Abstract
The principal risk factor for developing most adult onset neurodegenerative diseases is aging, with incidence rising significantly after age 50. Despite research efforts, the causes of Parkinson's disease (PD) remain unknown. As neurons age, they show signs of diminished lysosomal and mitochondrial function, including increased oxidative stress and accumulation of misfolded proteins, and these changes become exacerbated PD. We show that activity of the lysosomal hydrolase glucocerebrosidase gradually diminishes with age in the substantia nigra and putamen of healthy controls. This reduction is comparable to glucocerebrosidase activity in GBA1-mutation carrier PD patients. These data, demonstrate for the first time that an age-dependent reduction in glucocerebrosidase activity may lower the threshold for developing PD.
Introduction
Parkinson's disease (PD) is relatively rare before age 40, after age 50 the prevalence rises almost exponentially.1 By the eighth decade, the estimated prevalence in European and North American populations is between 1000 and 3000 cases per 100,000 population.1 This age-related risk may be related to the convergence of genetic impairments and age-related decline in cellular function, which include deficits in degradation pathways and elevated mitochondrial damage. As neurons age, they show signs of increased oxidative stress, disturbances in mitochondrial function, and accumulation of misfolded proteins, which are exacerbated in PD. If the role of lysosomal function could be better defined during the aging process, this may lead to more effective therapeutic options to treat PD.
Neuronal aging is unique because neurons are postmitotic cells, and therefore they rely heavily on protein clearance mechanisms to maintain homeostasis. The two major proteolytic systems responsible for intracellular protein turnover are the autophagy-lysosomal system and the ubiquitin-proteasome system. Aging lysosomal systems undergo dramatic changes including, increased volume and decreased stability and accumulation of indigestible materials.2 This is significant given that lysosomes are responsible for the clearance of proteins, such as α-synuclein as well as the removal of damaged organelles, such as mitochondria.3,4 The gene GBA1 encodes for the lysosomal hydrolase glucocerebrosidase (GCase), and reductions in GCase activity results in the accumulation of the glycolipids glucosylceramide (GluCer) and glucosylsphingosine (GluSph). Heterozygous loss-of-function mutations in GBA1 occur at a frequency of 4–7% in idiopathic PD patients.5–7 Approximately, 6–7% of early onset PD (<51 years) are carriers of either the N370S or L444P GBA mutations.8 These patients also develop clinical symptoms at an earlier age than nonmutation carriers.9 Moreover, PD patients who harbor a loss-of-function GBA1 mutation usually develop more severe cognitive symptoms, are often diagnosed younger, and are associated with increased α-synuclein accumulation relative to non-GBA1 mutation carrier (nonGBA1) PD patients.7,10–12 A recent report demonstrated that early stage nonGBA1 PD patients are associated with a reduction in GCase activity and dysfunctional lysosomes.11 Despite the overwhelming evidence linking GBA1 and diminished GCase activity with PD,7,12,13 it is presently unclear when this reduction occurs and how it is related to cellular aging and therefore its role the initiation and pathogenesis of the disease.
Elucidating some of the intracellular changes that occur with normal aging may give insight into the early changes that occur in PD. Our data suggest that aging lysosomes may contribute to the early pathology in PD and offers a broader perspective of how aging can lower the threshold and contribute to the onset of the disease. Here, we present data that show that GCase activity is reduced in nonGBA1 PD patients in the sixth, seven, and eight decade of life, which is associated with an increase in GluSph levels. Remarkably, healthy subject controls exhibit a gradual age-related reduction in GCase activity in the substantia nigra and putamen, which became evident during the seventh and eighth decade of life, respectively. These data suggest that GCase activity or more broadly diminished lysosomal function may be a critical age-related risk factor that increases the vulnerability of dopamine neurons and lower the threshold for developing PD.
