Abstract
Internalization of some plasma membrane constituents, bacterial toxins, and viruses occurs via caveolae; however, the factors that regulate caveolar internalization are still unclear. Here, we demonstrate that a brief treatment of cultured cells with natural or synthetic glycosphingolipids (GSLs) or elevation of cholesterol (either by acute treatment with mβ-cyclodextrin/cholesterol or by alteration of growth conditions) dramatically stimulates caveolar endocytosis with little or no effect on other endocytic mechanisms. These treatments also stimulated the movement of GFP-labeled vesicles in cells transfected with caveolin-1-GFP and reduced the number of surface-connected caveolae seen by electron microscopy. In contrast, overexpression of caveolin-1 decreased caveolar uptake, but treatment with GSLs reversed this effect and stimulated caveolar endocytosis. Stimulation of caveolar endocytosis did not occur using ceramide or phosphatidylcholine and was not due to GSL degradation because similar results were obtained using a nonhydrolyzable GSL analog. Stimulated caveolar endocytosis required src kinase and PKC-α activity as shown by i) use of pharmacological inhibitors, ii) expression of kinase inactive src or dominant negative PKCα, and iii) stimulation of src kinase activity upon addition of GSLs or cholesterol. These results suggest that caveolar endocytosis is regulated by a balance of caveolin-1, cholesterol, and GSLs at the plasma membrane.
INTRODUCTION
Caveolae are plasma membrane (PM) specializations that are rich in cholesterol, sphingolipids, and caveolin-1 (Cav1), a cholesterol-binding protein (Smart et al., 1999). By electron microscopy, they appear as flask-shaped structures at the PM and as smooth uncoated vesicles near the PM. Caveolae have been implicated in signaling, endocytosis, transcytosis, and potocytosis (for reviews see Smart et al., 1999; Fielding and Fielding, 2001; Mineo and Anderson, 2001; Carver and Schnitzer, 2003; Parton, 2003). Recently caveolae have gained attention because they have been documented to play a role in the cellular uptake and intracellular delivery of some bacterial toxins, viruses, and bacteria (Lencer et al., 1999; Shin et al., 2000; Norkin, 2001; Pelkmans et al., 2001; Richterova et al., 2001; Duncan et al., 2002; Marjomaki et al., 2002). One of the first markers to be used for caveolar endocytosis was the cholera toxin B subunit (CtxB), which binds to GM1 ganglioside at the PM and is internalized through caveolae in some cell types (Orlandi and Fishman, 1998; Torgersen et al., 2001). SV40 virus has been shown to be internalized from the PM in small Cav1-containing vesicles (Pelkmans et al., 2001), and caveolar endocytosis of derivatized albumin has also been reported (Schnitzer et al., 1994; Shubert et al., 2001; Sharma et al., 2003; Singh et al., 2003). We previously showed that fluorescent glycosphingolipid (GSL) analogs (lactosylceramide [LacCer] and globoside) are selectively internalized via caveolae in human skin fibroblasts (HSFs) and other cell types using pathway-specific inhibitors and colocalization with endocytic markers and DsRed-Cav1 (Puri et al., 2001; Sharma et al., 2003; Singh et al., 2003).
Although the internalization of various markers through caveolae can be demonstrated, the molecular machinery underlying caveolar endocytosis is not fully described and the essential components required for caveolar uptake are unclear. For example, some studies suggest that Cav1 overexpression negatively regulates uptake through caveolae (Minshall et al., 2000; Le et al., 2002), whereas our own studies showed that internalization of BODIPY-LacCer through caveolae is stimulated by increased Cav1 expression (Singh et al., 2003). In addition, the rates of internalization and the subsequent endocytic itinerary of different caveolar markers can vary markedly (e.g., SV40 vs. fluorescent GSLs; Pelkmans et al., 2001, 2002; Sharma et al., 2003; Singh et al., 2003). These observations raise the possibility that there may be multiple forms of caveolar endocytosis (Mineo and Anderson, 2001; Le and Nabi, 2003).
In our previous studies of caveolar endocytosis in HSFs, we found that CtxB internalization was very robust in cells simultaneously labeled with fluorescent LacCer (Puri et al., 2001; Singh et al., 2003), but were surprised to find that its internalization in the absence of LacCer was barely detectable on the same time scale (seconds to minutes at 37°C; unpublished observations). This led us to consider the possibility that the addition of LacCer, and perhaps other GSLs, to the PM might stimulate caveolar uptake. In the current study we test this possibility as well as the more general hypothesis that caveolar endocytosis can be modulated by altering the balance of cholesterol, SLs, and Cav1 at the PM. Our data demonstrate that elevation in cellular cholesterol, or acute treatment of cells with exogenous natural or synthetic GSLs selectively stimulates caveolar endocytosis and does so in a src- and PKC-dependent manner. This study provides insights into the regulation of caveolar endocytosis and also helps reconcile discrepancies in the literature concerning the vastly different rates of caveolar uptake seen for different cargo and the conflicting reports suggesting that Cav1 can act as either a negative regulator (Le et al., 2002) or a stimulator of endocytosis (Singh et al., 2003).
MATERIALS AND METHODS
Lipids, Fluorescent Probes, and Miscellaneous Reagents
BODIPY-LacCer(Martin and Pagano, 1994) and the nondegradable(Albrecht et al., 1995) analog, BODIPY-Lac-S-Cer (Puri et al., 2003), were synthesized as described. Nonfluorescent synthetic lipids, d-lactosyl-β1-1′-N-octanoyl-D-erythro-sphingosine (C8-LacCer), di-C8-phosphatidylcholine, and C8-ceramide were from Avanti Polar Lipids (Alabaster, AL). Bovine lactosylceramide and monosialoganglioside GM1 were from Matreya Inc. (State College, PA). Nonfluorescent lipids were complexed with defatted BSA as described (Martin and Pagano, 1994) or dissolved in DMSO.
AlexaFluor 594 (AF594)-labeled transferrin (Tfn), dextran, and albumin were from Molecular Probes (Eugene, OR). Tyrosine kinase (PP2, PP3, herbimycin, and genistein) and Protein kinase C inhibitors (chelerytherine chloride [CC] and Gö 6976) were from Calbiochem (San Diego, CA). Src activation assay kit was from Upstate (Charlottesville, VA). Unless otherwise indicated, all other reagents were from Sigma Chemical Co. (St. Louis, MO).
Cell Culture and Transfections
Normal HSFs (GM-5659D; Coriell Institute, Camden, NJ) were grown in EMEM with 10% FBS; HeLa cells (American Type Culture Collection, Rockville, MD) were grown in DMEM with 10% FBS. Cholesterol levels were altered by growth in medium containing LPDS and/or an excess of LDL as described (Martin et al., 1993; Puri et al., 1999) or by incubation with a complex of mβ-CD and cholesterol (Christian et al., 1997). Cav1-mRed was generated by fusion of the mRed construct (gift from R.Y. Tsien) to the C terminus of the human Cav1 in a pcDNA 3.1 vector. Cotransfection of this construct with Cav1-GFP resulted in >90% colocalization (unpublished data).
Cells were transfected with DN Eps15-GFP (Benmerah et al., 1999), Cav1-GFP (Pelkmans et al., 2001), or Cav1-mRed or cotransfected with pDsRed2-Nuc and DN PKCα by electroporation. Briefly cells were trypsinized, pelleted, and resuspended in electroporation buffer (128 mM l-glutamine; 2 mM magnesium acetate; 10 μM calcium acetate; 20 mM PIPES, pH 7). DNA (15 μg) was then added to 1-1.5 × 106 cells in 400 μL buffer, incubated for 10 min at 4°C, followed by electroporation using GenePulser Xcell (Bio-Rad Laboratories, Hercules, CA) operating at 400 V and 800 μF. The cells were then diluted and cultured in EMEM including 10% FBS and used in experiments 48-72 h later.
