Abstract
Aims
To determine the efficacy of MGMT depletion plus BCNU (carmustine) therapy and the impact of methylation status in adults with glioblastoma (GBM) and gliosarcoma.
Methods
Methylation analysis was performed on GBM patients with adequate tissue samples. Patients with newly-diagnosed GBM or gliosarcoma were eligible for this Phase III open-label clinical trial. At registration patients were randomized to Arm 1: O6-BG + BCNU (reduced dose) plus radiation therapy (RT) or Arm 2: BCNU plus radiation therapy.
Results
One hundred eighty-three patients with newly diagnosed GBM or gliosarcoma were enrolled from 42 United States institutions; 90 eligible patients received O6-BG + BCNU plus radiation therapy (RT), and 89 received BCNU plus RT. The trial was halted at first interim analysis per stopping guidelines due to futility (less than 40% improvement on O6BG + BCNU arm). Following adjustment for stratification factors, there was no significant difference in overall (OS) or progression-free survival (PFS) between the two groups (one sided p=0.94 and p=0.88 respectively). Median OS was 11 months (95% c.i. 8 – 13 months) for patients on the O6BG+BCNU arm and 10 months (95% c.i. 8 – 12) for the BCNU arm. PFS was 4 months for patients in each arm. Adverse events were reported in both arms, with significantly more grade 4 and 5 events in the experimental arm.
Conclusions
The addition of O6-BG to the standard regimen of radiation and BCNU for treatment of newly-diagnosed glioblastoma and gliosarcoma did not offer added benefit and in fact caused additional toxicity.
Keywords: glioblastoma, gliosarcoma, phase III trial, SWOG, methylation, AGT, MGMT, BCNU, Carmustine, O6BG
Introduction
Glioblastoma (GBM) is the highest grade and most frequent primary adult brain tumor. Standard radiation therapy doubles median survival,1, 2 and the addition of chemotherapy plays a significant role in further enhancing longevity.3, 4 In recent years, median overall survival (OS) for has increased to 14.6 months with first-line therapy of radiation and temozolomide (TMZ).3 Over the past decade, certain tumor molecular and epigenetic characteristics such as methylguanine methyltransferase (MGMT) methylation have been identified as important predictive factors for survival and treatment response in glioma.5–7 It has long been recognized that approximately 30% of patients with GBM respond favorably to alkylating chemotherapy.8, 9 Later work has shown that this percentage correlates with promoter methylation of the MGMT enzyme, which repairs tumor DNA damaged by alkylating therapy.10 Patients whose tumors lack MGMT methylation are less likely to respond to standard alkylating chemotherapy.
O6-benzylguanine (O6-BG), which is inert and nontoxic when administered alone, is a potent inhibitor of MGMT. In animal models with MGMT-active (nonmethylated) BCNU-resistant tumors, MGMT activity is inhibited for several hours after exposure to O6-BG, during which time the tumor becomes highly sensitive to BCNU.11 Likewise, MGMT-deficient human CNS tumor xenografts are more sensitive to alkylating drugs.12
MGMT expression has been shown to play an important role in human CNS tumors. Several retrospective studies of patients with anaplastic gliomas who were treated on various protocols with radiation therapy and BCNU showed strong correlation with low MGMT activity (stronger than other prognostic factors such as age) and improved survival.13
Friedman conducted a Phase I trial to define the presurgical dose required to deplete tumor MGMT activity in patients with malignant glioma. In this study, O6-BG was not toxic when administered as a single agent.14
Subsequently, Spiro performed a dose escalation clinical trial in 30 patients to determine the dose of O6-BG required to deplete AGT to undetectable levels with acceptable toxicity. Sequential CT-guided biopsies were performed before and 18 hours after O6-BG.15 MGMT depletion below the level of detection was demonstrated at 120 mg/M2, hence the recommended dose of 120 mg/M2 of O6-BG infused over 1 hour in Phase II trials.
Improved survival correlated with low MGMT levels, and O6-BG could be administered at doses without significant toxicity while effectively depleting MGMT. The intent of our study was to determine whether there is benefit to MGMT depletion plus BCNU in patients with grade IV astrocytomas.
Patients and Methods
Eligibility
Patients from 42 institutions with a histologically confirmed diagnosis of GBM or gliosarcoma (World Health Organization [WHO] grade IV astrocytoma) were enrolled in S0001 (ClinicalTrials.gov Identifier: NCT00017147) between 2001 and 2005. Biopsy or surgical resection was required within 28 days prior to registration. Eligible patients were ≥ 18 years of age with a Zubrod performance status ≤ 2. Documentation of adequate renal function was required (serum creatinine ≤ 1.5 × the institutional upper limit of normal or creatinine clearance ≥ 60ml/min) as well as PT/PTT of ≤120 % of upper limit of normal within 28 days of registration. Documentation of pulmonary function (DLCO ≥ 70% of predicted) within 42 days of registration was required.
