Abstract
Introduction: Bone marrow-derived autologous human mesenchymal stem cells (MSCs) are one of the most promising cell sources for cell therapy to treat heart failure. The cell sheet technique has allowed transplantation of a large number of cells and enhanced the efficacy of cell therapy. We hypothesized that the transplantation of MSC sheets may be a feasible, safe, and effective treatment for ischemic cardiomyopathy (ICM).
Methods and Results: Human MSCs acquired from bone marrow were positive for CD73, CD90, and CD105 and negative for CD11b and CD45 by flow cytometry. Ten MSC sheets were created from a total cell number of 1×108 MSCs using temperature-responsive culture dishes. These were successfully transplanted over the infarct myocardium of porcine ICM models induced by placing an ameroid constrictor on the left anterior descending coronary artery without any procedural-related complications (MSC group=6: sheet transplantation; sham group=6, oral intake of tacrolimus in both groups). Premature ventricular contractions were rarely detected by Holter electrocardiogram (ECG) in the MSC group in the first week after transplantation. On echocardiography, the cardiac performance of the MSC group was significantly better than that of the sham group at 8 weeks after transplantation. On histological examination 8 weeks after transplantation, left ventricular (LV) remodeling was significantly attenuated compared with the sham group (cardiomyocyte size and interstitial fibrosis were measured). Immunohistochemistry of the von Willebrand factor showed that the vascular density in the infarct border area was significantly greater in the MSC group than the sham group. Expression of angiogenesis-related factors in the infarct border area of the MSC group was significantly greater than that of the sham group, as measured by real-time polymerase chain reaction.
Conclusions: Bone marrow-derived MSC sheets improved cardiac function and attenuated LV remodeling in ICM without major complications, indicating that this strategy would be applicable in clinical settings.
Introduction
Ischemic heart disease is a major cause of mortality and morbidity worldwide, and loss of the myocardium due to myocardial infarction (MI) may lead to heart failure. Although significant advances in pharmacological, interventional, and surgical treatments have improved patient symptoms and prolonged life, end-stage heart failure remains a poor prognosis.1,2 Recently, remarkable progress has been made in myocardial regeneration therapy using cell transplantation.3–5 The myocardium has a limited regenerative capacity and therefore is one of the most important targets in regenerative medicine. Numerous preclinical and clinical studies have demonstrated the feasibility, safety, and efficacy of various cell transplantation methods to improve cardiac function and attenuate left ventricular (LV) remodeling in heart failure.3–5 Thus, cell transplantation has emerged as a promising new option for treatment for heart failure.3–5
Bone marrow-derived mesenchymal stem cells (MSCs) have a multipotent capacity, differentiating not only into osteoblasts and chondrocytes but also into vascular endothelial cells or cardiomyocytes in vitro.6–8 As MSCs, in contrast to their hematopoietic components, are adherent and expandable in plastic culture dishes, MSCs can easily be prepared for cell transplantation. Many recent studies have shown that transplantation of bone marrow-derived MSCs has potential in the treatment of heart failure. However, differentiation of MSCs to cardiomyocytes rarely occurs in significant numbers in vivo, and the main mechanism of the therapeutic effect of MSC transplantation is considered to be the secretion of growth factors and cytokines, known as the paracrine effect.3–5 Given these findings, MSCs are considered a promising cell source, and clinical trials of cell transplantation using MSCs in patients with ischemic heart disease through the intramyocardial injection are currently underway.
The method of cell delivery is one of the most important concerns in cell transplantation and may influence the therapeutic effects of cell transplantation for heart failure.3–5 The recently developed cell sheet engineering technique that uses temperature-responsive culture dishes is applicable in cell transplantation as a cell delivery method.9,10 In contrast to the direct intramyocardial or intracoronary injection of dissociated single cells, which has been used in most clinical studies, the cell sheet technique can deliver a large number of cells to impaired myocardium without the loss of transplanted cells or injury to the host myocardium.10 We have previously reported that use of the cell sheet technique with autologous skeletal myoblasts improves cardiac function in ischemic cardiomyopathy (ICM) models,11 and importantly, this method has already demonstrated feasibility and safety in a clinical study.12 The combination of MSCs and the cell sheet technique has been demonstrated in cardiac function recovery in a rodent heart failure model13; however, the efficacy and safety of MSC sheet transplantation in a large animal model has not been reported.
In this study, we hypothesized that transplantation of bone marrow-derived mesenchymal cell sheets might improve cardiac function and attenuate LV remodeling in ICM. This preclinical study was designed to evaluate the therapeutic effectiveness of human bone marrow-derived MSC sheets in a porcine ICM model, aiming to translate to clinical application.
Materials and Methods
All experimental procedures were approved by the institutional ethics committee. Animal care was conducted humanely in compliance with the Principles of Laboratory Animal Care formulated by the National Society for Medical Research and the Guide for the Care and Use of Laboratory Animals prepared by the Institute of Animal Resources and published by the National Institutes of Health (Publication No 85-23, revised 1996).
Culture of human MSCs
Human bone marrow-derived MSCs were acquired from the Lonza Group AG (Basel, Switzerland) and used in this study. MSCs from passage 5 or earlier were used for experiments and cultured in multilayered flasks (Nunc TripleFlasks; Thermo Fisher Scientific, Inc., Waltham, MA) in MSC growth medium (MSCGM-CD™, BulletKit™; Lonza), which is intended to support MSC maintenance, but not differentiation. The cultures were maintained in a humidified atmosphere with 5% CO2 at 37°C, and the culture medium was changed twice a week. When cells reached 80–90% confluence, they were detached using a recombinant trypsin-like protease (TrypLE Select; Invitrogen Ltd., Carlsbad, CA), and then replated at 5000 cells per cm2. MSC basal medium without supplements was conditioned by MSCs for 48 h. A total of 48 cytokines and growth factors were measured by the Bio-Plex human cytokine assay (Bio-Rad Laboratories Ltd., Hertfordshire, United Kingdom) for in vitro screening.
