Abstract
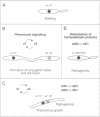
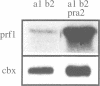
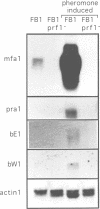
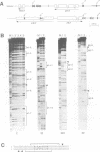
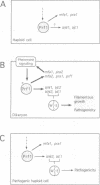
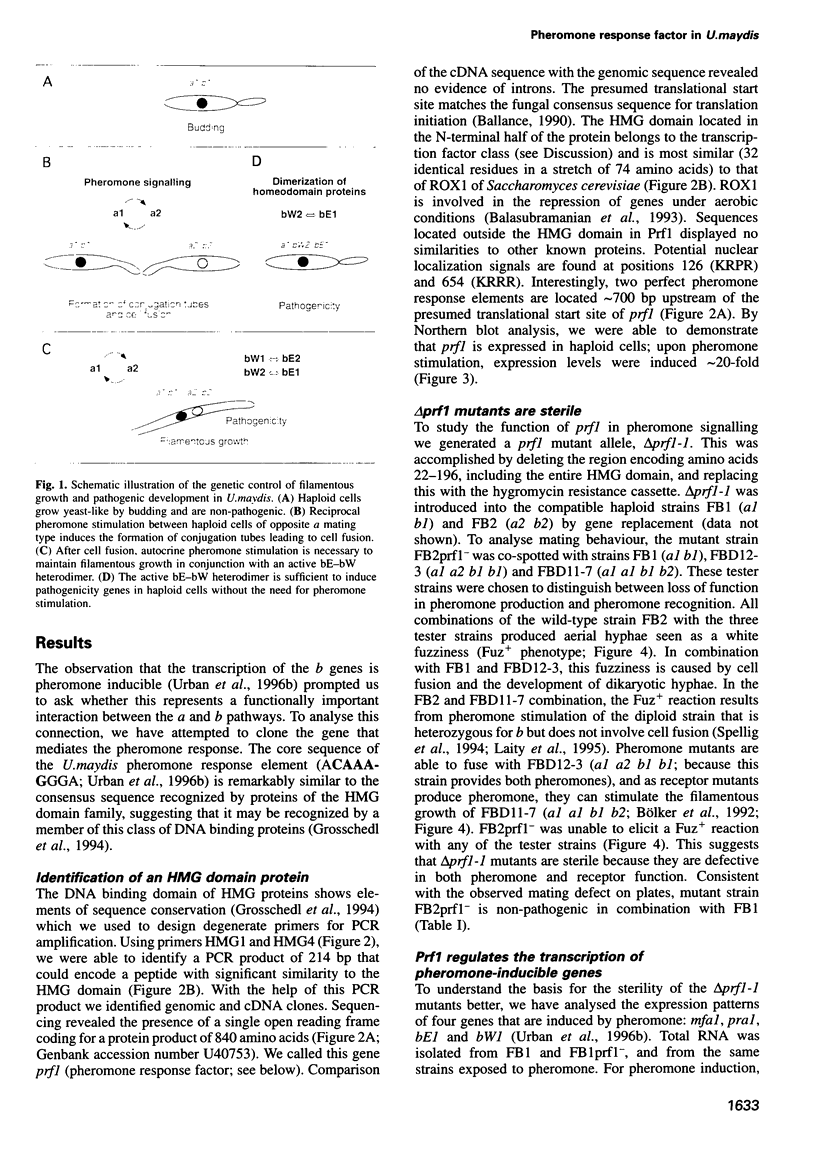
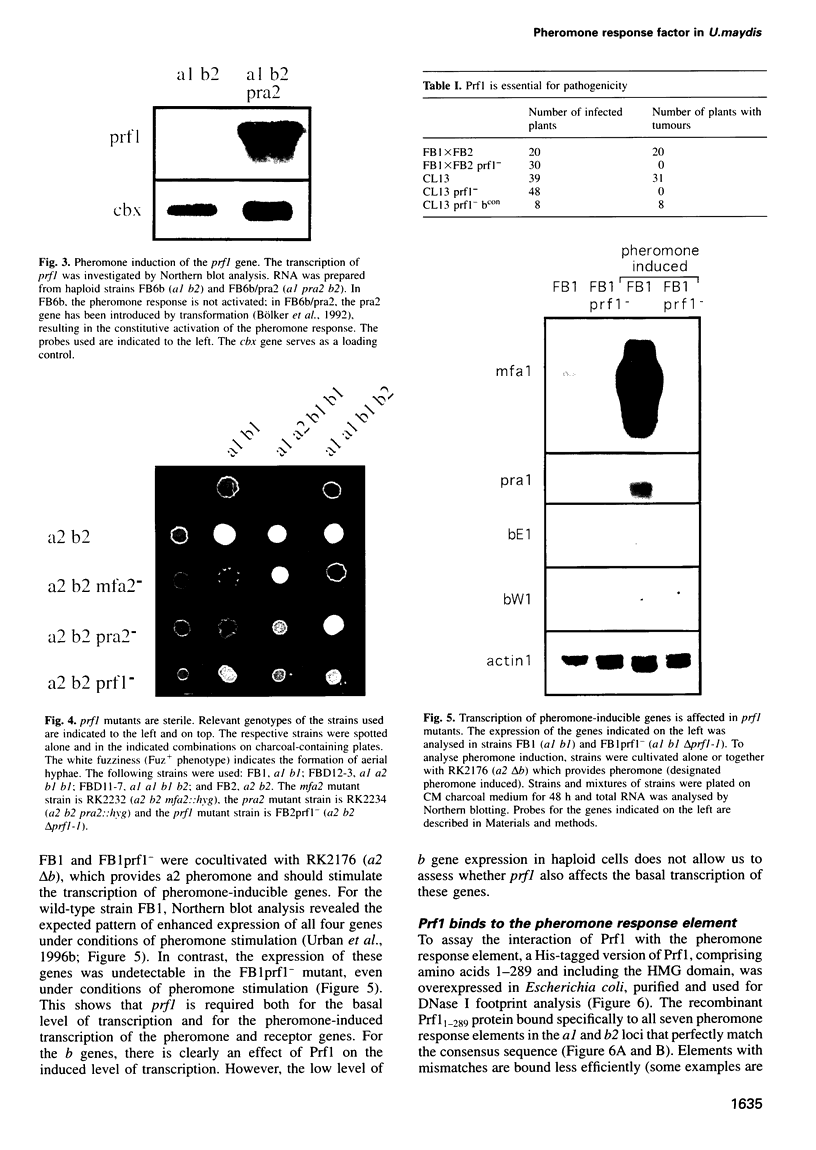
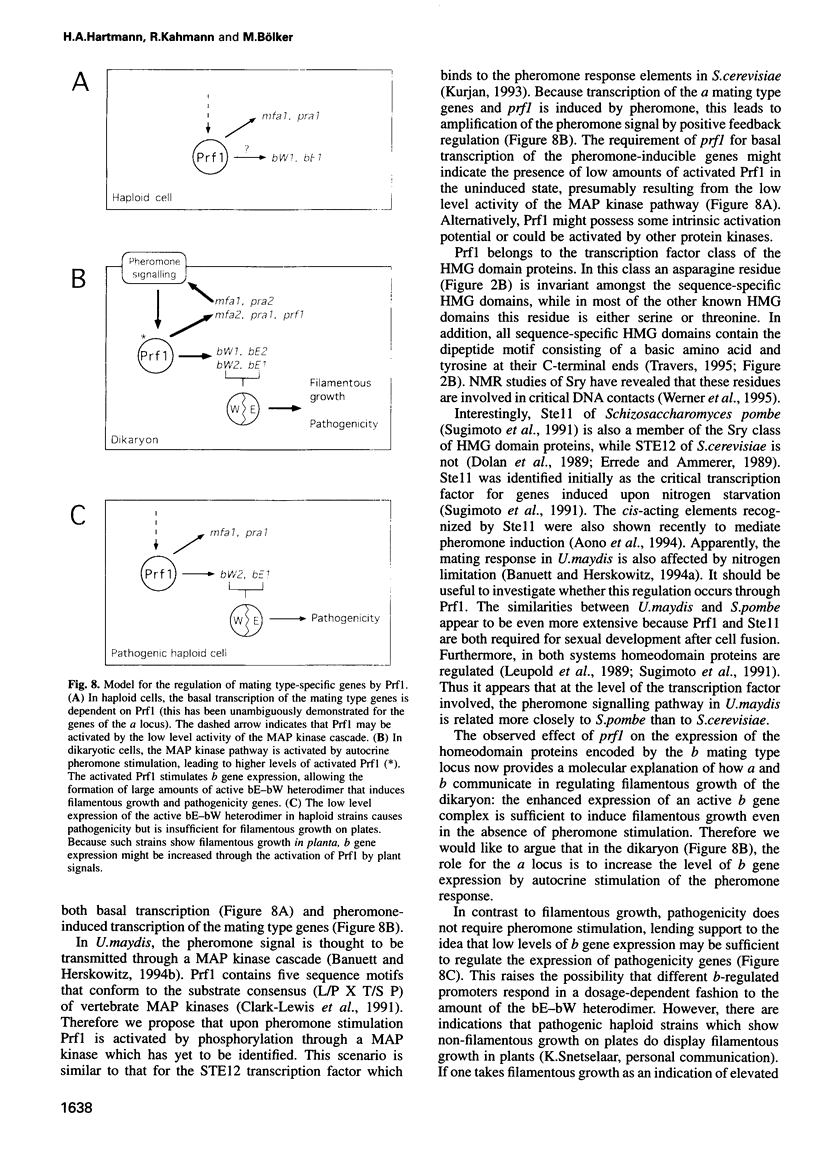
In Ustilago maydis, the a and b mating type loci regulate cell fusion, filamentous growth and pathogenicity. The a locus encodes a pheromone-based cell recognition system, and the b locus specifies two homeodomain proteins. The expression of all genes in the a and b loci is induced by pheromone. We have identified a HMG protein (Prf1) that binds sequence specifically to pheromone response elements present in the a and b loci. prf1 mutants do not express the a and b genes and are sterile. The disruption of prf1 in pathogenic haploid strains results in a loss of pathogenicity. The constitutive expression of the b genes restores pathogenicity and induces filamentous growth in the absence of the pheromone signal. These results provide evidence that pheromone signalling, filamentous growth and pathogenic development are linked through Prf1.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aono T., Yanai H., Miki F., Davey J., Shimoda C. Mating pheromone-induced expression of the mat1-Pm gene of Schizosaccharomyces pombe: identification of signalling components and characterization of upstream controlling elements. Yeast. 1994 Jun;10(6):757–770. doi: 10.1002/yea.320100607. [DOI] [PubMed] [Google Scholar]
- Balasubramanian B., Lowry C. V., Zitomer R. S. The Rox1 repressor of the Saccharomyces cerevisiae hypoxic genes is a specific DNA-binding protein with a high-mobility-group motif. Mol Cell Biol. 1993 Oct;13(10):6071–6078. doi: 10.1128/mcb.13.10.6071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banuett F., Herskowitz I. Different a alleles of Ustilago maydis are necessary for maintenance of filamentous growth but not for