Abstract
Humans produce endogenous cannabinoids (endocannabinoids), a group of molecules that activate the same receptors as tetrahydrocannabinol. Endocannabinoids play important roles in reproduction in multiple species, but data in human endometrium are limited. Because endocannabinoids such as anandamide (AEA) and 2-arachidonoyl glycerol (2-AG) often act within tissues as paracrine factors, their effects can be modulated by changes in expression of locally produced synthetic and degradative/oxidative enzymes. The objective of this study was to localize and quantify expression of these key synthetic and degradative/oxidative enzymes for AEA and 2-AG in human endometrium throughout the menstrual cycle. Key synthetic enzymes include N-arachidonyl-phosphatidylethanolamine phospholipase-D (NAPE-PLD), diacylglycerol-lipase a (DAGL-α, and DAGL-β. Key degradative enzymes include fatty acid amide hydrolase (FAAH) and monoacylglycerol lipase (MAGL); cyclooxygenase 2 (COX2) is an oxidative enzyme. Endometrial samples were collected in 49 regularly cycling, normal women. Protein localization and expression were achieved by immunohistochemistry and messenger RNA (mRNA) expression by real-time reverse transcriptase polymerase chain reaction. No significant cycle-dependent mRNA expression was observed except that of COX2 (P = .002), which demonstrated maximum expression in the proliferative phase. During the secretory phase, NAPE-PLD protein had increased expression in luminal (P = .001), stromal (P = .007), and glandular (P = .04) epithelia, while FAAH had increased glandular (P = .009) and luminal (P = .01) expression. Increased expression in glandular epithelia was identified for MAGL (P = .03). The COX2 had increased luminal expression during the early secretory phase (P < .0001). In conclusion, maximal expression of degradatory/oxidative enzymes in the secretory phase may foster decreased endocannabinoid tone during implantation.
Keywords: endometrium, fatty-acid amide hydrolase, diacylglycerol-lipase, monoacylglycerol lipase, cyclooxygenase 2
Introduction
Cannabinoids are a class of over 60 compounds derived from the plant Cannabis sativa as well as the synthetic or endogenous versions of these compounds.1 The first cannabinoid to be intensively studied was trans-Δ9-tetrahydrocannabinol (Δ9-THC).2 In humans, Δ9-THC exposure has been associated with luteinizing hormone (LH) suppression, anovulation,3 and pregnancy complications including preterm labor and intrauterine growth restriction. However, these associations have unclear mechanistic explanations, and no clear association with early pregnancy loss has been found.4–6
Humans and many other animals also produce endogenous cannabinoids (endocannabinoids). A role for cannabinoids in human reproduction is suggested by studies on women using marijuana, murine reproductive studies, and correlation between lymphocyte endocannabinoid regulation and reproductive status. However, there has been no direct evidence of endocannabinoid action in the process of human embryo implantation or human endometrial function.
The best characterized endocannabinoids are the unsaturated fatty acid derivatives, N-arachidonoylethanolamide (anandamide [AEA]) and 2-arachidonoyl glycerol (2-AG). Both AEA and 2-AG are synthesized by various tissues in many species. The local and steady state levels of AEA and 2-AG are tightly regulated by the local synthetic and degradative/oxidative enzymes described in Figure 1.7–10 Endocannabinoid systems have been implicated in a variety of physiological and pathological processes.11 Subsequent studies in the murine female reproductive tract have suggested roles in decidualization, embryo development, and implantation.12
Figure 1.
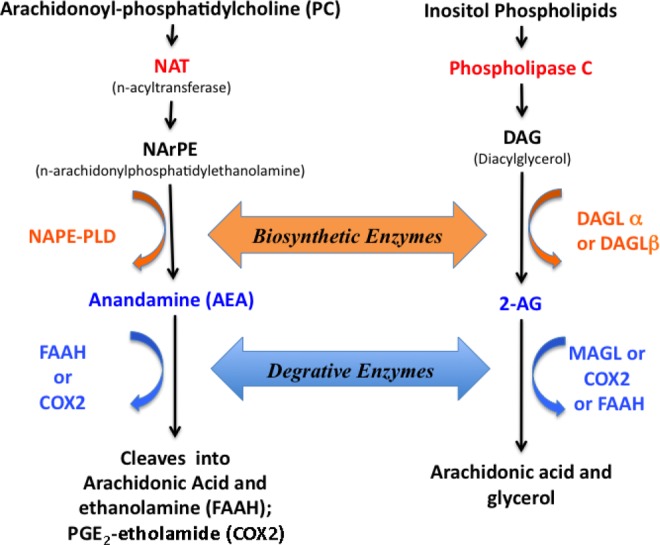
A simplified representation of endocannabinoid synthesis and degradation pathway. NAT indicates arylamine N-acetyltransferase; NAPE-PLD, N-arachidonyl-phosphatidylethanolamine phospholipase-D; FAAH, fatty acid amide hydrolase; COX, cyclooxygenase; MAGL, monoacylglycerol lipase; 2-AG, 2-arachidonoyl glycerol; DAGL, diacylglycerol-lipase; AA, arachidonic acid.
Endocannabinoid signaling plays a critical role in murine embryonic implantation.13 In the mouse, AEA, 2-AG, and their regulatory enzymes are produced in endometrium, and their levels fluctuate significantly over the estrus cycle.14 The cyclic changes in regulatory enzymes create spatiotemporal gradients of AEA and 2-AG concentrations in mouse endometrium, with low levels seen at implantation sites and high levels seen at interimplantation sites during nonreceptive endometrial phases.14 Furthermore, mouse blastocysts secrete a product that induces uterine expression of fatty acid amide hydrolase (FAAH), a degradative enzyme of AEA.15 Additionally, mouse embryos express cannabinoid receptors, and ligation of those receptors with high doses of AEA causes dose-dependent arrest of embryo development and inhibition of blastocyst hatching.16 On the other hand, mouse embryos with cannabinoid receptor deficiencies demonstrate delayed development, resulting in dysynchrony with timing of uterine receptivity.17 Taken together, the data strongly suggest that cannabinoid signaling regulates implantation between the embryo and the endometrium in the mouse.