Flow cytometry
Evaluation of various cell markers on MSCs was performed by flow cytometry. MSCs were dissociated by incubation with TrypLE Select (Invitrogen) and resuspended in FACS staining buffer (phosphate-buffered saline supplemented with 5% fetal bovine serum). Antibodies used for FACS analysis were mouse anti-human CD11b, CD45, CD73, CD90, CD105, and the corresponding mouse IgG1 isotype conjugated to fluorescein isothiocyanate or phycoerythrin. The cells were stained for 15 min at room temperature, and then washed and examined using a BD FACSCanto II instrument (BD Biosciences, San Jose, CA). The data were analyzed using the BD FACSDiva Software (BD Biosciences).
Creation of human MSC sheets
MSCs were detached from multilayered flasks with TrypLE Select and seeded at a density of 1×107 cells/dish in 6-cm temperature-responsive culture dishes (UpCell; Cellseed, Tokyo, Japan). The following day, the dishes were incubated at room temperature, which caused the cells to detach spontaneously to form scaffold-free MSC sheets, 10 of which were prepared for cell transplantation for each minipig.
Study protocol
MI was induced in 12 female minipigs (Crown minipig; Japan Farm, Kagoshima, Japan), weighing 20–25 kg. Four weeks after MI induction, either MSC sheet transplantation or a sham operation was performed. Echocardiography was serially performed at pretreatment (baseline), 4 weeks, and 8 weeks after cell sheet transplantation. The minipigs were randomly divided into two treatment groups, either MSC sheet transplantation (MSC group) or sham operation (n=6 each). The sham operation indicated that MI was induced in minipigs, and after 4 weeks, the chest was opened without MSC sheet transplantation. At the endpoint of this study, the animals were humanely sacrificed 8 weeks after cell transplantation for histologic and biochemical analyses of the heart tissue. All animals were immunosuppressed with a daily dose of tacrolimus (0.75 mg/kg; Astellas, Tokyo, Japan) from 5 days before transplantation until sacrifice (Fig. 1).
FIG. 1.
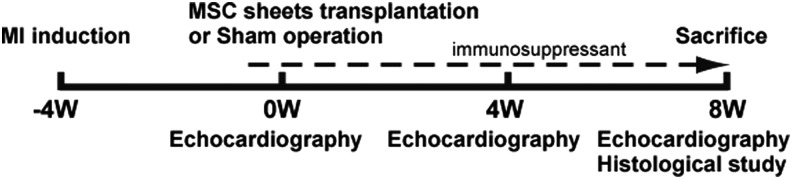
Study protocol of the minipig experiment and the evaluation of cardiac function and histological analysis.
Porcine ICM model generation and MSC sheet transplantation
Twelve female minipigs (Japan Farm) weighing 20–25 kg were preanesthetized with ketamine hydrochloride (20 mg/kg; DAIICHI SANKYO, Tokyo, Japan) and xylazine (2 mg/kg; Bayer HealthCare, Leverkusen, Germany), intubated endotracheally, and maintained under general anesthesia by a continuous infusion of propofol (6 mg/kg/h; Astra-Zeneca K.K., Osaka, Japan) and vecuronium bromide (0.05 mg/kg/h; DAIICHI SANKYO). The pericardial space was exposed by left thoracotomy through the fourth intercostal space. The distal portion of the left anterior descending coronary artery (LAD) was ligated directly, and an ameroid constrictor (COR-2.50-SS; Research Instruments SW, Escondido, CA) was placed around the LAD just distal to the branch point of the left circumflex coronary artery, as previously described.14 The muscle and skin were closed in layers. The minipigs were then allowed to recover in temperature-controlled individual cages.
Four weeks after MI induction, either MSC sheet transplantation or a sham operation was performed through median sternotomy under general anesthesia. The area of the myocardial infarct was identified visually on the basis of surface scarring and abnormal wall motion. In the MSC group, 10 MSC sheets were transplanted over the infarcted myocardium. MSC sheets were stacked over the broad surface of the myocardium. The minipigs were then allowed to recover in temperature-controlled individual cages and were later humanely sacrificed for analysis.
Echocardiography
The minipigs were anesthetized as mentioned above. Echocardiography was performed with a commercially available echocardiograph, the SONOS 5500 (PHILIPS Electronics, Tokyo, Japan). An 8.0-MHz annular array transducer was used for cardiac evaluation. The minipigs were examined in a shallow left lateral decubitus position. The LV end-diastolic and end-systolic diameters (LVDd and LVDs, respectively) were measured, while the LV end-diastolic and end-systolic volumes (LVEDV and LVESV, respectively) were calculated from the Teichholz formula.15 The LV ejection fraction (LVEF) was calculated from the following formula: LVEF (%)=100×(LVEDV−LVESV)/(LVEDV).
Holter ECG
Holter ECG was performed for 24 h in both groups (n=6 each). The arrhythmogenesis associated with MSC sheet transplantation was evaluated based on the number of premature ventricular contractions.
