Abstract
Opioid receptor antagonists increase hyperalgesia in humans and animals, indicating that endogenous activation of opioid receptors provides relief from acute pain; however, the mechanisms of long-term opioid inhibition of pathological pain have remained elusive. We found that tissue injury produced μ-opioid receptor constitutive activity (MORCA) that repressed spinal nociceptive signaling for months. Pharmacological blockade during the post-hyperalgesia state with MOR inverse agonists reinstated central pain sensitization, and precipitated hallmarks of opioid withdrawal (including cAMP overshoot and hyperalgesia) that required N-methyl-D-aspartate receptor activation of adenylyl cyclase type 1 (AC1). Thus, MORCA initiates both analgesic signaling as well as a compensatory opponent process that generates endogenous opioid dependence. Tonic MORCA suppression of withdrawal hyperalgesia may prevent the transition from acute to chronic pain.
Chronic pain is determined by facilitatory mechanisms such as long-term potentiation (LTP) of synaptic strength in dorsal horn neurons (1–3). While exogenously applied opioids prevent (4, 5) and/or erase (6) spinal LTP, and spinal enkephalin release exerts inhibitory control of acute pain intensity soon after tissue injury (7, 8), it remains unclear how the endogenous opioid system might persistently repress pathological pain. Opiate administration provides powerful pain relief, but repeated administration leads to the development of compensatory neuroadaptations underlying opiate tolerance and dependence (9), including the selective upregulation of calcium-sensitive AC isoforms (10, 11). Cessation of opiates leads to cellular and behavioral symptoms of withdrawal (12–16). An intriguing hypothesis of drug addiction suggests that chronic opiates increase MOR constitutive activity (MORCA) to preserve physical and psychological dependence (17–21), which is enhanced by enkephalins (22). Whether MORs adopt constitutive signaling states in other disease syndromes, such as chronic pain, is unknown. We tested the hypothesis that tissue injury increases MORCA in the spinal cord. With sufficient time after injury, enhanced basal MOR signaling should produce endogenous cellular and physical dependence in the CNS.
We first discovered that spinal opioid signaling promotes the intrinsic recovery of acute inflammatory pain and orchestrates long-lasting antinociception. In mice, a unilateral intraplantar injection of complete Freund’s adjuvant (CFA) produced mechanical hyperalgesia that resolved within 10 d (Fig. 1A). Subcutaneous chronic minipump infusion of naltrexone hydrochloride (NTX), a non-selective opioid receptor antagonist, prolonged hyperalgesia throughout the 14 d infusion period in CFA-injured mice (F3,17 = 25.4; P < 0.0001; Fig. 1B), while having no effect in sham-injured mice. Upon NTX-pump removal, hyperalgesia rapidly declined. NTX did not alter the induction phase of CFA-induced hyperalgesia (fig. S1A–B; Supplementary Note 1); however, when delivered 21d after CFA, in the complete absence of pain, systemic NTX reinstated hyperalgesia (F1,21 = 41, P < 0.0001;Fig. 1C) in a dose-dependent manner with no effect in shams (Fig. 1D). By contrast, systemic injection of naltrexone methobromide (NMB), an opioid receptor antagonist that does not cross the blood brain barrier, failed to alter mechanical thresholds at either the ipsilateral or contralateral paws (both P > 0.05; Fig. 1E). Intrathecal administration of either NTX or NMB precipitated robust hyperalgesia in CFA-21d mice at both the injured ipsilateral paw (P < 0.05; Fig. 1F) and uninjured contralateral paw (P < 0.05; Fig. 1F), with no effect in shams (Fig. 1G). NTX also induced heat hyperalgesia (P < 0.05; Fig. 1H) as well as spontaneous pain in males (P < 0.05; Fig. 1I) and females (fig S3). Intrathecal NTX reinstated hyperalgesia in a model of post-surgical pain (P < 0.05; Fig. 1J) (23), several other models of inflammatory and neuropathic pain, and in multiple mouse strains (not shown).
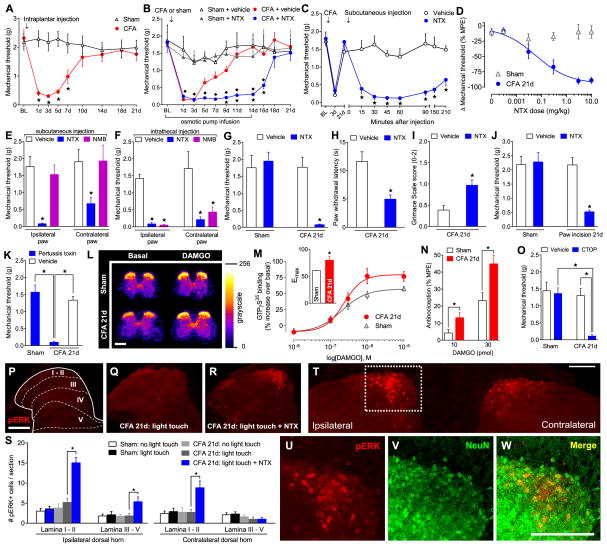
Fig. 1. Injury-induced pain sensitization is tonically opposed by spinal MOR-G-protein signaling.
