play a role in kidney function.(13) Therefore, we tested the EphB6 KO mice for some of the altered urinary parameters found in the Trpv6 KO mice to determine the contribution of each deleted gene. Our results show that the EphB6 KO mice have normal urinary Ca2+ excretion (Fig. 6D), normal urine volume (Fig. 6E), and normal urine creatinine (Fig. 6F), indicating that the alterations in volume, osmolality, and Ca2+ excretion in the Trpv6 KO mice are caused by the Trpv6 deletion.
Bone parameters
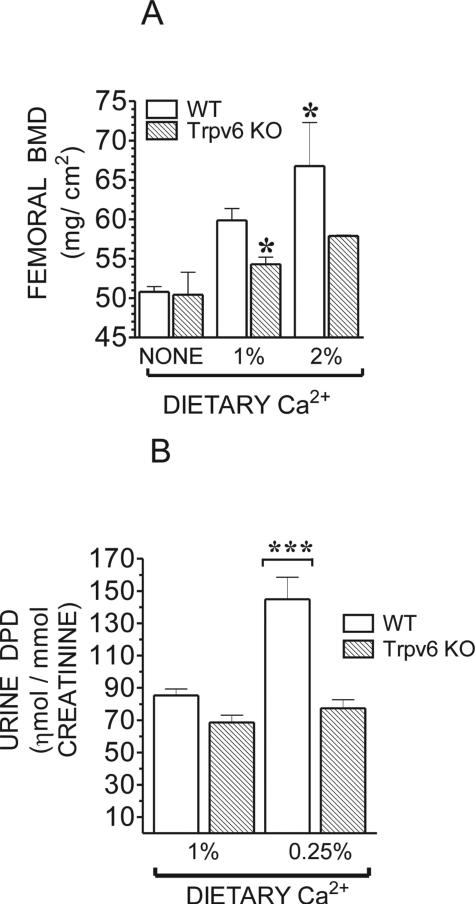
The results of the DXA revealed that, at 95 days of age, Trpv6 KO mice on regular diet exhibited a 9.3% decrease in femoral mineral density, which was not restored after treatment with the high (2%) Ca2+ diet for 1 month (Fig. 7A). In fact, this regimen increased the difference in femoral mineral density between the WT and Trpv6 KO mice to 13%. This was unexpected because PTH and 1,25-dihydroxyvitamin D are suppressed on the high Ca2+ diet, and the expression of Trpv6 is very low in this condition.(14) The WT group increased their bone mass by 11.5% after high Ca2+ treatment, whereas the increase in Trpv6 KO mice was much lower. There was no significant difference in femoral mineral density among mice maintained on the no added Ca2+ diet for 1 month (Fig. 7A).
FIG. 7.
(A) BMD in adult Trpv6 KO and WT littermates fed a no added Ca2+, regular (1%), or high (2%) Ca2+ diet. BMD was assessed by DXA in the femora of 95-day-old male mice. Values are mean ± SE of three mice. (B) DPD concentration in urine samples from adult WT or Trpv6 KO mice housed overnight in individual metabolic cages and fed a regular (1%) or low (0.25%) Ca2+ diet. Values are the mean ± SE of 10–15 samples collected from 10 mice. *p < 0.05 or ***p < 0.0001 vs. WT in regular diet.
Urinary DPD, a marker of bone resorption, was not increased in Trpv6 KO mice fed the regular diet, even though their serum PTH was elevated. To the contrary, DPD levels tended to decrease in the KO mice. Even when fed the low Ca2+ diet, Trpv6 KO mice did not respond by increasing their bone resorption as would have been expected. These findings are in contrast to those observed with the WT mice, which promptly responded to the low Ca2+ by increasing DPD levels by ~80% (Fig. 7B), probably contributing to the elevated serum Ca2+ observed in these WT mice.
