Abstract
Inflammatory CD4+ T cell responses to self or commensal bacteria underlie the pathogenesis of autoimmunity and inflammatory bowel disease (IBD), respectively. While selection of self-specific T cells in the thymus limits responses to tissue antigens, the mechanisms that control selection of commensal bacteria-specific T cells remain poorly understood. Here we demonstrate that group 3 innate lymphoid cell (ILC3)-intrinsic expression of major histocompatibility complex class II (MHCII) is regulated similarly to thymic epithelial cells, and that MHCII+ ILC3s directly induce cell death of activated commensal bacteria-specific T cells. Further, MHCII on human colonic ILC3s was reduced in pediatric IBD patients. Collectively, these results define a selection pathway for commensal bacteria-specific CD4+ T cells in the intestine, and suggest that this process is dysregulated in human IBD.
Keywords: Commensal bacteria, T cell selection, innate lymphoid cell, inflammatory bowel disease
Pathologic CD4+ T cell responses to self are limited by presentation of self-antigens in the thymus on MHCII+ thymic epithelial cells (TECs) and dendritic cells (DCs), resulting in clonal deletion (1-6). In contrast, commensal bacteria-specific CD4+ T cells, which have been implicated in the pathogenesis of inflammatory bowel disease (IBD) (7-11), do not encounter cognate antigen in the thymus and therefore are not subject to negative selection prior to entering the periphery (12, 13). While physical and biochemical barriers separate the immune system from intestinal commensal bacteria (7, 13-17), antigens derived from commensal bacteria are continuously sampled from the intestinal lumen and presented by DCs in the draining lymph nodes (7, 10, 15, 18, 19). Regulatory T cells (Treg) can in part limit dysregulated CD4+ T cell responses to commensal bacteria (20, 21). However, whether other mechanisms control commensal bacteria-specific CD4+ T cells in lieu of thymic negative selection is poorly defined. In recent studies, ILC3s were found to express MHCII and genetic deletion of ILC3-intrinsic MHCII resulted in spontaneous CD4+ T cell-dependent intestinal inflammation (22), suggesting additional antigen-presentation pathways control commensal bacteria-specific CD4+ T cell populations.
CCR6-expressing lymphoid tissue inducer (LTi)-like ILC3s (CCR6+ ILC3s), are a major ILC subset present in the mesenteric lymph node (mLN) (Fig. 1A) and colon lamina propria (cLPL) (fig. S1A) of healthy mice, and constitutively express retinoic acid-related orphan receptor gamma t (RORγt) and MHCII, relative to ST2+ group 2 ILCs (ILC2) (fig. S1B-C). ILC3s are regulated by various cytokine, environmental and microbial factors (23, 24). However, interleukin (IL)-23p19, the aryl hydrocarbon receptor (Ahr) or the intestinal microbiota were not required for CCR6+ ILC3 expression of MHCII (fig. S1D-F), although ILC3 frequencies were reduced in the intestine of Ahr-/- mice, as previously described (fig. S1E) (25). Further, in contrast to a recent report (26), expression of MHCII, CD80 and CD86 on CCR6+ ILC3s were unaffected by ex vivo stimulation with microbial or inflammatory stimuli, or by the absence of MyD88 or Caspase 1/11 in vivo (fig. S2).
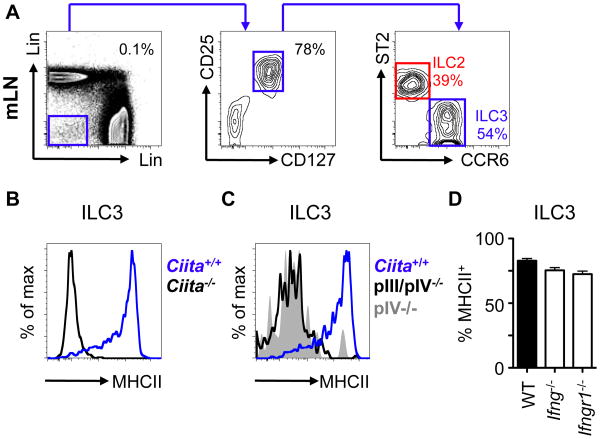
Fig. 1. ILC3 expression of MHCII is controlled by a transcriptional pathway previously associated with thymic epithelial cells.
A) Mesenteric lymph node (mLN) cells from naïve mice were gated as CD45+ lineage (x-axis; CD3, CD5, CD8, NK1.1, y-axis; B220, CD11c, CD11b) negative, CD25+ and CD127+ and further divided by expression of ST2 (ILC2; red) or CCR6 (ILC3; blue). MHCII expression was determined on ILC3s in mice deficient in B) CIITA, C) CIITA promoter regions (pIII/pIV, pIV) or D) IFN-γ and IFN-γR1. All data representative of at least 3 independent experiments with n=2-3 mice per group. SPF, specific pathogen free.
Next we examined whether expression of ILC3-intrinsic MHCII was dependent on the class II transactivator (CIITA), a master transcriptional regulator of MHCII expression (27). MHCII expression was absent on CCR6+ ILC3s from Ciita-/- mice, relative to Ciita+/+ control mice (Fig. 1B and fig. S3A).
Transcription of CIITA in mice is driven via distinct promoter elements, termed pI, pIII and pIV, that are indicative of the upstream signaling events that induce MHCII expression (27). In contrast to B cells and DCs, CCR6+ ILC3-intrinsic MHCII expression was absent in the mLN of both pIII/pIV-/- and pIV-/- mice (Fig 1C, fig. S3B-C), indicating that the pIV promoter of Ciita is required for MHCII expression on CCR6+ ILC3s. The pIV promoter of Ciita is utilized by multiple cell types, such as epithelial cells, in response to interferon (IFN)-γ signaling (27). However, expression of MHCII on CCR6+ ILC3s was not impaired in the absence of IFN-γ, IFN-γR1 or STAT-1 (Fig. 1D, fig. S3D-E). MHCII expression in TECs is also dependent upon the pIV promoter of Ciita (fig. S3A-B) and pIV-dependent, IFN-γ-independent CIITA expression has previously only been described in TECs (27-29), suggesting a previously unappreciated link between these cell types.
These data provoked the hypothesis that TECs and MHCII+ ILC3s share similar functional roles in the selection of CD4+ T cells. To test this, we examined CD4+ T cells in the intestine of mice with an ILC3-intrinsic deletion in MHCII (MHCIIΔILC3 mice). As we previously reported (22), frequencies and cell numbers of CD44hi CD4+ T cells (Teff) in the cLPL of MHCIIΔILC3 mice were increased relative to H2-Ab1fl/fl controls (fig. S4A-B). In contrast, the total numbers of CD44lo naïve T cells and FoxP3+ Treg were unchanged (fig. S4B). Teff cell populations utilized a broad range of T cell receptor (TCR) Vβ chains and Teff expansion was detected in the cLPL but not in the thymus (fig. S4C). Further, sort-purified CD4+ T cells from MHCIIΔILC3 mice responded to fecal-derived antigen, but not mammalian tissue-derived antigens (fig. S4D).
These data, along with previous studies (20, 30, 31), suggest the majority of CD4+ T cells in the steady-state intestine are specific for commensal bacteria and that ILC3-intrinsic MHCII controls commensal bacteria-specific CD4+ T cell responses through direct presentation of microbiota-derived antigens. To test this, we crossed MHCIIΔILC3 mice with either TCR transgenic mice specific for CBir1, an antigen expressed by Clostridia species constitutively present in the murine and human microbiota (13, 32), or TCR transgenic mice specific for ovalbumin (OT-II). Loss of ILC3-intrinsic MHCII had no effect on the frequencies or cell numbers of OT-II T cells or CBir1 T cells in the thymus (Fig. 2A). In contrast, numbers of CBir1, but not OT-II, T cells were increased in the cLPL and mLN (Fig. 2B and fig. S5A), and CBir1MHCIIΔILC3 mice exhibited increased frequencies of antigen-specific IFN-γ+ and tumor necrosis factor (TNF)-α+ colonic CD4+ T cells, colonic inflammation and neutrophil infiltration, which could be prevented by administration of antibiotics (ABX), and was not observed in Rag1-/- MHCIIΔILC3 mice (Fig. 2B-D and fig. S5A-D). Finally, similar to polyclonal MHCIIΔILC3 mice, the population expansion and increased cytokine production of CBir1 CD4+ T cells was associated with a selective increase in CD44+CD62Llo Teff, whereas numbers of FoxP3+ Treg remained unchanged (fig. S5E). These data suggest that CCR6+ ILC3s selectively limit the expansion of commensal bacteria-specific CD4+ effector T cells through presentation of antigen derived from endogenous commensal bacteria. In support of this hypothesis and consistent with recent findings (33), MHCII+ ILC3s localized in distinct clusters in the mLN at the interface between the B- and T- cell zones in the marginal and subcapsular sinus (Fig. 2E), a site through which antigen-experienced T cells traffic.
