Abstract
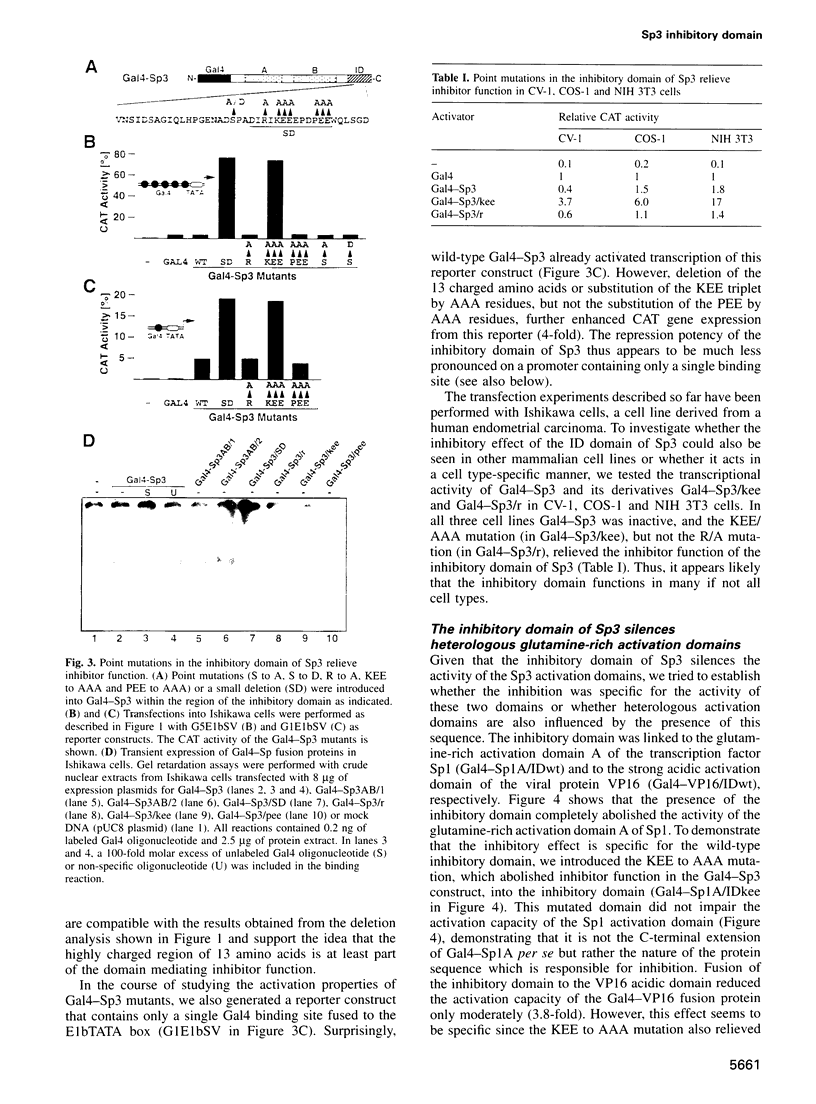
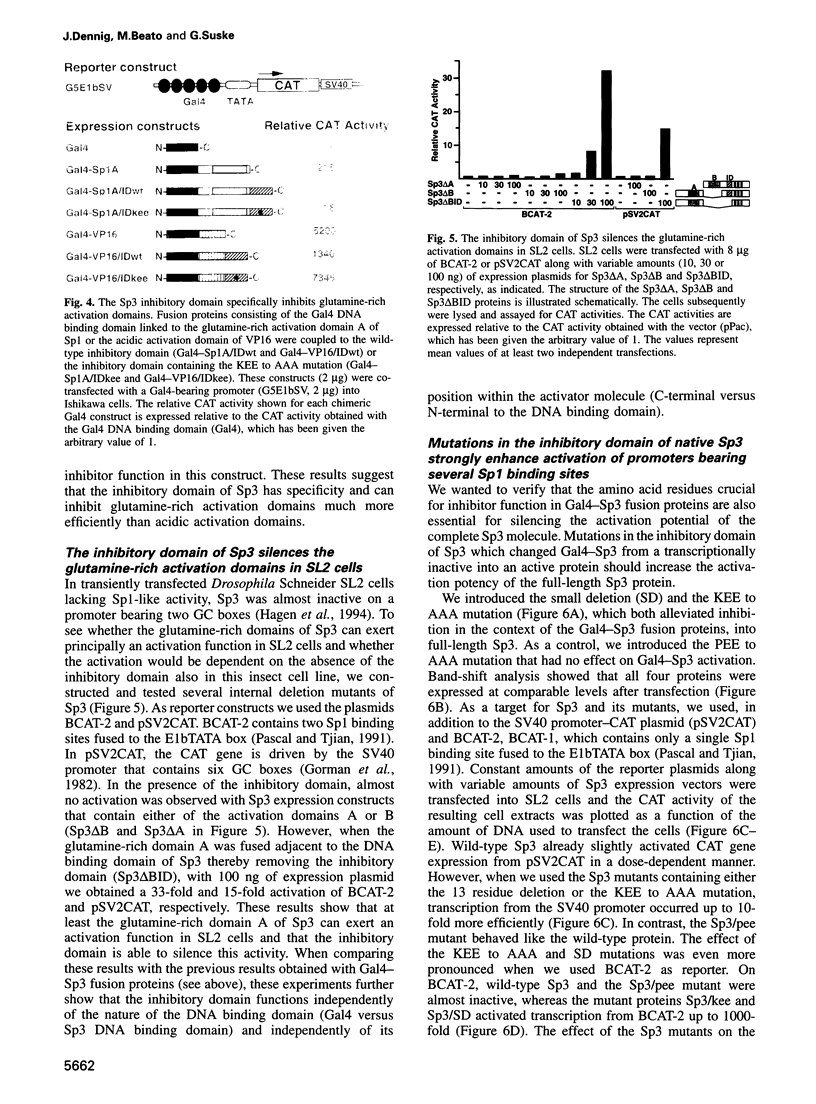
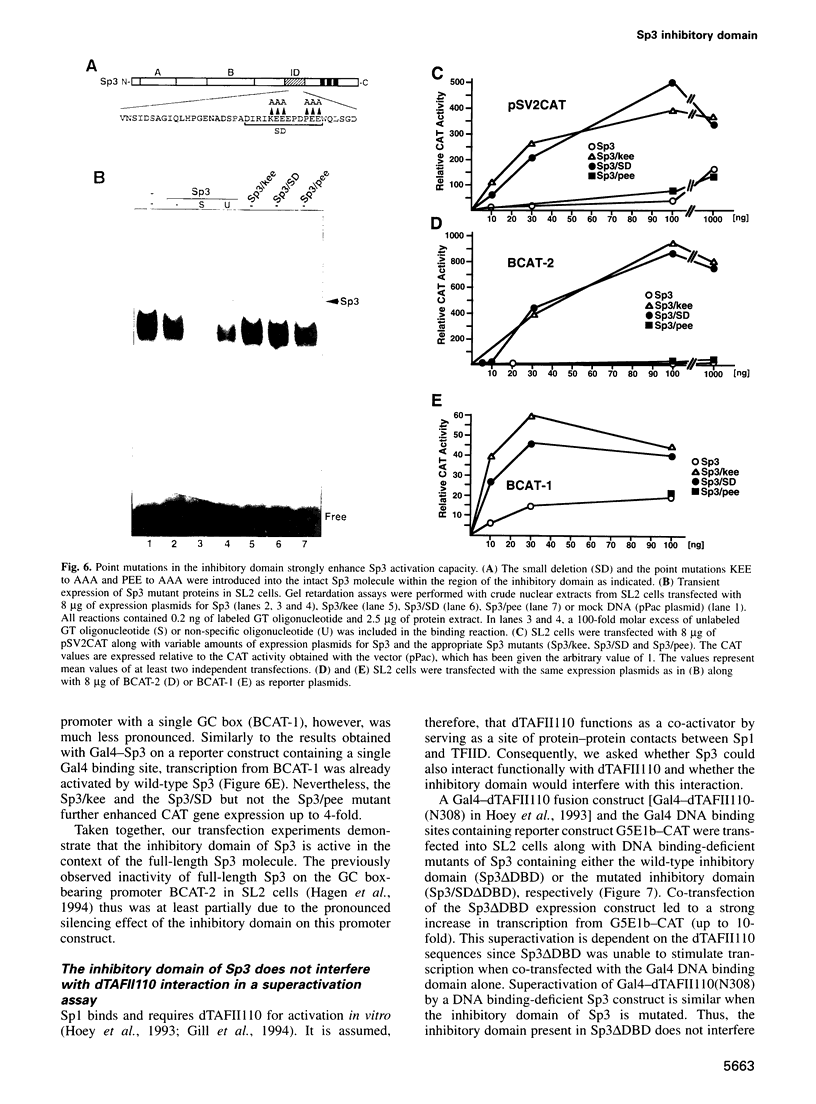
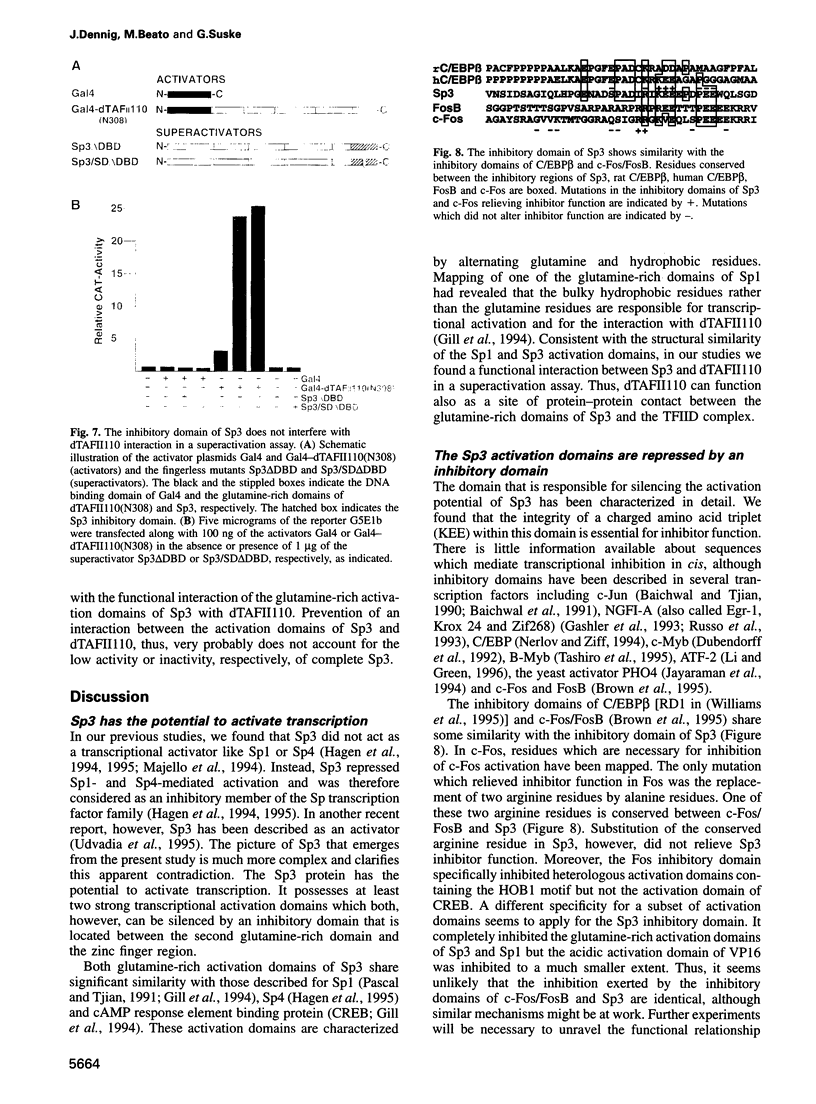
Sp3 is a ubiquitously expressed human transcription factor closely related to Sp1 and Sp4. All three proteins contain a highly conserved DNA binding domain and two glutamine-rich regions, suggesting that they possess similar activation functions. In our previous experiments, however, Sp3 failed to activate transcription. Instead, it repressed Sp1-mediated transcriptional activation, suggesting that it is an inhibitory member of this family of regulatory factors. Here we show that Sp3 can also act as a positive regulator of transcription. The glutamine-rich domains on their own have a strong activation function and interact with the TATA box binding protein (TBP)-associated factor dTAFII110. However, in full-length Sp3 as well as in Gal4-Sp3 fusion proteins, both activation domains are silenced by an inhibitory domain located between the second glutamine-rich region and the DNA binding domain. The inhibitory domain completely suppressed transcriptional activation when fused to a heterologous glutamine-rich domain but only moderately suppressed transcription when linked to an acidic activation domain. Site-directed mutagenesis identified a stretch of highly charged amino acid residues essential for inhibitor function. Substitution of the amino acid triplet KEE by alanine residues within this region changed the almost transcriptionally inactive Sp3 into a strong activator. Our results suggest that the transcriptional activity of Sp3 might be regulated in vivo by relief of inhibition.