meiosis. Proc Natl Acad Sci U S A. 1989 Aug;86(15):5878–5882. doi: 10.1073/pnas.86.15.5878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banuett F., Herskowitz I. Identification of fuz7, a Ustilago maydis MEK/MAPKK homolog required for a-locus-dependent and -independent steps in the fungal life cycle. Genes Dev. 1994 Jun 15;8(12):1367–1378. doi: 10.1101/gad.8.12.1367. [DOI] [PubMed] [Google Scholar]
- Banuett F. Ustilago maydis, the delightful blight. Trends Genet. 1992 May;8(5):174–180. doi: 10.1016/0168-9525(92)90220-x. [DOI] [PubMed] [Google Scholar]
- Bölker M., Böhnert H. U., Braun K. H., Görl J., Kahmann R. Tagging pathogenicity genes in Ustilago maydis by restriction enzyme-mediated integration (REMI). Mol Gen Genet. 1995 Sep 20;248(5):547–552. doi: 10.1007/BF02423450. [DOI] [PubMed] [Google Scholar]
- Bölker M., Urban M., Kahmann R. The a mating type locus of U. maydis specifies cell signaling components. Cell. 1992 Feb 7;68(3):441–450. doi: 10.1016/0092-8674(92)90182-c. [DOI] [PubMed] [Google Scholar]
- Clark-Lewis I., Sanghera J. S., Pelech S. L. Definition of a consensus sequence for peptide substrate recognition by p44mpk, the meiosis-activated myelin basic protein kinase. J Biol Chem. 1991 Aug 15;266(23):15180–15184. [PubMed] [Google Scholar]
- Day P. R., Anagnostakis S. L., Puhalla J. E. Pathogenicity resulting from mutation at the b locus of Ustilago maydis. Proc Natl Acad Sci U S A. 1971 Mar;68(3):533–535. doi: 10.1073/pnas.68.3.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debuchy R., Coppin E. The mating types of Podospora anserina: functional analysis and sequence of the fertilization domains. Mol Gen Genet. 1992 May;233(1-2):113–121. doi: 10.1007/BF00587568. [DOI] [PubMed] [Google Scholar]
- Dolan J. W., Kirkman C., Fields S. The yeast STE12 protein binds to the DNA sequence mediating pheromone induction. Proc Natl Acad Sci U S A. 1989 Aug;86(15):5703–5707. doi: 10.1073/pnas.86.15.5703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Errede B., Ammerer G. STE12, a protein involved in cell-type-specific transcription and signal transduction in yeast, is part of protein-DNA complexes. Genes Dev. 1989 Sep;3(9):1349–1361. doi: 10.1101/gad.3.9.1349. [DOI] [PubMed] [Google Scholar]