Several studies have found correlations between human lymphocyte and plasma FAAH and AEA concentrations and pregnancy outcomes.18–21 Collectively, these data strongly suggest that endocannabinoid signaling may be a regulator of human embryo implantation and early pregnancy maintenance in humans.
Although significant data exist demonstrating the importance of endocannabinoid signaling in mouse reproduction and human peripheral plasma, data related to FAAH and monoacylglycerol lipase (MAGL) expression in human endometrium are scant. One prior study, using immunohistochemical technique, described the expression and localization of N-arachidonyl-phosphatidyl ethanolamine phospholipase D (NAPE-PLD) and FAAH, the regulatory enzymes for AEA, in endometrial biopsies of women who were under investigation for benign conditions, such as menorrhagia, leiomyomata, adnexal mass, pelvic pain, and cervical intraepithelial neoplasia.22 The NAPE-PLD, the synthetic enzyme, has increased expression in endometrial glands during the menstrual, early proliferative, and late secretory (LSE) phases of the menstrual cycle. The FAAH, a degrative enzyme, has a relatively low immunoreactivity in glands throughout the cycle, but it is increased during the LSE phase. However, these data were limited by use of tissues from women undergoing surgery for various gynecologic disorders that might affect the expression of the endocannabinoid system components.22
The purpose of our study was to assess the location and amount of expression of synthetic and degradative/oxidative enzymes for both AEA and 2-AG, more specifically, NAPE-PLD, FAAH, cyclooxygenase 2 (COX2), MAGL, and diacylglycerol-lipase (DAGL) α and β, in human endometrium through the menstrual cycle in volunteer women without any gynecological disorders.
Materials and Methods
This study was approved by the institutional review board of University of North Carolina at Chapel Hill. Timed endometrial biopsies were performed across the menstrual cycle in 49 regularly cycling volunteers without known reproductive abnormality. Biopsies were performed under sterile conditions using Pipelle cannulas. Samples were dated by cycle day (proliferative phase—Prolif) or days after urinary LH surge detection (secretory phase; early secretory [ESE]—LH + 1 to LH + 6; mid-secretory [MSE]—LH + 7 to LH + 10; and LSE—LH + 11 to LH + 14). Samples were snap frozen in liquid nitrogen for later RNA isolation (Prolif, n = 11; ESE, n = 5; MSE, n = 6; and LSE, n = 4) or fixed in formalin for immunohistochemistry analysis (Prolif, n = 6; ESE, n = 6; MSE, n = 7; and LSE, n = 4).
RNA Isolation and Quantification
Total endometrial RNA was extracted from tissue samples using the Ambion RNAqueous-4PCR kit (Applied Biosystems, Ann Arbor, Michigan) according to manufacturer’s instructions and stored at −80°C. RNA was quantitated using RiboGreen (Molecular Probes, Carlsbad, California) with a ribosomal RNA standard curve. First strand complimentary DNA was synthesized from 500 ng of total RNA with Avian Myeloblastosis Virus reverse transcriptase using manufacturer’s instructions (Roche, Indianapolis, Indiana). An equivalent volume of water was substituted for RNA for each reaction as a “no template” negative control. The total reaction volume was 20 μL, and reverse transcription conditions were 25°C for 10 minutes, 42°C for 60 minutes, and 99°C for 5 minutes.
Complimentary DNA samples were then diluted 1:5 and plated in triplicate with TaqMan Master Mix (Applied Biosystems, Foster City, California) and sterile water. Predesigned TaqMan probe and primer sets (Applied Biosystems) for NAPE-PLD (Hs00419593_m1), FAAH (Hs01038660), MAGL (Hs00200752_m1), cyclooxygenase-2 (COX2; Hs00153133_m1), –DAGL-a (Hs00391374_m1), and DAGL-β (Hs00373700_m1) genes were used to perform reverse transcriptase polymerase chain reaction (RT-PCR), with their expression normalized to peptidyl-prolyl cis-trans isomerase A (PPIA; Hs04194521_s1), a constitutive control. The PPIA was chosen as housekeeping gene based on previous work where 12 housekeeping genes were validated in the endometrium. The Normfinder average expression stability value23 for PPIA in endometrium was 0.31. The PPIA was the best normalization gene among a set of candidates for human endometrial samples.24 The total reaction volume for all RT-PCR experiments was 20 μL, and reactions were performed in 96-well plates on a Stratagene MX3000 thermocycler (Agilent Technologies) for 40 two-step cycles (95°C for 25 seconds and then 60°C for 1 minute). All experiments were conducted in triplicate.
Immunohistochemistry
Protein immunolocalization was performed for NAPE-PLD, FAAH, MAGL, and COX2 with commercially available antibodies (all from Cayman Chemical, Ann Arbor, Michigan), and specificity was determined with the respective blocking peptides (Cayman Chemical) as shown in Table 1. Endometrial samples were deparaffinized in xylene, rehydrated in decreasing concentrations of ethanol, and rinsed in phosphate-buffered saline (PBS). Samples were incubated with 0.3% H2O2 in methanol for 30 minutes to block endogenous peroxidase activity and then with 0.1 mol/L citrate buffer (pH 6.0) at 100°C for 15 minutes for antigen retrieval. Slides were washed in water and incubated with 10% goat serum in PBS for 1 hour at room temperature to block nonspecific binding. Tissue sections were then incubated overnight in a humid chamber at 4°C with rabbit polyclonal antibodies diluted by blocking solution (anti-NAPE-PLD, 1:80; anti-FAAH, 1:400; anti-MAGL, 1:40; and anti-COX2, 1:250). After a PBS rinse and immersion in blocking solution for 10 minutes, the slides were incubated with biotintylated goat anti-rabbit immunoglobulin G (Cayman Chemical) at 1:250 dilution for 1 hour and then with avidin-biotinylated horseradish peroxidase complex (Vector Laboratories, Burlingame, California) for 1 hour. Additional samples incubated without primary antibody were used as controls. Slides were placed in a diaminobenzidine solution for 10 minutes, incubated in 2% osmium at room temperature for 10 minutes, rinsed in PBS, then counterstained with toluidine blue, and dehydrated using increasing ethanol concentrations. Protein staining intensity and distribution were assessed using HSCORE.25 Briefly, the whole sample (all microscopic fields) was analyzed under a 200 and 400× magnification and the following formula was used: HSCORE = S Pi (I + 1), where i = intensity of staining (values are 1, 2, or 3; weak, moderate or strong, respectively) and Pi is the percentage of stained cells. Human testis was used as external positive and negative control. Our group has recently documented the high correlation (r [s] .86-.79 to .9; P < .0001) of HSCORE and image analysis software results.26
Table 1.
