Version Changes
Revised. Amendments from Version 1
In this version, a table was included listing past and ongoing clinical trials using nelfinavir in cancer treatment.
Abstract
Objective: To review the mechanisms of anti-cancer activity of nelfinavir and other protease inhibitors (PIs) based on evidences reported in the published literature.
Methods: We extensively reviewed the literature concerning nelfinavir (NFV) as an off target anti-cancer drug and other PIs. A classification of PIs based on anti-cancer mode of action was proposed. Controversies regarding nelfinavir mode of action were also addressed.
Conclusions: The two main mechanisms involved in anti-cancer activity are endoplasmic reticulum stress-unfolded protein response pathway and Akt inhibition. However there are many other effects, partially dependent and independent of those mentioned, that may be useful in cancer treatment, including MMP-9 and MMP-2 inhibition, down-regulation of CDK-2, VEGF, bFGF, NF-kB, STAT-3, HIF-1 alfa, IGF, EGFR, survivin, BCRP, androgen receptor, proteasome, fatty acid synthase (FAS), decrease in cellular ATP concentration and upregulation of TRAIL receptor DR5, Bax, increased radiosensitivity, and autophagy. The end result of all these effects is slower growth, decreased angiogenesis, decreased invasion and increased apoptosis, which means reduced proliferation and increased cancer cells death.
PIs may be classified according to their anticancer activity at clinically achievable doses, in AKT inhibitors, ER stressors and Akt inhibitors/ER stressors.
Beyond the phase I trials that have been recently completed, adequately powered and well-designed clinical trials are needed in the various cancer type settings, and specific trials where NFV is tested in association with other known anti-cancer pharmaceuticals should be sought, in order to find an appropriate place for NFV in cancer treatment.
The analysis of controversies on the molecular mechanisms of NFV hints to the possibility that NFV works in a different way in tumor cells and in hepatocytes and adipocytes.
Keywords: Nelfinavir, protease inhibitor, cancer, endoplasmic reticulum stress, unfolded protein response, Akt
Abbreviations
NFV: Nelfinavir
PI: HIV Protease Inhibitors
ERS: Endoplasmic reticulum stress
UPR: Unfolded protein response
BCRP: Breast cancer resistance protein
FAS: Fatty Acid Synthase
Introduction
In March 1997, the United States Food and Drug Administration (FDA) approved Nelvinavir (NFV, brand name Viracept) for HIV treatment in humans 1. NFV is a safe, orally available, and potent drug against HIV-1 and HIV-2 2. This protease inhibitor (PI) was developed by the private pharmaceutical sector and was a big success in the treatment of AIDS in association with other anti-retroviral drugs 3. The introduction of PIs combined with HIV reverse transcriptase inhibitors started the era of HAART (highly active anti-retroviral treatment) and is nowadays the standard of care in HIV-AIDS 4.
PIs inhibit HIV-1 and HIV-2 proteases (which are aspartate proteases), impeding virus replication and release of infecting viral particles from diseased cells. The mechanism of action of protease inhibitors involves competitive binding to the enzyme 5.
NFV is being progressively displaced from HIV therapeutics by second generation HIV PIs, but has shown interesting off target actions in cancer.
The possible use of anti-HIV drugs against cancer is not new: in the 1990s AZT (zidovudine or azidothymidine) was proposed as anti-neoplastic drug, but clinical trials did not confirm the preliminary good results obtained in vitro 6.
That HIV PIs target other molecules besides the HIV protease is quite evident if we examine adverse effects like insulin resistance and lipodystrophy. These and other evidences such as inhibition of tumor cell production of cytokines, anti-angiogenesis, induction of apoptosis and others, suggest off targets effects for PIs, and hints to the concept of a new class of drugs against cancer with multiple anti-cancer effects 6.
NFV, the most important anti-cancer drug of the PI family, if repurposed for cancer treatment, would have an important advantage: it has been used for more than 15 years in HIV treatment and its safety, pharmacokinetics, and adverse events are well known. Serious adverse events are not common with the exception of diarrhea when used at high doses.
Research on NFV as a potentially useful drug for cancer treatment 6 started in 2009.
In this article, we thoroughly review the literature published in this matter and analyze mainly the anti-cancer mechanisms of action of NFV.
Certain controversies regarding NFV activity in lipid metabolism will be considered in depth.
Evidences of nelfinavir anti-cancer activity
A partial response of Kaposi’s sarcoma patients to PIs was published in 1998 7 and good results with regression (six complete responses out of 10 patients) 8. In 1999 Niehues et al. 9 published complete regression of Kaposi’s sarcoma in a child treated with highly active anti-retroviral therapy (HAART). Sgadari et al. (2003) described also the inhibition of Kaposi’s sarcoma with protease inhibitors and they also mention that these drugs can antagonize vital properties of tumor cells like growth, invasion, tissue remodelling, angiogenesis and survival. They consider these effects to be a consequence of inhibition of invasion, matrix metalloprotease, proteasome and NF-κB signaling 10. The possible mechanisms of PIs off target activity on tumor cells were described by pioneering work of Schmidtke et al. in 1999 11: they observed that ritonavir was a modulator of proteasomal activity, allowed normal proliferation when used at low concentrations, but affected protein degradation when present at higher concentrations, and cell cycle was arrested.
Ikezoe et al. 12 described that protease inhibitors increased cellular growth inhibition of all transretinoic acid (ATRA) on cell cultures of myelocitytic leukaemia lines. Protease inhibitors also increased differentiation of acute myeloid leukemia cell lines.
In 2004, Ikezoe 13 described the mechanisms involved in anti-cancer activity of protease inhibitors in myeloma cells.
The mechanisms involved in PIs anti-cancer activity are summarized in chronological order on Table 1.
Table 1. Mechanisms of action of Nelvinavir and other PIs in cancer.
| Author | Study performed in | Results |
|---|---|---|
| Andre, 1998 14 | Mice infected with lymphocytic
choriomeningitis virus receiving ritonavir. |
Ritonavir inhibits chymotrypsin-like activity of the 20S proteasome. Nelfinavir
does not inhibit chymotrypsin like activity. |
| Gaedicke, 2002 15 | Thymoma cells growing in
syngeneic mouse |
Ritonavir produces growth inhibition of tumors, apoptosis and affects
proteosomal proteolysis. Non-transformed cell lines were relatively resistant to this activity. Accumulation of p21 (due to inhibition of proteolytic degradation). |
| Ikezoe, 2004 13 | Human Multiple Myeloma cells | Growth arrest, apoptosis, blocked IL6 stimulated phosphorylation of STAT 3
and ERK ½. Decreased VEGF production. |
| Sgadari, 2002 16 | Kaposi sarcoma cell lesions in
nude mice |
Anti-angiogenesis. Decrease of VEGF, bFGF. Decrease MMP2 activation. |
| Pajonk, 2002 17 | PC-3 and DU-145 prostate
cancer, U373 glioblastoma, and K562 and Jurkat leukemia cells |
Saquinavir inhibited activation of NF-κB. Inhibited 20s and 26s proteasome
activity. Sensitized the surviving cells to ionizing radiation. In a previous paper 18, this same group showed that proteasome is a direct target of radiation. This explains the synergism between proteasome inhibitors and ionizing radiation. |
| Olson, 2002 19 | Cell culture of UMCC-1/VP cells
which over-express MRP-1 |
Ritonavir inhibits the functional activity of the multidrug resistance related-
protein 1 (MRP-1). This characteristic was not shared by other PIs. |
| Zhou, 2004 20 | Insulinoma cells | Nelfinavir decreased insulin stimulated phosphorylation of IRS-2 and Akt-
Thr(308) in a dose-dependent manner. For 10 micromol/L of nelfinavir, the decrease in Akt phosphorylation was 55%. |
| Gupta, 2004 21 | Human embryonic kidney cells | HIV PIs are breast cancer resistance protein inhibitors. This applies to
ritonavir, saquinavir, and nelfinavir. Indinavir and amprenavir showed no inhibition on BCRP. |
| Piccinini, 2005 22 | HL60 cells incubated with and
without drug. |
Saquinavir and nelfinavir inhibited proteasome activity at therapeutic
dosages. Retroviral medication had no effect on proteasome. |
| Gupta, 2005 23 | Tumor cell culture and
xenografts. |
Amprenavir, nelfinavir, and saquinavir inhibited Akt phosphorylation and
exerted synergistic effects with radiotherapy. |
| Yang, 2005 24 | Prostate Cancer Cells: LNCaP,
DU145, PC3 and LNCaP xenografts in nude mice |
NFV induces growth arrest and apoptosis of prostate cancer cells and
blockade of androgen receptor, STAT3 and AKT. It also inhibits proliferation of LNCaP xenografts. |
| Yang, 2006 25 | NSCLC cell culture and
xenografts in nude mice. |
NFV induces growth arrest, reduces Akt signalling, apoptosis and docetaxel
sensitisation. It is responsible for up-regulation of p21, p27 and p53, down- regulation of Bcl-2 and MMP-2. NFV slowed proliferation and induced apoptosis in tumour xenografts mice without adverse systemic effects. Of the 3 PIs tested (saquinavir, ritonavir and NFV) NFV exerted the strongest inhibition on proliferation. |
| Chow, 2006 26 | Liposarcoma and non
liposarcoma cell lines |
NFV induces apoptosis of liposarcoma cell through up-regulation of SREBP-1.