(A) Progression of mechanical hyperalgesia following intraplantar CFA (5 μl) (n = 10). (B) Resolution of hyperalgesia during and 14d after infusion of NTX (10 mg/kg/d, s.c.) in Sham and CFA mice (n = 5–6). ★ P < 0.05 compared to CFA+saline, ◇ P < 0.05 compared to Sham+NTX. (C) Time course of reinstatement of hyperalgesia following subcutaneous NTX (3 mg/kg) in CFA-21d mice (n = 6–13). (D) Dose-response effects of NTX on hyperalgesia (n = 6 per dose). MPE: maximal possible effect. (E–F) Effect on hyperalgesia of (E) subcutaneous or (F) intrathecal NTX (3 mg/kg or 1 μg) or NMB (3 mg/kg or 0.3 μg) (n = 5–10). (G–J) Effect of NTX (1 μg, i.t.) on reinstatement of (G) mechanical hyperalgesia in Sham and CFA mice (n = 5–8), (H) heat hyperalgesia (n = 5–10), (I) spontaneous pain (n = 4–8), and (J) post-operative pain (n = 6–11). (K) Effect of pertussis toxin (0.5 μg, i.t.) on hyperalgesia (n = 6). (L) Representative radiograms and (M) dose-response effects of DAMGO-stimulated GTPγS35 binding in lumbar spinal cord; inset: binding Emax (n = 7–9). (N) Effect of DAMGO (i.t.) on hotplate latency (n = 8). (O) Effect of CTOP (100 ng, i.t.) on hyperalgesia (n = 6–7). (P–R) Representative images and (S) dorsal horn laminar quantification (I–II and III–V) of light touch-evoked pERK after NTX (1μg, i.t.) (n = 5–7). (T) Confocal image of pERK+ cells. (U–W) From boxed region in panel T: Co-localization of pERK with NeuN. All scale bars = 200 μm. ★ P < 0.05 for all panels. All data shown as mean±s.e.m. See fig. S1 for full time course data of panels E–J, O.
Whether MOR-G-protein signaling can be maintained for sufficient duration to oppose chronic pain is unknown. First, we found that disruption of Gαi/o signaling with intrathecal injection of pertussis toxin precipitated hyperalgesia in CFA-21d but not shams (P < 0.05; Fig. 1K). Second, we assessed guanosine-5′-O-(3-[35S]thio)triphosphate (GTPγS35) binding in fresh spinal cord slices (Fig. 1L and 1M). In control slices, the MOR-selective agonist DAMGO elicited a stimulation of GTPγS35 binding with an Emax and EC50 of 58.02 ± 0.67% and 0.24 ± 0.01 μM, respectively (Fig. 1M). Emax was potentiated in CFA-21d slices, not only in the ipsilateral (79.85 ± 7.35%; P < 0.05 compared to sham; Fig. 1M) but also the contralateral (74.05 ± 4.13%; P < 0.05 compared to sham; fig. S4) dorsal horns, with no change in the EC50. Third, the antinociceptive effects of intrathecal DAMGO were potentiated in CFA-21d mice (P < 0.05; Fig. 1N), reflecting increases in receptor number, receptor affinity, or descending modulatory circuits. Fourth, intrathecal injection of Phe-Cys-Tyr-Trp-Orn-Thr-Pen-Thr-NH2 (CTOP), a MOR-selective antagonist, reinstated hyperalgesia in CFA-21d mice but not shams (P < 0.05; Fig. 1M).
We next asked whether central sensitization (increased responsiveness of CNS nociceptive neurons to normal or sub-threshold afferent input) persists in the post-hyperalgesia state and remain under the control of endogenous MOR inhibitory mechanisms. Tested 21 days after CFA, innocuous light-touch of the injured hindpaw did not increase the dorsal horn expression of phosphorylated extracellular signal-regulated kinase (pERK) (Fig. 1Q and 1S). However, intrathecal NTX increased touch-evoked pERK in lamina I–II (P < 0.05; Fig 1R and 1S, fig. S4) and III–V (P < 0.05; Fig. 1R and 1S). NTX also increased pERK at the contralateral dorsal horn (7.34 ± 1.37 cells per slice, P < 0.05; Fig. 1S and 1T). Confocal microscopy revealed that pERK was expressed in neurons (Fig. 1U–W, fig. S5), but not in microglia or astrocytes (fig. S5).
We next tested the hypothesis that N-methyl-D-aspartate receptor (NMDA-R)–Ca2+-dependent mechanisms of central sensitization (1, 24) continue to operate after the resolution of inflammatory pain. Using live-cell Fura-2 ratiometric analysis in adult spinal cord slices (25), we found that glutamate-evoked [Ca2+]i in lamina II neurons was potentiated at 3 d after CFA and then resolved by day 21 (F3,17 = 15, P < 0.0001; Fig. 2A); this coincides with the temporal onset and resolution of inflammatory hyperalgesia. Perfusion of either CTOP or NTX increased the peak amplitude of glutamate-evoked [Ca2+]i in CFA-21d but not sham slices (Lamina II: P < 0.05; Fig. 2B and 2C and fig. S6; Lamina I: P < 0.05, fig. S7) or CFA-24hr slices (fig. S1C). The activity-dependent NMDA-R blocker, MK-801, prevented the NTX-mediated rise in [Ca2+]i (F1,16 = 4.6, P < 0.05; Fig. 2C), hyperalgesia (F3,22 = 6.5, P < 0.005; Fig. 2D) and dorsal horn pERK levels (P < 0.05; Fig. 2E, ipsilateral and fig. S8, contralateral).
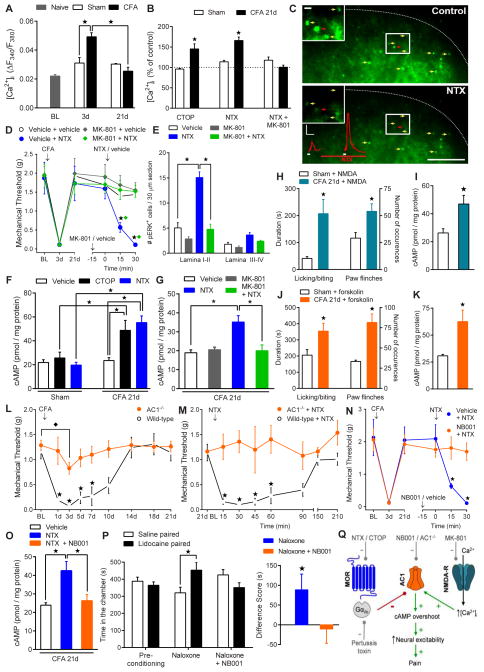
Fig. 2. Pain reinstatement requires potentiated N-methyl-D-aspartate receptor activation of calcium-sensitive adenylyl cyclase type 1.