Antibodies Used in the Study.
| Antibody | Manufacturer | Dilution |
|---|---|---|
| FAAH—fatty acid amide hydrolase—polyclonal antibody rabbit anti-human | Cayman Chemical N#101600 | 1:400 |
| MAGL—polyclonal antibody rabbit anti-human | Cayman Chemical N#100035 | 1:40 |
| COX2—polyclonal antibody rabbit anti-human | Cayman Chemical N#160126 | 1:250 |
| NAPE-PLD—polyclonal antibody rabbit anti-human | Cayman Chemical N#10305 | 1:80 |
Statistical Analysis
Data were grouped into 4 menstrual cycle phases for analysis: Prolif, ESE, MSE, and LSE. They were also grouped by endometrial compartment: lumen, glands, and stroma. Statistical analysis of the messenger RNA (mRNA) expressions of the different genes and proteins was performed using 1-way analysis of variance (ANOVA) tests (with Tukey post hoc test) if data were normally distributed (D'Agostino & Pearson omnibus normality test); otherwise, data were normalized using a logarithmic formula. Immunohistochemical expression was analyzed with 2-way ANOVA, considering the different compartments (luminal, glandular, and stroma) and different phases of the menstrual cycle. Fisher least significant difference was used as a post hoc test. Significance was set at P < .05. Analyses were performed using Prism 6.0 (GraphPad, San Diego, California).
Results
Endocannabinoid Synthetic Enzymes (NAPE-PLD, DAGL-α, and DAGL-β)
Coding mRNA for all 3 synthetic enzymes, NAPE-PLD, DAGL-α, and DAGL-β, was detected throughout the menstrual cycle (Figures 2 and 3), and no significant difference was found among their mRNA levels.
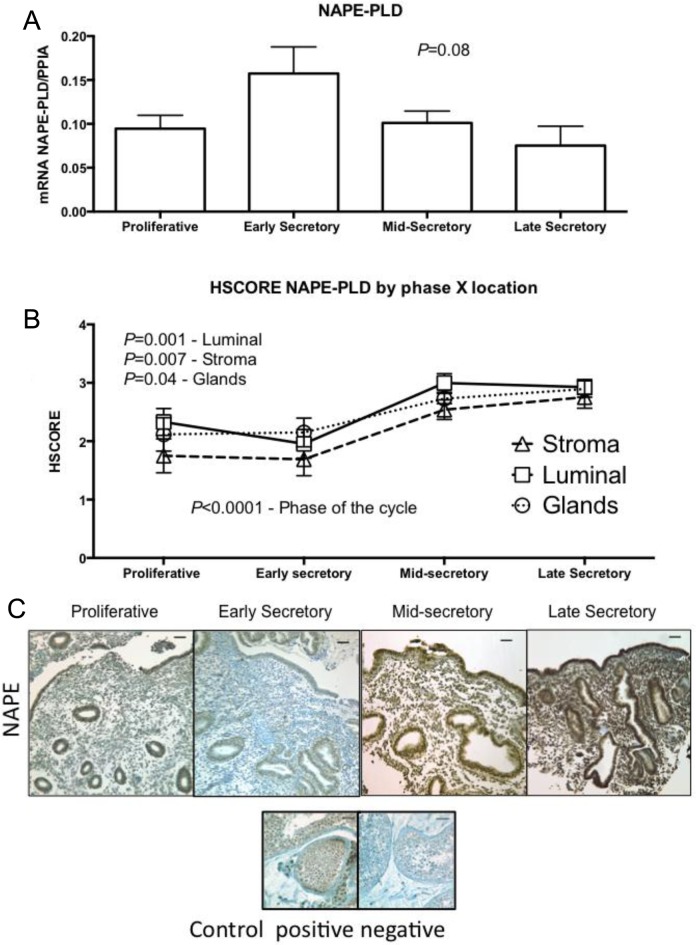
Figure 2.
Expression of N-arachidonyl-phosphatidylethanolamine phospholipase-D (NAPE-PLD) across different phases of the menstrual cycle, using (A) reverse transcriptase polymerase chain reaction (RT-PCR; proliferative, n = 11; early secretory, n = 5; mid-secretory, n = 6; and late secretory, n = 4). One-way analysis of variance (ANOVA) was used for analysis. B, HSCORE at different compartment of the endometrium; analysis was done with 2-way ANOVA, with Fisher least significant difference as post hoc test. Variables were phase of the cycle and compartment of the endometrium. Values in graph are mean ± standard error of the mean (SEM; proliferative, n = 6; early secretory, n = 6; mid-secretory, n = 7; and late secretory, n = 4). C, Representative pictures of each phase of the menstrual cycle and its immunolocalization. Human testis was used as positive and negative control. Bars represent 50 µm.
Figure 3.