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews N. C., Faller D. V. A rapid micropreparation technique for extraction of DNA-binding proteins from limiting numbers of mammalian cells. Nucleic Acids Res. 1991 May 11;19(9):2499–2499. doi: 10.1093/nar/19.9.2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baichwal V. R., Park A., Tjian R. v-Src and EJ Ras alleviate repression of c-Jun by a cell-specific inhibitor. Nature. 1991 Jul 11;352(6331):165–168. doi: 10.1038/352165a0. [DOI] [PubMed] [Google Scholar]
- Baichwal V. R., Tjian R. Control of c-Jun activity by interaction of a cell-specific inhibitor with regulatory domain delta: differences between v- and c-Jun. Cell. 1990 Nov 16;63(4):815–825. doi: 10.1016/0092-8674(90)90147-7. [DOI] [PubMed] [Google Scholar]
- Birnbaum M. J., van Wijnen A. J., Odgren P. R., Last T. J., Suske G., Stein G. S., Stein J. L. Sp1 trans-activation of cell cycle regulated promoters is selectively repressed by Sp3. Biochemistry. 1995 Dec 19;34(50):16503–16508. doi: 10.1021/bi00050a034. [DOI] [PubMed] [Google Scholar]
- Brown H. J., Sutherland J. A., Cook A., Bannister A. J., Kouzarides T. An inhibitor domain in c-Fos regulates activation domains containing the HOB1 motif. EMBO J. 1995 Jan 3;14(1):124–131. doi: 10.1002/j.1460-2075.1995.tb06982.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cato A. C., Miksicek R., Schütz G., Arnemann J., Beato M. The hormone regulatory element of mouse mammary tumour virus mediates progesterone induction. EMBO J. 1986 Sep;5(9):2237–2240. doi: 10.1002/j.1460-2075.1986.tb04490.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courey A. J., Tjian R. Analysis of Sp1 in vivo reveals multiple transcriptional domains, including a novel glutamine-rich activation motif. Cell. 1988 Dec 2;55(5):887–898. doi: 10.1016/0092-8674(88)90144-4. [DOI] [PubMed] [Google Scholar]
