Abstract
Metastasis is a 100-year-old research topic. Technological advancements during the past few decades have led to significant progress in our understanding of metastatic disease. However, metastasis remains the leading cause of cancer-related mortalities. The lack of appropriate clinical trials for metastasis preventive drugs and incomplete understanding of the molecular machinery are major obstacles in metastasis prevention and treatment. A number of processes, factors, and signaling pathways are involved in regulating metastasis. Here, we discuss recent progress in metastasis research, including epithelial-mesenchymal plasticity, cancer stem cells, emerging molecular determinants and therapeutic targets, and the link between metastasis and therapy resistance.
Keywords: Metastasis, epithelial-mesenchymal plasticity, cancer stem cell, circulating tumor cell, therapy resistance
Hurdles in eliminating metastasis-associated mortality
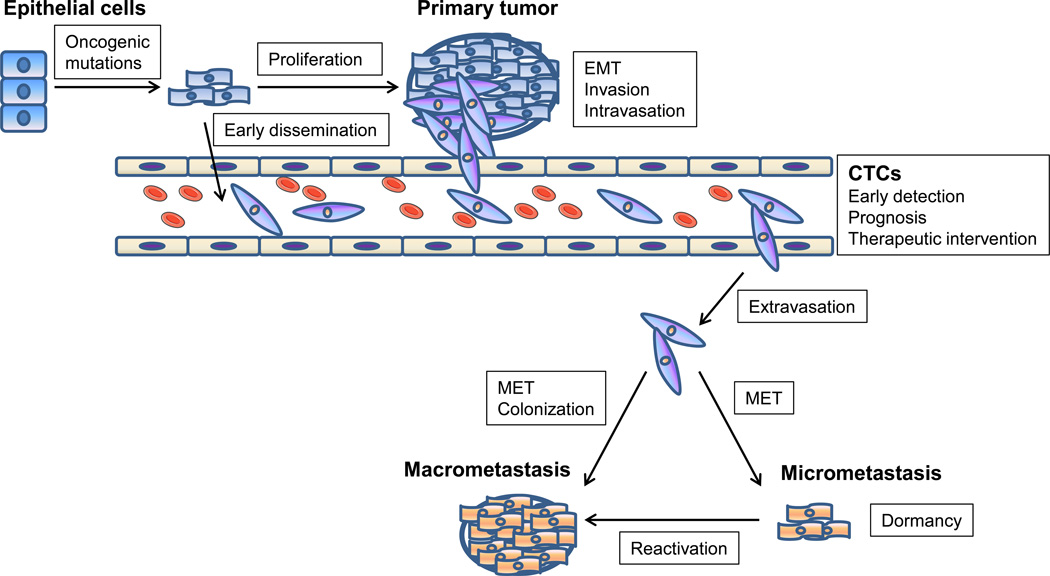
Metastasis is a multi-step process which begins when primary tumor cells break away from their neighboring cells, such as nearby stromal cells, and invade through the basement membrane. Subsequently, metastasizing cells enter the circulation (intravasate), either directly or via lymphatics, and then home to distant organs where they exit the vasculature (extravasate). Eventually, tumor cells that successfully adapt to the new microenvironment proliferate from micrometastases into clinical detectable metastatic tumors (Figure 1) [1]. Although great advances have been made in combating cancer, particularly in its early stages, metastasis remains a formidable and frequently fatal challenge [2–4]. It is becoming increasingly clear that the seeds of metastasis are present in many cases of early disease [3, 5], leading to deaths that might be prevented. A number of processes, factors, and signaling pathways have been implicated in regulating metastasis, including epithelial-mesenchymal plasticity, cancer stem cells, non-coding RNAs, cytokines, hormones, and receptor tyrosine kinase (RTK) pathways, with the list of determinants of metastasis still expanding. However, few molecules have been translated into effective metastasis prevention or treatment in the clinic.
Figure 1. Schematic of the invasion-metastasis cascade.
Metastasis involves a succession of discrete steps, beginning with local invasion, then intravasation of cancer cells into blood and lymphatic vessels, transit of circulating tumor cells (CTCs) through the vasculature, followed by extravasation to the parenchyma of distant organs, and finally proliferation from micrometastases into macrometastases.
Over 90% of cancer-related deaths are caused by metastasis. For instance, breast cancer, the most common malignant disease in women, begins as a local disease and later metastasizes to lymph nodes and other organs. The most common sites of breast cancer metastasis are vital organs, such as the lung, liver, bone, and to a lesser extent, the brain [6, 7]. National Cancer Institute (NCI) Surveillance, Epidemiology, and End Results (SEER) data indicate that the percentages of patients with localized, regional, metastatic, or unstaged breast cancer at diagnosis are 61%, 32%, 5%, or 2%. Their corresponding five-year survival rates are 98.5%, 84.6%, 25%, and 49.8%, respectively. However, many patients with localized or regional cancers show evidence of local invasion or disseminated, micrometastatic tumor cells at diagnosis, meaning that it is too late to stop the early steps of metastasis [8]. Therefore, for those 93% of patients, preventing metastatic colonization – the growth from disseminated, micrometastatic tumor cells to macroscopic metastases – holds the most therapeutic promise. For those 5% of patients with metastatic disease at diagnosis, shrinking established metastases must be the goal.
Surgery, radiation therapy, and chemotherapy can eliminate many primary tumors, and thus approaches to preventing metastatic colonization should be most effective as adjuvant therapy [8]. The major roadblock to devising adjuvant metastasis prevention treatments is that the current clinical trial system is not designed to test metastasis preventive drugs [9]. In the current setting, running metastasis prevention trials on patients with early-stage cancer would be prohibitively lengthy and costly and would require many thousands of patients. Therefore, drugs today have to induce regression of established metastatic tumors in late-stage cancer patients who failed standard treatment, if those drugs are to receive regulatory approval and to be advanced to adjuvant metastasis prevention trials [2, 9]. This is in contrast to the preclinical setting, where most anti-metastatic agents that have been tested prevent the formation of metastases but do not shrink established metastatic tumors [2, 9]. It has been suggested that the format of clinical trials be changed to accommodate metastasis preventive drugs [9], but to do so would require a new approach to defining patient eligibility and to predicting drug response.
Ideally, trials of metastasis preventive drugs would enroll patients with early-stage disease who are at high risk of developing metastases as well as those who already have metastases and are at risk of developing more [9]. One major obstacle to this ideal, however, is that we have no good means of identifying these high-risk patients. We also do not know how to select patients who might benefit from specific metastasis preventive agents. To surmount these barriers and facilitate metastasis prevention trials, we need to find better prognostic markers for metastasis, effective anti-metastatic drugs, and predictive markers for drug response.
In addition to the lack of appropriate clinical trials for metastasis prevention, the heterogeneity of metastatic tumor cells may account for the failure in therapeutic targeting of a specific pathway, since different subpopulations of metastatic tumor cells could employ distinct molecular machinery. For instance, treatment of patients with triple-negative breast cancer (TNBC), which metastasizes more frequently than other breast cancer subtypes and is associated with poor clinical outcomes, has been challenging due to the heterogeneity of this disease and the lack of well-defined therapeutic targets [10]. Thus, there is a pressing need to understand tumor heterogeneity and elucidate the mechanisms by which different metastases originate from different subpopulations of cancer cells co-existing within a tumor.
In this review, we dissect the processes of metastatic progression. These processes depend on genetic and epigenetic aberrations in tumor cells and alterations in the associated microenvironment. In addition to reviewing the emerging molecular determinants and therapeutic targets at each step of the invasion-metastasis cascade, we discuss the molecular basis of the cellular plasticity of tumor cells. Such plasticity is likely to underlie therapy resistance and metastatic relapse, which suggests the importance of understanding tumor heterogeneity and the need to develop new combination therapies to target all types of cancer cell subpopulations, including cancer stem cells (CSCs), circulating tumor cells (CTCs), disseminated tumor cells (DTCs), and differentiated cancer cells.
Role of epithelial-mesenchymal plasticity and cancer stem cells in metastasis
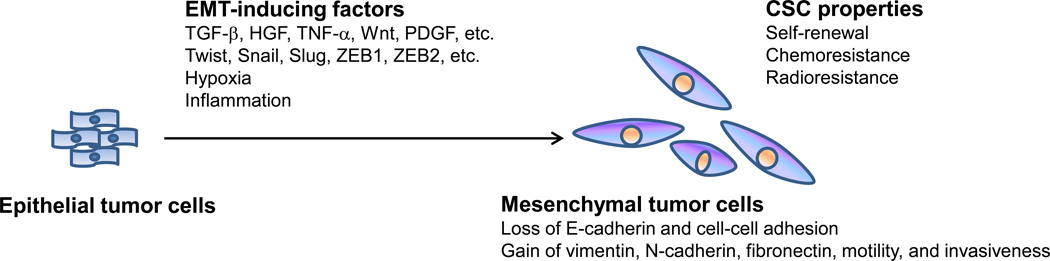
The ability of cancer cells to metastasize depends on their genetic and epigenetic alterations as well as the microenvironmental cues they receive. Recent studies suggest that many of the properties associated with invasion and metastasis do not arise as purely cell-autonomous processes; instead, the surrounding tumor stroma becomes ‘activated’ during primary tumor progression and begins to release signals, such as transforming growth factor (TGF)-β, hepatocyte growth factor (HGF), tumor necrosis factor (TNF)-α, Wnt, and platelet-derived growth factor (PDGF). Subsequent adaptation of carcinoma cells to these heterotypic signals can lead to acquisition of highly-malignant, cell-biological traits through processes such as the epithelial-mesenchymal transition, EMT (Figure 2) [11].