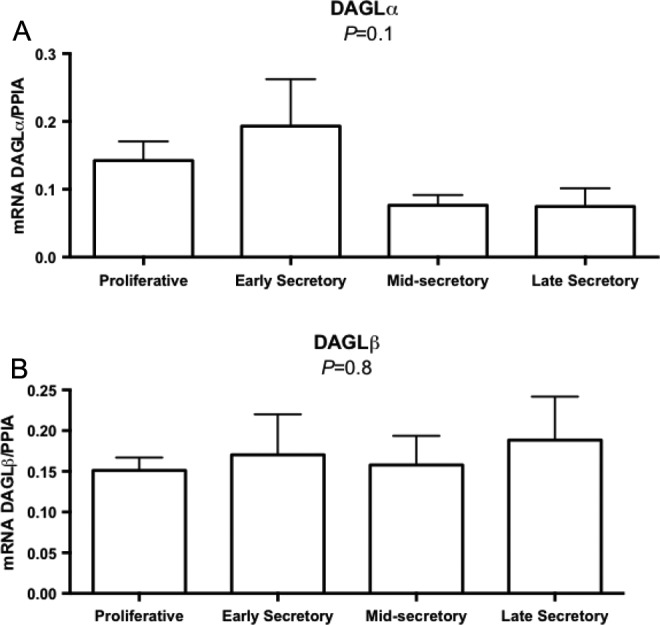
Quantitative reverse transcriptase polymerase chain reaction (RT-PCR) evaluation of endometrial messenger RNA expression for diacylglycerol-lipase (DAGL) α (A) and DAGL β (B) enzymes involved in endocannabinoid metabolism across the menstrual cycle (proliferative, n = 11; early secretory, n = 5; mid-secretory, n = 6; and late secretory, n = 4). One-way analysis of variance (ANOVA). Graphs represent mean ± standard error of the mean (SEM). All groups passed the normality test distribution.
The NAPE-PLD protein was immunolocalized to luminal, glandular, and stroma cells, with higher intensity seen during the secretory phase (Figure 2B). A significant cyclic variability in the luminal (P = .001), stroma (P = .007), and glands (P = 0.04) was seen during the MSE and LSE phases.
Endocannabinoid Degradatives/Oxidative Enzymes (FAAH, MAGL, and COX2)
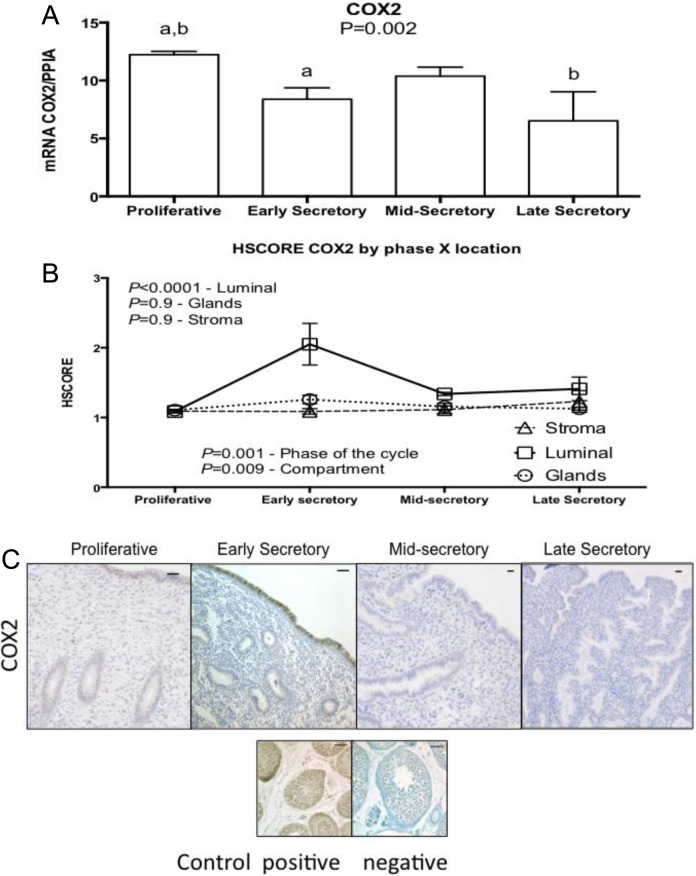
Messenger RNA encoding the degradative (FAAH and MAGL) and oxidative (COX2) enzymes of endocannabinoids was detected throughout the menstrual cycle (Figures 4 –6). Among these enzymes, only mRNA expression of COX2 exhibited a significant difference over the menstrual cycle. Low and maximal mRNA expression was seen in the LSE and Prolif phases, respectively (Figure 5A; P = .002). There was a significant increase in COX2 protein expression in luminal endometrium during the ESE (Figure 5B and C).
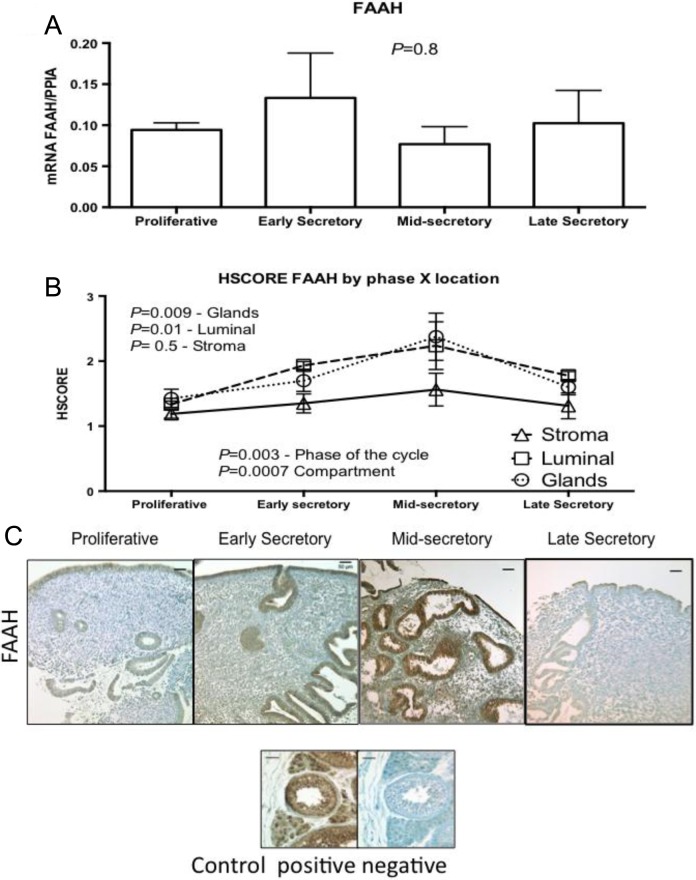
Figure 4.