- De Luca P., Majello B., Lania L. Sp3 represses transcription when tethered to promoter DNA or targeted to promoter proximal RNA. J Biol Chem. 1996 Apr 12;271(15):8533–8536. doi: 10.1074/jbc.271.15.8533. [DOI] [PubMed] [Google Scholar]
- Di Nocera P. P., Dawid I. B. Transient expression of genes introduced into cultured cells of Drosophila. Proc Natl Acad Sci U S A. 1983 Dec;80(23):7095–7098. doi: 10.1073/pnas.80.23.7095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubendorff J. W., Whittaker L. J., Eltman J. T., Lipsick J. S. Carboxy-terminal elements of c-Myb negatively regulate transcriptional activation in cis and in trans. Genes Dev. 1992 Dec;6(12B):2524–2535. doi: 10.1101/gad.6.12b.2524. [DOI] [PubMed] [Google Scholar]
- Fried M., Crothers D. M. Equilibria and kinetics of lac repressor-operator interactions by polyacrylamide gel electrophoresis. Nucleic Acids Res. 1981 Dec 11;9(23):6505–6525. doi: 10.1093/nar/9.23.6505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gashler A. L., Swaminathan S., Sukhatme V. P. A novel repression module, an extensive activation domain, and a bipartite nuclear localization signal defined in the immediate-early transcription factor Egr-1. Mol Cell Biol. 1993 Aug;13(8):4556–4571. doi: 10.1128/mcb.13.8.4556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill G., Pascal E., Tseng Z. H., Tjian R. A glutamine-rich hydrophobic patch in transcription factor Sp1 contacts the dTAFII110 component of the Drosophila TFIID complex and mediates transcriptional activation. Proc Natl Acad Sci U S A. 1994 Jan 4;91(1):192–196. doi: 10.1073/pnas.91.1.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagen G., Dennig J., Preiss A., Beato M., Suske G. Functional analyses of the transcription factor Sp4 reveal properties distinct from Sp1 and Sp3. J Biol Chem. 1995 Oct 20;270(42):24989–24994. doi: 10.1074/jbc.270.42.24989. [DOI] [PubMed] [Google Scholar]