Histology
The MSC sheet and the excised heart specimens were fixed with 10% buffered formalin and embedded in paraffin. The paraffin-embedded sections were stained with hematoxylin and eosin (HE) and visualized using standard light microscopy. Picrosirius red or periodic acid-Schiff (PAS) staining was performed to assess interstitial fibrosis or cardiomyocyte hypertrophy, respectively.16,17 The paraffin-embedded sections were also immunolabeled with anti-human von Willebrand factor antibody (Dako, Glostrup, Denmark) and visualized with the horseradish peroxidase-based EnVision kit (Dako), according to the manufacturer's instructions. Ten different fields were randomly selected, and the number of stained vascular endothelial cells in each field was counted using a light microscope under high-power magnification (200×). The number of stained blood vessels from the 10 fields was averaged and the results are expressed as vascular density (per square millimeter).
Real-time polymerase chain reaction
One heart specimen was excised from each minipig heart to perform real-time polymerase chain reaction (RT-PCR). Immediately after sacrifice, the samples were extracted and fixed with RNA stabilization reagent (RNAlater; QIAGEN, Inc., Tokyo, Japan). Total RNA was extracted from cardiac tissue, reverse transcribed using TaqMan reverse transcription reagents (Applied Biosystems, Stockholm, Sweden), and RT-PCR was performed with the ABI PRISM 7700 (Applied Biosystems)18 system, using pig-specific primers for vascular endothelial growth factor (VEGF) and basic fibroblast growth factor (bFGF). Complementary DNA samples were prepared and assayed in triplicate. The average copy number of gene transcripts was normalized to that of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) for each sample.
Statistical analyses
Statistical analyses were performed using JMP 9.02 (SAS Institute, Cary, NC). Data are expressed as mean±standard deviation (SD). Comparisons between two groups were made using Welch's t-test. Cardiac functions by echocardiography were assessed by repeated measures analysis of variance (ANOVA) with group, time, and group×time interaction effects. All probability values were two-sided, and values of p<0.05 were considered to indicate statistical significance.
Results
Characteristics of human bone marrow-derived MSCs and generation of MSC sheets
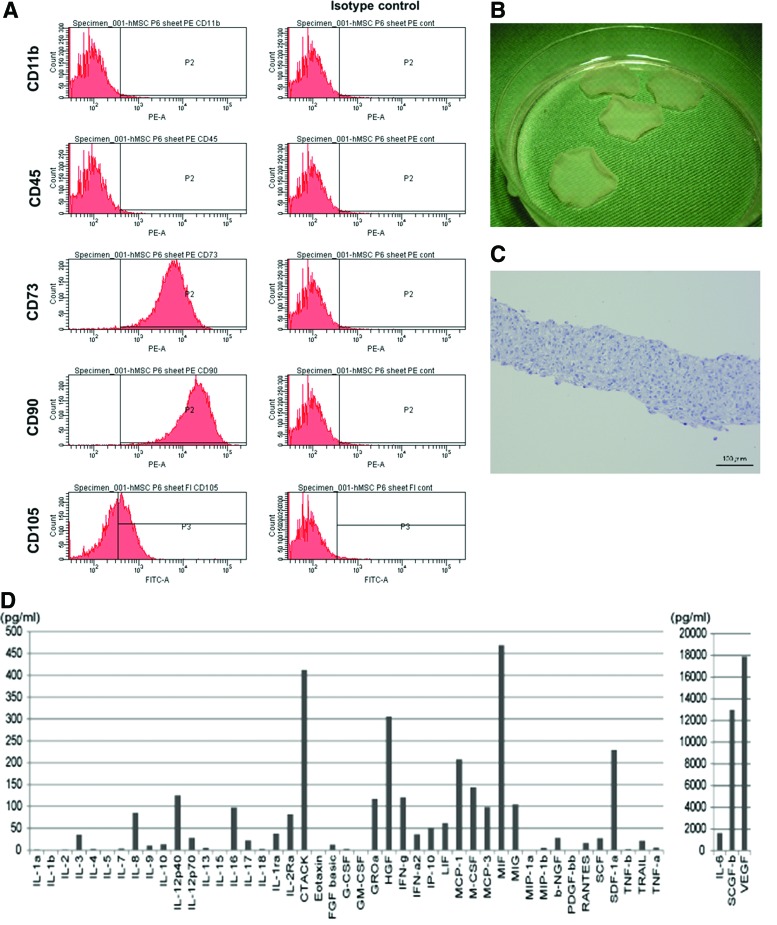
Immunophenotyping of undifferentiated human bone marrow-derived MSCs for various cell surface markers was carried out by flow cytometry, which revealed that the fluorescent intensity and distribution of the cells stained for CD11b and CD45 were not significantly different from those of the cells stained with isotype controls (Fig. 2A). These results indicate that these cultures were devoid of any hematopoietic stem and/or progenitor cells. In contrast, human bone marrow-derived MSCs displayed a high expression of CD73 (98.5%), CD90 (99.6%), and CD105 (84.6%) surface antigens (Fig. 2A). The expression profiles of these surface molecules were consistent with previous reports.10
FIG. 2.
Characteristics of bone marrow-derived mesenchymal stem cells (MSCs) and the MSC sheet. (A) Immunophenotypic characterization of MSCs by flow cytometry. MSCs stained positive for CD73, CD90, and CD105, whereas they were negative for CD11b and CD45. (B) The MSC sheets in a 10-cm dish. The size of the MSC sheet was ∼3 cm in diameter. (C) Hematoxylin and eosin (HE) staining. The MSC sheet was 100–200 μm thick. Scale bar=100 μm (C). (D) In vitro screening for cytokines and growth factors. Several factors that may potentially be involved in cardiac repair were detected at relatively high concentrations in the medium. Color images available online at www.liebertpub.com/tea
Serum-free conditioned media from human bone marrow-derived MSCs were screened for secreted factors using enzyme-linked immunosorbent assay (Fig. 2D). The media contained high concentrations of various factors, such as VEGF, hepatocyte growth factor, stromal cell-derived factor-1a, interleukin-6, macrophage inhibitory factor, and monocyte chemoattractant protein-1, which have previously been reported as cardiac protective factors.19
Subsequently, culture in the temperature-responsive dishes yielded round-shaped scaffold-free MSC sheets (Fig. 2B). The cell sheet spontaneously detached from the culture surface after 10–20 min of incubation at room temperature. Cross-sectional analysis of the MSC sheet with HE staining revealed a 100–200-μm-thick regular structure (Fig. 2C).