Expression of fatty acid amide hydrolase (FAAH) across the different phases of the menstrual cycle, using (A) reverse transcriptase polymerase chain reaction (RT-PCR; proliferative, n = 11; early secretory, n = 5; mid-secretory, n = 6; and late secretory, n = 4). One-way analysis of variance (ANOVA) was used for analysis. B, HSCORE at different compartment of the endometrium; analysis was done with 2-way ANOVA, with Fisher least significant difference as post hoc test. Variables were phase of the cycle and compartment of the endometrium. Values in graph are mean ± standard error of the mean (SEM; proliferative, n = 6; early secretory, n = 6; mid-secretory, n = 7; and late secretory, n = 4). All samples passed normality distribution. C, Representative pictures of each phase of the menstrual cycle and its immunolocalization. Human testis was used as positive and negative control. Bars represent 50 µm.
Figure 5.
Expression of cyclooxygenase 2 (COX2) across the different phases of the menstrual cycle, using (A) reverse transcriptase polymerase chain reaction (RT-PCR; proliferative, n = 11; early secretory, n = 5; mid-secretory, n = 6; and late secretory, n = 4). Values were not normally distributed and therefore underwent logarithmic transformation to allow parametric analysis. One-way analysis of variance (ANOVA) was used for analysis, with Tukey as post hoc test, (a) P = 0.02; (b) P = 0.04. B, HSCORE at different compartment of the endometrium; analysis was done with 2-way ANOVA, with Fisher least significant difference as post hoc test. Variables were phase of the cycle and compartment of the endometrium. Values in graph are mean ± standard error of the mean (SEM; proliferative, n = 6; early secretory, n = 6; mid-secretory, n = 7; and late secretory, n = 4). All samples passed normality distribution. C, Representative pictures of each phase of the menstrual cycle and its immunolocalization. Human testis was used as positive and negative control. Bars represent 50 µm.
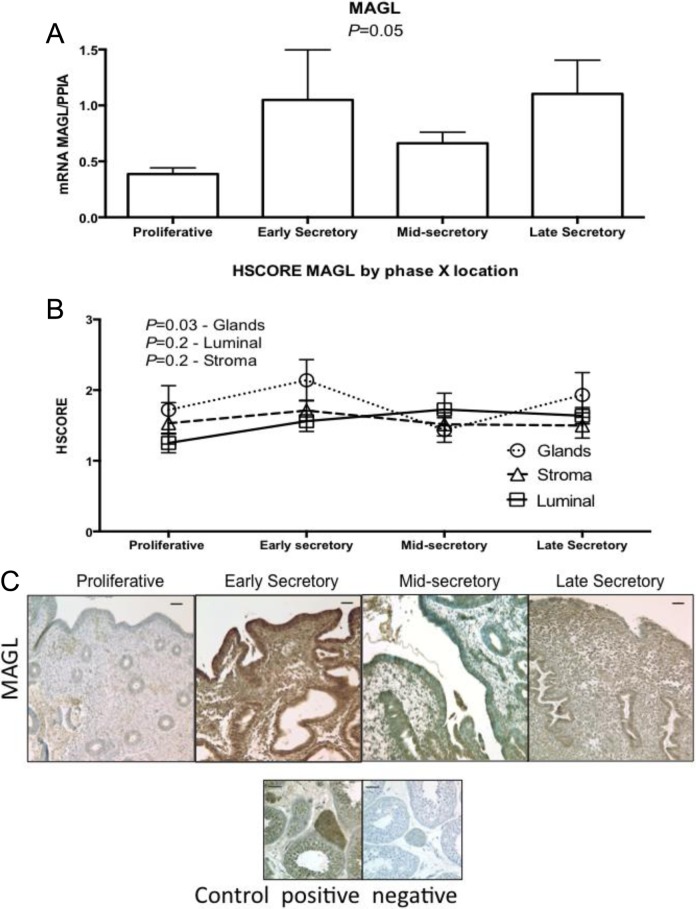
Figure 6.
Expression of monoacylglycerol lipase (MAGL) across the different phases of the menstrual cycle, using (A) reverse transcriptase polymerase chain reaction (RT-PCR; proliferative, n = 11; early secretory, n = 5; mid-secretory, n = 6; and late secretory, n = 4). One-way analysis of variance (ANOVA) was used for analysis. B, HSCORE at different compartment of the endometrium; analysis was done with 2-way ANOVA, with Fisher least significant difference as post hoc test. Variables were phase of the cycle and compartment of the endometrium. Values in graph are mean ± standard error of the mean (SEM). (proliferative, n = 6; early secretory, n = 6; mid-secretory, n = 7; and late secretory, n = 4). All samples passed normality distribution. C, Representative pictures of each phase of the menstrual cycle and its immunolocalization. Human testis was used as positive and negative control. Bars represent 50 µm.
The FAAH protein was detected in endometrial lumen, glands, and stroma. Immunostaining of FAAH was significantly higher in endometrial glands and luminal epithelium in the MSE phase (Figure 4B and C).
Monoacylglycerol lipase protein did not stain strongly in any cycle phase, except for the ESE phase, where a significant difference was found in the glands (Figure 6B and C).