Authors consider that NFV is a new class of anti-liposarcoma agent. |
| Pore, 2006 27 | Glioblastoma cells | NFV decreased VEGF expression and secretion under normoxia. NFV
decreases VEGF through the PI3K/Akt pathway. NFV also decreased the hypoxic induction of VEGF and the hypoxic induction of HIF-1alpha. NFV’s effect was a decreased angiogenesis |
| Pore, 2006 28 | In vivo Matrigel plug assay | NFV decreases VEGF expression through the transcription factor Sp1,
which regulates VEGF promoter. It down-regulates HIF-1 alfa by decreasing translation. |
| Hampson, 2006 29 | HPV transformed cervix
carcinoma cells |
Protease inhibitors inhibit S 26 proteasome blocking p53 degradation. |
| Ben-Romano,
2006 30 |
Cell culture of 3T3-L1 adipocytes | NFV induces oxidative stress that may lead to apoptosis (in adipocytes). |
| Gupta, 2007 31 | Meningioma cells | Combination therapy with imatinib and NFV potentiated anti-proliferative
activity of imatinib due to decrease in survivin and increase of Bax. |
| Jiang, 2007 32 | Melanoma cells | NFV produces cell cycle arrest and apoptosis through inhibition of CDK2. |
| Jiang and Pore,
2007 33 |
Glioblastoma cells | NFV decreased Akt expression and enhanced radiosensitization in PTEN
deficient glioblastoma cells. |
| Gills, 2007 34 | NSCLC xenografts and breast
cancer resistant cell lines |
NFV induced caspase dependent apoptosis and also caspase independent
apoptosis via ER stress and autophagy. |
| Cuneo, 2007 35 | HUVEC and tumor vascular
endothelium |
NFV enhance the effects of irradiation on endothelial cells. |
| Pyrko, 2007 36 | Glioblastoma cell lines | Endoplasmic reticulum stress (ERS) response, as shown by increased
expression of ERS markers, GRP78 and CHOP, and activation of ERS- associated caspase-4. Proteasome inhibition. |
| De Barros, 2007 37 | Human subcutaneous abdominal
white adipose tissue |
PIs inhibited proteasome and differentiation of human preadipocytes in
culture, reducing expression and production of matrix metalloproteinase 9 (MMP-9). |
| Gills, 2008 38 | 60 different cancer lines | NFV causes apoptosis and non-apoptotic death, ERS and autophagy. Blocks
growth factor receptor activation and decreases growth factor-induced and endogenous Akt signaling. In vivo, NFV inhibits tumor growth. |
| Plastaras, 2008 39 | Leucocytes of HIV patients
receiving PI (peripheral blood biomarker assay) |
Decreased Akt activation at clinically achievable doses. Increases sensibility
of cancer cells to radiotherapy. PIs do not increase toxicity in patients receiving radiotherapy. |
| Brüning, 2008 40 | Ovarian cancer cells | NFV upregulates TRAIL receptor DR5 which is an apoptosis inducing
receptor. |
| Giri, 2009 41 | MDCKII wild-type and Bcrp1-
transfected cell lines |
NFV may act as a breast cancer resistance protein (BCRP) inhibitor with
certain substrates. |
| Brüning, 2009 42 | Ovarian cancer cell lines. Ascites
samples of cancer patients. |
NFV induced cell death in carboplatin-sensitive resistant ovarian cancer
cell lines. NFV induced formation of ER-derived vacuoles and induced up- regulation of the hsp70 heat shock family member GRP78. It induced the unfolded protein response, which causes cell cycle arrest and apoptosis. Down-regulation of cell cycle regulatory proteins, especially cyclin D3. |
| Dewan, 2009 43 | Lymphoblastoid B cells
in vitro
and in mice model |
Ritonavir induced cell cycle arrest and apoptosis by down-regulation of cyclin
D2 and surviving and suppressed transcriptional activation of NF-κB. |
| Wang, 2010 44 | Tumor vascular network | NFV improved vascular network. |
| Xie, 2011 45 | Chemical systems biology | Weak inhibition of multiple kinases is one of the causes of NFV anti-cancer
activity, without severe side effects, but still having an impact on the system. Off targets of NFV are possibly: EGFR, IGF-1R, Akt2, Abl, FGFR, CDK2, ARK2, Fak1, PDK1, Ephrin receptors. The concept behind this research is that the whole is greater than the sum of the parts. |
| Tian, 2011 46 | Glioblastoma cells | Authors describe a pathway NFV/ERS/CHOP/up regulation of trail receptor
DR5. This pathway clearly interconnects NFV with apoptosis. |
| Brüning, 2011 47 | Cervical cancer line | NFV and Bortezomib (a proteosomal inhibitor) show synergy as apoptosis
inducers. |
| Zeng, 2011 48 | Pituitary adenoma cells and
xenografted tumors |
Growth retardation
in vivo. Inhibition of PI3K-Akt-mTOR axis. Increased
sensitivity to radiation. |
| Guan, 2012 49 | Cell culture | NFV inhibits proteolysis of SREBP-1 by inhibiting site-2 protease (S2P).
Inhibition of autophagy with hydroxychloroquine enhanced apoptotic effect of nelfinavir. Accumulation of SREBP-1 produced ER stress. Decreases FAS. |
| Bono, 2012 50 | Human myeloma plasma cells
and xenografted SCID mice |
Inhibition of S26 proteasome, impaired proliferation and increased apoptosis.
Decreased phosphorylation of AKT, STAT3 and ERK ½. ER stress corroborated by PERK and CHOP. |
| Brüning, 2012 51 | HeLa cells and other cancer cells | NFV and other ER stressor upregulate inhibin Beta E which shows anti-
proliferative effects. |
| Barillari, 2012 52 | Cervical intraepithelial neoplasia
cells |
Saquinavir and ritonavir reduce MMP2 and 9, inhibiting cell invasion and
growth. |
| Timeus, 2012 53 | Neuroblastoma cells | Saquinavir showed anti-proliferative and anti-invasive activity which
was increased by the association with imatinib. Activation of NF-κB was decreased. Saquinavir also exerted a pro-apoptotic activity on NB lines, which was significantly increased by the association with imatinib. |
| Ismail, 2013 54 | Hamster ovary cells | Inhibits proximal insulin receptor signalling which may explain insulin
resistance. |
| Escalante, 2013 55 | Myeloma cells in culture and
mice xenografts. |
NFV is a calpain blocker. This activity enhances bortezomib (a proteosomal
inhibitor) citotoxicity in vivo and in vitro. Drugs that block the HIV1-associated aspartyl protease also show cross reactivity with the cysteine protease calpain. |
| Bociaga-Jasik,
2013 56 |
Pre-adipocytes and adipocytes
in culture |
Saquinavir decreased mitochondrial membrane potencial and intracellular
ATP in adipocytes. |
Nelfinavir and the ERS-UPR pathway
NFV inhibits the proteases S1P and S2P that are involved in SREBP-1 maturation and other proteases necessary for protein maturation and folding (yet not fully identified) in the endothelial reticulum 49.
Activation of the unfolded protein response (UPR) starts in the ER when abnormal accumulation of protein is detected 57. This was investigated thoroughly in yeasts where detection of abnormal protein occurs through Ire1p/Ern1p-mediated signaling from the ER (in mammals there are three sensor proteins IRE1α, PERK and ATF6 58). UPR activation leads to the specific removal of 252 nucleotides intron from a precursor mRNA of the transcription factor HAC-1p, and the resulting mature mRNA HAC-1p is translated to produce active HAC-1p. This transcription factor translocates to the nucleus and promotes the transcription of chaperones like GRP78 that facilitates removal of abnormal proteins from the ER through retrotranslocation and final disposal by the ubiquitin-proteasome pathway 59.
HAC1 precursor mRNA is constitutively expressed but not translated until Ire1p/Ern1p sensor removes the necessary nucleotides.
Thus the UPR is an intracellular signaling pathway where the ER “informs” the nucleus on the need to increase the levels of molecular chaperones and folding enzymes in order to maintain the ER homeostasis. Therefore UPR keeps unfolded proteins in the ER until they are correctly folded before they can go to their final destination. NFV seems to produce cellular stress by accumulation of misfolded or abnormal proteins in the ER, overwhelming the normal ER protein folding machinery 60. Chaperones bound to unfolded proteins in the ER initiate protein kinase cascades that inhibit translation, reverse translocation, activate ubiquitination enzymes, induce autophagia, and when stress is extreme, induce apoptosis.
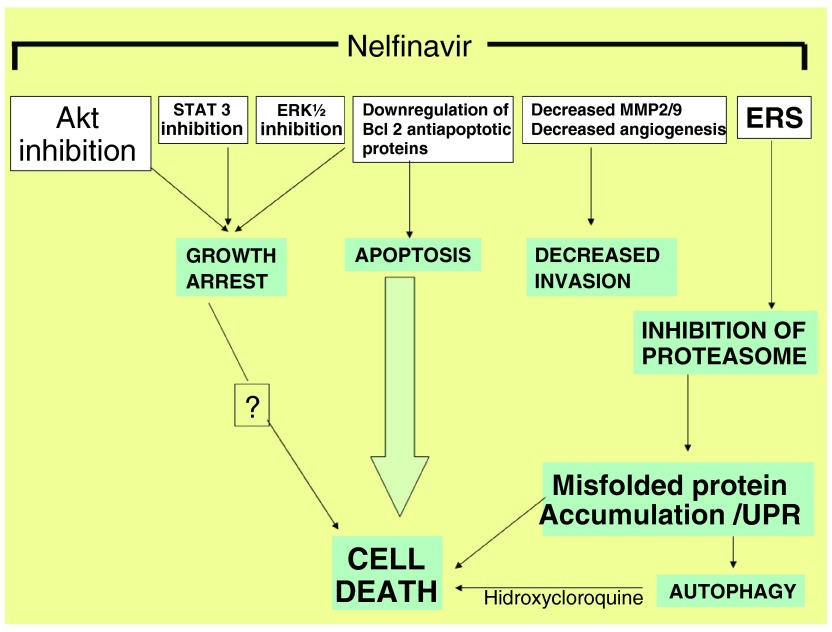
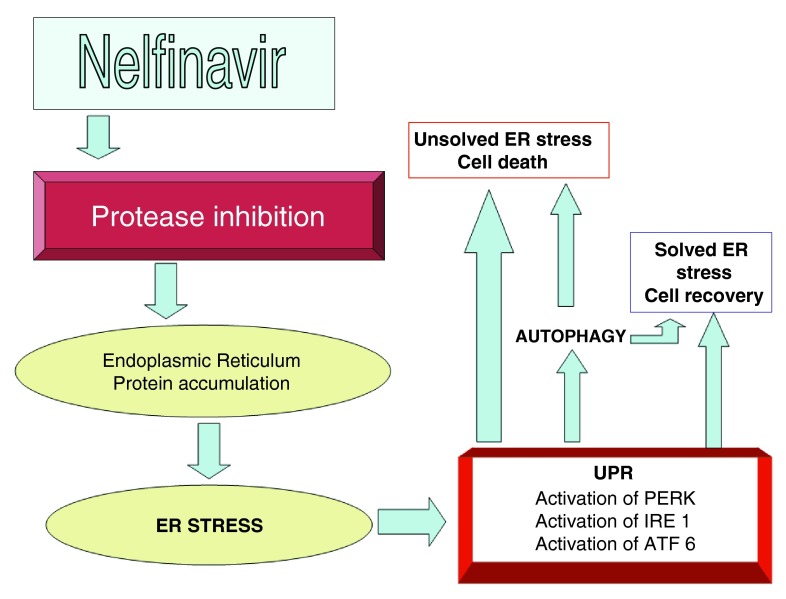
Mechanism of action of Nelfinavir in cancer
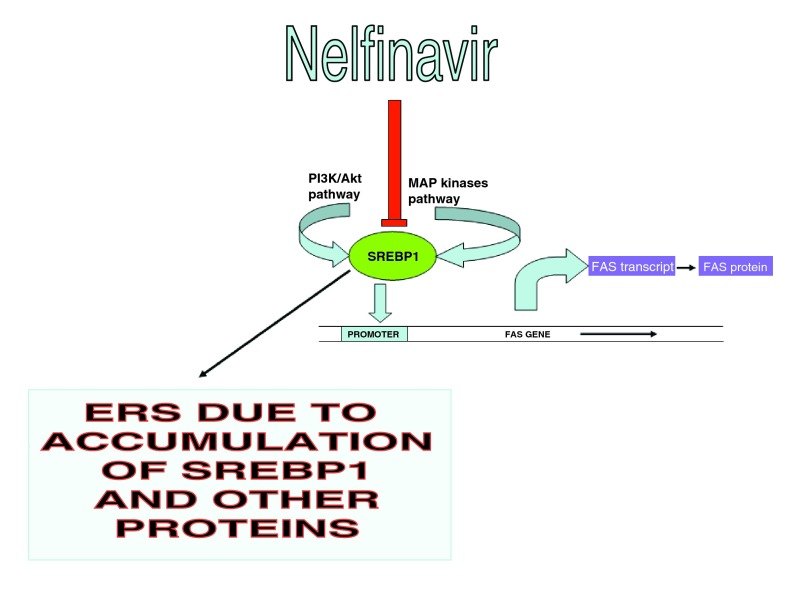
Figure 1. Simplified mechanisms of action of nelfinavir in cancer.