Patients with prior or concurrent cranial radiation or chemotherapy outside of the protocol treatment were not eligible. Patients with three or more noncontiguous sites of tumor on T2 magnetic resonance imaging (MRI) or computed tomography (CT) were excluded.
A post-operative MRI was required prior to registration for tumor removal greater than simple biopsy (pre-operative MRI was allowed for biopsy-only). Patients unable to undergo MRI for medical reasons were eligible if they underwent CT scan with intravenous contrast. Documentation of stable or decreasing corticosteroid usage was required prior to the preregistration MRI/CT.
Patients with known allergies to the study drugs, HIV-positive status or medical illnesses not adequately controlled with therapy were ineligible. History of prior malignancy (other than adequately treated basal cell or squamous cell skin cancer; in situ stage I or II cervical cancer in complete remission; or any other cancer from which the patient had been disease-free for five or more years) were criteria for ineligibility. Pregnant or nursing women were not eligible and women of reproductive potential needed to agree to effective contraception methods.
All participating centers had formal institutional review board approval of the protocol and all participants provided signed informed consent prior to registration and treatment.
Central pathology review for eligibility determination was performed by the Neuropathology Coordinator (EJR). Specifically, microscopic preparations, which consisted of hematoxylin and eosin (H&E) stained sections were reviewed and classified according to WHO criteria.16
Study design and treatment plan
At registration, patients were randomized by the SWOG Statistical Center to receive O6-BG + BCNU (at low dose) plus radiation therapy (RT) or BCNU (at standard dose) plus RT using a dynamic balancing algorithm program stratified by age (< 50 vs ≥ 50), performance status (0–1 vs 2) and surgery (biopsy only vs resection). Treatment was begun within five working days of registration.
Patients were randomized to Arm 1: O6-BG + BCNU plus radiation therapy or Arm 2: BCNU plus radiation therapy. Chemotherapy began concurrently with radiation therapy. The Arm 1 treatment group received 40mg/m2 BCNU six hours after the administration of 120mg/m2 O6-BG intravenously over one hour every six weeks. The Arm 2 group received BCNU 200 mg/m2 intravenously over 1 hour every six weeks. A maximum of seven cycles were allowed.
Radiation therapy was administered once per day, five days per week with a linear accelerator using x-ray energy of at least 4MV. The initial gross target volume (GTV1) was defined as T2 signal abnormality on a postoperative MRI. The boost GTV (GTV2) was defined by the resection cavity plus gadolinium enhancement on T1 MRI. Multiple conformal fields (without intensity modulation) were used, with field margins encompassing GTV1 and GTV2 by 2 and 2.5 cm, respectively. The initial volume was treated to 5040 cGy in 28 fractions, and the boost volume was treated for an additional 1080 cGy in 6 fractions (cumulative dose of 6120 cGy). Doses were prescribed to the 100% isodose at the isocenter, and each GTV was covered by at least the 95% isodose. Central radiation review for adherence to treatment protocol was performed by the Radiation Therapy Study Coordinator (KJS).
Dose modifications were specified for hematologic, non-hematologic, and pulmonary toxicities: BCNU was held for hematologic toxicity ≥ CTC (common toxicity criteria) grade 2, until recovery to ≤ CTC grade 1. For CTC grade 3 toxicity resolving within 8 weeks of dosing, BCNU was continued without dose reduction once ≤ CTC grade 1. If recovery to normal bone marrow function exceeded 8 weeks, BCNU dose was reduced by 25%. For hematologic CTC grade 4, BCNU dose was reduced by 50% (after recovery to CTC grade ≤1). Failure to recover to CTC grade 1 within 10 weeks mandated removal from protocol treatment. Similar dose reduction criteria were dictated for non-hematologic toxicities: 25% dose reduction of BCNU for CTC grade 2 toxicity delaying treatment more than 2 weeks, and 50% dose reduction for any CTC grade ≥ 3 toxicity. Patients were removed from protocol treatment for clinical or radiographic evidence of pulmonary fibrosis or DLCO < 50% of the upper limit of normal. Radiation therapy was withheld for WBC < 1,000/ul or platelets < 20,000/ul until recovered.
Patients were removed from the study for completion of therapy (7 cycles), tumor progression (defined as a 25% increase in the bidimensional sum of the tumor areas; clear worsening of evaluable, but not measurable disease; appearance of a new lesion; or reappearance of a prior lesion); unacceptable toxicities not manageable with dose reduction; delay in treatment greater than four weeks due to toxicity; administration of other antitumor treatment; or patient-initiated withdrawal for any reason. Patients were followed for 5 years after randomization or until death.