Discussion
In this study, we analyzed mRNA coding for 5 regulatory enzymes to be expressed throughout the menstrual cycle. Only COX-2 mRNA varied consistently with cycle phase, with an increase in the ESE endometrium (Figure 5). Data on human endometrial COX2 mRNA expression over different cycle phases are novel. Porcine endometrial COX2 mRNA expression is increased on days 10 to 12 of the estrous cycle, that is, before ovulation.27 The mean levels of other genes did not differ across the menstrual cycle (Figures 2, 3, 4, and 6, Similarly, Gebeh et al did not find a difference in NAPE-PLD mRNA expression between follicular and secretory phases of endometrium obtained from normal women.28 The patterns of protein expression of FAAH, MAGL, COX2, and NAPE-PLD did not correlate with the mRNA levels. Possible explanations for these findings could be related to the fact that the mRNA levels were obtained from whole endometrial biopsy, while immunohistochemical analysis was performed in different compartments of the histological samples. Nevertheless, some correlation between mean mRNA levels and immunohistochemical expression can be observed in MAGL (Figure 6). Of note, recent data have demonstrated that there is substantial posttranscriptional regulation of protein abundance; only 40% of the protein concentration can be explained by abundance of mRNA.29
Maximal protein expression was seen in the secretory phase for all characterized enzymes (Figures 2 and 4-6). The NAPE-PLD protein expression in endometrium was significantly higher in the MSE and LSE phases (Figure 2). These results are different from Taylor et al. Taylor et al reported NAPE-PLD protein expression to be high in the Prolif phase, reaches a nadir at the ESE phase, and returns to increase gradually at MSE and LSE phases.22 In our study, NAPE-PLD is higher in MSE and LSE phases, compared to the Prolif phase. The major discrepancy is the expression in the Prolif phase. Possible explanations for these differences may be related to the selection of patients. We performed endometrial biopsy in normal, proven fertile volunteers without any reproductive abnormality, while Taylor et al obtained their samples from patients who underwent endometrial biopsies to investigate menorrhagia, leiomyoma, or pelvic pain. These conditions are often associated with abnormal levels of prostaglandins30–32 and infertility.33,34
Similarly, a major difference was found in FAAH protein expression in glandular compartment. Taylor et al found that FAAH has a nadir in MSE endometrium22; FAAH, in our study, reaches its highest expression in the MSE phase (Figure 4). Nevertheless, our findings on FAAH protein expression in the endometrium mirror those found by Maccarrone et al who measured lymphocyte levels of FAAH and AEA.19 Indeed, high levels of FAAH are found at the site of murine embryo implantation. These high levels are thought to be beneficial to implantation because AEA levels are reduced.35 Another possibility could be related to the differences in antibodies and to the methodology for image analysis.36 These differences warrant further investigation.
Monoacylglycerol lipase is the principal enzyme involved in the degradation of endogenous 2-AG. The degradative (MAGL) and synthetic enzymes (DAGL-α and β) for 2-AG have not been studied in all phases of the menstrual cycle in endometrium of women of reproductive age. We found MAGL protein expression to be increased in luminal endometrium during the ESE phase, similar to what has been found in the murine model.14 These findings suggest that 2-AG is degraded by MAGL in humans as in the murine model.14 On the other hand, we did not find DAGL-a mRNA expression to be upregulated during the window of implantation as has been shown in the mouse.14 Normal implantation occurs when low levels of 2-AG and AEA occur on the implantation site.14 Our findings on different levels of MAGL on the luminal site corroborate with those findings in the murine model. The differences in tissue analysis and compartments may explain the differences we found between MAGL mRNA levels and protein levels. Although not significant, the mean levels of MAGL mRNA correspond to those found in the glandular epithelium (Figure 6). The DAGL is an important player in 2-AG formation. The low mean levels of DAGL-a mRNA in MSE endometrium, although not significant, need further investigation in order to generate data about the balance of 2-AG levels during the implantation period (Figure 3).
Cyclooxygenase 2, which oxidizes both AEA and 2-AG, was found to have an increase in mRNA levels during the Prolif phase, followed by a reduction in ESE and LSE (Figure 5). Of note, protein expression did not correlate with the mRNA levels. The increased expression of COX2 in luminal epithelium as compared to stroma is similar to that found by other authors using immunohistochemistry to analyze human endometrium.37,38 Likewise, the low expression of COX2 in glandular epithelium is similar to those published by Jones et al.39 However, Stavreus-Evers et al did not find COX2 protein expression to vary during the luteal phase as we found.40 This is likely related to the staining score system; we used a parametric method, while Stavreus-Evers et al used a nonparametric method. Overall, these findings indicate that the role of COX2 in endocannabinoid regulation may be particularly important to understand in human reproduction. Further, the use of nonsteroidal anti-inflammatory medications (NSAIDs) is common and has been associated with ovulatory dysfunction in humans (eg, preventing follicle rupture),41 rats,42 and mice (eg, inhibition of ovulation, fertilization, decidualization, and implantation).43 Although the mechanism of COX2 inhibition by NSAIDs in reproductive dysfunction may be due to reduced prostaglandin production, another possibility is elevated endometrial levels of AEA and 2-AG. This mechanism may favor the debate on the risk of miscarriage in users of NSAIDs as advocated by many authors.44–47
The selection of normal healthy cycling women is the major strength of this study. By sampling normal volunteer women, we removed the bias that could be associated with benign gynecological conditions, such as pelvic pain, leiomyoma, and abnormal uterine bleeding. Unfortunately, we did not measure local levels of AEA, the immunoexpression of DAGL-α and β, CB1, CB2, TRPV1, and the lipoxygenase isoenzymes in our sample to complement our findings.
Human research investigating the role of endocannabinoid signaling in reproduction has largely concentrated on studies of peripheral blood and circulating lymphocytes. For example, plasma AEA levels fluctuate during the menstrual cycle, with peak levels in the ovulatory phase and a nadir in the LSE phase.48 Also, low lymphocyte FAAH expression and high plasma AEA levels have been associated with lower odds of successful pregnancy after in vitro fertilization20 and increased risk of miscarriage.49
Overall, our findings as well as those previously done using human peripheral blood samples reflect increased endocannabinoid hydrolysis during the MSE phase, supporting the hypothesized role of endocannabinoid regulation in embryo implantation. In conclusion, we have presented the description of protein and mRNA levels for some of the major synthetic and degradative/oxidative enzymes regulating endocannabinoid (AEA and 2-AG) levels in human endometrium across the menstrual cycle. Our findings establish a foundation for further investigation of other endocannabinoid signaling receptors in human embryo implantation.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Grant support: This work was supported by the Eunice Kennedt Shriver NICHD/NIH through cooperative agreement U54 HD-30476 (SLY) as part of the Specialized Cooperative Centers Program in Reproduction Research and Infertility Research (SLY) and R01 HD067721 (SLY), and by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) 240239/2012-1 (RFS).