ERS: Endoplasmic reticulum stress.
Figure 2. A more detailed view of nelfinavir’s action on ER stress.
Table 2. Nelfinavir anti-cancer activity in different tumor tissues*.
| Tumor | Reference | Level |
|---|---|---|
| Hepatocarcinoma | Sun, 2014 62 | Cell culture |
| Diffuse B cell lymphoma | Petrich, 2012 63 | Cell culture |
| Glioblastoma | Kast, 2012 64 | Cell culture |
| Liposarcoma | Pan, 2012 65 | Clinical Trial (Phase I) |
| HER 2 positive, breast cancer cells | Shim, 2012 66 | Cell culture |
| Acute myeloid leukemia | Kraus, 2014 67 | Cell culture |
| Acute myeloid leukemia | Kraus, 2013 68 | Cell culture |
| Cancer stem cells expressing
embryonic genes** |
Darini, 2013 69 | Cell culture |
| Glioblastoma | Kas, 2013 70 | Clinical |
| Castration resistant prostate cancer | Mathur, 2014 71 | Cell culture |
| Medullary thyroid cancer | Kushchayeva, Jensen,
Recupero 2014 72 |
Cell culture |
| Medullary thyroid cancer | Kushchayeva, Jensen,
Burman, 2014 73 |
Cell culture |
| Glioblastoma | Alonso-Basanta,
2014 74 |
Clinical Trial, Phase I |
| Rectal cancer | Buijsen J, 2013 75 | Clinical Trial, Phase I |
| Refractary adenoid cystic carcinoma | Hoover, 2014 76 | Clinical Trial, Phase II |
*Saquinavir-NO has been tested in human melanoma cells with good results 77.
**It is necessary to underscore the finding that cancer stem cells expressing embryonic genes like Oct4, Sox2 and others, are particularly prone to apoptosis when PIs are used, particularly iopinavir (nelfinavir and saquinavir are also effective in this matter).
Table 3. Summary of clinical trials performed with NFV as an anti-cancer drug.
| PROTOCOL | CHARACTERISTICS |
|---|---|
| NCT 0145106 | SOLID TUMORS: A phase I trial of nelfinavir (Viracept ) in adults with solid tumors |
| NCT 00436735 |
SOLID TUMORS: Nelfinavir in treating patients with metastatic, refractory, or recurrent solid
tumors |
| NCT 02080416 | Non-Hodgkin Lymphoma, Hodgkin Lymphoma, Kaposi Sarcoma, Gastric Cancer,
Nasopharyngeal Cancer, EBV, Castleman Disease: nelfinavir for the treatment of gammaherpesvirus-related tumors |
| NCT 00589056 |
NSCLC: NFV, radiation therapy, cisplatin, and etoposide in treating patients with stage III non-
small cell lung cancer that cannot be removed by surgery |
| NCT 01068327 |
Pancreatic cancer: stereotactic radiation therapy, nelfinavir mesylate, gemcitabine
hydrochloride, leucovorin calcium, and fluorouracil in treating patients with locally advanced pancreatic cancer |
| NCT 01925378 |
Cervical intraepithelial neoplasia: a phase II single-arm intervention trial of nelfinavir in
patients with grade 2/3 or 3 cervical intraepithelial neoplasia |
| NCT 01959672 |
Pancreatic cancer: nelfinavir mesylate in treating patients with locally advanced pancreatic
cancer |
| NCT 01079286 | Renal cell cancer: study of nelfinavir and temsirolimus in patients with advanced cancers |
| NCT 00704600 | Rectal cancer: nelfinavir, a phase I/phase II rectal cancer study |
| NCT 01065844 | Adenoid cystic cancer : nelfinavir in recurrent adenoid cystic cancer of the head and neck |
| NCT 01164709 |
Hematologic cancer: nelfinavir mesylate and bortezomib in treating patients with relapsed or
progressive advanced hematologic cancer |
| NCT 01086332 | Pancreatic cancer: evaluation of nelfinavir and chemoradiation for pancreatic cancer |
| NCT 01485731 |
Cervical cancer: safety study of nelfinavir + cisplatin + pelvic radiation therapy to treat cervical
cancer |
| NCT 02024009 |
Pancreatic cancer: systemic therapy and chemoradiation in advanced localised pancreatic
cancer - 2 |
| NCT 00915694 |
Glioblastoma: nelfinavir, radiation therapy, and temozolomide in treating patients with
glioblastoma multiforme |
| NCT 01108666 |
NSCLC: proton beam radiation with concurrent chemotherapy and nelfinavir for inoperable stage
III non small cell lung cancer (NSCLC) |
| NCT 00791336 | Study to evaluate using nelfinavir with chemoradiation for NSCLC |
| NCT 02207439 |
Larynx carcinoma: A phase II of nelfinavir, given with definitive, concurrent chemoradiotherapy
(CTRT) in patients with locally-advanced, human papilloma virus (HPV) negative, squamous cell carcinoma larynx |
| NCT 01020292 |
Glioma: nelfinavir and concurrent radiation and temozolomide in patients with WHO grade IV
glioma |
| NCT 01555281 |
Myeloma: nelfinavir and lenalidomide/dexamethasone in patients with progressive multiple
myeloma that have failed lenalidomide-containing therapy |
| NCT 01728779 |
Metastatic lesions of the lung, liver or bone: stereotactic body radiation with nelfinavir for
oligometastases. |
| NCT 00233948 |
Liposarcoma: nelfinavir mesylate in treating patients with recurrent, metastatic, or unresectable
liposarcoma |
| NCT 02188537 |
Myeloma: nelfinavir as bortezomib-sensitizing drug in patients with proteasome inhibitor-
nonresponsive myeloma |
| EudraCT Number:
2008-006302-42 University of Oxford |
Pancreatic cancer: a phase II study in patients with locally advanced pancreatic carcinoma:
ARC-II – Akt-inhibition by nelfinavir plus chemoradiation with gemcitabine and cisplatin |
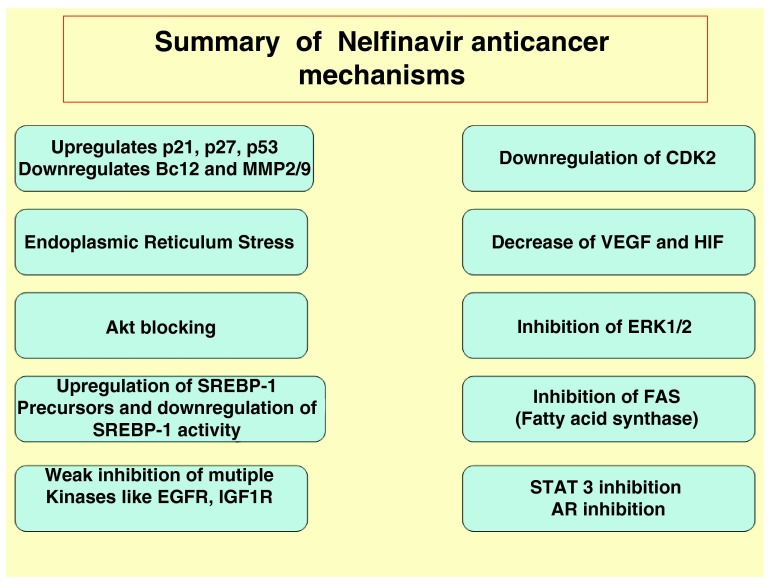
Figure 5. Summary of NFV anti-cancer mechanisms.
Interactions of NFV and PIs with other drugs
Indinavir and NFV increase anti-malarial action of artemisinin in vitro on Plasmodium falciparum 78, but artemisinin has also an off target anti-cancer activity. Thus it is reasonable to raise the question: may the association of artesiminin with NFV increase anti-cancer activity?
Another research team 70 has included both, NFV and artesiminin, in a multidrug repurposed protocol (CUSP 9) for the treatment of relapsed glioblastoma.
Celecoxib is an ER stressor that may enhance NFV anti-tumor activity 79.
Chloroquine and hydroxicloquine are autophagy inhibitors and may work synergistically with NFV, down-regulating autophagy and increasing apoptosis 70, 80.
Nelfinavir may produce overproduction of mcl1 through upregulation of Erk ½, which would reduce apoptosis. The problem can be solved adding sorafenib 81, 82.
In breast cancer cells, tamoxifen enhances anti-cancer activity of NFV 83. This synergism was independent of the estrogen receptor status so that the authors consider that the association of NFV and tamoxifen may be advantageous even in patients with no hormone responsive tumors.
Saquinavir has an interesting off target effect: it decreases intracellular ATP in adipocytes 56. If this effect is similar in tumor cells, an association with metformin and 2-deoxyglucose may produce anti-cancer activity 84– 86. There is growing interest on metabolic perturbators in cancer therapy and saquinavir may play a role in this field.
Clinical trials
A phase I dose escalation trial performed in 2014 87 established a MTD (maximum tolerated dose) of 3125 mg twice daily and described that 45% of patients with solid tumors treated with this dose decreased AKT activity and increased ERS indicators. This indicated a possible benefit in neuroendocrine tumor patients and also established that dose limiting toxicity consists in neutropenia.
The dose (3125 mg bid) is more than twice the dose used in HIV treatment. But lower doses, in the range of those used in HIV treatment have been tested, combining nelfinavir with chemoradiotherapy in pancreatic cancer with evidence of efficacy 88. No control group was used in this research, so comparison was established with known data from previous publications, mainly the favourable possibility of tumor resection after treatment.