- Hagen G., Müller S., Beato M., Suske G. Cloning by recognition site screening of two novel GT box binding proteins: a family of Sp1 related genes. Nucleic Acids Res. 1992 Nov 11;20(21):5519–5525. doi: 10.1093/nar/20.21.5519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagen G., Müller S., Beato M., Suske G. Sp1-mediated transcriptional activation is repressed by Sp3. EMBO J. 1994 Aug 15;13(16):3843–3851. doi: 10.1002/j.1460-2075.1994.tb06695.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall C. V., Jacob P. E., Ringold G. M., Lee F. Expression and regulation of Escherichia coli lacZ gene fusions in mammalian cells. J Mol Appl Genet. 1983;2(1):101–109. [PubMed] [Google Scholar]
- Hoey T., Weinzierl R. O., Gill G., Chen J. L., Dynlacht B. D., Tjian R. Molecular cloning and functional analysis of Drosophila TAF110 reveal properties expected of coactivators. Cell. 1993 Jan 29;72(2):247–260. doi: 10.1016/0092-8674(93)90664-c. [DOI] [PubMed] [Google Scholar]
- Jayaraman P. S., Hirst K., Goding C. R. The activation domain of a basic helix-loop-helix protein is masked by repressor interaction with domains distinct from that required for transcription regulation. EMBO J. 1994 May 1;13(9):2192–2199. doi: 10.1002/j.1460-2075.1994.tb06496.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingsley C., Winoto A. Cloning of GT box-binding proteins: a novel Sp1 multigene family regulating T-cell receptor gene expression. Mol Cell Biol. 1992 Oct;12(10):4251–4261. doi: 10.1128/mcb.12.10.4251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X. Y., Green M. R. Intramolecular inhibition of activating transcription factor-2 function by its DNA-binding domain. Genes Dev. 1996 Mar 1;10(5):517–527. doi: 10.1101/gad.10.5.517. [DOI] [PubMed] [Google Scholar]