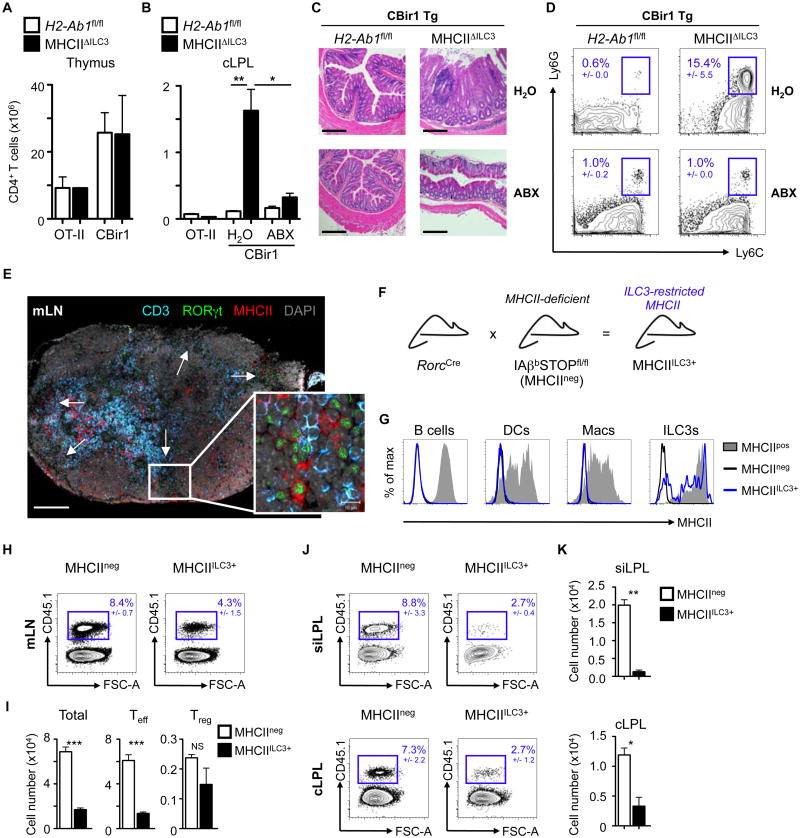
Fig. 2. MHCII+ ILC3s induce deletion of commensal bacteria-specific CD4+ T effector cells in the intestine and associated lymph nodes.
Total Vβ5+ (OT-II) or Vβ8.3+ (CBir1) CD4+ T cell numbers determined in A) the thymus and B) cLPL of control (H20) or antibiotic (ABX)-treated OT-II and CBir1 transgenic mice crossed with MHCIIΔILC3 or H2-Ab1fl/fl littermate controls. C) Colon histology (scale bar 200μm) and D) frequencies of (CD45+ B220- CD3-) Ly6C+ Ly6G+ neutrophils in the cLPL of CBir1MHCIIΔILC3 and CBir1H2-Ab1fl/fl mice. E) Immunofluorescence imaging of mLN sections stained for CD3 (blue), RORγt (green), MHCII (red) or DAPI (grey). White arrows indicate RORγt+ cell clusters. Scale bar = 200μm. Insert demonstrates colocalization of RORγt+ MHCII+ ILCs and CD3+ T cells. Insert scale bar = 10μm. F) MHCII expression was restricted to RORγt+ ILC3s (MHCIIILC3+ mice) by crossing RorcCre mice with IAβbSTOPfl/fl (MHCIIneg) mice and G) MHCII levels were determined on B220+ B cells, CD11b+ CD11chi dendritic cells (DCs), CD11b+ F4/80+ macrophages (Macs) or Linneg CD25+ CD127+ CCR6+ ILC3s in the mLN of heterozygote littermates (IAβbSTOP+/fl, MHCIIpos), MHCIIneg or MHCIIILC3+ mice. (H-K) MHCIIneg and MHCIIILC3+ received pre-activated CD45.1+ CBir1 transgenic CD4+ T cells and were injected with CBir1 peptide i.p. every 2 days. Frequencies and numbers of transferred T cells were analyzed in the mLN (H,I), siLPL and cLPL 9 days post transfer (J,K). All data representative of at least 4 independent experiments with n=2-3 mice per group. Results are shown as the mean +/- s.e.m. * p < 0.05, ** p < 0.01, *** p < 0.001 (two-tailed students t-test).
To investigate the in vivo mechanisms through which MHCII+ ILC3s control commensal bacteria-specific CD4+ T cells, mice were generated in which MHCII expression was restricted to only ILC3s. This was accomplished by utilizing mice with a floxed-STOP sequence cassette inserted between the first and second IAβb exons (IAβbSTOPfl/fl mice) (34). IAβbSTOPfl/fl mice lack MHCII on antigen-presenting cells in the absence of Cre recombinase (MHCIIneg) (Fig. 2G). In contrast, IAβbSTOPfl/fl mice crossed with RorcCre mice demonstrated a partial restoration of MHCII on CCR6+ ILC3s (MHCIIILC3+ mice) (Fig. 2F and G), but not B cells, DCs and macrophages (Fig. 2G). As MHCIIneg and MHCIIILC3+ mice lack MHCII on TECs and have disrupted endogenous T cell selection (34), we first employed an adoptive transfer approach with naïve CFSE-labeled CD45.1+ CBir1 T cells. In mice with normal MHCII expression (MHCIIpos) administration of CBir1 peptide resulted in dilution of CFSE in transferred CBir1 T cells, expansion of CD44hiCD62Llo CBir1 Teff cells and an increase in FoxP3+ Treg in the mLN (fig. S6A). In contrast, naïve CBir1 CD4+ T cells transferred into control MHCIIneg and MHCIIILC3+ mice failed to proliferate or differentiate into Teff or Treg following peptide administration (fig. S6A), suggesting that ILC3-intrinsic MHCII does not influence naïve CD4+ T cells. As MHCII+ ILC3s localize at lymphoid sites through which antigen-experienced CD4+ T cells traffic (Fig. 2E) (33), CBir1 CD4+ T cells were pre-activated overnight prior to transfer into MHCIIneg or MHCIIILC3+ mice. Following CBir1 peptide administration, MHCIIILC3+ mice exhibited reduced frequencies and numbers of pre-activated CBir1 CD4+ T cells in the mLN (Fig. 2H-I), which was due to a selective decrease in effector, but not regulatory CBir1 T cells (Fig. 2I). MHCII+ ILC3-mediated effects were antigen-specific, could be driven by endogenous microbiota-derived antigen alone and were specific to ILC3s (fig. S6B-D and fig. S7). Further, in complementary loss-of-function studies, CBir1 CD4+ Teff expanded at higher frequencies and numbers in the mLN and cLPL of mice lacking ILC3-intrinsic MHCII (MHCIIΔILC3 mice), despite exhibiting comparable proliferation (fig. S8). Finally, in line with ILC3-mediated control of antigen-experienced T cells, the frequency and number of activated CBir1 CD4+ T cells reaching the small intestine lamina propria (siLPL) and cLPL of MHCIIILC3+ mice were significantly decreased relative to MHCIIneg controls (Fig. 2J-K).
We hypothesized that the loss of CBir1 CD4+ T cells from the mLN and intestine of MHCIIILC3+ mice could be the result of altered migration, proliferation or through induction of cell death. However, pre-activated CBir1 T cells did not accumulate in peripheral organs, such as the spleen (fig. S9A) and CBir1 T cells recovered from MHCIIILC3+ mice exhibited comparable proliferation, relative to MHCIIneg mice (fig. S9B). To further investigate this question, we developed an in vitro ILC3-CD4+ T cell co-culture system. Consistent with our in vivo findings, co-culture of activated CBir1 T cells and sort-purified MHCII+ CCR6+ ILC3s resulted in a significant reduction in T cell numbers following culture in the presence of cognate antigen, which could be reversed by administration of an MHCII-blocking antibody (Fig. 3A). Reduced cell recovery was associated with an antigen and MHCII-dependent increase in Caspase-3 activation (Fig. 3B) and increased Annexin V staining (Fig. 3C) in the remaining CBir1 T cells, indicative of programmed cell death. Despite selectively regulating commensal bacteria-specific CD4+ T cells in the steady state, MHCII+ ILC3s were also sufficient to influence T cells with other antigen-specificities only if antigen was systemically provided (fig. S10).