MGMT promoter methylation assay
Unstained slides prepared from formalin-fixed paraffin-embedded tissue blocks were used for MGMT activity assays. H & E stained slides were marked by a neuropathologist (EJR) to demarcate tumor from uninvolved tissue on each slide. DNA was isolated from archived paraffin embedded formalin fixed unstained slides from tumor areas identified by the pathologist. DNA was subsequently bisulfite-treated and then used for methylation sensitive PCR (polymerase chain reaction) using bisulfite and methylation sensitive DNA primers.17
Statistical analysis
The primary endpoint was OS. Assuming a 12 month median survival for standard therapy, a total enrollment of 375 patients was expected to show a 40% improvement with the addition of O6-BG (Arm 1) for a one-sided .05 level test with 92.5% power.
Interim analysis was planned after entrance of half the patients, and a second interim analysis was planned after complete accrual. Early termination of the trial and conclusion that O6BG+BCNU arm is not superior would occur if the alternative hypothesis of a 40% improvement in survival with the combination arm is rejected at the 0.005 level using an extension of the log-rank test.
All eligible patients were included in the survival analyses by assigned treatment according to the intent-to-treat principle. Patients who were never treated were not included in toxicity analyses. All survival curves and estimates were calculated using the Kaplan-Meier product limit method.18
Response was assessed in a post-hoc fashion by the study chair for all patients who had evidence of measurable or non-measurable disease using the Macdonald criteria.19
Additional analysis of the prognostic value of MGMT expression as assessed by immunohistochemical assay of MGMT on overall survival and progression-free survival was planned. Subgroup analysis (based on MGMT levels) of treatment groups also was planned. Based on data from SWOG -9218, an assumption of 70% MER expression was expected, and statistical models and sample sizes were calculated accordingly.8
Results
This study was terminated November 2005 at the time of the initial interim analyses per recommendation of the Data and Safety Monitoring Committee. The hypothesis that O6-BG added benefit to BCNU+RT was judged to be untenable. A 40% OS improvement in the O6-BG+BCNU arm was ruled out (p=0.002).
At the time of study closure, 183 patients were registered. Four patients were ineligible due to prior diagnosis of low-grade brain tumors (two), surgery more than 28 days prior to registration (one) and pre-study MRI done without contrast (one). Patient characteristics were balanced between the two treatment arms (tables 1 and 2).
Table 1.
Patient, Tumor and Treatment Characteristics
| O6BG+BCNU+RT (N=90) | BCNU+RT (N=89) | |||
|---|---|---|---|---|
| Age (years) | 55 (19 – 73) | 56 (24 – 76) | ||
| Median (range) | ||||
| ≤ 50 yrs | 25 | 28% | 27 | 30 |
| > 50 yrs | 65 | 72% | 62 | 70 |
|
| ||||
| Sex | ||||
| Male | 56 | 62% | 51 | 57% |
| Female | 34 | 38% | 38 | 43 |
|
| ||||
| Race | ||||
| White | 88 | 98% | 87 | 98% |
| Black | 1 | 1% | 1 | 1% |
| Asian | 0 | 0% | 1 | 1% |
| Native American | 1 | 1% | 0 | 0% |
|
| ||||
| Ethnicity | ||||
| Non-hispanic | 85 | 94% | 84 | 94% |
| Hispanic | 2 | 2% | 2 | 2% |
| 3 | 3% | 3 | 3% | |
|
| ||||
| Performance Status | ||||
| 0–1 | 79 | 88% | 77 | 87% |
| 2 | 11 | 12% | 12 | 13% |
|
| ||||
| Type of Initial Surgery Stratification | ||||
| Biopsy Only | 20 | 22% | ||
| Resection | 70 | 78% | 16 | 18% |
| Institutional report | 73 | 82% | ||
| Excisional biopsy | 20 | 22% | ||
| Partial Resection | 29 | 32% | 16 | 18% |
| Complete Resection | 38 | 42% | 32 | 36% |
| Other | 0 | 0% | 36 | 40% |
| Not Reported | 3 | 3% | 4 | 4% |
| Central review of Operative Report® | 1 | 1% | ||
| Biopsy only | 20 | 22% | 13 | 15% |
| Sub-total resection | 52 | 58% | 50 | 56% |
| Gross Total resection | 17 | 19% | 22 | 25% |
| Insufficient information | 1 | 1% | 4 | 4% |
|
| ||||
| Subsequent Surgery | 3 | 3% | 7 | 8% |
|
| ||||
| Histology | ||||
| Glioblastoma Multiforme | 88 | 98% | 88 | 99% |
| Gliosarcoma | 0 | 0% | 0 | 0% |
| Other | 2 | 2% | 1 | 1% |
|
| ||||
|
O6BG+BCNU+RT (N=90) |
BCNU+RT (N=89) |
|||
|
| ||||
| Hemisphere of Tumor | ||||
| Right | 42 | 47% | 43 | 48% |
| Left | 41 | 46% | 39 | 44% |
| Both | 2 | 2% | 5 | 6% |
| Midline | 5 | 6% | 0 | 0% |
| Infratentorial | 0 | 0% | 1 | 1% |
| Not Reported | 0 | 0% | 1 | 1% |
|
| ||||
| Baseline MMSE Score* | ||||
| 30 | 24 | 29% | 37 | 45% |
| 27 – 29 | 36 | 44% | 31 | 37% |
| < 26 | 22 | 27% | 15 | 18% |
| Unknown | ||||
|
| ||||
| Disease Status at Baseline® | ||||
| Measurable | 32 | 36% | 28 | 32% |
| Non-measurable | 49 | 55% | 57 | 65% |
| Unknown | 9 | 10% | 4 | 5% |
|
| ||||
| Best Response®# | ||||
| Complete Response | 1 | 1% | 0 | 0% |
| Partial Response | 9 | 11% | 4 | 5% |
| Stable Disease | 25 | 31% | 33 | 39% |
| Increasing Disease | 33 | 41% | 27 | 32% |
| Not Assessable | 13 | 16% | 21 | 25% |
Maximum score on the mini-Mental Stat Examination (MMSE) is 30, and scores above 26 are considered to indicate normal mental status.