- Majello B., De Luca P., Hagen G., Suske G., Lania L. Different members of the Sp1 multigene family exert opposite transcriptional regulation of the long terminal repeat of HIV-1. Nucleic Acids Res. 1994 Nov 25;22(23):4914–4921. doi: 10.1093/nar/22.23.4914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majello B., De Luca P., Suske G., Lania L. Differential transcriptional regulation of c-myc promoter through the same DNA binding sites targeted by Sp1-like proteins. Oncogene. 1995 May 4;10(9):1841–1848. [PubMed] [Google Scholar]
- Nerlov C., Ziff E. B. Three levels of functional interaction determine the activity of CCAAT/enhancer binding protein-alpha on the serum albumin promoter. Genes Dev. 1994 Feb 1;8(3):350–362. doi: 10.1101/gad.8.3.350. [DOI] [PubMed] [Google Scholar]
- Pascal E., Tjian R. Different activation domains of Sp1 govern formation of multimers and mediate transcriptional synergism. Genes Dev. 1991 Sep;5(9):1646–1656. doi: 10.1101/gad.5.9.1646. [DOI] [PubMed] [Google Scholar]
- Russo M. W., Matheny C., Milbrandt J. Transcriptional activity of the zinc finger protein NGFI-A is influenced by its interaction with a cellular factor. Mol Cell Biol. 1993 Nov;13(11):6858–6865. doi: 10.1128/mcb.13.11.6858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadowski I., Ma J., Triezenberg S., Ptashne M. GAL4-VP16 is an unusually potent transcriptional activator. Nature. 1988 Oct 6;335(6190):563–564. doi: 10.1038/335563a0. [DOI] [PubMed] [Google Scholar]
- Sauer F., Fondell J. D., Ohkuma Y., Roeder R. G., Jäckle H. Control of transcription by Krüppel through interactions with TFIIB and TFIIE beta. Nature. 1995 May 11;375(6527):162–164. doi: 10.1038/375162a0. [DOI] [PubMed] [Google Scholar]
- Schneider I. Cell lines derived from late embryonic stages of Drosophila melanogaster. J Embryol Exp Morphol. 1972 Apr;27(2):353–365. [PubMed] [Google Scholar]