(A) Time course of glutamate-evoked (0.3 mM) [Ca2+]i in spinal cord slices from sham and CFA mice (n = 4–7 mice). (B) Effect of CTOP (1 μM), NTX (10 μM) or NTX+MK-801 (100 μM) on [Ca2+]i. Values are relative to pre-drug control responses (n = 3–5 mice). (C) Representative F380 nm image of dorsal horn neurons from a CFA-21d slice responding to glutamate before (top) and after NTX (10 μM, bottom) (yellow arrows). Decrease in fluorescence intensity corresponds to increase in [Ca2+]i. The red traces illustrate the rise in [Ca2+]i for the indicated cell (red arrow). Insets depict area in white box. Scale bars: 0.02 ΔF/F (vertical) and 3 min (horizontal), and 100 μm and 10 μm (inset). Effect of MK-801 (1 μg, i.t.) on NTX-precipitated (1 μg, i.t.) (D) hyperalgesia and (E) touch-evoked dorsal horn pERK expression (n = 5–10). (F) Spinal cord cAMP levels after intrathecal vehicle (n = 14–18), CTOP (100 ng; n = 6) or NTX (1 μg; n = 6–10). (G) Effect of MK-801 (1 μg, i.t.) on NTX-precipitated spinal cAMP overshoot (n = 5). (H–K) Effect of intrathecal NMDA (3 pmol; n = 5–7) or forskolin (1.5 μg; n = 6) on (H and J) spontaneous nocifensive behaviors and (I and K) spinal cAMP levels. (L) Progression of mechanical hyperalgesia and (M) effect of intrathecal NTX in AC1−/− and wild-type mice 21d after CFA (n = 5–9). Effect of NB001 (1.5 μg, i.t.) on NTX-precipitated (N) hyperalgesia (n = 4–7), (O) spinal cAMP levels (n = 6–9), and (P) affective pain (n = 6–12). (Q) Schematic of cellular pathways involved in endogenous opioid withdrawal and pain reinstatement. ★ P < 0.05.
Opioids produce their acute actions in part through inhibition of adenylyl cyclases (ACs), whereas chronic opiate exposure produces a homeostatic upregulation of ACs (9, 14). In this opioid-dependent state, receptor antagonists produce cellular withdrawal, characterized by an adenosine 3′,5′-cyclic monophosphate (cAMP) overshoot response. To determine whether similar homeostatic mechanisms operate in the setting of tonic opioid receptor signaling after injury, we sampled intracellular cAMP content from ex vivo lumbar spinal tissue. Basal spinal cAMP levels were comparable in sham and CFA-21d mice, suggestive of a return to baseline AC function (Fig. 2F; Supplementary Note 2). In CFA-21d mice, however, intrathecal CTOP or NTX increased cAMP levels in CFA-21d mice (P < 0.05; Fig. 2F), indicative of AC superactivation. Because the Ca2+ stimulated isoforms of ACs are activated by NMDA-Rs (26), we hypothesized that NMDA-R signaling contributes to this cAMP overshoot. Intrathecal MK-801 abolished the NTX-precipitated increases in cAMP (P < 0.05 compared to NTX group; Fig. 2G). Moreover, direct activation of spinal NMDA-Rs and ACs by intrathecal NMDA or forskolin, respectively, increased nocifensive behaviors (P < 0.05; Fig. 2H and 2J) and spinal cAMP levels (P < 0.05; Fig. 2I and 2K) in CFA-21d mice as compared to shams, suggesting latent upregulation, but not occlusion, of NMDA-R–AC1 pathways.
Adenylyl cyclase type 1 (AC1) in the brain is intricately linked to morphine dependence (27, 28) and chronic pain (29), while in the spinal cord it contributes to activity-dependent LTP (30). Baseline mechanical thresholds were similar in wild-type and AC1 knockout mice (AC1−/−) (29) (Fig. 2L). However, AC1 gene deletion reduced inflammatory hyperalgesia (3d vs. baseline: P < 0.05, t test; F1,11 = 31.5, P < 0.0005, Genotype X Time; Fig. 2L), without affecting edema (fig. S9). At day 21 after CFA, NTX reinstatement was lost in AC1−/− mice (F1,7 = 20.3, P < 0.005; Fig. 2M). Furthermore, intrathecal NB001, a selective AC1 inhibitor (30), prevented NTX-reinstatement of hyperalgesia (F1,9 = 6.6, P < 0.05; Fig. 2N), and cAMP overshoot and spontaneous pain (P < 0.05; Fig. 2O–P). These data suggest that withdrawal from tonic MOR signaling increases pronociceptive neural excitability consequent to AC1 superactivation (Fig. 2Q).
Tonic MOR signaling arises from either continuous agonist stimulation or constitutive (agonist-independent) activity (31–34). MORCA develops with chronic morphine administration, leading to physical and affective signs of opiate dependence and addiction (17, 19–22). To determine the existence and physiologic significance of MORCA in pathological pain processing, we utilized the neutral antagonist 6β-naltrexol, a structural analog of NTX (35). Intrathecal 6β-naltrexol alone did not change Ca2+ levels in sham or CFA-21d spinal slices (Fig. 3A), and failed to precipitate a cAMP overshoot (Fig. 3B) or hyperalgesia (Fig. 3C). 6β-naltrexol abolished the ability of NTX to produce Ca2+ mobilization (P < 0.05; Fig. 3A), cAMP overshoot (P < 0.05; Fig. 3B), and hyperalgesia in CFA-21d mice (P < 0.05; Fig. 3C). 6β-naltrexol also abolished NTX-induced reinstatement of mechanical hyperalgesia in a postoperative pain model (23) (Fig 3D). These data suggest that NTX acts as an inverse agonist to inactivate MORCA in multiple models of inflammatory pain (Supplementary Notes 3 and 4) (36, 37).