Incubation with Inhibitors
Cells were preincubated in HEPES-buffered MEM (HMEM) containing PP2 (10 nM), PP3 (10 μM), genistein (50 μM), herbimicin (1 μM), CC (2.5 μM), Gö 6976 (10 nM), or Clostridium dificile Toxin B (100 μM) for 1 h at 37°C, or with nystatin (25 μg/ml) for 30 min at 37°C. Inhibitors were present in all subsequent steps of the experiments.
Incubation with Fluorescent Lipids and Proteins
Cells were incubated for 30 min at 10°C with 0.5-2.5 μM BODIPY-LacCer/BSA, washed twice with HMEM, and further incubated for 5 min at 37°C, followed by back-exchange with 5% DF-BSA (6 times for 10 min at 10°C) to remove any fluorescent lipid remaining at the PM after endocytosis (Martin and Pagano, 1994; Chen et al., 1997). For labeled proteins, cells were preincubated with 5 μg/ml AF594-Tfn or 50 μg/ml AF-594 albumin for 30 min at 10°C, further incubated for 5 min at 37°C, and acid-stripped (Singh et al., 2003) to remove labeled protein remaining at the cell surface. For fluid phase uptake, cells were incubated with 1 mg/ml AF594-dextran for 5 min at 37°C without preincubation or acid stripping.
Incubation with C8-LacCer and Other Nonfluorescent Lipids
Cells were incubated with various concentrations of nonfluorescent lipids for 30 min at 10°C followed by internalization for 5 min at 37°C as described for fluorescent lipids and proteins. The exogenous lipids were added from a complex with DF-BSA (Martin and Pagano, 1994) or from DMSO and were present during an initial preincubation step with the fluorescent marker at 10°C as well as during the subsequent incubation at 37°C. In the case of AF594-dextran, cells were pretreated with the exogenous lipid at 10°C, but dextran was only present during the 37°C incubation.
Adenoviral Infection
Cells were infected with Ad-Cav1, Ad-KI-src, Ad-DynK44A, or “empty virus” (Ad-empty) in culture medium at the indicated multiplicity of infection (MOI). Infection was allowed to proceed for 4 h, followed by washing with fresh medium and further incubation for 24 h before use. The authenticity of Ad-KI-src and in vitro studies of src kinase were determined in cell lysates using a commercial kit (Upstate Biotechnology, Inc., Lake Placid, NY) to measure phosphorylation of model src peptide substrate.
Microscopy
Fluorescence microscopy was carried out using an Olympus IX70 fluorescence microscope and the “Metamorph” image-processing program (Universal Imaging Corp., Downingtown, PA). In any given experiment, all photomicrographs were exposed and processed identically for a given fluorophore. Intracellular fluorescence was quantified by analyzing images of 10 or more cells in at least three independent experiments.
Samples were prepared for electron microscopy as described using Ruthenium Red to identify caveolae connected to the cell surface exactly as described previously (Parton et al., 2002). The number of caveolae, defined as uncoated surface invaginations of between 50 and 100 nm in diameter, and coated pits per cell profile were counted in a double-blind manner with a code that was only broken after the final quantitation was completed.
RESULTS
Cholesterol Stimulates Endocytosis of Caveolar Markers
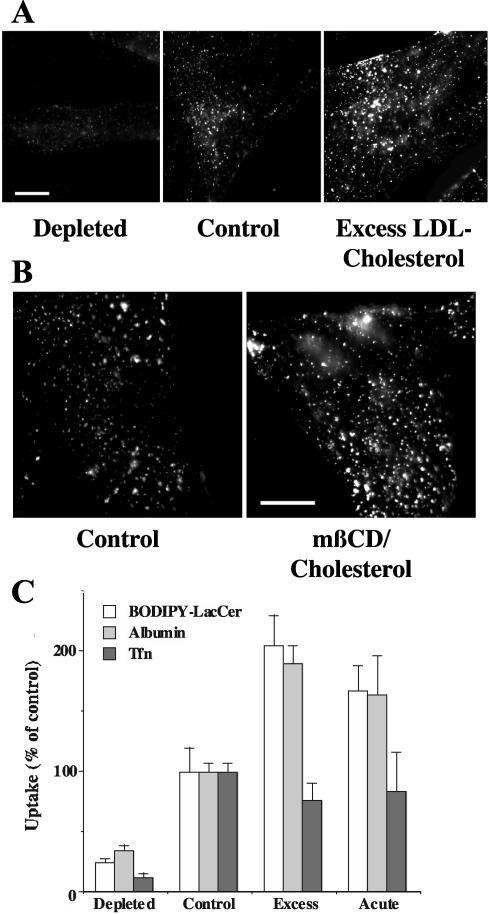
HSFs were cultured under various conditions to modulate cellular cholesterol levels (Martin et al., 1993; Puri et al., 1999) and subsequently used to study the initial internalization of BODIPY-LacCer, a marker for caveolar endocytosis (Puri et al., 2001; Sharma et al., 2003; Singh et al., 2003). Growth in lipoprotein deficient serum (LPDS) for 3 days resulted in approximately a 40% reduction in cellular cholesterol and inhibited the uptake of LacCer by 75-80%, relative to control cells grown under normal conditions (Figure 1, A and C). In contrast, when cells were grown in the presence of an excess of LDL, cellular-free cholesterol was increased twofold relative to control cells (Martin et al., 1993; Puri et al., 1999, 2003), resulting in about a twofold stimulation of LacCer internalization. Cholesterol also regulated the internalization of albumin (Figure 1C), another marker for caveolar endocytosis in some cell types (Minshall et al., 2000; Shubert et al., 2001; Sharma et al., 2003; Singh et al., 2003), in a similar manner to that seen using BODIPY-LacCer. However, excess cholesterol did not stimulate the uptake of fluorescent Tfn, a marker for clathrin-mediated endocytosis (Figure 1C).
Figure 1.
Cholesterol positively regulates the uptake of caveolar markers. HSFs were (A) grown under various conditions to obtain cells with low (“depleted”), normal (“control”), or elevated (“excess”) levels of cholesterol or (B) were briefly incubated at 10°C ± mβ-CD/cholesterol to increase PM cholesterol (see MATERIALS AND METHODS). The cells were then incubated with various concentrations (e.g., 5, 2, or 0.6 μM in A) of BODIPY-LacCer for 30 min at 10°C to achieve “equal labeling” of the PM. Samples were then incubated for 5 min at 37°C to initiate endocytosis, chilled to 10°C, and back-exchanged with DF-BSA to remove any fluorescent lipid remaining at the PM (see MATERIALS AND METHODS), and observed under the fluorescence microscope. Bars, 10 μm. (C) HSFs were pulse-labeled with BODIPY-LacCer as in A and B, or with AF594 albumin or AF594-Tfn, and endocytosis was quantified by image analysis. The concentrations of fluorescent albumin or Tfn to achieve “equal loading” of the PM were determined empirically by image analysis. After a subsequent endocytic pulse (5 min at 37°C), noninternalized fluorescence was removed by acid stripping before image analysis to quantify uptake. All values are normalized to untreated control samples. At least 15 cells were quantified for each condition in three or more independent experiments.
In addition to altering cell growth conditions, we also elevated cellular cholesterol in an acute manner by incubating HSFs with a complex of methyl-β-cyclodextrin (mβ-CD) and cholesterol (Christian et al., 1997). After this treatment we stained the cells with filipin, a polyene antibiotic that fluoresces when bound to cholesterol (Neufeld et al., 1999) and found an approximately 25% increase in PM fluorescence (unpublished data). Treatment of cells with mβ-CD/cholesterol stimulated the internalization of BODIPY-LacCer and AF549 albumin, but had little or no effect on Tfn uptake (Figure 1, B and C). Together these results demonstrate that increasing cellular cholesterol by two independent methods results in a stimulation of the internalization of caveolar markers with little or no effect on the uptake of a marker for clathrin-mediated endocytosis.
