Abstract
Staphylococcus aureus causes many types of human infections and syndromes—most notably skin and soft tissue infections. Abscesses are a frequent manifestation of S. aureus skin and soft tissue infections and are formed, in part, to contain the nidus of infection. Polymorphonuclear leukocytes (neutrophils) are the primary cellular host defense against S. aureus infections and a major component of S. aureus abscesses. These host cells contain and produce many antimicrobial agents that are effective at killing bacteria, but can also cause non-specific damage to host tissues and contribute to the formation of abscesses. By comparison, S. aureus produces several molecules that also contribute to the formation of abscesses. Such molecules include those that recruit neutrophils, cause host cell lysis, and are involved in the formation of the fibrin capsule surrounding the abscess. Herein, we review our current knowledge of the mechanisms and processes underlying the formation of S. aureus abscesses, including the involvement of polymorphonuclear leukocytes, and provide a brief overview of therapeutic approaches.
Staphylococcus aureus is a widespread commensal bacterium and pathogen. Approximately 50% to 60% of individuals are intermittently or permanently colonized with S. aureus and, thus, there is relatively high potential for infections.1,2 Indeed, S. aureus is among the most prominent causes of bacterial infections in the United States and other industrialized countries.3,4 For example, S. aureus was the most frequently recovered bacterium from inpatients among 300 clinical microbiology laboratories in the United States from 1998 to 2005.5 Staphylococcus aureus ranked second (after Escherichia coli) among bacterial isolates recovered from bacteremias in Europe in 2008, and the prevalence of S. aureus bacteremias increased from 2002 to 2008.4 Recently, S. aureus has been reported to be second only to Clostridium difficile as a cause of health care–associated infections in the United States.6
In addition to its high prevalence, S. aureus is well known for its ability to acquire resistance to antibiotics. Notably, antibiotic resistance in S. aureus has occurred in epidemic waves.7 Penicillin-resistant S. aureus emerged in the late 1940s, and by the mid-1950s, penicillin resistance was so prevalent that the antibiotic was no longer effective for treatment of infections. Methicillin-resistant S. aureus (MRSA) was reported in the early 1960s and then ultimately spread worldwide over the next several decades. MRSA is now endemic in health care facilities in virtually all industrialized countries, although recent data indicate a decrease in the number of invasive MRSA infections in US health care facilities.8 Community-associated MRSA (CA-MRSA) appeared inexplicably in the 1990s and is currently a major problem in many countries worldwide, including the United States.8,9 Unlike health care–associated MRSA infections, which occur in individuals with predisposing risk factors, CA-MRSA typically causes disease in otherwise healthy individuals. Although resistance to β-lactam antibiotics is arguably the greatest problem for treatment of S. aureus infections, the pathogen can develop resistance to multiple antibiotics beyond β-lactams, including vancomycin, an important therapeutic agent for severe MRSA infections.9 Taking these attributes collectively, it is not surprising that there is a high prevalence of S. aureus infections globally or that it remains a leading cause of pathogen-associated morbidity and mortality in the United States.6,8,10,11
Although S. aureus causes a wide range of diseases and syndromes, including bacteremia, pneumonia, cellulitis, and osteomyelitis, most community-associated infections in the United States are those that affect skin and soft tissues.9,11,12 Of all military personnel, 4% to 6% ultimately acquire a skin and soft tissue infection (SSTI), and 91% of these infections are caused by S. aureus (70% are MRSA).10 A CA-MRSA strain known as pulsed-field type USA300 (referred to herein as USA300) was the most frequently recovered bacterial isolate from community-associated SSTIs in the early-to-mid 2000s.3,13 This particular S. aureus strain gained additional notoriety after it caused skin abscesses in several US professional football players.14 USA300 has remained the most frequent organism recovered from individuals reporting to hospital emergency departments for purulent SSTIs,11 with infections classified as abscesses in 85% of these cases.11 Many SSTIs are relatively minor and self-limiting, but complicated SSTIs can be life threatening. There are several defining features or clinical manifestations of complicated S. aureus SSTIs, and these often include formation of large abscesses.15
Herein, we review our current knowledge of the pathogenesis of S. aureus abscesses, with emphasis on the involvement of polymorphonuclear leukocytes (PMNs; or neutrophils) and selected bacterial molecules.
S. aureus SSTIs
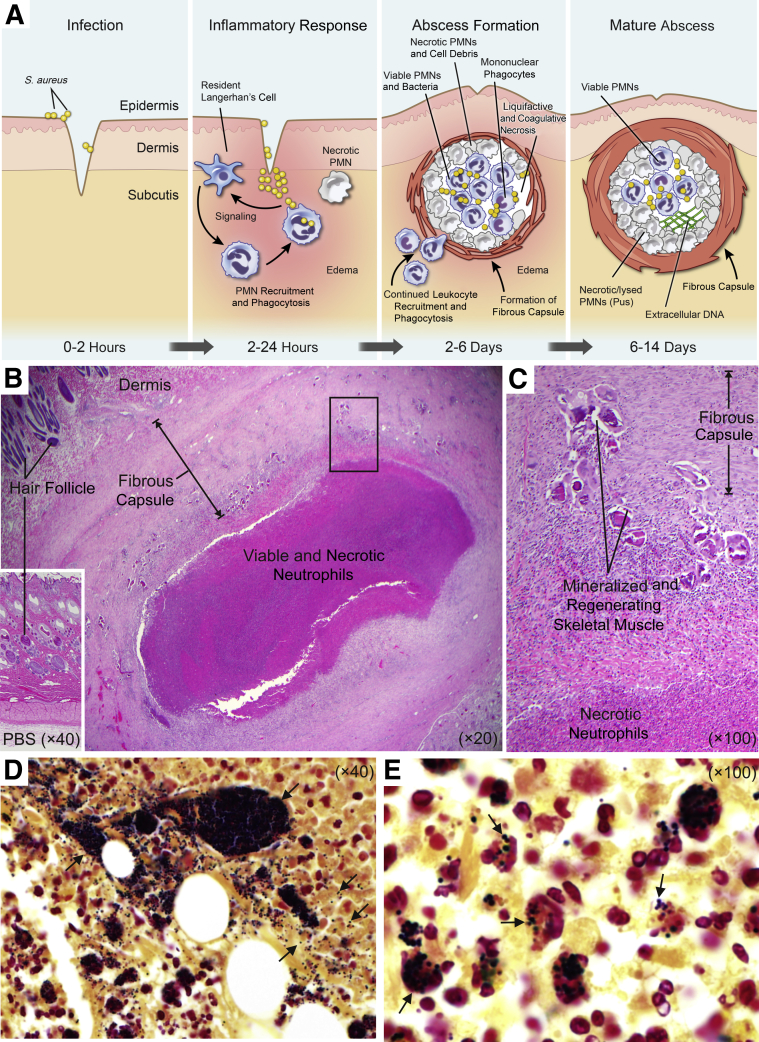
The skin is an essential first line of defense against invading bacterial pathogens, including those present in the external environment and opportunistic skin microbes. At the most basic level, the skin serves as a physical barrier to prevent entry of bacteria into deeper layers of tissue and/or dissemination to internal organ systems. Keratinocytes form this important physical barrier. Traumatic breech of the skin enables entry of pathogenic microorganisms into the underlying tissue and initiates a complex cellular response that includes mobilization of immune cells to the site of infection (Figure 1A). The clinical presentation of bacterial SSTIs can vary from superficial to highly invasive and/or disseminated disease. The importance of S. aureus in SSTIs has long been appreciated since Alexander Ogston first unveiled the role of the pathogen in the etiology of the pyogenic abscess in the late 19th century.16 Although a diversity of bacteria are currently implicated in SSTIs, S. aureus is overwhelmingly the most prominent cause of infection (eg, a recent study of a large US health care delivery system found approximately 80% of SSTIs to be associated with S. aureus),17 with the most common clinical presentation being abscess and cellulitis (63%).
Figure 1.
Staphylococcus aureus skin abscesses. A: Formation of a S. aureus skin abscess. B: Representative histopathological section of a typical rabbit skin abscess at day 14 after infection. C: Increased magnification of the boxed area shown in B. D and E: Gram stains of histological sections of a rabbit abscess. Arrows in D indicate S. aureus. The dark area is a colony of S. aureus. Arrows in E indicate S. aureus associated with leukocytes within the abscess. These studies conformed to the guidelines set forth by the NIH and were approved by the Institutional Animal Care and Use Committee at Rocky Mountain Laboratories, National Institute of Allergy and Infectious Diseases (Hamilton, Montana). PBS, phosphate-buffered saline; PMN, polymorphonuclear leukocyte.