- Grosschedl R., Giese K., Pagel J. HMG domain proteins: architectural elements in the assembly of nucleoprotein structures. Trends Genet. 1994 Mar;10(3):94–100. doi: 10.1016/0168-9525(94)90232-1. [DOI] [PubMed] [Google Scholar]
- Gubbay J., Collignon J., Koopman P., Capel B., Economou A., Münsterberg A., Vivian N., Goodfellow P., Lovell-Badge R. A gene mapping to the sex-determining region of the mouse Y chromosome is a member of a novel family of embryonically expressed genes. Nature. 1990 Jul 19;346(6281):245–250. doi: 10.1038/346245a0. [DOI] [PubMed] [Google Scholar]
- Herskowitz I. MAP kinase pathways in yeast: for mating and more. Cell. 1995 Jan 27;80(2):187–197. doi: 10.1016/0092-8674(95)90402-6. [DOI] [PubMed] [Google Scholar]
- Hoffman C. S., Winston F. A ten-minute DNA preparation from yeast efficiently releases autonomous plasmids for transformation of Escherichia coli. Gene. 1987;57(2-3):267–272. doi: 10.1016/0378-1119(87)90131-4. [DOI] [PubMed] [Google Scholar]
- Holden D. W., Kronstad J. W., Leong S. A. Mutation in a heat-regulated hsp70 gene of Ustilago maydis. EMBO J. 1989 Jul;8(7):1927–1934. doi: 10.1002/j.1460-2075.1989.tb03596.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahmann R., Romeis T., Bölker M., Kämper J. Control of mating and development in Ustilago maydis. Curr Opin Genet Dev. 1995 Oct;5(5):559–564. doi: 10.1016/0959-437x(95)80023-9. [DOI] [PubMed] [Google Scholar]
- Kelly M., Burke J., Smith M., Klar A., Beach D. Four mating-type genes control sexual differentiation in the fission yeast. EMBO J. 1988 May;7(5):1537–1547. doi: 10.1002/j.1460-2075.1988.tb02973.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keon J. P., White G. A., Hargreaves J. A. Isolation, characterization and sequence of a gene conferring resistance to the systemic fungicide carboxin from the maize smut pathogen, Ustilago maydis. Curr Genet. 1991 Jun;19(6):475–481. doi: 10.1007/BF00312739. [DOI] [PubMed] [Google Scholar]