DISCUSSION
Mice with deletion of the Trpv6 gene have a normal life span, although they exhibit multiple signs of Ca2+ deficiency and abnormal development. One of the hallmarks of the Trpv6 KO mice is their slower rate of weight gain (Fig. 3A), even when the litter is being breastfed. The Trpv6 KO mice show certain striking similarities to the vitamin D knockout mice (VDR KO), despite the significantly elevated serum 1,25-dihydroxyvitamin D and normal expression of the VDR in the Trpv6 KO mice. These similarities include decreased intestinal Ca2+ absorption even with ample dietary Ca2+ availability, secondary hyperparathyroidism (increased serum PTH and 1,25-dihydroxyvitamin D), decreased BMD, alopecia, and other skin abnormalities revealed by electron microscopy. For instance, a significant percentage of the Trpv6 KO mice develop persistent alopecia (Fig. 3B) and dermatitis (Fig. 3C), phenotypes associated with the vitamin D resistance caused by inactivating mutations of the VDR.(15–18) The detection of Trpv6 expression in normal skin tissue (Fig. 3F) may explain the skin phenotype in both the VDR and the Trpv6 KO mice. Accordingly, recent studies found multiple vitamin D–responsive elements in the promoter region of the TRPV6 gene,(19,20) and alopecia is the only Ca2+-related phenotype of the VDR KO mouse not rescued by a high Ca2+ diet, suggesting a tissue-specific deficiency. These findings suggest that there is a link between TRPV6 and vitamin D–regulated Ca2+ transport, normal hair growth, and the modulation of keratinocyte proliferation and differentiation. The Ca2+ concentration in the upper stratum granulosum is >1.4 mM,(21) which is above the free serum Ca2+, indicating the presence of an active mechanism of Ca2+ transport. Loss of skin Ca2+ is observed in the abrogation of the epidermal permeability barrier,(22) as well as in skin diseases, such as psoriasis and Hailey-Hailey disease.(23,24) The Trpv6 KO mice, however, have a disrupted skin Ca2+ gradient in the absence of dermatitis or alopecia (Fig. 3Eb), indicating an intrinsic inability to properly concentrate Ca2+ and suggesting that TRPV6 is involved in transporting Ca2+ into the epidermal keratinocytes. Further studies are needed to establish the exact role of TRPV6 in epidermal function.
The TRPV6 channel plays an important role in the vitamin D–stimulated intestinal Ca2+ absorption and its intestinal expression is stimulated by VDR activation.(4) Consistent with this, Trpv6 expression in two strains of VDR KO mice is <10% of the normal levels,(14) and the intestinal Ca2+ absorption in VDR KO mice is substantially decreased, suggesting that disruption of TRPV6 impairs vitamin D–dependent stimulation of intestinal Ca2+ absorption. Accordingly, the Trpv6 KO mice exhibit a substantial decrease in intestinal Ca2+ absorption under all conditions tested (Figs. 4A and 4B), despite their elevated serum 1,25-dihydroxyvitamin D levels. However, Trpv6 KO mice were still able to proportionally increase Ca2+ absorption when switched to the low (0.25%) Ca2+ diet (compare Fig. 4B with 4A), suggesting that TRPV6-independent mechanisms also play a role. However, these TRPV6-independent mechanisms are not sufficient to maintain normal Ca2+ homeostasis, as indicated by the substantial secondary hyperparathyroidism and the inability of the Trpv6 KO mice to respond to further Ca2+ challenges.
The purpose of the 0.25% Ca2+ diet was to disrupt Ca2+ balance in the Trpv6 KO mice while being relatively mild and easily compensated for by the WT mice, providing at least one half of the daily Ca2+ requirement for WT mice. Accordingly, the WT mice promptly responded to this diet by elevating their serum PTH and 1,25-dihydroxyvitamin D levels, as well as increasing bone resorption and intestinal Ca2+ absorption. Conversely, Trpv6 KO mice fed this low Ca2+ diet were unable to further elevate their serum PTH and 1,25-dihydroxyvitamin D levels, showed insufficient intestinal Ca2+ absorption, and did not increase their bone resorption. Interestingly, the WT mice on this diet also exhibited increased serum Ca2+. This increase was consistently observed in all WT mice fed this diet. It should be noted that studies looking at the effects of low Ca2+ are usually performed with no or extremely low Ca2+ concentrations added to the diet.(5,14,25) Accordingly, WT mice fed the no added Ca2+ diet showed no increase in serum Ca2+. Thus, we used serum from these mice as internal controls.