Materials and Methods
Patients
Frozen postmortem brain tissue from male and female neurologically unaffected patients (healthy subject controls) and sporadic PD patients were provided by the Harvard Brain Tissue Resource Center (HBTRC; McLean Hospital, Belmont, MA). All PD cases met a pathological diagnosis of PD made by the brain bank, which was based on the extent of neuronal (pigment) loss in the substantia nigra and locus coeruleus (0–4), and Braak staging (0–6). Postmortem brains from healthy subject controls and PD patients were closely matched for age, sex, and postmortem interval (Table1). All of the PD patient brain tissue was sequenced for GBA1 mutations (the GBA1 pseudogene was taken into account) at Beckman Coulter Genomics (Danvers, MA) and any PD patient with a GBA1 mutation was removed from further analysis.
Table 1.
Parkinson's disease and control case information.
| Patient ID | Diagnosis | Age | Sex | PMI |
|---|---|---|---|---|
| AN15395 | Control | 74 | Male | 18.58 |
| AN18990 | Control | 71 | Male | 17.41 |
| AN15515 | Control | 73 | Male | 20.88 |
| AN17230 | Control | 68 | Male | 26.52 |
| AN06318 | Control | 66 | Male | 17.83 |
| AN13687 | Control | 73 | Female | 24.88 |
| AN06749 | Control | 75 | Male | 23.97 |
| AN04648 | Control | 73 | Male | 32.68 |
| AN08704 | Control | 70 | Male | 23.5 |
| AN06429 | Control | 77 | Male | 23.25 |
| AN05049 | Control | 78 | Male | 35.17 |
| AN01717 | Control | 79 | Male | 20.6 |
| AN01234 | Control | 85 | Male | 20.83 |
| AN01219 | Control | 83 | Male | 8.86 |
| AN16467 | Control | 79 | Female | 26.67 |
| AN06669 | Control | 81 | Male | 23.33 |
| AN06400 | Control | 91 | Male | 23.48 |
| AN05090 | Control | 87 | Male | 21.12 |
| 8201 | Control | 74 | Male | 24.62 |
| 8155 | Control | 58 | Male | 20.47 |
| 8136 | Control | 76 | Male | 12.25 |
| 8134 | Control | 77 | Female | 20.61 |
| 8113 | Control | 51 | Male | 28.82 |
| 7559 | Control | 78 | Male | 19 |
| 7542 | Control | 76 | Male | 24 |
| 7519 | Control | 55 | Male | 24 |
| 7516 | Control | 63 | Male | 20 |
| 7508 | Control | 62 | Male | 26 |
| 7458 | Control | 77 | Male | 29 |
| AN01987 | Parkinson's disease | 74 | Male | 21.08 |
| AN08649 | Parkinson's disease | 71 | Male | 12.8 |
| AN01756 | Parkinson's disease | 69 | Male | 7.1 |
| AN01131 | Parkinson's disease | 68 | Male | 16.82 |
| AN06530 | Parkinson's disease | 64 | Male | 17.75 |
| AN02428 | Parkinson's disease | 75 | Male | 19.42 |
| AN18072 | Parkinson's disease | 73 | Male | 4.5 |
| AN12683 | Parkinson's disease | 77 | Male | 20.67 |
| AN02532 | Parkinson's disease | 79 | Male | 21.12 |
| AN12710 | Parkinson's disease | 87 | Male | 5.58 |
| AN01459 | Parkinson's disease | 87 | Male | 12.3 |
| AN15147 | Parkinson's disease | 82 | Female | 18.08 |
| AN02821 | Parkinson's disease | 81 | Male | 23 |
| AN11926 | Parkinson's disease | 84 | Male | 13.82 |
| AN13686 | Parkinson's disease | 88 | Male | 24.25 |
| 8118 | Parkinson's disease | 83 | Male | 15.03 |
| 8095 | Parkinson's disease | 70 | Male | 22.92 |
| 8091 | Parkinson's disease | 71 | Male | 23.42 |
| 8067 | Parkinson's disease | 75 | Male | 26.08 |
| AN05419 | Parkinson's disease | 76 | Male | 13 |
| AN05440 | Parkinson's disease | 77 | Male | 18 |
| AN06461 | Parkinson's disease | 87 | Male | 28 |
| AN18571 | Parkinson's disease | 70 | Male | 20 |
| AN10634 | Parkinson's disease | 86 | Male | 20 |
| AN10333 | Parkinson's disease | 74 | Male | 30 |
PMI, postmortem interval.