Ascertained by Study Chair by post hoc chart review of clinical notes and imaging reports
Among patients with measurable or non-measurable disease.
Table 2.
Characteristics of pre-Op MRI*
| BCNU+RT (N=89) |
O6BG+BCNU+RT (N=90) |
|||
|---|---|---|---|---|
| Evidence of Necrosis | ||||
| Yes | 59 | 68% | 50 | 60% |
| No | 28 | 32% | 33 | 40% |
|
| ||||
| Evidence of Cyst/cystic | ||||
| Yes | 14 | 16% | 17 | 20% |
| No | 71 | 84% | 66 | 80% |
|
| ||||
| Evidence of hemorrhage/blood | ||||
| Yes | 9 | 11% | 8 | 10% |
| No | 76 | 89% | 75 | 90% |
Ascertained by Study Chair
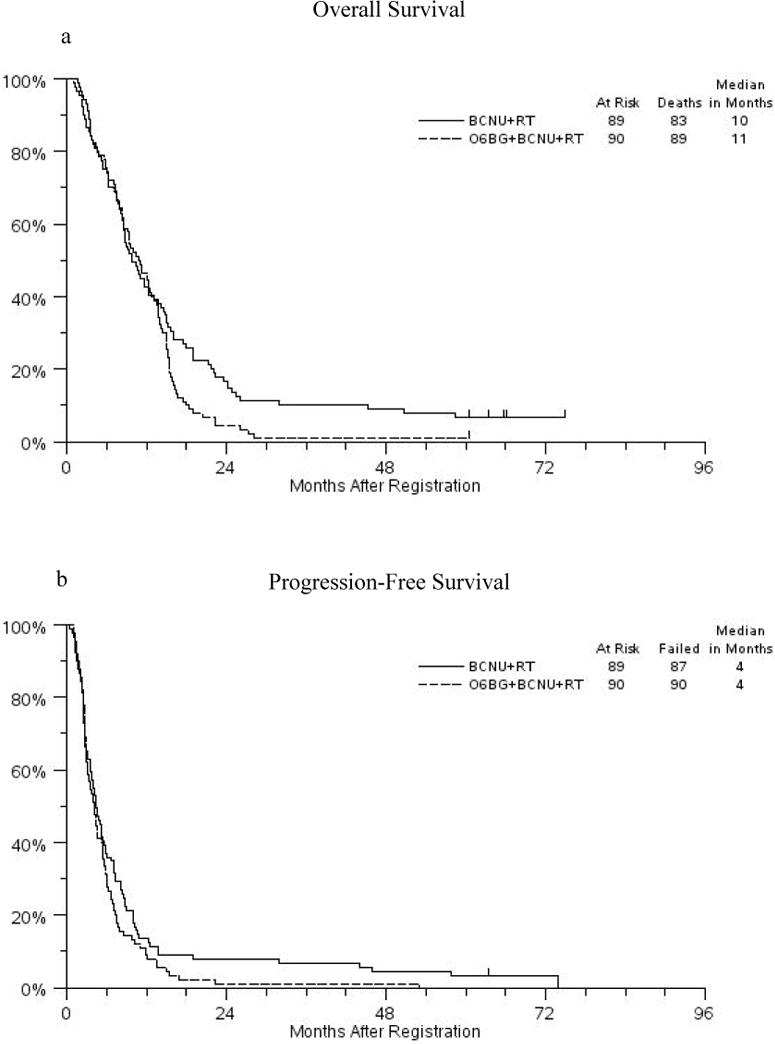
Response data are shown in Table 1. BCNU+RT patients had a median PFS of 4 months (95% confidence interval [ci] 4 – 5 months) and OS of 10 months (95% ci 8 – 12 months). O6-BG+BCNU patients had a median PFS of 4 months (95% ci 4 – 5 months) and OS of 11 months (95% ci 8–13).