In addition to SSTIs, pyogenic bacterial abscesses can form in deeper tissues, such as underlying muscle, and bacteria can disseminate to form abscesses at distal sites and affect virtually any internal organ system. The overall structure of S. aureus abscesses is consistent regardless of anatomical location, based, at least in part, on lesion histopathology from experimental animal models of infection (eg, rabbit SSTIs18 and murine skin,19 kidney,20 and brain).21 Similarities aside, it is unclear if there are variations in organ-specific immune response and/or bacterial response that may govern the process of abscess formation, depending on anatomical location. Staphylococcus aureus kidney abscesses in mice have features not found in S. aureus skin abscesses in rabbits. For example, Cheng et al20 found a large mass of replicating S. aureus at the center of the kidney abscess that was surrounded by an eosinophilic pseudocapsule—a feature not observed in rabbit skin abscesses.18 Inasmuch as S. aureus can produce molecules that promote abscess formation (see below), it is possible there is species and tissue specificity conferred by these molecules.
The pyogenic abscess begins as a localized host acute inflammatory response to bacterial infection. In addition to serving as a physical barrier to protect against microbes, keratinocytes possess pattern recognition receptors that detect invading microbes and, in turn, signal the proinflammatory response.22 These host cells also produce antimicrobial peptides that have direct activity against S. aureus.23,24 As an abscess forms, it acquires several characteristic features. The center of the abscess contains an acute inflammatory exudate composed of many viable and necrotic PMNs, tissue debris, fibrin, and live bacteria (Figure 1).25 Maturation of the abscess is accompanied by fibroblastic proliferation and tissue repair at the abscess margin and formation of a fibrous capsule at the periphery (Figure 1). SSTIs that present as bacterial abscesses form in the dermis, epidermis, or subcutaneous tissue and are often accompanied by cellulitis. Abscess formation is a mechanism used by the host to contain and ultimately eliminate the pathogen. Indeed, some SSTIs resolve spontaneously in the absence of treatment. Notably, PMNs play a prominent role in the formation and resolution of abscesses.
Circulating PMNs are elicited from the vasculature to the infection site in response to tissue damage,26 host proinflammatory molecules, and signals imparted directly by bacteria.27 For example, S. aureus induces expression of many host proinflammatory factors in vitro or during experimental infection in mice, including IL-1α,28 IL-1β,29 IL-6,30 IL-8,31 IL-17,32 leukotriene B4,31 tumor necrosis factor-α,33 CXCL1,34 and CXCL2.34 These factors are known to promote PMN extravasation and recruitment to infected tissues. Keratinocytes,35 T cells,34 PMNs,32 and macrophages36 produce chemotactic factors that contribute to the large influx of neutrophils that occurs in response to S. aureus SSTIs. In addition, experimental animal models provide evidence that S. aureus SSTIs result in increased numbers of PMNs in circulation,37 and myeloid progenitor cells are recruited to infection sites where they undergo granulopoiesis.37,38 The accumulation and persistence of PMNs, followed by necrotic cell lysis, contribute to the overall pathology of S. aureus SSTIs.
Innate Host Defense against S. aureus Infections—the Role of PMNs
PMNs are arguably the most important cellular defense against invading bacteria, such as S. aureus. Indeed, genetic disorders that negatively affect PMN function typically predispose individuals to severe (and frequent) bacterial and fungal infections. For example, individuals with chronic granulomatous disease, a genetic disorder characterized by the inability of PMNs and other phagocytes to produce superoxide, often acquire severe and recurrent S. aureus infections. These infections often manifest as abscesses that can ultimately transform into granulomas, which obstruct organ function and must be surgically removed. Inasmuch as PMNs play a central role in S. aureus abscess formation and the resolution of infection, it is important to understand basic functions of these prominent host cells.
Phagocytosis of Bacteria
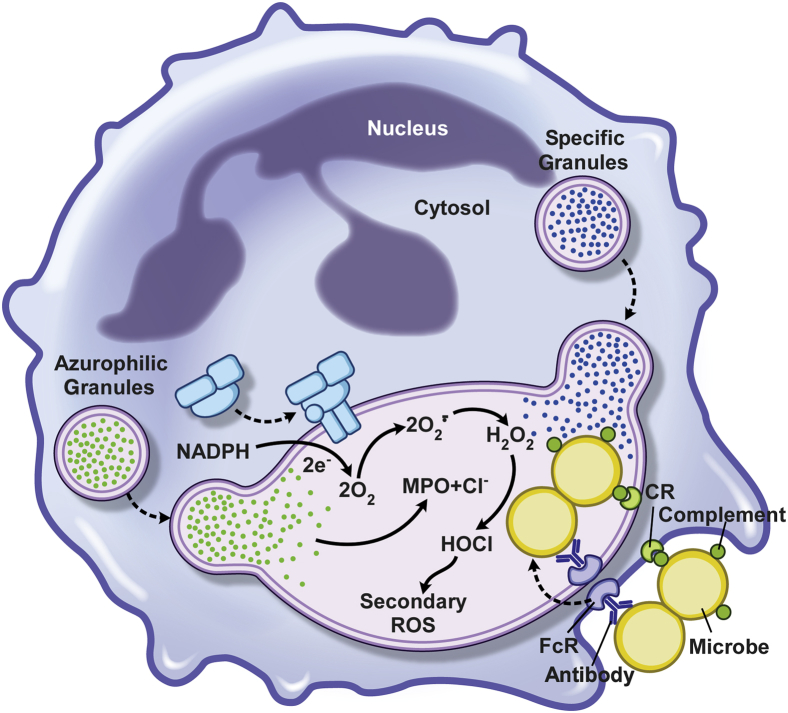
Neutrophils are recruited rapidly to the site of infection and remove invading microorganisms through a process known as phagocytosis (Figure 2). Bacteria express a litany of molecules on their surface, such as lipopolysaccharide, lipoprotein, and lipoteichoic acid, and these pathogen-associated molecular patterns interact with receptors on the surface of neutrophils. In general, ligation of the neutrophil pattern recognition receptors (eg, Toll-like receptors and CD14) activates signal transduction pathways that ultimately contribute to bactericidal activity. PMN phagocytosis is most efficiently promoted by opsonization of bacteria with antibody and complement. Specific antibody binds to epitopes on the surface of bacteria and enables the deposition of complement. Antibodies bound to the bacterial surface are recognized by neutrophil receptors specific for the Fc region, including CD64 (FcγRI, IgG receptor), CD32 (FcγRIIa, low-affinity IgG receptor), CD16 (FcγRIIIb, low-affinity IgG receptor), CD89 (FcαR, IgA receptor), and CD23 (FcεRI, IgE receptor). Bacteria opsonized with complement are recognized by PMN surface receptors, including ClqR, CD35, CD11b/CD18 (CR3), and CD11c/CD18 (CR4). Ingested bacteria are sequestered within membrane-bound vacuoles called phagosomes (Figure 2).
Figure 2.
Polymorphonuclear leukocyte phagocytosis and microbicidal processes. Surface receptors for host opsonins, such as complement and antibody, promote ingestion of S. aureus, which, in turn, activates the microbicidal processes that operate in a bacteria-containing phagosome (the cytoplasmic vacuole containing bacteria). The enzyme complex responsible for generation of superoxide—NADPH oxidase—is depicted by the blue cluster of shapes on the phagosome membrane. CR, complement receptor; HOCl, hypochlorous acid; MPO, myeloperoxidase; ROS, reactive oxygen species.
Killing of Bacteria
PMN phagocytosis is followed by the execution of bactericidal mechanisms, including the production of superoxide radicals and other reactive oxygen species (ROS), and enrichment of antimicrobial peptides, proteins, and degradative enzymes in the phagosome (Figure 2). ROS are generated by a multicomponent membrane-bound complex known as the NADPH-dependent oxidase,39 which is defective in individuals with chronic granulomatous disease. In resting neutrophils, components of the NADPH oxidase are either cytosolic (p40phox, p47phox, p67phox, and the GTPase Rac2) or located in membranes (flavocytochrome b558). NADPH oxidase assembly involves translocation of the cytosolic protein components to the plasma or phagosome membrane and their subsequent association with flavocytochrome b558, a transmembrane heterodimer that serves as the nidus of the assembling enzyme complex. After activation of the NADPH oxidase, electrons are transported from cytosolic NADPH to molecular oxygen, thereby generating superoxide anion.39 Multiple oxygen metabolites, including hydrogen peroxide, superoxide anion, and hypochlorous acid, contribute to neutrophil bactericidal activity.40
In addition to activation of PMN oxygen-dependent bactericidal mechanisms, phagocytosis triggers degranulation, which involves fusion of cytoplasmic granules with the phagosome membrane (Figure 2).41,42 Peroxidase-negative granules, including secretory vesicles, gelatinase granules, and specific granules, are a reservoir of functionally important membrane proteins, such as CR3, formyl peptide receptor, flavocytochrome b558, and β2-integrins.40,43 Peroxidase-positive granules (primary/azurophilic granules) contain the bulk of oxygen-independent antimicrobial agents of neutrophils, including α-defensins, cathepsins, proteinase-3, elastase, azurocidin, lysozyme, and bactericidal permeability–increasing protein.43 Thus, fusion of azurophilic granules with phagosomes enriches these microbe-containing vacuoles with a relatively large repertoire of antimicrobial agents. PMN antimicrobial activity, composed of ROS and a broad range of antimicrobial peptides and enzymes, is sufficient to kill most invading bacteria. Notwithstanding, bacterial pathogens, such as S. aureus, have the ability to evade the host innate immune response to promote disease. Indeed, there are numerous S. aureus molecules that can contribute to destruction of PMNs, and these molecules are discussed below in more detail.