Feasibility and safety of MSC sheet transplantation for a porcine ICM model
Transplantation of 10 MSC sheets was successfully performed through median sternotomy under general anesthesia in six immunosuppressed minipigs, with LVEF values of 30–45% due to induced chronic MI. There was no mortality related to the procedure or other factors before the planned euthanasia. Twenty-four-hour electrocardiography monitoring only rarely identified ventricular arrhythmias in either group before the planned euthanasia (data not shown). Calcified tissues were not detected in any specimens from the heart with MSC sheet transplantation (data not shown).
Cardiac function recovery following MSC sheet transplantation
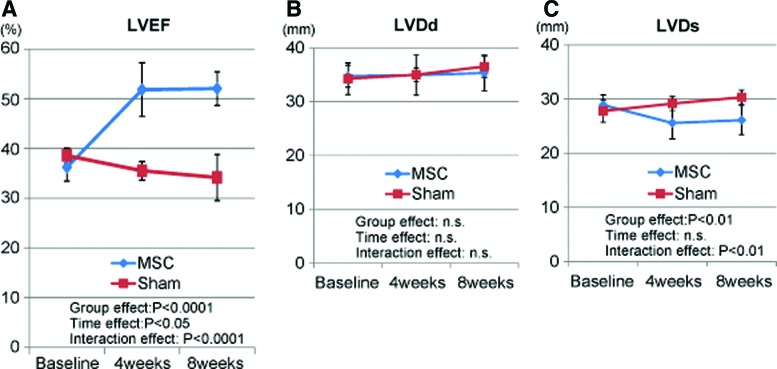
Serial standard transthoracic echocardiography was performed before and 4 and 8 weeks after the cell sheet transplantation or sham surgery. The baseline LV end-diastolic diameter, LV end-systolic diameter, and LVEF did not differ significantly between the two groups. The sham-operated minipigs demonstrated a nonsignificant upward trend in LV end-diastolic diameter and LV end-systolic diameter, and a downward trend in LVEF between 4 and 8 weeks after surgery (Fig. 3A–C). LVEF was significantly greater in the MSC group than in the sham group 4 weeks (51.8%±5.4% vs. 35.5%±1.9%) and 8 weeks (52.1%±3.4% vs. 34.2%±4.7%) after the treatment (p<0.0001 for interaction effect of time and group in the repeated ANOVA). The LV end-systolic diameter was significantly smaller in the MSC group than in the sham group 4 weeks (25.6±2.9 mm vs. 29.2±1.3 mm) and 8 weeks (26.1±2.7 mm vs. 30.3±1.3 mm) after the treatment (p<0.001 for interaction effect of time and group in the repeated ANOVA), whereas LV end-diastolic diameter did not differ significantly between the two groups.
FIG. 3.
Echocardiographic evaluation. (A) The global cardiac function as assessed by the left ventricular ejection fraction (LVEF) was significantly better in the MSC group. (B) The left ventricular end-diastolic diameter (LVDd) did not differ significantly between the MSC and sham groups. (C) The left ventricular end-systolic diameter (LVDs) was significantly smaller in the MSC group than in the sham group. The red line indicates the sham group and the blue line the MSC group. Color images available online at www.liebertpub.com/tea
Pathological hypertrophy, interstitial fibrosis, and vascular density
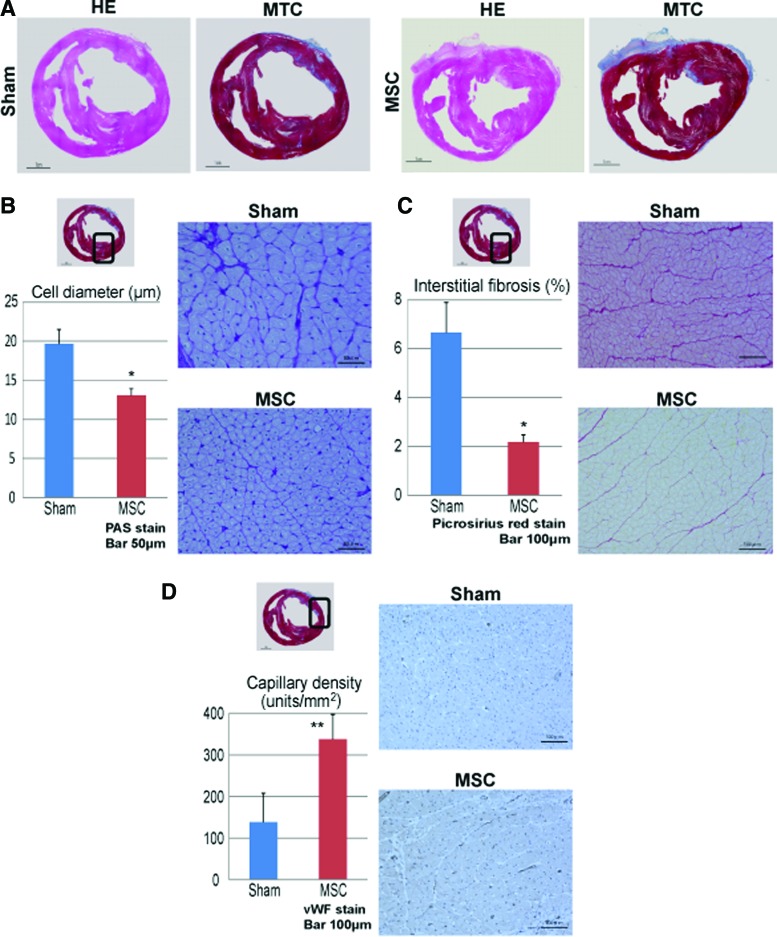
Heart tissues excised 8 weeks after transplantation were assessed by histology. Representative whole heart specimens from both groups are shown in Figure 4A, and the thickness of the myocardium in the MSC group was found to be maintained compared with that in the sham group (Fig. 4A). The pathological cardiomyocyte hypertrophy, interstitial fibrosis, and vascular density 8 weeks after treatment were assessed semiquantitatively by PAS staining, Picrosirius red staining, and immunohistochemistry for von Willebrand factor, respectively (Fig. 4B–D). The diameters of the cardiomyocytes in the remote area were significantly smaller in the MSC group than in the sham group (13±1 μm vs. 20±2 μm, p<0.0001). There was consistently significantly less accumulation of interstitial fibrosis in the remote area in the MSC group than in the sham group (2.2%±0.3% vs. 6.7%±1.2%, p<0.0001). In addition, the vascular density in the border area was significantly greater in the MSC group than in the sham group (338±59 units/mm2 vs. 139±69 units/mm2, p<0.001).