Figure 2. The epithelial-mesenchymal transition (EMT).
Hypoxia, inflammation, and extracellular factors present in the tumor stroma, such as TGF-β, HGF, TNF-α, Wnt, and PDGF, can activate the expression of transcription factors including Twist, Snail, Slug, ZEB1, and ZEB2, which are regarded as the core EMT regulators. Inducing EMT in carcinoma cells leads to loss of epithelial markers and cell-cell adhesion and acquisition of mesenchymal markers, motility, invasiveness, and cancer-stem-cell (CSC) properties including self-renewal, chemoresistance, and radioresistance.
EMT is characterized by repression of epithelial marker expression, acquisition of mesenchymal markers, loss of cell adhesion, and increased cell motility and invasiveness (Figure 2) [12, 13]. During development, the EMT program, together with its reverse process, mesenchymal-epithelial transition (MET), enables cells to move from one part of the embryo to another and then differentiate, which contributes to the formation of various organs [13, 14]. In adult cells, the EMT program is usually silent but can be reactivated in processes such as wound healing [15]. Recent studies suggest that cancer cells can resurrect this developmental program: while inducing EMT in epithelial tumor cells facilitates migration, invasion, and dissemination, the MET process enables metastatic colonization [16, 17]. Microenvironmental stimuli emanating from the tumor stroma, such as TGF-β, can activate the expression of several master regulators of embryogenesis, including transcription factors Twist [18], Snail [19, 20], Slug [21], ZEB1 [22], and ZEB2 [23], which have been identified as inducers of EMT and tumor metastasis (Figure 2). Despite initial skepticism, in vivo models and studies investigating EMT features in clinical tumor samples have provided strong evidence for the involvement of EMT and MET in metastasis [24, 25]. In an elegant study, Yang and colleagues generated mice with skin-specific, doxycycline-inducible Twist transgene and induced skin tumors using chemical carcinogens. Then either oral (to induce Twist in both primary and disseminated skin tumor cells) or topical (to induce Twist in only primary skin tumor cells) administration of doxycycline promoted EMT, tumor invasion, and dissemination. Strikingly, mice receiving topical doxycycline had much more lung metastases than mice receiving oral doxycycline, and the metastatic tumors from mice treated with oral or topical doxycycline lost Twist expression and had epithelial features, indicating reversion of EMT [16]. These findings suggest that both EMT and MET are essential for tumor cells to accomplish the invasion-metastasis cascade in certain cancers. However, it should be noted that EMT and MET may not be the prerequisite for metastasis in all tumor types; alternative mechanisms, such as “collective invasion” [26] and “amoeboid movement” [27], have been proposed.
Another model proposes that cancer stem cells (CSCs), which are defined operationally as tumor-initiating cells, are responsible for generating secondary tumors [28]. Interestingly, induction of the EMT program in carcinoma cells can generate cells with properties of CSCs (Figure 2) [29, 30]. Hence, the invasion and intravasation steps of metastasis may involve EMT, which confers both motility and ‘stemness’ on carcinoma cells, while the metastatic colonization step may require the MET program, which facilitates the differentiation of CSCs into non-CSCs. Moreover, the epithelial-mesenchymal plasticity may underlie the non-CSC-to-CSC plasticity. For instance, a recent study demonstrated that TGF-β-induced expression of ZEB1 can drive basal breast cancer cells to undergo EMT and convert from non-CSC state to CSC state [31], while ZEB1-targeting microRNAs (miRNAs), such as miR-205 and the miR-200 family, have been found to promote MET and suppress CSC properties [32–34]. Interestingly, ZEB1 binds to the promoter region of miR-200 genes and represses their transcription, forming a doublenegative feedback loop [35]. Consistent with its MET-inducing effect, the miR-200 family has been found to suppress cancer cell migration and invasion [33, 35] but enhance metastatic colonization after tumor cells have already disseminated [36, 37].
The implication of EMT and CSCs in metastasis has offered potential opportunities for therapeutic intervention [24, 25]. Small-molecule inhibitors of ALK5, MEK, and Src were found to block EMT induction by HGF, epidermal growth factor (EGF), or insulin-like growth factor (IGF)-1 [38], while rapamycin (mTOR inhibitor) and 17-allylamino-17-demethoxygeldanamycin (17-AAG; HSP90 inhibitor) were identified as inhibitors of TGF-β-induced EMT, migration, and invasion [39]. These approaches designed to inhibit EMT induction will likely block tumor cell invasion in early-stage carcinomas; however, in patients with disseminated, micrometastatic tumor cells, killing mesenchymal cancer cells or preventing MET should be the goal. For instance, salinomycin was identified as a compound that induced selective killing of mesenchymal-type breast cancer cells and reduced the proportion of breast CSCs [40]. To date, the signals that trigger MET at the metastatic site remain unclear. Identifying such signals may reveal new therapeutic targets to prevent metastatic colonization.
Molecular determinants of the metastatic process
Oncoproteins and oncomirs: therapeutic targets for both primary tumors and metastases
A primary tumor can be initiated by various alternative oncogenic mutations or amplifications. Certain cancer-causing proteins and miRNAs (oncomirs) also confer advantages for migration, invasion, or metastatic colonization, and thus targeting these tumor-initiating molecules could be beneficial even in advanced cancer, including metastatic disease. One of the most important advances in cancer treatment is the development of drugs that inhibit oncogenic kinases. The monoclonal human epidermal growth factor receptor 2 (HER2) antibody Herceptin® and smallmolecule HER2 inhibitors are effective in treating breast cancers driven by the receptor tyrosine kinase HER2. HER2 serves not only as a drug target, but also as a predictive marker to select responsive patients [41]. Herceptin® in combination with first-line chemotherapy significantly increased the survival of women with metastatic breast cancer that overexpressed HER2 [42]. Similarly, agents targeting mutant ALK kinase in advanced non-small-cell lung cancer [43] or mutant BRAF kinase in metastatic melanoma [44] also showed clinical benefits.
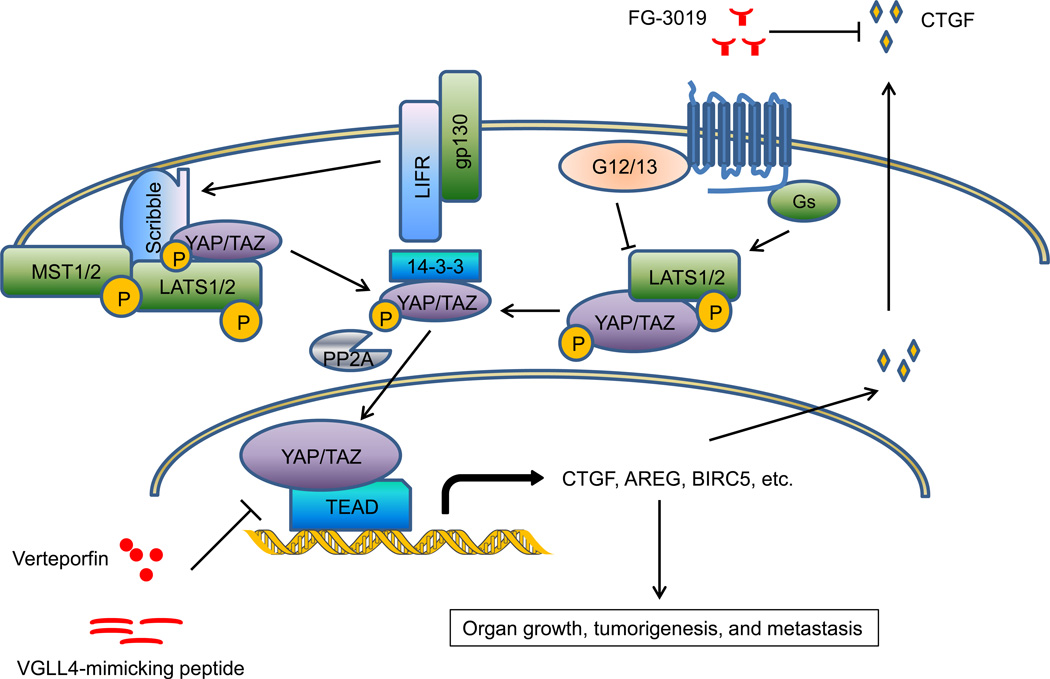
To determine whether targeting specific oncoproteins can also benefit patients with metastatic disease, it will be of great interest to define the role of known oncogenic signaling pathways in metastasis, including RTK signaling cascades, cell cycle regulators, and DNA repair pathways. In addition, recent evidence indicates that deregulation of signaling pathways that control organ size, such as the Hippo pathway (Figure 3), can lead to tumorigenesis and metastasis. As the core component of mammalian Hippo signaling, mammalian Ste20-like kinase (MST, which is the mammalian Hippo homolog) phosphorylates and activates large tumor suppressor kinase (LATS), and LATS kinase in turn phosphorylates two mammalian Yorkie homologs, YES-associated protein (YAP) and transcriptional co-activator with PDZ-binding motif (TAZ), leading to cytoplasmic retention and functional inactivation of these two transcriptional co-activators [45]. Genetic ablation of Mst1/2 [46] or transgenic overexpression of Yap [47] in mice increased liver size and ultimately induced hepatocellular carcinoma, demonstrating a critical role of Hippo signaling in organ growth and tumorigenesis. Moreover, deletion of Yap in the mouse mammary gland strongly suppressed oncogene-induced mammary tumorigenesis and metastasis [48], while overexpression of YAP in breast cancer and melanoma cells promoted tumor growth and metastasis [49]. Several upstream regulators provide inputs feeding into the core Hippo-YAP pathway [45]; among them G-protein-coupled receptors (GPCRs) have been found to regulate LATS and YAP phosphorylation, although they act in a Hippo-independent manner and do not regulate MST [50]. Recently, leukemia inhibitory factor receptor (LIFR) was identified as a cell membrane receptor that inhibits breast cancer metastasis by activating the MST-LATS-YAP phosphorylation cascade [51]. Mechanistically, LIFR promotes cell membrane recruitment of the adaptor protein Scribble, which in turn bridges MST, LATS, and YAP together and facilitates this phosphorylation cascade [51]. Therapeutic agents targeting the Hippo-YAP pathway, including the small-molecule YAP inhibitor verteporfin [52], the peptide mimicking the YAP antagonist vestigial-like family member 4 (VGLL4) [53], and FG-3019, a monoclonal antibody that neutralizes the functional YAP target connective tissue growth factor (CTGF) [54, 55], have shown anti-tumor or anti-metastatic effect in preclinical models (Figure 3). Notably, a Phase II study of FG-3019 treated in combination with gemcitabine demonstrated a dose-dependent increase in the survival of patients with pancreatic cancer (66 of 75 with stage 4 metastatic disease; 2014 American Society of Clinical Oncology Annual Meeting, Abstract #4138: http://meetinglibrary.asco.org/content/134242-144). It would be of interest to determine whether the blood level of CTGF can serve as a predictive marker for anti-CTGF therapy response; if so, this would resemble the HER2 paradigm and facilitate biomarker-driven personalization of metastasis prevention or treatment.