The one-sided tests of differences between arms were not significant for OS (p=.94 adjusted for stratification factors of age, performance status and type of surgery) or in PFS (p=.88 adjusted for stratification factors). The BCNU/O6BG+BCNU hazard ratio for overall survival is 0.77 with a 99% ci from .51 to 1.18 (i.e., upper bound of improvement due to addition of O6-BG is 18%). The BCNU+RT/O6-BG+BCNU+RT hazard ratio for PFS is 0.83 with a 99% confidence interval from .54 to 1.26.
MGMT analysis
Tissue from 84 GBM patients was evaluated for methylation status of MGMT. Results were obtainable in 41 cases (49%), with one ineligible, leaving 40 cases. One of these cases was from an ineligible patient, so methylation results are shown for 40 patients. Thirteen (33%) were found to be methylated and 27 (67%) were unmethylated.
Regardless of treatment arm, patients with methylated DNA had a median OS of 13 months (95% ci 8 – 16 months) and median PFS of 4 months (95% ci 3 – 6 months). Patients with unmethylated MGMT had OS of OS of 11 months (95% ci 9 – 13 months) and median PFS of 3 months (95% ci 3 – 5 months).
OS and PFS by methylation status and arm are shown in Table 3.
Table 3.
OS and PFS by MGMT Methylation Status and Arm
| Overall Survival | Progression-Free Survival | |||
|---|---|---|---|---|
| Methylated (N=13) |
Unmethylated (N=27) |
Methylated (N=13) |
Unmethylated (N=27) |
|
| Arms Combined | 13 months (8 – 16 months) |
11 months (9 – 13 months) |
4 months (3 – 6 months) |
3 months (3 – 5 months) |
| O6BG+BCNU | 13 months (9 – 14 months) n=9 |
13 months (8 – 14 months) n=14 |
4 months (4 – 6 months) n=9 |
3 months (3 – 4 months) n=14 |
| BCNU | 19 months (3 – 19 months) n=4 |
10 months (8 – 11 months) n=13 |
5 months (1 – 5 months) n=4 |
4 months (3 – 8 months) n=13 |
Treatment delivery and tolerability
Of the 90 eligible O6-BG+BCNU patients, 10 patients were removed from the O6-BG arm due to adverse events, primarily hematologic in nature. Four patients on this arm had major protocol deviations (two due to chemotherapy dosing errors and two due to deviations in radiation therapy). Of the 90 patients assessed for adverse events, there were three treatment-related deaths (sepsis, febrile neutropenia, renal failure and acute respiratory distress syndrome). Forty-five additional patients reported grade 4 toxicities, primarily hematologic events (Table 4).
Table 4. Adverse Events.
Adverse Events Unlikely or Not Related to Treatment Excluded
| O6BG+BCNU+RT (n=90) |
BCNU+RT (n=82) |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Grade
|
Grade
|
|||||||||||||
| ADVERSE EVENT | Unk | 0 | 1 | 2 | 3 | 4 | 5 | Unk | 0 | 1 | 2 | 3 | 4 | 5 |
|
|
|
|
||||||||||||
| ADR | 0 | 90 | 0 | 0 | 0 | 0 | 0 | 0 | 81 | 0 | 0 | 0 | 0 | 1 |
| Cardiovascular | 0 | 67 | 10 | 10 | 3 | 0 | 0 | 0 | 63 | 4 | 10 | 4 | 1 | 0 |
| Clotting | 0 | 89 | 0 | 0 | 1 | 0 | 0 | 0 | 82 | 0 | 0 | 0 | 0 | 0 |
| Dermatologic | 0 | 38 | 24 | 27 | 1 | 0 | 0 | 0 | 33 | 30 | 16 | 3 | 0 | 0 |
| Ear | 0 | 85 | 2 | 2 | 1 | 0 | 0 | 0 | 72 | 9 | 1 | 0 | 0 | 0 |
| Endocrine | 0 | 85 | 0 | 5 | 0 | 0 | 0 | 0 | 78 | 1 | 3 | 0 | 0 | 0 |
| Eye | 0 | 70 | 6 | 14 | 0 | 0 | 0 | 0 | 71 | 3 | 7 | 1 | 0 | 0 |
| Flu-like Symptoms | 0 | 27 | 23 | 31 | 8 | 1 | 0 | 0 | 23 | 26 | 28 | 5 | 0 | 0 |
| Gastrointestinal | 0 | 28 | 32 | 28 | 2 | 0 | 0 | 0 | 27 | 31 | 20 | 3 | 1 | 0 |
| Hematologic | 0 | 2 | 4 | 7 | 34 | 43 | 0 | 0 | 2 | 12 | 24 | 30 | 14 | 0 |