The kidneys of the Trpv6 KO mice do not respond to the elevated PTH and vitamin D levels by upregulating renal Ca2+ reabsorption (Fig. 2B), as would have been expected. As a result, Trpv6 KO mice waste Ca2+ in the urine (Fig. 6B), suggesting abnormal renal handling of Ca2+. We detected only moderate levels of Trpv6 in the kidney,(5) (Fig. 2B), and another study found significant amounts of protein and RNA along the nephron.(26) However, all data published to date indicate that Trpv5 is the gatekeeper of the active Ca2+ reabsorption by the kidney.(2,27,28) Accordingly, Hoenderop et al.(29) showed that Trpv5 KO mice waste Ca2+ in the urine, excreting as much as 6-fold more than their WT littermates. Trpv5 KO mice, however, can compensate for this renal wasting with a 6-fold increase in the duodenal expression of Trpv6 and a resultant marked increase in intestinal Ca2+ absorption. Although exhibiting a 3-fold increase in serum 1,25-dihydroxyvitamin D, Trpv5 KO mice had no significant changes in PTH levels and no profound bone abnormalities.(29) On the other hand, the kidney of the Trpv6 KO mice does not respond to the Ca2+ wasting, showing no apparent signs of compensation. It is possible that other Trpv5-independent mechanisms of Ca2+ handling may also be impaired. Alternatively, Trpv6 expression in early kidney development could play a role. Although Trpv6 expression in adult kidney is lower,(5,30) the situation during embryonic development is quite different: whereas Trpv6 is expressed at high levels, Trpv5 expression is much lower. It is around the time of weaning that this expression pattern is reversed: Trpv6 expression falls, whereas Trpv5 expression increases.(5) Alternatively, it is possible that the deficient Ca2+ reabsorption in Trpv6 KO mice is exacerbated by direct effects of the marked hypercalciuria on the renal tubule (e.g., inhibition of water reabsorption mediated through the Ca2+-sensing receptor) and that deposition of Ca2+ salts within the parenchyma may promote abnormal tubular function.
The Trpv6 KO mice also carry a partial deletion of a second gene located adjacent to and aligned tail to tail with the Trpv6 gene. This adjacent gene encodes EphB6, a kinase-defective EphB member of the tyrosine kinase EphB family.(12) This deletion was not intentional; however, we used the EphB6 KO mice to measure some of the altered parameters found in our Trpv6 KO mice for comparison. Mice with deletion of the EphB6 gene do not have any obvious abnormalities related to Ca2+ homeostasis or development up to 24 months of age.(8,9) EphB6 is highly expressed in the developing and adult brain and thymus, and at much lower levels in other tissues such as lung and kidney.(31) Members of the EphB family with tyrosine kinase activity have been shown to play a role in brain development and function, whereas the kinase-deficient EphB6 has been shown to promote cell adhesion and migration in transfected HEK cells(31) and in the immune responses in mice.(9) EphB6 KO mice have normal thymocytes subpopulations, thought to be caused by compensation by the overlapping expression of other members of the EphB family,(8,9) although the immune response of their mature T cells is compromised.(9) On the other hand, the EphB6 KO mice have no signs of an abnormal skin phenotype.(7) They have normal Ca2+ excretion, urine volume, and creatinine concentration (Figs. 6D, 6E, and 6F, respectively), even though EphB6 has been shown to be expressed in adult mouse kidney, and was suggested to contribute to the regulation of ion transport along the nephron.
The significant lower BMD observed in the Trpv6 KO mice on regular diet (Fig. 7A) would be an expected consequence of an increased bone resorption caused by the elevated circulating PTH and 1,25-dihyrdoxyvitamin D levels found in these mice, thereby generating a negative Ca2+ balance in their bones. Conversely, urine levels of DPD were not altered in Trpv6 KO mice, suggesting skeletal resistance to activation of bone resorption by PTH and vitamin D. This, in turn, suggests that the decreased BMD in the Trpv6 KO mice may not be caused by enhanced bone resorption. Interestingly, Nijenhuis et al.(26) detected Trpv6 expression in bone tissue, raising the possibility of a role of Trpv6 in bone homeostasis that could explain the lower BMD in this mouse model. In addition, the failure of the high (2%) Ca2+ “rescue” diet to normalize the BMD in these KO mice suggests that they might have a bone formation/mineralization defect. Even when taking into account that the KO mice started the rescue diet with a lower absolute BMD, the relative increase in their BMD after the high Ca2+ treatment was not comparable with that of their WT littermates. Furthermore, when challenged with a low Ca2+ diet, Trpv6 KO mice did not increase their urinary DPD, thereby contributing to their subsequent hypocalcemia, whereas their WT littermates almost doubled their urinary DPD levels (Fig. 7B). These results indicate that the bones and kidneys of the Trpv6 KO mice do not respond properly to PTH and 1,25-dihydroxyvitamin D, making these mice a unique model of bone and kidney resistance to PTH and vitamin D.