References
- 1. Alexander A, Smith P, Rosengren R. Cannabinoids in the treatment of cancer. Cancer Lett. 2009;285 (1):6–12. [DOI] [PubMed] [Google Scholar]
- 2. Gaoni Y, Mechoulam R. Isolation, structure, and partial synthesis of an active constituent of hashish. J Am Chem Soc. 1964;86 (8):1646–1647. [Google Scholar]
- 3. Mendelson J, Mello N, Ellingboe J, Skupny A, Lex B, Griffin M. Marihuana smoking suppresses luteinizing hormone in women. J Pharmacol Exp Ther. 1986;237 (3):862–866. [PubMed] [Google Scholar]
- 4. Gibson G, Baghurst P, Colley D. Maternal alcohol, tobacco and cannabis consumption and the outcome of pregnancy. Aust N Z J Obstet Gynaecol. 1983;23 (1):15–19. [DOI] [PubMed] [Google Scholar]
- 5. Frank D, Bauchner H, Parker S. et al. Neonatal body proportionality and body composition after in utero exposure to cocaine and marijuana. J Pediatr. 1990;117 (4):622–626. [DOI] [PubMed] [Google Scholar]
- 6. Sherwood R, Keating J, Kavvadia V, Greenough A, Peters T. Substance misuse in early pregnancy and relationship to fetal outcome. Eur J Pediatr. 1999;158 (6):488–492. [DOI] [PubMed] [Google Scholar]
- 7. Mouslech Z, Valla V. Endocannabinoid system: an overview of its potential in current medical practice. Neuro Endocrinol Lett. 2009;30 (2):153–179. [PubMed] [Google Scholar]
- 8. Natarajan V, Schmid P, Reddy P, Schmid H. Catabolism of n-acylethanolamine phospholipids by dog brain preparations. J Neurochem. 1984;42 (6):1613–1619. [DOI] [PubMed] [Google Scholar]
- 9. Cravatt B, Giang D, Mayfield S, Boger D, Lerner R, Gilula N. Molecular characterization of an enzyme that degrades neuromodulatory fatty-acid amides. Nature. 1996;384 (6604):83–87. [DOI] [PubMed] [Google Scholar]
- 10. Cravatt B, Lichtman A. The enzymatic inactivation of the fatty acid amide class of signaling lipids. Chem Phys Lipids. 2002;121 (1-2):135–148. [DOI] [PubMed] [Google Scholar]
- 11. Pacher P, Kunos G. Modulating the endocannabinoid system in human health and disease--successes and failures. FEBS J. 2013;280 (9):1918–1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Di Blasio AM, Vignali M, Gentilini D. The endocannabinoid pathway and the female reproductive organs. J Mol Endocrinol. 2013;50 (1):R1–R9. [DOI] [PubMed] [Google Scholar]
- 13. Sun X, Dey S. Cannabinoid/endocannabinoid signaling impact on early pregnancy events. Curr Top Behav Neurosci. 2009;1:255–273. [DOI] [PubMed] [Google Scholar]
- 14. Wang H, Xie H, Sun X. et al. Differential regulation of endocannabinoid synthesis and degradation in the uterus during embryo implantation. Prostaglandins Other Lipid Mediat. 2007;83 (1-2):62–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Maccarrone M, Defelici M, Klinger F. et al. Mouse blastocysts release a lipid which activates anandamide hydrolase in intact uterus. Mol Hum Reprod. 2004;10 (4):215–221. [DOI] [PubMed] [Google Scholar]
- 16. Wang H, Xie H, Guo Y. et al. Fatty acid amide hydrolase deficiency limits early pregnancy events. J Clin Invest. 2006;116 (8):2122–2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Paria B, Song H, Wang X. et al. Dysregulated cannabinoid signaling disrupts uterine receptivity for embryo implantation. J Biol Chem. 2001;276 (23):20523–20528. [DOI] [PubMed] [Google Scholar]
- 18. Maccarrone M, Valensise H, Bari M, Lazzarin N, Romanini C, Finazzi-Agro A. Relation between decreased anandamide hydrolase concentrations in human lymphocytes and miscarriage. Lancet. 2000;355 (9212):1326–1329. [DOI] [PubMed] [Google Scholar]
- 19. Maccarrone M, Valensise H, Bari M, Lazzarin N, Romanini C, Finazzi-Agro A. Progesterone up-regulates anandamide hydrolase in human lymphocytes: Role of cytokines and implications for fertility. J Immunol. 2001;166 (12):7183–7189. [DOI] [PubMed] [Google Scholar]
- 20. Maccarrone M, Bisogno T, Valensise H. et al. Low fatty acid amide hydrolase and high anandamide levels are associated with failure to achieve an ongoing pregnancy after ivf and embryo transfer. Mol Hum Reprod. 2002;8 (2):188–195. [DOI] [PubMed] [Google Scholar]
- 21. Habayeb O, Taylor A, Finney M, Evans M, Konje J. Plasma anandamide concentration and pregnancy outcome in women with threatened miscarriage. JAMA. 2008;299 (10):1135–1136. [DOI] [PubMed] [Google Scholar]
- 22. Taylor A, Abbas M, Habiba M, Konje J. Histomorphometric evaluation of cannabinoid receptor and anandamide modulating enzyme expression in the human endometrium through the menstrual cycle. Histochem Cell Biol. 2010;133 (5):557–565. [DOI] [PubMed] [Google Scholar]
- 23. Andersen C, Jensen J, Orntoft T. Normalization of real-time quantitative reverse transcription-pcr data: A model-based variance estimation approach to identify genes suited for normalization, applied to bladder and colon cancer data sets. Cancer Res. 2004;64 (15):5245–5250. [DOI] [PubMed] [Google Scholar]
- 24. Plante B, Lessey B, Taylor R. et al. G protein-coupled estrogen receptor (gper) expression in normal and abnormal endometrium. Reprod Sci. 2012;19 (7):684–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Budwit-Novotny DA, Mccarty K, Cox E. et al. Immunohistochemical analyses of estrogen receptor in endometrial adenocarcinoma using a monoclonal antibody. Cancer Res. 1986;46 (10):5419–5425. [PubMed] [Google Scholar]
