Summary
The molecular mechanisms that regulate the rapid transcriptional changes that occur during cytotoxic T lymphocyte (CTL) proliferation and differentiation in response to infection are poorly understood. We have utilised ChIP-seq to assess histone H3 methylation dynamics within naïve, effector and memory virus-specific T cells isolated directly ex vivo after influenza A virus infection. Our results show that within naïve T cells, co-deposition of the permissive H3K4me3 and repressive H3K27me3 modifications is a signature of gene loci associated with gene transcription, replication and cellular differentiation. Upon differentiation into effector and/or memory CTL, the majority of these gene loci lose the repressive H3K27me3 while retaining the permissive H3K4me3 modification. In contrast, immune-related effector gene promoters within naïve T cells lacked the permissive H3K4me3 modification, with acquisition of this modification occurring upon differentiation into effector/memory CTL. Thus, coordinate transcriptional regulation of CTL genes with related functions is achieved using distinct epigenetic mechanisms.
Introduction
A cardinal feature of adaptive T cell responses to infection is the rapid initiation of a proliferative response that coincides with acquisition of lineage-specific functions by pathogen-specific T cells (Kaech et al., 2002; van Stipdonk et al., 2003). Antigen-dependent activation of naïve cytotoxic T lymphocytes (CTLs) initiates a program of differentiation that has been shown to be largely autonomous (van Stipdonk et al., 2003) and results in the acquisition of effector mechanisms including the production of pro-inflammatory cytokines such as interferon (IFN)-γ and tumour necrosis factor (TNF)-α (La Gruta et al., 2004), and expression of cytolytic effector molecules including perforin (PFP) (Kagi et al., 1994) and the granule enzymes (granzymes, GZM) A, B and K (Jenkins et al., 2007; Moffat et al., 2009; Peixoto et al., 2007). Once an infection is cleared, the expanded effector CTL population contracts, leaving a pool of long-lived, pathogen-specific memory T cells. In contrast to naïve CD8+ T cells, virus-specific memory CTLs are able to respond more readily and rapidly to subsequent infections, and without the need for further differentiation (Kaech et al., 2002; La Gruta et al., 2004; Lalvani et al., 1997; Oehen and Brduscha-Riem, 1998; Veiga-Fernandes et al., 2000). Whilst naïve, effector and memory CD8+ T cells have distinct molecular, phenotypic and functional characteristics, the molecular mechanisms that underpin the initiation and maintenance of CD8+ T cell differentiation in response to infection are not well understood.
Within eukaryotic cells, DNA is wrapped around a complex of histone proteins known as a nucleosome, with the nucleosome-DNA complex termed chromatin. Post-translational modification (PTM) of histones is an important mechanism for regulation and inheritance of gene transcriptional activity, regulating differentiation in an array of cellular contexts (Kouzarides, 2007). Histone PTMs contribute to regulation of transcription by providing a platform that promotes binding of transcription factors (TFs) and chromatin remodelling proteins (Kouzarides, 2007). Histones can be modified by a vast array of covalent modifications, particularly on the solvent-exposed N-terminus, and the combination of histone PTMs and their genomic location is a predictor of transcriptional activity (Wang et al., 2008). For example tri-methylation of histone 3 at lysine 4 (H3K4me3) is typically enriched within gene promoters, and correlates with active transcription (Santos-Rosa et al., 2002). In contrast, deposition of tri-methylated H3K27 (H3K27me3) at gene promoters typically correlates with transcriptional repression (Wang et al., 2008).
It is becoming increasingly evident that both the combination and extent of histone PTMs at specific genomic locations control gene transcriptional activity (Wang et al., 2008). In particular, studies in embryonic stem cells (ESCs) have demonstrated that transcriptional potential is maintained by co-enrichment of both active (H3K4me3) and repressive (H3K27me3) modifications at developmentally important gene loci (termed bivalent loci) (Araki et al., 2009; Bernstein et al., 2006; Hawkins et al., 2010). Importantly, upon differentiation, the vast majority of bivalent loci in ESCs are permanently repressed by maintenance of H3K27me3 and loss of H3K4me3, ensuring that only cell lineage-specific genes are expressed (Hawkins et al., 2010). These data suggest that bivalency is an epigenetic state from which a gene can be rapidly activated or repressed depending on the differentiation pathway initiated. However, it remains to be determined whether these findings extend beyond embryonic development and represent a general mechanism for regulating cell lineage commitment.
Upon activation, naïve CD4+ T cells can be directed to differentiate into a variety of T cell subsets, including TH1 or TH2 lineages, each characterised by their ability to express a distinct set of cytokines. It is now apparent that CD4+ T cell lineage commitment correlates with dynamic changes in histone PTM deposition at gene loci associated with directing subset specific CD4+ T cell effector function (Allan et al., 2012; Ansel et al., 2003; Wei et al., 2009). Whether changes in histone PTMs also play a role in directing naïve CD8+ T cell fate is less clear. Analysis of histone PTMs at single effector CTL loci has demonstrated that changes in H3K4me3 and H3K27me3 deposition correlate with dynamic changes in effector function during naïve to effector and memory differentiation (Denton et al., 2011; Zediak et al., 2011). Consistent with these studies, genome-wide analysis of H3K4me3 and H3K27me3 deposition within human, polyclonal, naïve and memory CTLs showed that specific methylation patterns correlated with subset specific gene expression (Araki et al., 2009). However, it is difficult to link changes in the global epigenetic patterns within polyclonal CTL populations to those induced specifically by infection. To that end we utilised a mouse model of adoptive transfer of T cell receptor transgenic CD8+ T cells and recombinant influenza A virus infection so that naïve, effector and memory virus-specific CD8+ T cells could be analysed.
Here we report genome-wide mapping of histone H3 methylation patterns within antigen-specific naïve, effector and memory CTLs elicited by an acute viral infection. This, combined with global transcriptional analyses of resting and stimulated CTLs, demonstrated that establishment of permissive chromatin domains at CTL-lineage defining genes correlates with observed phenotypic and functional differences between virus-specific CTLs at each phase of the immune response. Surprisingly, rather than a broad increase in the activating H3K4me3 histone PTM, effector and memory CTLs could be defined on the basis of a large-scale but focused reduction in the repressive H3K27me3 PTM, suggesting that naïve CTLs are maintained in a state of restraint until activation. Strikingly, the particular dynamics of PTM loss and gain during differentiation identified functionally distinct classes of genes, providing a mechanistic basis for the coordinate regulation of each group. These data suggest that upon naïve CTL activation, distinct epigenetic histone methylation patterns are established to tightly co-regulate transcription of gene modules that in turn ensure programmed CTL differentiation and establishment of effective cellular immunity.
Results
Different epigenetic PTM patterns associated with distinct cellular functions during CTL differentiation
To investigate the dynamics of H3K4me3 and H3K27me3 histone modifications during CTL differentiation in response to infection, we utilised an infection model where naïve (CD44lowCD62Lhigh) OT-I TCR transgenic CD8+ T cells, specific for the ovalbumin peptide (OVA257–264), were adoptively transferred into congenic C57BL/6J (B6) hosts, followed by intranasal (i.n.) infection with the A/HKx31-OVA virus (Jenkins et al., 2006). We then carried out ChIP-Seq analysis on nuclei isolated from sort purified (>99% purity) naïve (day 0), effector (day 10) and memory (>60) OTI CTLs after immunoprecipitation using antibodies specific for either H3K4me3 or H3K27me3 modifications. We were able to map a total of 28.9 × 106, 44.5 × 106 and 48.5 × 106 H3K4me3 associated sequence tags for naïve, effector and memory CTLs, respectively. For H3K27me3 associated sequences, a total of 18.9 × 106, 42.8 × 106 and 26 × 106 sequences tags were mapped from naïve, effector and memory CTLs. To validate our data, we examined sequences mapping to the constitutively expressed housekeeping gene, Oaz1, and the constitutively repressed Krt8 (keratin protein) gene (Supplementary Fig. 1). As expected, there was a permissive epigenetic signature around the promoter and transcriptional start site (TSS) of the Oaz1 gene with high levels of H3K4me3 deposition (red histogram) and little or no H3K27me3 (blue histogram) (Supplementary Fig. 1a). This contrasted with the Krt8 locus that had a repressive epigenetic signature with little or no H3K4me3, but extensive H3K27me3 deposition (Supplementary Fig. 1b). Thus, these data were suitable for identifying patterns of genome-wide methylation in virus-specific naïve, effector and memory CD8+ T cells.
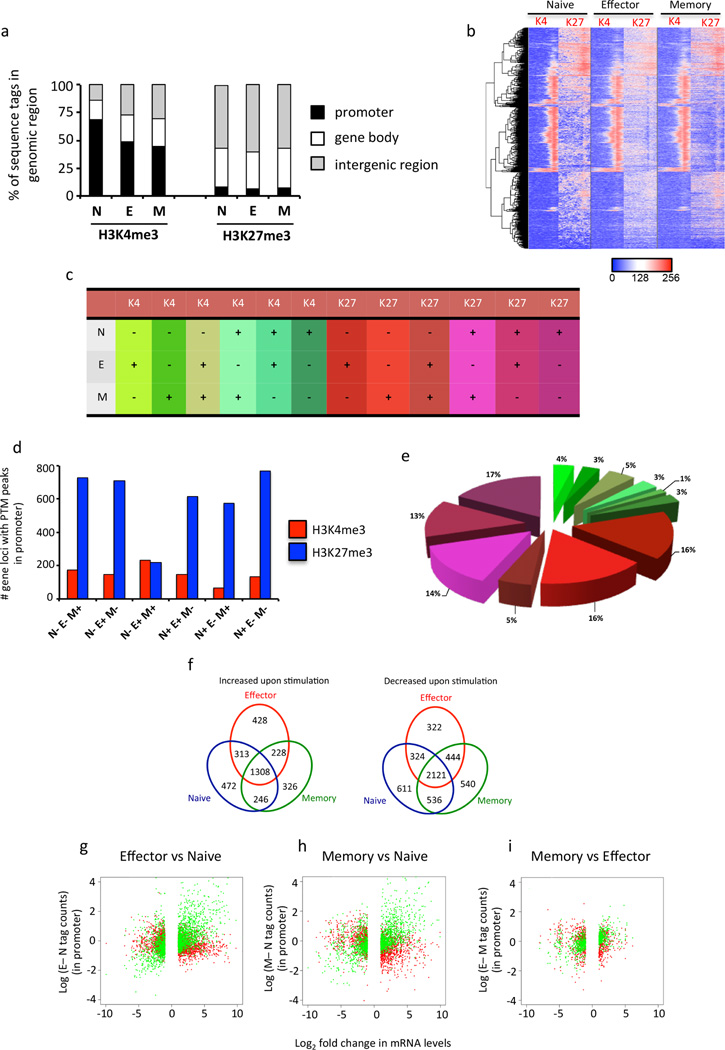
We next determined the proportion of H3K4me3 and H3K27me3 sequence tags associated with the promoter (defined as −3 kb and +1 kb of the TSS of genes, Supplementary Table 1), gene body (+1kb to 3’UTR of genes) and intergenic regions. As expected, the majority of H3K4me3 sequence tags were associated within gene promoters and the gene bodies of loci within naïve, effector and memory CTLs (Fig. 1a). Interestingly, there was an increase in the proportion of H3K4me3 sequence tags associated with intergenic regions within effector and memory CTLs (Fig. 1a) demonstrating there is modulation of H3K4me3 at non-coding genomic regions upon naïve CTL activation. We observed that the majority of H3K27me3 sequence tags were predominantly associated with intergenic regions and gene bodies within naïve, effector and memory CTLs (Fig. 1a), with a small proportion of sequence tags (approx. 10 %) associated with our defined promoter region of 4kb. Interestingly, there was little change in the proportionality of H3K27me3 deposition within these regions between naïve, effector and memory CTLs.