Figure 3. The Hippo-YAP pathway regulates organ growth, tumorigenesis, and metastasis.
Cell membrane receptors LIFR and GPCRs regulate the MST-LATS-YAP/TAZ phosphorylation cascade. Phosphorylation of YAP leads to its cytoplasmic retention and functional inactivation, whereas dephosphorylated YAP translocates to the nucleus and acts as a transcriptional co-activator. Therapeutic agents targeting the Hippo-YAP pathway include the small-molecule YAP inhibitor verteporfin, the peptide mimicking the YAP antagonist VGLL4, and FG-3019, a monoclonal antibody that neutralizes the functional YAP target CTGF.
Several oncomirs are also pro-metastatic [56, 57]. In the Tet-Off miR-21 transgenic mice, miR-21-driven tumors regressed completely in a few days after doxycycline treatment [58], providing a proof-of-principle for oncomir addiction that might be exploited therapeutically. In addition to its oncogenic role, miR-21 also promotes invasion and metastasis by targeting PDCD4, TPM1, and Maspin [59, 60]. Another example is miR-373, which was originally identified as an oncomir targeting the tumor suppressor LATS2 [61]. Later, miR-373 was found to promote migration, invasion, and metastasis of otherwise non-metastatic breast cancer cells [62]. To date, no miRNA has been approved by the Food and Drug Administration (FDA) as a drug. The challenges associated with miRNA therapeutics include off-target effects, difficulty in delivery of the therapeutic agent to the target tissues, immune response, and toxicity [63]. Notwithstanding these obstacles, miRNA-targeting agents are in the developmental pipelines of several pharmaceutical companies, and a liposome-formulated miR-34 mimic (MRX34) entered Phase I clinical trials to treat liver cancer [63]. miRNA-based agents with improved specificity, efficacy, and safety may emerge as new cancer drugs in the near future.
Drivers of migration, invasion, and intravasation: tumor-intrinsic regulators and extracellular/microenvironmental factors
Cancer cells that disseminate from a primary solid tumor can switch between individual and collective movement modes; these cells need to break through physical barriers including the extracellular matrix, the basement membrane, and vasculatures [64]. Regulators of cell motility and invasiveness include integrins, matrix-degrading proteases, cell-cell adhesion molecules, small GTPases (Rho, Rac, and CDC42), and EMT inducers, many of which contribute to metastatic progression [24, 64]. For instance, an in vivo selection approach combined with gene expression analysis identified RhoC as a pro-metastatic protein [65]. Interestingly, the EMT inducer Twist can activate the transcription of a metastasis-promoting miRNA, miR-10b, which in turn targets the mRNA encoding HOXD10, a transcriptional repressor of RhoC [66]. Treatment with the antisense inhibitors of miR-10b blocked metastasis in a mouse mammary tumor model [67].
Intravasation requires tumor cells to cross the walls of vessels made of endothelial cells and pericytes. Pathways regulating tumor-endothelial interaction, trans-endothelial migration, and intravasation include integrin signaling [68] and Notch signaling [69]. Moreover, induction of EMT facilitates carcinoma cell intravasation into the blood circulation, as evidenced by increased numbers of circulating tumor cells (CTCs) in mice bearing skin tumors with induced expression of the Twist transgene; these CTCs were negative for epithelial markers but positive for mesenchymal markers [16]. Consistently, CTCs from human cancer patients also exhibit features of EMT [70, 71].
The crosstalk between tumor cells and their surrounding microenvironment profoundly influences the invasion-metastasis cascade. Hypoxia and inflammation, which are often found in the tumor microenvironment, can induce EMT and dissemination of cancer cells [72, 73]. Various types of stromal cells, including fibroblasts, myofibroblasts, endothelial cells, adipocytes, and bone marrow-derived cells (such as mesenchymal stem cells, macrophages, and other immune cells), provide a repertoire of pro-inflammatory and pro-invasive molecules, such as cytokines, chemokines, and growth factors [74]. Chemokine (C-X-C motif) ligand 12 (CXCL12) secreted by cancer-associated fibroblasts acts on its cognate receptor expressed by tumor cells, chemokine (C-X-C motif) receptor 4 (CXCR4), to enhance cancer cell proliferation, migration, and invasion [75]. Chemokine (C-C motif) ligand 5 (CCL5) secreted by mesenchymal stem cells [76] or interleukin-6 (IL-6) secreted by adipocytes [77] induces breast cancer invasion and metastasis. Migration of carcinoma cells in the primary tumor can be stimulated by a paracrine loop, in which macrophages secrete EGF, engaging EGF receptor expressed by tumor cells, and tumor cells secrete colony stimulating factor 1 (CSF1), engaging CSF1 receptor expressed by macrophages, thereby creating a chemotactic relay system [78]. It should be noted that certain stromal cells, such as fibroblasts, T lymphocytes, and macrophages, can either promote or inhibit tumor progression, depending on their functional state [74]. Therefore, the bidirectional interactions between tumor cells and stromal cells require systematic functional dissection, which may open new avenues for therapeutic intervention.
Circulating tumor cells (CTCs) and disseminated tumor cells (DTCs): emerging biomarkers and therapeutic targets
In most cancer patients, CTCs are rare cells in circulation (a few to a few hundred CTCs per 10 ml blood) and are extremely difficult to detect. Since the presence of CTCs is associated with tumor progression, metastatic relapse, and poor survival outcome, the use of CTCs as early detection or prognostic biomarkers and therapeutic targets are currently under extensive evaluation [79–81]. At clinicaltrials.gov, over 600 registered clinical trials involve CTCs.
Of note, recent evidence suggests that CTCs are present in early-stage cancers. In a mouse model of pancreatic cancer, fluorescently labeled mesenchymal-like pancreatic cancer cells entered the blood and seeded the liver even before any primary tumor was detectable [73], indicating that dissemination from the primary site can be an early event. Therefore, CTCs might serve as a potential biomarker for early detection. This would be particularly important for pancreatic cancer and ovarian cancer, because patients with these cancers usually do not exhibit any obvious symptom until the disease becomes advanced and metastatic. To date, the low incidence of CTCs still represents a major obstacle in developing CTCs as biomarkers; however, as the sensitivity of CTC analyses increases, false positive results may become another challenge.
CTCs have two forms, single-cell CTCs and CTC clusters (Figure 4). CTC clusters are associated with poor prognosis in lung cancer [82]. Recently, by using fluorescent protein-tagged mouse mammary tumor models, Aceto et al. found that CTC clusters, formed in a plakoglobin-dependent manner, exhibit 23- to 50-fold higher metastatic potential than single-cell CTCs [83]. Improvement in CTC enrichment and single-cell sequencing will expedite molecular characterization of CTCs. In addition, development of CTC-derived explant (CDX) models and ex vivo CTC culture systems will enable CTCs to facilitate delivery of personalized medicine and testing of drug sensitivity [84, 85].