FIG. 4.
Histological evaluation after MSC sheet transplantation. (A) Macroscopic images of the whole heart at the mid-level by HE and Masson's trichrome (MTC) staining. (B) The diameter of the cardiomyocytes was measured at an area remote from the infarct; cardiomyocyte hypertrophy was significantly lower in the MSC group than in the sham group. Photomicrographs of periodic acid-Schiff (PAS)-stained sections are shown in (B). (C) The proportions of fibrosis-occupied area (%) at a site remote from the infarct; Picrosirius red staining demonstrated significantly less interstitial fibrosis in the MSC group than in the sham group. Photomicrographs of the Picrosirius red-stained sections are shown in (C). (D) Capillary density in an area bordering the infarct; the capillary density as assessed by immunostaining with an anti-von Willebrand factor antibody was significantly better in the MSC group. Photomicrographs of immunostaining for von Willebrand factor are shown in (D). Scale bar=1 cm (A), scale bar=50 μm (B), and scale bar=100 μm (C, D). *p<0.0001, **p<0.001 versus sham. Color images available online at www.liebertpub.com/tea
Upregulated VEGF and bFGF in the infarct border area after treatment
The expression levels of growth factors that are expressed in the myocardium and are potentially related to neovascularization were quantified by RT-PCR 8 weeks after treatment. The expression levels of VEGF and bFGF in the infarct border area were significantly greater in the MSC group than in the sham group (VEGF; 0.006±0.004 vs. 0.0005±0.0004, p<0.05, bFGF; 0.05±0.02 vs. 0.005±0.004, p<0.01 Fig. 5A, B).
FIG. 5.
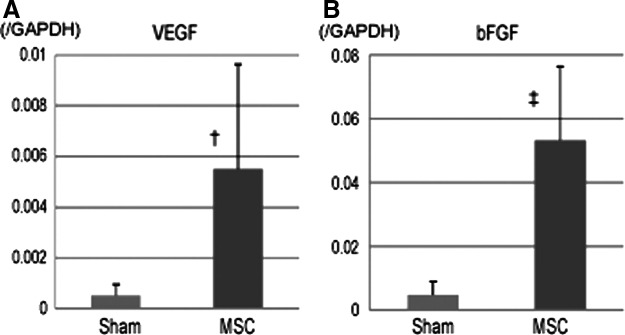
Angiogenesis-related mRNA expression in an area bordering the infarct, as measured by real-time polymerase chain reaction (RT-PCR). (A, B) The mRNA expression levels of vascular endothelial growth factor (VEGF; A) and basic fibroblast growth factor (bFGF; B) were significantly higher in the MSC group than in the sham group. †p<0.05, ‡p<0.01 versus sham.
Discussion
This study demonstrated that the transplantation of human bone marrow-derived MSC sheets was feasible, safe, and effective in treating heart failure in this preclinical study. The MSC sheets were created using temperature-responsive culture dishes, and total of 1×108 cells were transplanted into the injured heart. There was no mortality related to the procedure, and life-threatening arrhythmia was not detected by Holter electrocardiography. After the treatment, cardiac functions were improved by echocardiography, and histological examination indicated that LV remodeling was attenuated, and neovascularization in the infarct border area was increased.
Several studies demonstrated that bone marrow-derived MSC therapy had therapeutic effects in a porcine model of ICM. Allogeneic or autologous MSCs delivered by transendocardial injection or direct surgical injection resulted in an improvement in cardiac function, and ejection fractions were gradually increased over 12 weeks.20,21 In the present study, a similar porcine model of ICM was treated with cell sheets of MSCs, and marked improvement of cardiac function in the treated animals was demonstrated 4 weeks after MSC sheet transplantation. This difference in the pattern of cardiac improvement might be caused partially by the difference in the cell delivery method. The cell sheet technique applied in the current study makes it possible to harvest cells for transplant as a sheet, thus preserving the extracellular matrix (ECM). Preserving ECM in the transplanted cells has the benefit of preventing anoikis, apoptosis occurring due to cell detachment from the ECM.22 Preventing anoikis might reduce initial cell loss after cell transplantation compared with dissociated cell injection and results in early improvement of cardiac function. On the other hand, three-dimensional (3D) cell-seeded scaffold methods, which are made of biocompatible or degradable materials, might also prevent anoikis and have been developed to efficiently deliver cells into the heart.23,24 However, scaffold degradation after transplantation would have negative impacts on the survival and functionality of the transplanted cell constructs25 and thus on therapeutic effects as well. Moreover, from the standpoint of regulatory science, use of the scaffold would inevitably be subjected to extensive investigation of the materials to ensure safety in clinical settings. Given this, the scaffoldless cell sheet technique, which does not use any materials, would have an advantage in clinical applications.