Figure 1. Global histone methylation and transcriptional patterns of naïve, effector and memory OT-I CD8+ T cells.
(f) Naïve (CD44loCD62Lhi) CD45.1+ CD8+ OT-I CTLs were sort purified prior to adoptive transfer into CD45.2+ congenic mice. Mice that had received naïve OT-Is were infected with A/HKx31-OVA and effector (CD44hiCD62Llo), and memory (CD44hi) OT-Is were isolated and sort purified either 10 or 60 days after infection, respectively. (a–e) ChIP-seq using an Illumina HiSeq2000 was performed on naïve, effector and memory CD8+ OT-I T cells for both H3K4me3 and H3K27me3 histone PTMs. Data were mapped back to the mouse genome (version mm10). (a) Shown is the proportion of H3K4me3 or H3K27me3 sequence tags that mapped to promoter regions (−3 kb/+1 kb around the TSS, black bars), the gene body (+1kb TSS to 3’UTR, white bars) or intergenic regions (grey bars). (b) Gene promoters that exhibited differences in H3K4me3 or H3K27me3 enrichment were identified and the data transformed (log2) and converted into a heatmap (described in methods). Hierarchical clustering was then used to determine the relationship of histone methylation patterns observed in the promoter region between naïve, effector and memory CTL subsets. (c–e) The number of gene loci that exhibited differences in histone methylation patterns (c) within the promoter region was determined as described in the methods. Shown is the number (d) of genes and proportion (e) that exhibited distinct patterns of H3K4me3 (blue) or H3K27me3 (red) peaks between naïve, effector and memory OT-Is. Shown is the number of gene loci that exhibited specific patterns of H3K4me3 or H3K27me3 deposition between naïve, effector and memory OT-Is. (f) RNA-seq was performed on an Illumina HiSeq2000 and the reads mapped back to exons on the mouse genome (version mm10) to determine the transcriptional profiles of naïve, effector and memory CD8+ CD45.1+ OT-I before and after 5 hrs of OVA257 peptide stimulation. The number of differentially expressed (DE, log2>1.7) genes that were either upregulated or down-regulated were identified for naïve (blue), effector (red) and memory (green) CTL subsets. (g–i) The difference in H3K4me3 (green) or H3K27me3 (red) sequence tag density was determined for promoters identified with distinct patterns of peak enrichment (d, e) for effector vs naïve (g), memory vs naïve (h) and memory vs effector (i) CTL subsets. This was then correlated against the log2FC of DE gene expression between the same groups (effector vs naïve (g), memory vs naïve (h) and memory vs effector (i)).
While H3K4me3 is primarily associated with short domains of enrichment, typically associated with promoter regions (Wang et al., 2008), H3K27me3 typically associated with broad domains of enrichment that are associated with transcriptional repression (Jung et al., 2014; Young et al., 2011). Further, recent analyses have demonstrated that H3K27me3 can also be associated with promoter regions where it coincides with H3K4me3 and transcriptional activation (Bernstein et al., 2006; Roh et al., 2006; Young et al., 2011). Moreover it has been demonstrated that during T cell development, H3K27me3 deposition within promoter regions is dynamic and not always linked to transcriptional repression (Zhang et al., 2012). We could demonstrate that both H3K4me3 and H3K27me3 deposition was associated with a bimodal peak of enrichment around the TSS of gene loci (Supplementary Fig 2). As such, we were interested in the dynamics of H3K4me3 and H3K27me3 enrichment within gene promoters during T cell differentiation in response to infection. We first established the distribution of gene loci that had high (hi), intermediate (int) and low (lo) levels of H3K4me3 and H3K27me3 enrichment within gene promoters (Supplementary Fig. 3 a–d), and then determined the number of gene promoters with distinct patterns of H3K4me3 or H3K27me3 enrichment within naïve, effector and memory CTL (Supplementary Fig. 3e, f). There was very little change in the levels of H3K4me3 enrichment within the gene promoters between naïve, effector and memory CTL, regardless of the starting level (high, intermediate or low) (Supplementary Fig. 3e). It was therefore of interest to note that there were clearly changes in H3K27me3 deposition between naïve, effector and memory CTL (Supplementary Fig. 3f). Hence, in a similar fashion to H3K27me3 marking in developing thymocytes, H3K27me3 marking within the promoters of genes during virus-specific CTL differentiation is a dynamic process.
To further explore the dynamics of K4me3 and H3K27me3 during virus-specific differentiation we identified genes for which the presence or absence of peaks for one or both PTMs changed across the phases of differentiation, again focusing on the promoter region (−3 kb to +1 kb around the TSS). Overall, we identified 4,516 genes that exhibited changes in peaks of either H3K4me3 or H3K27me3 between naïve, effector and memory CTLs (Fig. 1b). We then determined the proportion and number of genes that shared distinct patterns of H3K4me3 or H3K27me3 deposition within the naïve, effector and memory CTL subsets (Fig. 1c–e). Strikingly, of these, 3,616 gene promoters (81%) exhibited modulation of the repressive H3K27me3 modification, with only 900 (19%) exhibiting modulation of H3K4me3 (Fig. 1d, e). To further validate our approach of identifying H3K27me3 peaks within gene promoters, we selected 6 gene loci that were identified as having a K27me3 peak within naïve CTLs that was then lost upon differentiation into effector and memory CTL, but had a range of sequence tags that mapped to the promoter (Supplementary Fig. 4). Importantly, the change in peak patterns within the promoters of these 6 gene loci between naïve and effector CTL (N27+E27- or N27-E27+) correlated with changes in the normalised count of H3K27me3 sequence tags (Supplementary Fig. 4a). To further validate that changes in H3K27me3 deposition determined by changes in H3K27me3 peaks reflected actual changes in H3K27me3 enrichment, we performed H3K27me3 ChIP on naïve and in vitro activated OT-I CTLs. The ChIP analysis on the promoters of the selected genes largely reflected the differences in H3K27me3 tag counts observed between naïve and effector CTLs. Hence, we concluded that our peak was a valid means of examining how changes in histone methylation within promoter regions influences virus-specific CTL differentiation in response to infection.
The loss of H3K27me3 or gain of H3K4me3 represent two distinct epigenetic mechanisms that could lead to transcriptional activation upon naïve CD8+ T cell differentiation. Therefore, we investigated whether these alternative mechanisms regulated functionally distinct gene groupings. Gene ontology (GO) analysis (Huang da et al., 2009) identified that the majority of genes that were activated by gaining H3K4me3 were involved specifically in T cell immune function and regulation (Table 1). In contrast, genes that were activated by loss of H3K27me3 had broader biological functions, being related to cellular differentiation, transcription and proliferation (Table 1). Thus, these data suggest that distinct histone methylation patterns are used to promote transcriptional activity of gene modules that have particular roles to play during naïve CD8+ T cell differentiation.
Table 1.
GO analysis of genes regulated via gain of H3K4me3 or loss of H3K27me3 modifications.
| H3K4me3 | H3K27me3 | ||||
|---|---|---|---|---|---|
| N− E+ M+ | N+ E− M− | ||||
| GO TERM | Number of genes |
Fisher Exact |
GO TERM | Number of genes |
Fisher Exact |
| chemotaxis | 10 | 6.1E-7 | peptide metabolic process | 8 | 1.3E-05 |
| immune response | 19 | 2.8E-6 | glutathione metabolic process | 6 | 5.8E-05 |
| locomotry behavior | 13 | 5.0E-6 | cellular amino acid derivative metabolic process | 13 | 3.1E-04 |
| positive regulation of immune system process | 11 | 3.2E-5 | carbohydrate phosphorylation | 3 | 4.1E-04 |
| regulation of lymphocyte activation | 9 | 5.1E-5 | positive regulation of macromolecule metabolic process | 33 | 1.4E-03 |
| positive regulation of leukocyte activation | 9 | 8.6E-5 | DNA metabolic process | 24 | 1.9E-03 |
| positive regulation of cell proliferation | 12 | 1.4E-5 | superoxide metabolic process | 4 | 2.1E-03 |
| regulation of immune effector process | 7 | 7.9E-5 | regulation of transcriptional pre-initiation complex assembly | 2 | 2.6E-03 |
| regulation of lymphocyte mediated immunity | 6 | 1.1E-4 | cellular macromolecule catabolic process | 31 | 2.7E-03 |
| positive regulation of cell activation | 7 | 1.9E-4 | transcription | 72 | 3.7E-03 |
| regulation of T cell activation | 7 | 2.8E-4 | co-enzyme metabolic process | 11 | 3.8E-03 |
| positive regulation of lymphocyte proliferation | 6 | 2.9E-04 | establishment of protein localisation | 32 | 4.4E-03 |
| positive regulation of cell communication | 9 | 4.0E-4 | positive regulation of macromolecule biosynthetic process | 27 | 4.9E-03 |
| positive regulation of signal transduction | 9 | 9.8E-04 | cellular response to stress | 22 | 5.1E-03 |
| defense response | 13 | 2.5E-03 | cell cycle | 29 | 9.4E-03 |
Naïve, effector and memory CTLs exhibit distinct transcriptional signatures both before and antigen specific stimulation
Virus-specific T cell differentiation is characterised by changes in transcriptional signatures that distinguish naïve, effector and memory CTLs (Best et al., 2013; Kaech et al., 2002). Importantly, however, these studies have only looked at the transcriptional profiles of CTLs in the resting state. To gain further understanding of the association between the distribution of histone PTMs in resting state CTLs, and gene transcription in resting and recently activated cells, we performed RNA-seq on naïve, effector (day 10) and memory (day 60) OT-I CTLs, before and after a brief (5 h) peptide stimulation (Supplementary Table 2, and Fig. 1f, total number of reads listed in supplemental methods). We identified 8,219 genes that were differentially expressed (DE) following stimulation, with 3,321 genes up-regulated, and 4,898 genes down-regulated, with the number of specific DE genes between each subset outlined in Figure 1f. Next, we performed multi-dimensional scaling on the RNA-seq data to determine the extent of similarity in gene transcription between each condition (Supplementary Fig. 5). To summarize these data, each CTL subset could be distinguished using this approach, regardless of their activation state (Supplementary Fig. 5), however, when the data were viewed as a whole, effector and memory CTLs were more similar to each other than either was to the naïve, both before and after stimulation.