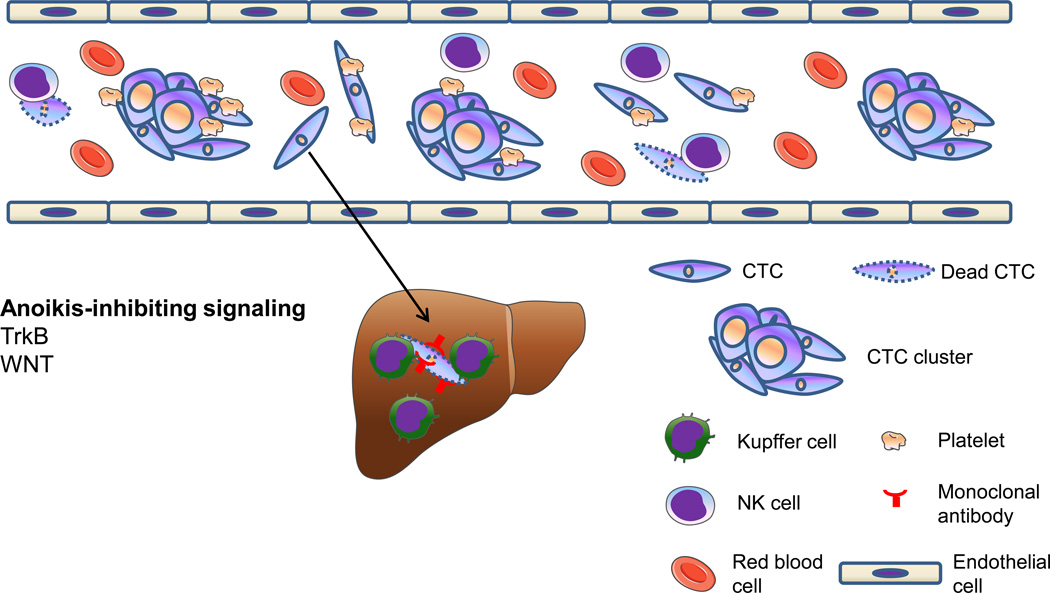
Figure 4. Circulating tumor cells (CTCs) exist as single-cell CTCs and CTC clusters.
Platelets can protect CTCs from NK cell-mediated lysis, whereas Kupffer cells (specialized macrophages in the liver) activated by anti-tumor monoclonal antibodies can eliminate CTCs through phagocytosis.
CTCs encounter several stresses, including hemodynamic shear forces, killing by immune cells, and detachment from matrix. Tumor cells can shield themselves from shear forces and natural killer (NK) cell-mediated lysis by co-opting platelets and forming microthrombi. Higher levels of activated circulating platelets are associated with advanced malignancy, and treatment with anti-coagulants has been found to reduce metastasis and increase survival in experimental and clinical settings [86]. In addition, anti-tumor monoclonal antibody treatment has been found to activate liver macrophages (Kupffer cells) that eliminate CTCs through phagocytosis [87]. In circulation, metastasizing cells also need to overcome anoikis, a form of programmed cell death that is induced by detachment from the surrounding extracellular matrix. TrkB, a neurotrophic tyrosine kinase receptor for brain-derived neurotrophic factor (BDNF), was identified as an anoikis suppressor in a genome-wide functional screen [88]. TrkB inhibits anoikis by activating the PI3K-AKT pathway, leading to survival of tumor cells in lymphatics and blood circulation and increased metastasis [88]. Recently, Yu et al. reported that WNT2 is upregulated in CTCs isolated from a mouse model of pancreatic cancer, and that non-canonical WNT signaling suppresses anoikis and promotes CTC survival and metastasis [89].
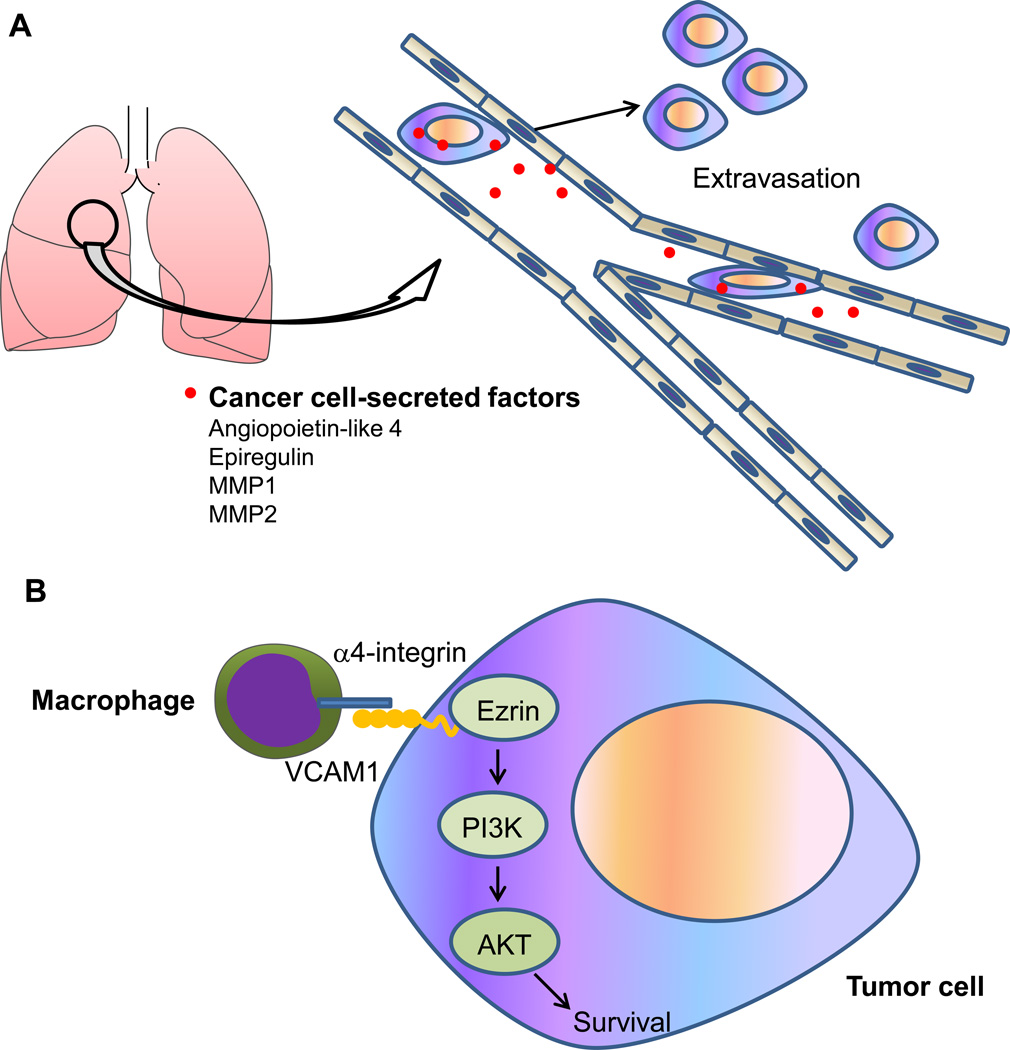
The relatively large diameter of carcinoma cells is estimated to be 20–30 µm, whereas the luminal diameter of capillaries is approximately 8 µm [90]. As might be expected, this size constraint causes CTCs to be arrested in capillary beds at distant anatomic sites, where they extravasate and enter the foreign microenvironment. Of interest, angiopoietin-like 4 (ANGPTL4), the epidermal growth factor receptor (EGFR) ligand epiregulin, the cyclooxygenase COX2, and the matrix metalloproteinases 1 and 2 (MMP1 and MMP2) expressed by breast cancer cells can increase vascular permeability and facilitate extravasation by disrupting pulmonary endothelial cell-cell junctions (Figure 5A), and combined pharmacologic inhibition of these factors by the anti-EGFR antibody cetuximab, the COX2 inhibitor celecoxib, and the broad-spectrum MMP inhibitor GM6001 suppressed lung metastasis in experimental metastasis models [91, 92]. Having breached the vasculature at the site of extravasation, disseminated tumor cells (DTCs) need to adapt to the new milieu for survival and proliferation. In the lung, vascular cell adhesion molecule 1 (VCAM1) expressed on the surface of breast DTCs tethers macrophages to cancer cells via counter receptor α4-integrin, which triggers AKT activation through Ezrin and protects DTCs from pro-apoptotic cytokines such as TNF-related apoptosis-inducing ligand (TRAIL) (Figure 5B) [93]. In the bone marrow, Src kinase is dispensable for homing to the bone but essential for the survival and outgrowth of breast DTCs; mechanistically, Src potentiates CXCL12-CXCR4-AKT pro-survival signaling and dampens TRAIL-mediated pro-apoptotic signaling in the bone marrow microenvironment [94]. Interestingly, treatment with the Src kinase inhibitor dasatinib prevented breast cancer bone metastasis in an experimental metastasis model [94].
Figure 5. Regulation of extravasation and survival of disseminated tumor cells (DTCs) in the lung.
(A) Breast cancer cells can secrete factors, including angiopoietin-like 4, epiregulin, MMP1, and MMP2, which increase vascular permeability and facilitate extravasation by disrupting pulmonary endothelial cell-cell junctions.
(B) DTCs expressing VCAM1 interact with pulmonary macrophages via counter receptor α4-intergrin, which triggers activation of a VCAM1–Ezrin–PI3K–AKT pro-survival pathway.
Determinants of metastatic colonization: key regulators of the bottleneck of metastasis
The organ distribution of metastases not only depends on the vascular pattern, but also reflects the adaptability of tumor cells to specific organ microenvironment. In a pioneering study, Kang, Massagué, and colleagues compared gene expression profiles of MDA-MB-231 human breast cancer cells and the bone metastatic subline derived from intra-cardiac injection of the parental cells, and identified a set of four genes (IL11, CTGF, CXCR4, and MMP1) that act collectively to facilitate metastatic colonization in the bone [95]. Similar approaches have been used to identify genes that regulate breast cancer colonization in the lung [96] and brain [97], which revealed the molecular basis of organ tropism.