A previous study demonstrated that transplanted MSCs were not converted to structurally and functionally integrated cardiomyocytes in vivo,26 although MSCs have shown potential for cardiomyogenic differentiation in vitro.27 Another study demonstrated that transplantation of autologous MSC sheet fragments improved postinfarct cardiac function through transdifferentiation of MSCs into vascular lineages other than cardiomyocytes.28 However, in the present study, neither human MSC-derived cardiomyocytes nor vascular lineage cells were detected in the porcine heart at 2 months after the transplant (data not shown). Transplanted human MSCs might have been chronically rejected due to xenotransplant mismatch in our study. A recent study demonstrated that a modified cell delivery method successfully enhanced the engraftment rate of transplanted cells in a xenotransplant model (human cell transplanted into porcine heart).29 In this study, a combination of transplanted cells with insulin-like growth factor additive 3D fibrin patch was applied for the cell delivery method. This indicates that optimization of cell preparation and cell delivery method could improve the therapeutic effects of cell therapy through enhanced survival of the transplanted cells. Nevertheless, an increase in blood perfusion and cardiac improvement were maintained after human MSC sheet transplant during the observation period, although no cells survived in the long term. In addition, our data confirmed that MSCs secrete multiple paracrine factors that possibly contribute to angiogenesis and endogenous cardiomyogenesis.18 The main mechanism of cardiac improvement from MSC sheet transplantation was considered to have occurred through the paracrine effect. Recent studies support the idea that transplanted cells induce myocardial repair by releasing signals (multiple cytokines, chemokines, and growth factors) into the host tissue. These signals then promote and enhance the injury healing process and lead to functional recovery through the activation of endogenous cardiac stem cells (CSCs), angiogenesis, inhibition of apoptosis, inhibition of hypertrophy, and favorable alterations of the ECM.5 A recent study revealed that transplantation of human induced pluripotent stem (iPS) cell-derived vascular cells, which do not contain cardiomyocytes, reduced scar size in a porcine model of acute MI.30 In this study, noncardiomyocyte transplantation stimulated endogenous progenitor cells and neovascularization and led to a reduced scar size. This indicated that enhancement of endogenous progenitor cells would be one of the most important targets in cell therapy as well as promotion of neovascularization. Another study reported that bone marrow-derived MSCs stimulated the proliferation and cardiomyogenic differentiation of CSCs and promoted endogenous cardiac regeneration.31 MSC and CSC combination therapy produced greater infarct size reduction compared with either cell administered alone after MI.32 This suggests that there may be important interactions between MSCs and CSCs in the process of cardiac regeneration. Other studies have demonstrated that a source of endogenous resident cardiac progenitor cells with regenerative potential existed in the epicardium.33,34 Our strategy of treating the damaged myocardium using cell sheets that implant on the epicardium might have the potential to enhance resident cardiac progenitor cells and promote endogenous cardiac regeneration, which is another mechanism to improve failing hearts.
The potential to induce immunologic tolerance is a well-known feature of MSCs. It is attributed to their lack of major histocompatibility complex class II and costimulatory molecules, the prevention of T-cell responses, and the secretion of soluble mediators, which induce a suppressive local microenvironment.35,36 Therefore, MSCs may play a unique and important role in mixed cell transplantation strategies, such as allogeneic iPS cell therapy to treat heart failure. Human iPS cells have great potential for cardiomyogenic differentiation and are therefore one of the most promising sources of cells for cardiac regeneration therapy.37 Future clinical applications of human iPS cells for regeneration therapy may involve allogeneic and HLA type-matched transplantation as some acute injuries, such as MI, stroke, and spinal cord trauma, may be targeted for regeneration therapy.38 Successful regeneration therapy using human iPS cells requires that their derivatives remain in the recipient long term or permanently. The transplanted cells will inevitably be rejected by the immune system if the cells are allogeneic. Therefore, it is necessary to establish an optimal immunosuppressant regimen or a strategy of inducing immunologic tolerance. Mixtures or simultaneous transplantation of MSCs with the derivatives of human iPS cells might be a useful strategy to help control allorejection and guarantee their therapeutic effects.
In conclusion, the transplantation of bone marrow-derived MSC sheets could improve cardiac function and attenuate LV remodeling in ICM. The cell sheet technique may provide a feasible, safe, and efficacious means of MSC transplantation in clinical settings.
Acknowledgments
The authors appreciate Shigeru Matsumi, Tsuyoshi Ishikawa, and Akima Harada for their excellent technical assistance. This work was supported by the JSPS Core-to-Core Program (JSPS; Japan Society for the Promotion of Science), and by the Research Project for Practical Applications of Regenerative Medicine from the Japan Agency for Medical Research and Development, AMED.
Disclosure Statement
Dr. T.S. is a consultant for CellSeed, Inc. Dr. T.O. is an advisory board member in CellSeed, Inc., and an inventor/developer designated on the patent for temperature-responsive culture surfaces.