We then sought to determine the correlation between differential gene expression (DE) and the extent of histone methylation patterns within gene promoters (Fig. 1g–i). We correlated the difference in gene transcripts between effector and naïve, memory and naïve or memory and effector CTL subsets, with the differences in normalised tag counts for H3K4me3 (Fig 1g–i, green plots) and H3K27me3 (Fig. 1g–i, red plots) for each pair. As expected, we observed that increased mRNA levels in effector and memory CTLs, compared to naïve CTLs, correlated with higher H3K4me3 enrichment within the promoter of effector and memory CTLs (Fig. 1g, h). Moreover, given the close relationship identified between memory and effector CTLs by MDS (Supplementary Fig. 5), it was not surprising to observe there was less difference in H3K4me3 levels between effector and memory CTLs (Fig. 5g). In contrast, we observed that greater levels of H3K27me3 within the promoter of effector and memory CTLs was associated with decreased mRNA levels compared to naïve CTLs (Fig. 5h, i). Of particular interest was the fact that rather than a direct correlation, differences in mRNA levels between naïve and effector and naïve and memory CTLs was associated with either the presence (Fig 5i, h, upper left quadrant) or absence (Fig. 5i, h, lower right quadrant) of H3K27me3 at the promoter. Thus, this analysis demonstrates that while increased H3K4me3 deposition correlates with increased transcriptional activation, it is more the presence or absence of H3K27me3 within the promoter that correlates with transcriptional repression and activation, rather than any differences in the degree of K27me3 enrichment.
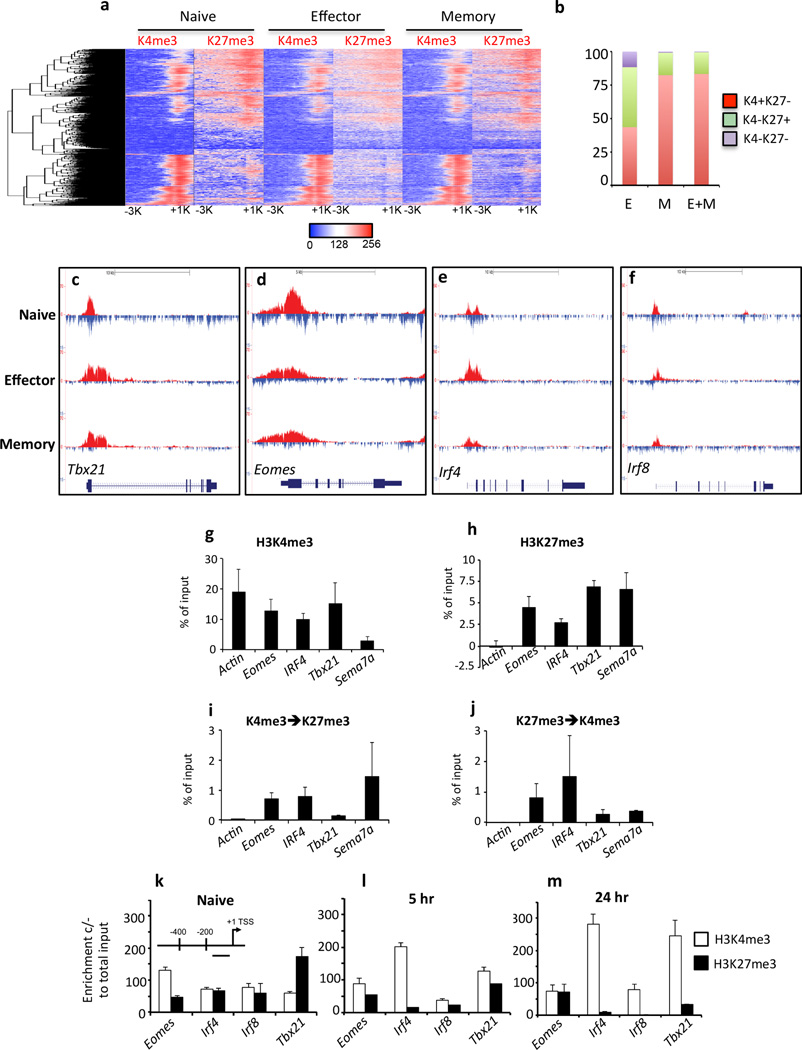
Figure 5. Bivalent loci within naïve CTLs rapidly resolve to a permissive H3K4me3+/H3K27me3-methylation signature.
(a) Bivalent loci were identified in naïve OT-I as having overlapping H3K4me3/H3K27me3 peaks within 400 bp of each other. Clustering analysis, based on log2 enrichment levels of histone methylation patterns within the promoter region was performed to generate a hierarchical list and the histone methylation patterns in effector and memory OT-I determined. (b) Methylation patterns of genes that were bivalent in naïve cells were determined in effector and memory CTL populations. Shown is the proportion of genes that lost H3K27me3 (K4+/K27-, red); lost H3K4me3 (K4−/K27+, green); or lost both methylation marks (K4−/K27−, puple) in effector (E), memory (M) or both effector and memory (E+M) subsets (c–f) Shown is the pattern of H3K4me3 (red, above line) and H3K27me3 (blue, below line) enrichment within the promoter, gene body and 3’UTR of bivalent genes within naïve, effector and memory OT-Is. (g–j) Sequential ChIP was performed on sort purified naïve (CD44loCD62Lhi) OTI by first immunoprecipitation with antibodies specific for H3K4me3 (g) or H3K27me3 (h) with samples probed for enrichment by RT-PCR using primers that targeted a 400 bp region just upstream of the TSS. These samples were then immunoprecipitated a second time with antibodies specific for either H3K27me3 (i) or H3K4me3 (j) and samples probed for enrichment as above. (k–m) ChIP for either H3K4me3 (white bars) or H3K27me3 (black bars) was performed on naïve OT-Is (k), or OT-Is stimulated with OVA257 peptide for 5 (l) or 24 (m) hrs. Enrichment for H3K4me3 or H3K27me3 was determined by Q-PCR, using primers that targeted the proximal promoter just upstream of the TSS (inset).
Hierarchical clustering of CTL genes identifies distinct epigenetic methylation patterns employed during CTL differentiation
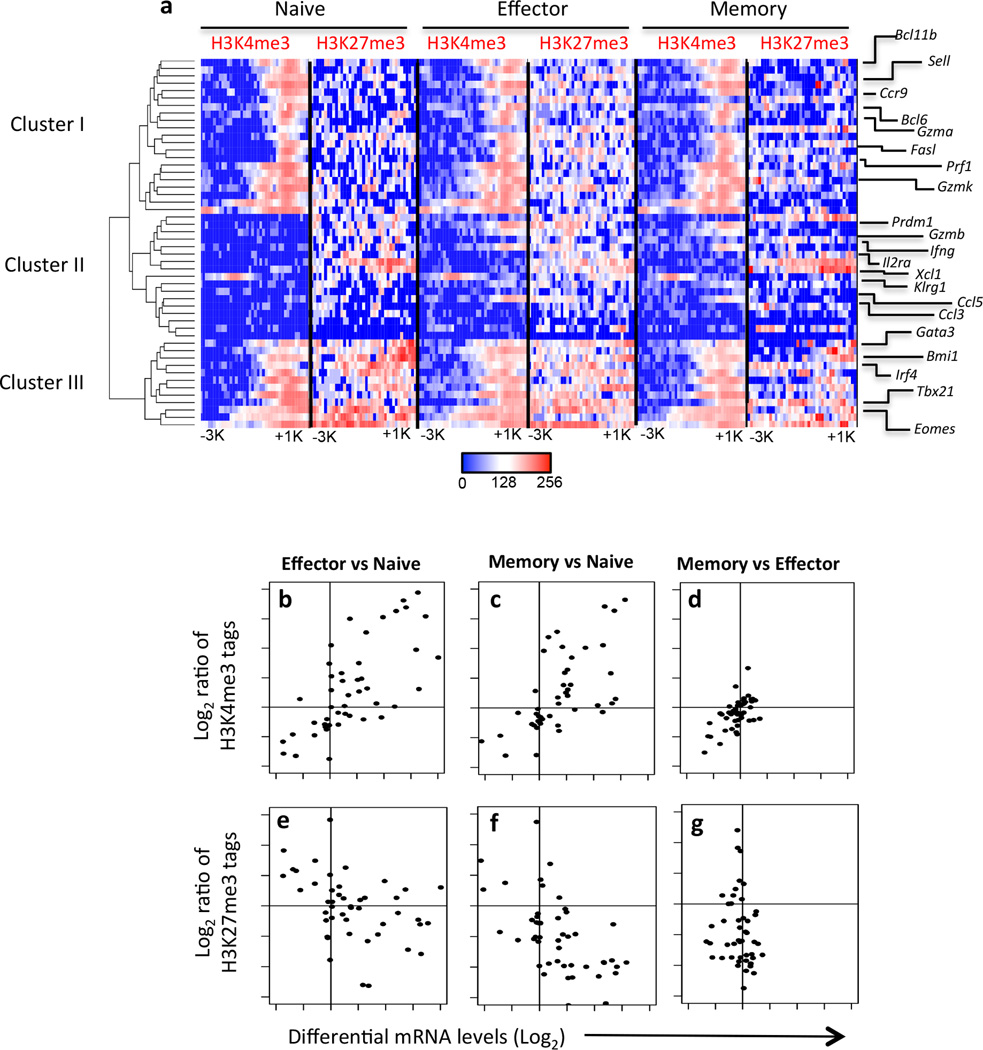
To gain a deeper understanding of how the genomic location and degree of H3K4me3 or H3K27me3 deposition may influence gene regulation during CTL differentiation, we generated heat maps showing the location and degree of enrichment of H3K4me3 and H3K27me3 PTMs within our defined promoter region (−3 kb/+1 kb around the TSS) of genes known to regulate various aspects of virus-specific CTL differentiation and effector function (listed in Supplementary Table 3). The heat maps were then organised by hierarchical clustering, with the clusters rooted to the naïve CTL subset (Fig. 2a).
Figure 2. Histone methylation patterns identify gene cohorts with distinct functional roles during CTL differentiation.