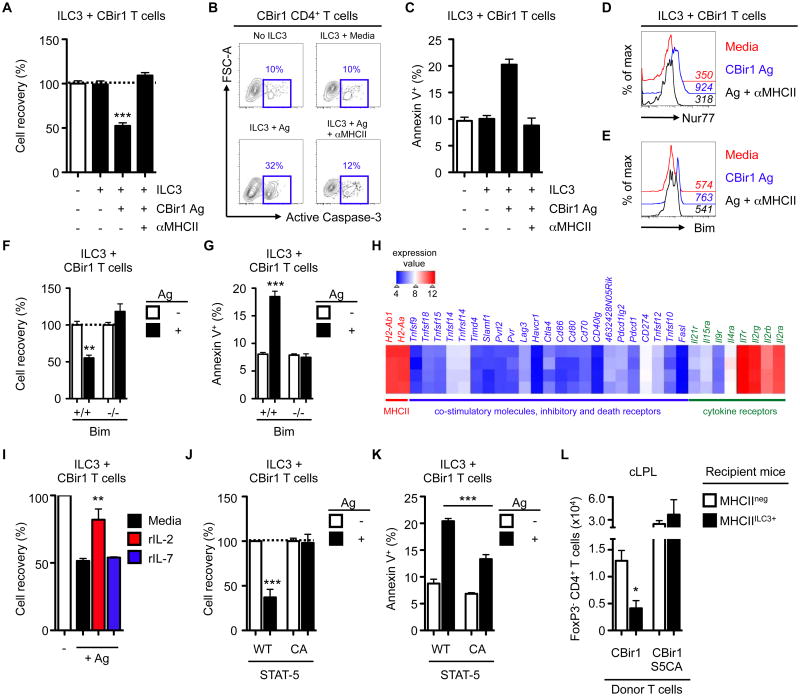
Fig. 3. MHCII+ ILC3s directly induce cell death of commensal bacteria-specific CD4+ T cells.
(A-D) Activated CBir1 CD4+ T cells were co-cultured with sort-purified CCR6+ ILC3s in the presence or absence of CBir1 antigen or an anti-MHCII neutralizing antibody and A) relative cell recovery (%) was quantified relative to T cells cultured alone, B) frequency of active Caspase-3 expressing cells were assessed and C) frequencies of Annexin V+ Dead cell exclusion dye-negative (pre-apoptotic) cells were quantified. D) Expression of Nur77 by CBir1 CD4+ T cells and E) expression of Bim by pre-activated CBir1 CD4+ T cells following 24h (D) or 48h (E) co-culture with antigen-pulsed MHCII+ ILC3s in the presence or absence of an anti-MHCII neutralizing antibody. Mean fluorescent intensity (MFI) values are shown in italics. (F-G) Bim+/+ or Bim-/- CBir1 T cells were co-cultured with ILC3s in the presence or absence of CBir1 antigen for 48h and F) relative cell recovery (%) and G) frequencies of Annexin V+ pre-apoptotic cells were quantified. H) Heat map of selected candidate genes from mLN-derived CCR6+ ILC3s. I) Relative cell recovery (%) of wildtype CBir1 CD4+ T cells co-cultured with antigen-pulsed ILC3s in the presence or absence of exogenous rIL-2 or rIL-7. (J-K) Activated CBir1 CD4+ T cells with wildtype or (WT) or constitutively active (CA) STAT-5 signaling were co-cultured with ILC3s in the presence or absence of CBir1 antigen for 48h and J) relative cell recovery (%) and K) frequencies of Annexin V+ pre-apoptotic cells were quantified. L) WT CBir1 CD4+ T cells or CBir1 STAT5-CA CD4+ T cells were adoptively transferred into MHCIIneg or MHCIIILC3+ mice. Mice were administered CBir1 antigen and numbers of FoxP3- CD45.1+ CBir1 T cells in the cLPL were quantified 9 days post-transfer. In vitro assay data are representative of at least 2-3 independent experiments with 2-3 biological replicates per experiment. Array data are representative of a single experiment with 4 biological replicates. All in vivo data are representative of at least 2 independent experiments with at least n=3 mice per group. Results are shown as the mean +/- s.e.m. * p < 0.05, ** p < 0.01, *** p < 0.001 (two-tailed students t-test).
Negative selection in the thymus has been shown to be associated with induction of Nur77 and subsequent upregulation of the pro-apoptotic molecule Bim (35). Antigen-dependent interactions between CBir1 T cells by MHCII+ ILC3s also resulted in the upregulation of Nur77 as well as Bim, which was required for ILC3-mediated induction of cell death (Fig. 3D-G). Antigen-presentation by ILC3s in vitro selectively led to Teff death, but did not affect Treg numbers (fig. S11A). We next analyzed mLN-derived CCR6+ ILC3 for expression of surface molecules that directly influence antigen-specific CD4+ T cell responses (Fig. 3H). ILC3s demonstrated high levels of MHCII-associated transcripts, but had negligible expression of transcripts for canonical co-stimulatory molecules and inhibitory or death receptors (Fig. 3H). Indeed, CCR6+ ILC3s lacked expression of FasL by flow cytometry and antibody-mediated neutralization of FasL did not influence ILC3-induced CBir1 T cell death (fig. S11B-D). Moreover, in contrast to Bim-/- mice, FasLgld/gld mice did not exhibit increased frequencies of endogenous commensal bacteria-specific CD4+ T cells in gut-associated lymphoid tissues (fig. S11E).
Bim-dependent apoptotic cell death may also be induced via cytokine or growth factor starvation (36, 37). As CCR6+ ILC3s constitutively express high levels of the common gamma chain cytokine receptors CD25 (IL-2R) and CD127 (IL-7Rα) (Fig. 1, fig. S1 and Fig. 3H), we hypothesized that MHCII+ ILC3s may induce cell death of commensal bacteria-specific CD4+ T cells synergistically through TCR-induction of an apoptotic program in concert with cytokine withdrawal. Consistent with this, cell death could be reduced upon addition of exogenous recombinant (r)IL-2, but not rIL-7, to in vitro co-cultures (Fig. 3I). MHCII+ ILC3s exhibited over twofold higher capacity to bind IL-2 as compared to activated CBir1 CD4+ T cells (fig. S11F-G), suggesting MHCII+ ILC3s out-compete activated T cells for pro-survival cytokines. The requirement for IL-2 was T cell-intrinsic as activated CBir1 CD4+ cells expressing a constitutively active STAT-5 molecule (S5CA), were resistant to Bim upregulation and ILC3-induced cell death in vitro and in vivo (Fig. 3J-L and fig. S11H-I). Taken together these data indicate that MHCII+ ILC3s mediate a negative selection process through antigen-presentation and withdrawal of IL-2 from the local milieu, resulting in deletion of activated commensal bacteria-specific T cells.
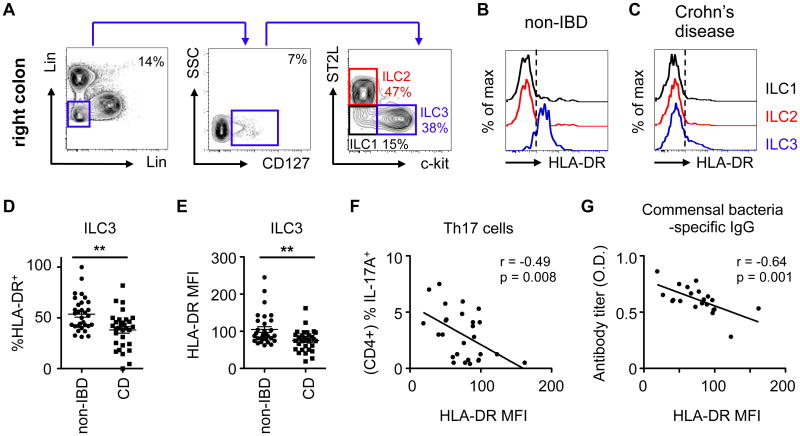
Inflammatory CD4+ T cell responses against commensal bacteria are causally associated with the pathogenesis of IBD (7-11). Furthermore, inflammatory T cells derived from Crohn's disease patients also exhibit reduced Bim-mediated cell death and cytokine-withdrawal-mediated apoptosis (38, 39). Therefore, we next examined whether ILC3-intrinsic MHCII may be dysregulated in the context of human IBD. Using a previously defined gating strategy (40), all ILC subsets could be identified in intestinal biopsies of pediatric Crohn's disease patients (Fig. 4A), including CD127+ c-kit+ ST2L- ILC3s, that expressed NKp44 and RORγt (fig. S12A-B). Human ILC3s expressed MHCII (HLA-DR) in intestinal biopsies from non-IBD controls, whereas MHCII expression was largely absent on other ILC subsets (Fig. 4B). Although no alterations in the total frequency of ILC3s were observed between patient cohorts (fig. S12C), MHCII expression was significantly reduced on ILC3s (Fig. 4C-E), but not CD4+ T cells or professional antigen-presenting cells (fig. S12D-E), from pediatric Crohn's disease patients in comparison to non-IBD controls. Moreover, we observed an inverse correlation between MHCII levels on ILC3s and frequencies of effector T helper 17 (Th17) cells (Fig. 4F), and circulating commensal bacteria-specific immunoglobulin (Ig)G titers (Fig. 4G), in pediatric Crohn's disease patients. Taken together, these data indicate alterations in MHCII on human ILC3s are associated with elevated commensal bacteria-specific inflammatory responses.