GSLs Stimulate Endocytosis of Caveolar Markers
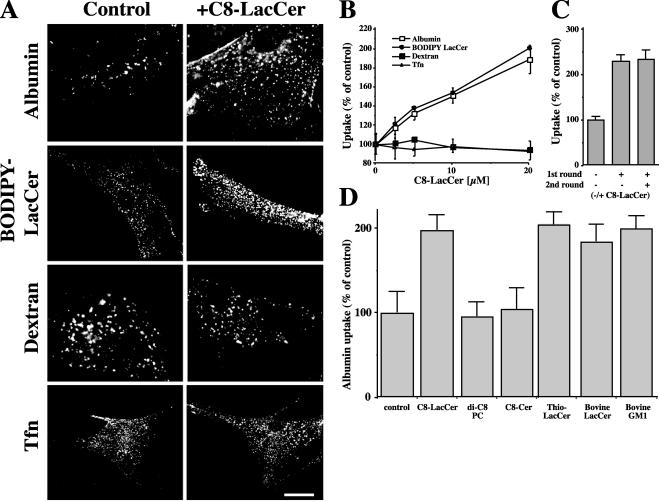
To examine the effect of exogenous GSLs on endocytosis, HSFs were coincubated with a nonfluorescent, short-chain analog of LacCer (C8-LacCer) and various endocytic markers (see MATERIALS AND METHODS). As seen in Figure 2A, 20 μM C8-LacCer markedly stimulated the endocytosis of the caveolar markers, BODIPY-LacCer, and labeled albumin as well as CtxB (unpublished data), but had no effect on the uptake of Tfn, a marker for the clathrin pathway, or on the uptake of a fluid phase marker, fluorescent dextran. There was approximately a linear relationship between the concentration of C8-LacCer (0-20 μM) used and the internalization of BODIPY-LacCer or AF594-albumin, with no obvious saturation effect (Figure 2B); higher concentrations of C8-LacCer were not used because they reduced cell viability. Endocytosis of LacCer or albumin was restored to basal levels when C8-LacCer was removed from the PM by back-exchange (unpublished data). We also found that a second incubation with C8-LacCer (added immediately after the first round of stimulated endocytosis) did not further stimulate caveolar endocytosis above the level seen after the initial incubation with this lipid (Figure 2C). The stimulation of uptake by C8-LacCer was not due to its degradation as shown by lipid extraction and analysis (unpublished data). Furthermore, similar results were obtained using a nondegradable thio analog of LacCer (Albrecht et al., 1995; Puri et al., 2003; Figure 2D). We also screened a number of other lipids to learn whether they could also stimulate caveolar endocytosis (Figure 2D). Treatment of HSFs with naturally occurring LacCer or GM1 ganglioside increased the internalization of fluorescent albumin to a similar extent as that seen using C8-LacCer. Interestingly, short-chain analogs of ceramide or phosphatidylcholine had no effect on this uptake.
Figure 2.
Pretreatment of HSFs with C8-LacCer stimulates the uptake of fluorescent albumin or BODIPY-LacCer, but not fluorescent dextran or Tfn. (A) HSFs were coincubated with 20 μM C8-LacCer/BSA or BSA alone (Control) and the indicated fluorescent marker for 30 min at 10°C, washed, and further incubated for 5 min at 37°C (see MATERIALS AND METHODS). Samples were then cooled to 10°C and back-exchanged (for BODIPY-LacCer) or acid-stripped before fluorescence microscopy. Bar, 10 μm. (B) Experiments were performed as in A except that the concentration of C8-LacCer was varied. Internalization of indicated marker after 5 min at 37°C was quantified by image analysis. Values are expressed as % relative to control cells that were not treated with C8-LacCer. (C) Cells were incubated through a first round with 1 μM BODIPY-LacCer without (-) or with (+) 20 μM C8-LacCer for 30 min at 4°C followed by 5 min at 37°C as in A. Samples were then rinsed and incubated through a second round (30 min at 4°C followed by 5 min at 37°C) with or without 20 μM C8-LacCer. For incubations without C8-LacCer, cells were incubated with vehicle alone (i.e., 20 μM BSA). (D) Stimulation of albumin uptake by C8-LacCer vs. other lipids. HSFs were coincubated as in A with the indicated natural or synthetic lipid (20 μM) and the internalization of AF594-albumin evaluated after 5 min at 37°C. Results in B and C are expressed as % internalization relative to control cells. Values are mean ± SD (20 cells for each experimental condition; 3 independent experiments).
Exogenous GSLs or Cholesterol Do Not Induce Endocytosis by an Alternate Mechanism
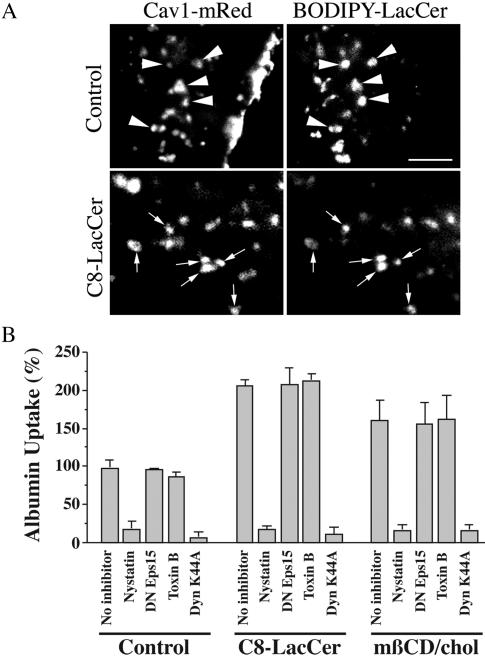
In a previous study we showed that BODIPY-LacCer internalization was Cav1-dependent in multiple cell types and colocalized with DsRed-Cav1 after a short period of endocytosis (Puri et al., 2001; Sharma et al., 2003; Singh et al., 2003). To determine whether exogenous GSLs or cholesterol altered the mechanism of internalization of caveolar markers such that they became internalized by an alternate endocytic mechanism, we studied the localization of BODIPY-LacCer in HSFs that were expressing Cav1-mRed. Extensive colocalization (80-95%) of BODIPY-LacCer with Cav1-mRed was seen in punctate structures at short times of endocytosis (e.g., 30 s at 37°C), both in the absence and presence of C8-LacCer (Figure 3A). Interestingly, this colocalization was reduced to 10-20% at a 2-min time point (unpublished data), suggesting that BODIPY-LacCer rapidly exits the Cav1-positive compartment. This result is in contrast to our previous study in which ∼90% overlap was seen between BODIPY-LacCer and DsRed-Cav1 at the 2-min time point in rat fibroblasts (Singh et al., 2003). This difference could be due to the reduced tendency of mRed to oligomerize (Campbell et al., 2002), to the position of DsRed (N-terminus) vs. mRed (C-terminus) relative to Cav1, or to the different cell types studied. Further evidence that treatment with exogenous GSLs did not alter the mechanism of endocytosis was provided by Cav1 RNAi studies. BODIPY-LacCer and albumin internalization were each inhibited 80-90% by Cav1 RNAi under both stimulated and unstimulated conditions in HSFs (unpublished data).
Figure 3.