Molecules Produced by S. aureus that Affect/Alter PMN Function and Viability
S. aureus Immune Evasion Molecules
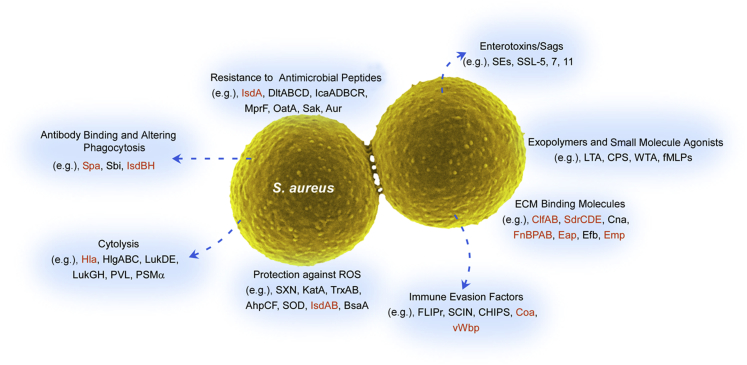
Staphylococcus aureus produces an array of potential virulence factors that play an important role on every level of host-pathogen interactions, including immune evasion molecules that allow bacteria to circumvent host innate and adaptive immunity. A multitude of these virulence factors protects S. aureus from bactericidal activity of PMNs or directly alters neutrophil function.44 These molecules can be categorized according to their functions, and include those that do the following: i) affect PMN recruitment, ii) moderate the effects of phagocyte microbicides, iii) alter phagocytosis, and iv) cause host cell lysis (cytolytic toxins) (Figure 3).
Figure 3.
Staphylococcus aureus virulence molecules. Staphylococcus aureus can produce multiple types of molecules that contribute to virulence and pathogenesis. Many of these molecules have been linked to the pathogenesis of abscesses (red text). AhpCF, alkyl hydroperoxide reductase subunits C and F; Aur, aureolysin; BsaA, glutathione peroxidase; CHIPS, chemotaxis inhibitory protein of staphylococcus; Clf, clumping factor; Cna, collagen adhesin; Coa, coagulase; CPS, capsule; Eap, extracellular adherence protein; Efb, extracellular fibrinogen binding protein; FLIPr, formyl peptide receptor-like 1 inhibitory protein; fMLP, N-formyl-methionyl-leucyl-phenylalanine; FnBPAB, fibronectin binding protein A and B; Hla, α-hemolysin; HlgABC, gamma-hemolysin subunits A, B, and C; IcaADBCR, intercellular adhesin subunits A, D, B, C, and R; Isd, iron-regulated surface determinant; KatA, catalase; LTA, lipoteichoic acid; Luk, leukocidin; MprF, multiple peptide resistance factor; OatA, O-acetyltransferase A; PSM, phenol-soluble modulin; PVL, Panton-Valentine leukocidin; ROS, reactive oxygen species; Sak, staphylokinase; Sbi, staphylococcal IgG-binding protein; SCIN, staphylococcal complement inhibitor; SdrCDE, Ser-Asp rich fibrinogen/bone sialoprotein-binding protein subunits C, D, and E; SE, staphylococcal enterotoxin; SOD, superoxide dismutase; Spa, staphylococcal protein A; SSL, staphylococcal superantigen-like protein; SXN, staphyloxanthin; TrxAB, thioredoxin (TrxA) and thioredoxin reductase (TrxB); vWbp, von Willebrand factor binding protein; WTA, wall techoic acid.
As an example, S. aureus secretes short N-formylated peptides, which are produced during protein biosynthesis or released during bacterial cytolysis. These peptides generate a chemotactic gradient for PMNs.45 N-formylated peptides, along with other bacteria-derived molecules, also signal resident host cells to produce proinflammatory molecules (chemoattractants) that signal PMN recruitment. The battle between S. aureus and PMNs begins early during infection, during which time, for example, secreted staphylococcal superantigen-like protein-5 and protein-11 obstruct interaction of PSGL-1 on the PMN surface and P-selectin on the endothelial lining, thereby blocking PMN rolling in the vessel.46,47 Extracellular adherence protein hinders association of Mac-1 and intercellular adhesion molecule-1 or binding of lymphocyte function-associated antigen-1 to intercellular adhesion molecule-1, which negatively affects PMN adhesion and diapedesis through the endothelium of the blood vessel.48 After extravasation, PMNs migrate toward infection sites on the basis of an increasing gradient of chemoattractants, which involve, in part, the formyl peptide receptor, C5a receptor, and formyl peptide receptor like-1. To counter this process, the chemotaxis inhibitory protein of staphylococcus and staphylococcal complement inhibitor are directed to inhibit chemotaxis dependent on C5a and formyl peptide receptor, and formyl peptide receptor-like 1 inhibitory protein impedes formyl peptide receptor like-1–dependent migration of PMNs.49–52 At the site of S. aureus infection, PMNs encounter secreted cytolytic toxins that can permeabilize host cell plasma membranes and/or cause rapid cytolysis and must overcome the effects of molecules that potentially inhibit bacterial uptake. Among these antiphagocytic molecules are protein A, which binds the Fc region of IgG (thereby blocking opsonization with specific antibody), clumping factor A, and extracellular fibrinogen binding protein, which blocks phagocytosis by depositing fibrinogen on the bacterial surface.53–55 Despite these obstacles, S. aureus is readily engulfed by PMNs—especially by those that are adherent.
Bacterial pathogens have also evolved mechanisms to protect against oxygen-dependent and oxygen-independent killing by human PMNs (Figure 3). For example, S. aureus uses alkyl hydroperoxide reductase, catalase, and superoxide dismutase to protect against ROS.56 Moreover, staphylococcal golden pigment or staphyloxanthin functions as an antioxidant and is additional protection against ROS.57 Staphylococcus aureus has multiple, redundant molecules/systems that promote resistance to antimicrobial peptides, and such resistance typically involves modification of the cell wall.44
Thus, given the prominent role played by PMNs in host defense against S. aureus infections, and considering the pathogen has many molecules that can potentially contribute to evasion of neutrophil function, it is not surprising that PMNs play a major role in the formation of abscesses.
Lysis of PMNs and the Role of S. aureus Secreted Toxins
To maintain proper homeostasis, the host immune system is subject to constant turnover of cells, including neutrophils. Typically, aging PMNs undergo apoptosis and are removed by macrophages in a process known as efferocytosis.58 However, bacteria such as S. aureus have the ability to alter and/or circumvent this process and cause PMN lysis.59 Because PMNs contain numerous cytotoxic and proinflammatory molecules, uncontrolled lysis can have pivotal consequences to host health and additionally can promote dissemination of bacteria previously contained within phagosomes. Recent studies revealed that after PMN engulfment, S. aureus is able to divert PMNs from conventional apoptotic pathways and cause subsequent lysis of these host cells by means of a process termed programmed necrosis.60 In vitro studies have shown that within 3 to 4 hours after phagocytosis of S. aureus, neutrophils initiate morphological changes, such as blebbing, increase exposure of phosphatidylserine on the surface of the cell, and nuclear condensation, which are hallmarks of PMN apoptosis. Although the initial steps of programmed necrosis are similar to apoptosis, S. aureus–induced programmed necrosis is a receptor-interacting protein 1–dependent process that does not result in activation of caspases 2, 3, 8, and 9.60 Moreover, PMN phagocytosis of S. aureus is accompanied by increased expression of CD47 (a don't eat me signal), a molecule that has been shown to prevent efferocytosis of apoptotic PMNs by macrophages.60 Notably, bacterial burden plays an essential role in directing PMNs toward programmed necrosis. Inasmuch as a relatively high bacteria/PMN ratio (10:1) is sufficient to induce the process, a low bacterial burden (1:1) requires additional caspase inhibition. Furthermore, engulfment of S. aureus by PMNs alters macrophage production of cytokines, such as IL-6, IL-8, or tumor necrosis factor-α, and lowers secretion of IL-1β, which is an essential cytokine in subcutaneous infections.29,60,61
In addition to triggering programmed necrosis, S. aureus secretes virulence factors that promote direct lysis of neutrophils (Figure 3). Among them are leukotoxins, such as Panton-Valentine leukocidin, leukocidin GH, or leukocidin DE, and α-type phenol-soluble modulins, γ-hemolysin, and δ-toxin.62–72 Permeabilization of the cell membrane by Panton-Valentine leukocidin or leukocidin GH can cause neutrophil lysis that results in the formation of structures called neutrophil extracellular traps, which are web-like structures of nuclear DNA to which histones and other cationic molecules are bound non-specifically.73–75 Whether these structures play a direct role in the formation of abscesses is not clear, although there is no question that abscesses contain a bolus of lysed PMNs and PMN debris, which includes extracellular DNA.