(a) The number of H3K4me3 or H3K27me3 sequences tags within −3 kg/+1 kb around the TSS for genes known to have roles in CTL differentiation (listed in Supplementary Table 2) was transformed (log2) and converted into a heatmap (described in methods). Hierarchical clustering was then used to determine the relationship of histone methylation patterns observed in the promoter regions between the listed genes. Shown is the list order of genes after clustering. (b–g) The ratio of sequence tags for H3K4me3 (panels b–d) or H3K27me3 (panels e–g) were correlated with the log2 fold change in transcriptional mRNA levels between naiive, effector and memory CTL subsets as described in Figure 1 for listed the gene listed in Supplementary Table 2.
Three major clusters were identified. The first cluster included genes such as Sell (CD62L), Bcl11b, Gzma, GzmK and Bcl2, each characterised by a permissive methylation signature (H3K4me3high/H3K27me3low) in naïve and memory CTLs, but with increased H3K27me3 deposition in the effector subset (Fig. 2a). The second cluster of genes was characterised by a repressive signature (H3K4me3−/H3K27me3+ in naïve CTLs that was altered to a permissive signature (H3K4me3+/H3K27me3+/−) in the memory and effector population (Fig. 2a). The majority of these genes encode effector molecules such as CCL3, CCL5, XCL1, IFNG, GZMB, or markers of effector CTL differentiation such as KLRG1, IL-2Rα (CD25) and PRDM1 (Blimp-1). The third and final cluster was characterised by co-localisation of H3K4me3 and H3K27me3 deposition at the same genomic location in the naïve subset. This cluster included transcription factors known to be important regulators of CTL differentiation such as TBX21 (T-bet), IRF4, BMII, GATA3 and EOMES (Fig. 2a). Strikingly, virus-specific CTL differentiation was associated with loss of the repressive H3K27me3 from these bivalent loci, and resolution to a permissive histone methylation pattern (H3K4me3+/H3K27me3−) (Fig. 2a). Thus, it appears that distinct epigenetic regulatory mechanisms are utilised to co-regulate the transcriptional activity of distinct gene modules that serve to underpin CTL differentiation and effector function.
To better define the relationship between histone PTMs and transcriptional activation, we utilised a similar approach, as described for Fig. 1g–i, and enumerated the number of H3K4me3 (Fig. 2b–d) and H3K27me3 (Fig. 2e–g) sequence tags within the promoters of the genes listed in Fig. 2a, and then determined the ratios of normalised tag counts between effector vs naïve, memory vs naïve and memory vs effector CTL populations. We then correlated this with the Log2FC in mRNA levels 5 hrs after peptide stimulation for the same comparisons. Similar to our global analysis of histone PTMs and differences between naïve, effector and memory CTL in the resting state (Fig. 1g–i), transcriptional upregulation of lineage-specific CTL gene expression after peptide stimulation was associated with a greater ratio of H3K4me3, and lower ratio of H3K27me3 in the promoters of effector and memory CTL compared to naïve CTL (Fig. 2bcef). In contrast, lower levels of mRNA transcription after 5hrs of peptide stimulation by effector and memory CTL when compared to naïve CTL was associated with a low ration of H3K4me3 and high ratio of H3K27me3 associated with gene promoters (Fig. 2bcef). There was little difference between effector and memory mRNA levels and either H3K4me3 (Fig. 2d) and H3K27me3 (Fig. 2g) ratios supporting the notion that both these CTL subsets respond similarly to peptide stimulation. Overall, these data demonstrate that despite distinct patterns of histone PTM deposition within the promoter of naïve, CTL lineage-specific gene loci, transcriptional activation is associated with establishment of a permissive histone signature upon differentiation in response to infection.
Changes in histone methylation across effector gene loci correlate with acquisition of lineage-specific CTL effector gene expression
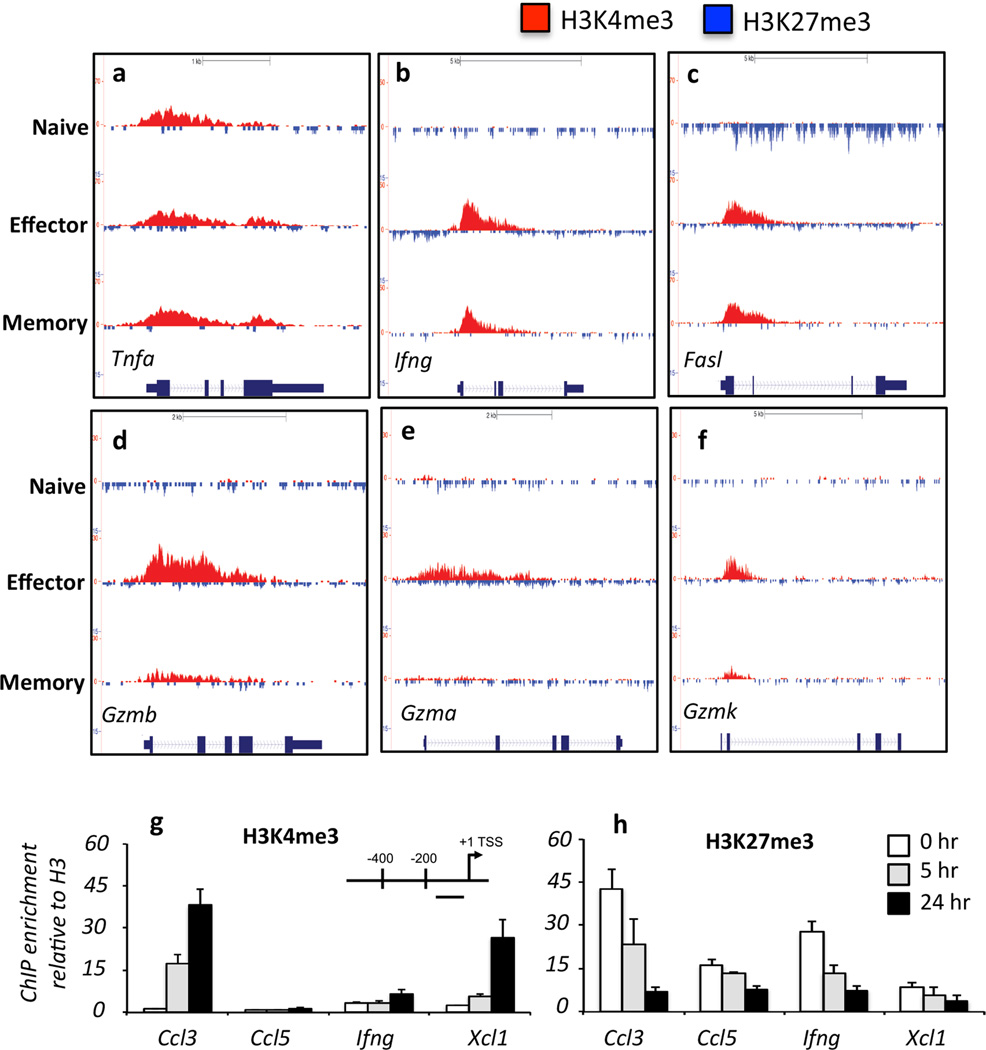
To gain a better insight into the dynamics of epigenetic remodelling across the gene body of key CTL gene loci, we mapped the methylation signatures across the gene body for selected effector genes in naïve, effector and memory CTLs (Fig. 3a–h; Supplementary Fig. 6). Extending previous observations, naïve OT-I CD8+ T cells were H3K4me3high/H3K27me3low at the Tnfa locus, consistent with the ability of naïve T cells to express TNF-α rapidly after stimulation (Brehm et al., 2005; Denton et al., 2011). Interestingly, upon differentiation, H3K4me3 levels at the Tnfa locus were enriched in the middle of the gene body, in effector and memory CTLs (Fig. 3a). A similar pattern was observed for Cd44 at the TSS, suggesting that transcribed genes may undergo further H3K4me3 methylation upon naïve CTL activation to reinforce active transcription (Supplementary Fig 6a). Other effector genes, such as Ifng and Fasl, had a repressive signature (H3K4me3low/H3K27me3med/high) in naive OT-I CD8+ T cells and acquired an active signature (H3K4me3high/H3K27me3low) at the TSS and 5’ region of the gene body upon differentiation into effector and memory CTLs (Fig. 3b, c). While a similar pattern was observed for the Gzm loci (Fig. 3d–f), the increased H3K4me3 deposition seen in effector CTLs was not as evident in memory CTLs (Fig. 3d–f). This diminished permissive histone methylation pattern correlates with the lower levels of Gzm expression observed in memory than effector CTLs (Jenkins et al., 2007; Moffat et al., 2009; Peixoto et al., 2007). Thus, the idea that permissive histone PTM signatures acquired upon memory CTL differentiation are maintained in the resting state at effector gene loci does not extend to all lineage-specific effector gene loci.
Figure 3.
(a–f) Shown is the pattern of H3K4me3 (red, above line) and H3K27me3 (blue, below line) peaks within the promoter, gene body and 3’UTR regions of CTL effector gene loci within naïve, effector and memory OT-Is. (g, h) ChIP for either H3K4me3 or H3K27me3 was performed on naïve OT-Is (white bars), or OT-Is stimulated with OVA257 peptide for 5 (grey bars) or 24 (black bars) hours. Enrichment for H3K4me3 or H3K27me3 was determined by Q-PCR, using primers that targeted the proximal promoter just upstream of the TSS (inset) of the specific gene loci.