Even lower doses (625 mg and 1250 mg bid) were tested in a phase I trial of NSCLC in stages IIIA/IIIB combined with chemoradiotherapy 89. In nine out of 12 patients a PET scan was available post-treatment with 100% overall response (56% complete response and 44% partial response). Unfortunately, in this trial there was no control group; 50% of the patients (six out of 12) lived for more than 22 months after treatment; 25% (three out of 12) lived without disease for more than 32 months. The results may be considered favourable, even without a control group.
Buijsen et al. 75 recommend a dose of 750 mg NFV bid for a phase II trial of this drug in combination with chemoradiotherapy in locally advanced rectal cancer.
Ongoing phase II trials are mainly in myeloma (associated with bortezomib or lenalomide), glioblastoma patients (associated with chemoradiation), pancreas (associated with gemcitabine and radiation) and lung. (See http://www.clinicaltrials.gov).
The first results of clinical trials did not show a meaningful improvement in the outcome of patients with refractory adenoid cystic carcinoma which is a malignant salivary gland tumor which usually has a poor prognosis. In this case NFV was used as monotherapy 76.
Why nelfinavir has a role to play in cancer therapy?
Akt activation is an important step in cancer phenotype and is a key player in acquisition and maintenance of cancer hallmarks. Akt is a nodal regulator of cellular survival pathways 90. There are no drugs at the present time that can inhibit this protein with a good safety profile. Wortmannin, perifosine and other chemicals designed for PI3K/Akt inhibition were too toxic for clinical use or have shown disappointing results, so they did not enter the medical practice. Insulin stimulation of Akt phosphorylation was reduced by 55% at achievable doses 20. At the same time there is clear evidence that it favours apoptosis and growth inhibition at clinically tolerable and achievable doses.
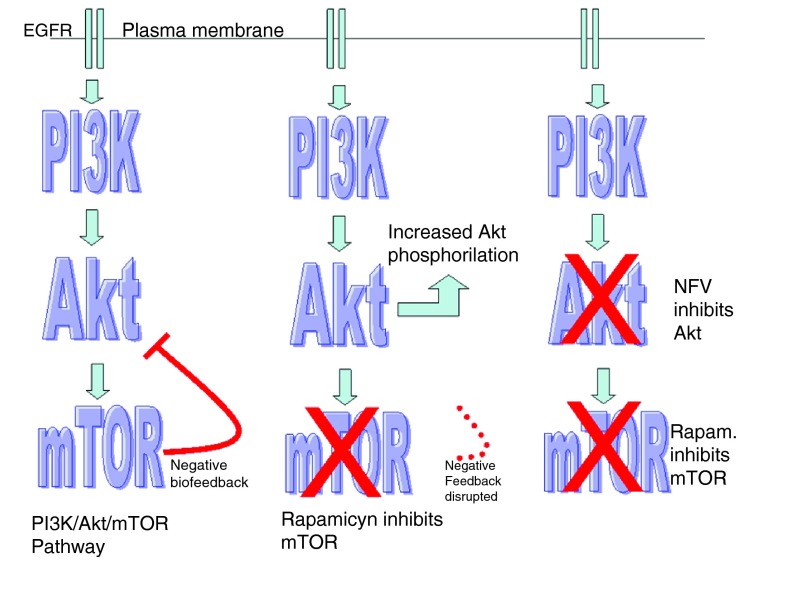
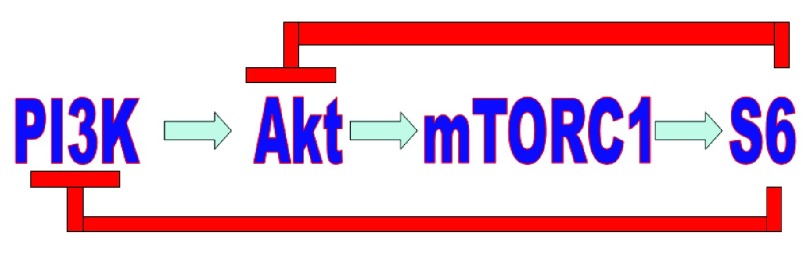
This anti-Akt activity of NFV can be reinforced by concomitant mTOR inhibition which results in synergistic cytotoxicity 63. This may be due to the fact that mTOR inhibition without Akt inhibition eliminates a negative biofeedback loop on Akt, producing increased phoshorilation of Akt 91. According to Sarbassov 92 this negative feedback is born in the mTORC2 complex.
According to Carracedo 93, this negative feedback loop goes as far as PI3K ( Figure 6).
Figure 6. Negative feedback loop.
mTOR inhibitors have become a new and important tool against cancer, for example in renal cell carcinoma. But the negative biofeedback loop on Akt must be solved to achieve really good results. NFV could be the clue.
Figure 7. Mechanism of synergy between NFV and mTOR inhibitors.
At the left is depicted the pathway under normal or pathological circumstances; in the middle drawing mTOR inhibition is counterbalanced by Akt activation due to loss of the negative biofeedback circuit; on the right, inhibition of both, mTOR and Akt may result in increased anti-cancer results.
But the most important anti-tumor activity of NVR is not limited to Akt inhibition but ER stress and UPR which may be one of the pathways leading to apoptosis 94.
Additional features of NFV and other protease inhibitors are
-
1)
the ability to sensitize cancer cells to chemoradiotherapy,
-
2)
anti-angiogenesis by decreasing VEGF/HIF expression,
-
3)
decreased expression of FAS (fatty acid synthase),
-
4)
the combination of radiation and PIs is well tolerated 39, 94,
-
5)
NFV cancer cell killing ability can easily be enhanced with other ER stressors like celecoxib 79. Cho et al. found enhanced killing of chemoresistant breast cancer cells after celecoxib treatment that aggravated ER stress; perillyl alcohol is another stress aggravator that has been used with that purpose 96,
-
6)
In head and neck cancer related to HPV, NFV produced down-regulation of Akt and radiosensitization 97,
-
7)
NFV not only down-regulates Akt but also MAPK (in adenoid cystic cancer) 98, and retards oral cell proliferation including normal keratinocytes and squamous cell cancer 99,
-
8)
There are evidences, at least in pancreatic cancer, that NFV dependent down-regulation of Akt is independent of the mutational status of K-ras 100,
-
9)
There is clear evidence (in glioblastoma) of the relation between NFV and apoptosis through the following pathway 46:
Possible controversies
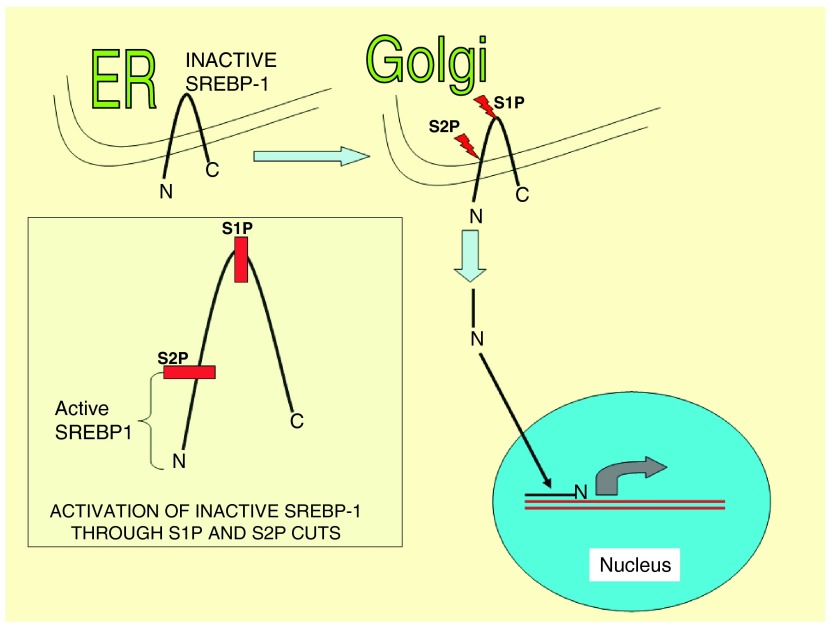
The SREBP pathway for regulation of fat metabolism is initiated through proteolytic cleavage of precursor forms of the SREBPs (125 Kd protein) in ER membranes. When cells are in need of sterol, the precursor SREBPs are hydrolyzed by a 2-step mechanism involving membrane-bound serine protease S1P and a metalloprotease S2P. The N-terminal fragment of SREBP (nSREBP) is a 68 Kd protein that translocates to the nucleus where it works as a promoter-enhancer, binding to sterol regulatory elements located in DNA and activates gene transcription ( Figure 4). The nuclear SREBP can be rapidly degraded by a proteasome-mediated mechanism. This provides regulation of gene transcriptional activities 103.
Figure 4. SREBP is synthesized as an ER transmembrane protein and transported to the Golgi upon appropriate stimulus.
For activation of SREBP it is necessary that luminal S1P (a protease) cleaves first, followed by intramembrane S2P (another protease) to liberate the transcriptionally active amino-terminal segments of nSREBP. NFV inhibits S1P and S2P, so that transcriptionally active SREBP is not produced. Accumulation of inactive SREBP is one of the UPR initiators.
Transgenic mice over-expressing the constitutively active nuclear forms of the SREBPs (nSREBPs) revealed that overexpression of SREBP-1 or SREBP-2 leads to activation of genes involved in the cholesterol and fatty acid biosynthesis cascades. These transgenic mice displayed the classical features of generalized lipodystrophy, similar to those found in patients under PI therapy 104.
Riddle et al. in 2001 105 found that PI therapy (they used ritonavir) induced the accumulation of activated SREBP-1 and SREBP-2 in the nucleus of liver and adipose tissues. As a consequence, fatty acid and cholesterol biosynthesis were increased in these tissues. The authors consider that lipodystrophy, hyperlipidemia, and insulin resistance, are the consequence of activated SREBP-1 and SREBP-2 accumulation in the nucleus of liver and adipose tissues. The possible mechanism for these events, according to their criteria is PI suppression of activated SREBP degradation in the nucleus. In summary, Riddles’s study showed that ritonavir induced lipid metabolism abnormalities through stabilization of activated SREBP-1 and SREBP-2 in the nucleus of liver and adipose tissues.
These findings are in contrast with those of Guan 49, 106 where NFV inhibited the nuclear translocation of the sterol regulatory element binding protein-1 (SREBP-1) in castration resistant prostate cancer and liposarcoma through inhibition of S1P. This led to accumulation of unprocessed SREBP-1.
Riddle et al. described accumulation of processed SREBP-1 in the liver and adipose tissue while Guan found accumulation of unprocessed SREBP1 in ER and Golgi with no translocation to nucleus in liposarcoma and castration resistant prostate cancer tissue.
The controversy may be explained in the following way:
-
1)
There are three different isoforms of SREBP: SREBP-1a, SREBP-1c and SREBP-2.