Hla and Dermonecrosis
α-Hemolysin (Hla; α-toxin) is one of the earliest studied staphylococcal virulence factors.76,77 This pore-forming cytotoxin is freely secreted by S. aureus as a water-soluble monomer, and then binds the surface of target cells, forming a heptameric transmembrane pore.78 Formation of functional pores generates ion imbalance, including efflux of potassium cations and ATP or influx of calcium ions, and ultimately leads to cell death. Hla targets many different cell types, including epithelial and endothelial cells, blood cells, and platelets.79
Hla plays a crucial role in the pathogenesis of S. aureus SSTIs and, in particular, promotes dermonecrosis in animal infection models. Functional inactivation of the gene encoding Hla by mutagenesis or deletion, or passive or active immunization against this toxin, significantly reduces size of abscesses and virtually eliminates dermonecrosis in animal infection models.18,19,80–82 The relatively recent discovery of an Hla receptor—a disintegrin and metalloprotease 10—was a major advance for our understanding of the role played by Hla during SSTIs.83 By activating a disintegrin and metalloprotease 10, Hla contributes to proteolysis of E-cadherin, which leads to the disruption of the adherens junction in the epithelial layer, thereby prompting potential remodeling of the epithelial layer and consequently pathogen dissemination.83,84 In a similar manner, Hla contributes to the breach of blood vessel endothelium integrity by causing proteolysis of the extracellular domain of vascular endothelial cadherin.85 The toxin also promotes a vigorous host inflammatory response, and this response has been linked to increased morbidity and mortality in animal infection models (eg, in S. aureus pneumonia).86 Hla also acts directly or indirectly with intracellular host sensor molecules, notably, members of the nucleotide-binding domain leucine-rich repeat containing (NLR) family, such as NLRC2 and NLRP3.87–89 Activation of the NLRP3 inflammasome by Hla and costimulation of NLRC2 by Hla and muramyl dipeptide trigger downstream activation of caspase 1, which subsequently leads to activation of the potent proinflammatory cytokine IL-1β that largely contributes to PMN influx to the site of infection.87,88
The ability of Hla to cause host cell cytolysis (and thus destabilize the dermis) and elicit neutrophil recruitment likely plays a central role in the pathogenesis of SSTIs. More notably, Hla can promote dermonecrosis in animal skin infection models, a more severe manifestation of SSTIs. Whether this virulence attribute of Hla is recapitulated in human SSTIs remains unknown, but the toxin is potentially a target for therapeutics designed to moderate the severity of disease. Key features of mature abscesses in experimental animal models and those of humans have many similar attributes, and thus the experimental abscesses in animals seem to be a reasonable approximation of S. aureus skin abscesses in humans. However, there are clear differences between experimental S. aureus abscesses in animals and human S. aureus abscesses. These differences include those in the innate immune systems of experimental animals and humans; for example, there are known differences in the ability of leukocidins, such as Panton-Valentine leukocidin, to cause cytolysis of rodent and human PMNs.90
Coagulases
Although the contribution of Hla to S. aureus SSTIs is clear in animal infection models, there is little known about the contribution of additional staphylococcal factors to abscess formation and development. Notwithstanding, several recent studies provide evidence that S. aureus coagulase (Coa) and von Willebrand factor binding protein (vWbp) facilitate abscess formation in a mouse model of disseminated infection.91–93 Coa and vWbp are perhaps best known for their ability to alter host defense by promoting coagulation and altering normal hemostasis, and thus contribute to S. aureus pathogenesis.91,94 Both Coa and vWbp activate prothrombin in a non-proteolytic manner, which diverts prothrombin activation away from host regulation.95,96 Furthermore, the C-terminal domain of Coa binds fibrinogen and subsequently enables proteolytic conversion of fibrinogen to fibrin, and the deposition of fibrin. Coagulase and high levels of vWbp accumulate at the abscess peripheries, and these molecules likely contribute to abscess development via formation of a pseudocapsule (also called fibrous capsule) and microcolony-associated meshwork.97 These structures generate a mechanical barrier that hinders the recognition and phagocytosis of bacteria by host neutrophils and other immune cells.98 Single Coa or vWbp deletion mutants alter bacterial survival and/or decrease abscess formation, but the greatest decrease in abscess formation occurs with coa/vwbp double deletion mutant strains in animal infection models. This phenomenon is likely correlated with functional redundancy of Coa and vWbp during prothrombin activation and fibrin deposition.93,97
The importance of Coa and vWbp in the formation of S. aureus abscesses is best illustrated by recent vaccine studies, in which active and passive immunization with antibodies against Coa and vWbp significantly reduced number of lesions in a murine kidney abscess model.93,97 Whether such an approach would be successful in treatment or prevention of severe/complicated SSTIs remains unclear.
Treatment and Future Perspective
Staphylococcus aureus is a human commensal microbe and has been a cause of infections throughout recorded history. There is no question that it will continue to be a significant cause of human infections. Many of the infections caused by S. aureus are nonsevere SSTIs that are self-limiting or resolve without therapeutic intervention. However, severe or complicated SSTIs require some type of therapy or treatment. Indeed, skin infections often led to invasive disease or death before the antibiotic era.65
The Infectious Diseases Society of America has set forth specific guidelines for treatment of S. aureus SSTIs.99 Incision and drainage alone is the recommended treatment for cutaneous abscesses, whereas antibiotics are recommended only for abscesses associated with severe and/or disseminated disease or those that fail to respond to incision and drainage.100 That said, outpatients with minor abscesses can be treated with empirical antibiotic therapy that is effective against CA-MRSA.100 Such antibiotics include trimethoprim-sulfamethoxazole, clindamycin, and doxycycline or minocycline.99,100 By comparison, vancomycin, linezolid, daptomycin, televancin, and clindamycin are among the antibiotics recommended for hospitalized patients with complicated SSTIs, which include major abscesses.99
Given the ability of S. aureus to develop resistance to antibiotics rapidly, it is worthwhile to consider alternative therapies for—or prophylactic measures to prevent—severe S. aureus disease. A potential therapeutic and/or prophylactic approach is the use of active or passive vaccination against S. aureus molecules known to promote severe SSTIs. There has been significant effort put forth in recent years to develop a vaccine designed to protect against S. aureus infection, but these vaccines have failed in human clinical trials.101 One confounding issue is our lack of knowledge about the factors (both host and microbe) that contribute to protective immunity against S. aureus infections. Recently, Fritz et al102 reported that anti-Hla antibody titers correlate with protection against subsequent S. aureus infection, albeit SSTIs elicit a limited protective immune response. Consistent with those findings, work in mouse infection models has demonstrated that antibodies directed against Hla, Coa, and vWbp protect against severe S. aureus skin disease.19,93 Sampedro et al103 took these models one step further by testing the ability of anti-Hla approaches, which include direct toxin neutralization or receptor blocking, to moderate or prevent recurrent S. aureus SSTIs in a mouse model.
Collectively, these studies suggest that it should be possible to use a vaccine or similar (eg, receptor blocking) approach for treatment, moderation, or prevention of severe SSTIs. Although significant progress has been made (eg, use of incision and drainage as a treatment approach), more work is needed in this general area to develop therapies that are not dependent on antibiotics.
Acknowledgments
We thank Dr. Donald J. Gardner [National Institute of Allergy and Infectious Diseases (NIAID)], for analysis of histopathology images and Anita Mora (NIAID) and Ryan Kissinger (NIAID) for assistance with photography and graphic illustration.
Infectious Disease Theme Issue
Footnotes
Supported by the National Institute of Allergy and Infectious Diseases, NIH, Intramural Research Program (S.D.K., N.M., and F.R.D.).