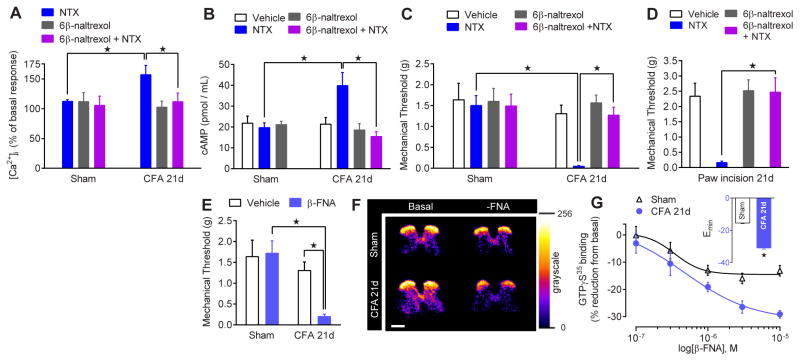
Fig. 3. Spinal MORs acquire constitutive activity after injury.
(A,B,C) Effects of NTX (1μM or 1 μg), 6β-naltrexol (1μM or 10 μg) or co-administration of 6β-naltrexol+NTX in sham and CFA-21d mice on (A) [Ca2+]i in spinal cord slices (n = 5–6), (B) spinal cAMP levels (n = 6–11) and (C) hyperalgesia (n = 6–7). (D) Effect of intrathecal 6β-naltrexol and/or NTX on hyperalgesia in Paw Incision-21d mice (n = 6–7). (E) Effect of intrathecal β-funaltrexamine (β-FNA; 2.5 μg) on hyperalgesia (n = 6–7). (F) Representative radiograms and (G) dose-response effects of β-FNA on basal GTPγS35 binding in lumbar ipsilateral dorsal horn; inset: binding Emax (n = 7–9). ★ P < 0.05. All data shown as mean ± s.e.m. See fig. S9 for full time course data of panels C–E.
Intrathecal administration of an alternative μ-selective inverse agonist, β-funaltrexamine (β-FNA) (38), reinstated hyperalgesia in CFA-21d but not sham mice (P < 0.05; Fig. 3E). Because MORCA results in elevated basal G-protein cycling (19, 38), we determined whether β-FNA could promote the MOR-inactive state and thereby decrease spontaneous basal GDP/GTPγS35 exchange. β-FNA concentration-dependently reduced basal GTPγS35 binding in dorsal horn sections from CFA-21d mice and, to a significantly lesser degree, sham-injured mice, in both ipsilateral and contralateral dorsal horns (Fig. 3F and 3G and fig. S11).
Pain comprises sensory (hyperalgesia) and affective (aversiveness) components; the latter can be identified by changes in the rewarding property of analgesics and associated motivational behavior. In a conditioned place preference paradigm (39–41), the negative reinforcing capacity of intrathecal lidocaine (motivation to seek pain relief) demonstrates the presence of aversive pain 1d after CFA (40). This aversive component was absent at 21d (Fig. 4A; ‘CFA-21d+saline’ group). CFA-21d but not sham mice responded to systemic naloxone by spending more time in the chamber paired with intrathecal lidocaine (538 ± 39 s) than with intrathecal saline (283 ± 28 s; P < 0.001; Fig. 4A). Systemic NTX, but not saline or NMB, precipitated numerous escape and somato-motor behaviors analogous to classical morphine withdrawal (42, 43) in CFA-21d mice, with no effect in shams (Fig. 4B and 4C).
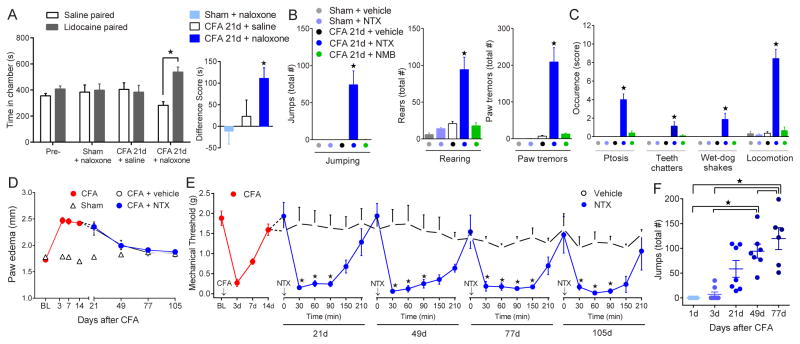
Fig. 4. Prolonged endogenous MOR signaling generates psychological and physical dependence.
(A) Behavioral signs of psychological withdrawal (aversion associated with spontaneous pain), reflected by place preference for intrathecal lidocaine upon naloxone administration. Left: Intragroup chamber analysis for intrathecal saline (5 μl) or lidocaine (0.04%) in sham and CFA-21d mice treated with intraperitoneal saline or naloxone (3 mg/kg). Right: Intergroup difference score analysis illustrating time spent in intrathecal lidocaine-paired chambers. (n = 6 per group). (B,C) Behavioral signs of physical withdrawal, recorded for 60 min after injection of NTX (3 mg/kg), NMB (3 mg/kg) or vehicle. (n = 6 – 7). (D) Progression paw edema and (E) effects of repeated subcutaneous vehicle or NTX (3 mg/kg) on hyperalgesia over 105 days after CFA. (n = 7 per group) (F) Effect of repeated NTX (3 mg/kg) on the number of precipitated escape jumps over 77 days after CFA. (n = 8). ★ P < 0.05. All data shown as mean ± s.e.m.
To determine if pain sensitization and endogenous opioid physical dependence persist beyond tissue healing, we gave periodic injections of NTX during and after the course of inflammatory edema, which subsided within 77 d after CFA (Fig. 4D). NTX, but not saline, reinstated hyperalgesia for at least 105d post-CFA (21d: F1,80 = 8.5, P < 0.05; 49d: F1,72 = 59, P < 0.0001; 77d: F1,72 = 76, P < 0.0001; 105d: F1,64 = 33, P < 0.0001; Fig. 4E). This was true at 200d post-CFA (fig. S12; Supplementary Note 5), and after a single intrathecal injection 105d post CFA, without prior exposure of the animal to the testing environment, of NTX or CTOP (fig. S12). NTX-precipitated escape-jump frequency increased with time after the injury (F4,29 = 14, P < 0.0001; Fig. 4F), suggesting that intensifying opioidergic and compensatory neuroadaptations create a physical and psychological dependence that greatly outlasts acute pain and tissue injury (Supplementary Note 6).