Fig. 4. ILC3-intrinsic MHCII is dysregulated in pediatric Crohn's disease patients and is associated with increased intestinal Th17 cells.
A) Lamina propria cells were isolated from colon biopsies from non-IBD control patients and ILCs were identified as CD45+ and lineage (x-axis; CD3, CD5, CD14, FcεRI, y-axis; CD11b CD11c, and CD19) negative, CD127+ and further divided by expression of ST2L (ILC2s; red) and c-kit (ILC3s; blue) or as lacking expression of both markers (ILC1s; black). Expression of MHC class II (HLA-DR) was then determined on ILC subsets in representative biopsies from B) non-IBD patients or C) pediatric Crohn's disease (CD) patients, and the D) frequencies and E) mean fluorescent intensity of HLA-DR expression on ILC3s was quantified. ILC3 HLA-DR MFI was correlated with F) frequencies of IL-17A+ CD4+ Th17 cells in colon biopsies and G) commensal bacterial-specific IgG was quantified in the sera of pediatric Crohn's disease patients. (B-E) Representative of n=31 non-IBD and n=31 CD patients or F) n=27 and G) n=21 pediatric Crohn's disease patients. Results are shown as the mean +/- s.e.m. Statistical analyses between patient groups are performed using a Mann-Whitney test * p < 0.05, ** p < 0.01, *** p < 0.001. Correlative analyses were compared by parametric Pearson's rank correlation coefficient (r).
The mammalian gastrointestinal tract is colonized with trillions of beneficial commensal bacteria that regulate host nutrient metabolism, immune cell homeostasis and protect from pathogen infection (7, 11, 15). As such, commensal bacteria are an essential component of the mammalian “superorganism” required for the host to thrive (41). It is well characterized that self-specific CD4+ T cells with the potential to cause pathologic inflammation in mammalian tissues are controlled through antigen-dependent thymic selection (1-5). Here, MHCII-expressing CCR6+ ILC3s were found to control intestinal homeostasis through induction of apoptotic cell death and deletion of activated commensal bacteria-specific T cells, a process with multiple similarities to negative selection in the thymus, which we propose to call “intestinal selection” (fig. S13). Thus, intestinal selection controls the peripheral commensal bacteria-specific CD4+ T cell pool in concert with other previously described tolerogenic pathways, including Treg, production of IgA and active maintenance of intestinal barrier function (7, 15, 17, 23). Dysregulated ILC3-intrinsic MHCII in pediatric Crohn's disease patients suggests a possible role for alterations in this pathway in the onset and/or progression of human IBD. Thus, MHCII+ ILC3 may represent a novel therapeutic target to control pathologic CD4+ T cell responses in chronic human inflammatory disorders associated with dysregulated host-commensal bacteria relationships (7, 10, 15).
Supplementary Material
fig. S1. MHCII expression of mLN and cLPL group 3 ILCs is independent of IL-23, Ahr and the intestinal microbiota. A) Colonic lamina propria cells from naïve mice were gated as CD45+ and lineage (x-axis; CD3, CD5, CD8, NK1.1, y-axis; B220, CD11c, CD11b) negative and CD127+ and further divided by expression of ST2 (ILC2s; red) or CCR6 (ILC3s; blue) and expression of B) RORγteGFP or C) MHC class II was determined on mLN and cLPL ILC3s. Expression of MHCII on ILC2s (red) and ILC3s (blue) in mice deficient in D) IL-23p19 E) Aryl hydrocarbon receptor (Ahr) or F) intestinal microbiota from the mLN (top panel) and cLPL (bottom panel). Gate shows frequency of MHCII expression amongst gated ILC3s (blue). All data representative of at least 3 independent experiments with at least n=3 mice per group. SPF, specific pathogen free. Results are shown as the mean +/- s.e.m.
fig. S2. MHCII and co-stimulatory molecule expression of mLN ILC3s is unaffected by TLR ligands or pro-inflammatory cytokines. A) Mean fluorescent intensity of ILC3 MHCII, CD80 and CD86 from mLN or cLPL cells stimulated with media alone, TLR ligands (LPS, Poly I:C) or pro-inflammatory cytokines (IL-23, IL-1β, IFN-γ). B) Frequency of MHCII+ (Lin- CD127+ CCR6+) ILC3s in the mLN (top panel) and cLPL (bottom panel) of Capase 1/11-/- and MyD88-/- mice. C) Representative histograms depicting expression of MHCII, CD80 and CD86 on WT C57BL/6 DCs (black line), WT ILC3s (blue line) or Capase 1/11-/- ILC3s (red line) in the mLN (top panel) or cLPL (bottom panel). All data representative of at least 3 independent experiments with 3-4 mice per group or 3 biological replicates. Results are shown as the mean +/- s.e.m.
fig. S3. CIITA transcriptional control of MHCII expression on B cells, DCs and TECs and IFN-γ dependence of MHCII expression in colonic ILC3s. Expression of MHCII was determined on B220+ CD11c- B cells or CD11b+ CD11chi DCs from the mLN or CD45- EpCAM+ Ly51-/low mTECs or CD45- EpCAM+ Ly51+ cTECs from the thymus of mice deficient in A) CIITA and B) CIITA-specific promoters (pIII/pIV, pIV). MHCII expression on C) mLN CCR6+ ILC3s from mice deficient CIITA in promoter regions (pIII/pIV, pIV) D) cLPL CCR6+ ILC3s from IFN-γ or IFN-γR1-deficient mice and E) mLN and cLPL CCR6+ ILC3s from STAT-1 deficient mice. All data representative of at least 3 independent experiments with n=2-3 mice per group. Results are shown as the mean +/- s.e.m.
fig. S4. ILC3-intrinsic MHCII selectively controls commensal bacteria-specific CD4+ T effector cells in the intestine. A) Relative frequencies and B) total cell numbers of naïve (CD44lo), Teff (CD44hi) and Treg (FoxP3+) CD4+ T cells in the colonic lamina propria of MHCIIΔILC3 mice or H2-Ab1fl/fl littermate controls. C) Analysis of the frequencies of naïve (grey), Teff (blue) and Treg (green) amongst CD4+ T cells expressing commonly utilized TCR Vβ chains in the thymus and colonic lamina propria of MHCIIΔILC3 mice or H2-Ab1fl/fl littermate controls. D) Frequency of proliferating cells (CFSEdim) in CD4+ T cells derived from MHCIIΔILC3 mice or H2-Ab1fl/fl littermate controls and stimulated with fecal and tissue-derived homogenate antigens in vitro for 72 h. All data representative of at least 2 independent experiments with 3 biological replicates or n=3 mice per group. Results are shown as the mean +/- s.e.m. Data was analyzed by student's t-test (B) or one-way ANOVA (D). ** p≤0.01 and *** p≤0.001, ˆˆˆ indicates p≤0.001 for H2-Ab1fl/fl comparisons versus matched media control.
fig. S5. ILC3-intrinsic MHCII selectively controls CBir1 CD4+ T effector cells in the intestine. OT-II or CBir1 TCR transgenic mice were crossed with either MHCIIΔILC3 mice or H2-Ab1fl/fl littermate controls and total Vβ5+ (OT-II) or Vβ8.3+ (CBir1) CD4+ T cell numbers were determined. A) CBir1 CD4+ T cell numbers in the mLN of conventional or ABX-treated CBir1 transgenic mice crossed with either MHCIIΔILC3 mice or H2-Ab1fl/fl littermate controls. B-C) Frequencies of IFN-γ+ and/or TNF-α+ T cells following stimulation with cognate antigen, OVA peptide (OT-II) or CBir1 peptide (CBir1), for 5 h in the presence of Brefeldin A. D) Frequencies of CD45+ CD3- B220- Ly6C+ Ly6G+ neutrophils in the cLPL of Rag1-/- MHCIIΔILC3 mice or Rag1-/- H2-Ab1fl/fl littermate controls. E) Number of CD4+ Teff or Treg in the colonic lamina propria of CBir1 transgenic mice crossed with either MHCIIΔILC3 mice or H2-Ab1fl/fl littermate controls. All data representative of at least 3 independent experiments with n=2-3 mice per group. Results are shown as the mean +/- s.e.m. * p < 0.05, ** p < 0.01, *** p < 0.001 (two-tailed students t-test).