Disclosures: None declared.
This article is part of a review series on infectious disease.
References
- 1.Gorwitz R.J., Kruszon-Moran D., McAllister S.K., McQuillan G., McDougal L.K., Fosheim G.E., Jensen B.J., Killgore G., Tenover F.C., Kuehnert M.J. Changes in the prevalence of nasal colonization with Staphylococcus aureus in the United States, 2001-2004. J Infect Dis. 2008;197:1226–1234. doi: 10.1086/533494. [DOI] [PubMed] [Google Scholar]
- 2.Wertheim H.F., Melles D.C., Vos M.C., van Leeuwen W., van Belkum A., Verbrugh H.A., Nouwen J.L. The role of nasal carriage in Staphylococcus aureus infections. Lancet Infect Dis. 2005;5:751–762. doi: 10.1016/S1473-3099(05)70295-4. [DOI] [PubMed] [Google Scholar]
- 3.Moran G.J., Krishnadasan A., Gorwitz R.J., Fosheim G.E., McDougal L.K., Carey R.B., Talan D.A., EMERGENcy ID Net Study Group Methicillin-resistant S. aureus infections among patients in the emergency department. N Engl J Med. 2006;355:666–674. doi: 10.1056/NEJMoa055356. [DOI] [PubMed] [Google Scholar]
- 4.de Kraker M.E., Jarlier V., Monen J.C., Heuer O.E., van de Sande N., Grundmann H. The changing epidemiology of bacteraemias in Europe: trends from the European Antimicrobial Resistance Surveillance System. Clin Microbiol Infect. 2013;19:860–868. doi: 10.1111/1469-0691.12028. [DOI] [PubMed] [Google Scholar]
- 5.Styers D., Sheehan D.J., Hogan P., Sahm D.F. Laboratory-based surveillance of current antimicrobial resistance patterns and trends among Staphylococcus aureus: 2005 status in the United States. Ann Clin Microbiol Antimicrob. 2006;5:2. doi: 10.1186/1476-0711-5-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Magill S.S., Edwards J.R., Bamberg W., Beldavs Z.G., Dumyati G., Kainer M.A., Lynfield R., Maloney M., McAllister-Hollod L., Nadle J., Ray S.M., Thompson D.L., Wilson L.E., Fridkin S.K., Emerging Infections Program Healthcare-Associated Infections and Antimicrobial Use Prevalence Survey Team Multistate point-prevalence survey of health care-associated infections. N Engl J Med. 2014;370:1198–1208. doi: 10.1056/NEJMoa1306801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chambers H.F., DeLeo F.R. Waves of resistance: staphylococcus aureus in the antibiotic era. Nat Rev Microbiol. 2009;7:629–641. doi: 10.1038/nrmicro2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dantes R., Mu Y., Belflower R., Aragon D., Dumyati G., Harrison L.H., Lessa F.C., Lynfield R., Nadle J., Petit S., Ray S.M., Schaffner W., Townes J., Fridkin S., Emerging Infections Program–Active Bacterial Core Surveillance MRSA Surveillance Investigators National burden of invasive methicillin-resistant Staphylococcus aureus infections, United States, 2011. JAMA Intern Med. 2013;173:1970–1978. doi: 10.1001/jamainternmed.2013.10423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.DeLeo F.R., Otto M., Kreiswirth B.N., Chambers H.F. Community-associated meticillin-resistant Staphylococcus aureus. Lancet. 2010;375:1557–1568. doi: 10.1016/S0140-6736(09)61999-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Landrum M.L., Neumann C., Cook C., Chukwuma U., Ellis M.W., Hospenthal D.R., Murray C.K. Epidemiology of Staphylococcus aureus blood and skin and soft tissue infections in the US military health system, 2005-2010. JAMA. 2012;308:50–59. doi: 10.1001/jama.2012.7139. [DOI] [PubMed] [Google Scholar]
- 11.Talan D.A., Krishnadasan A., Gorwitz R.J., Fosheim G.E., Limbago B., Albrecht V., Moran G.J., EMERGENcy ID Net Study Group Comparison of Staphylococcus aureus from skin and soft-tissue infections in US emergency department patients, 2004 and 2008. Clin Infect Dis. 2011;53:144–149. doi: 10.1093/cid/cir308. [DOI] [PubMed] [Google Scholar]
- 12.Fridkin S.K., Hageman J.C., Morrison M., Sanza L.T., Como-Sabetti K., Jernigan J.A., Harriman K., Harrison L.H., Lynfield R., Farley M.M., Active Bacterial Core Surveillance Program of the Emerging Infections Program Network Methicillin-resistant Staphylococcus aureus disease in three communities. N Engl J Med. 2005;352:1436–1444. doi: 10.1056/NEJMoa043252. [DOI] [PubMed] [Google Scholar]
- 13.McDougal L.K., Steward C.D., Killgore G.E., Chaitram J.M., McAllister S.K., Tenover F.C. Pulsed-field gel electrophoresis typing of oxacillin-resistant Staphylococcus aureus isolates from the United States: establishing a national database. J Clin Microbiol. 2003;41:5113–5120. doi: 10.1128/JCM.41.11.5113-5120.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kazakova S.V., Hageman J.C., Matava M., Srinivasan A., Phelan L., Garfinkel B., Boo T., McAllister S., Anderson J., Jensen B., Dodson D., Lonsway D., McDougal L.K., Arduino M., Fraser V.J., Killgore G., Tenover F.C., Cody S., Jernigan D.B. A clone of methicillin-resistant Staphylococcus aureus among professional football players. N Engl J Med. 2005;352:468–475. doi: 10.1056/NEJMoa042859. [DOI] [PubMed] [Google Scholar]
- 15.Bae I.G., Tonthat G.T., Stryjewski M.E., Rude T.H., Reilly L.F., Barriere S.L., Genter F.C., Corey G.R., Fowler V.G., Jr. Presence of genes encoding the panton-valentine leukocidin exotoxin is not the primary determinant of outcome in patients with complicated skin and skin structure infections due to methicillin-resistant Staphylococcus aureus: results of a multinational trial. J Clin Microbiol. 2009;47:3952–3957. doi: 10.1128/JCM.01643-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Classics in infectious diseases: “on abscesses”: Alexander Ogston (1844-1929) Rev Infect Dis. 1984;6:122–128. [PubMed] [Google Scholar]
- 17.Ray G.T., Suaya J.A., Baxter R. Microbiology of skin and soft tissue infections in the age of community-acquired methicillin-resistant Staphylococcus aureus. Diagn Microbiol Infect Dis. 2013;76:24–30. doi: 10.1016/j.diagmicrobio.2013.02.020. [DOI] [PubMed] [Google Scholar]
- 18.Kobayashi S.D., Malachowa N., Whitney A.R., Braughton K.R., Gardner D.J., Long D., Bubeck Wardenburg J., Schneewind O., Otto M., Deleo F.R. Comparative analysis of USA300 virulence determinants in a rabbit model of skin and soft tissue infection. J Infect Dis. 2011;204:937–941. doi: 10.1093/infdis/jir441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kennedy A.D., Bubeck Wardenburg J., Gardner D.J., Long D., Whitney A.R., Braughton K.R., Schneewind O., DeLeo F.R. Targeting of alpha-hemolysin by active or passive immunization decreases severity of USA300 skin infection in a mouse model. J Infect Dis. 2010;202:1050–1058. doi: 10.1086/656043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cheng A.G., Kim H.K., Burts M.L., Krausz T., Schneewind O., Missiakas D.M. Genetic requirements for Staphylococcus aureus abscess formation and persistence in host tissues. FASEB J. 2009;23:3393–3404. doi: 10.1096/fj.09-135467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kielian T., Bearden E.D., Baldwin A.C., Esen N. IL-1 and TNF-alpha play a pivotal role in the host immune response in a mouse model of Staphylococcus aureus-induced experimental brain abscess. J Neuropathol Exp Neurol. 2004;63:381–396. doi: 10.1093/jnen/63.4.381. [DOI] [PubMed] [Google Scholar]
- 22.Krishna S., Miller L.S. Innate and adaptive immune responses against Staphylococcus aureus skin infections. Semin Immunopathol. 2012;34:261–280. doi: 10.1007/s00281-011-0292-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Braff M.H., Zaiou M., Fierer J., Nizet V., Gallo R.L. Keratinocyte production of cathelicidin provides direct activity against bacterial skin pathogens. Infect Immun. 2005;73:6771–6781. doi: 10.1128/IAI.73.10.6771-6781.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harder J., Bartels J., Christophers E., Schroder J.M. A peptide antibiotic from human skin. Nature. 1997;387:861. doi: 10.1038/43088. [DOI] [PubMed] [Google Scholar]