| Hemorrhage | 0 | 79 | 7 | 3 | 1 | 0 | 0 | 0 | 79 | 2 | 0 | 1 | 0 | 0 |
| Immunological | 0 | 87 | 1 | 2 | 0 | 0 | 0 | 0 | 81 | 1 | 0 | 0 | 0 | 0 |
| Infection | 0 | 57 | 2 | 5 | 17 | 7 | 2 | 0 | 69 | 0 | 4 | 6 | 1 | 2 |
| Liver | 0 | 44 | 34 | 7 | 3 | 2 | 0 | 0 | 61 | 12 | 7 | 2 | 0 | 0 |
| Lung | 0 | 57 | 10 | 13 | 8 | 1 | 1 | 1 | 61 | 4 | 8 | 5 | 2 | 1 |
| Metabolic | 0 | 57 | 21 | 4 | 6 | 2 | 0 | 0 | 64 | 13 | 2 | 2 | 1 | 0 |
| Musculoskeletal | 0 | 79 | 3 | 7 | 0 | 1 | 0 | 0 | 76 | 2 | 2 | 2 | 0 | 0 |
| Neurologic | 0 | 42 | 11 | 24 | 12 | 1 | 0 | 2 | 40 | 6 | 17 | 14 | 3 | 0 |
| Pain | 0 | 36 | 23 | 24 | 7 | 0 | 0 | 1 | 38 | 19 | 16 | 7 | 1 | 0 |
| Renal/Bladder | 0 | 74 | 10 | 4 | 1 | 0 | 1 | 0 | 76 | 4 | 1 | 0 | 1 | 0 |
| Sexual/Reproductive Function | 0 | 87 | 0 | 1 | 2 | 0 | 0 | 0 | 81 | 0 | 1 | 0 | 0 | 0 |
| MAXIMUM GRADE ANY ADVERSE EVENT | ||||||||||||||
| Number | 0 | 0 | 0 | 9 | 33 | 45 | 3 | 0 | 0 | 1 | 24 | 35 | 18 | 4 |
Of the 89 eligible BCNU+RT patients, 7 refused protocol treatment. Two additional patients experienced major protocol deviations with regard to radiation therapy and one received decreased dose chemotherapy. Nine patients were removed due to toxicity. Eighty-two BCNU+RT patients were assessed for toxicity, with four treatment-related deaths (infection, ARDS and sudden death possibly related to infection). Eighteen additional patients had grade 4 toxicities.
Grade 4 or higher toxicities were seen in 53% of the O6BG+BCNU patients compared to 27% in the BCNU arm (χ2 p=0.0004), primarily hematologic. There was no difference between arms with respect to Grade 4 and 5 non-hematologic events (χ2 p=0.40).
Discussion
Historically, approximately a third of GBM patients seem to benefit from treatment with alkylating chemotherapy. This minority may correspond to tumor status of O6-methylguanine-methyltransferase enzyme (O6-MGMT) activity, which repairs tumor DNA damaged by chemotherapy and allows tumors to progress after exposure to treatment. Patients with inactive MGMT are more likely to respond favorably to treatment with alkylating agents, as tumor DNA is not repaired by the enzyme.20
Since hypermethylation of the MGMT promoter region had been shown to be associated with improved outcome and treatment response in GBM patients, we attempted to exogenously influence MGMT- methylation status. Our hypothesis was that the addition of O6-BG would render unmethylated tumors functionally “methylated” and improve response to alkylating chemotherapy with BCNU. At its inception, this phase III study was designed to accrue 375 patients; however, the study was halted at the interim analysis due to negative results.
Although the study protocol proved non-beneficial for GBM patients, there may still be an application for O6-BG at the proper dose and in the appropriate clinical setting. We may have been limited in showing efficacy by using too small a dose; BCNU dosing was limited by systemic toxicity (primarily myelosuppression) and capped at a maximum of 7 cycles due to concerns of pulmonary and bone marrow toxicities from cumulative nitrosourea toxicity21, 22, 23
Although many pathways of cell-survival and repair mechanisms are involved in the process of GBM proliferation, the strongest prospectively-confirmed pathway is methylation status of MGMT.24 For over 60% of GBM patients with unmethylated MGMT, the optimal strategy to improve outcome would be by effectively changing their biologic functional status to that of their methylated counterparts.