fig. S6. ILC3-restricted MHCII expression is not sufficient to induce proliferation, activation or Treg differentiation of naïve CBir1 CD4+ T cells, but induces antigen-specific deletion of activated T cells in vivo. A) MHCIIpos, MHCIIneg and MHCIIILC3+ mice received sort-purified naive CFSE-labeled CD45.1+ CBir1 CD4+ T cells and were injected with CBir1 peptide i.p. and analyzed for proliferation (CFSE dilution; upper panel) and frequencies of CD4+ CD45.1+ CD44hiCD62Llo effector T cells (Teff; middle panel) or CD4+ CD45.1+ FoxP3+ regulatory T cells (Treg; lower panel) in the mLN. B) Frequencies and C) numbers of activated congenic CD90.1+ OT-II and CD45.1+ Cbir1 T cells transferred at a 1:1 ratio in the mLN and cLPL of recipient MHCIIneg or MHCIIILC3+ mice which received CBir1 peptide. D) Cell numbers of transferred CBir1 T cells in the spleen, mLN and cLPL of recipient MHCIIneg or MHCIIILC3+ mice one month post-transfer in the absence of exogenously administered CBir1 peptide. All data representative of at least 3 independent experiments with n=2-3 mice per group. Results are shown as the mean +/- s.e.m.
fig. S7. CD11c+ DC-restricted expression of MHCII drives expansion of commensal bacteria-specific Teff and Treg. A) Expression of MHCII on B220+ B cells or CD11c+ DCs in the mLN of CD11c transgenic (CD11cTg) mice (blue line) and MHCII-/- mice (grey fill). B) Numbers of total CD45.1+ CBir1 CD4+ T cells, CD44+ CD62Llo (Teff) CBir1 CD4+ T cells and FoxP3+ CBir1 CD4+ Treg cells in the mLN (top panel) and cLPL (bottom panel) of CD11cTg mice or MHCII-/- mice. All data representative of 2 independent experiments with n=3 mice per group. Results are shown as the mean +/- s.e.m. * p < 0.05, ** p < 0.01 (two-tailed students t-test).
fig. S8. ILC3-intrinsic deletion of MHCII results in selective expansion of CD4+ CBir1 effector T cells in the intestine and associated lymphoid tissue. (A-C) MHCIIΔILC3 mice and H2-Ab1fl/fl littermate controls received CD45.1+ CBir1 Tg CD4+ T cells that had been sort-purified following pre-activation and were injected with CBir1 peptide i.p. on day 0 and day 1 post transfer. A) Frequencies, B) numbers and C) proliferation (Ki-67) of transferred CBir1 T cells were quantified 0-6 days following antigen administration in the mLN and cLPL. (D-E) MHCIIΔILC3 mice and H2-Ab1fl/fl littermate controls received CD45.1+ CBir1 Tg CD4+ T cells that had been sort-purified following pre-activation and were injected with CBir1 peptide i.p. every 2 days post transfer. Frequencies and numbers of total transferred CD4+ CD45.1+ T cells, CD4+ CD45.1+ CD44hi CD62Llo effector T cells (Teff) or CD4+ CD45.1+ FoxP3+ regulatory T cells (Treg) were analyzed in D) the colonic lamina propria (cLPL) and E) the mLN. All data representative of at least 3 independent experiments with n=2-3 mice per group. N/D = not detected. Results are shown as the mean +/- s.e.m. * p < 0.05 (two-tailed students t-test).
fig. S9. MHCII+ ILC3 control of commensal bacteria-specific CD4+ T cells is not mediated via altered homing or suppression of proliferation. A) Frequencies of adoptively transferred activated CD45.1+ CBir1 and CD90.1+ OT-II CD4+ T cells in the spleen of MHCIIneg and MHCIIILC3+ mice administered CBir1 antigen at day 9 post transfer. B) CFSE dilution and Ki-67 expression of transferred activated CD45.1+ CBir1 CD4+ T cells in the mLN of MHCIIneg (grey fill) or MHCIIILC3+ mice (blue line) at day 9 post transfer. Data are representative of at least 2 independent experiments with n=3 mice per group.
fig. S10. MHCII+ ILC3s may also control expansion of non-commensal bacteria-specific T cells upon systemic administration of exogenous cognate antigen. A) Frequencies of congenic CD90.1+ OT-II cells in the mLN of recipient mice that received OVA peptide i.p. every 2 days following transfer. B) Total numbers of OT-II T cells in the mLN, siLPL and cLPL of recipient mice. C) Relative cell recovery (%) and frequency of Annexin V+ cells during in vitro co-culture of OT-II T cells and antigen-pulsed ILC3s in the presence or absence of an anti-MHCII neutralizing antibody. Data are representative of 2 independent experiments with 3 mice per group (A-B) or 2 independent experiments with 2-3 biological replicates per group (C). Results are shown as the mean +/- s.e.m. ** p < 0.01 (two-tailed students t-test).
fig. S11. MHCII+ ILC3s induce apoptotic cell death in CBir1 CD4+ T cells. A) Activated CBir1 CD4+ T cells were co-cultured with ILC3s in the presence or absence of CBir1 antigen and relative cell recovery (%) of either total CD45.1+ CBir1 CD4+ T cells or FoxP3+ CBir1 Treg cells were quantified after 48 hours. B) Expression of FasL by CCR6+ ILC3s and C) relative cell recovery (%) and D) frequency of Annexin V+ cells during in vitro co-culture of CBir1 T cells and ILC3s in the presence or absence of anti- FasL neutralizing antibody. E) mLN, PP, siLPL and cLPL lymphocytes (GALT) were pooled and labeled with an SFB-specific MHCII tetramer and frequencies of SFBspecific CD4+ T cells quantified in C57BL/6, Fasgld/gld or Bim-/- mice. F) Representative histograms demonstrating IL-2 binding capacity and G) quantification of bound IL-2 MFI of naïve CBir1 CD4+ T cells (grey), antigen-activated CBir1 CD4+ T cells (black) and CCR6+ MHCII+ ILC3s (blue). H) Expression of Bim by wild type CBir1 CD4+ T cells or CBir1 CD4+ T cells with a constitutively active STAT-5 signaling molecule following co-culture with Ag-pulsed MHCII+ ILC3. I) Wild type CBir1 CD4+ T cells or CBir1 STAT5-CA CD4+ T cells were adoptively transferred into MHCIIneg or MHCIIILC3+ mice. Mice were administered CBir1 antigen and numbers of FoxP3- CD45.1+ CBir1 T cells in the mLN were quantified 9 days post transfer. In vitro data representative of at least 2 independent experiments with 2-3 biological replicates per culture condition, in vivo data representative of at least 2 independent experiments with 3 mice per group. Results are shown as the mean +/- s.e.m. ** p < 0.01 (two-tailed students t-test).
fig. S12. ILC3 phenotype and frequencies in the intestine of non-IBD and pediatric Crohn's disease patients. ILC subsets as gated in Fig. 4A were analyzed for expression of the human ILC3 markers A) NKp44 and B) RORγt. C) Frequencies of c-kit+ ST2L- ILC3s within the Linneg CD127+ ILC gate were analyzed in right colon biopsies from pediatric Crohn's disease (CD) and non-IBD controls. (D-E) HLA-DR expression (MFI) was quantified on D) CD4+ T cells or E) CD19+ CD11c+ APCs in the same cohort of non-IBD or Crohn's disease patients as depicted in Figure 4. Results are shown as the mean +/- s.e.m. Statistical analyses between patient groups are performed using a Mann- Whitney test * p < 0.05, ** p < 0.01, *** p < 0.001.
fig. S13. Intestinal selection of commensal bacteria-specific CD4+ T cells. Thymocytes with specificity for either self or commensal bacteria undergo positive selection through interactions with cortical thymic epithelial cells (cTECs), subsequently differentiate into mature CD4+ T cells and migrate into the thymic medulla. Once in the medulla self-specific CD4+ T cells are subject to negative selection through interactions with MHCII+ medullary thymic epithelial cells (mTECs) and dendritic cells presenting self-antigen. This process of negative selection is mediated by programmed cell death, associated with Nur77 signaling and Bim-dependent apoptosis, which results in the deletion of self antigen-specific CD4+ T cells prior to entering the periphery. In contrast, CD4+ T cells specific for commensal bacteria fail to undergo negative selection due to the absence of commensal bacteria-derived antigen in the thymus. Rather, mature commensal bacteria-specific CD4+ T cells enter the peripheral circulation where they may encounter professional antigen-presenting cells, such as dendritic cells, presenting commensal bacteria-derived antigen via MHCII. Antigen-experienced Teff then migrate to distinct niches within the lymph node, which are enriched for MHCII+ ILC3s, resulting in the deletion of activated commensal bacteria-specific Teff cells from the peripheral pool. Commensal bacteria-specific T cells receive TCR signals from ILC3 MHCII-Ag complexes in the absence of co-stimulation, which is associated with upregulation of the signaling molecule Nur77. In addition, ILC3s efficiently consume growth factor cytokines, in particular IL-2, resulting in T cell cytokine withdrawal. The net effect of both TCR signaling and cytokine withdrawal results in upregulation of the pro-apoptotic Bim and induction of Caspase-3 associated apoptotic cell death, promoting deletion of activated commensal bacteria-specific T cells in a process we term “intestinal selection”, which parallels thymic selection. In contrast, commensal bacteria-specific CD4+ T regulatory cells (Treg) are not subject to ILC3-mediated deletion, potentially due to their ability to compete for IL-2, and migrate to the intestine whereby they further maintain tissue homeostasis in the presence of the commensal microflora.