- 25.Kumar V., Abbas A.K., Fausto N. Robbins and Cotran Pathologic Basis of Disease. ed 7. In: Kumar V., Abbas A.K., Fausto N., editors. Elsevier Saunders; Philadelphia: 2005. [Google Scholar]
- 26.Kolaczkowska E., Kubes P. Neutrophil recruitment and function in health and inflammation. Nat Rev Immunol. 2013;13:159–175. doi: 10.1038/nri3399. [DOI] [PubMed] [Google Scholar]
- 27.Miller L.S., Cho J.S. Immunity against Staphylococcus aureus cutaneous infections. Nat Rev Immunol. 2011;11:505–518. doi: 10.1038/nri3010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Olaru F., Jensen L.E. Staphylococcus aureus stimulates neutrophil targeting chemokine expression in keratinocytes through an autocrine IL-1alpha signaling loop. J Invest Dermatol. 2010;130:1866–1876. doi: 10.1038/jid.2010.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cho J.S., Guo Y., Ramos R.I., Hebroni F., Plaisier S.B., Xuan C., Granick J.L., Matsushima H., Takashima A., Iwakura Y., Cheung A.L., Cheng G., Lee D.J., Simon S.I., Miller L.S. Neutrophil-derived IL-1beta is sufficient for abscess formation in immunity against Staphylococcus aureus in mice. PLoS Pathog. 2012;8:e1003047. doi: 10.1371/journal.ppat.1003047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Puel A., Picard C., Lorrot M., Pons C., Chrabieh M., Lorenzo L., Mamani-Matsuda M., Jouanguy E., Gendrel D., Casanova J.L. Recurrent staphylococcal cellulitis and subcutaneous abscesses in a child with autoantibodies against IL-6. J Immunol. 2008;180:647–654. doi: 10.4049/jimmunol.180.1.647. [DOI] [PubMed] [Google Scholar]
- 31.Konig B., Prevost G., Piemont Y., Konig W. Effects of Staphylococcus aureus leukocidins on inflammatory mediator release from human granulocytes. J Infect Dis. 1995;171:607–613. doi: 10.1093/infdis/171.3.607. [DOI] [PubMed] [Google Scholar]
- 32.Cho J.S., Pietras E.M., Garcia N.C., Ramos R.I., Farzam D.M., Monroe H.R., Magorien J.E., Blauvelt A., Kolls J.K., Cheung A.L., Cheng G., Modlin R.L., Miller L.S. IL-17 is essential for host defense against cutaneous Staphylococcus aureus infection in mice. J Clin Invest. 2010;120:1762–1773. doi: 10.1172/JCI40891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Prabhakara R., Foreman O., De Pascalis R., Lee G.M., Plaut R.D., Kim S.Y., Stibitz S., Elkins K.L., Merkel T.J. Epicutaneous model of community-acquired Staphylococcus aureus skin infections. Infect Immun. 2013;81:1306–1315. doi: 10.1128/IAI.01304-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McLoughlin R.M., Solinga R.M., Rich J., Zaleski K.J., Cocchiaro J.L., Risley A., Tzianabos A.O., Lee J.C. CD4+ T cells and CXC chemokines modulate the pathogenesis of Staphylococcus aureus wound infections. Proc Natl Acad Sci U S A. 2006;103:10408–10413. doi: 10.1073/pnas.0508961103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Minegishi Y., Saito M., Nagasawa M., Takada H., Hara T., Tsuchiya S., Agematsu K., Yamada M., Kawamura N., Ariga T., Tsuge I., Karasuyama H. Molecular explanation for the contradiction between systemic Th17 defect and localized bacterial infection in hyper-IgE syndrome. J Exp Med. 2009;206:1291–1301. doi: 10.1084/jem.20082767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Abtin A., Jain R., Mitchell A.J., Roediger B., Brzoska A.J., Tikoo S., Cheng Q., Ng L.G., Cavanagh L.L., von Andrian U.H., Hickey M.J., Firth N., Weninger W. Perivascular macrophages mediate neutrophil recruitment during bacterial skin infection. Nat Immunol. 2014;15:45–53. doi: 10.1038/ni.2769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim M.H., Granick J.L., Kwok C., Walker N.J., Borjesson D.L., Curry F.R., Miller L.S., Simon S.I. Neutrophil survival and c-kit(+)-progenitor proliferation in Staphylococcus aureus-infected skin wounds promote resolution. Blood. 2011;117:3343–3352. doi: 10.1182/blood-2010-07-296970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Granick J.L., Falahee P.C., Dahmubed D., Borjesson D.L., Miller L.S., Simon S.I. Staphylococcus aureus recognition by hematopoietic stem and progenitor cells via TLR2/MyD88/PGE2 stimulates granulopoiesis in wounds. Blood. 2013;122:1770–1778. doi: 10.1182/blood-2012-11-466268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Quinn M.T., Gauss K.A. Structure and regulation of the neutrophil respiratory burst oxidase: comparison with nonphagocyte oxidases. J Leukoc Biol. 2004;76:760–781. doi: 10.1189/jlb.0404216. [DOI] [PubMed] [Google Scholar]
- 40.Nauseef W.M. How human neutrophils kill and degrade microbes: an integrated view. Immunol Rev. 2007;219:88–102. doi: 10.1111/j.1600-065X.2007.00550.x. [DOI] [PubMed] [Google Scholar]
- 41.Sengelov H., Kjeldsen L., Borregaard N. Control of exocytosis in early neutrophil activation. J Immunol. 1993;150:1535–1543. [PubMed] [Google Scholar]
- 42.DeLeo F.R., Allen L.A., Apicella M., Nauseef W.M. NADPH oxidase activation and assembly during phagocytosis. J Immunol. 1999;163:6732–6740. [PubMed] [Google Scholar]
- 43.Borregaard N., Sorensen O.E., Theilgaard-Monch K. Neutrophil granules: a library of innate immunity proteins. Trends Immunol. 2007;28:340–345. doi: 10.1016/j.it.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 44.Rigby K.M., DeLeo F.R. Neutrophils in innate host defense against Staphylococcus aureus infections. Semin Immunopathol. 2012;34:237–259. doi: 10.1007/s00281-011-0295-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Durr M.C., Kristian S.A., Otto M., Matteoli G., Margolis P.S., Trias J., van Kessel K.P., van Strijp J.A., Bohn E., Landmann R., Peschel A. Neutrophil chemotaxis by pathogen-associated molecular patterns–formylated peptides are crucial but not the sole neutrophil attractants produced by Staphylococcus aureus. Cell Microbiol. 2006;8:207–217. doi: 10.1111/j.1462-5822.2005.00610.x. [DOI] [PubMed] [Google Scholar]
- 46.Bestebroer J., Poppelier M.J., Ulfman L.H., Lenting P.J., Denis C.V., van Kessel K.P., van Strijp J.A., de Haas C.J. Staphylococcal superantigen-like 5 binds PSGL-1 and inhibits P-selectin-mediated neutrophil rolling. Blood. 2007;109:2936–2943. doi: 10.1182/blood-2006-06-015461. [DOI] [PubMed] [Google Scholar]
- 47.Chung M.C., Wines B.D., Baker H., Langley R.J., Baker E.N., Fraser J.D. The crystal structure of staphylococcal superantigen-like protein 11 in complex with sialyl Lewis X reveals the mechanism for cell binding and immune inhibition. Mol Microbiol. 2007;66:1342–1355. doi: 10.1111/j.1365-2958.2007.05989.x. [DOI] [PubMed] [Google Scholar]
- 48.Chavakis T., Hussain M., Kanse S.M., Peters G., Bretzel R.G., Flock J.I., Herrmann M., Preissner K.T. Staphylococcus aureus extracellular adherence protein serves as anti-inflammatory factor by inhibiting the recruitment of host leukocytes. Nat Med. 2002;8:687–693. doi: 10.1038/nm728. [DOI] [PubMed] [Google Scholar]
- 49.Rooijakkers S.H., Ruyken M., Roos A., Daha M.R., Presanis J.S., Sim R.B., van Wamel W.J., van Kessel K.P., van Strijp J.A. Immune evasion by a staphylococcal complement inhibitor that acts on C3 convertases. Nat Immunol. 2005;6:920–927. doi: 10.1038/ni1235. [DOI] [PubMed] [Google Scholar]
- 50.Rooijakkers S.H., Ruyken M., van Roon J., van Kessel K.P., van Strijp J.A., van Wamel W.J. Early expression of SCIN and CHIPS drives instant immune evasion by Staphylococcus aureus. Cell Microbiol. 2006;8:1282–1293. doi: 10.1111/j.1462-5822.2006.00709.x. [DOI] [PubMed] [Google Scholar]