Glucocerebrosidase activity analysis
Brain tissue was resuspended in ice-cold MilliQ water and homogenized for 15 sec. A portion of the homogenate was used for protein determination (BCA Assay, Pierce, Pierce Biotechnology, Rockford/IL, USA). To determine GCase activity, brain homogenates were diluted in a 2 mg/mL bovine serum albumin, citric acid sodium phosphate buffer (pH 5). Ten microliter of sample was added to 75 μL of 10 mmol/L 4-Mu-β-d-glucopyranoside (Sigma, Sigma-Aldrich, St. Louis, MO, USA) substrate. After incubation with the substrate for 60 min at 37°C, the reaction was terminated using a stop solution (0.3 mol/L glycine/0.2 mol/L sodium carbonate, pH 10.7). Plates were read (Ex 360/Em 460) using a Molecular Devices, Sunnyvale, CA SPECTRAmax plate reader. Enzymatic activity was assessed from a 4-Mu standard curve and normalized to protein content in each sample.
LC-MS/MS analysis GluSph
A 50-μL aliquot of homogenate was used to prepare sample for the quantitation of GluSph. Eight hundred microliter of acetonitrile and methanol 1:1 solvent was used to extract GluSph from the tissue. The samples were sonicated, vortexed, and centrifuged before being transferred to injection plate for Liquid chromatography/tandem mass spectrometry (LC-MS/MS) analysis. For quantification of GluSph, 2 μL of sample was injected onto a Supelco 2.1 mm i.d. × 25 cm LC-Si HPLC (high-performance liquid chromatography) column (Sigma) connected to a Shimadzu LC Prominence UFLC solvent delivery system. The isocratic solvent system consisted of 0.08 mL/min mobile phase A (water) and 1.0 mL/min mobile phase B (CH3CN/CH3OH/CH3COOH 97:2:1 with 5 mmol/L of ammonium formate). The Selected Reaction Monitoring transitions monitored for GluSph and the internal standard plant GluSph were m/z 462 to m/z 282 and m/z 460 to m/z 280, respectively.
Results
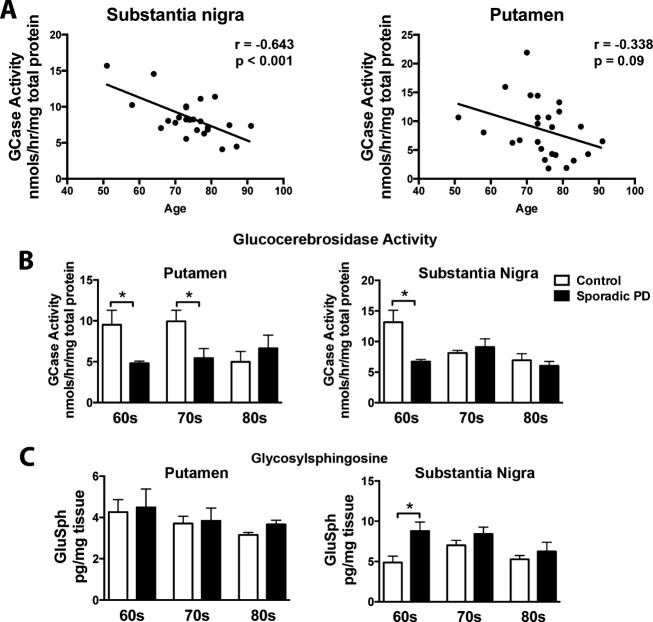
Glucocerebrosidase activity progressively declines in the substantia nigra with normal aging
Brain tissue from nonGBA1 mutation carrying sporadic PD patients was collected and GCase activity was measured in substantia nigra and putamen. Our data show a clear age-dependent decrease in GCase activity in the substantia nigra that spans the sixth to eighth decade of life (r = −0.643, P < 0.001) in healthy subject controls (Fig.1A). A similar trend was also observed in the putamen across the sixth to eighth decade of life in healthy subject controls. This gradual decline in GCase activity in healthy subject controls became similar to nonGBA1 mutation carrying PD patients by the seventh decade in the substantia nigra (F2,35 = 28.26, P < 0.0001) and the eighth decade in the putamen (F2,32 = 7.16, P < 0.05). GluSph levels were increased in sporadic PD patients at the sixth decade of life (F2,35 = 14.52, P < 0.001) in the substantia nigra. We measured both GluCer and GluSph in sporadic PD patient tissue, however, due to an interfering peak, only GluSph was analyzed.