Acknowledgments
Members of the Sonnenberg laboratory are thanked for discussions and critical reading of the manuscript. The authors thank C Hunter and S Wagage (University of Pennsylvania) for the Ahr-deficient mice, I Brodsky (University of Pennsylvania) for the Caspase 1/11-deficient mice, and M Jenkins, J Walter and T Dileepan (University of Minnesota) for tetramer reagents and protocols. Data presented in this manuscript are tabulated in the main paper and in the supplementary materials. Microarray data is accessible at GEO (http://www.ncbi.nlm.nih.gov/geo/) via accession number GSE67076. We thank the University of Alabama, Institut Pasteur, University of Minnesota and Janssen Research and Development LLC for sharing mouse strains by material transfer agreement. Research in the Sonnenberg laboratory is supported by the National Institutes of Health (DP5OD012116), the NIAID Mucosal Immunology Studies Team (MIST) Scholar Award in Mucosal Immunity and the Institute for Translational Medicine and Therapeutics Transdisciplinary Program in Translational Medicine and Therapeutics (UL1-RR024134 from the US National Center for Research Resources). MRH is supported by a research fellowship from the Crohn's and Colitis Foundation of America (CCFA, #297365). TCF is supported by a Cancer Research Institute Student Training and Research in Tumor immunology (STaRT) grant. DRW is supported by a Wellcome Trust Research Career Development Fellowship. COE is supported by the National Institutes of Health (DK071176).
References and Notes
- 1.Klein L, Kyewski B, Allen PM, Hogquist KA. Positive and negative selection of the T cell repertoire: what thymocytes see (and don't see) Nat Rev Immunol. 2014;14:377–391. doi: 10.1038/nri3667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.von Boehmer H, Melchers F. Checkpoints in lymphocyte development and autoimmune disease. Nat Immunol. 2010;11:14–20. doi: 10.1038/ni.1794. [DOI] [PubMed] [Google Scholar]
- 3.Laufer TM, Glimcher LH, Lo D. Using thymus anatomy to dissect T cell repertoire selection. Semin Immunol. 1999;11:65–70. doi: 10.1006/smim.1998.9997. [DOI] [PubMed] [Google Scholar]
- 4.Mathis D, Benoist C. Aire. Annu Rev Immunol. 2009;27:287–312. doi: 10.1146/annurev.immunol.25.022106.141532. [DOI] [PubMed] [Google Scholar]
- 5.Sprent J, Kishimoto H. The thymus and negative selection. Immunol Rev. 2002;185:126–135. doi: 10.1034/j.1600-065x.2002.18512.x. [DOI] [PubMed] [Google Scholar]
- 6.Blackman M, Kappler J, Marrack P. The role of the T cell receptor in positive and negative selection of developing T cells. Science (New York, N Y. 1990;248:1335–1341. doi: 10.1126/science.1972592. [DOI] [PubMed] [Google Scholar]
- 7.Belkaid Y, Hand TW. Role of the microbiota in immunity and inflammation. Cell. 2014;157:121–141. doi: 10.1016/j.cell.2014.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaser A, Zeissig S, Blumberg RS. Inflammatory bowel disease. Annu Rev Immunol. 2010;28:573–621. doi: 10.1146/annurev-immunol-030409-101225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khor B, Gardet A, Xavier RJ. Genetics and pathogenesis of inflammatory bowel disease. Nature. 2011;474:307–317. doi: 10.1038/nature10209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maloy KJ, Powrie F. Intestinal homeostasis and its breakdown in inflammatory bowel disease. Nature. 2011;474:298–306. doi: 10.1038/nature10208. [DOI] [PubMed] [Google Scholar]
- 11.Maynard CL, Elson CO, Hatton RD, Weaver CT. Reciprocal interactions of the intestinal microbiota and immune system. Nature. 2012;489:231–241. doi: 10.1038/nature11551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ai TL, Solomon BD, Hsieh CS. T-cell selection and intestinal homeostasis. Immunol Rev. 2014;259:60–74. doi: 10.1111/imr.12171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cong Y, Feng T, Fujihashi K, Schoeb TR, Elson CO. A dominant, coordinated T regulatory cell-IgA response to the intestinal microbiota. Proc Natl Acad Sci U S A. 2009;106:19256–19261. doi: 10.1073/pnas.0812681106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Macpherson AJ, Slack E, Geuking MB, McCoy KD. The mucosal firewalls against commensal intestinal microbes. Seminars in immunopathology. 2009;31:145–149. doi: 10.1007/s00281-009-0174-3. [DOI] [PubMed] [Google Scholar]
- 15.Hooper LV, Littman DR, Macpherson AJ. Interactions between the microbiota and the immune system. Science (New York, N Y. 2012;336:1268–1273. doi: 10.1126/science.1223490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bollrath J, Powrie FM. Controlling the frontier: regulatory T-cells and intestinal homeostasis. Semin Immunol. 2013;25:352–357. doi: 10.1016/j.smim.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 17.Peterson LW, Artis D. Intestinal epithelial cells: regulators of barrier function and immune homeostasis. Nat Rev Immunol. 2014;14:141–153. doi: 10.1038/nri3608. [DOI] [PubMed] [Google Scholar]
- 18.Macpherson AJ, Uhr T. Induction of protective IgA by intestinal dendritic cells carrying commensal bacteria. Science (New York, N Y. 2004;303:1662–1665. doi: 10.1126/science.1091334. [DOI] [PubMed] [Google Scholar]
- 19.Rescigno M. Intestinal dendritic cells. Adv Immunol. 2010;107:109–138. doi: 10.1016/B978-0-12-381300-8.00004-6. [DOI] [PubMed] [Google Scholar]
- 20.Lathrop SK, et al. Peripheral education of the immune system by colonic commensal microbiota. Nature. 2012;478:250–254. doi: 10.1038/nature10434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cebula A, et al. Thymus-derived regulatory T cells contribute to tolerance to commensal microbiota. Nature. 2013;497:258–262. doi: 10.1038/nature12079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hepworth MR, et al. Innate lymphoid cells regulate CD4+ T-cell responses to intestinal commensal bacteria. Nature. 2013;498:113–117. doi: 10.1038/nature12240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sonnenberg GF, Artis D. Innate lymphoid cell interactions with microbiota: implications for intestinal health and disease. Immunity. 2012;37:601–610. doi: 10.1016/j.immuni.2012.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Spits H, et al. Innate lymphoid cells--a proposal for uniform nomenclature. Nat Rev Immunol. 2013;13:145–149. doi: 10.1038/nri3365. [DOI] [PubMed] [Google Scholar]
- 25.Kiss EA, et al. Natural aryl hydrocarbon receptor ligands control organogenesis of intestinal lymphoid follicles. Science (New York, N Y. 2011;334:1561–1565. doi: 10.1126/science.1214914. [DOI] [PubMed] [Google Scholar]
- 26.von Burg N, et al. Activated group 3 innate lymphoid cells promote T-cell-mediated immune responses. Proc Natl Acad Sci U S A. 2014;111:12835–12840. doi: 10.1073/pnas.1406908111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reith W, Leibund Gut-Landmann S, Waldburger JM. Regulation of MHC class II gene expression by the class II transactivator. Nat Rev Immunol. 2005;5:793–806. doi: 10.1038/nri1708. [DOI] [PubMed] [Google Scholar]
- 28.Waldburger JM, et al. Promoter IV of the class II transactivator gene is essential for positive selection of CD4+ T cells. Blood. 2003;101:3550–3559. doi: 10.1182/blood-2002-06-1855. [DOI] [PubMed] [Google Scholar]