These data indicate that blockade of MORCA unmasks a silent AC1 central sensitization pathway that persists beyond the resolution of pain and inflammation, reflective of hyperalgesic priming (44). The presence of contralateral spinal MORCA and neural sensitization illustrates the spread of this pathology to areas of the CNS beyond those directly innervated by the injured tissue. Thus MORCA might tonically repress wide-spread hyperalgesia (Supplemental Note 7). If true, then loss of MORCA antinociception (e.g. during stress) could lead to the emergence of rampant chronic pain (45, 46).
We have identified an injury-induced MORCA that promotes both endogenous analgesia and dependence. Our data suggest that long-term MORCA inhibition of AC1-mediated central sensitization drives a counter-adaptive, homeostatic increase in pronociceptive AC1 signaling cascades (29, 47), thereby paradoxically promoting the maintenance of latent central sensitization. Thus, injury produces a long-lasting dependence on MORCA that tonically prevents withdrawal hyperalgesia, consistent with proposed mechanisms of dependence to opiate drugs such as morphine (27, 48). We contend that loss of MORCA, and the ensuing reinstatement of pain reflects a process of spinal cellular withdrawal (NMDA-mediated AC1 superactivation) to enhance pronociceptive synaptic strength (Supplemental Note 8) (49, 50), as observed following NMDA-R-dependent spinal LTP at C-fiber synapses during withdrawal from exogenous opiates (12). Indeed, stress (46) or injury (51) escalates opposing inhibitory and excitatory influences on nociceptive processing, as a pathological consequence of increased endogenous opioid tone. This raises the prospect that opposing homeostatic interactions between MORCA analgesia and latent NMDA-R–AC1 pain sensitization create a lasting susceptibility to develop chronic pain.
Supplementary Material
Acknowledgments
The authors thank H.L. Fields, A. I. Basbaum, Lindsay Hough, Juan Carlos Marvizon, Ed Bilsky, Wolfgang Sadee, G. Scherrer, K. Westlund High, Mads Werner, Jorgen Dahl, and B. Solway for critical discussions, and J. Grasch, L. Martin, and R. Griggs for technical assistance and blinding. This work was supported by NIH grants F31DA032496 (G.C.), R01NS45954 (B.K.T.), and 5K02DA19656 (B.K.T.).
Footnotes
Author Contributions:
G.C. and B.K.T. formulated the hypotheses, designed, analyzed and coordinated all experiments. G.C. performed surgeries, behavioral pharmacology, histology, and fluorescence imaging and analyzed the data. G.C. and B.L.J. carried out the biochemical studies and GC analyzed the data. S.D. and G.C. carried out Ca2+ imagining experiments and analyzed the data. M.W., K.E.M., G.C., and B.K.T. designed the GTPγS35 binding studies, M.K.W. collected the data, and G.C., K.E.M. and B.K.T. analyzed the data. D.R.S. supplied the AC1−/− breeders. Z.J.W., G.C., and B.K.T. designed the conditioned place preference experiments, and Y.H. and X.H. conducted experiments and analyzed the data. J.M., J.W., G.C., and B.K.T. designed the Mouse Grimace Scale experiments, and J.W. conducted the experiments. G.C. and B.K.T. wrote the manuscript.
Materials and Methods
References and Notes
- 1.Latremoliere A, Woolf CJ. Central sensitization: a generator of pain hypersensitivity by central neural plasticity. J Pain. 2009;10:895. doi: 10.1016/j.jpain.2009.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ikeda H, et al. Synaptic amplifier of inflammatory pain in the spinal dorsal horn. Science. 2006;312:1659. doi: 10.1126/science.1127233. [DOI] [PubMed] [Google Scholar]
- 3.Ruscheweyh R, Wilder-Smith O, Drdla R, Liu XG, Sandkuhler J. Long-term potentiation in spinal nociceptive pathways as a novel target for pain therapy. Mol Pain. 2011;7:20. doi: 10.1186/1744-8069-7-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Terman GW, Eastman CL, Chavkin C. Mu opiates inhibit long-term potentiation induction in the spinal cord slice. J Neurophysiol. 2001;85:485. doi: 10.1152/jn.2001.85.2.485. [DOI] [PubMed] [Google Scholar]
- 5.Benrath J, Brechtel C, Martin E, Sandkuhler J. Low doses of fentanyl block central sensitization in the rat spinal cord in vivo. Anesthesiology. 2004;100:1545. doi: 10.1097/00000542-200406000-00030. [DOI] [PubMed] [Google Scholar]
- 6.Drdla-Schutting R, Benrath J, Wunderbaldinger G, Sandkuhler J. Erasure of a spinal memory trace of pain by a brief, high-dose opioid administration. Science. 2012;335:235. doi: 10.1126/science.1211726. [DOI] [PubMed] [Google Scholar]
- 7.Basbaum AI, Fields HL. Endogenous pain control systems: brainstem spinal pathways and endorphin circuitry. Annu Rev Neurosci. 1984;7:309. doi: 10.1146/annurev.ne.07.030184.001521. [DOI] [PubMed] [Google Scholar]
- 8.Ossipov MH, Dussor GO, Porreca F. Central modulation of pain. J Clin Invest. 2010;120:3779. doi: 10.1172/JCI43766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Christie MJ. Cellular neuroadaptations to chronic opioids: tolerance, withdrawal and addiction. Br J Pharmacol. 2008;154:384. doi: 10.1038/bjp.2008.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Avidor-Reiss T, Nevo I, Saya D, Bayewitch M, Vogel Z. Opiate-induced adenylyl cyclase superactivation is isozyme-specific. J Biol Chem. 1997;272:5040. doi: 10.1074/jbc.272.8.5040. [DOI] [PubMed] [Google Scholar]