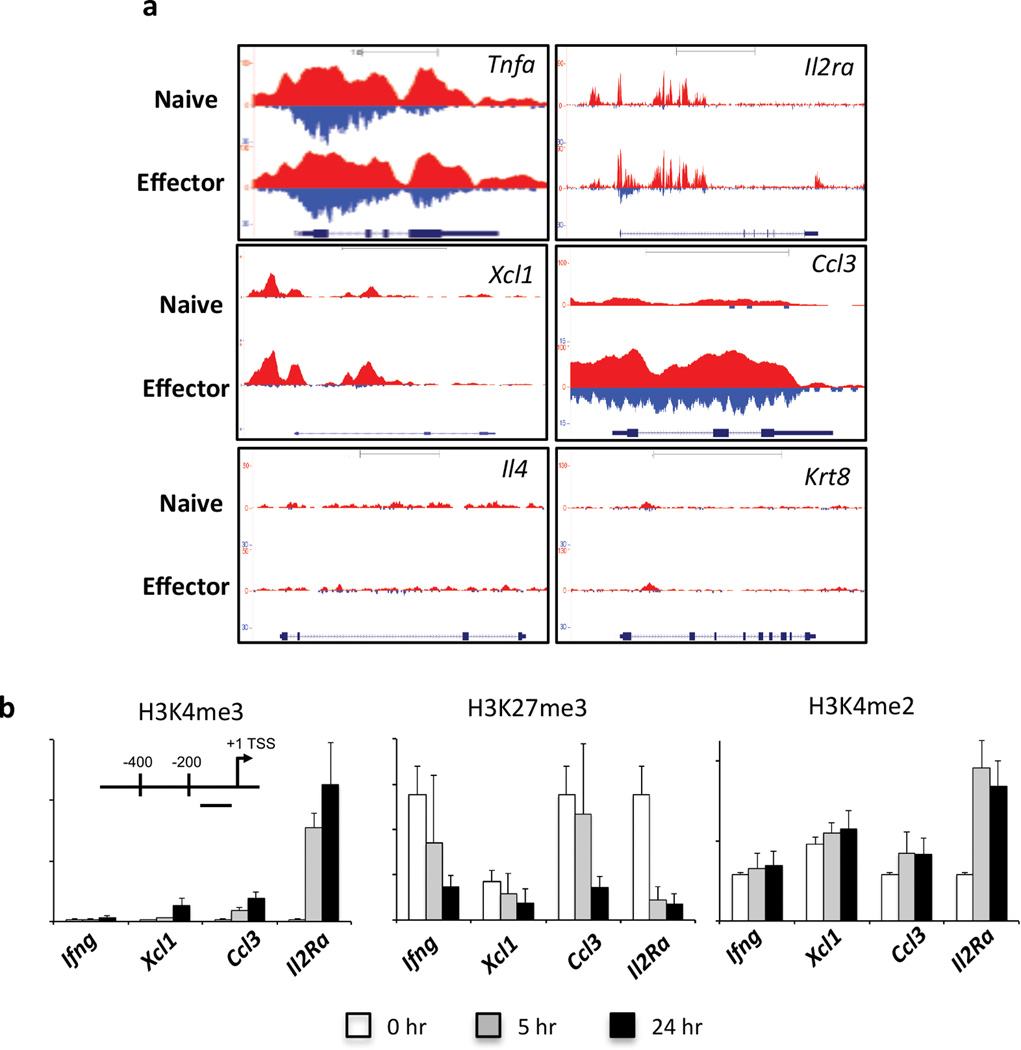
Naïve CD8+ T cells can exhibit rapid changes in cell surface marker expression and effector function upon activation, and prior to division. This includes up-regulation of activation markers such as CD69 and IL-2Rα (CD25) and expression of TNF-α, CCL3, CCL4 and XCL1 (Supplementary Fig. 7a–d). In agreement with previous studies, naïve CD8+ T cells did not demonstrate significant expression of IFN-γ (Supplementary Fig. 7b), IL-4, IL-17 or GZMB after short term in vitro stimulation (data not shown). We have previously demonstrated that the Tnfa locus of naïve CD8+ T cells exhibits a permissive H3K4me3+/H3K27me3− methylation signature (Denton et al., 2011), an observation confirmed by our ChIP-Seq analysis (Fig. 3a). It was therefore of interest to observe that despite being strongly transcribed within 5 hrs of stimulation, there was little H3K4me3 detected at the Ccl3, Xcl1 and Il2ra promoters of naïve CD8+ T cells (Supplementary Fig. 6b–d). This contrasted with another rapidly expressed cell surface marker, CD69, which exhibited H3K4me3 deposition (Supplementary Fig. 6e) and was highly expressed within 5 hrs of naïve CTL activation (Supplementary Fig. 7b).
To determine whether H3K4me3 deposition occurred rapidly after naïve T cell activation, we performed ChIP analysis for H3K4me3 (Fig. 3g) and H3K27me3 (Fig. 3h) at the proximal promoter region of selected effector gene loci shown to be rapidly transcribed after naïve CTL activation. Deposition of the permissive H3K4me3 PTM was apparent at the Ccl3 and Xcl1 promoters at 5 hrs after peptide stimulation, with further increases at 24 hrs post activation (Fig. 3g). This coincided with loss of the repressive H3K27me3 PTM within these same gene promoters at these time points (Fig. 3h). This rapid change in the histone signature correlated with rapid transcriptional upregulation of Ccl3 and Xcl1 upon naïve CTL activation (Supplementary Fig. 7). Despite loss of the repressive H3K27me3 modification at the Ifng promoter, there was little gain of H3K4me3 (Fig. 3g, h). These data are consistent with the observation that IFN-γ is not expressed rapidly upon activation of naïve CD8+ T cells (Supplementary Fig. 7), or indeed prior to cellular division (Denton et al., 2011; Lawrence and Braciale, 2004). Thus, the loss of the repressive H3K27me3 modification is not sufficient to enable Ifng gene expression. Thus, these data demonstrate that rapid epigenetic reprogramming occurs within naïve CD8+ T cells at selected gene loci upon activation and this correlates with rapid effector gene transcription before onset of cell division.
H3K4me2 patterns are predictive of rapid effector function upon naïve CD8+ T cell activation
In multi-potent hematopoietic stem cells, di-methylation of H3K4 (H3K4me2) has been reported to mark genes that become transcriptionally active upon lineage-commitment (Orford et al., 2008). Importantly, these lineage-specific genes, maintained as H3K4me2+/H3K4me3− in the undifferentiated state, acquire H3K4me3+ following differentiation suggesting that H3K4me2 may act a substrate enabling rapid transition to a permissive H3K4me3 PTM (Orford et al., 2008). We performed ChIP-Seq to determine the distribution of H3K4me2 in naïve OT-I CD8+ T cells and effector OT-I CTLs, and examined the extent of H3K4me2 and H3K4me3 deposition at effector genes rapidly up-regulated upon stimulation (Fig. 4a). H3K4me2 was observed in naïve CD8+ T cells at the Tnfa, Ccl3, Xcl1 and Il2Ra loci, all of which were rapidly transcribed and/or expressed after stimulation. The Tnfa locus was me2+/me3+ in both naïve and effector CTLs, whilst Il2Ra and Ccl3 were me2+/me3− in naïve CTLs, only becoming me2+/me3+ upon differentiation into effector CTLs. Interestingly, Xcl1 remained me2+/me3− in both naïve and effector CTLs (Fig. 4a). Importantly, genes not expressed by naïve or effector CTLs (e.g. Il4 and Krt8) remained H3K4me2−/me3− in both differentiation states (Fig. 4a).
Figure 4. H3K4me2 marks a subset of rapidly transcribed effector gene loci in naïve CTLs.
(a) Shown are the patterns of H3K4me2 (red, above line) and H3K4me3 (blue, below line) peaks within the promoter, gene body and 3’UTR of genes within naïve and effector OT-Is. (b) ChIP for either H3K4me3, H3K27me3 and H3K4me2 was performed on naïve OT-Is (white bars), or OT-Is stimulated with OVA257 peptide for 5 (grey bars) or 24 (black bars) hrs. Enrichment for H3K4me3, H3K27me3 or H3K4me2 was determined by Q-PCR, using primers that targeted the proximal promoter just upstream of the TSS (inset) of the specific genes.
Given that antigen stimulation of naïve CD8+ T cells resulted in expression of Ccl3, Xcl1 and Il2ra within 5 hrs despite there being a lack of the permissive H3K4me3 modification, it was of interest to determine how rapidly these loci became H3K4me3+. We stimulated naïve OT-I CD8+ T cells for 5 and 24 hrs and carried out ChIP to determine the levels of H3K4me2, H3K4me3 and H3K27me3 at the TSSs of the Ifng, Il2ra, Ccl3 and Xcl1 genes (Fig. 4b). There were changes in the epigenetic signatures observed at the Il2ra, Ccl3 and Xcl1 gene promoters, 5 and 24 hrs after activation (Fig. 4b). All three loci exhibited loss of H3K27me3, and increases in H3K4me3 deposition, particularly at 24 hrs after stimulation, with the greatest levels of H3K4me3 deposition being observed at the Il2ra promoter. Interestingly, there were also increased levels of H3K4me2 deposition at both 5 and 24 hrs after activation (Fig. 4b), most apparent at the Ccl3 and Il2ra promoters. There was also a trend for increased H3K4me2 at the Xcl1 promoter with little change observed at the Ifng promoter. Overall, these data demonstrate that within naive OT-I CD8+ T cells, a subset of effector gene loci that are marked with H3K4me2, are differentially regulated via dynamic changes in H3K4 methylation status, consistent with a role for H3K4me2 enrichment in maintaining selected gene loci in a transcriptionally ready state (Orford et al., 2008).
Identification and resolution of bivalent loci during virus-specific CTL differentiation
Previous studies have shown that genes involved in human ESC pluripotency and cell fate decisions upon differentiation exhibit co-localisation of the permissive H3K4me3 and the repressive H3K27me3 modifications (Araki et al., 2009; Bernstein et al., 2006). Upon ESC differentiation, the majority of these “bivalent” loci become heritably silenced, losing H3K4me3 while maintaining H3K27me3, ensuring that only lineage-specific genes are expressed. Our hierarchical clustering analysis of CTL lineage associated gene loci (Fig. 2a) and quantitation of H3K4me3 and H3K27me3 read counts (Supplementary Table 1) found that genes such as Tbx21, Irf4 and Eomes, TFs known to play a key role in virus-specific CTL differentiation (Cruz-Guilloty et al., 2009; Intlekofer et al., 2005; Man et al., 2013) were bivalent for both H3K4me3 and H3K27me3 histone methylation modifications in the naïve state but resolved to H3K4me3+/H3K27me3− upon differentiation. To further explore the role of promoter bivalency in regulating virus-specific CTL differentiation, we identified genes that were bivalent in naïve CTLs, and performed hierarchical clustering based on their enrichment patterns around the TSS (−3 kb/+1 kb) (Fig. 5a). We limited our analysis to H3K4me3 and H3K27me3 peaks that occurred within 400 bp of each other, reasoning that these modifications occurred on either the same or proximal nucleosomes. From this analysis we identified 2,254 genes that were bivalent in naïve CTLs. Upon naïve CTL differentiation, 43% of bivalent loci lost one or both modifications in effector and/or memory CTLs (Fig. 5b). However, in contrast to what is observed upon differentiation of ESCs, of those bivalent genes that resolved from naïve to effector/memory states, approximately 80% of gene loci that had resolved in either memory, or in effector and memory had lost H3K27me3 while maintaining H3K4me3 (Fig. 5b). Many of these genes were found to encode transcription factors including Tbx21, Eomes, Irf4 and Irf8 (Fig. 5c–f), Prdm1 (BLIMP1), Socs7, Zbtb7b (ThPOK) and Nfil3, as well as other genes such as Nrp1, Sema7a (Supplementary Fig. 8b–g). Thus, analysis of bivalent histone methylation patterns can be used to identify gene loci that are likely key for naïve CD8+ T cell lineage commitment into the effector/memory CTL subsets.