Some of the abnormalities observed in the Trpv6 KO mice may be a result of Ca2+ deficiency during embryonic development through deficient maternal–fetal transfer of Ca2+. The placenta exhibits exceptionally high levels of Trpv6 expression in humans and mice.(3,32,33) It is well known that, in mammals, the Ca2+ concentration in the blood of the fetus is always higher than that in the maternal circulation.(34) To maintain this relative hypercalcemia, the transfer of Ca2+ from mother to fetus takes place against a Ca2+ gradient. In this context, Trpv6 expression in the placenta is expected to enhance the rate of maternal transfer of Ca2+ to the fetus during pregnancy. In addition, Trpv6 is known to be the target of the estrogen-induced increase in intestinal Ca2+ absorption during pregnancy to compensate for the higher maternal demand.(35) The pregnancy-specific deficiencies, combined with a decrease in intestinal Ca2+ absorption in the mother may lead to Ca2+ deficiency throughout embryonic development in Trpv6 KO fetuses. A detailed study of fetal development in Trpv6 KO mice is required and may uncover new roles of Trpv6 in tissue differentiation and eventually help the understanding and prevention of diseases caused by Ca2+ deficiency and other abnormalities of Ca2+ homeostasis.
ACKNOWLEDGMENTS
This work was partially supported by NIH Grant CA101075. EMB was supported by NIH Grants DK52005 and DK67155. TM was supported by NIH Grant PO1AR39488.
Footnotes
The authors state that they have no conflicts of interest.
REFERENCES
- 1.Frick KK, Bushinsky DA. Molecular mechanisms of primary hypercalciuria. J Am Soc Nephrol. 2003;14:1082–1095. doi: 10.1097/01.asn.0000062960.26868.17. [DOI] [PubMed] [Google Scholar]
- 2.Peng JB, Brown EM, Hediger MA. Epithelial Ca2+ entry channels: Transcellular Ca2+ transport and beyond. J Physiol. 2003;551:729–740. doi: 10.1113/jphysiol.2003.043349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peng JB, Brown EM, Hediger MA. Structural conservation of the genes encoding cat1, cat2, and related cation channels. Genomics. 2001;76:99–109. doi: 10.1006/geno.2001.6606. [DOI] [PubMed] [Google Scholar]
- 4.Vennekens R, Hoenderop JG, Prenen J, Stuiver M, Willems PH, Droogmans G, Nilius B, Bindels RJ. Permeation and gating properties of the novel epithelial Ca(2+) channel. J Biol Chem. 2000;275:3963–3969. doi: 10.1074/jbc.275.6.3963. [DOI] [PubMed] [Google Scholar]
- 5.Song Y, Peng X, Porta A, Takanaga H, Peng JB, Hediger MA, Fleet JC, Christakos S. Calcium transporter 1 and epithelial calcium channel messenger ribonucleic acid are differentially regulated by 1,25 dihydroxyvitamin D3 in the intestine and kidney of mice. Endocrinology. 2003;144:3885–3894. doi: 10.1210/en.2003-0314. [DOI] [PubMed] [Google Scholar]
- 6.Zhuang L, Peng JB, Tou L, Takanaga H, Adam RM, Hediger MA, Freeman MR. Calcium-selective ion channel, CaT1, is apically localized in gastrointestinal tract epithelia and is aberrantly expressed in human malignancies. Lab Invest. 2002;82:1755–1764. doi: 10.1097/01.lab.0000043910.41414.e7. [DOI] [PubMed] [Google Scholar]
- 7.Hafner C, Schmitz G, Meyer S, Bataille F, Hau P, Langmann T, Dietmaier W, Landthaler M, Vogt T. Differential gene expression of Eph receptors and ephrins in benign human tissues and cancers. Clin Chem. 2004;50:490–499. doi: 10.1373/clinchem.2003.026849. [DOI] [PubMed] [Google Scholar]
- 8.Shimoyama M, Matsuoka H, Nagata A, Iwata N, Tamekane A, Okamura A, Gomyo H, Ito M, Jishage K, Kamada N, Suzuki H, Tetsuo NT, Matsui T. Developmental expression of EphB6 in the thymus: Lessons from EphB6 knockout mice. Biochem Biophys Res Commun. 2002;298:87–94. doi: 10.1016/s0006-291x(02)02399-9. [DOI] [PubMed] [Google Scholar]