- 11.Lane-Ladd SB, et al. CREB (cAMP response element-binding protein) in the locus coeruleus: biochemical, physiological, and behavioral evidence for a role in opiate dependence. J Neurosci. 1997;17:7890. doi: 10.1523/JNEUROSCI.17-20-07890.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Drdla R, Gassner M, Gingl E, Sandkuhler J. Induction of synaptic long-term potentiation after opioid withdrawal. Science. 2009;325:207. doi: 10.1126/science.1171759. [DOI] [PubMed] [Google Scholar]
- 13.Heinl C, Drdla-Schutting R, Xanthos DN, Sandkuhler J. Distinct mechanisms underlying pronociceptive effects of opioids. J Neurosci. 2011;31:16748. doi: 10.1523/JNEUROSCI.3491-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nestler EJ, Aghajanian GK. Molecular and cellular basis of addiction. Science. 1997;278:58. doi: 10.1126/science.278.5335.58. [DOI] [PubMed] [Google Scholar]
- 15.Kauer JA, Malenka RC. Synaptic plasticity and addiction. Nat Rev Neurosci. 2007;8:844. doi: 10.1038/nrn2234. [DOI] [PubMed] [Google Scholar]
- 16.Angst MS, Clark JD. Opioid-induced hyperalgesia: a qualitative systematic review. Anesthesiology. 2006;104:570. doi: 10.1097/00000542-200603000-00025. [DOI] [PubMed] [Google Scholar]
- 17.Wang Z, Bilsky EJ, Porreca F, Sadee W. Constitutive mu opioid receptor activation as a regulatory mechanism underlying narcotic tolerance and dependence. Life Sci. 1994;54:PL339. doi: 10.1016/0024-3205(94)90022-1. [DOI] [PubMed] [Google Scholar]
- 18.Liu JG, Prather PL. Chronic exposure to mu-opioid agonists produces constitutive activation of mu-opioid receptors in direct proportion to the efficacy of the agonist used for pretreatment. Mol Pharmacol. 2001;60:53. doi: 10.1124/mol.60.1.53. [DOI] [PubMed] [Google Scholar]
- 19.Wang D, et al. Basal signaling activity of mu opioid receptor in mouse brain: role in narcotic dependence. J Pharmacol Exp Ther. 2004;308:512. doi: 10.1124/jpet.103.054049. [DOI] [PubMed] [Google Scholar]
- 20.Shoblock JR, Maidment NT. Constitutively active micro opioid receptors mediate the enhanced conditioned aversive effect of naloxone in morphine-dependent mice. Neuropsychopharmacology. 2006;31:171. doi: 10.1038/sj.npp.1300782. [DOI] [PubMed] [Google Scholar]
- 21.Meye FJ, van Zessen R, Smidt MP, Adan RA, Ramakers GM. Morphine withdrawal enhances constitutive mu-opioid receptor activity in the ventral tegmental area. J Neurosci. 2012;32:16120. doi: 10.1523/JNEUROSCI.1572-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shoblock JR, Maidment NT. Enkephalin release promotes homeostatic increases in constitutively active mu opioid receptors during morphine withdrawal. Neuroscience. 2007;149:642. doi: 10.1016/j.neuroscience.2007.05.011. [DOI] [PubMed] [Google Scholar]
- 23.Pogatzki EM, Raja SN. A mouse model of incisional pain. Anesthesiology. 2003;99:1023. doi: 10.1097/00000542-200310000-00041. [DOI] [PubMed] [Google Scholar]
- 24.Luo C, Seeburg PH, Sprengel R, Kuner R. Activity-dependent potentiation of calcium signals in spinal sensory networks in inflammatory pain states. Pain. 2008;140:358. doi: 10.1016/j.pain.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 25.Doolen S, Blake CB, Smith BN, Taylor BK. Peripheral nerve injury increases glutamate-evoked calcium mobilization in adult spinal cord neurons. Mol Pain. 2012;8:56. doi: 10.1186/1744-8069-8-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chetkovich DM, Sweatt JD. nMDA receptor activation increases cyclic AMP in area CA1 of the hippocampus via calcium/calmodulin stimulation of adenylyl cyclase. J Neurochem. 1993;61:1933. doi: 10.1111/j.1471-4159.1993.tb09836.x. [DOI] [PubMed] [Google Scholar]
- 27.Zachariou V, et al. Distinct roles of adenylyl cyclases 1 and 8 in opiate dependence: behavioral, electrophysiological, and molecular studies. Biol Psychiatry. 2008;63:1013. doi: 10.1016/j.biopsych.2007.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mazei-Robison MS, Nestler EJ. Opiate-induced molecular and cellular plasticity of ventral tegmental area and locus coeruleus catecholamine neurons. Cold Spring Harb Perspect Med. 2012;2:a012070. doi: 10.1101/cshperspect.a012070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wei F, et al. Genetic elimination of behavioral sensitization in mice lacking calmodulin-stimulated adenylyl cyclases. Neuron. 2002;36:713. doi: 10.1016/s0896-6273(02)01019-x. [DOI] [PubMed] [Google Scholar]
- 30.Wang H, et al. Identification of an adenylyl cyclase inhibitor for treating neuropathic and inflammatory pain. Sci Transl Med. 2011;3:65ra3. doi: 10.1126/scitranslmed.3001269. [DOI] [PubMed] [Google Scholar]
- 31.Kenakin T. Inverse, protean, and ligand-selective agonism: matters of receptor conformation. FASEB J. 2001;15:598. doi: 10.1096/fj.00-0438rev. [DOI] [PubMed] [Google Scholar]
- 32.Kenakin T. Principles: receptor theory in pharmacology. Trends Pharmacol Sci. 2004;25:186. doi: 10.1016/j.tips.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 33.Costa T, Herz A. Antagonists with negative intrinsic activity at delta opioid receptors coupled to GTP-binding proteins. Proc Natl Acad Sci U S A. 1989;86:7321. doi: 10.1073/pnas.86.19.7321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Seifert R, Wenzel-Seifert K. Constitutive activity of G-protein-coupled receptors: cause of disease and common property of wild-type receptors. Naunyn Schmiedebergs Arch Pharmacol. 2002;366:381. doi: 10.1007/s00210-002-0588-0. [DOI] [PubMed] [Google Scholar]