Figure 1.
GCase activity is gradually decreased in the substantia nigra and putamen during normal aging. Brain tissue homogenates from frozen postmortem brain samples were used for measurements of GCase using a 4-methylumbelliferyl activity assay. (A) GCase activity gradually decreased in the substantia nigra and the putamen over the sixth to eighth decade of life in healthy subjects. (B) GCase activity remained consistently low in non-GBA mutation carrying PD patients across the sixth to eighth decade of life in comparison to healthy age-matched controls. (C) By the seventh and eighth decade of life GCase activity levels in healthy subject controls looked similar to PD patients in the substantia nigra and putamen, respectively. GluSph levels were increased in sporadic PD patients at the sixth decade of life in the substantia nigra, but did not change in the putamen. *P < 0.05, two-way ANOVA with Bonferroni post hoc analysis, correlation analysis completed by Pearson test. N = 23/disease cohort. Graphs are expressed as mean ± SEM. GCase, glucocerebrosidase; GluSph, glucosylsphingosine; PD, Parkinson's disease; SEM, standard error of the mean; ANOVA, analysis of variance.
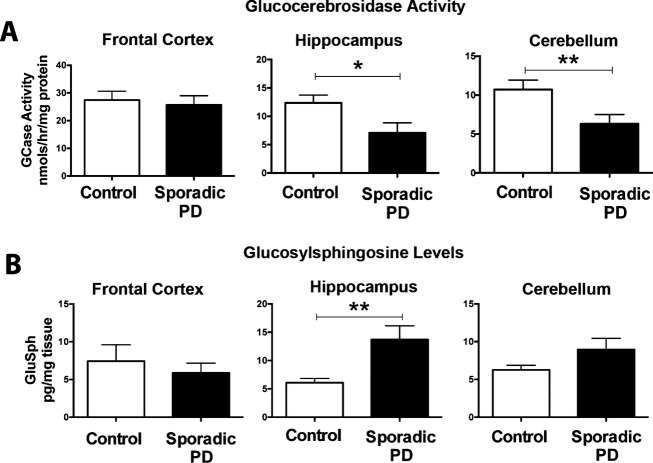
Glucocerebrosidase activity is decreased in the brains of sporadic PD patients coinciding with an accumulation of GluSph
GCase activity and levels of GluSph were also assayed in the frontal cortex, hippocampus, and cerebellum of nonGBA1 PD patients and age-matched healthy controls between their 70 and 80 years of age. GCase activity was significantly decreased in the hippocampus (T1,8 = 2.405, P < 0.05) and cerebellum (T1,16 = 2.439, P < 0.05) in nonGBA1 PD patients in comparison to age-matched controls. We found a significant increase in GluSph in the hippocampus (T1,10 = 3.19, P < 0.05) of nonGBA1 PD patients. There was a trend toward an increase for GluSph in the cerebellum of nonGBA1 PD patients in comparison to age-matched controls (Fig.2B).
Figure 2.