Pretreatment with C8-LacCer or mβ-CD/cholesterol does not alter the mechanism of albumin internalization in HSFs. (A) HSFs were transfected with Cav1-mRed and incubated for 30 min at 10°C without (control) or with C8-LacCer in the presence of 1 μM BODIPY-LacCer. The cells were then rinsed and further incubated for 30 s at 37°C, cooled to 10°C, and back-exchanged with defatted BSA as in Figure 1 to remove any BODIPY-LacCer that was not internalized. Cav1-mRed and BODIPY-LacCer were then imaged in red and green channels, respectively. Bar, 2 μm. (B) Cells were untreated (no inhibitor) or treated with C8-LacCer/BSA or mβ-CD/cholesterol as in Figures 1 and 2, and the uptake of AF594-albumin was quantified after 5 min of internalization at 37°C. To assess the mechanism of internalization, cells were pretreated with nystatin, Clostridium dificile Toxin B, transfected with DN Eps15-GFP, or infected with Ad-DynK44A. Data are expressed relative to the value obtained in the absence of inhibitors for the untreated Control sample. Note that pretreatment with C8-LacCer or mβ-CD/cholesterol stimulated albumin uptake, but did not affect the inhibition (or lack of inhibition) by the various inhibitors. Values are mean ± SD (10 cells for each experimental condition; 5 independent experiments).
We also investigated the effect of various inhibitors that block different mechanisms of endocytosis on the stimulated uptake (Figure 3B). In control cells that were not coincubated with C8-LacCer, albumin uptake was dynamin- and nystatin-sensitive, but was not affected by dominant negative (DN) Eps15 (blocks clathrin internalization; Benmerah et al., 1999) or by Clostridium dificile toxin B (Toxin B) (broad range Rho GTPase inhibitor that inhibits fluid phase endocytosis and phagocytosis; Aktories et al., 2000; Sabharanjak et al., 2002). Virtually identical results were obtained when C8-LacCer was coincubated with albumin, except that the absolute amount of albumin uptake (in the absence of inhibitors) was stimulated about twofold. Namely, the stimulated uptake of albumin continued to be dynamin- and nystatin-sensitive, but was unaffected by DN Eps15 or Toxin B. Control experiments were carried out to verify that DN Eps15 inhibited internalization of AF549-Tfn and that Toxin B inhibited fluorescent dextran uptake under the incubation conditions used (unpublished data and Sharma et al., 2003; Singh et al., 2003). Similar results to those with C8-LacCer were also obtained in cells treated with mβ-CD/cholesterol, except that the stimulation of albumin uptake by cholesterol (in the absence of inhibitors) was somewhat less than that seen using C8-LacCer (Figure 3B). BODIPY-LacCer internalization exhibited the same sensitivities to inhibitors as AF594 albumin, both with and without stimulation (unpublished data).
Thus under both unstimulated and C8-LacCer- or cholesterol-stimulated conditions, i) BODIPY-LacCer colocalized with fluorescent protein tagged Cav1, and ii) AF594 albumin and BODIPY-LacCer showed the same spectrum of sensitivities to various endocytic inhibitors, similar to that observed in our previous studies of BODIPY-LacCer in unstimulated cells and consistent with uptake via caveolae.
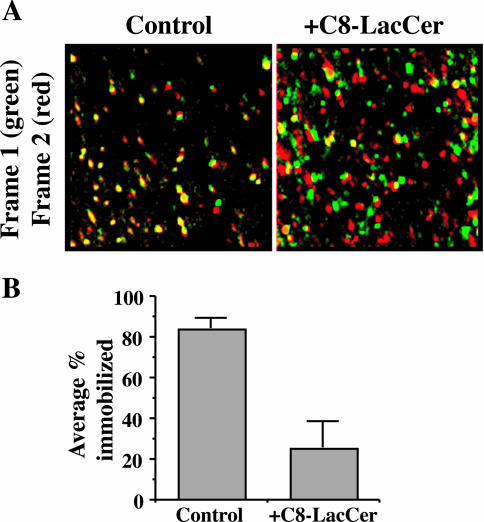
Exogenous GSLs Stimulate Movement of Cav1-GFP
GFP-tagged Cav1 has previously been shown to be relatively immobile at the PM of cells, suggesting that Cav1 is trapped and immobilized at stable, membrane-associated caveolae (Thomsen et al., 2002). In the present study, we transfected HSFs with Cav1-GFP and acquired images at or near the cell surface every second. In the absence of C8-LacCer treatment, movement of the Cav1-GFP was relatively slow and many of the fluorescent puncta appeared immobile during the time course of the observations (∼1 min). In striking contrast, Cav1-GFP puncta became highly mobile upon stimulation with C8-LacCer, and many of the fluorescent puncta disappeared from the plane of focus, suggesting they had been internalized from the PM. This movement was analyzed in several different ways and is shown in Figure 4 and in Supplemental Data. Similar results were obtained when cells were treated with mβ-CD/cholesterol (unpublished data).
Figure 4.
C8-LacCer stimulates the movement of Cav1-GFP. HSFs were transfected with Cav1-GFP and incubated for 30 min at 10°C without (Control) or with C8-LacCer. The samples were then warmed to 37°C and fluorescence images acquired every second for 1 min. See Supplemental Data. (A) Sequential frames were rendered in pseudocolor (frame 1, green; frame 2, red) and then merged. Yellow puncta indicate immobile structures, whereas green and red puncta indicate Cav1-GFP that has moved between frames. Note the high fraction of yellow puncta in the Control cells relative to the +C8-LacCer-treated sample. (B) Successive frames were examined as in A and the % of fluorescent puncta in the overlay images which were immobile (i.e., yellow) was quantified.
Effect of Cav1 Overexpression and Exogenous GSLs on Caveolar Endocytosis
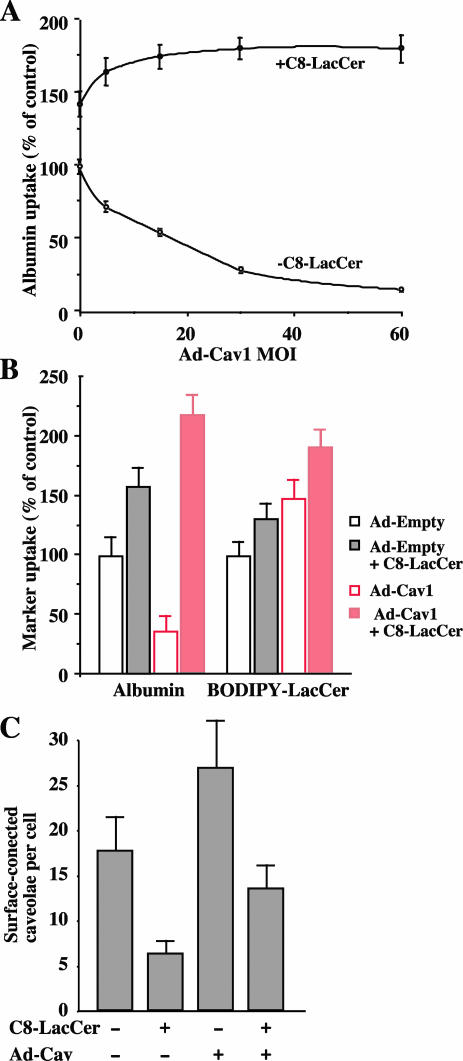
To examine the effect of Cav1 expression on caveolar endocytosis, we used HeLa cells, which have lower levels of Cav1 than HSFs (Torgersen et al., 2001; Singh et al., 2003) and fewer caveolae at the PM (unpublished data). Cells were infected with Ad-Cav1 at various MOI for 24 h and then uptake of AF594 albumin (5 min at 37°C) was quantified. With increasing MOI, albumin uptake gradually decreased (Figure 5A), consistent with previous studies showing that Cav1 is a negative regulator of endocytosis (Le et al., 2002; Le and Nabi, 2003). Interestingly this effect was reversed and the uptake of albumin was significantly stimulated above control values when cells were incubated in the presence of C8-LacCer (Figure 5A). In contrast, when BODIPY-LacCer was used as a marker for caveolar internalization, we found that infection with Ad-Cav stimulated uptake of the fluorescent marker (Figure 5B). This stimulation was further enhanced when C8-LacCer was also present during the incubations (Figure 5B). We also performed a quantitative double-blind analysis of surface caveolae levels by electron microscopy under several of the conditions noted above (Figure 5C). As expected, infection with Ad-Cav1 caused an increase in density of caveolae at the cell surface. Interestingly, treatment with C8-LacCer decreased the number of surface-connected caveolae regardless of whether the levels of Cav1 were increased by Ad-Cav1 viral infection (Figure 5C). In the same cells, there was no significant difference in clathrin-coated pit density under all the conditions tested (unpublished data). On the basis of these results, we suggest that in HeLa cells, increasing Cav1 by itself does not stimulate caveolar endocytosis (i.e., reduce the number of surface connected caveolae) unless additional GSLs are also added to the PM.