- 9.Luo H, Yu G, Tremblay J, Wu J. EphB6-null mutation results in compromised T cell function. J Clin Invest. 2004;114:1762–1773. doi: 10.1172/JCI21846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Menon GK, Grayson S, Elias PM. Ionic calcium reservoirs in mammalian epidermis: Ultrastructural localization by ion-capture cytochemistry. J Invest Dermatol. 1985;84:508–512. doi: 10.1111/1523-1747.ep12273485. [DOI] [PubMed] [Google Scholar]
- 11.Kunchaparty S, Palcso M, Berkman J, Velazquez H, Desir GV, Bernstein P, Reilly RF, Ellison DH. Defective processing and expression of thiazide-sensitive Na-Cl cotransporter as a cause of Gitelman's syndrome. Am J Physiol. 1999;277:F643–F649. doi: 10.1152/ajprenal.1999.277.4.F643. [DOI] [PubMed] [Google Scholar]
- 12.Matsuoka H, Iwata N, Ito M, Shimoyama M, Nagata A, Chihara K, Takai S, Matsui T. Expression of a kinase-defective Eph-like receptor in the normal human brain. Biochem Biophys Res Commun. 1997;235:487–492. doi: 10.1006/bbrc.1997.6812. [DOI] [PubMed] [Google Scholar]
- 13.Ogawa K, Wada H, Okada N, Harada I, Nakajima T, Pasquale EB, Tsuyama S. EphB2 and ephrin-B1 expressed in the adult kidney regulate the cytoarchitecture of medullary tubule cells through Rho family GTPases. J Cell Sci. 2006;119:559–570. doi: 10.1242/jcs.02777. [DOI] [PubMed] [Google Scholar]
- 14.Van Cromphaut SJ, Dewerchin M, Hoenderop JG, Stockmans I, Van Herck E, Kato S, Bindels RJ, Collen D, Carmeliet P, Bouillon R, Carmeliet G. Duodenal calcium absorption in vitamin D receptor-knockout mice: Functional and molecular aspects. Proc Natl Acad Sci USA. 2001;98:13324–13329. doi: 10.1073/pnas.231474698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hochberg Z. Vitamin-D-dependent rickets type 2. Horm Res. 2002;58:297–302. doi: 10.1159/000066442. [DOI] [PubMed] [Google Scholar]
- 16.Sultan A, Vitale P. Vitamin D-dependent rickets Type II with alopecia: Two case reports and review of the literature. Int J Dermatol. 2003;42:682–685. doi: 10.1046/j.1365-4362.2003.01816.x. [DOI] [PubMed] [Google Scholar]
- 17.Zlotogorski A, Hochberg Z, Mirmirani P, Metzker A, Ben Amitai D, Martinez-Mir A, Panteleyev AA, Christiano AM. Clinical and pathologic correlations in genetically distinct forms of atrichia. Arch Dermatol. 2003;139:1591–1596. doi: 10.1001/archderm.139.12.1591. [DOI] [PubMed] [Google Scholar]
- 18.Bergman R, Schein-Goldshmid R, Hochberg Z, Ben Izhak O, Sprecher E. The alopecias associated with vitamin D-dependent rickets type IIA and with hairless gene mutations: A comparative clinical, histologic, and immunohistochemical study. Arch Dermatol. 2005;141:343–351. doi: 10.1001/archderm.141.3.343. [DOI] [PubMed] [Google Scholar]
- 19.Wang TT, Tavera-Mendoza LE, Laperriere D, Libby E, Macleod NB, Nagai Y, Bourdeau V, Konstorum A, Lallement B, Zhang R, Mader S, White JH. Large-scale in silico and microarray-based identification of direct 1,25-dihydroxyvitamin D3 target genes. Mol Endocrinol. 2005;19:2685–2695. doi: 10.1210/me.2005-0106. [DOI] [PubMed] [Google Scholar]
- 20.Meyer MB, Watanuki M, Kim S, Shevde NK, Pike JW. The human transient receptor potential vanilloid type 6 distal promoter contains multiple vitamin D receptor binding sites that mediate activation by 1,25-dihydroxyvitamin D3 in intestinal cells. Mol Endocrinol. 2006;20:1447–1461. doi: 10.1210/me.2006-0031. [DOI] [PubMed] [Google Scholar]