- Kronstad J. W., Leong S. A. The b mating-type locus of Ustilago maydis contains variable and constant regions. Genes Dev. 1990 Aug;4(8):1384–1395. doi: 10.1101/gad.4.8.1384. [DOI] [PubMed] [Google Scholar]
- Kurjan J. The pheromone response pathway in Saccharomyces cerevisiae. Annu Rev Genet. 1993;27:147–179. doi: 10.1146/annurev.ge.27.120193.001051. [DOI] [PubMed] [Google Scholar]
- Kämper J., Reichmann M., Romeis T., Bölker M., Kahmann R. Multiallelic recognition: nonself-dependent dimerization of the bE and bW homeodomain proteins in Ustilago maydis. Cell. 1995 Apr 7;81(1):73–83. doi: 10.1016/0092-8674(95)90372-0. [DOI] [PubMed] [Google Scholar]
- Laity C., Giasson L., Campbell R., Kronstad J. Heterozygosity at the b mating-type locus attenuates fusion in Ustilago maydis. Curr Genet. 1995 Apr;27(5):451–459. doi: 10.1007/BF00311215. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Nossal G. J. The molecular and cellular basis of affinity maturation in the antibody response. Cell. 1992 Jan 10;68(1):1–2. doi: 10.1016/0092-8674(92)90198-l. [DOI] [PubMed] [Google Scholar]
- Schauwecker F., Wanner G., Kahmann R. Filament-specific expression of a cellulase gene in the dimorphic fungus Ustilago maydis. Biol Chem Hoppe Seyler. 1995 Oct;376(10):617–625. doi: 10.1515/bchm3.1995.376.10.617. [DOI] [PubMed] [Google Scholar]
- Schulz B., Banuett F., Dahl M., Schlesinger R., Schäfer W., Martin T., Herskowitz I., Kahmann R. The b alleles of U. maydis, whose combinations program pathogenic development, code for polypeptides containing a homeodomain-related motif. Cell. 1990 Jan 26;60(2):295–306. doi: 10.1016/0092-8674(90)90744-y. [DOI] [PubMed] [Google Scholar]