Figure 5.
Cav1 overexpression attenutates caveolar endocytosis in HeLa cells, but this effect is overcome by treatment with C8-LacCer. (A) Cells were infected with Ad-Cav1 at various MOI for 24 h. The cells were then incubated with AF594 albumin without (○) or with C8-LacCer/BSA (•) for 5 min at 37°C as in Figure 2, and the uptake was quantified by image analysis. All values are relative to the uptake obtained in the absence of C8-LacCer treatment using cells that were not infected with Ad-Cav1. (B) Cells were incubated with Ad-Empty or Ad-Cav1 virus at an MOI of 30 for 24 h and then incubated with AF594 albumin or BODIPY-LacCer as in Figure 2. Values for each marker are quantified relative to control values obtained using cells infected with Ad-empty virus. Values are mean ± SD (≥10 cells for each experimental condition; 3 independent experiments). (C) Cells were infected with Ad-Empty or Ad-Cav1, followed by incubations with BSA or C8-LacCer/BSA at 10°C. Samples were then warmed for 5 min at 37°C, fixed, stained with Ruthenium Red, and embedded for electron microscopy. Cells were sectioned parallel to the substratum through the nucleus and 20 random profiles from different cells were examined for each treatment in a blinded study. The number of surface connected caveolae were quantified and plotted for each experimental condition (see MATERIALS AND METHODS).
Src Kinase and PKCα Are Required for GSL or Cholesterol Stimulation of Caveolar Endocytosis
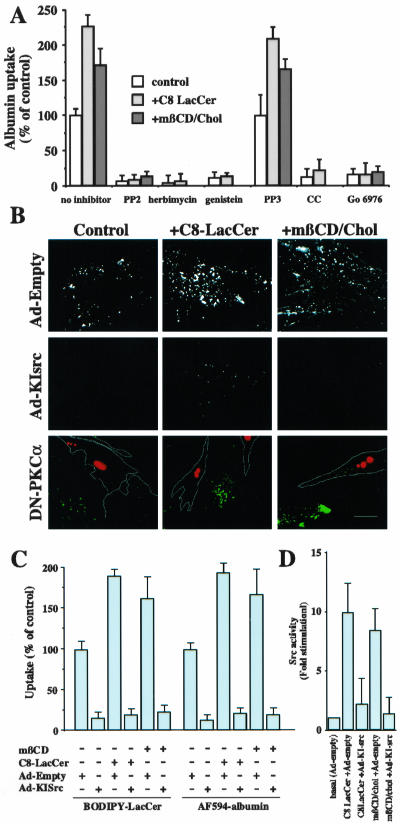
To obtain further information on the mechanism by which LacCer stimulates caveolar endocytosis, we examined the effect of several inhibitors of src kinase and PKCα, two kinases that have been shown to be involved in the regulated internalization through caveolae (Smart et al., 1995; Mineo et al., 1999; Mineo and Anderson, 2001). As shown in Figure 6A, two src kinase inhibitors, herbimycin and PP2, blocked the endocytosis of fluorescent albumin in intact cells, with or without stimulation by C8-LacCer or mβ-CD/cholesterol, whereas PP3, a negative control for PP2, had no effect on albumin uptake. Genistein, a more general tyrosine kinase inhibitor, also inhibited both the stimulated and unstimulated internalization of albumin.
Figure 6.
C8-LacCer or mβ-CD/cholesterol stimulation of endocytosis in human skin fibroblasts is dependent on src and PKC-α. (A) Uptake of AF594 albumin (5 min at 37°C) was assessed in the absence or presence of the src kinase inhibitors, PP2 (PP3 is a negative control for PP2), herbimycin, or genestein, or the PKC inhibitors, chelerytherine chloride (CC) or Gö 6976. Values are expressed as % of uptake in untreated control cells. (B) Inhibition of stimulated endocytosis by Ad-KI-Src and DN-PKC-α. Cells were infected with Ad-empty or Ad-KI-src for 24 h or cotransfected with DsRed-Nuc and DN-PKC-α (cells outlined in white) for 24 h. Samples were subsequently incubated with or without C8-LacCer or mβ-CD/cholesterol and AF594-albumin for 5 min at 37°C as in Figure 2. Bar, 10 μm. (C) Quantitation of inhibition of stimulated endocytosis by Ad-KI-Src. Cells were infected with Ad-KI src or Ad-empty for 24 h and subsequently treated with C8-LacCer or mβCD/cholesterol (see Figures 1 and 2). Internalization of AF594-albumin or BODIPY-LacCer internalization (5 min at 37°C) was then quantified by image analysis. (D) C8-LacCer and cholesterol stimulate src kinase activity. Cells were infected with Ad-KI src or Ad-empty and subsequently treated with C8-LacCer or mβCD/Cholesterol as C. Cells were then lysed and src kinase activity was quantified by measuring the phosphorylation of a src peptide substrate (see MATERIALS AND METHODS). In A and C, values are the means ± SD (≥10 cells per experimental condition; 3 independent experiments). In D, values are means ± SD, expressed as fold stimulation over basal measurements obtained for Ad-empty infected cells without C8-LacCer or cholesterol treatment.
We also examined the stimulated endocytosis of fluorescent albumin or BODIPY-LacCer in HSFs which were infected with an adenovirus expressing a Kinase Inactive src (Ad-KI-src) and found that both the unstimulated and the C8-LacCer- or mβ-CD/cholesterol-stimulated uptake of AF594 albumin (Figure 6, B and C) and of BODIPY-LacCer (Figure 6C) were inhibited. Interestingly, when src kinase activity was measured in cell lysates by measuring the phosphorylation of a src kinase peptide substrate, there was ∼10-fold increase in src activity using cells pretreated with C8-LacCer and ∼8-fold increase in src activity using cells pretreated with mβ-CD/cholesterol relative to untreated controls (Figure 6D). Little stimulation was seen in control samples in which C8-LacCer was added to the cell lysate rather than to intact cells, or in cell lysates after infection with Ad-KI-src (unpublished data).
Similarly, chelerytherine chloride (CC) and Gö 6976, general and specific inhibitors of PKC, inhibited albumin internalization, with or without C8-LacCer or mβ-CD/cholesterol treatment (Figure 6A). In addition, we transfected cells with DN PKCα and found that AF594 albumin internalization was inhibited in the transfected cells and that this inhibition was not affected by treatment with C8-LacCer or mβ-CD/cholesterol (Figure 6B). Together, these results demonstrate a requirement for both src kinase and PKCα in the stimulation of caveolar uptake by C8-LacCer or mβ-CD/cholesterol and show that src activity is greatly increased by the addition of these lipids.