- 35.Raehal KM, et al. In vivo characterization of 6beta-naltrexol, an opioid ligand with less inverse agonist activity compared with naltrexone and naloxone in opioid-dependent mice. J Pharmacol Exp Ther. 2005;313:1150. doi: 10.1124/jpet.104.082966. [DOI] [PubMed] [Google Scholar]
- 36.Sadee W, Wang D, Bilsky EJ. Basal opioid receptor activity, neutral antagonists, and therapeutic opportunities. Life Sci. 2005;76:1427. doi: 10.1016/j.lfs.2004.10.024. [DOI] [PubMed] [Google Scholar]
- 37.Lam H, et al. Analgesic tone conferred by constitutively active mu opioid receptors in mice lacking beta-arrestin 2. Mol Pain. 2011;7:24. doi: 10.1186/1744-8069-7-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu JG, Ruckle MB, Prather PL. Constitutively active mu-opioid receptors inhibit adenylyl cyclase activity in intact cells and activate G-proteins differently than the agonist [D-Ala2,N-MePhe4,Gly-ol5]enkephalin. J Biol Chem. 2001;276:37779. doi: 10.1074/jbc.M106104200. [DOI] [PubMed] [Google Scholar]
- 39.King T, et al. Unmasking the tonic-aversive state in neuropathic pain. Nat Neurosci. 2009;12:1364. doi: 10.1038/nn.2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.He Y, Tian X, Hu X, Porreca F, Wang ZJ. Negative reinforcement reveals non-evoked ongoing pain in mice with tissue or nerve injury. J Pain. 2012;13:598. doi: 10.1016/j.jpain.2012.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sufka KJ. Conditioned place preference paradigm: a novel approach for analgesic drug assessment against chronic pain. Pain. 1994;58:355. doi: 10.1016/0304-3959(94)90130-9. [DOI] [PubMed] [Google Scholar]
- 42.Koob GF, Maldonado R, Stinus L. Neural substrates of opiate withdrawal. Trends Neurosci. 1992;15:186. doi: 10.1016/0166-2236(92)90171-4. [DOI] [PubMed] [Google Scholar]
- 43.Kest B, et al. Naloxone-precipitated withdrawal jumping in 11 inbred mouse strains: evidence for common genetic mechanisms in acute and chronic morphine physical dependence. Neuroscience. 2002;115:463. doi: 10.1016/s0306-4522(02)00458-x. [DOI] [PubMed] [Google Scholar]
- 44.Asiedu MN, et al. Spinal protein kinase M zeta underlies the maintenance mechanism of persistent nociceptive sensitization. J Neurosci. 2011;31:6646. doi: 10.1523/JNEUROSCI.6286-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.De Felice M, et al. Triptan-induced enhancement of neuronal nitric oxide synthase in trigeminal ganglion dural afferents underlies increased responsiveness to potential migraine triggers. Brain. 2010;133:2475. doi: 10.1093/brain/awq159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rivat C, et al. Non-nociceptive environmental stress induces hyperalgesia, not analgesia, in pain and opioid-experienced rats. Neuropsychopharmacology. 2007;32:2217. doi: 10.1038/sj.npp.1301340. [DOI] [PubMed] [Google Scholar]
- 47.Zhuo M. Targeting neuronal adenylyl cyclase for the treatment of chronic pain. Drug Discov Today. 2012;17:573. doi: 10.1016/j.drudis.2012.01.009. [DOI] [PubMed] [Google Scholar]
- 48.Li S, et al. Calmodulin-stimulated adenylyl cyclase gene deletion affects morphine responses. Mol Pharmacol. 2006;70:1742. doi: 10.1124/mol.106.025783. [DOI] [PubMed] [Google Scholar]
- 49.Lu HC, et al. Adenylyl cyclase I regulates AMPA receptor trafficking during mouse cortical ‘barrel’ map development. Nat Neurosci. 2003;6:939. doi: 10.1038/nn1106. [DOI] [PubMed] [Google Scholar]
- 50.Xu H, et al. Presynaptic and postsynaptic amplifications of neuropathic pain in the anterior cingulate cortex. J Neurosci. 2008;28:7445. doi: 10.1523/JNEUROSCI.1812-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rivat C, et al. Fentanyl enhancement of carrageenan-induced long-lasting hyperalgesia in rats: prevention by the N-methyl-D-aspartate receptor antagonist ketamine. Anesthesiology. 2002;96:381. doi: 10.1097/00000542-200202000-00025. [DOI] [PubMed] [Google Scholar]
- 52.Fairbanks CA. Spinal delivery of analgesics in experimental models of pain and analgesia. Adv Drug Deliv Rev. 2003;55:1007. doi: 10.1016/s0169-409x(03)00101-7. [DOI] [PubMed] [Google Scholar]
- 53.Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. Journal of Neuroscience Methods. 1994;53:55. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- 54.Langford DJ, et al. Coding of facial expressions of pain in the laboratory mouse. Nat Methods. 2010;7:447. doi: 10.1038/nmeth.1455. [DOI] [PubMed] [Google Scholar]
- 55.Sotocinal SG, et al. The Rat Grimace Scale: a partially automated method for quantifying pain in the laboratory rat via facial expressions. Mol Pain. 2011;7:55. doi: 10.1186/1744-8069-7-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gao YJ, Ji RR. Light touch induces ERK activation in superficial dorsal horn neurons after inflammation: involvement of spinal astrocytes and JNK signaling in touch-evoked central sensitization and mechanical allodynia. J Neurochem. 2010;115:505. doi: 10.1111/j.1471-4159.2010.06946.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Abbadie C, Pan Y, Drake CT, Pasternak GW. Comparative immunohistochemical distributions of carboxy terminus epitopes from the mu-opioid receptor splice variants MOR-1D, MOR-1 and MOR-1C in the mouse and rat CNS. Neuroscience. 2000;100:141. doi: 10.1016/s0306-4522(00)00248-7. [DOI] [PubMed] [Google Scholar]