-
2)
SREBP-1a and -1c have different expression profiles: SREBP-1a is highly expressed in proliferating cells, such as cancer cells, while SREBP-1c is the predominant form in normal cells, particularly hepatocytes 104.
-
3)
The target genes for the three SREBP isoforms are different.
-
4)
Riddle et al. found increased SREBP-1 and two in the nucleus of liver and adipose tissues; these SREBPs are the active form (they make no difference between SREBP-1a and SREBP-1c).
-
5)
Guan et al. found increased SREBP in Golgi in the inactive form (precursor) of tumor tissues treated with NFV.
-
6)
It is possible that tumor tissues that overexpress SREBP-1a behave in a different way than liver and adipose tissue that overexpress SREBP-1c.
-
7)
Riddle et al. tested ritonavir and Guam et al. tested NFV, so the pharmacological effects between these PIs may differ.
A second controversy that stems from the one described above is on the effect of NFV on FAS:
-
1)
According to Guan et al. 106, NFV decreases expression of FAS in liposarcoma cells and castrate resistant prostate cancer as was depicted in Figure 3.
-
2)
According to Lenhard et al. 2000 107, NFV increases expression of FAS in HepG2 cells (which show many of the normal biochemical functions of non tumor liver parenchymal cells).
Figure 3. Nelfinavir inhibition of SREBP1 as a cause of endoplasmic reticulum stress (ERS).
For this figure the model of nelfinavir in liposarcoma was used.
May this difference be due to tissue-specific effects of NFV? Does NFV have different effects in tumor tissues and normal tissues?
To definitely solve these controversies, it is necessary to proceed with further experimental research, but the findings described above necessarily raise the doubt that mechanisms that work in tumor cells might be slightly different from those working in hepatocytes and adipocytes.
Possible negative aspects of PIs in cancer
Despite the anti-cancer activity of NFV and PIs, these drugs do not reduce the risk of developing cancer in HIV population 108 and also exert certain depression of immunological functions, interfering with the differentiation program of monocytes into dendritic cells 109.
PIs increase the expression of P-glycoprotein (ABCB1) in Kaposi’s sarcoma cell lines increasing the multidrug resistance phenotype 110. At the same time ABCB1 expression depends on Akt activation 111 and NFV inhibits partially Akt. The final result of the two antagonistic aspects requires further research.
There are well known undesirable side effects with HIV PIs, like hyperlipidemia, insulin resistance and lypodystrophy (peripheral fat wasting and excessive central fat deposition). One of the main responsible mechanisms of these side effects is the suppression of the breakdown of SREBP in the liver and adipose tissues resulting in increased fatty acid and cholesterol biosynthesis. SREBP accumulation in adipose tissue causes lipodystrophy.
PIs suppress proteasome-mediated breakdown of nascent apolipoprotein (apo) B, resulting in the overproduction of triglyceride. Finally, PIs also suppress the inhibition of the glucose transporter GLUT-4 activity in adipose tissue and muscle. This contributes directly to insulin resistance and diabetes 112.
Hepatomegaly and hepatic steatosis are direct consequences of the metabolic alterations explained above 105.
New PIs with anti-cancer activity
In 2010 You et al. 113 synthesized a new indinavir analogue with remarkable anti-cancer activity, similar to NFV: CH05-10. This drug achieved similar cytotoxity to NFV but at lower concentrations, against leukaemia, melanoma, ovarian and prostate cancer cell lines.
In 2009 Saquinavir-NO was introduced 114; it showed interesting anti-cancer properties in melanoma xenografts with significantly lower toxicity than saquinavir.
Conclusions
The most relevant mechanisms of PIs anti-cancer activity are Akt inhibition and ER stress.
Following our exhaustive analysis of the current medical literature we conclude that NFV anti-cancer activity is mainly dependent on ER stress-UPR.
Akt inhibition plays also a very important role but is not the unique or main source of anti-cancer effects.
The evidences that support these conclusions are:
-
1)
Even at very high doses of NFV (3,125 mg bid), Akt achieved a level of inhibition around 55% in cell culture 20.
-
2)
NFV is at the same time a strong ER stressor and an Akt inhibitor.
-
3)
Anti-cancer activity can be achieved at much lower doses than those necessary for Akt inhibition.
-
4)
Increasing ER stress by adding Celecoxib to NFV enhances cytotoxicity.
-
5)
Autophagy, which is one of the mechanisms cells use to survive increasing ER stress 115, is inhibited by adding chloroquine or hydroxychloroquine to NFV. In this case, apoptosis is significantly enhanced 80, 102.
-
6)
The PIs with anti-cancer activity like NFV, ritonavir 116, saquinavir 117, and the experimental drug CH05-10 113 are strong ER stressors. Amprenavir is a PI that induces no ER stress and its anti-cancer activity is significantly weaker than that of NFV, although it has Akt inhibiting effects.
-
7)
Ritonavir, which is ER stressor, shows anti-cancer activity although it does not down-regulate Akt at concentrarions usually found in HIV patients 23.
-
8)
Inhibition of proteasome with bortezomib has a synergistic effect with NFV apoptotic activity 118
-
9)PIs can be classified regarding anti-cancer activity at clinically achievable concentration in patients in
-
A)Akt inhibitors only: e.g. amprenavir
-
B)ER stressors only: e.g. ritonavir
-
C)Akt inhibitors and ER stressors: examples NFV and experimental PI CH05-10. Of course this is the group that shows stronger anti-cancer activity.
-
A)
There is enough evidence of NFV anti-cancer effects and there is adequate knowledge of how this activity works, so that NFV deserves well designed phase II clinical trials, as adjunct cancer therapy.
Associations with proteasomal inhibitors, celecoxib and other cell stressor should also be investigated in the clinical setting due to possible synergy. Tamoxifen with NFV may show interesting results in breast cancer.
Although a large amount of publications, including reviews, have been written on NFV and other PIs in cancer, none has been dedicated to a thorough examination and analysis of the mode of action of these pharmaceuticals as off target drugs (with the exception of the review by Gantt et al. 2). It is hoped that this review will encourage an increment adequately powered and well-designed clinical trials in the various cancer types, beyond the phase I trials that have been recently performed, and specifically trials where these compounds may be tested in association with other known anti-cancer pharmaceuticals like NFV associated to bortezomib and hydroxychloroquine in myeloma, or mTOR inhibitors with NFV in HNSCC and many other possible combinations where the dual feature of NFV, ER stressor and Akt inhibitor, are required.
In myeloma NFV increases proteasome inhibition by bortezomib and may overcome resistance to proteasomal inhibitors. This is an action exclusive of NFV and not shared with other PIs 67, with the additional advantage that NFV shows the highest cytotoxic activity against primary myeloma cells.
If apoptosis is described as a cascade, then apoptosis stimulator drugs like NFV should be viewed as enhancers of this cascade. An initiator of the cascade is still necessary, for example chemoradiotherapy. After this initial step, apoptosis stimulator drugs increase the amount of cells entering this pathway. This might be one of possible reasons why nelfinavir alone has shown poor results in a clinical trial used as monotherapy.
This does not mean that NFV cannot act as an initiator, but the evidences show that it is prone to be an enhancer of apoptosis rather than an initiator.
Future directions
All the evidences presented in this review reinforce the concept that NFV is a useful drug in cancer treatment. It should be considered in association with chemoradiotherapy in the design of new protocols for diseases like multiple myeloma (in association with bortezomib and hydroxychloroquine) and prostate, pancreas and lung cancer where clinical trials are ongoing. New PIs are being developed with better anti-cancer profile like CH05-10 and saquinavir-NO 77 and further development of new PIs with stronger anti-cancer activity, will probably go on in the future.
Funding Statement
The author(s) declared that no grants were involved in supporting this work.