DISCUSSION
In this study we examined caveolar endocytosis in cultured fibroblasts and show that internalization through caveolae is regulated by three essential components: cholesterol, GSLs, and Cav1. Acute treatment of cells with exogenous GSLs (natural or synthetic) or elevation of cellular cholesterol dramatically and selectively stimulated caveolar endocytosis without affecting other mechanisms of internalization. Consistent with the selective stimulation of caveolar uptake, treatment with exogenous GSLs or cholesterol markedly accelerated the movement of Cav1-GFP in living cells and decreased the number of surface connected caveolae seen by electron microscopy. No stimulation was observed when cells were treated with several other classes of lipids, suggesting this phenomenon is restricted to GSLs and cholesterol. These findings provide new insights into the regulation of caveolar endocytosis and are discussed below.
Regulation of Caveolar Endocytosis by Cholesterol, Cav1, and GSLs
Results from the current study suggest that a balance among cholesterol, GSLs, and Cav1 levels is important in regulating caveolar endocytosis. i) In the case of cholesterol, we found that endocytosis of two caveolar markers (labeled albumin or fluorescent LacCer) was increased when cells were grown under conditions that elevate free cholesterol by about twofold (Martin et al., 1993; Puri et al., 1999, 2003) or when PM cholesterol was increased acutely by incubation with mβ-CD/cholesterol (Figure 1). No such effect was seen using fluorescent Tfn, a marker for clathrin-mediated endocytosis. In contrast, and as shown previously by others, cholesterol depletion reduced the number of caveolae at the PM and reduced the internalization of caveolar markers (Figure 1 and Hailstones et al., 1998; Dreja et al., 2002). ii) In the case of GSLs, we found that an acute treatment of cells with natural LacCer or GM1 ganglioside, or with synthetic short-chain C8-LacCer selectively and dramatically stimulated the uptake of fluorescent albumin and BODIPY-LacCer, but did not affect internalization of markers internalized by fluid phase or clathrin-mediated endocytosis (Figure 2). Consistent with a stimulation of caveolar endocytosis, we observed that treatment of HSFs with C8-LacCer enhanced the movement of Cav1-GFP-labeled vesicles at or near the PM, relative to untreated cells in which such vesicles were relatively immobile (Figure 4 and Supplemental Data). We also found that incubation of HeLa cells with C8-LacCer reduced the number of surface connected caveolae seen by electron microscopy (Figure 5C), presumably reflecting an increase in internalization of vesicles derived from caveolae. This result may explain our inability to stimulate a second round of caveolar uptake by the immediate addition of a second bolus of C8-LacCer (Figure 2C) because the number of caveolae at the PM available for internalization is reduced by the first treatment with LacCer. iii) In the case of Cav1, we found that increasing Cav1 levels (by infection with Ad-Cav1) decreased caveolar uptake of albumin in HeLa cells, consistent with the findings of others who have shown that overexpression of Cav1 acts as a negative regulator of caveolar uptake (Le et al., 2002; Le and Nabi, 2003). Interestingly, incubation with C8-LacCer (Figure 5A) reversed this effect and actually further stimulated caveolar internalization (Figure 5A).
Our finding that BODIPY-LacCer is internalized through caveolae within seconds (Puri et al., 2001; Sharma et al., 2003; Singh et al., 2003) seems to be in contradiction to observations using other markers for caveolar endocytosis that are internalized very slowly through caveolae (e.g., SV40 virus, folate receptor; Mineo and Anderson, 2001; Pelkmans et al., 2001). However, this difference in internalization rates could be readily explained if BODIPY-LacCer, a GSL analog, stimulates its own internalization. Indeed this seems to be the case from the data in Figure 5B, where it was shown that infection of HeLa cells with Ad-Cav1 inhibited albumin internalization but stimulated the uptake of BODIPY-LacCer (Figure 5B). Furthermore, both albumin and BODIPY-LacCer uptake were increased in Ad-Cav1-infected HeLa cells when these markers were incubated with C8-LacCer (Figure 5B). This is also consistent with the stimulation of CtxB uptake seen in the presence of BODIPY-LacCer (unpublished observations). Thus, it seems likely that BODIPY-LacCer stimulates caveolar endocytosis in a manner analogous to C8-LacCer.
Another important point concerns the itineraries of BODIPY-LacCer and SV40 virus after their internalization from the PM. Although both agents are taken up through caveolae (i.e., they have similar sensitivities to cholesterol, dynamin, and src kinase, and both initially colocalize with Cav1), their subsequent fates are very different. Namely, BODIPY-LacCer is rapidly transported to EEA1-positive early endosomes (Sharma et al., 2003) and then moves to the Golgi apparatus in a rab7- and rab9-dependent manner (Choudhury et al., 2002). In contrast, SV40 virus is not associated with early endosomes, but rather is delivered to the endoplasmic reticulum from caveosomes (Pelkmans et al., 2001). One potential explanation for this discrepancy is that BODIPY-LacCer initially enters the caveosome but is rapidly sorted to early endosomes in a process that is stimulated by cholesterol or GSLs. This possibility could be examined in the future by simultaneously monitoring the transport of labeled virus and BODIPY-LacCer in the same cell over time.
Models for Stimulation of Caveolar Endocytosis by Exogenous GSLs or Cholesterol
Stimulation of caveolar endocytosis by cholesterol or exogenous GSLs required src kinase and PKCα activity. This was shown by use of (i) pharmacological inhibitors and (ii) expression of a kinase inactive src or DN PKCα (Figure 6). These results are consistent with the literature showing that one or more tyrosine kinases play roles in caveolar endocytosis or other aspects of caveolae function (Mineo et al., 1999; Minshall et al., 2000; Li et al., 2001; Mineo and Anderson, 2001; Wang et al., 2002). Importantly, in the current study we found that GSLs or cholesterol significantly stimulated src kinase activity, suggesting that stimulation of caveolar endocytosis by these lipids occurs via activation of src kinase. A number of previous studies have shown that long-term addition of exogenous GSLs or overexpression of GSL synthases can increase src activity (Li et al., 2001; Duchemin et al., 2002; Wang et al., 2002); however, effects on caveolar endocytosis were not assessed in those studies.
A major question posed by the current study concerns the mechanism by which addition of GSLs to the PM initiates a signaling process that stimulates caveolar uptake. We propose two alternate models that might be tested in future studies. In both of these, it is assumed that the exogenous GSLs are present only in the external leaflet of the PM bilayer. It is also assumed that transbilayer movement of these GSLs does not occur and that their degradation is not required for triggering uptake by caveolae. The former assumption is based on studies with a fluorescent LacCer analog showing that it requires endocytosis for internalization (Puri et al., 2001) and that it can be completely removed from the cell surface by back-exchange with BSA (unpublished data). The latter assumption is supported by our observation that a nonhydrolyzable thio-LacCer analog (Albrecht et al., 1995; Puri et al., 2003) also stimulates caveolar uptake of albumin.
In the first model we propose that addition of GSLs to the outer leaflet of the PM may cause a specific interaction of the GSL with a particular PM protein. This in turn could initiate a cascade of signaling events resulting in stimulation of caveolar endocytosis. In this model the GSL could interact with surface proteins either as a ligand or as a modifier of the protein environment. The data in Figure 2C demonstrate that with a second addition of C8-LacCer no further stimulation of caveolar uptake is observed. This data is consistent with a model in which the C8-LacCer exerts an effect on a specific membrane protein(s).