- 58.Liu-Chen LY, Li SX, Tallarida RJ. Studies on kinetics of [3H]beta-funaltrexamine binding to mu opioid receptor. Mol Pharmacol. 1990;37:243. [PubMed] [Google Scholar]
- 59.Aceto MD, Dewey WL, Portoghese PS, Takemori AE. Effects of beta-funaltrexamine (beta-FNA) on morphine dependence in rats and monkeys. Eur J Pharmacol. 1986;123:387. doi: 10.1016/0014-2999(86)90713-2. [DOI] [PubMed] [Google Scholar]
- 60.Le Guen S, Gestreau C, Besson JM. Morphine withdrawal precipitated by specific mu, delta or kappa opioid receptor antagonists: a c-Fos protein study in the rat central nervous system. Eur J Neurosci. 2003;17:2425. doi: 10.1046/j.1460-9568.2003.02678.x. [DOI] [PubMed] [Google Scholar]
- 61.Gmerek DE, Woods JH. Effects of beta-funaltrexamine in normal and morphine-dependent rhesus monkeys: observational studies. J Pharmacol Exp Ther. 1985;235:296. [PubMed] [Google Scholar]
- 62.Sirohi S, Dighe SV, Madia PA, Yoburn BC. The relative potency of inverse opioid agonists and a neutral opioid antagonist in precipitated withdrawal and antagonism of analgesia and toxicity. J Pharmacol Exp Ther. 2009;330:513. doi: 10.1124/jpet.109.152678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bohn LM, et al. Enhanced morphine analgesia in mice lacking beta-arrestin 2. Science. 1999;286:2495. doi: 10.1126/science.286.5449.2495. [DOI] [PubMed] [Google Scholar]
- 64.Zollner C, et al. Chronic morphine use does not induce peripheral tolerance in a rat model of inflammatory pain. J Clin Invest. 2008;118:1065. doi: 10.1172/JCI25911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bohn LM, Gainetdinov RR, Lin FT, Lefkowitz RJ, Caron MG. Mu-opioid receptor desensitization by beta-arrestin-2 determines morphine tolerance but not dependence. Nature. 2000;408:720. doi: 10.1038/35047086. [DOI] [PubMed] [Google Scholar]
- 66.Noble F, Turcaud S, Fournie-Zaluski MC, Roques BP. Repeated systemic administration of the mixed inhibitor of enkephalin-degrading enzymes, RB101, does not induce either antinociceptive tolerance or cross-tolerance with morphine. Eur J Pharmacol. 1992;223:83. doi: 10.1016/0014-2999(92)90821-k. [DOI] [PubMed] [Google Scholar]
- 67.Buchsbaum MS, Davis GC, Bunney WE. Naloxone alters pain perception and somatosensory evoked potentials in humans. Nature (London) 1977;270:620. doi: 10.1038/270620a0. [DOI] [PubMed] [Google Scholar]
- 68.Levine JD, Gordon NC, Fields HL. Naloxone dose dependently produces analgesia and hyperalgesia in postoperative pain. Nature. 1979;278:740. doi: 10.1038/278740a0. [DOI] [PubMed] [Google Scholar]
- 69.Gracely RH, Dubner R, Wolskee PJ, Deeter WR. Placebo and naloxone can alter post-surgical pain by separate mechanisms. Nature. 1983;306:264. doi: 10.1038/306264a0. [DOI] [PubMed] [Google Scholar]
- 70.Zubieta JK, et al. Regional mu opioid receptor regulation of sensory and affective dimensions of pain. Science. 2001;293:311. doi: 10.1126/science.1060952. [DOI] [PubMed] [Google Scholar]
- 71.Tambeli CH, Levine JD, Gear RW. Centralization of noxious stimulus-induced analgesia (NSIA) is related to activity at inhibitory synapses in the spinal cord. Pain. 2009;143:228. doi: 10.1016/j.pain.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kaur M, et al. Induction of withdrawal-like symptoms in a small randomized, controlled trial of opioid blockade in frequent tanners. J Am Acad Dermatol. 2006;54:709. doi: 10.1016/j.jaad.2005.11.1059. [DOI] [PubMed] [Google Scholar]
- 73.Chai B, Guo W, Wei F, Dubner R, Ren K. Trigeminal-rostral ventromedial medulla circuitry is involved in orofacial hyperalgesia contralateral to tissue injury. Mol Pain. 2012;8:78. doi: 10.1186/1744-8069-8-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tillu DV, Gebhart GF, Sluka KA. Descending facilitatory pathways from the RVM initiate and maintain bilateral hyperalgesia after muscle insult. Pain. 2008;136:331. doi: 10.1016/j.pain.2007.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hatashita S, Sekiguchi M, Kobayashi H, Konno S, Kikuchi S. Contralateral neuropathic pain and neuropathology in dorsal root ganglion and spinal cord following hemilateral nerve injury in rats. Spine (Phila Pa 1976) 2008;33:1344. doi: 10.1097/BRS.0b013e3181733188. [DOI] [PubMed] [Google Scholar]
- 76.Zhou HY, Chen SR, Chen H, Pan HL. Opioid-induced long-term potentiation in the spinal cord is a presynaptic event. J Neurosci. 2010;30:4460. doi: 10.1523/JNEUROSCI.5857-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Weyerbacher AR, Xu Q, Tamasdan C, Shin SJ, Inturrisi CE. N-Methyl-D-aspartate receptor (NMDAR) independent maintenance of inflammatory pain. Pain. 2010;148:237. doi: 10.1016/j.pain.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sandkuhler J, Gruber-Schoffnegger D. Hyperalgesia by synaptic long-term potentiation (LTP): an update. Curr Opin Pharmacol. 2012;12:18. doi: 10.1016/j.coph.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Liauw J, Wu LJ, Zhuo M. Calcium-stimulated adenylyl cyclases required for long-term potentiation in the anterior cingulate cortex. J Neurophysiol. 2005;94:878. doi: 10.1152/jn.01205.2004. [DOI] [PubMed] [Google Scholar]
- 80.Wei F, et al. Calcium calmodulin-stimulated adenylyl cyclases contribute to activation of extracellular signal-regulated kinase in spinal dorsal horn neurons in adult rats and mice. J Neurosci. 2006;26:851. doi: 10.1523/JNEUROSCI.3292-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.