- 29.Waldburger JM, Suter T, Fontana A, Acha-Orbea H, Reith W. Selective abrogation of major histocompatibility complex class II expression on extrahematopoietic cells in mice lacking promoter IV of the class II transactivator gene. J Exp Med. 2001;194:393–406. doi: 10.1084/jem.194.4.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goto Y, et al. Segmented filamentous bacteria antigens presented by intestinal dendritic cells drive mucosal Th17 cell differentiation. Immunity. 2014;40:594–607. doi: 10.1016/j.immuni.2014.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang Y, et al. Focused specificity of intestinal Th17 cells towards commensal bacterial antigens. Nature. 2014 doi: 10.1038/nature13279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lodes MJ, et al. Bacterial flagellin is a dominant antigen in Crohn disease. The Journal of clinical investigation. 2004;113:1296–1306. doi: 10.1172/JCI20295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mackley EC, et al. CCR7-dependent trafficking of RORgamma(+) ILCs creates a unique microenvironment within mucosal draining lymph nodes. Nature communications. 2015;6:5862. doi: 10.1038/ncomms6862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Archambault AS, et al. Cutting edge: Conditional MHC class II expression reveals a limited role for B cell antigen presentation in primary and secondary CD4 T cell responses. J Immunol. 2013;191:545–550. doi: 10.4049/jimmunol.1201598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stritesky GL, et al. Murine thymic selection quantified using a unique method to capture deleted T cells. Proc Natl Acad Sci U S A. 2014;110:4679–4684. doi: 10.1073/pnas.1217532110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pandiyan P, Zheng L, Ishihara S, Reed J, Lenardo MJ. CD4+CD25+Foxp3+ regulatory T cells induce cytokine deprivation-mediated apoptosis of effector CD4+ T cells. Nat Immunol. 2007;8:1353–1362. doi: 10.1038/ni1536. [DOI] [PubMed] [Google Scholar]
- 37.Bouillet P, et al. Proapoptotic Bcl-2 relative Bim required for certain apoptotic responses, leukocyte homeostasis, and to preclude autoimmunity. Science (New York, N Y. 1999;286:1735–1738. doi: 10.1126/science.286.5445.1735. [DOI] [PubMed] [Google Scholar]
- 38.Mudter J, Neurath MF. Apoptosis of T cells and the control of inflammatory bowel disease: therapeutic implications. Gut. 2007;56:293–303. doi: 10.1136/gut.2005.090464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Neurath MF, et al. Regulation of T-cell apoptosis in inflammatory bowel disease: to die or not to die, that is the mucosal question. Trends in immunology. 2001;22:21–26. doi: 10.1016/s1471-4906(00)01798-1. [DOI] [PubMed] [Google Scholar]
- 40.Bernink JH, et al. Human type 1 innate lymphoid cells accumulate in inflamed mucosal tissues. Nat Immunol. 2013;14:221–229. doi: 10.1038/ni.2534. [DOI] [PubMed] [Google Scholar]
- 41.Eberl G. A new vision of immunity: homeostasis of the superorganism. Mucosal Immunol. 2010;3:450–460. doi: 10.1038/mi.2010.20. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
fig. S1. MHCII expression of mLN and cLPL group 3 ILCs is independent of IL-23, Ahr and the intestinal microbiota. A) Colonic lamina propria cells from naïve mice were gated as CD45+ and lineage (x-axis; CD3, CD5, CD8, NK1.1, y-axis; B220, CD11c, CD11b) negative and CD127+ and further divided by expression of ST2 (ILC2s; red) or CCR6 (ILC3s; blue) and expression of B) RORγteGFP or C) MHC class II was determined on mLN and cLPL ILC3s. Expression of MHCII on ILC2s (red) and ILC3s (blue) in mice deficient in D) IL-23p19 E) Aryl hydrocarbon receptor (Ahr) or F) intestinal microbiota from the mLN (top panel) and cLPL (bottom panel). Gate shows frequency of MHCII expression amongst gated ILC3s (blue). All data representative of at least 3 independent experiments with at least n=3 mice per group. SPF, specific pathogen free. Results are shown as the mean +/- s.e.m.
fig. S2. MHCII and co-stimulatory molecule expression of mLN ILC3s is unaffected by TLR ligands or pro-inflammatory cytokines. A) Mean fluorescent intensity of ILC3 MHCII, CD80 and CD86 from mLN or cLPL cells stimulated with media alone, TLR ligands (LPS, Poly I:C) or pro-inflammatory cytokines (IL-23, IL-1β, IFN-γ). B) Frequency of MHCII+ (Lin- CD127+ CCR6+) ILC3s in the mLN (top panel) and cLPL (bottom panel) of Capase 1/11-/- and MyD88-/- mice. C) Representative histograms depicting expression of MHCII, CD80 and CD86 on WT C57BL/6 DCs (black line), WT ILC3s (blue line) or Capase 1/11-/- ILC3s (red line) in the mLN (top panel) or cLPL (bottom panel). All data representative of at least 3 independent experiments with 3-4 mice per group or 3 biological replicates. Results are shown as the mean +/- s.e.m.
fig. S3. CIITA transcriptional control of MHCII expression on B cells, DCs and TECs and IFN-γ dependence of MHCII expression in colonic ILC3s. Expression of MHCII was determined on B220+ CD11c- B cells or CD11b+ CD11chi DCs from the mLN or CD45- EpCAM+ Ly51-/low mTECs or CD45- EpCAM+ Ly51+ cTECs from the thymus of mice deficient in A) CIITA and B) CIITA-specific promoters (pIII/pIV, pIV). MHCII expression on C) mLN CCR6+ ILC3s from mice deficient CIITA in promoter regions (pIII/pIV, pIV) D) cLPL CCR6+ ILC3s from IFN-γ or IFN-γR1-deficient mice and E) mLN and cLPL CCR6+ ILC3s from STAT-1 deficient mice. All data representative of at least 3 independent experiments with n=2-3 mice per group. Results are shown as the mean +/- s.e.m.
fig. S4. ILC3-intrinsic MHCII selectively controls commensal bacteria-specific CD4+ T effector cells in the intestine. A) Relative frequencies and B) total cell numbers of naïve (CD44lo), Teff (CD44hi) and Treg (FoxP3+) CD4+ T cells in the colonic lamina propria of MHCIIΔILC3 mice or H2-Ab1fl/fl littermate controls. C) Analysis of the frequencies of naïve (grey), Teff (blue) and Treg (green) amongst CD4+ T cells expressing commonly utilized TCR Vβ chains in the thymus and colonic lamina propria of MHCIIΔILC3 mice or H2-Ab1fl/fl littermate controls. D) Frequency of proliferating cells (CFSEdim) in CD4+ T cells derived from MHCIIΔILC3 mice or H2-Ab1fl/fl littermate controls and stimulated with fecal and tissue-derived homogenate antigens in vitro for 72 h. All data representative of at least 2 independent experiments with 3 biological replicates or n=3 mice per group. Results are shown as the mean +/- s.e.m. Data was analyzed by student's t-test (B) or one-way ANOVA (D). ** p≤0.01 and *** p≤0.001, ˆˆˆ indicates p≤0.001 for H2-Ab1fl/fl comparisons versus matched media control.
fig. S5. ILC3-intrinsic MHCII selectively controls CBir1 CD4+ T effector cells in the intestine. OT-II or CBir1 TCR transgenic mice were crossed with either MHCIIΔILC3 mice or H2-Ab1fl/fl littermate controls and total Vβ5+ (OT-II) or Vβ8.3+ (CBir1) CD4+ T cell numbers were determined. A) CBir1 CD4+ T cell numbers in the mLN of conventional or ABX-treated CBir1 transgenic mice crossed with either MHCIIΔILC3 mice or H2-Ab1fl/fl littermate controls. B-C) Frequencies of IFN-γ+ and/or TNF-α+ T cells following stimulation with cognate antigen, OVA peptide (OT-II) or CBir1 peptide (CBir1), for 5 h in the presence of Brefeldin A. D) Frequencies of CD45+ CD3- B220- Ly6C+ Ly6G+ neutrophils in the cLPL of Rag1-/- MHCIIΔILC3 mice or Rag1-/- H2-Ab1fl/fl littermate controls. E) Number of CD4+ Teff or Treg in the colonic lamina propria of CBir1 transgenic mice crossed with either MHCIIΔILC3 mice or H2-Ab1fl/fl littermate controls. All data representative of at least 3 independent experiments with n=2-3 mice per group. Results are shown as the mean +/- s.e.m. * p < 0.05, ** p < 0.01, *** p < 0.001 (two-tailed students t-test).
fig. S6. ILC3-restricted MHCII expression is not sufficient to induce proliferation, activation or Treg differentiation of naïve CBir1 CD4+ T cells, but induces antigen-specific deletion of activated T cells in vivo. A) MHCIIpos, MHCIIneg and MHCIIILC3+ mice received sort-purified naive CFSE-labeled CD45.1+ CBir1 CD4+ T cells and were injected with CBir1 peptide i.p. and analyzed for proliferation (CFSE dilution; upper panel) and frequencies of CD4+ CD45.1+ CD44hiCD62Llo effector T cells (Teff; middle panel) or CD4+ CD45.1+ FoxP3+ regulatory T cells (Treg; lower panel) in the mLN. B) Frequencies and C) numbers of activated congenic CD90.1+ OT-II and CD45.1+ Cbir1 T cells transferred at a 1:1 ratio in the mLN and cLPL of recipient MHCIIneg or MHCIIILC3+ mice which received CBir1 peptide. D) Cell numbers of transferred CBir1 T cells in the spleen, mLN and cLPL of recipient MHCIIneg or MHCIIILC3+ mice one month post-transfer in the absence of exogenously administered CBir1 peptide. All data representative of at least 3 independent experiments with n=2-3 mice per group. Results are shown as the mean +/- s.e.m.