An alternative and more complicated scenario is that addition of GSLs to the outer leaflet of the PM bilayer could induce changes in the distribution and organization of lipids in both leaflets of the PM. Such a reorganization (e.g., clustering) of the lipids could induce a redistribution of lipidmodified signaling proteins on the inner leaflet of the PM. Coupling of the inner and outer leaflets of the lipid bilayer has been documented in artificial membranes (Edidin, 2003) and this phenomenon is also thought to occur at the PM of living cells, based on the localization of various lipid anchored proteins at the PM (Prior et al., 2001; Zacharias et al., 2002). If such a coupling occurs, changes in inner leaflet protein localization could lead to the activation of kinases (e.g., src or PKC) at the PM and initiate caveolar endocytosis. Indeed, recently it has been demonstrated that rac distribution is dependent on cholesterol/GSL domains at the PM (del Pozo et al., 2004).
In summary, our results may have important implications at both a basic and applied level. First, the observation that cells respond to increases in PM GSLs and cholesterol by inducing selective endocytosis of these constituents through caveolae suggests that cells are able to monitor the PM lipid composition and adjust it by regulating endocytosis. Second, caveolae have been proposed as potential sites for selective delivery of pharmaceuticals (e.g., to vascular endothelial cells, or lung alveolar cells; Carver and Schnitzer, 2003; Gumbleton et al., 2003). The regulation of caveolar internalization by modifying GSLs or cholesterol may thus be useful in further developing such strategies.
Supplementary Material
Acknowledgments
We thank the following for providing the indicated constructs or viruses: A. Benmerah and A. Dautry-Varsat (Eps15-GFP); L. Pelkmans and A. Helenius (Cav1-GFP); S.A. Trushin (DN PKCα); J.-I. Abe and C. Yan (Ad-KI-src); J. Pessin (Ad-Dyn1 K44A); and R.Y. Tsien (mRed). This work was supported by US Public Health Service Grants GM-22942 and GM-60934 to R.E.P., HL-65191 to R.D.S., and a grant from the Australian National Health Medical Research Council to R.G.P. D.K.S. was supported by a Mayo Kendall Fellowship and A.C. by a fellowship from the National Niemann Pick Disease Foundation. R.D.S. is an Established Investigator of the American Heart Association.
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E04-03-0189. Article and publication date are available at www.molbiolcell.org/cgi/doi/10.1091/mbc.E04-03-0189.
Online version of this article contains supporting material. Online version is available at www.molbiolcell.org.
References
- Aktories, K., Schmidt, G., and Just, I. (2000). Rho GTPases as targets of bacterial protein toxins. Biol. Chem. 381, 421-426. [DOI] [PubMed] [Google Scholar]
- Albrecht, B., Putz, U., and Schwarzmann, G. (1995). Synthesis of fluorescent and radioactive analogues of two lactosylceramides and glucosylceramide containing b-thioglycosidic bonds that are resistant to enzymatic degradation. Carbohydrate Res. 276, 289-308. [DOI] [PubMed] [Google Scholar]
- Benmerah, A., Bayrou, M., Cerf-Bensussan, N., and Dautry-Varsat, A. (1999). Inhibition of clathrin-coated pit assembly by an Eps15 mutant. J. Cell Sci. 112, 1303-1311. [DOI] [PubMed] [Google Scholar]
- Campbell, R.E., Tour, O., Palmer, A.E., Steinbach, P.A., Baird, G.S., Zacharias, D.A., and Tsien, R.Y. (2002). A monomeric red fluorescent protein. Proc. Natl. Acad. Sci. USA 99, 7877-7882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carver, L.A., and Schnitzer, J.E. (2003). Caveolae: mining little caves for new cancer targets. Nat. Rev. Cancer 3, 571-581. [DOI] [PubMed] [Google Scholar]
- Chen, C.S., Martin, O.C., and Pagano, R.E. (1997). Changes in the spectral properties of a plasma membrane lipid analog during the first seconds of endocytosis in living cells. Biophys. J. 72, 37-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhury, A., Dominguez, M., Puri, V., Sharma, D.K., Narita, K., Wheatley, C.W., Marks, D.L., and Pagano, R.E. (2002). Rab proteins mediate Golgi transport of caveola-internalized glycosphingolipids and correct lipid trafficking in Niemann-Pick C cells. J. Clin. Invest. 109, 1541-1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian, A.E., Haynes, M.P., Phillips, M.C., and Rothblat, G.H. (1997). Use of cyclodextrins for manipulating cellular cholesterol content. J. Lipid Res. 38, 2264-2272. [PubMed] [Google Scholar]
- del Pozo, M.A., Alderson, N.B., Klosses, W.B., Chiang, H.-H., Anderson, R.G.W., and Schwartz, M.A. (2004). Integrins regulate Rac targeting by internalization of membrane domains. Science 303, 839-842. [DOI] [PubMed] [Google Scholar]
- Dreja, K., Voldstedlund, M., Vinten, J., Tranum-Jensen, J., Hellstrand, P., and Sward, K. (2002). Cholesterol depletion disrupts caveolae and differentially impairs agonist-induced arterial contraction. Arterioscler. Thromb. Vasc. Biol. 22, 1267-1272. [DOI] [PubMed] [Google Scholar]
- Duchemin, A.M., Ren, Q., Mo, L., Neff, N.H., and Hadjiconstantinou, M. (2002). GM1 ganglioside induces phosphorylation and activation of Trk and Erk in brain. J. Neurochem. 81, 696-707. [DOI] [PubMed] [Google Scholar]
- Duncan, M.J., Shin, J.S., and Abraham, S.N. (2002). Microbial entry through caveolae: variations on a theme. Cell. Microbiol. 4, 783-791. [DOI] [PubMed] [Google Scholar]
- Edidin, M. (2003). The state of lipids rafts: From model membranes to cells. Annu. Rev. Biophys. Biomolec. Struct. 32, 257-283. [DOI] [PubMed] [Google Scholar]
- Fielding, C.J., and Fielding, P.E. (2001). Caveolae and intracellular trafficking of cholesterol. Adv. Drug Deliv. Rev. 49, 251-264. [DOI] [PubMed] [Google Scholar]
- Gumbleton, M., Hollins, A.J., Omidi, Y., Campbell, L., and Taylor, G. (2003). Targeting caveolae for vesicular drug transport. J. Control Release 87, 139-151. [DOI] [PubMed] [Google Scholar]
- Hailstones, D., Sleer, L.S., Parton, R.G., and Stanley, K.K. (1998). Regulation of caveolin and caveolae by cholesterol in MDCK cells. J. Lipid Res. 39, 369-379. [PubMed] [Google Scholar]
- Le, P.U., Guay, G., Altschuler, Y., and Nabi, I.R. (2002). Caveolin-1 is a negative regulator of caveolae-mediated endocytosis to the endoplasmic reticulum. J. Biol. Chem. 277, 3371-3379. [DOI] [PubMed] [Google Scholar]
- Le, P.U., and Nabi, I.R. (2003). Distinct caveolae-mediated endocytic pathways target the Golgi apparatus and the endoplasmic reticulum. J. Cell Sci. 116, 1059-1071. [DOI] [PubMed] [Google Scholar]
- Lencer, W.I., Hirst, T.R., and Holmes, R.K. (1999). Membrane traffic and the cellular uptake of cholera toxin. Biochim. Biophys. Acta 1450, 177-190. [DOI] [PubMed] [Google Scholar]
- Li, R., Liu, Y., and Ladisch, S. (2001). Enhancement of epidermal growth factor signaling and activation of SRC kinase by gangliosides. J. Biol. Chem. 276, 42782-42792. [DOI] [PubMed] [Google Scholar]