- Sjøttem E., Anderssen S., Johansen T. The promoter activity of long terminal repeats of the HERV-H family of human retrovirus-like elements is critically dependent on Sp1 family proteins interacting with a GC/GT box located immediately 3' to the TATA box. J Virol. 1996 Jan;70(1):188–198. doi: 10.1128/jvi.70.1.188-198.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slater E. P., Redeuihl G., Theis K., Suske G., Beato M. The uteroglobin promoter contains a noncanonical estrogen responsive element. Mol Endocrinol. 1990 Apr;4(4):604–610. doi: 10.1210/mend-4-4-604. [DOI] [PubMed] [Google Scholar]
- Southgate C. D., Green M. R. The HIV-1 Tat protein activates transcription from an upstream DNA-binding site: implications for Tat function. Genes Dev. 1991 Dec;5(12B):2496–2507. doi: 10.1101/gad.5.12b.2496. [DOI] [PubMed] [Google Scholar]
- Tashiro S., Takemoto Y., Handa H., Ishii S. Cell type-specific trans-activation by the B-myb gene product: requirement of the putative cofactor binding to the C-terminal conserved domain. Oncogene. 1995 May 4;10(9):1699–1707. [PubMed] [Google Scholar]
- Udvadia A. J., Templeton D. J., Horowitz J. M. Functional interactions between the retinoblastoma (Rb) protein and Sp-family members: superactivation by Rb requires amino acids necessary for growth suppression. Proc Natl Acad Sci U S A. 1995 Apr 25;92(9):3953–3957. doi: 10.1073/pnas.92.9.3953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams S. C., Baer M., Dillner A. J., Johnson P. F. CRP2 (C/EBP beta) contains a bipartite regulatory domain that controls transcriptional activation, DNA binding and cell specificity. EMBO J. 1995 Jul 3;14(13):3170–3183. doi: 10.1002/j.1460-2075.1995.tb07319.x. [DOI] [PMC free article] [PubMed] [Google Scholar]