- Spellig T., Bölker M., Lottspeich F., Frank R. W., Kahmann R. Pheromones trigger filamentous growth in Ustilago maydis. EMBO J. 1994 Apr 1;13(7):1620–1627. doi: 10.1002/j.1460-2075.1994.tb06425.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staben C., Yanofsky C. Neurospora crassa a mating-type region. Proc Natl Acad Sci U S A. 1990 Jul;87(13):4917–4921. doi: 10.1073/pnas.87.13.4917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimoto A., Iino Y., Maeda T., Watanabe Y., Yamamoto M. Schizosaccharomyces pombe ste11+ encodes a transcription factor with an HMG motif that is a critical regulator of sexual development. Genes Dev. 1991 Nov;5(11):1990–1999. doi: 10.1101/gad.5.11.1990. [DOI] [PubMed] [Google Scholar]
- Thompson J. D., Higgins D. G., Gibson T. J. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994 Nov 11;22(22):4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travers A. A. Reading the minor groove. Nat Struct Biol. 1995 Aug;2(8):615–618. doi: 10.1038/nsb0895-615. [DOI] [PubMed] [Google Scholar]
- Tsukuda T., Carleton S., Fotheringham S., Holloman W. K. Isolation and characterization of an autonomously replicating sequence from Ustilago maydis. Mol Cell Biol. 1988 Sep;8(9):3703–3709. doi: 10.1128/mcb.8.9.3703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turgeon B. G., Bohlmann H., Ciuffetti L. M., Christiansen S. K., Yang G., Schäfer W., Yoder O. C. Cloning and analysis of the mating type genes from Cochliobolus heterostrophus. Mol Gen Genet. 1993 Apr;238(1-2):270–284. doi: 10.1007/BF00279556. [DOI] [PubMed] [Google Scholar]
- Wang J., Holden D. W., Leong S. A. Gene transfer system for the phytopathogenic fungus Ustilago maydis. Proc Natl Acad Sci U S A. 1988 Feb;85(3):865–869. doi: 10.1073/pnas.85.3.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner M. H., Huth J. R., Gronenborn A. M., Clore G. M. Molecular basis of human 46X,Y sex reversal revealed from the three-dimensional solution structure of the human SRY-DNA complex. Cell. 1995 Jun 2;81(5):705–714. doi: 10.1016/0092-8674(95)90532-4. [DOI] [PubMed] [Google Scholar]