An important consideration is that the maximum effective alkylator dose used in combination with O6-BG is limited by systemic toxicities. A strategy to avoid systemic toxicity by combining O6-BG with a local therapy, Carmustine wafers implanted into the surgical cavity,25, 26 was studied with promising responses, but associated with complications including CSF leak and CNS/CSF infections.26
The rationale for the addition of O6-BG to alkylating chemotherapy in order to overcome MGMT resistance in malignant gliomas should apply to TMZ as well.
A phase II trial using TMZ with O6-BG in the setting of recurrent TMZ-resistant malignant glioma showed some response to the combination for anaplastic glioma, but not GBM, and nearly 50% grade 4 hematologic toxicity.27
More recent studies have shown that there may be an advantage to combining additional agents, concurrently or in rotation, with carmustine and O6-BG for optimal anti-tumor effect.25, 28
Conclusion
Based on the doses and treatments given to this cohort of patients, the results of SWOG S0001 do not support the hypothesis that extrinsic depletion of MGMT renders GBM more sensitive to alkylating therapy with BCNU.
Fig 1.
Acknowledgments
This investigation was supported in part by the following PHS Cooperative Agreement grant numbers awarded by the National Cancer Institute, DHHS: CA32102, CA38926, CA35431, CA58861, CA20319, CA35178, CA45560, CA35262, CA76462, CA42777, CA45807, CA74647, CA95860, CA67575, CA12644, CA45377, CA13612, CA04919, CA46368, CA35090, CA35176, CA52654, CA45450, CA35281, CA46113, CA35261, CA46282, CA45808, CA35128, CA37981, CA74811, CA35192 and CA22433
We would like to recognize the efforts of the late Dr. Alexander Spence, who set this protocol in motion and is responsible for its inception.
References
- 1.Walker MD, Alexander E, Jr, Hunt WE, et al. Evaluation of BCNU and/or radiotherapy in the treatment of anaplastic gliomas. A cooperative clinical trial. J Neurosurg. 1978;49:333–343. doi: 10.3171/jns.1978.49.3.0333. [DOI] [PubMed] [Google Scholar]
- 2.Walker MD, Strike TA, Sheline GE. An analysis of dose-effect relationship in the radiotherapy of malignant gliomas. International journal of radiation oncology, biology, physics. 1979;5:1725–1731. doi: 10.1016/0360-3016(79)90553-4. [DOI] [PubMed] [Google Scholar]
- 3.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 4.Stewart LA. Chemotherapy in adult high-grade glioma: a systematic review and meta-analysis of individual patient data from 12 randomised trials. Lancet. 2002;359:1011–1018. doi: 10.1016/s0140-6736(02)08091-1. [DOI] [PubMed] [Google Scholar]
- 5.Theeler BJ, Yung WK, Fuller GN, De Groot JF. Moving toward molecular classification of diffuse gliomas in adults. Neurology. 2012;79:1917–1926. doi: 10.1212/WNL.0b013e318271f7cb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.SongTao Q, Lei Y, Si G, et al. IDH mutations predict longer survival and response to temozolomide in secondary glioblastoma. Cancer science. 2012;103:269–273. doi: 10.1111/j.1349-7006.2011.02134.x. [DOI] [PubMed] [Google Scholar]
- 7.Ohgaki H, Kleihues P. The definition of primary and secondary glioblastoma. Clinical cancer research: an official journal of the American Association for Cancer Research. 2013;19:764–772. doi: 10.1158/1078-0432.CCR-12-3002. [DOI] [PubMed] [Google Scholar]
- 8.Jaeckle KA, Eyre HJ, Townsend JJ, et al. Correlation of tumor O6 methylguanine-DNA methyltransferase levels with survival of malignant astrocytoma patients treated with bis-chloroethylnitrosourea: a Southwest Oncology Group study. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 1998;16:3310–3315. doi: 10.1200/JCO.1998.16.10.3310. [DOI] [PubMed] [Google Scholar]
- 9.Esteller M, Garcia-Foncillas J, Andion E, et al. Inactivation of the DNA-repair gene MGMT and the clinical response of gliomas to alkylating agents. N Engl J Med. 2000;343:1350–1354. doi: 10.1056/NEJM200011093431901. [DOI] [PubMed] [Google Scholar]
- 10.Hegi ME, Diserens AC, Gorlia T, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352:997–1003. doi: 10.1056/NEJMoa043331. [DOI] [PubMed] [Google Scholar]
- 11.Felker GM, Friedman HS, Dolan ME, Moschel RC, Schold C. Treatment of subcutaneous and intracranial brain tumor xenografts with O6-benzylguanine and 1,3-bis(2-chloroethyl)-1-nitrosourea. Cancer chemotherapy and pharmacology. 1993;32:471–476. doi: 10.1007/BF00685892. [DOI] [PubMed] [Google Scholar]