- 51.de Haas C.J., Veldkamp K.E., Peschel A., Weerkamp F., Van Wamel W.J., Heezius E.C., Poppelier M.J., Van Kessel K.P., van Strijp J.A. Chemotaxis inhibitory protein of Staphylococcus aureus, a bacterial antiinflammatory agent. J Exp Med. 2004;199:687–695. doi: 10.1084/jem.20031636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Prat C., Bestebroer J., de Haas C.J., van Strijp J.A., van Kessel K.P. A new staphylococcal anti-inflammatory protein that antagonizes the formyl peptide receptor-like 1. J Immunol. 2006;177:8017–8026. doi: 10.4049/jimmunol.177.11.8017. [DOI] [PubMed] [Google Scholar]
- 53.Dossett J.H., Kronvall G., Williams R.C., Jr., Quie P.G. Antiphagocytic effects of staphylococcal protein A. J Immunol. 1969;103:1405–1410. [PubMed] [Google Scholar]
- 54.Forsgren A., Nordstrom K. Protein A from Staphylococcus aureus: the biological significance of its reaction with IgG. Ann N Y Acad Sci. 1974;236:252–266. doi: 10.1111/j.1749-6632.1974.tb41496.x. [DOI] [PubMed] [Google Scholar]
- 55.Ko Y.P., Kuipers A., Freitag C.M., Jongerius I., Medina E., van Rooijen W.J., Spaan A.N., van Kessel K.P., Hook M., Rooijakkers S.H. Phagocytosis escape by a Staphylococcus aureus protein that connects complement and coagulation proteins at the bacterial surface. PLoS Pathog. 2013;9:e1003816. doi: 10.1371/journal.ppat.1003816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cosgrove K., Coutts G., Jonsson I.M., Tarkowski A., Kokai-Kun J.F., Mond J.J., Foster S.J. Catalase (KatA) and alkyl hydroperoxide reductase (AhpC) have compensatory roles in peroxide stress resistance and are required for survival, persistence, and nasal colonization in Staphylococcus aureus. J Bacteriol. 2007;189:1025–1035. doi: 10.1128/JB.01524-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liu G.Y., Essex A., Buchanan J.T., Datta V., Hoffman H.M., Bastian J.F., Fierer J., Nizet V. Staphylococcus aureus golden pigment impairs neutrophil killing and promotes virulence through its antioxidant activity. J Exp Med. 2005;202:209–215. doi: 10.1084/jem.20050846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fadok V.A., Bratton D.L., Henson P.M. Phagocyte receptors for apoptotic cells: recognition, uptake, and consequences. J Clin Invest. 2001;108:957–962. doi: 10.1172/JCI14122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kobayashi S.D., Braughton K.R., Palazzolo-Ballance A.M., Kennedy A.D., Sampaio E., Kristosturyan E., Whitney A.R., Sturdevant D.E., Dorward D.W., Holland S.M., Kreiswirth B.N., Musser J.M., DeLeo F.R. Rapid neutrophil destruction following phagocytosis of Staphylococcus aureus. J Innate Immun. 2010;2:560–575. doi: 10.1159/000317134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Greenlee-Wacker M.C., Rigby K.M., Kobayashi S.D., Porter A.R., DeLeo F.R., Nauseef W.M. Phagocytosis of Staphylococcus aureus by human neutrophils prevents macrophage efferocytosis and induces programmed necrosis. J Immunol. 2014;192:4709–4717. doi: 10.4049/jimmunol.1302692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Miller L.S., Pietras E.M., Uricchio L.H., Hirano K., Rao S., Lin H., O'Connell R.M., Iwakura Y., Cheung A.L., Cheng G., Modlin R.L. Inflammasome-mediated production of IL-1beta is required for neutrophil recruitment against Staphylococcus aureus in vivo. J Immunol. 2007;179:6933–6942. doi: 10.4049/jimmunol.179.10.6933. [DOI] [PubMed] [Google Scholar]
- 62.Ventura C.L., Malachowa N., Hammer C.H., Nardone G.A., Robinson M.A., Kobayashi S.D., DeLeo F.R. Identification of a novel Staphylococcus aureus two-component leukotoxin using cell surface proteomics. PLoS One. 2010;5:e11634. doi: 10.1371/journal.pone.0011634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang R., Braughton K.R., Kretschmer D., Bach T.H., Queck S.Y., Li M., Kennedy A.D., Dorward D.W., Klebanoff S.J., Peschel A., DeLeo F.R., Otto M. Identification of novel cytolytic peptides as key virulence determinants for community-associated MRSA. Nat Med. 2007;13:1510–1514. doi: 10.1038/nm1656. [DOI] [PubMed] [Google Scholar]
- 64.Malachowa N., Kobayashi S.D., Braughton K.R., Whitney A.R., Parnell M.J., Gardner D.J., Deleo F.R. Staphylococcus aureus leukotoxin GH promotes inflammation. J Infect Dis. 2012;206:1185–1193. doi: 10.1093/infdis/jis495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Panton P.N., Valentine F.C.O. Staphylococcal toxin. Lancet. 1932;219:506–508. [Google Scholar]
- 66.Cooney J., Kienle Z., Foster T.J., O'Toole P.W. The gamma-hemolysin locus of Staphylococcus aureus comprises three linked genes, two of which are identical to the genes for the F and S components of leukocidin. Infect Immun. 1993;61:768–771. doi: 10.1128/iai.61.2.768-771.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Prevost G., Cribier B., Couppie P., Petiau P., Supersac G., Finck-Barbancon V., Monteil H., Piemont Y. Panton-Valentine leucocidin and gamma-hemolysin from Staphylococcus aureus ATCC 49775 are encoded by distinct genetic loci and have different biological activities. Infect Immun. 1995;63:4121–4129. doi: 10.1128/iai.63.10.4121-4129.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Graves S.F., Kobayashi S.D., Braughton K.R., Diep B.A., Chambers H.F., Otto M., Deleo F.R. Relative contribution of Panton-Valentine leukocidin to PMN plasma membrane permeability and lysis caused by USA300 and USA400 culture supernatants. Microbes Infect. 2010;12:446–456. doi: 10.1016/j.micinf.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Marks J., Vaughan A.C. Staphylococcal delta-haemolysin. J Pathol Bacteriol. 1950;62:597–615. doi: 10.1002/path.1700620411. [DOI] [PubMed] [Google Scholar]
- 70.Kreger A.S., Kim K.S., Zaboretzky F., Bernheimer A.W. Purification and properties of staphylococcal delta hemolysin. Infect Immun. 1971;3:449–465. doi: 10.1128/iai.3.3.449-465.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gravet A., Colin D.A., Keller D., Girardot R., Monteil H., Prevost G. Characterization of a novel structural member, LukE-LukD, of the bi-component staphylococcal leucotoxins family. FEBS Lett. 1998;436:202–208. doi: 10.1016/s0014-5793(98)01130-2. [DOI] [PubMed] [Google Scholar]
- 72.Alonzo F., 3rd, Benson M.A., Chen J., Novick R.P., Shopsin B., Torres V.J. Staphylococcus aureus leucocidin ED contributes to systemic infection by targeting neutrophils and promoting bacterial growth in vivo. Mol Microbiol. 2012;83:423–435. doi: 10.1111/j.1365-2958.2011.07942.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Malachowa N., Kobayashi S.D., Freedman B., Dorward D.W., DeLeo F.R. Staphylococcus aureus leukotoxin GH promotes formation of neutrophil extracellular traps. J Immunol. 2013;191:6022–6029. doi: 10.4049/jimmunol.1301821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pilsczek F.H., Salina D., Poon K.K., Fahey C., Yipp B.G., Sibley C.D., Robbins S.M., Green F.H., Surette M.G., Sugai M., Bowden M.G., Hussain M., Zhang K., Kubes P. A novel mechanism of rapid nuclear neutrophil extracellular trap formation in response to Staphylococcus aureus. J Immunol. 2010;185:7413–7425. doi: 10.4049/jimmunol.1000675. [DOI] [PubMed] [Google Scholar]
- 75.Brinkmann V., Reichard U., Goosmann C., Fauler B., Uhlemann Y., Weiss D.S., Weinrauch Y., Zychlinsky A. Neutrophil extracellular traps kill bacteria. Science. 2004;303:1532–1535. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 76.Burnet F.M. The exotoxins of Staphylococcus pyogenes aureus. J Pathol Bacteriol. 1929;32:717–734. [Google Scholar]
- 77.Burnet F.M. The production of staphylococcal toxin. J Pathol Bacteriol. 1930;33:1–16. [Google Scholar]
- 78.Song L., Hobaugh M.R., Shustak C., Cheley S., Bayley H., Gouaux J.E. Structure of staphylococcal alpha-hemolysin, a heptameric transmembrane pore. Science. 1996;274:1859–1866. doi: 10.1126/science.274.5294.1859. [DOI] [PubMed] [Google Scholar]