Certain physiological processes can be hijacked by cancer cells during metastatic colonization. The bone undergoes remodeling reflecting the balance between osteoclasts which degrade mineralized bone and osteoblasts which reconstruct the bone. Osteoblasts secrete: (1) receptor activator of NF-κB ligand (RANKL), which binds to its receptor (RANK) displayed by the osteoclast precursor to induce its maturation into the osteoclast, and (2) osteoprotegerin (OPG), a soluble decoy receptor that binds secreted RANKL, preventing it from interacting with the RANK receptor. Osteolytic cancer cells often overexpress osteoclast-inducing factors, such as parathyroid hormone-related protein (PTHrP), IL-1, IL-6, and IL-11, which act on osteoblasts to stimulate production of RANKL, leading to osteoclast maturation and bone degradation; on the other hand, matrix-embedded cytokines and growth factors released from the dissolved bone matrix, such as TGF-β and IGF, act on DTCs to stimulate production of osteoclast-promoting factors. This positive feedback loop is often referred to as “the vicious cycle of osteolytic bone metastasis” [98]. Approaches to breaking this vicious cycle and treating bone metastasis include bisphosphonates, OPG, and PTHrP-neutralizing antibodies; among them bisphosphonates are being used clinically to prevent or treat diseases of bone loss, including osteoporosis, Paget’s disease, and cancers that cause osteolytic metastasis. Bisphosphonates inhibit osteoclastic bone resorption by promoting osteoclasts to undergo apoptosis [98].
DTCs at the distant organ site can either grow into clinically significant metastases, or remain dormant due to the lack of proliferative signals and/or the presence of anti-proliferative signals in the new environment, obstacles that they need to overcome in order to proliferate from occult micrometastases into macroscopic secondary tumors. Dormant DTCs, the seed of distant relapse, are extremely difficult to eradicate because they are clinically asymptomatic and resistant to conventional or targeted therapies. Although evidence indicates that CSCs, cell cycle regulators, epigenetic factors, and microenvironment play important roles in regulating tumor cell dormancy, our understanding of this field is still limited. Due to its importance in metastatic recurrence, the mechanism of dormancy and reactivation of DTCs has become an awakening field of cancer research [99, 100]. Fibrosis can induce tumor progression and metastasis not only through tumor-stroma interaction at the primary site, but also by reactivating dormant DTCs at the metastatic site. For instance, the transition of breast DTCs from quiescence to proliferation is mediated by binding to fibronectin or type I collagen (Col-I) often found in the fibrotic metastatic lesions, which induces otherwise dormant breast cancer cells to proliferate through β1-integrin activation of Src and focal adhesion kinase (FAK); genetic or pharmacologic inhibition of this signaling cascade blocked cytoskeletal reorganization and cell proliferation in vitro and reduced metastatic outgrowth in vivo [101–103]. Fibrosis is associated with metastasis and poor prognosis in breast cancer, pancreatic cancer, and other cancers [2]. Anti-fibrotic drugs that have been developed for fibrotic diseases, such as the CTGF-neutralizing antibody (FG-3019) mentioned above, may prove useful as anti-metastatic agents. On the other hand, stromaderived growth-inhibitory signals, such as bone morphogenic protein (BMP) produced by the lung parenchyma, represent a barrier to metastatic colonization. Gain-of-expression of Coco, a secreted BMP antagonist, induces reactivation of otherwise dormant breast DTCs to proliferate and form metastases in the lung [104], suggesting that counteracting the anti-metastatic signals in the distant organ leads to metastatic outgrowth.
The link between metastasis and therapy resistance
Tumor cells with therapy resistance, including radioresistance and drug resistance, give rise to tumor recurrence and metastatic relapse [2]. Emerging evidence has suggested that some of the molecules that endow tumor cells with metastatic ability also confer treatment resistance. Therefore, targeting these molecules has the potential to overcome therapy resistance and to eliminate local and distant recurrence.
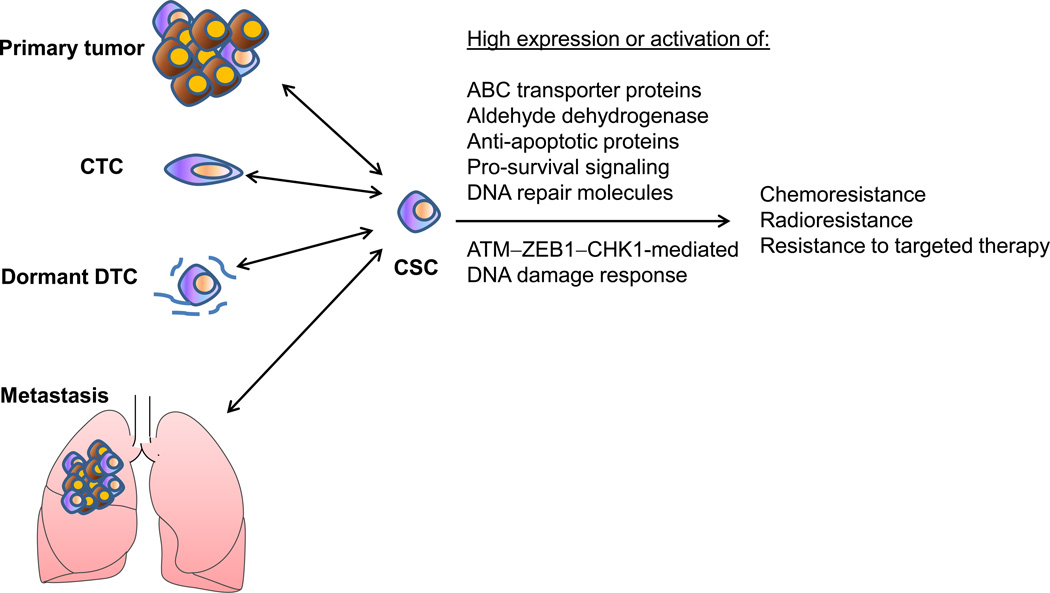
Recently, CSCs have been found to promote tumor radioresistance though activation of the DNA damage response. This was first reported in glioblastoma, in which glioma cells expressing the brain CSC marker CD133 are resistant to ionizing radiation because they are more efficient at repairing damaged DNA than the bulk of the tumor cells [105]. Later, similar findings were reported for other tumor types including breast cancer [106, 107]. CSCs are also believed to be resistant to chemotherapy due to high expression or activation of ATP-binding cassette (ABC) transporter proteins, aldehyde dehydrogenase (ALDH), anti-apoptotic proteins, pro-survival signaling components, and DNA repair molecules [108]. The association between EMT and CSC properties, including chemoresistance, radioresistance, and resistance to targeted therapies, has been reported by a number of studies (Figure 6) [109–117]. Does EMT itself or specific EMT regulators play a causal role in therapy resistance? Moreover, are all EMT inducers equal?
Figure 6. Cancer stem cells (CSCs) and therapy resistance.
CSCs exhibit chemoresistance, radioresistance, and resistance to targeted therapies, and are responsible for generating primary and metastatic tumors. Plasticity is likely to exist between non-CSCs and CSCs. For instance, induced expression of ZEB1 can drive differentiated epithelial cancer cells to undergo EMT and convert from non-CSC state to CSC state, and promote DNA damage response, radioresistance, and drug resistance.
A recent study demonstrated that it is not the epithelial or mesenchymal state itself that dictates tumor radioresistance; instead, it is a specific EMT inducer, ZEB1, that regulates the response to radiation, whereas Twist and Snail do not affect radiosensitivity [117]. Mechanistically, radiation-induced activation of ATM kinase phosphorylates and stabilizes ZEB1, which in turn recruits USP7 and enhances its ability to deubiquitinate and stabilize CHK1, leading to increased DNA repair and radioresistance independent of EMT [117]. In parallel, ZEB1 represses its own negative regulator, miR-205, resulting in further increased levels of ZEB1 [118]. Radiation-induced upregulation of ZEB1 has been observed in breast cancer cells [117], lung cancer cells [119], and nasopharyngeal cancer cells [120]. These studies suggest that radiation treatment may cause therapy-induced radioresistance through ZEB1, leading to local and distant recurrence eventually. In support of this notion, among patients who received radiotherapy, those with high ZEB1 expression or low miR-205 expression in their breast tumors had much worse metastatic relapse-free survival outcome than those with low ZEB1 expression or high miR-205 expression [117, 118]. Moreover, therapeutic delivery of ZEB1-targeting miRNAs, including miR-205 [118] and miR-200c [121], sensitized tumors to radiation treatment in preclinical models. As a driver of EMT, ZEB1 can promote tumor metastasis and stemness by repressing E-cadherin and stemness-inhibiting miRNAs [22, 122], or by repressing other target genes including HUGL2 (also named LGL2, lethal giant larvae homolog 2), PATJ (Pals1-associated tight junction protein), and Crumbs3 [123, 124]. In addition, depending on the specific tumor type and treatment type, ZEB1 can employ EMTdependent and EMT-independent mechanisms to regulate resistance to chemotherapeutic agents (such as temozolomide, gemcitabine, 5-fluorouracil, cisplatin, and docetaxel) [110, 114, 115] and targeted therapies (such as the PI3K inhibitor and the EGFR inhibitor) [116, 125]. Taken together, ZEB1 represents a pleiotropically acting transcription factor that links EMT, metastasis, and therapy resistance. ZEB1-targeting agents, such as miR-200c and miR-205 mimics, may provide new therapeutic opportunities [126].