- 26. Fuhrich D, Lessey B, Savaris R. Comparison of hscore assessment of endometrial beta3 integrin subunit expression with digital hscore using computerized image analysis (imagej). Anal Quant Cytol Histol. 2013;35 (4):210–216. [PMC free article] [PubMed] [Google Scholar]
- 27. Blitek A, Waclawik A, Kaczmarek M, Stadejek T, Pejsak Z, Ziecik A. Expression of cyclooxygenase-1 and -2 in the porcine endometrium during the oestrous cycle and early pregnancy. Reprod Domest Anim. 2006;41 (3):251–257. [DOI] [PubMed] [Google Scholar]
- 28. Gebeh A, Marczylo E, Amoako A, Willets J, Konje J. Variation in stability of endogenous reference genes in fallopian tubes and endometrium from healthy and ectopic pregnant women. Int J Mol Sci. 2012;13 (3):2810–2826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Vogel C, Marcotte E. Insights into the regulation of protein abundance from proteomic and transcriptomic analyses. Nat Rev Genet. 2012;13 (4):227–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Maia HJ, Haddad C, Pinheiro N, Casoy J. The effect of oral contraceptives on aromatase and cox-2 expression in the endometrium of patients with idiopathic menorrhagia or adenomyosis. Int J Womens Health. 2013;5:293–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Smith O, Jabbour H, Critchley H. Cyclooxygenase enzyme expression and e series prostaglandin receptor signalling are enhanced in heavy menstruation. Hum Reprod. 2007;22 (5):1450–1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Koike H, Ikenoue T, Mori N. [Studies on prostaglandin production relating to the mechanism of dysmenorrhea in endometriosis]. Nihon Naibunpi Gakkai Zasshi. 1994;70 (1):43–56. [DOI] [PubMed] [Google Scholar]
- 33. Brady P, Stanic A, Styer A. Uterine fibroids and subfertility: an update on the role of myomectomy. Curr Opin Obstet Gynecol. 2013;25 (3):255–259. [DOI] [PubMed] [Google Scholar]
- 34. Diagnostic evaluation of the infertile female: a committee opinion. Fertil Steril. 2012;98(2):302–307. [DOI] [PubMed] [Google Scholar]
- 35. Melford S, Taylor A, Konje J. Of mice and (wo)men: factors influencing successful implantation including endocannabinoids. Hum Reprod Update. 2014;20 (3):415–428. [DOI] [PubMed] [Google Scholar]
- 36. Baek J, Darlington C, Smith P, Ashton J. Antibody testing for brain immunohistochemistry: brain immunolabeling for the cannabinoid cb(2) receptor. J Neurosci Methods. 2013;216 (2):87–95. [DOI] [PubMed] [Google Scholar]
- 37. Marions L, Danielsson K. Expression of cyclo-oxygenase in human endometrium during the implantation period. Mol Hum Reprod. 1999;5 (10):961–965. [DOI] [PubMed] [Google Scholar]
- 38. Tokyol C, Aktepe F, Dilek F, Sahin O, Arioz D. Expression of cyclooxygenase-2 and matrix metalloproteinase-2 in adenomyosis and endometrial polyps and its correlation with angiogenesis. Int J Gynecol Pathol. 2009;28 (2):148–156. [DOI] [PubMed] [Google Scholar]
- 39. Jones R, Kelly R, Critchley H. Chemokine and cyclooxygenase-2 expression in human endometrium coincides with leukocyte accumulation. Hum Reprod. 1997;12 (6):1300–1306. [DOI] [PubMed] [Google Scholar]
- 40. Stavreus-Evers A, Koraen L, Scott J, Zhang P, Westlund P. Distribution of cyclooxygenase-1, cyclooxygenase-2, and cytosolic phospholipase a2 in the luteal phase human endometrium and ovary. Fertil Steril. 2005;83 (1):156–162. [DOI] [PubMed] [Google Scholar]
- 41. Smith G, Roberts R, Hall C, Nuki G. Reversible ovulatory failure associated with the development of luteinized unruptured follicles in women with inflammatory arthritis taking non-steroidal anti-inflammatory drugs. Br J Rheumatol. 1996;35 (5):458–462. [DOI] [PubMed] [Google Scholar]
- 42. Diao H, Zhu H, Ma H. et al. Rat ovulation, implantation and decidualization are severely compromised by cox-2 inhibitors. Front Biosci. 2007;12:3333–3342. [DOI] [PubMed] [Google Scholar]
- 43. Lim H, Paria B, Das S. et al. Multiple female reproductive failures in cyclooxygenase 2-deficient mice. Cell. 1997;91 (2):197–208. [DOI] [PubMed] [Google Scholar]
- 44. Nakhai-Pour HR, Broy P, Sheehy O, Berard A. Use of nonaspirin nonsteroidal anti-inflammatory drugs during pregnancy and the risk of spontaneous abortion. CMAJ. 2011;183 (15):1713–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Li D, Liu L, Odouli R. Exposure to non-steroidal anti-inflammatory drugs during pregnancy and risk of miscarriage: Population based cohort study. BMJ. 2003;327 (7411):368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Nielsen G, Sorensen H, Larsen H, Pedersen L. Risk of adverse birth outcome and miscarriage in pregnant users of non-steroidal anti-inflammatory drugs: population based observational study and case-control study. BMJ. 2001;322 (7281):266–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Keim S, Klebanoff M. Aspirin use and miscarriage risk. Epidemiology. 2006;17 (4):435–439. [DOI] [PubMed] [Google Scholar]
- 48. El-Talatini MR, Taylor A, Konje J. The relationship between plasma levels of the endocannabinoid, anandamide, sex steroids, and gonadotrophins during the menstrual cycle. Fertil Steril. 2010;93 (6):1989–1996. [DOI] [PubMed] [Google Scholar]
- 49. Trabucco E, Acone G, Marenna A. et al. Endocannabinoid system in first trimester placenta: low FAAH and high cb1 expression characterize spontaneous miscarriage. Placenta. 2009;30(6):516–522. [DOI] [PubMed] [Google Scholar]