- 21.Lansdown AB. Calcium: A potential central regulator in wound healing in the skin. Wound Repair Regen. 2002;10:271–285. doi: 10.1046/j.1524-475x.2002.10502.x. [DOI] [PubMed] [Google Scholar]
- 22.Menon GK, Elias PM, Feingold KR. Integrity of the permeability barrier is crucial for maintenance of the epidermal calcium gradient. Br J Dermatol. 1994;130:139–147. doi: 10.1111/j.1365-2133.1994.tb02892.x. [DOI] [PubMed] [Google Scholar]
- 23.Menon GK, Elias PM. Ultrastructural localization of calcium in psoriatic and normal human epidermis. Arch Dermatol. 1991;127:57–63. [PubMed] [Google Scholar]
- 24.Hu Z, Bonifas JM, Beech J, Bench G, Shigihara T, Ogawa H, Ikeda S, Mauro T, Epstein EH., Jr Mutations in ATP2C1, encoding a calcium pump, cause Hailey-Hailey disease. Nat Genet. 2000;24:61–65. doi: 10.1038/71701. [DOI] [PubMed] [Google Scholar]
- 25.Bouillon R, Van Cromphaut S, Carmeliet G. Intestinal calcium absorption: Molecular vitamin D mediated mechanisms. J Cell Biochem. 2003;88:332–339. doi: 10.1002/jcb.10360. [DOI] [PubMed] [Google Scholar]
- 26.Nijenhuis T, Hoenderop JG, Van Der Kemp AW, Bindels RJ. Localization and regulation of the epithelial Ca2+ channel TRPV6 in the kidney. J Am Soc Nephrol. 2003;14:2731–2740. doi: 10.1097/01.asn.0000094081.78893.e8. [DOI] [PubMed] [Google Scholar]
- 27.Hoenderop JG, Nilius B, Bindels RJ. Calcium absorption across epithelia. Physiol Rev. 2005;85:373–422. doi: 10.1152/physrev.00003.2004. [DOI] [PubMed] [Google Scholar]
- 28.Nijenhuis T, Hoenderop JG, Bindels RJ. TRPV5 and TRPV6 in Ca(2+) (re)absorption: Regulating Ca(2+) entry at the gate. Pflugers Arch. 2005;451:181–192. doi: 10.1007/s00424-005-1430-6. [DOI] [PubMed] [Google Scholar]
- 29.Hoenderop JG, van Leeuwen JP, van der Eerden BC, Kersten FF, Van Der Kemp AW, Merillat AM, Waarsing JH, Rossier BC, Vallon V, Hummler E, Bindels RJ. Renal Ca2+ wasting, hyperabsorption, and reduced bone thickness in mice lacking TRPV5. J Clin Invest. 2003;112:1906–1914. doi: 10.1172/JCI19826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van Abel M, Hoenderop JG, Bindels RJ. The epithelial calcium channels TRPV5 and TRPV6: Regulation and implications for disease. Naunyn Schmiedebergs Arch Pharmacol. 2005;371:295–306. doi: 10.1007/s00210-005-1021-2. [DOI] [PubMed] [Google Scholar]
- 31.Matsuoka H, Obama H, Kelly ML, Matsui T, Nakamoto M. Biphasic functions of the kinase-defective Ephb6 receptor in cell adhesion and migration. J Biol Chem. 2005;280:29355–29363. doi: 10.1074/jbc.M500010200. [DOI] [PubMed] [Google Scholar]
- 32.Peng JB, Chen XZ, Berger UV, Weremowicz S, Morton CC, Vassilev PM, Brown EM, Hediger MA. Human calcium transport protein CaT1. Biochem Biophys Res Commun. 2000;278:326–332. doi: 10.1006/bbrc.2000.3716. [DOI] [PubMed] [Google Scholar]
- 33.Hirnet D, Olausson J, Fecher-Trost C, Bodding M, Nastainczyk W, Wissenbach U, Flockerzi V, Freichel M. The TRPV6 gene, cDNA and protein. Cell Calcium. 2003;33:509–518. doi: 10.1016/s0143-4160(03)00066-6. [DOI] [PubMed] [Google Scholar]
- 34.Kovacs CS, Kronenberg HM. Maternal-fetal calcium and bone metabolism during pregnancy, puerperium, and lactation. Endocr Rev. 1997;18:832–872. doi: 10.1210/edrv.18.6.0319. [DOI] [PubMed] [Google Scholar]
- 35.Van Cromphaut SJ, Rummens K, Stockmans I, Van Herck E, Dijcks FA, Ederveen AG, Carmeliet P, Verhaeghe J, Bouillon R, Carmeliet G. Intestinal calcium transporter genes are upregulated by estrogens and the reproductive cycle through vitamin D receptor-independent mechanisms. J Bone Miner Res. 2003;18:1725–1736. doi: 10.1359/jbmr.2003.18.10.1725. [DOI] [PubMed] [Google Scholar]