Concluding remarks
Metastasis is the leading cause of cancer-related death. Although significant progress has been made in understanding the mechanisms of tumor progression and metastasis during the past century, the knowledge of the molecular machinery governing metastasis is still incomplete. Heterogeneity that exists within both the primary tumor and the metastatic tumor may underlie the failure of cancer treatment, and thus it is critical to improve molecular characterization of heterogeneous metastatic cells. In addition, a number of approaches have been established for metastasis research, but until recently, there was a paucity of technologies for studying CTCs, DTCs, and metastatic dormancy and reactivation. CTC enrichment methods, single-cell sequencing techniques, patient-derived xenograft models, and other new tools will facilitate metastasis research and clinical development. Furthermore, despite the emerging new regulators of metastasis, the knowledge is rarely translated into clinical advances. There is a pressing need to develop novel biomarker-driven clinical trials for metastasis prevention and treatment.
Highlights.
We highlight the challenges in making translational advance on metastasis research.
We delineate the role of epithelial-mesenchymal plasticity and cancer stem cells.
We review emerging molecular determinants and therapeutic targets of metastasis.
We discuss the link between metastasis and therapy resistance.
Acknowledgments
The authors’ research is supported by the US National Institutes of Health grants R01CA166051 and R01CA181029 (to L.M.) and Cancer Prevention and Research Institute of Texas grants R1004 and RP150319 (to L.M.). L.M. is an R. Lee Clark Fellow (supported by the Jeanne F. Shelby Scholarship Fund) of The University of Texas MD Anderson Cancer Center. We thank Ashley Siverly for critical reading of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fidler IJ. The pathogenesis of cancer metastasis: the 'seed and soil' hypothesis revisited. Nature reviews. Cancer. 2003;3:453–458. doi: 10.1038/nrc1098. [DOI] [PubMed] [Google Scholar]
- 2.Brabletz T, et al. Roadblocks to translational advances on metastasis research. Nature medicine. 2013;19:1104–1109. doi: 10.1038/nm.3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wan L, et al. Tumor metastasis: moving new biological insights into the clinic. Nature medicine. 2013;19:1450–1464. doi: 10.1038/nm.3391. [DOI] [PubMed] [Google Scholar]
- 4.Eccles SA, Welch DR. Metastasis: recent discoveries and novel treatment strategies. Lancet. 2007;369:1742–1757. doi: 10.1016/S0140-6736(07)60781-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Talmadge JE, Fidler IJ. AACR centennial series: the biology of cancer metastasis: historical perspective. Cancer research. 2010;70:5649–5669. doi: 10.1158/0008-5472.CAN-10-1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee YT. Breast carcinoma: pattern of metastasis at autopsy. Journal of surgical oncology. 1983;23:175–180. doi: 10.1002/jso.2930230311. [DOI] [PubMed] [Google Scholar]
- 7.Weigelt B, et al. Breast cancer metastasis: markers and models. Nature reviews. Cancer. 2005;5:591–602. doi: 10.1038/nrc1670. [DOI] [PubMed] [Google Scholar]
- 8.Steeg PS, Theodorescu D. Metastasis: a therapeutic target for cancer. Nature clinical practice. Oncology. 2008;5:206–219. doi: 10.1038/ncponc1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Steeg PS. Perspective: The right trials. Nature. 2012;485:S58–S59. doi: 10.1038/485S58a. [DOI] [PubMed] [Google Scholar]
- 10.Lehmann BD, et al. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. The Journal of clinical investigation. 2011;121:2750–2767. doi: 10.1172/JCI45014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scheel C, et al. Adaptation versus selection: the origins of metastatic behavior. Cancer research. 2007;67:11476–11479. doi: 10.1158/0008-5472.CAN-07-1653. discussion 11479–11480. [DOI] [PubMed] [Google Scholar]
- 12.Thiery JP. Epithelial-mesenchymal transitions in tumour progression. Nature reviews. Cancer. 2002;2:442–454. doi: 10.1038/nrc822. [DOI] [PubMed] [Google Scholar]
- 13.Thiery JP, et al. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139:871–890. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 14.Yang J, Weinberg RA. Epithelial-mesenchymal transition: at the crossroads of development and tumor metastasis. Dev Cell. 2008;14:818–829. doi: 10.1016/j.devcel.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 15.Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119:1420–1428. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tsai JH, et al. Spatiotemporal regulation of epithelial-mesenchymal transition is essential for squamous cell carcinoma metastasis. Cancer cell. 2012;22:725–736. doi: 10.1016/j.ccr.2012.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ocana OH, et al. Metastatic colonization requires the repression of the epithelial-mesenchymal transition inducer Prrx1. Cancer cell. 2012;22:709–724. doi: 10.1016/j.ccr.2012.10.012. [DOI] [PubMed] [Google Scholar]
- 18.Yang J, et al. Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell. 2004;117:927–939. doi: 10.1016/j.cell.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 19.Batlle E, et al. The transcription factor snail is a repressor of E-cadherin gene expression in epithelial tumour cells. Nat Cell Biol. 2000;2:84–89. doi: 10.1038/35000034. [DOI] [PubMed] [Google Scholar]
- 20.Cano A, et al. The transcription factor snail controls epithelial-mesenchymal transitions by repressing E-cadherin expression. Nat Cell Biol. 2000;2:76–83. doi: 10.1038/35000025. [DOI] [PubMed] [Google Scholar]
- 21.Hajra KM, et al. The SLUG zinc-finger protein represses E-cadherin in breast cancer. Cancer research. 2002;62:1613–1618. [PubMed] [Google Scholar]
- 22.Eger A, et al. DeltaEF1 is a transcriptional repressor of E-cadherin and regulates epithelial plasticity in breast cancer cells. Oncogene. 2005;24:2375–2385. doi: 10.1038/sj.onc.1208429. [DOI] [PubMed] [Google Scholar]
- 23.Comijn J, et al. The two-handed E box binding zinc finger protein SIP1 downregulates E-cadherin and induces invasion. Mol Cell. 2001;7:1267–1278. doi: 10.1016/s1097-2765(01)00260-x. [DOI] [PubMed] [Google Scholar]
- 24.Tsai JH, Yang J. Epithelial-mesenchymal plasticity in carcinoma metastasis. Genes Dev. 2013;27:2192–2206. doi: 10.1101/gad.225334.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Davis FM, et al. Targeting EMT in cancer: opportunities for pharmacological intervention. Trends in pharmacological sciences. 2014;35:479–488. doi: 10.1016/j.tips.2014.06.006. [DOI] [PubMed] [Google Scholar]
- 26.Friedl P, Wolf K. Tube travel: the role of proteases in individual and collective cancer cell invasion. Cancer research. 2008;68:7247–7249. doi: 10.1158/0008-5472.CAN-08-0784. [DOI] [PubMed] [Google Scholar]
- 27.Sabeh F, et al. Protease-dependent versus -independent cancer cell invasion programs: three-dimensional amoeboid movement revisited. The Journal of cell biology. 2009;185:11–19. doi: 10.1083/jcb.200807195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brabletz T, et al. Opinion: migrating cancer stem cells - an integrated concept of malignant tumour progression. Nature reviews. Cancer. 2005;5:744–749. doi: 10.1038/nrc1694. [DOI] [PubMed] [Google Scholar]
- 29.Mani SA, et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133:704–715. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morel AP, et al. Generation of breast cancer stem cells through epithelial-mesenchymal transition. PloS one. 2008;3:e2888. doi: 10.1371/journal.pone.0002888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chaffer CL, et al. Poised chromatin at the ZEB1 promoter enables breast cancer cell plasticity and enhances tumorigenicity. Cell. 2013;154:61–74. doi: 10.1016/j.cell.2013.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gregory PA, et al. The miR-200 family and miR-205 regulate epithelial to mesenchymal transition by targeting ZEB1 and SIP1. Nat Cell Biol. 2008;10:593–601. doi: 10.1038/ncb1722. [DOI] [PubMed] [Google Scholar]
- 33.Park SM, et al. The miR-200 family determines the epithelial phenotype of cancer cells by targeting the E-cadherin repressors ZEB1 and ZEB2. Genes Dev. 2008;22:894–907. doi: 10.1101/gad.1640608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shimono Y, et al. Downregulation of miRNA-200c links breast cancer stem cells with normal stem cells. Cell. 2009;138:592–603. doi: 10.1016/j.cell.2009.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Burk U, et al. A reciprocal repression between ZEB1 and members of the miR-200 family promotes EMT and invasion in cancer cells. EMBO reports. 2008;9:582–589. doi: 10.1038/embor.2008.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Korpal M, et al. Direct targeting of Sec23a by miR-200s influences cancer cell secretome and promotes metastatic colonization. Nature medicine. 2011;17:1101–1108. doi: 10.1038/nm.2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dykxhoorn DM, et al. miR-200 enhances mouse breast cancer cell colonization to form distant metastases. PloS one. 2009;4:e7181. doi: 10.1371/journal.pone.0007181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chua KN, et al. A cell-based small molecule screening method for identifying inhibitors of epithelial-mesenchymal transition in carcinoma. PloS one. 2012;7:e33183. doi: 10.1371/journal.pone.0033183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reka AK, et al. Identifying inhibitors of epithelial-mesenchymal transition by connectivity map-based systems approach. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer. 2011;6:1784–1792. doi: 10.1097/JTO.0b013e31822adfb0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gupta PB, et al. Identification of selective inhibitors of cancer stem cells by high-throughput screening. Cell. 2009;138:645–659. doi: 10.1016/j.cell.2009.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pegram MD, et al. HER-2/neu as a predictive marker of response to breast cancer therapy. Breast cancer research and treatment. 1998;52:65–77. doi: 10.1023/a:1006111117877. [DOI] [PubMed] [Google Scholar]