References
- 1.Jessup M., and Brozena S. Heart failure. N Engl J Med 348, 2007, 2003 [DOI] [PubMed] [Google Scholar]
- 2.Roger V.L., Weston S.A., Redfield M.M., Hellermann-Homan J.P., Killian J., Yawn B.P., and Jacobsen S.J. Trends in heart failure incidence and survival in a community-based population. JAMA 292, 344, 2004 [DOI] [PubMed] [Google Scholar]
- 3.Menasché P. Cardiac cell therapy: lessons from clinical trials. J Mol Cell Cardiol 50, 258, 2011 [DOI] [PubMed] [Google Scholar]
- 4.Hsiao L.C., Carr C., Chang K.C., Lin S.Z., and Clarke K. Review article: stem cell-based therapy for ischemic heart disease. Cell Transplant 22, 663, 2013 [DOI] [PubMed] [Google Scholar]
- 5.Sanganalmath S.K., and Bolli R. Cell therapy for heart failure: a comprehensive overview of experimental and clinical studies, current challenges, and future directions. Circ Res 113, 810, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Makino S., Fukuda K., Miyoshi S., Konishi F., Kodama H., Pan J., Sano M., Takahashi T., Hori S., Abe H., Hata J., Umezawa A., and Ogawa S. Cardiomyocytes can be generated from marrow stromal cells in vitro. J Clin Invest 103, 697, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oswald J., Boxberger S., Jørgensen B., Feldmann S., Ehninger G., Bornhäuser M., and Werner C. Mesenchymal stem cells can be differentiated into endothelial cells in vitro. Stem Cells 22, 377, 2004 [DOI] [PubMed] [Google Scholar]
- 8.Williams A.R., and Hare J.M. Mesenchymal stem cells: biology, pathophysiology, translational findings, and therapeutic implications for cardiac disease. Circ Res 109, 923, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shimizu T., Yamato M., Isoi Y., Akutsu T., Setomaru T., Abe K., Kikuchi A., Umezu M., and Okano T. Fabrication of pulsatile cardiac tissue grafts using a novel 3-dimensional cell sheet manipulation technique and temperature-responsive cell culture surfaces. Circ Res 90, e40, 2002 [DOI] [PubMed] [Google Scholar]
- 10.Miyagawa S., Roth M., Saito A., Sawa Y., and Kostin S. Tissue-engineered cardiac constructs for cardiac repair. Ann Thorac Surg 91, 320, 2011 [DOI] [PubMed] [Google Scholar]
- 11.Memon I.A., Sawa Y., Fukushima N., Matsumiya G., Miyagawa S., Taketani S., Sakakida S.K., Kondoh H., Aleshin A.N., Shimizu T., Okano T., and Matsuda H. Repair of impaired myocardium by means of implantation of engineered autologous myoblast sheets. J Thorac Cardiovasc Surg 130, 1333, 2005 [DOI] [PubMed] [Google Scholar]
- 12.Sawa Y., Miyagawa S., Sakaguchi T., Fujita T., Matsuyama A., Saito A., Shimizu T., and Okano T. Tissue engineered myoblast sheets improved cardiac function sufficiently to discontinue LVAS in a patient with DCM: report of a case. Surg Today 42, 181, 2012 [DOI] [PubMed] [Google Scholar]
- 13.Narita T., Shintani Y., Ikebe C., Kaneko M., Campbell N.G., Coppen S.R., Uppal R., Sawa Y., Yashiro K., and Suzuki K. The use of scaffold-free cell sheet technique to refine mesenchymal stromal cell-based therapy for heart failure. Mol Ther 21, 860, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Teramoto N., Koshino K., Yokoyama I., Miyagawa S., Zeniya T., Hirano Y., Fukuda H., Enmi J., Sawa Y., Knuuti J., and Iida H. Experimental pig model of old myocardial infarction with long survival leading to chronic left ventricular dysfunction and remodeling as evaluated by PET. J Nucl Med 52, 761, 2011 [DOI] [PubMed] [Google Scholar]
- 15.Teichholz L.E., Kreulen T., Herman M.V., and Gorlin R. Problems in echocardiographic volume determinations: echocardiographic-angiographic correlations in the presence or absence of asynergy. Am J Cardiol 37, 7, 1976 [DOI] [PubMed] [Google Scholar]
- 16.Fukui S., Kitagawa-Sakakida S., Kawamata S., Matsumiya G., Kawaguchi N., Matsuura N., and Sawa Y. Therapeutic effect of midkine on cardiac remodeling in infarcted rat hearts. Ann Thorac Surg 85, 562, 2008 [DOI] [PubMed] [Google Scholar]
- 17.Zou Y., Liang Y., Gong H., Zhou N., Ma H., Guan A., Sun A., Wang P., Niu Y., Jiang H., Takano H., Toko H., Yao A., Takeshima H., Akazawa H., Shiojima I., Wang Y., Komuro I., and Ge J. Ryanodine receptor type 2 is required for the development of pressure overload-induced cardiac hypertrophy. Hypertension 58, 1099, 2011 [DOI] [PubMed] [Google Scholar]
- 18.Horiguchi K., Kitagawa-Sakakida S., Sawa Y., Li Z.Z., Fukushima N., Shirakura R., and Matsuda H. Selective chemokine and receptor gene expressions in allografts that develop transplant vasculopathy. J Heart Lung Transplant 21, 1090, 2002 [DOI] [PubMed] [Google Scholar]