It was formally possible that the presence of overlapping H3K4me3 and H3K27me3 peaks identified by ChIP-seq represented heterogeneity within the CTL populations analysed. Thus, to determine whether the peaks we observed in our ChIP-seq data represented the presence of both modifications on the same nucleosome, we performed sequential ChIP (Fig 5g–j). We purified naïve (CD44lo CD62Lhi) OT-Is and initially immunoprecipitated chromatin with H3K4me3 antibody and were able to detect H3K4me3 deposition within the Actin, Eomes, IRF4, Tbx21 and Sema7a gene promoters (Fig. 5g). When we did a second immunoprecipitation using H3K27me3 antibodies on the material from the initial H3K4me3 pull-down, we were able to demonstrate that the H3K27me3 modification was enriched at the Eomes, IRF4, Tbx21 and Sema7a, but not the Actin promoter (Fig. 5i). Moreover, when we reversed the order of immunoprecipitation (H3K27me3, then H3K4me3) to validate our initial observations (Fig. 5h, j), we observed after the initial pull-down, enrichment of H3K27me3 at the Eomes, IRF4, Tbx21 and Sema7a, but not Actin gene promoter. As expected from our earlier observations, we observed evidence of H3K4me3 deposition at the Eomes, IRF4, Tbx21 and Sema7a promoters upon secondary immunoprecipitation with H3K4me3 antibody (Fig. 5j). There was no signal in the Actin control as it would not have been pulled-down with the initial H3K27me3 immunoprecipitation. Together these data suggest that identification of bivalent gene loci via the ChIPseq data accurately reflects the epigenetic state within naïve CTLs. To examine the dynamics of resolution of bivalent gene loci, we used ChIP to examine the level of H3K4me3 and H3K27me3 deposition within gene promoter regions for Tbx21, Eomes, Irf4 and Irf8 in naïve OT-I CD8+ T cells stimulated with cognate peptide for 0, 5 and 24 hrs (Fig. 5 k–m). All gene loci had measurable levels of both H3K4me3 and H3K27me3 within naïve CD8+ T cells (Fig. 5k). Upon T cell activation, there was loss of H3K27me3, with a concomitant increase in H3K4me3 deposition, within the Tbx21, Irf4 and Irf8 promoters at the 5 hr time point, and this was exacerbated at 24 hrs (Fig. 5l, m). There was little change in H3K27me3 levels at the Eomes promoter (Fig. 5l) correlating with its reported expression late after T cell activation (Cruz-Guilloty et al., 2009). The resolution to an active methylation signature (H3K4me3+/H3K27me3−) correlated with the rapid up-regulation of Tbx21, Irf4 and Irf8 mRNA levels 5 hrs after stimulation (Supplementary Table 2). These data suggest that within naïve CD8+ T cells, bivalent loci such as Tbx21, Irf8 and Irf4, are poised for activation and rapidly acquire a permissive histone methylation signature upon receipt of TCR signals. Thus, in a manner similar to ESCs, maintenance of bivalency at key gene loci in naïve CTL is an epigenetic mechanism that helps ensure rapid transcriptional activation of factors that are known early drivers of CTL lineage commitment and is a key step in initiating programmed CTL differentiation.
Discussion
Our genome-wide mapping of H3K4me3 and H3K27me3 deposition during CTL differentiation demonstrated that T cell activation results in rapid changes in histone methylation patterns and the establishment of transcriptionally permissive states. Moreover, the specific pattern of histone methylation appeared to distinguish genes associated with distinct functional outcomes. Thus, the data suggest that, upon T cell activation, distinct histone methylation regulatory mechanisms are rapidly engaged to regulate distinct transcriptional signatures during CTL differentiation and help ensure the establishment of effective cellular immunity.
Given the large number of gene loci that underwent transcriptional activation upon stimulation, it was surprising that deposition of H3K4me3 and subsequent establishment of a permissive histone methylation signatures accounted for approximately 20% of the changes in histone methylation patterns observed between naïve, effector and memory CTLs. Strikingly, this mechanism for enabling transcriptional activation was most prominent for genes associated with lineage-specific T cell function. The presence of H3K4me3, and a lack of H3K27me3 PTMs at gene promoters are typically associated with a permissive transcriptional state. Our genome-wide analysis of H3K4me3 and H3K27me3 patterns in naïve, effector and memory CTLs supported previous single gene analyses (Araki et al., 2008; Denton et al., 2011; Northrop et al., 2006; Zediak et al., 2011) demonstrating that acquisition and maintenance of CTL specific effector function upon naïve T cell differentiation correlated with establishment of a permissive epigenetic signature at gene promoters.
There were observable differences in H3K27me3 and H3K4me3 patterns within the different effector genes. For example, whilst naïve CD8+ T cells exhibited little H3K4me3 enrichment at the Ifng and Fasl promoters, the levels of H3K4me3 deposition were similar between the effector and memory subsets. This supports earlier observations that memory CTL maintain a permissive histone PTM signature at effector genes enabling rapid gene expression upon re-activation(Denton et al., 2011; Zediak et al., 2011). In contrast, the GzmA, GzmB and GzmK gene promoters exhibited much lower levels of the permissive H3K4me3 modification in memory CTLs compared to effector CTLs. The lack of a permissive histone methylation signature at these genes in memory CTLs supports the observed low levels of mRNA and protein expression in resting, memory virus-specific CTLs (Jenkins et al., 2007; Moffat et al., 2009). The presence of distinct regulatory mechanisms that control cytokine versus cytolytic gene expression likely reflects a need for tighter control of GZM expression. It has been reported that both GZMA and GZMK expression within effector CTLs requires extended differentiation with GZMA most highly expressed by effector CTLs at the site of infection (Jenkins et al., 2007; Moffat et al., 2009). This would help ensure appropriate expression of such potent effector molecules is found primarily at the site of infection, where it is likely to contribute to effective pathogen clearance whilst avoiding potential immunopathology.
Interestingly, our data demonstrated that modulation of H3K27me3 deposition was the primary regulatory mechanism for activation of genes within naïve, effector and memory CTLs. In particular, a large proportion of genes that possessed H3K27me3 peaks in naïve OT-I CD8+ T cells also had H3K4me3 peaks within the same promoter region. In a recent study assessing changes in chromatin structure during T cell development (Zhang et al., 2012), it was demonstrated that H3K27me3, typically a histone PTM associated with transcriptional repression, was often deposited at genes after transcription had already been shut-down, indicating that the likely role of this modification is not to directly regulate transcription, as is generally accepted, but rather to stabilise repression. Further, there appeared to be multiple mechanisms of transcriptional repression, since only approximately a third of genes that were developmentally repressed during thymic differentiation were associated H3K27me3. Finally, by mapping genome wide H3K27me3 enrichment at different stages of thymocyte development, this study showed that marking of gene loci was highly dynamic and reversible. This finding is consistent with our observations that data clearly show that loss of H3K27me3 occurred rapidly after T cell activation (5–24 hrs), suggesting that transcriptional activation can be initiated after loss of the repressive H3K27me3 modification without the need for de novo deposition of H3K4me3 and that this occurs prior to initiation of cell division.
An important consideration for this study is what represents a biologically relevant difference in H3K4me3 or H3K27me3 sequence tag deposition between naïve, effector and memory CTL. To that end, we validated that change in peaks correlated with changes in tag density, and that such changes could be validated by ChIP analysis (Supplementary Fig. 4). From this were able to determine that while increased H3K4me3 correlated with increased transcriptional activation, it as more the presence or absence of H3K27me3 within the promoter that correlated with transcriptional repression or activation, rather than any differences in the degree of K27me3 enrichment. Further, we were able to determine that specific changes in histone PTMs during CTL differentiation identified distinct functional gene groupings (Table 1). Moreover, the observation that genuine epigenetic bivalency for H3K4me3 and H3K27me3 within the promoters identified a subset of gene loci key for CD8+ T cell lineage commitment, and that the this bivalent state resolved quickly upon activation prior to cellular division also provides biological insights into the how histone PTMs can co-ordinate regulated gene transcription upon T cell activation.
Thus, we hypothesise that H3K27me3 deposition within naïve CD8+ T cells acts as a brake on transcription at specific genes already marked with the permissive H3K4me3 modification. We speculate that this pattern of histone methylation within naïve CD8+ T cells represents an epigenetic state whereby rapid removal of the repressive signature enables rapid transcriptional activation of genes that underpin the reported instructional differentiation program induced by TCR signals (Kaech and Ahmed, 2001; van Stipdonk et al., 2003). To that end, gene ontology analysis demonstrated that genes regulated in this manner played roles in cellular processes such as transcriptional activation, cellular division and cellular metabolism, all processes required for sustaining a rapid proliferative cellular response, a cardinal feature of adaptive T cell immunity in response to infection.
Of particular interest was the identification of gene promoters that exhibited overlapping peaks of H3K4me3 and H3K27me3 deposition, so called bivalent genes (Bernstein et al., 2006). This specific pattern of histone methylation was observed within the gene promoters of Tbx21 and Eomes, two transcription factors important for effector and memory CTL differentiation (Cruz-Guilloty et al., 2009; Intlekofer et al., 2005; Joshi et al., 2007). Further, this bivalent pattern also identified other transcription factors known to play key roles in T cell differentiation, such as Prdm1 (Kallies et al., 2009) and Irf4 (Cretney et al., 2011; Nayar et al., 2012). Analysis of our ChIP-seq data identified other bivalent loci within naïve CTL, such as Irf8 and Zbtb7b (ThPOK), both recently implicated in promoting effective CTL differentiation (Miyagawa et al., 2012; Setoguchi et al., 2009). Other bivalent genes that may have an as yet unknown role in effector/memory CTL differentiation and function included Nrp1 (implicated in the formation of T cell-dendritic cell conjugates (Romeo et al., 2002)), Sema7a (key for promoting T cell dependent inflammatory responses (Suzuki et al., 2007)) and Socs7 (able to inhibit nuclear translocation of STAT5, a key mediator of IL-2 signalling; (Martens et al., 2005)). Thus, further interrogation of our data set may lead to the identification of genes that have previously undescribed roles in determining effector and/or memory CTL differentiation.
H3K4me3/H3K27me3 bivalency is present within the promoters of developmentally important genes within ESCs, whereby the majority of bivalent loci resolve to a repressive methylation signature by losing the active H3K4me3 modification. In this way, bivalent loci within ESCs are either rapidly shut-down, ensuring appropriate cell lineage-specific patterns of gene expression (Bernstein et al., 2006; Hawkins et al., 2010). However, in contrast to ESC differentiation, the majority of bivalent loci within naïve CD8+ T cells resolved to a permissive histone methylation pattern upon effector/memory CTL differentiation. Moreover, we demonstrated that resolution of bivalent domains within Tbx21, Irf4 and Irf8 promoter regions occurred within 24 hrs, prior to cellular division, and coinciding with rapid transcription of these genes. Therefore, these data support the notion that naïve CD8+ T cells are epigenetically pre-programmed for rapid expression of key genes that underpin the instructional differentiation program enacted upon receipt of TCR activation signals. Importantly, rapid resolution of the bivalent Prdm1 and Eomes gene promoters was not observed, and supports the observation that these genes are expressed, and play a role at later stages of effector and memory CTL differentiation (Cruz-Guilloty et al., 2009; Intlekofer et al., 2005). During T cell development, immature T cells undergo extensive changes to their epigenetic and transcriptional signatures resulting in a molecular blueprint that serves to direct lineage-specific T cell fate (Zhang et al., 2012). In particular, a recent analysis demonstrated that dynamic changes in H3K4me3/H3K27me3 bivalency within developing thymocytes occurs at promoters that mark transcriptional regulators (Vigano et al., 2013). In a manner similar to ESCs, resolution of bivalent loci during thymocyte development was mostly due to loss of H3K4me3 suggesting that many loci are repressed. That said, other loci were observed to establish bivalency at the later stages of T cell development and are therefore likely maintained within mature, naïve T cells once in the periphery. Therefore, the molecular blueprint apparent within naïve CD8+ T cells enabling rapid differentiation upon activation, including bivalency, is programmed during T cell development and subsequent commitment to the CD8+ T cell lineage.