- 42.Slamon DJ, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. The New England journal of medicine. 2001;344:783–792. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 43.Kwak EL, et al. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. The New England journal of medicine. 2010;363:1693–1703. doi: 10.1056/NEJMoa1006448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chapman PB, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. The New England journal of medicine. 2011;364:2507–2516. doi: 10.1056/NEJMoa1103782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yu FX, Guan KL. The Hippo pathway: regulators and regulations. Genes Dev. 2013;27:355–371. doi: 10.1101/gad.210773.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lu L, et al. Hippo signaling is a potent in vivo growth and tumor suppressor pathway in the mammalian liver. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:1437–1442. doi: 10.1073/pnas.0911427107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dong J, et al. Elucidation of a universal size-control mechanism in Drosophila and mammals. Cell. 2007;130:1120–1133. doi: 10.1016/j.cell.2007.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen Q, et al. A temporal requirement for Hippo signaling in mammary gland differentiation, growth, and tumorigenesis. Genes Dev. 2014;28:432–437. doi: 10.1101/gad.233676.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lamar JM, et al. The Hippo pathway target, YAP, promotes metastasis through its TEAD-interaction domain. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:E2441–E2450. doi: 10.1073/pnas.1212021109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yu FX, et al. Regulation of the Hippo-YAP pathway by G-protein-coupled receptor signaling. Cell. 2012;150:780–791. doi: 10.1016/j.cell.2012.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen D, et al. LIFR is a breast cancer metastasis suppressor upstream of the Hippo-YAP pathway and a prognostic marker. Nature medicine. 2012;18:1511–1517. doi: 10.1038/nm.2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu-Chittenden Y, et al. Genetic and pharmacological disruption of the TEAD-YAP complex suppresses the oncogenic activity of YAP. Genes Dev. 2012;26:1300–1305. doi: 10.1101/gad.192856.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jiao S, et al. A peptide mimicking VGLL4 function acts as a YAP antagonist therapy against gastric cancer. Cancer cell. 2014;25:166–180. doi: 10.1016/j.ccr.2014.01.010. [DOI] [PubMed] [Google Scholar]
- 54.Dornhofer N, et al. Connective tissue growth factor-specific monoclonal antibody therapy inhibits pancreatic tumor growth and metastasis. Cancer research. 2006;66:5816–5827. doi: 10.1158/0008-5472.CAN-06-0081. [DOI] [PubMed] [Google Scholar]
- 55.Finger EC, et al. CTGF is a therapeutic target for metastatic melanoma. Oncogene. 2014;33:1093–1100. doi: 10.1038/onc.2013.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang J, Ma L. MicroRNA control of epithelial-mesenchymal transition and metastasis. Cancer Metastasis Rev. 2012;31:653–662. doi: 10.1007/s10555-012-9368-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pencheva N, Tavazoie SF. Control of metastatic progression by microRNA regulatory networks. Nat Cell Biol. 2013;15:546–554. doi: 10.1038/ncb2769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Medina PP, et al. OncomiR addiction in an in vivo model of microRNA-21-induced pre-B-cell lymphoma. Nature. 2010;467:86–90. doi: 10.1038/nature09284. [DOI] [PubMed] [Google Scholar]
- 59.Asangani IA, et al. MicroRNA-21 (miR-21) post-transcriptionally downregulates tumor suppressor Pdcd4 and stimulates invasion, intravasation and metastasis in colorectal cancer. Oncogene. 2008;27:2128–2136. doi: 10.1038/sj.onc.1210856. [DOI] [PubMed] [Google Scholar]
- 60.Zhu S, et al. MicroRNA-21 targets tumor suppressor genes in invasion and metastasis. Cell Res. 2008;18:350–359. doi: 10.1038/cr.2008.24. [DOI] [PubMed] [Google Scholar]
- 61.Voorhoeve PM, et al. A genetic screen implicates miRNA-372 and miRNA-373 as oncogenes in testicular germ cell tumors. Cell. 2006;124:1169–1181. doi: 10.1016/j.cell.2006.02.037. [DOI] [PubMed] [Google Scholar]
- 62.Huang Q, et al. The microRNAs miR-373 and miR-520c promote tumour invasion and metastasis. Nat Cell Biol. 2008;10:202–210. doi: 10.1038/ncb1681. [DOI] [PubMed] [Google Scholar]
- 63.Ling H, et al. MicroRNAs and other non-coding RNAs as targets for anticancer drug development. Nature reviews. Drug discovery. 2013;12:847–865. doi: 10.1038/nrd4140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Friedl P, Wolf K. Tumour-cell invasion and migration: diversity and escape mechanisms. Nature reviews. Cancer. 2003;3:362–374. doi: 10.1038/nrc1075. [DOI] [PubMed] [Google Scholar]
- 65.Clark EA, et al. Genomic analysis of metastasis reveals an essential role for RhoC. Nature. 2000;406:532–535. doi: 10.1038/35020106. [DOI] [PubMed] [Google Scholar]
- 66.Ma L, et al. Tumour invasion and metastasis initiated by microRNA-10b in breast cancer. Nature. 2007;449:682–688. doi: 10.1038/nature06174. [DOI] [PubMed] [Google Scholar]
- 67.Ma L, et al. Therapeutic silencing of miR-10b inhibits metastasis in a mouse mammary tumor model. Nature biotechnology. 2010;28:341–347. doi: 10.1038/nbt.1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Reymond N, et al. Cdc42 promotes transendothelial migration of cancer cells through beta1 integrin. The Journal of cell biology. 2012;199:653–668. doi: 10.1083/jcb.201205169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sonoshita M, et al. Suppression of colon cancer metastasis by Aes through inhibition of Notch signaling. Cancer cell. 2011;19:125–137. doi: 10.1016/j.ccr.2010.11.008. [DOI] [PubMed] [Google Scholar]
- 70.Kallergi G, et al. Epithelial to mesenchymal transition markers expressed in circulating tumour cells of early and metastatic breast cancer patients. Breast cancer research : BCR. 2011;13:R59. doi: 10.1186/bcr2896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yu M, et al. Circulating breast tumor cells exhibit dynamic changes in epithelial and mesenchymal composition. Science. 2013;339:580–584. doi: 10.1126/science.1228522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lu X, Kang Y. Hypoxia and hypoxia-inducible factors: master regulators of metastasis. Clinical cancer research : an official journal of the American Association for Cancer Research. 2010;16:5928–5935. doi: 10.1158/1078-0432.CCR-10-1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rhim AD, et al. EMT and dissemination precede pancreatic tumor formation. Cell. 2012;148:349–361. doi: 10.1016/j.cell.2011.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Joyce JA, Pollard JW. Microenvironmental regulation of metastasis. Nature reviews. Cancer. 2009;9:239–252. doi: 10.1038/nrc2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Allinen M, et al. Molecular characterization of the tumor microenvironment in breast cancer. Cancer cell. 2004;6:17–32. doi: 10.1016/j.ccr.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 76.Karnoub AE, et al. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature. 2007;449:557–563. doi: 10.1038/nature06188. [DOI] [PubMed] [Google Scholar]
- 77.Dirat B, et al. Cancer-associated adipocytes exhibit an activated phenotype and contribute to breast cancer invasion. Cancer research. 2011;71:2455–2465. doi: 10.1158/0008-5472.CAN-10-3323. [DOI] [PubMed] [Google Scholar]
- 78.Wyckoff J, et al. A paracrine loop between tumor cells and macrophages is required for tumor cell migration in mammary tumors. Cancer research. 2004;64:7022–7029. doi: 10.1158/0008-5472.CAN-04-1449. [DOI] [PubMed] [Google Scholar]
- 79.Krebs MG, et al. Molecular analysis of circulating tumour cells-biology and biomarkers. Nat Rev Clin Oncol. 2014;11:129–144. doi: 10.1038/nrclinonc.2013.253. [DOI] [PubMed] [Google Scholar]
- 80.Yap TA, et al. Circulating tumor cells: a multifunctional biomarker. Clinical cancer research : an official journal of the American Association for Cancer Research. 2014;20:2553–2568. doi: 10.1158/1078-0432.CCR-13-2664. [DOI] [PubMed] [Google Scholar]
- 81.Miyamoto DT, et al. Circulating tumour cells-monitoring treatment response in prostate cancer. Nat Rev Clin Oncol. 2014;11:401–412. doi: 10.1038/nrclinonc.2014.82. [DOI] [PubMed] [Google Scholar]
- 82.Hou JM, et al. Clinical significance and molecular characteristics of circulating tumor cells and circulating tumor microemboli in patients with small-cell lung cancer. J Clin Oncol. 2012;30:525–532. doi: 10.1200/JCO.2010.33.3716. [DOI] [PubMed] [Google Scholar]