v2; ref status: indexed
References
- 1. Pai VB, Nahata MC: Nelfinavir mesylate: a protease inhibitor. Ann Pharmacother. 1999;33(3):325–339. 10.1345/aph.18089 [DOI] [PubMed] [Google Scholar]
- 2. Gantt S, Casper C, Ambinder RF: Insights into the broad cellular effects of nelfinavir and the HIV protease inhibitors supporting their role in cancer treatment and prevention. Curr Opin Oncol. 2013;25(5):495–502. 10.1097/CCO.0b013e328363dfee [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Flexner C: HIV-protease inhibitors. N Engl J Med. 1998;338(18):1281–1292. 10.1056/NEJM199804303381808 [DOI] [PubMed] [Google Scholar]
- 4. Volberding PA, Deeks SG: Antiretroviral therapy for HIV infection: promises and problems. JAMA. 1998;279(17):1343–1344. 10.1001/jama.279.17.1343 [DOI] [PubMed] [Google Scholar]
- 5. Zhang KE, Wu E, Patick AK, et al. : Circulating metabolites of the human immunodeficiency virus protease inhibitor nelfinavir in humans: structural identification, levels in plasma, and antiviral activities. Antimicrob Agents Chemother. 2001;45(4):1086–1093. 10.1128/AAC.45.4.1086-1093.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chow WA, Jiang C, Guan M: Anti-HIV drugs for cancer therapeutics: back to the future? Lancet Oncol. 2009;10(1):61–71. 10.1016/S1470-2045(08)70334-6 [DOI] [PubMed] [Google Scholar]
- 7. Krischer J, Rutschmann O, Hirschel B, et al. : Regression of Kaposi’s sarcoma during therapy with HIV-1 protease inhibitors: a prospective pilot study. J Am Acad Dermatol. 1998;38(4):594–598. 10.1016/S0190-9622(98)70124-0 [DOI] [PubMed] [Google Scholar]
- 8. Lebbé C, Blum L, Pellet C, et al. : Clinical and biological impact of antiretroviral therapy with protease inhibitors on HIV-related Kaposi’s sarcoma. AIDS. 1998;12(7):F45–9. 10.1097/00002030-199807000-00002 [DOI] [PubMed] [Google Scholar]
- 9. Niehues T, Horneff G, Megahed M, et al. : Complete regression of AIDS-related Kaposi’s sarcoma in a child treated with highly active antiretroviral therapy. AIDS. 1999;13(9):1148–9. 10.1097/00002030-199906180-00026 [DOI] [PubMed] [Google Scholar]
- 10. Sgadari C, Monini P, Barillari G, et al. : Use of HIV protease inhibitors to block Kaposi's sarcoma and tumour growth. Lancet Oncol. 2003;4(9):537–47. Review. 10.1016/S1470-2045(03)01192-6 [DOI] [PubMed] [Google Scholar]
- 11. Schmidtke G, Holzhutter HG, Bogyo M, et al. : How an inhibitor of the HIV-I protease modulates proteasome activity. J Biol Chem. 1999;274(50):35734–40. 10.1074/jbc.274.50.35734 [DOI] [PubMed] [Google Scholar]
- 12. Ikezoe T, Daar ES, Hisatake J, et al. : HIV-1 protease inhibitors decrease proliferation and induce differentiation of human myelocytic leukemia cells. Blood. 2000;96(10):3553–9. [PubMed] [Google Scholar]
- 13. Ikezoe T, Saito T, Bandobashi K, et al. : HIV-1 protease inhibitor induces growth arrest and apoptosis of human multiple myeloma cells via inactivation of signal transducer and activator of transcription 3 and extracellular signal-regulated kinase 1/2. Mol Cancer Ther. 2004;3(4):473–9. [PubMed] [Google Scholar]
- 14. Andre P, Groettrup M, Klenerman P, et al. : An inhibitor of HIV-1 protease modulates proteasome activity, antigen presentation, and T cell responses. Proc Natl Acad Sci U S A. 1998;95(22):13120–13124. 10.1073/pnas.95.22.13120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gaedicke S, Firat-Geier E, Constantiniu O, et al. : Antitumor effect of the human immunodeficiency virus protease inhibitor ritonavir: induction of tumor-cell apoptosis associated with perturbation of proteasomal proteolysis. Cancer Res. 2002;62(23):6901–8. [PubMed] [Google Scholar]
- 16. Sgadari C, Barillari G, Toschi E, et al. : HIV protease inhibitors are potent anti-angiogenic molecules and promote regression of Kaposi sarcoma. Nat Med. 2002;8(3):225–32. 10.1038/nm0302-225 [DOI] [PubMed] [Google Scholar]
- 17. Pajonk F, Himmelsbach J, Riess K, et al. : The human immunodeficiency virus (HIV)-1 protease inhibitor saquinavir inhibits proteasome function and causes apoptosis and radiosensitization in non-HIV-associated human cancer cells. Cancer Res. 2002;62(18):5230–5. [PubMed] [Google Scholar]
- 18. Pajonk F, McBride WH: Ionizing radiation affects 26s proteasome function and associated molecular responses, even at low doses. Radiother Oncol. 2001;59(2):203–212. 10.1016/S0167-8140(01)00311-5 [DOI] [PubMed] [Google Scholar]
- 19. Olson DP, Scadden DT, D’Aquila RT, et al. : The protease inhibitor ritonavir inhibits the functional activity of the multidrug resistance related-protein 1 (MRP-1). AIDS. 2002;16(13):1743–1747. 10.1097/00002030-200209060-00005 [DOI] [PubMed] [Google Scholar]
- 20. Zhou JQ, Xiang Z, Schutt M: [Impairment of IRS-2 signaling in rat insulinoma INS-1 cells by nelfinavir]. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2004;33(4):311–4. Chinese. [DOI] [PubMed] [Google Scholar]
- 21. Gupta A, Zhang Y, Unadkat JD, et al. : HIV protease inhibitors are inhibitors but not substrates of the human breast cancer resistance protein (BCRP/ABCG2). J Pharmacol Exp Ther. 2004;310(1):334–341. 10.1124/jpet.104.065342 [DOI] [PubMed] [Google Scholar]
- 22. Piccinini M, Rinaudo MT, Anselmino A, et al. : The HIV protease inhibitors nelfinavir and saquinavir, but not a variety of HIV reverse transcriptase inhibitors, adversely affect human proteasome function. Antivir Ther. 2005;10(2):215–23. [PubMed] [Google Scholar]
- 23. Gupta AK, Cerniglia GJ, Mick R, et al. : HIV protease inhibitors block Akt signaling and radiosensitize tumor cells both in vitro and in vivo. Cancer Res. 2005;65(18):8256–65. 10.1158/0008-5472.CAN-05-1220 [DOI] [PubMed] [Google Scholar]
- 24. Yang Y, Ikezoe T, Takeuchi T, et al. : HIV-1 protease inhibitor induces growth arrest and apoptosis of human prostate cancer LNCaP cells in vitro and in vivo in conjunction with blockade of androgen receptor STAT3 and AKT signaling. Cancer Sci. 2005;96(7):425–33. 10.1111/j.1349-7006.2005.00063.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yang Y, Ikezoe T, Nishioka C, et al. : NFV, an HIV-1 protease inhibitor, induces growth arrest, reduced Akt signalling, apoptosis and docetaxel sensitisation in NSCLC cell lines. Br J Cancer. 2006;95(12):1653–1662. 10.1038/sj.bjc.6603435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chow WA, Guo S, Valdes-Albini F: Nelfinavir induces liposarcoma apoptosis and cell cycle arrest by upregulating sterol regulatory element binding protein-1. Anticancer Drugs. 2006;17(8):891–903. 10.1097/01.cad.0000224448.08706.76 [DOI] [PubMed] [Google Scholar]
- 27. Pore N, Gupta AK, Cerniglia GJ, et al. : HIV protease inhibitors decrease VEGF/HIF-1alpha expression and angiogenesis in glioblastoma cells. Neoplasia. 2006;8(11):889–95. 10.1593/neo.06535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pore N, Gupta AK, Cerniglia GJ, et al. : Nelfinavir down-regulates hypoxia-inducible factor 1alpha and VEGF expression and increases tumor oxygenation: implications for radiotherapy. Cancer Res. 2006;66(18):9252–9. 10.1158/0008-5472.CAN-06-1239 [DOI] [PubMed] [Google Scholar]
- 29. Hampson L, Kitchener HC, Hampson IN: Specific HIV protease inhibitors inhibit the ability of HPV16 E6 to degrade p53 and selectively kill E6-dependent cervical carcinoma cells in vitro. Antivir Ther. 2006;11(6):813–25. [PubMed] [Google Scholar]
- 30. Ben-Romano R, Rudich A, Etzion S, et al. : Nelfinavir induces adipocyte insulin resistance through the induction of oxidative stress: differential protective effect of antioxidant agents. Antivir Ther. 2006;11(8):1051–1060. [PubMed] [Google Scholar]
- 31. Gupta V, Samuleson CG, Su S, et al. : Nelfinavir potentiation of imatinib cytotoxicity in meningioma cells via survivin inhibition. Neurosurg Focus. 2007;23(4):E9. 10.3171/FOC-07/10/E9 [DOI] [PubMed] [Google Scholar]
- 32. Jiang W, Mikochik PJ, Ra JH, et al. : HIV protease inhibitor nelfinavir inhibits growth of human melanoma cells by induction of cell cycle arrest. Cancer Res. 2007;67(3):1221–7. 10.1158/0008-5472.CAN-06-3377 [DOI] [PubMed] [Google Scholar]
- 33. Jiang Z, Pore N, Cerniglia GJ, et al. : Phosphatase and tensin homologue deficiency in glioblastoma confers resistance to radiation and temozolomide that is reversed by the protease inhibitor nelfinavir. Cancer Res. 2007;67(9):4467–73. 10.1158/0008-5472.CAN-06-3398 [DOI] [PubMed] [Google Scholar]
- 34. Gills JJ, Lopiccolo J, Tsurutani J: Nelfinavir, A lead HIV protease inhibitor, is a broad-spectrum, anticancer agent that induces endoplasmic reticulum stress, autophagy, and apoptosis in vitro and in vivo. Clin Cancer Res. 2007;13(17):5183–94. 10.1158/1078-0432.CCR-07-0161 [DOI] [PubMed] [Google Scholar]
- 35. Cuneo KC, Tu T, Geng L, et al. : HIV protease inhibitors enhance the efficacy of irradiation. Cancer Res. 2007;67(10):4886–93. 10.1158/0008-5472.CAN-06-3684 [DOI] [PubMed] [Google Scholar]
- 36. Pyrko P, Kardosh A, Wang W, et al. : HIV-1 protease inhibitors nelfinavir and atazanavir induce malignant glioma death by triggering endoplasmic reticulum stress. Cancer Res. 2007;67(22):10920–8. 10.1158/0008-5472.CAN-07-0796 [DOI] [PubMed] [Google Scholar]
- 37. De Barros S, Zakaroff-Girard A, Lafontan M, et al. : Inhibition of human preadipocyte proteasomal activity by HIV protease inhibitors or specific inhibitor lactacystin leads to a defect in adipogenesis, which involves matrix metalloproteinase-9. J Pharmacol Exp Ther. 2007;320(1):291–299. 10.1124/jpet.106.111849 [DOI] [PubMed] [Google Scholar]
- 38. Gills JJ, Lopiccolo J, Dennis PA: Nelfinavir, a new anti-cancer drug with pleiotropic effects and many paths to autophagy. Autophagy. 2008;4(1):107–109. 10.4161/auto.5224 [DOI] [PubMed] [Google Scholar]
- 39. Plastaras JP, Vapiwala N, Ahmed MS, et al. : Validation and toxicity of PI3K/Akt pathway inhibition by HIV protease inhibitors in humans. Cancer Biol Ther. 2008;7(5):628–635. 10.4161/cbt.7.5.5728 [DOI] [PubMed] [Google Scholar]
- 40. Brüning A, Vogel M, Burger P, et al. : Nelfinavir induces TRAIL receptor upregulation in ovarian cancer cells. Biochem Biophys Res Commun. 2008;377(4):1309–1314. 10.1016/j.bbrc.2008.10.167 [DOI] [PubMed] [Google Scholar]