fig. S7. CD11c+ DC-restricted expression of MHCII drives expansion of commensal bacteria-specific Teff and Treg. A) Expression of MHCII on B220+ B cells or CD11c+ DCs in the mLN of CD11c transgenic (CD11cTg) mice (blue line) and MHCII-/- mice (grey fill). B) Numbers of total CD45.1+ CBir1 CD4+ T cells, CD44+ CD62Llo (Teff) CBir1 CD4+ T cells and FoxP3+ CBir1 CD4+ Treg cells in the mLN (top panel) and cLPL (bottom panel) of CD11cTg mice or MHCII-/- mice. All data representative of 2 independent experiments with n=3 mice per group. Results are shown as the mean +/- s.e.m. * p < 0.05, ** p < 0.01 (two-tailed students t-test).
fig. S8. ILC3-intrinsic deletion of MHCII results in selective expansion of CD4+ CBir1 effector T cells in the intestine and associated lymphoid tissue. (A-C) MHCIIΔILC3 mice and H2-Ab1fl/fl littermate controls received CD45.1+ CBir1 Tg CD4+ T cells that had been sort-purified following pre-activation and were injected with CBir1 peptide i.p. on day 0 and day 1 post transfer. A) Frequencies, B) numbers and C) proliferation (Ki-67) of transferred CBir1 T cells were quantified 0-6 days following antigen administration in the mLN and cLPL. (D-E) MHCIIΔILC3 mice and H2-Ab1fl/fl littermate controls received CD45.1+ CBir1 Tg CD4+ T cells that had been sort-purified following pre-activation and were injected with CBir1 peptide i.p. every 2 days post transfer. Frequencies and numbers of total transferred CD4+ CD45.1+ T cells, CD4+ CD45.1+ CD44hi CD62Llo effector T cells (Teff) or CD4+ CD45.1+ FoxP3+ regulatory T cells (Treg) were analyzed in D) the colonic lamina propria (cLPL) and E) the mLN. All data representative of at least 3 independent experiments with n=2-3 mice per group. N/D = not detected. Results are shown as the mean +/- s.e.m. * p < 0.05 (two-tailed students t-test).
fig. S9. MHCII+ ILC3 control of commensal bacteria-specific CD4+ T cells is not mediated via altered homing or suppression of proliferation. A) Frequencies of adoptively transferred activated CD45.1+ CBir1 and CD90.1+ OT-II CD4+ T cells in the spleen of MHCIIneg and MHCIIILC3+ mice administered CBir1 antigen at day 9 post transfer. B) CFSE dilution and Ki-67 expression of transferred activated CD45.1+ CBir1 CD4+ T cells in the mLN of MHCIIneg (grey fill) or MHCIIILC3+ mice (blue line) at day 9 post transfer. Data are representative of at least 2 independent experiments with n=3 mice per group.
fig. S10. MHCII+ ILC3s may also control expansion of non-commensal bacteria-specific T cells upon systemic administration of exogenous cognate antigen. A) Frequencies of congenic CD90.1+ OT-II cells in the mLN of recipient mice that received OVA peptide i.p. every 2 days following transfer. B) Total numbers of OT-II T cells in the mLN, siLPL and cLPL of recipient mice. C) Relative cell recovery (%) and frequency of Annexin V+ cells during in vitro co-culture of OT-II T cells and antigen-pulsed ILC3s in the presence or absence of an anti-MHCII neutralizing antibody. Data are representative of 2 independent experiments with 3 mice per group (A-B) or 2 independent experiments with 2-3 biological replicates per group (C). Results are shown as the mean +/- s.e.m. ** p < 0.01 (two-tailed students t-test).
fig. S11. MHCII+ ILC3s induce apoptotic cell death in CBir1 CD4+ T cells. A) Activated CBir1 CD4+ T cells were co-cultured with ILC3s in the presence or absence of CBir1 antigen and relative cell recovery (%) of either total CD45.1+ CBir1 CD4+ T cells or FoxP3+ CBir1 Treg cells were quantified after 48 hours. B) Expression of FasL by CCR6+ ILC3s and C) relative cell recovery (%) and D) frequency of Annexin V+ cells during in vitro co-culture of CBir1 T cells and ILC3s in the presence or absence of anti- FasL neutralizing antibody. E) mLN, PP, siLPL and cLPL lymphocytes (GALT) were pooled and labeled with an SFB-specific MHCII tetramer and frequencies of SFBspecific CD4+ T cells quantified in C57BL/6, Fasgld/gld or Bim-/- mice. F) Representative histograms demonstrating IL-2 binding capacity and G) quantification of bound IL-2 MFI of naïve CBir1 CD4+ T cells (grey), antigen-activated CBir1 CD4+ T cells (black) and CCR6+ MHCII+ ILC3s (blue). H) Expression of Bim by wild type CBir1 CD4+ T cells or CBir1 CD4+ T cells with a constitutively active STAT-5 signaling molecule following co-culture with Ag-pulsed MHCII+ ILC3. I) Wild type CBir1 CD4+ T cells or CBir1 STAT5-CA CD4+ T cells were adoptively transferred into MHCIIneg or MHCIIILC3+ mice. Mice were administered CBir1 antigen and numbers of FoxP3- CD45.1+ CBir1 T cells in the mLN were quantified 9 days post transfer. In vitro data representative of at least 2 independent experiments with 2-3 biological replicates per culture condition, in vivo data representative of at least 2 independent experiments with 3 mice per group. Results are shown as the mean +/- s.e.m. ** p < 0.01 (two-tailed students t-test).
fig. S12. ILC3 phenotype and frequencies in the intestine of non-IBD and pediatric Crohn's disease patients. ILC subsets as gated in Fig. 4A were analyzed for expression of the human ILC3 markers A) NKp44 and B) RORγt. C) Frequencies of c-kit+ ST2L- ILC3s within the Linneg CD127+ ILC gate were analyzed in right colon biopsies from pediatric Crohn's disease (CD) and non-IBD controls. (D-E) HLA-DR expression (MFI) was quantified on D) CD4+ T cells or E) CD19+ CD11c+ APCs in the same cohort of non-IBD or Crohn's disease patients as depicted in Figure 4. Results are shown as the mean +/- s.e.m. Statistical analyses between patient groups are performed using a Mann- Whitney test * p < 0.05, ** p < 0.01, *** p < 0.001.
fig. S13. Intestinal selection of commensal bacteria-specific CD4+ T cells. Thymocytes with specificity for either self or commensal bacteria undergo positive selection through interactions with cortical thymic epithelial cells (cTECs), subsequently differentiate into mature CD4+ T cells and migrate into the thymic medulla. Once in the medulla self-specific CD4+ T cells are subject to negative selection through interactions with MHCII+ medullary thymic epithelial cells (mTECs) and dendritic cells presenting self-antigen. This process of negative selection is mediated by programmed cell death, associated with Nur77 signaling and Bim-dependent apoptosis, which results in the deletion of self antigen-specific CD4+ T cells prior to entering the periphery. In contrast, CD4+ T cells specific for commensal bacteria fail to undergo negative selection due to the absence of commensal bacteria-derived antigen in the thymus. Rather, mature commensal bacteria-specific CD4+ T cells enter the peripheral circulation where they may encounter professional antigen-presenting cells, such as dendritic cells, presenting commensal bacteria-derived antigen via MHCII. Antigen-experienced Teff then migrate to distinct niches within the lymph node, which are enriched for MHCII+ ILC3s, resulting in the deletion of activated commensal bacteria-specific Teff cells from the peripheral pool. Commensal bacteria-specific T cells receive TCR signals from ILC3 MHCII-Ag complexes in the absence of co-stimulation, which is associated with upregulation of the signaling molecule Nur77. In addition, ILC3s efficiently consume growth factor cytokines, in particular IL-2, resulting in T cell cytokine withdrawal. The net effect of both TCR signaling and cytokine withdrawal results in upregulation of the pro-apoptotic Bim and induction of Caspase-3 associated apoptotic cell death, promoting deletion of activated commensal bacteria-specific T cells in a process we term “intestinal selection”, which parallels thymic selection. In contrast, commensal bacteria-specific CD4+ T regulatory cells (Treg) are not subject to ILC3-mediated deletion, potentially due to their ability to compete for IL-2, and migrate to the intestine whereby they further maintain tissue homeostasis in the presence of the commensal microflora.