- 83.Aceto N, et al. Circulating tumor cell clusters are oligoclonal precursors of breast cancer metastasis. Cell. 2014;158:1110–1122. doi: 10.1016/j.cell.2014.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hodgkinson CL, et al. Tumorigenicity and genetic profiling of circulating tumor cells in small-cell lung cancer. Nature medicine. 2014;20:897–903. doi: 10.1038/nm.3600. [DOI] [PubMed] [Google Scholar]
- 85.Yu M, et al. Cancer therapy. Ex vivo culture of circulating breast tumor cells for individualized testing of drug susceptibility. Science. 2014;345:216–220. doi: 10.1126/science.1253533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Nash GF, et al. Platelets and cancer. The Lancet. Oncology. 2002;3:425–430. doi: 10.1016/s1470-2045(02)00789-1. [DOI] [PubMed] [Google Scholar]
- 87.Gul N, et al. Macrophages eliminate circulating tumor cells after monoclonal antibody therapy. J Clin Invest. 2014;124:812–823. doi: 10.1172/JCI66776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Douma S, et al. Suppression of anoikis and induction of metastasis by the neurotrophic receptor TrkB. Nature. 2004;430:1034–1039. doi: 10.1038/nature02765. [DOI] [PubMed] [Google Scholar]
- 89.Yu M, et al. RNA sequencing of pancreatic circulating tumour cells implicates WNT signalling in metastasis. Nature. 2012;487:510–513. doi: 10.1038/nature11217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Valastyan S, Weinberg RA. Tumor metastasis: molecular insights and evolving paradigms. Cell. 2011;147:275–292. doi: 10.1016/j.cell.2011.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Padua D, et al. TGFbeta primes breast tumors for lung metastasis seeding through angiopoietin-like 4. Cell. 2008;133:66–77. doi: 10.1016/j.cell.2008.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gupta GP, et al. Mediators of vascular remodelling co-opted for sequential steps in lung metastasis. Nature. 2007;446:765–770. doi: 10.1038/nature05760. [DOI] [PubMed] [Google Scholar]
- 93.Chen Q, et al. Macrophage binding to receptor VCAM-1 transmits survival signals in breast cancer cells that invade the lungs. Cancer cell. 2011;20:538–549. doi: 10.1016/j.ccr.2011.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhang XH, et al. Latent bone metastasis in breast cancer tied to Src-dependent survival signals. Cancer cell. 2009;16:67–78. doi: 10.1016/j.ccr.2009.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kang Y, et al. A multigenic program mediating breast cancer metastasis to bone. Cancer cell. 2003;3:537–549. doi: 10.1016/s1535-6108(03)00132-6. [DOI] [PubMed] [Google Scholar]
- 96.Minn AJ, et al. Genes that mediate breast cancer metastasis to lung. Nature. 2005;436:518–524. doi: 10.1038/nature03799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bos PD, et al. Genes that mediate breast cancer metastasis to the brain. Nature. 2009;459:1005–1009. doi: 10.1038/nature08021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mundy GR. Metastasis to bone: causes, consequences and therapeutic opportunities. Nature reviews. Cancer. 2002;2:584–593. doi: 10.1038/nrc867. [DOI] [PubMed] [Google Scholar]
- 99.Sosa MS, et al. Mechanisms of disseminated cancer cell dormancy: an awakening field. Nature reviews. Cancer. 2014;14:611–622. doi: 10.1038/nrc3793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Giancotti FG. Mechanisms governing metastatic dormancy and reactivation. Cell. 2013;155:750–764. doi: 10.1016/j.cell.2013.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Barkan D, et al. Inhibition of metastatic outgrowth from single dormant tumor cells by targeting the cytoskeleton. Cancer research. 2008;68:6241–6250. doi: 10.1158/0008-5472.CAN-07-6849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Barkan D, et al. Metastatic growth from dormant cells induced by a col-I-enriched fibrotic environment. Cancer research. 2010;70:5706–5716. doi: 10.1158/0008-5472.CAN-09-2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Shibue T, Weinberg RA. Integrin beta1-focal adhesion kinase signaling directs the proliferation of metastatic cancer cells disseminated in the lungs. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:10290–10295. doi: 10.1073/pnas.0904227106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Gao H, et al. The BMP inhibitor Coco reactivates breast cancer cells at lung metastatic sites. Cell. 2012;150:764–779. doi: 10.1016/j.cell.2012.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bao S, et al. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444:756–760. doi: 10.1038/nature05236. [DOI] [PubMed] [Google Scholar]
- 106.Phillips TM, et al. The response of CD24(−/low)/CD44+ breast cancer-initiating cells to radiation. Journal of the National Cancer Institute. 2006;98:1777–1785. doi: 10.1093/jnci/djj495. [DOI] [PubMed] [Google Scholar]
- 107.Baumann M, et al. Exploring the role of cancer stem cells in radioresistance. Nature reviews. Cancer. 2008;8:545–554. doi: 10.1038/nrc2419. [DOI] [PubMed] [Google Scholar]
- 108.Holohan C, et al. Cancer drug resistance: an evolving paradigm. Nature reviews. Cancer. 2013;13:714–726. doi: 10.1038/nrc3599. [DOI] [PubMed] [Google Scholar]
- 109.Wang Z, et al. Pancreatic cancer: understanding and overcoming chemoresistance. Nat Rev Gastroenterol Hepatol. 2011;8:27–33. doi: 10.1038/nrgastro.2010.188. [DOI] [PubMed] [Google Scholar]
- 110.Arumugam T, et al. Epithelial to mesenchymal transition contributes to drug resistance in pancreatic cancer. Cancer research. 2009;69:5820–5828. doi: 10.1158/0008-5472.CAN-08-2819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Sui H, et al. Epithelial-mesenchymal transition and drug resistance: role, molecular mechanisms, and therapeutic strategies. Oncol Res Treat. 2014;37:584–589. doi: 10.1159/000367802. [DOI] [PubMed] [Google Scholar]
- 112.Dave B, et al. Epithelial-mesenchymal transition, cancer stem cells and treatment resistance. Breast cancer research : BCR. 2012;14:202. doi: 10.1186/bcr2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Adam L, et al. miR-200 expression regulates epithelial-to-mesenchymal transition in bladder cancer cells and reverses resistance to epidermal growth factor receptor therapy. Clinical cancer research : an official journal of the American Association for Cancer Research. 2009;15:5060–5072. doi: 10.1158/1078-0432.CCR-08-2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Siebzehnrubl FA, et al. The ZEB1 pathway links glioblastoma initiation, invasion and chemoresistance. EMBO Mol Med. 2013;5:1196–1212. doi: 10.1002/emmm.201302827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ren J, et al. Inhibition of ZEB1 reverses EMT and chemoresistance in docetaxel-resistant human lung adenocarcinoma cell line. J Cell Biochem. 2013;114:1395–1403. doi: 10.1002/jcb.24481. [DOI] [PubMed] [Google Scholar]
- 116.Haddad Y, et al. Delta-crystallin enhancer binding factor 1 controls the epithelial to mesenchymal transition phenotype and resistance to the epidermal growth factor receptor inhibitor erlotinib in human head and neck squamous cell carcinoma lines. Clinical cancer research : an official journal of the American Association for Cancer Research. 2009;15:532–542. doi: 10.1158/1078-0432.CCR-08-1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zhang P, et al. ATM-mediated stabilization of ZEB1 promotes DNA damage response and radioresistance through CHK1. Nat Cell Biol. 2014;16:864–875. doi: 10.1038/ncb3013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zhang P, et al. miR-205 acts as a tumour radiosensitizer by targeting ZEB1 and Ubc13. Nat Commun. 2014;5:5671. doi: 10.1038/ncomms6671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Liu W, et al. Inhibition of TBK1 attenuates radiation-induced epithelial-mesenchymal transition of A549 human lung cancer cells via activation of GSK-3beta and repression of ZEB1. Lab Invest. 2014;94:362–370. doi: 10.1038/labinvest.2013.153. [DOI] [PubMed] [Google Scholar]
- 120.Chen W, et al. Effect of AKT inhibition on epithelial-mesenchymal transition and ZEB1-potentiated radiotherapy in nasopharyngeal carcinoma. Oncology letters. 2013;6:1234–1240. doi: 10.3892/ol.2013.1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Cortez MA, et al. Therapeutic delivery of miR-200c enhances radiosensitivity in lung cancer. Mol Ther. 2014;22:1494–1503. doi: 10.1038/mt.2014.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wellner U, et al. The EMT-activator ZEB1 promotes tumorigenicity by repressing stemness-inhibiting microRNAs. Nat Cell Biol. 2009;11:1487–1495. doi: 10.1038/ncb1998. [DOI] [PubMed] [Google Scholar]
- 123.Spaderna S, et al. The transcriptional repressor ZEB1 promotes metastasis and loss of cell polarity in cancer. Cancer research. 2008;68:537–544. doi: 10.1158/0008-5472.CAN-07-5682. [DOI] [PubMed] [Google Scholar]
- 124.Aigner K, et al. The transcription factor ZEB1 (deltaEF1) promotes tumour cell dedifferentiation by repressing master regulators of epithelial polarity. Oncogene. 2007;26:6979–6988. doi: 10.1038/sj.onc.1210508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Yang Y, et al. ZEB1 sensitizes lung adenocarcinoma to metastasis suppression by PI3K antagonism. J Clin Invest. 2014;124:2696–2708. doi: 10.1172/JCI72171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Zhang P, et al. ZEB1: At the crossroads of epithelial-mesenchymal transition, metastasis and therapy resistance. Cell cycle. 2015;14:481–487. doi: 10.1080/15384101.2015.1006048. [DOI] [PMC free article] [PubMed] [Google Scholar]