- 41. Giri N, Agarwal S, Shaik N, et al. : Substrate-dependent breast cancer resistance protein (Bcrp1/Abcg2)-mediated interactions: consideration of multiple binding sites in in vitro assay design. Drug Metab Dispos. 2009;37(3):560–70. 10.1124/dmd.108.022046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Brüning A, Burger P, Vogel M, et al. : Nelfinavir induces the unfolded protein response in ovarian cancer cells, resulting in ER vacuolization, cell cycle retardation and apoptosis. Cancer Biol Ther. 2009;8(3):226–32. 10.4161/cbt.8.3.7339 [DOI] [PubMed] [Google Scholar]
- 43. Dewan MZ, Tomita M, Katano H, et al. : An HIV protease inhibitor, ritonavir targets the nuclear factor-kappaB and inhibits the tumor growth and infiltration of EBV-positive lymphoblastoid B cells. Int J Cancer. 2009;124(3):622–9. 10.1002/ijc.23993 [DOI] [PubMed] [Google Scholar]
- 44. Wang P, Kelly C, Harvey A, et al. : Quantitative analysis of tumor vascular structure after drug treatment. Conf Proc IEEE Eng Med Biol Soc. 2010;2010:726–729. 10.1109/IEMBS.2010.5626274 [DOI] [PubMed] [Google Scholar]
- 45. Xie L, Evangelidis T, Xie L, et al. : Drug discovery using chemical systems biology: weak inhibition of multiple kinases may contribute to the anti-cancer effect of nelfinavir. PLoS Comput Biol. 2011;7(4):e1002037. 10.1371/journal.pcbi.1002037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Tian X, Ye J, Alonso-Basanta M, et al. : Modulation of CCAAT/enhancer binding protein homologous protein (CHOP)-dependent DR5 expression by nelfinavir sensitizes glioblastoma multiforme cells to tumor necrosis factor-related apoptosis-inducing ligand (TRAIL). J Biol Chem. 2011;286(33):29408–16. 10.1074/jbc.M110.197665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Brüning A, Vogel M, Mylonas I, et al. : Bortezomib targets the caspase-like proteasome activity in cervical cancer cells, triggering apoptosis that can be enhanced by nelfinavir. Curr Cancer Drug Targets. 2011;11(7):799–809. 10.2174/156800911796798913 [DOI] [PubMed] [Google Scholar]
- 48. Zeng J, See AP, Aziz K, et al. : Nelfinavir induces radiation sensitization in pituitary adenoma cells. Cancer Biol Ther. 2011;12(7):657–63. 10.4161/cbt.12.7.17172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Guan M, Fousek K, Chow WA: Nelfinavir inhibits regulated intramembrane proteolysis of sterol regulatory element binding protein-1 and activating transcription factor 6 in castration-resistant prostate cancer. FEBS J. 2012;279(13):2399–411. 10.1111/j.1742-4658.2012.08619.x [DOI] [PubMed] [Google Scholar]
- 50. Bono C, Karlin L, Harel S, et al. : The human immunodeficiency virus-1 protease inhibitor nelfinavir impairs proteasome activity and inhibits the proliferation of multiple myeloma cells in vitro and in vivo. Haematologica. 2012;97(7):1101–1109. 10.3324/haematol.2011.049981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Brüning A, Matsingou C, Brem GJ, et al. : Inhibin beta E is upregulated by drug-induced endoplasmic reticulum stress as a transcriptional target gene of ATF4. Toxicol Appl Pharmacol. 2012;264(2):300–304. 10.1016/j.taap.2012.08.011 [DOI] [PubMed] [Google Scholar]
- 52. Barillari G, Iovane A, Bacigalupo I, et al. : Ritonavir or saquinavir impairs the invasion of cervical intraepithelial neoplasia cells via a reduction of MMP expression and activity. AIDS. 2012;26(8):909–919. 10.1097/QAD.0b013e328351f7a5 [DOI] [PubMed] [Google Scholar]
- 53. Timeus F, Crescenzio N, Doria A, et al. : in vitro anti-neuroblastoma activity of saquinavir and its association with imatinib. Oncol Rep. 2012;27(3):734–740. 10.3892/or.2011.1582 [DOI] [PubMed] [Google Scholar]
- 54. Ismail WI, King JA, Anwar K, et al. : Indinavir and nelfinavir inhibit proximal insulin receptor signaling and salicylate abrogates inhibition: potential role of the NFkappa B pathway. J Cell Biochem. 2013;114(8):1729–37. 10.1002/jcb.24513 [DOI] [PubMed] [Google Scholar]
- 55. Escalante AM, McGrath RT, Karolak MR, et al. : Preventing the autophagic survival response by inhibition of calpain enhances the cytotoxic activity of bortezomib in vitro and in vivo. Cancer Chemother Pharmacol. 2013;71(6):1567–76. 10.1007/s00280-013-2156-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Bociaga-Jasik M, Polus A, Goralska J, et al. : Metabolic effects of the HIV protease inhibitor--saquinavir in differentiating human preadypocytes. Pharmacol Rep. 2013;65(4):937–950. 10.1016/s1734-1140(13)71075-2 [DOI] [PubMed] [Google Scholar]
- 57. Cox JS, Walter P: A novel mechanism for regulating activity of a transcription factor that controls the unfolded protein response. Cell. 1996;87(3):391–404. 10.1016/S0092-8674(00)81360-4 [DOI] [PubMed] [Google Scholar]
- 58. Tsai YC, Weissman AM: The Unfolded Protein Response, Degradation from Endoplasmic Reticulum and Cancer. Genes Cancer. 2010;1(7):764–778. 10.1177/1947601910383011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Mori K, Ogawa N, Kawahara T, et al. : mRNA splicing-mediated C-terminal replacement of transcription factor Hac1p is required for efficient activation of the unfolded protein response. Proc Natl Acad Sci U S A. 2000;97(9):4660–4665. 10.1073/pnas.050010197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Schönthal AH: Endoplasmic reticulum stress: its role in disease and novel prospects for therapy. Scientifica (Cairo). 2012;2012:857516. Review. 10.6064/2012/857516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Simmons SO, Fan CY, Ramabhadran R: Cellular stress response pathway system as a sentinel ensamble in toxicological screening. Toxicol Sci. 2009;111(2):202–225. (Review). 10.1093/toxsci/kfp140 [DOI] [PubMed] [Google Scholar]
- 62. Sun L, Niu L, Zhu X, et al. : Antitumour effects of a protease inhibitor, nelfinavir, in hepatocellular carcinoma cancer cells. J Chemother. 2012;24(3):161–6. 10.1179/1973947812Y.0000000011 [DOI] [PubMed] [Google Scholar]
- 63. Petrich AM, Leshchenko V, Kuo PY, et al. : Akt inhibitors MK-2206 and nelfinavir overcome mTOR inhibitor resistance in diffuse large B-cell lymphoma. Clin Cancer Res. 2012;18(9):2534–44. 10.1158/1078-0432.CCR-11-1407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Kast RE, Halatsch ME: Matrix metalloproteinase-2 and -9 in glioblastoma: a trio of old drugs-captopril, disulfiram and nelfinavir-are inhibitors with potential as adjunctive treatments in glioblastoma. Arch Med Res. 2012;43(3):243–7. Review. 10.1016/j.arcmed.2012.04.005 [DOI] [PubMed] [Google Scholar]
- 65. Pan J, Mott M, Xi B, et al. : Phase I study of nelfinavir in liposarcoma. Cancer Chemother Pharmacol. 2012;70(6):791–799. 10.1007/s00280-012-1961-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Shim JS, Rao R, Beebe K, et al. : Selective inhibition of HER2-positive breast cancer cells by the HIV protease inhibitor nelfinavir. J Natl Cancer Inst. 2012;104(20):1576–90. 10.1093/jnci/djs396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Kraus M, Bader J, Overkleeft H, et al. : Nelfinavir augments proteasome inhibition by bortezomib in myeloma cells and overcomes bortezomib and carfilzomib resistance. Blood Cancer J. 2013;3:e103. 10.1038/bcj.2013.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Kraus M, Müller-Ide H, Rückrich T, et al. : Ritonavir, nelfinavir, saquinavir and lopinavir induce proteotoxic stress in acute myeloid leukemia cells and sensitize them for proteasome inhibitor treatment at low micromolar drug concentrations. Leuk Res. 2014;38(3):383–92. 10.1016/j.leukres.2013.12.017 [DOI] [PubMed] [Google Scholar]
- 69. Darini CY, Martin P, Azoulay S, et al. : Targeting cancer stem cells expressing an embryonic signature with anti-proteases to decrease their tumor potential. Cell Death Dis. 2013;4:e706. 10.1038/cddis.2013.206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Kast RE, Boockvar JA, Brüning A, et al. : A conceptually new treatment approach for relapsed glioblastoma: coordinated undermining of survival paths with nine repurposed drugs (CUSP9. by the International Initiative for Accelerated Improvement of Glioblastoma Care. Oncotarget. 2013;4(4):502–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Mathur A, Abd Elmageed ZY, Liu X, et al. : Subverting ER-stress towards apoptosis by nelfinavir and curcumin coexposure augments docetaxel efficacy in castration resistant prostate cancer cells. PLoS One. 2014;9(8):e103109. 10.1371/journal.pone.0103109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Kushchayeva Y, Jensen K, Recupero A, et al. : The HIV protease inhibitor nelfinavir down-regulates RET signaling and induces apoptosis in medullary thyroid cancer cells. J Clin Endocrinol Metab. 2014;99(5):E734–45. 10.1210/jc.2013-3369 [DOI] [PubMed] [Google Scholar]
- 73. Kushchayeva Y, Jensen K, Burman KD, et al. : Repositioning therapy for thyroid cancer: new insights on established medications. Endocr Relat Cancer. 2014;21(3):R183–94. 10.1530/ERC-13-0473 [DOI] [PubMed] [Google Scholar]
- 74. Alonso-Basanta M, Fang P, Maity A, et al. : A phase I study of nelfinavir concurrent with temozolomide and radiotherapy in patients with glioblastoma multiforme. J Neurooncol. 2014;116(2):365–72. 10.1007/s11060-013-1303-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Buijsen J, Lammering G, Jansen RL, et al. : Phase I trial of the combination of the Akt inhibitor nelfinavir and chemoradiation for locally advanced rectal cancer. Radiother Oncol. 2013;107(2):184–188. 10.1016/j.radonc.2013.03.023 [DOI] [PubMed] [Google Scholar]
- 76. Hoover AC, Milhem MM, Anderson CM, et al. : Efficacy of nelfinavir as monotherapy in refractory adenoid cystic carcinoma: Results of a phase II clinical trial. Head Neck. 2014. 10.1002/hed.23664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Donia M, Mangano K, Fagone P, et al. : Unique antineoplastic profile of Saquinavir-NO, a novel NO-derivative of the protease inhibitor Saquinavir, on the in vitro and in vivo tumor formation of A375 human melanoma cells. Oncol Rep. 2012;28(2):682–688. 10.3892/or.2012.1840 [DOI] [PubMed] [Google Scholar]
- 78. Mishra LC, Bhattacharya A, Sharma M, et al. : HIV protease inhibitors, indinavir or nelfinavir, augment antimalarial action of artemisinin in vitro. Am J Trop Med Hyg. 2010;82(1):148–150. 10.4269/ajtmh.2010.09-0427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Cho HY, Thomas S, Golden EB, et al. : Enhanced killing of chemo-resistant breast cancer cells via controlled aggravation of ER stress. Cancer Lett. 2009;282(1):87–97. 10.1016/j.canlet.2009.03.007 [DOI] [PubMed] [Google Scholar]