- 12.Schold SC, Jr, Brent TP, von Hofe E, et al. O6-alkylguanine-DNA alkyltransferase and sensitivity to procarbazine in human brain-tumor xenografts. J Neurosurg. 1989;70:573–577. doi: 10.3171/jns.1989.70.4.0573. [DOI] [PubMed] [Google Scholar]
- 13.Belanich M, Pastor M, Randall T, et al. Retrospective study of the correlation between the DNA repair protein alkyltransferase and survival of brain tumor patients treated with carmustine. Cancer research. 1996;56:783–788. [PubMed] [Google Scholar]
- 14.Friedman HS, Kokkinakis DM, Pluda J, et al. Phase I trial of O6-benzylguanine for patients undergoing surgery for malignant glioma. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 1998;16:3570–3575. doi: 10.1200/JCO.1998.16.11.3570. [DOI] [PubMed] [Google Scholar]
- 15.Spiro TP, Gerson SL, Liu L, et al. O6-benzylguanine: a clinical trial establishing the biochemical modulatory dose in tumor tissue for alkyltransferase-directed DNA repair. Cancer research. 1999;59:2402–2410. [PubMed] [Google Scholar]
- 16.Louis DN, Ohgaki H, Wiestler OD, et al. The 2007 WHO classification of tumours of the central nervous system. Acta neuropathologica. 2007;114:97–109. doi: 10.1007/s00401-007-0243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Watanabe T, Katayama Y, Komine C, et al. O6-methylguanine-DNA methyltransferase methylation and TP53 mutation in malignant astrocytomas and their relationships with clinical course. International journal of cancer Journal international du cancer. 2005;113:581–587. doi: 10.1002/ijc.20625. [DOI] [PubMed] [Google Scholar]
- 18.Kaplan EL, Meier R. Nonparametric estimation from incomplete observation. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 19.Macdonald DR, Cascino TL, Schold SC, Jr, Cairncross JG. Response criteria for phase II studies of supratentorial malignant glioma. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 1990;8:1277–1280. doi: 10.1200/JCO.1990.8.7.1277. [DOI] [PubMed] [Google Scholar]
- 20.Silber JR, Bobola MS, Blank A, Chamberlain MC. O(6)-methylguanine-DNA methyltransferase in glioma therapy: promise and problems. Biochimica et biophysica acta. 2012;1826:71–82. doi: 10.1016/j.bbcan.2011.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reithmeier T, Graf E, Piroth T, Trippel M, Pinsker MO, Nikkhah G. BCNU for recurrent glioblastoma multiforme: efficacy, toxicity and prognostic factors. BMC cancer. 2010;10:30. doi: 10.1186/1471-2407-10-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.O’Driscoll BR, Kalra S, Gattamaneni HR, Woodcock AA. Late carmustine lung fibrosis. Age at treatment may influence severity and survival. Chest. 1995;107:1355–1357. doi: 10.1378/chest.107.5.1355. [DOI] [PubMed] [Google Scholar]
- 23.Brandes AA, Tosoni A, Amista P, et al. How effective is BCNU in recurrent glioblastoma in the modern era? A phase II trial. Neurology. 2004;63:1281–1284. doi: 10.1212/01.wnl.0000140495.33615.ca. [DOI] [PubMed] [Google Scholar]
- 24.Gilbert MR, Wang M, Aldape KD, et al. Dose-dense temozolomide for newly diagnosed glioblastoma: a randomized phase III clinical trial. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2013;31:4085–4091. doi: 10.1200/JCO.2013.49.6968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Affronti ML, Heery CR, Herndon JE, 2nd, et al. Overall survival of newly diagnosed glioblastoma patients receiving carmustine wafers followed by radiation and concurrent temozolomide plus rotational multiagent chemotherapy. Cancer. 2009;115:3501–3511. doi: 10.1002/cncr.24398. [DOI] [PubMed] [Google Scholar]
- 26.Bota DA, Desjardins A, Quinn JA, Affronti ML, Friedman HS. Interstitial chemotherapy with biodegradable BCNU (Gliadel) wafers in the treatment of malignant gliomas. Therapeutics and clinical risk management. 2007;3:707–715. [PMC free article] [PubMed] [Google Scholar]
- 27.Quinn JA, Jiang SX, Reardon DA, et al. Phase II trial of temozolomide plus o6-benzylguanine in adults with recurrent, temozolomide-resistant malignant glioma. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2009;27:1262–1267. doi: 10.1200/JCO.2008.18.8417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Friedman HS, Keir S, Pegg AE, et al. O6-benzylguanine-mediated enhancement of chemotherapy. Molecular cancer therapeutics. 2002;1:943–948. [PubMed] [Google Scholar]