Within naïve CD8+ T cells, we also identified a number of genes, such as the Il2ra, Ccl3 and Xcl1 that lacked the active H3K4me3 modification, but were rapidly transcribed and expressed upon TCR activation. Genome-wide analysis of H3K4 methylation patterns within pluripotent erythroid progenitor cells demonstrated that whilst there is strong concordance with H3K4me2 and H3K3me3 deposition at most genes, a subset of hematopoietic-specific genes were H3K4me2+/H3K4me3− in the undifferentiated state (Orford et al., 2008). Upon receipt of differentiation signals, these genes became H3K4me2+/H3K4me3+ and transcriptionally active. Thus, this specific histone methylation signature appears to mark genes that are poised for expression in a cell-lineage specific manner. A similar pattern emerges upon genome-wide analysis of H3K4 methylation patterns between naïve CD8+ T cells and effector CTLs. The Il2ra and Ccl3 loci only become concordant for both H3K4 di- and tri-methylation upon differentiation into effector CTLs. As has been consistently observed for the other dynamic changes in histone methylation patterns in this study, the acquisition of H3K4me3 at these genes occurred rapidly after naïve CD8+ T cell activation. H3K4me2 deposition within promoters of genes may therefore represent a substrate allowing for rapid transition to a permissive histone methylation pattern upon receipt of appropriate signals, enabling rapid gene transcription. However, we noted that the number of gene loci that exhibited this transition from me2+me3- in naïve to me2+/me3+ was small (308 genes, data not shown). Nevertheless, a preliminary analysis demonstrated enrichment for genes involved in cell adhesion, immune response and immune defence (data not shown). Thus, this may represent yet another epigenetic regulatory mechanism that ensures appropriate gene expression to promote effective T cell activation and function in response to infection.
This study adds to a growing appreciation of how specific histone PTMs underpin regulation of gene transcription within T cells. Using a CD4 TH1 versus TH2 model system, Allan and colleagues (Allan et al.) recently demonstrated that epigenetic silencing of the TH1 associated Tbx21 gene locus was dependent on deposition of H3K9me3, a process mediated by the histone methyltransferase, Suv39H1. This process of Suv39H1-mediated trimethylation of H3K9 is an initial step that triggers histone deacetylation and binding of transcriptional repressor protein complexes that stably silence targeted loci (Fujita et al., 2003). Importantly, Suv39H1 deficient T cells were unable to stably repress TH1 gene expression, even after TH2 differentiation, demonstrating that this particular histone modification plays a key role in gene silencing during T cell differentiation, and this is a mechanism that ensures lineage specific effector gene expression. Another study examined the dynamics of genome wide modulation of H3 acetylation, H3K4me2 and H3K27me3 during T cell development and concluded that diverse epigenetic mechanisms were utilised to regulate gene transcriptional activation or repression at distinct stages of T cell development (Zhang et al., 2012). For example, acetylated histones appeared to rate-limit transcription, probably by directly regulating promoter accessibility, while H3K27me3 appeared to contribute to gene repression by operating "after the fact" by stabilising a transcriptionally silent signature, rather than directly repressing transcription. Moreover, H3K4me2 functioned as an intermediate between unmethylated H3K4, and the activating trimethylated state at gene promoters, thus allowing rapid transcriptional change following differentiation signals. This latter finding in developing T cells aligns with our observations that a subset of rapidly transcribed genes within naïve OTI CD8+T cells are enriched for H3K4me2 within their promoters, and that this is rapidly converted to H3K4me3 upon activation. Other genome wide studies have demonstrated that histone PTMs such as H3K27Ac and H3K4me1 can be used to identify enhancer regulatory elements within non-coding regions of the genome. It will be of particular interest to examine the dynamics of these specific epigenetic marks within intergenic regions as these may identify enhancers that are differentially regulated during CD8+ T cell differentiation. It will be of particular interest to examine the dynamics of these specific epigenetic marks within intergenic regions as these may identify enhancers that are differentially utilised during CD8+ T cell differentiation.
An intriguing question arising from this study is why is there a need for distinct histone methylation patterns to regulate distinct transcriptional signatures upon naïve CTL activation? One hypothesis is that each histone methylation pattern forms a unique platform that allows the co-ordinated regulation of a cohort of gene loci. This may involve the recruitment of protein complexes that facilitate rapid changes in transcriptional activity (Kouzarides, 2007). For example Tbx21 determines CD4+ TH1 cell differentiation via recruitment of H3K27 demethylases to the Ifng gene promoter, resulting in establishment of a permissive histone methylation signature (Miller et al., 2010). Thus, a similar mechanism may also be involved in CTL differentiation, and this is currently being investigated. Identification of such pathways and molecular targets may in the future lead to a better understanding of the molecular mechanisms that underpin acquisition and establishment of effective CTL immunity. This in turn has the potential to provide specific targets that can be modulated to alter the dynamics of histone PTMs, thus stably promoting or repressing CTL immune function where appropriate.
Methods
Methods and any associated references are available from the online version of the paper.
Supplementary Material
Highlights.
Specific H3K4me3/H3K27me3 patterns in CTL identify functionally distinct gene groups.
Many CTL specific transcription factors are bivalent for H3K4me3 and H3K27me3.
Bivalent loci largely resolve to a permissive signature rapidly after activation.
Acknowledgements
The authors would like to thank Drs Nicole La Gruta and Peter Doherty for discussion and helpful suggestions. This work was supported by grants from the National Health and Medical Research Council of Australia (Program Grant #5671222 awarded to awarded to S.J.T. and A.K.; Project grant # APP1003131 (awarded to S.J.T and S.R.). S.J.T is supported by an Australian Research Council Future Fellowship; The Melbourne WHO Collaborating Centre for Reference and Research on Influenza is supported by the Australian Government Department of Health and Ageing.
References
- Allan RS, Zueva E, Cammas F, Schreiber HA, Masson V, Belz GT, Roche D, Maison C, Quivy JP, Almouzni G, Amigorena S. An epigenetic silencing pathway controlling T helper 2 cell lineage commitment. Nature. 2012;487:249–253. doi: 10.1038/nature11173. [DOI] [PubMed] [Google Scholar]
- Ansel KM, Lee DU, Rao A. An epigenetic view of helper T cell differentiation. Nat Immunol. 2003;4:616–623. doi: 10.1038/ni0703-616. [DOI] [PubMed] [Google Scholar]
- Araki Y, Fann M, Wersto R, Weng NP. Histone acetylation facilitates rapid and robust memory CD8 T cell response through differential expression of effector molecules (eomesodermin and its targets: perforin and granzyme B) J Immunol. 2008;180:8102–8108. doi: 10.4049/jimmunol.180.12.8102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araki Y, Wang Z, Zang C, Wood WH, 3rd, Schones D, Cui K, Roh TY, Lhotsky B, Wersto RP, Peng W, et al. Genome-wide analysis of histone methylation reveals chromatin state-based regulation of gene transcription and function of memory CD8+ T cells. Immunity. 2009;30:912–925. doi: 10.1016/j.immuni.2009.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein BE, Mikkelsen TS, Xie X, Kamal M, Huebert DJ, Cuff J, Fry B, Meissner A, Wernig M, Plath K, et al. A bivalent chromatin structure marks key developmental genes in embryonic stem cells. Cell. 2006;125:315–326. doi: 10.1016/j.cell.2006.02.041. [DOI] [PubMed] [Google Scholar]
- Best JA, Blair DA, Knell J, Yang E, Mayya V, Doedens A, Dustin ML, Goldrath AW. Transcriptional insights into the CD8(+) T cell response to infection and memory T cell formation. Nat Immunol. 2013;14:404–412. doi: 10.1038/ni.2536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brehm MA, Daniels KA, Welsh RM. Rapid production of TNF-alpha following TCR engagement of naive CD8 T cells. J Immunol. 2005;175:5043–5049. doi: 10.4049/jimmunol.175.8.5043. [DOI] [PubMed] [Google Scholar]
- Cretney E, Xin A, Shi W, Minnich M, Masson F, Miasari M, Belz GT, Smyth GK, Busslinger M, Nutt SL, Kallies A. The transcription factors Blimp-1 and IRF4 jointly control the differentiation and function of effector regulatory T cells. Nature immunology. 2011;12:304–311. doi: 10.1038/ni.2006. [DOI] [PubMed] [Google Scholar]
- Cruz-Guilloty F, Pipkin ME, Djuretic IM, Levanon D, Lotem J, Lichtenheld MG, Groner Y, Rao A. Runx3 and T-box proteins cooperate to establish the transcriptional program of effector CTLs. The Journal of experimental medicine. 2009;206:51–59. doi: 10.1084/jem.20081242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denton AE, Russ BE, Doherty PC, Rao S, Turner SJ. Differentiation-dependent functional and epigenetic landscapes for cytokine genes in virus-specific CD8+ T cells. Proc Natl Acad Sci U S A. 2011;108:15306–15311. doi: 10.1073/pnas.1112520108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita N, Watanabe S, Ichimura T, Tsuruzoe S, Shinkai Y, Tachibana M, Chiba T, Nakao M. Methyl-CpG binding domain 1 (MBD1) interacts with the Suv39h1-HP1 heterochromatic complex for DNA methylation-based transcriptional repression. J Biol Chem. 2003;278:24132–24138. doi: 10.1074/jbc.M302283200. [DOI] [PubMed] [Google Scholar]