- 80. Mahoney E, Maddocks K, Flynn J, et al. : Identification of endoplasmic reticulum stress-inducing agents by antagonizing autophagy: a new potential strategy for identification of anti-cancer therapeutics in B-cell malignancies. Leuk Lymphoma. 2013;54(12):2685–92. 10.3109/10428194.2013.781168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Brüning A, Burger P, Vogel M, et al. : Nelfinavir induces mitochondria protection by ERK ½-mediated mcl-1 stabilization that can be overcome by sorafenib. Invest New Drugs. 2010;28(5):535–42. 10.1007/s10637-009-9281-1 [DOI] [PubMed] [Google Scholar]
- 82. Brüning A, Rahmeh M, Gingelmaier A, et al. : The mitochondria-independent cytotoxic effect of nelfinavir on leukemia cells can be enhanced by sorafenib-mediated mcl-1 downregulation and mitochondrial membrane destabilization. Mol Cancer. 2010;9:19. 10.1186/1476-4598-9-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Brüning A, Friese K, Burges A, et al. : Tamoxifen enhances the cytotoxic effects of nelfinavir in breast cancer cells. Breast Cancer Res. 2010;12(4):R45. 10.1186/bcr2602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Cheng G, Zielonka J, McAllister D, et al. : Profiling and targeting of cellular bioenergetics: inhibition of pancreatic cancer cell proliferation. Br J Cancer. 2014;111(1):85–93. 10.1038/bjc.2014.272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Cheong JH, Park ES, Liang J, et al. : Dual inhibition of tumor energy pathway by 2-deoxyglucose and metformin is effective against a broad spectrum of preclinical cancer models. Mol Cancer Ther. 2011;10(12):2350–62. 10.1158/1535-7163.MCT-11-0497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Ben Sahra I, Laurent K, Giuliano S, et al. : Targeting cancer cell metabolism: the combination of metformin and 2-deoxyglucose induces p53-dependent apoptosis in prostate cancer cells. Cancer Res. 2010;70(6):2465–75. 10.1158/0008-5472.CAN-09-2782 [DOI] [PubMed] [Google Scholar]
- 87. Blumenthal GM, Gills JJ, Ballas MS, et al. : A phase I trial of the HIV protease inhibitor nelfinavir in adults with solid tumors. Oncotarget. 2014;5(18):8161–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Brunner TB, Geiger M, Grabenbauer GG, et al. : Phase I trial of the human immunodeficiency virus protease inhibitor nelfinavir and chemoradiation for locally advanced pancreatic cancer. J Clin Oncol. 2008;26(16):2699–2706. 10.1200/JCO.2007.15.2355 [DOI] [PubMed] [Google Scholar]
- 89. Rengan R, Mick R, Pryma D, et al. : A phase I trial of the HIV protease inhibitor nelfinavir with concurrent chemoradiotherapy for unresectable stage IIIA/IIIB non-small cell lung cancer: a report of toxicities and clinical response. J Thorac Oncol. 2012;7(4):709–15. 10.1097/JTO.0b013e3182435aa6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Gills JJ, Dennis PA: Perifosine: update on a novel Akt inhibitor. Curr Oncol Rep. 2009;11(2):102–10. Review. 10.1007/s11912-009-0016-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Wan X, Harkavy B, Shen N, et al. : Rapamycin induces feedback activation of Akt signaling through an IGF-1R-dependent mechanism. Oncogene. 2007;26(13):1932–40. 10.1038/sj.onc.1209990 [DOI] [PubMed] [Google Scholar]
- 92. Sarbassov DD, Guertin DA, Ali SM, et al. : Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307(5712):1098–1101. 10.1126/science.1106148 [DOI] [PubMed] [Google Scholar]
- 93. Carracedo A, Bacelga J, Pandolfi PP: Deconstructing feedback-signaling networks to improve anticancer therapy with mTORC1 inhibitors. Cell Cycle. 2008;7(24):3805–9. 10.4161/cc.7.24.7244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Bernstein WB, Dennis PA: Repositioning HIV protease inhibitors as cancer therapeutics. Curr Opin HIV AIDS. 2008;3(6):666–75. 10.1097/COH.0b013e328313915d [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Bernhard EJ, Brunner TB: Progress towards the use of HIV protease inhibitors in cancer therapy. Cancer Biol Ther. 2008;7(5):636–637. 10.4161/cbt.7.5.6087 [DOI] [PubMed] [Google Scholar]
- 96. Cho HY, Wang W, Jhaveri N, et al. : Perillyl alcohol for the treatment of temozolomide-resistant gliomas. Mol Cancer Ther. 2012;11(11):2462–72. 10.1158/1535-7163.MCT-12-0321 [DOI] [PubMed] [Google Scholar]
- 97. Gupta AK, Lee JH, Wilke WW, et al. : Radiation response in two HPV-infected head-and-neck cancer cell lines in comparison to a non-HPV-infected cell line and relationship to signaling through AKT. Int J Radiat Oncol Biol Phys. 2009;74(3):928–33. 10.1016/j.ijrobp.2009.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Gupta AK, Wilke WW, Taylor EN, et al. : Signaling pathways in adenoid cystic cancers: implications for treatment. Cancer Biol Ther. 2009;8(20):1947–51. 10.4161/cbt.8.20.9596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Danaher RJ, Wang C, Roland AT, et al. : HIV protease inhibitors block oral epithelial cell DNA synthesis. Arch Oral Biol. 2010;55(2):95–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Kimple RJ, Vaseva AV, Cox AD, et al. : Radiosensitization of epidermal growth factor receptor/HER2-positive pancreatic cancer is mediated by inhibition of Akt independent of ras mutational status. Clin Cancer Res. 2010;16(3):912–23. 10.1158/1078-0432.CCR-09-1324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Bourlier V, Zakaroff-Girard A, De Barros S, et al. : Protease inhibitor treatments reveal specific involvement of matrix metalloproteinase-9 in human adipocyte differentiation. J Pharmacol Exp Ther. 2005;312(3):1272–1279. 10.1124/jpet.104.077263 [DOI] [PubMed] [Google Scholar]
- 102. Thomas S, Sharma N, Golden EB, et al. : Preferential killing of triple-negative breast cancer cells in vitro and in vivo when pharmacological aggravators of endoplasmic reticulum stress are combined with autophagy inhibitors. Cancer Lett. 2012;325(1):63–71. 10.1016/j.canlet.2012.05.030 [DOI] [PubMed] [Google Scholar]
- 103. Wang X, Sato R, Brown MS, et al. : SREBP-1, a membrane-bound transcription factor released by sterol-regulated proteolysis. Cell. 1994;77(1):53–62. 10.1016/0092-8674(94)90234-8 [DOI] [PubMed] [Google Scholar]
- 104. Shimomura I, Hammer RE, Richardson JA, et al. : Insulin resistance and diabetes mellitus in transgenic mice expressing nuclear SREBP-1c in adipose tissue: model for congenital generalized lipodystrophy. Genes Dev. 1998;12(20):3182–3194. 10.1101/gad.12.20.3182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Riddle TM, Kuhel DG, Woollett LA, et al. : HIV protease inhibitor induces fatty acid and sterol biosynthesis in liver and adipose tissues due to the accumulation of activated sterol regulatory element-binding proteins in the nucleus. J Biol Chem. 2001;276(40):37514–9. 10.1074/jbc.M104557200 [DOI] [PubMed] [Google Scholar]
- 106. Guan M, Fousek K, Jiang C, et al. : Nelfinavir induces liposarcoma apoptosis through inhibition of regulated intramembrane proteolysis of SREBP-1 and ATF6. Clin Cancer Res. 2011;17(7):1796–806. 10.1158/1078-0432.CCR-10-3216 [DOI] [PubMed] [Google Scholar]
- 107. Lenhard JM, Croom Dk, Weiel JE, et al. : HIV protease inhibitors stimulate hepatic triglyceride sinthesis. Arterioscler Thromb Vasc Biol. 2000;20(12):2625–2629. 10.1161/01.ATV.20.12.2625 [DOI] [PubMed] [Google Scholar]
- 108. Crum-Cianflone NF, Hullsiek KH, Marconi V, et al. : The impact of nelfinavir exposure on cancer development among a large cohort of HIV-infected patients. J Acquir Immune Defic Syndr. 2009;51(3):305–9. 10.1097/QAI.0b013e3181aa13c7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Giardino Torchia ML, Ciaglia E, Masci AM, et al. : Dendritic cells/natural killer cross-talk: a novel target for human immunodeficiency virus type-1 protease inhibitors. PLoS One. 2010;5(6):e11052. 10.1371/journal.pone.0011052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Lucia MB, Anu R, Handley M, et al. : Exposure to HIV-protease inhibitors selects for increased expression of P-glycoprotein (ABCB1) in Kaposi’s sarcoma cells. Br J Cancer. 2011;105(4):513–22. 10.1038/bjc.2011.275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Garcia MG, Alaniz LD, Cordo Russo RI, et al. : PI3K/Akt inhibition modulates multidrug resistance and activates NF-κB in murine lymphoma cell lines. Leuk Res. 2009;33(2):288–296. 10.1016/j.leukres.2008.06.010 [DOI] [PubMed] [Google Scholar]
- 112. Hui DY: Effects of HIV protease inhibitor therapy on lipid metabolism. Prog Lipid Res. 2003;42(2):81–92. 10.1016/S0163-7827(02)00046-2 [DOI] [PubMed] [Google Scholar]
- 113. You J, He Z, Chen L, et al. : CH05-10, a novel indinavir analog, is a broad-spectrum antitumor agent that induces cell cycle arrest, apoptosis, endoplasmic reticulum stress and autophagy. Cancer Sci. 2010;101(12):2644–51. 10.1111/j.1349-7006.2010.01724.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Maksimovic-Ivanic D, Mijatovic S, Miljkovic D: The antitumor properties of a nontoxic, nitric oxide-modified version of saquinavir are independent of Akt. Mol Cancer Ther. 2009;8(5):1169–78. 10.1158/1535-7163.MCT-08-0998 [DOI] [PubMed] [Google Scholar]
- 115. Yorimitsu T, Nair U, Yang Z, et al. : Endoplasmic reticulum stress triggers autophagy. J Biol Chem. 2006;281(40):30299–304. 10.1074/jbc.M607007200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Sato A, Asano T, Ito K: Ritonavir interacts with bortezomib to enhance protein ubiquitination and histone acetylation synergistically in renal cancer cells. Urology. 2012;79(4):966.e13–966.e21. 10.1016/j.urology.2011.11.033 [DOI] [PubMed] [Google Scholar]
- 117. McLean K, VanDeVen NA, Sorenson DR, et al. : The HIV protease inhibitor saquinavir induces endoplasmic reticulum stress, autophagy, and apoptosis in ovarian cancer cells. Gynecol Oncol. 2009;112(3):623–30. 10.1016/j.ygyno.2008.11.028 [DOI] [PubMed] [Google Scholar]
- 118. Kawabata S, Gills JJ, Mercado-Matos JR, et al. : Synergistic effects of nelfinavir and bortezomib on proteotoxic death of NSCLC and multiple myeloma cells. Cell Death Dis. 2012;3:e353. 10.1038/cddis.2012.87 [DOI] [PMC free article] [PubMed] [Google Scholar]