- 79.Berube B.J., Bubeck Wardenburg J. Staphylococcus aureus alpha-toxin: nearly a century of intrigue. Toxins (Basel) 2013;5:1140–1166. doi: 10.3390/toxins5061140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kernodle D.S., Voladri R.K., Menzies B.E., Hager C.C., Edwards K.M. Expression of an antisense hla fragment in Staphylococcus aureus reduces alpha-toxin production in vitro and attenuates lethal activity in a murine model. Infect Immun. 1997;65:179–184. doi: 10.1128/iai.65.1.179-184.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Menzies B.E., Kernodle D.S. Site-directed mutagenesis of the alpha-toxin gene of Staphylococcus aureus: role of histidines in toxin activity in vitro and in a murine model. Infect Immun. 1994;62:1843–1847. doi: 10.1128/iai.62.5.1843-1847.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Menzies B.E., Kernodle D.S. Passive immunization with antiserum to a nontoxic alpha-toxin mutant from Staphylococcus aureus is protective in a murine model. Infect Immun. 1996;64:1839–1841. doi: 10.1128/iai.64.5.1839-1841.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wilke G.A., Bubeck Wardenburg J. Role of a disintegrin and metalloprotease 10 in Staphylococcus aureus alpha-hemolysin-mediated cellular injury. Proc Natl Acad Sci U S A. 2010;107:13473–13478. doi: 10.1073/pnas.1001815107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Maretzky T., Reiss K., Ludwig A., Buchholz J., Scholz F., Proksch E., de Strooper B., Hartmann D., Saftig P. ADAM10 mediates E-cadherin shedding and regulates epithelial cell-cell adhesion, migration, and beta-catenin translocation. Proc Natl Acad Sci U S A. 2005;102:9182–9187. doi: 10.1073/pnas.0500918102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Powers M.E., Kim H.K., Wang Y., Bubeck Wardenburg J. ADAM10 mediates vascular injury induced by Staphylococcus aureus alpha-hemolysin. J Infect Dis. 2012;206:352–356. doi: 10.1093/infdis/jis192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bartlett A.H., Foster T.J., Hayashida A., Park P.W. Alpha-toxin facilitates the generation of CXC chemokine gradients and stimulates neutrophil homing in Staphylococcus aureus pneumonia. J Infect Dis. 2008;198:1529–1535. doi: 10.1086/592758. [DOI] [PubMed] [Google Scholar]
- 87.Hruz P., Zinkernagel A.S., Jenikova G., Botwin G.J., Hugot J.P., Karin M., Nizet V., Eckmann L. NOD2 contributes to cutaneous defense against Staphylococcus aureus through alpha-toxin-dependent innate immune activation. Proc Natl Acad Sci U S A. 2009;106:12873–12878. doi: 10.1073/pnas.0904958106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Craven R.R., Gao X., Allen I.C., Gris D., Bubeck Wardenburg J., McElvania-Tekippe E., Ting J.P., Duncan J.A. Staphylococcus aureus alpha-hemolysin activates the NLRP3-inflammasome in human and mouse monocytic cells. PLoS One. 2009;4:e7446. doi: 10.1371/journal.pone.0007446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ting J.P., Lovering R.C., Alnemri E.S., Bertin J., Boss J.M., Davis B.K., Flavell R.A., Girardin S.E., Godzik A., Harton J.A., Hoffman H.M., Hugot J.P., Inohara N., Mackenzie A., Maltais L.J., Nunez G., Ogura Y., Otten L.A., Philpott D., Reed J.C., Reith W., Schreiber S., Steimle V., Ward P.A. The NLR gene family: a standard nomenclature. Immunity. 2008;28:285–287. doi: 10.1016/j.immuni.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Spaan A.N., Henry T., van Rooijen W.J., Perret M., Badiou C., Aerts P.C., Kemmink J., de Haas C.J., van Kessel K.P., Vandenesch F., Lina G., van Strijp J.A. The staphylococcal toxin Panton-Valentine Leukocidin targets human C5a receptors. Cell Host Microbe. 2013;13:584–594. doi: 10.1016/j.chom.2013.04.006. [DOI] [PubMed] [Google Scholar]
- 91.Lam G.T., Sweeney F.J., Jr., Witmer C.M., Wise R.I. Abscess-forming factor(s) produced by Staphylococcus aureus, II: abscess formation and immunity by a Staphylococcus and its mutants. J Bacteriol. 1963;86:87–91. doi: 10.1128/jb.86.1.87-91.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Vanassche T., Kauskot A., Verhaegen J., Peetermans W.E., van Ryn J., Schneewind O., Hoylaerts M.F., Verhamme P. Fibrin formation by staphylothrombin facilitates Staphylococcus aureus-induced platelet aggregation. Thromb Haemost. 2012;107:1107–1121. doi: 10.1160/TH11-12-0891. [DOI] [PubMed] [Google Scholar]
- 93.McAdow M., DeDent A.C., Emolo C., Cheng A.G., Kreiswirth B.N., Missiakas D.M., Schneewind O. Coagulases as determinants of protective immune responses against Staphylococcus aureus. Infect Immun. 2012;80:3389–3398. doi: 10.1128/IAI.00562-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bjerketorp J., Nilsson M., Ljungh A., Flock J.I., Jacobsson K., Frykberg L. A novel von Willebrand factor binding protein expressed by Staphylococcus aureus. Microbiology. 2002;148:2037–2044. doi: 10.1099/00221287-148-7-2037. [DOI] [PubMed] [Google Scholar]
- 95.Friedrich R., Panizzi P., Fuentes-Prior P., Richter K., Verhamme I., Anderson P.J., Kawabata S., Huber R., Bode W., Bock P.E. Staphylocoagulase is a prototype for the mechanism of cofactor-induced zymogen activation. Nature. 2003;425:535–539. doi: 10.1038/nature01962. [DOI] [PubMed] [Google Scholar]
- 96.Kroh H.K., Panizzi P., Bock P.E. Von Willebrand factor-binding protein is a hysteretic conformational activator of prothrombin. Proc Natl Acad Sci U S A. 2009;106:7786–7791. doi: 10.1073/pnas.0811750106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Cheng A.G., McAdow M., Kim H.K., Bae T., Missiakas D.M., Schneewind O. Contribution of coagulases towards Staphylococcus aureus disease and protective immunity. PLoS Pathog. 2010;6:e1001036. doi: 10.1371/journal.ppat.1001036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Guggenberger C., Wolz C., Morrissey J.A., Heesemann J. Two distinct coagulase-dependent barriers protect Staphylococcus aureus from neutrophils in a three dimensional in vitro infection model. PLoS Pathog. 2012;8:e1002434. doi: 10.1371/journal.ppat.1002434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Liu C., Bayer A., Cosgrove S.E., Daum R.S., Fridkin S.K., Gorwitz R.J., Kaplan S.L., Karchmer A.W., Levine D.P., Murray B.E., J Rybak M., Talan D.A., Chambers H.F., Infectious Diseases Society of America Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis. 2011;52:e18–e55. doi: 10.1093/cid/ciq146. [DOI] [PubMed] [Google Scholar]
- 100.Singer A.J., Talan D.A. Management of skin abscesses in the era of methicillin-resistant Staphylococcus aureus. N Engl J Med. 2014;370:1039–1047. doi: 10.1056/NEJMra1212788. [DOI] [PubMed] [Google Scholar]
- 101.Jansen K.U., Girgenti D.Q., Scully I.L., Anderson A.S. Vaccine review: “Staphyloccocus aureus vaccines: problems and prospects”. Vaccine. 2013;31:2723–2730. doi: 10.1016/j.vaccine.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 102.Fritz S.A., Tiemann K.M., Hogan P.G., Epplin E.K., Rodriguez M., Al-Zubeidi D.N., Bubeck Wardenburg J., Hunstad D.A. A serologic correlate of protective immunity against community-onset Staphylococcus aureus infection. Clin Infect Dis. 2013;56:1554–1561. doi: 10.1093/cid/cit123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sampedro G.R., DeDent A.C., Becker R.E., Berube B.J., Gebhardt M.J., Cao H., Bubeck Wardenburg J. Targeting Staphylococcus aureus α-toxin as a novel approach to reduce severity of recurrent skin and soft-tissue infections. J Infect Dis. 2014;210:1012–1018. doi: 10.1093/infdis/jiu223. [DOI] [PMC free article] [PubMed] [Google Scholar]