- Marjomaki, V., Pietianinen, V., Matilainen, H., Upla, P., Ivaska, J., Nissinen, L., Reunanen, H., Huttunen, P., Hyypia, T., and Heino, J. (2002). Internalization of echovirus 1 in caveolae. J. Virol. 76, 1856-1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin, O.C., Comly, M.E., Blanchette-Mackie, E.J., Pentchev, P.G., and Pagano, R.E. (1993). Cholesterol deprivation affects the fluorescence properties of a ceramide analog at the Golgi apparatus of living cells. Proc. Natl. Acad. Sci. USA 90, 2661-2665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin, O.C., and Pagano, R.E. (1994). Internalization and sorting of a fluorescent analog of glucosylceramide to the Golgi apparatus of human skin fibroblasts: utilization of endocytic and nonendocytic transport mechanisms. J. Cell Biol. 125, 769-781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mineo, C., and Anderson, R.G. (2001). Potocytosis. Robert Feulgen Lecture. Histochem. Cell Biol. 116, 109-118. [DOI] [PubMed] [Google Scholar]
- Mineo, C., Gill, G.N., and Anderson, R.G. (1999). Regulated migration of epidermal growth factor receptor from caveolae. J. Biol. Chem. 274, 30636-30643. [DOI] [PubMed] [Google Scholar]
- Minshall, R.D., Tiruppathi, C., Vogel, S.M., Niles, W.D., Gilchrist, A., Hamm, H.E., and Malik, A.B. (2000). Endothelial cell-surface gp60 activates vesicle formation and trafficking via G(i)-coupled Src kinase signaling pathway. J. Cell Biol. 150, 1057-1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neufeld, E.B. et al. (1999). The Niemann-Pick C1 protein resides in a vesicular compartment linked to retrograde transport of multiple lysosomal cargo. J. Biol. Chem. 274, 9627-9635. [DOI] [PubMed] [Google Scholar]
- Norkin, L.C. (2001). Caveolae in the uptake and targeting of infectious agents and secreted toxins. Adv. Drug Deliv. Rev. 49, 301-315. [DOI] [PubMed] [Google Scholar]
- Orlandi, P.A., and Fishman, P.H. (1998). Filipin-dependent inhibition of cholera toxin: evidence for toxin internalization and activation through caveolae-like domains. J. Cell Biol. 141, 905-915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parton, R.G. (2003). Caveolae—from ultrastructure to molecular mechanisms. Nat. Rev. Mol. Cell. Biol. 4, 162-167. [DOI] [PubMed] [Google Scholar]
- Parton, R.G., Molero, J.C., Floetenmeyer, M., Green, K.M., and James, D.E. (2002). Characterization of a distinct plasma membrane macrodomain in differentiated adipocytes. J. Biol. Chem. 277, 46769-46778. [DOI] [PubMed] [Google Scholar]
- Pelkmans, L., Kartenbeck, J., and Helenius, A. (2001). Caveolar endocytosis of simian virus 40 reveals a new two-step vesicular-transport pathway to the ER. Nat. Cell Biol. 3, 473-483. [DOI] [PubMed] [Google Scholar]
- Pelkmans, L., Püntener, D., and Helenius, A. (2002). Local actin polymerization and dynamin recruitment in SV40-induced internalization of caveolae. Science 296, 535-539. [DOI] [PubMed] [Google Scholar]
- Prior, I.A., Harding, A., Yan, J., Sluimer, J., Parton, R.G., and Hancock, J.F. (2001). GTP-dependent segregation of H-ras from lipid rafts is required for biological activity. Nat. Cell Biol. 3, 368-375. [DOI] [PubMed] [Google Scholar]
- Puri, V., Jefferson, J.R., Singh, R.D., Wheatley, C.L., Marks, D.L., and Pagano, R.E. (2003). Sphingolipid storage induces accumulation of intracellular cholesterol by stimulating SREBP-1 cleavage. J. Biol. Chem. 278, 20961-20970. [DOI] [PubMed] [Google Scholar]
- Puri, V., Watanabe, R., Dominguez, M., Sun, X., Wheatley, C.L., Marks, D.L., and Pagano, R.E. (1999). Cholesterol modulates membrane traffic along the endocytic pathway in sphingolipid storage diseases. Nat. Cell Biol. 1, 386-388. [DOI] [PubMed] [Google Scholar]
- Puri, V., Watanabe, R., Singh, R.D., Dominguez, M., Brown, J.C., Wheatley, C.L., Marks, D.L., and Pagano, R.E. (2001). Clathrin-dependent and -independent internalization of plasma membrane sphingolipids initiates two Golgi targeting pathways. J. Cell Biol. 154, 535-547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richterova, Z., Liebl, D., Horak, M., Palkova, Z., Stokrova, J., Hozak, P., Korb, J., and Forstova, J. (2001). Caveolae are involved in the trafficking of mouse polyomavirus virions and artificial VP1 pseudocapsids toward cell nuclei. J. Virol. 75, 10880-10891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabharanjak, S., Sharma, P., Parton, R.G., and Mayor, S. (2002). GPI-anchored proteins are delivered to recycling endosomes via a distinct cdc42-regulated, clathrin-independent pinocytic pathway. Develop. Cell 2, 411-423. [DOI] [PubMed] [Google Scholar]
- Schnitzer, J.E., Oh, P., Pinney, E., and Allard, J. (1994). Filipin-sensitive caveolae-mediated transport in endothelium: reduced transcytosis, scavenger endocytosis, and capillary permeability of select macromolecules. J. Cell Biol. 127, 1217-1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma, D.K., Choudhury, A., Singh, R.D., Wheatley, C.L., Marks, D.L., and Pagano, R.E. (2003). Glycosphingolipids internalized via caveolar-related endocytosis rapidly merge with the clathrin pathway in early endosomes and form microdomains for recycling. J. Biol. Chem. 278, 7564-7572. [DOI] [PubMed] [Google Scholar]
- Shin, J.-S., Gao, Z., and Abraham, N. (2000). Involvement of cellular caveolae in bacterial entry into mast cells. Science 289, 785-788. [DOI] [PubMed] [Google Scholar]
- Shubert, W., Frank, P.G., Razani, B., Park, D.S., Chow, C.-W., and Lisanti, M.P. (2001). Caveolae-deficient endothelial cells show defects in the uptake and transport of albumin in vivo. J. Biol. Chem. 276, 48619-48622. [DOI] [PubMed] [Google Scholar]
- Singh, R.D., Puri, V., Valiyaveettil, J.T., Marks, D.L., Bittman, R., and Pagano, R.E. (2003). Selective caveolin-1-dependent endocytosis of glycosphingolipids. Mol. Biol. Cell 14, 3254-3265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smart, E., Graf, G., McNiven, M., Sessa, W., Elgelman, J., Scherer, P., Okamoto, T., and Lisanti, M. (1999). Caveolins, liquid-ordered domains, and signal transduction. Mol. Cell. Biol. 19, 7289-7304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smart, E.J., Ying, Y.S., and Anderson, R.G. (1995). Hormonal regulation of caveolae internalization. J. Cell Biol. 131, 929-938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen, P., Roepstorff, K., Stahlhut, M., and van Deurs, B. (2002). Caveolae are highly immobile plasma membrane microdomains, which are not involved in constitutive endocytic trafficking. Mol. Biol. Cell 13, 238-250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torgersen, M.L., Skretting, G., van Deurs, B., and Sandvig, K. (2001). Internalization of cholera toxin by different endocytic mechanisms. J. Cell Sci. 114, 3737-3742. [DOI] [PubMed] [Google Scholar]
- Wang, X.Q., Sun, P., and Paller, A.S. (2002). Ganglioside induces caveolin-1 redistribution and interaction with the epidermal growth factor receptor. J. Biol. Chem. 277, 47028-47034. [DOI] [PubMed] [Google Scholar]
- Zacharias, D.A., Violin, J.D., Newton, A.C., and Tsien, R.Y. (2002). Partitioning of lipid-modified monomeric GFPs into membrane microdomains of live cells. Science 296, 913-916. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.