- 19.Gnecchi M., Zhang Z., Ni A., and Dzau V.J. Paracrine mechanisms in adult stem cell signaling and therapy. Circ Res 103, 1204, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Quevedo H.C., Hatzistergos K.E., Oskouei B.N., Feigenbaum G.S., Rodriguez J.E., Valdes D., Pattany P.M., Zambrano J.P., Hu Q., McNiece I., Heldman A.W., and Hare J.M. Allogeneic mesenchymal stem cells restore cardiac function in chronic ischemic cardiomyopathy via trilineage differentiating capacity. Proc Natl Acad Sci U S A 106, 14022, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schuleri K.H., Feigenbaum G.S., Centola M., Weiss E.S., Zimmet J.M., Turney J., Kellner J., Zviman M.M., Hatzistergos K.E., Detrick B., Conte J.V., McNiece I., Steenbergen C., Lardo A.C., and Hare J.M. Autologous mesenchymal stem cells produce reverse remodelling in chronic ischaemic cardiomyopathy. Eur Heart J 30, 2722, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zvibel I., Smets F., and Soriano H. Anoikis: roadblock to cell transplantation? Cell Transplant 11, 621, 2002 [DOI] [PubMed] [Google Scholar]
- 23.Jawad H., Ali N.N., Lyon A.R., Chen Q.Z., Harding S.E., and Boccaccini A.R. Myocardial tissue engineering: a review. J Tissue Eng Regen Med 1, 327, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Martinez E.C., and Kofidis T. Myocardial tissue engineering: the quest for the ideal myocardial substitute. Expert Rev Cardiovasc Ther 7, 921, 2009 [DOI] [PubMed] [Google Scholar]
- 25.Akhyari P., Kamiya H., Haverich A., Karck M., and Lichtenberg A. Myocardial tissue engineering: the extracellular matrix. Eur J Cardiothorac Surg 34, 229, 2008 [DOI] [PubMed] [Google Scholar]
- 26.Caplan A.I., and Dennis J.E. Mesenchymal stem cells as trophic mediators. J Cell Biochem 98, 1076, 2006 [DOI] [PubMed] [Google Scholar]
- 27.Makino S., Fukuda K., Miyoshi S., Konishi F., Kodama H., Pan J., Sano M., Takahashi T., Hori S., Abe H., Hata J., Umezawa A., and Ogawa S. Cardiomyocytes can be generated from marrow stromal cells in vitro. J Clin Invest 103, 697, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang C.C., Tsai H.W., Lee W.Y., Lin W.W., Chen D.Y., Hung Y.W., Chen J.W., Hwang S.M., Chang Y., and Sung H.W. A translational approach in using cell sheet fragments of autologous bone marrow-derived mesenchymal stem cells for cellular cardiomyoplasty in a porcine model. Biomaterials 34, 4582, 2013; [DOI] [PubMed] [Google Scholar]
- 29.Ye L., Chang Y.H., Xiong Q., Zhang P., Zhang L., Somasundaram P., Lepley M., Swingen C., Su L., Wendel J.S., Guo J., Jang A., Rosenbush D., Greder L., Dutton J.R., Zhang J., Kamp T.J., Kaufman D.S., Ge Y., and Zhang J. Cardiac repair in a porcine model of acute myocardial infarction with human induced pluripotent stem cell-derived cardiovascular cells. Cell Stem Cell 15, 750, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xiong Q., Ye L., Zhang P., Lepley M., Tian J., Li J., Zhang L., Swingen C., Vaughan J.T., Kaufman D.S., and Zhang J. Functional consequences of human induced pluripotent stem cell therapy: myocardial ATP turnover rate in the in vivo swine heart with postinfarction remodeling. Circulation 127, 997, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hatzistergos K.E., Quevedo H., Oskouei B.N., Hu Q., Feigenbaum G.S., Margitich I.S., Mazhari R., Boyle A.J., Zambrano J.P., Rodriguez J.E., Dulce R., Pattany P.M., Valdes D., Revilla C., Heldman A.W., McNiece I., and Hare J.M. Bone marrow mesenchymal stem cells stimulate cardiac stem cell proliferation and differentiation. Circ Res 107, 913, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Williams A.R., Hatzistergos K.E., Addicott B., McCall F., Carvalho D., Suncion V., Morales A.R., Da Silva J., Sussman M.A., Heldman A.W., and Hare J.M. Enhanced effect of combining human cardiac stem cells and bone marrow mesenchymal stem cells to reduce infarct size and to restore cardiac function after myocardial infarction. Circulation 127, 213, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Limana F., Zacheo A., Mocini D., Mangoni A., Borsellino G., Diamantini A., De Mori R., Battistini L., Vigna E., Santini M., Loiaconi V., Pompilio G., Germani A., and Capogrossi M.C. Identification of myocardial and vascular precursor cells in human and mouse epicardium. Circ Res 101, 1255, 2007 [DOI] [PubMed] [Google Scholar]
- 34.Smart N., Risebro C.A., Melville A.A., Moses K., Schwartz R.J., Chien K.R., and Riley P.R. Thymosin beta4 induces adult epicardial progenitor mobilization and neovascularization. Nature 445, 177, 2007 [DOI] [PubMed] [Google Scholar]
- 35.Ryan J.M., Barry F.P., Murphy J.M., and Mahon B.P. Mesenchymal stem cells avoid allogeneic rejection. J Inflamm 2, 8, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Uccelli A., Pistoia V., and Moretta L. Mesenchymal stem cells: a new strategy for immunosuppression? Trends Immunol 28, 219, 2007 [DOI] [PubMed] [Google Scholar]
- 37.Yoshida Y., and Yamanaka S. iPS cells: a source of cardiac regeneration. J Mol Cell Cardiol 50, 327, 2011 [DOI] [PubMed] [Google Scholar]
- 38.Pearl J.I., Lee A.S., Leveson-Gower D.B., Sun N., Ghosh Z., Lan F., Ransohoff J., Negrin R.S., Davis M.M., and Wu J.C. Short-term immunosuppression promotes engraftment of embryonic and induced pluripotent stem cells. Cell Stem Cell 8, 309, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]