- Hawkins RD, Hon GC, Lee LK, Ngo Q, Lister R, Pelizzola M, Edsall LE, Kuan S, Luu Y, Klugman S, et al. Distinct epigenomic landscapes of pluripotent and lineage-committed human cells. Cell Stem Cell. 2010;6:479–491. doi: 10.1016/j.stem.2010.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang da W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nature protocols. 2009;4:44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- Intlekofer AM, Takemoto N, Wherry EJ, Longworth SA, Northrup JT, Palanivel VR, Mullen AC, Gasink CR, Kaech SM, Miller JD, et al. Effector and memory CD8+ T cell fate coupled by T-bet and eomesodermin. Nature immunology. 2005;6:1236–1244. doi: 10.1038/ni1268. [DOI] [PubMed] [Google Scholar]
- Jenkins MR, Kedzierska K, Doherty PC, Turner SJ. Heterogeneity of effector phenotype for acute phase and memory influenza A virus-specific CTL. J Immunol. 2007;179:64–70. doi: 10.4049/jimmunol.179.1.64. [DOI] [PubMed] [Google Scholar]
- Jenkins MR, Webby R, Doherty PC, Turner SJ. Addition of a prominent epitope affects influenza A virus-specific CD8+ T cell immunodominance hierarchies when antigen is limiting. Journal of Immunology. 2006;177:2917–2925. doi: 10.4049/jimmunol.177.5.2917. [DOI] [PubMed] [Google Scholar]
- Joshi NS, Cui W, Chandele A, Lee HK, Urso DR, Hagman J, Gapin L, Kaech SM. Inflammation directs memory precursor and short-lived effector CD8(+) T cell fates via the graded expression of T-bet transcription factor. Immunity. 2007;27:281–295. doi: 10.1016/j.immuni.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung YL, Luquette LJ, Ho JW, Ferrari F, Tolstorukov M, Minoda A, Issner R, Epstein CB, Karpen GH, Kuroda MI, Park PJ. Impact of sequencing depth in ChIP-seq experiments. Nucleic acids research. 2014;42:e74. doi: 10.1093/nar/gku178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaech SM, Ahmed R. Memory CD8+ T cell differentiation: initial antigen encounter triggers a developmental program in naive cells. Nat Immunol. 2001;2:415–422. doi: 10.1038/87720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaech SM, Hemby S, Kersh E, Ahmed R. Molecular and functional profiling of memory CD8 T cell differentiation. Cell. 2002;111:837–851. doi: 10.1016/s0092-8674(02)01139-x. [DOI] [PubMed] [Google Scholar]
- Kagi D, Ledermann B, Burki K, Seiler P, Odermatt B, Olsen KJ, Podack ER, Zinkernagel RM, Hengartner H. Cytotoxicity mediated by T cells and natural killer cells is greatly impaired in perforin-deficient mice. Nature. 1994;369:31–37. doi: 10.1038/369031a0. [DOI] [PubMed] [Google Scholar]
- Kallies A, Xin A, Belz GT, Nutt SL. Blimp-1 transcription factor is required for the differentiation of effector CD8(+) T cells and memory responses. Immunity. 2009;31:283–295. doi: 10.1016/j.immuni.2009.06.021. [DOI] [PubMed] [Google Scholar]
- Kouzarides T. Chromatin modifications and their function. Cell. 2007;128:693–705. doi: 10.1016/j.cell.2007.02.005. [DOI] [PubMed] [Google Scholar]
- La Gruta NL, Turner SJ, Doherty PC. Hierarchies in cytokine expression profiles for acute and resolving influenza virus-specific CD8+ T cell responses: correlation of cytokine profile and TCR avidity. J Immunol. 2004;172:5553–5560. doi: 10.4049/jimmunol.172.9.5553. [DOI] [PubMed] [Google Scholar]
- Lalvani A, Brookes R, Hambleton S, Britton WJ, Hill AV, McMichael AJ. Rapid effector function in CD8+ memory T cells. J Exp Med. 1997;186:859–865. doi: 10.1084/jem.186.6.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence CW, Braciale TJ. Activation, differentiation, and migration of naive virus-specific CD8+ T cells during pulmonary influenza virus infection. J Immunol. 2004;173:1209–1218. doi: 10.4049/jimmunol.173.2.1209. [DOI] [PubMed] [Google Scholar]
- Man K, Miasari M, Shi W, Xin A, Henstridge DC, Preston S, Pellegrini M, Belz GT, Smyth GK, Febbraio MA, et al. The transcription factor IRF4 is essential for TCR affinity-mediated metabolic programming and clonal expansion of T cells. Nat Immunol. 2013;14:1155–1165. doi: 10.1038/ni.2710. [DOI] [PubMed] [Google Scholar]
- Martens N, Uzan G, Wery M, Hooghe R, Hooghe-Peters EL, Gertler A. Suppressor of cytokine signaling 7 inhibits prolactin, growth hormone, and leptin signaling by interacting with STAT5 or STAT3 and attenuating their nuclear translocation. The Journal of biological chemistry. 2005;280:13817–13823. doi: 10.1074/jbc.M411596200. [DOI] [PubMed] [Google Scholar]
- Miller SA, Mohn SE, Weinmann AS. Jmjd3 and UTX play a demethylaseindependent role in chromatin remodeling to regulate T-box family member-dependent gene expression. Molecular cell. 2010;40:594–605. doi: 10.1016/j.molcel.2010.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyagawa F, Zhang H, Terunuma A, Ozato K, Tagaya Y, Katz SI. Interferon regulatory factor 8 integrates T-cell receptor and cytokine-signaling pathways and drives effector differentiation of CD8 T cells. Proc Natl Acad Sci U S A. 2012;109:12123–12128. doi: 10.1073/pnas.1201453109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffat JM, Gebhardt T, Doherty PC, Turner SJ, Mintern JD. Granzyme A expression reveals distinct cytolytic CTL subsets following influenza A virus infection. Eur J Immunol. 2009;39:1203–1210. doi: 10.1002/eji.200839183. [DOI] [PubMed] [Google Scholar]
- Nayar R, Enos M, Prince A, Shin H, Hemmers S, Jiang JK, Klein U, Thomas CJ, Berg LJ. TCR signaling via Tec kinase ITK and interferon regulatory factor 4 (IRF4) regulates CD8+ T-cell differentiation. Proc Natl Acad Sci U S A. 2012;109:E2794–E2802. doi: 10.1073/pnas.1205742109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northrop JK, Thomas RM, Wells AD, Shen H. Epigenetic remodeling of the IL-2 and IFN-gamma loci in memory CD8 T cells is influenced by CD4 T cells. J Immunol. 2006;177:1062–1069. doi: 10.4049/jimmunol.177.2.1062. [DOI] [PubMed] [Google Scholar]
- Oehen S, Brduscha-Riem K. Differentiation of naive CTL to effector and memory CTL: correlation of effector function with phenotype and cell division. J Immunol. 1998;161:5338–5346. [PubMed] [Google Scholar]
- Orford K, Kharchenko P, Lai W, Dao MC, Worhunsky DJ, Ferro A, Janzen V, Park PJ, Scadden DT. Differential H3K4 methylation identifies developmentally poised hematopoietic genes. Dev Cell. 2008;14:798–809. doi: 10.1016/j.devcel.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peixoto A, Evaristo C, Munitic I, Monteiro M, Charbit A, Rocha B, Veiga-Fernandes H. CD8 single-cell gene coexpression reveals three different effector types present at distinct phases of the immune response. J Exp Med. 2007;204:1193–1205. doi: 10.1084/jem.20062349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roh TY, Cuddapah S, Cui K, Zhao K. The genomic landscape of histone modifications in human T cells. Proc Natl Acad Sci U S A. 2006;103:15782–15787. doi: 10.1073/pnas.0607617103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romeo PH, Lemarchandel V, Tordjman R. Neuropilin-1 in the immune system. Advances in experimental medicine and biology. 2002;515:49–54. doi: 10.1007/978-1-4615-0119-0_4. [DOI] [PubMed] [Google Scholar]
- Santos-Rosa H, Schneider R, Bannister AJ, Sherriff J, Bernstein BE, Emre NC, Schreiber SL, Mellor J, Kouzarides T. Active genes are tri-methylated at K4 of histone H3. Nature. 2002;419:407–411. doi: 10.1038/nature01080. [DOI] [PubMed] [Google Scholar]
- Setoguchi R, Taniuchi I, Bevan MJ. ThPOK derepression is required for robust CD8 T cell responses to viral infection. Journal of immunology. 2009;183:4467–4474. doi: 10.4049/jimmunol.0901428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki K, Okuno T, Yamamoto M, Pasterkamp RJ, Takegahara N, Takamatsu H, Kitao T, Takagi J, Rennert PD, Kolodkin AL, et al. Semaphorin 7A initiates T-cell-mediated inflammatory responses through alpha1beta1 integrin. Nature. 2007;446:680–684. doi: 10.1038/nature05652. [DOI] [PubMed] [Google Scholar]
- van Stipdonk MJ, Hardenberg G, Bijker MS, Lemmens EE, Droin NM, Green DR, Schoenberger SP. Dynamic programming of CD8+ T lymphocyte responses. Nat Immunol. 2003;4:361–365. doi: 10.1038/ni912. [DOI] [PubMed] [Google Scholar]
- Veiga-Fernandes H, Walter U, Bourgeois C, McLean A, Rocha B. Response of naive and memory CD8+ T cells to antigen stimulation in vivo. Nat Immunol. 2000;1:47–53. doi: 10.1038/76907. [DOI] [PubMed] [Google Scholar]
- Vigano MA, Ivanek R, Balwierz P, Berninger P, van Nimwegen E, Karjalainen K, Rolink A. An epigenetic profile of early T-cell development from multipotent progenitors to committed T-cell descendants. Eur J Immunol. 2013 doi: 10.1002/eji.201344022. [DOI] [PubMed] [Google Scholar]
- Wang Z, Zang C, Rosenfeld JA, Schones DE, Barski A, Cuddapah S, Cui K, Roh TY, Peng W, Zhang MQ, Zhao K. Combinatorial patterns of histone acetylations and methylations in the human genome. Nat Genet. 2008;40:897–903. doi: 10.1038/ng.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei G, Wei L, Zhu J, Zang C, Hu-Li J, Yao Z, Cui K, Kanno Y, Roh TY, Watford WT, et al. Global mapping of H3K4me3 and H3K27me3 reveals specificity and plasticity in lineage fate determination of differentiating CD4+ T cells. Immunity. 2009;30:155–167. doi: 10.1016/j.immuni.2008.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young MD, Willson TA, Wakefield MJ, Trounson E, Hilton DJ, Blewitt ME, Oshlack A, Majewski IJ. ChIP-seq analysis reveals distinct H3K27me3 profiles that correlate with transcriptional activity. Nucleic acids research. 2011;39:7415–7427. doi: 10.1093/nar/gkr416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zediak VP, Johnnidis JB, Wherry EJ, Berger SL. Cutting edge: persistently open chromatin at effector gene loci in resting memory CD8+ T cells independent of transcriptional status. J Immunol. 2011;186:2705–2709. doi: 10.4049/jimmunol.1003741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JA, Mortazavi A, Williams BA, Wold BJ, Rothenberg EV. Dynamic transformations of genome-wide epigenetic marking and transcriptional control establish T cell identity. Cell. 2012;149:467–482. doi: 10.1016/j.cell.2012.01.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.