GCase activity is decreased and GluSph is increased in sporadic PD patient brains. GCase is a lysosomal enzyme responsible for the hydrolysis of the lipid substrates GluCer into ceramide and glucose, and GluSph into sphingosine and glucose. Data show that GBA activity is diminished in the putamen, hippocampus, substantia nigra, and cerebellum of sporadic PD patients in comparison to age-matched controls (A). Diminished GBA activity corresponded to accumulation of GluSph in the hippocampus (B). *P < 0.05, unpaired t-test. N = 6–12/group. Graphs are expressed as mean ± SEM. GCase, glucocerebrosidase; GluSph, glucosylsphingosine; GluCer, glucosylceramide; PD, Parkinson's disease; SEM, standard error of the mean; ANOVA, analysis of variance.
Discussion
Age-dependent reductions in GCase activity occur in normal aging and in sporadic PD patients
The involvement of GCase in the pathogenesis of PD is still not understood. Postmortem brain samples from nonGBA1 PD patients show a significant decrease in GCase activity and protein levels in the substantia nigra and cerebellum.14 We demonstrate for the first time that GCase activity gradually declines in the substantia nigra of healthy subject controls, eventually becoming comparable to PD patients by the seventh decade of life. Similarly, GCase activity also progressively declines in the putamen becoming comparable to PD patients by the eighth decade of life. Reductions in GCase activity may represent a progressive reduction in lysosomal function with normal aging, which may eventually become pathological to the cell. This is supported by recent evidence demonstrating that a reduction in GCase activity accelerates the progression and symptoms of PD.12,15 This reduction in GCase activity is accompanied by an accumulation of GluSph in the substantia nigra. This is the first report, to the best of our knowledge that has measured glycolipids in the brain of PD patients. We observed the accumulation in GluSph in the substantia nigra may reflect diminished lysosomal clearance capacity.
Although PD is rare before age 40, the prevalence rises almost exponentially after 50 years of age.1 It is possible that when the amount of functioning GCase is reduced below a critical level, it may directly influence lysosomal clearance capacity causing inefficient protein degradation, and may eventually become pathological promoting the accumulation of misfolded proteins, and dysfunctional mitochondria GBA1 mutations are twice as common in early onset PD in comparison to late onset PD cases.8 PD patients with a heterozygous GBA1 mutation are associated with 30–50% reduction in GCase activity.12 These patients usually develop the disease earlier and are associated with more advanced α-synuclein pathology.6,8,11 The motor impairments among GBA1 PD patients usually occur 6–7 years earlier in comparison to nonGBA1 PD patients.8,16 The data reported here indicate that with age, the levels of GCase activity in healthy subjects eventually reach a level of GCase reduction that is similar to GBA1 haploid insufficient PD patients. Given that the cell biological processes caused by GBA1 haploid-insufficiency predispose individuals to PD by leading to early degeneration and α-synuclein pathology with onset of the disease in the sixth decade, it is therefore reasonable to assume that a 30–50% age-dependent reduction in GCase activity in the seventh and eighth decade of life (during normal aging) would significantly increase the risk for developing PD in a nonGBA1 carrier. Moreover, sporadic PD is likely caused by a combination of convergent mechanisms that include age-related risk factors that contribute to DA neuron vulnerability such as accumulation of dysfunctional mitochondria, deficits in protein degradation systems, and aggregated α-synuclein accumulation. These age-related biological deficits likely contribute to the exponential rise in PD in the seventh and eighth decade of life. Tracking GCase activity levels during aging and during the preclinical phase of the disease may provide a unique opportunity to identify changes that occur prior to the pathophysiology that leads to the onset of PD symptoms.
Diminished GCase activity and GluSph accumulation occur in sporadic non-GBA mutation PD patients
We found widespread reduction in GCase activity in the cerebellum and hippocampus, in addition to the putamen and substantia nigra of sporadic PD patients that are non-GBA mutation carriers, which coincided with an increase in GluSph. These results show that the GluSph lipid substrate is accumulated in the brain of sporadic PD patients. It is possible that this accumulation of the GluSph disrupts intralysosomal homeostasis and promotes aggregation of α-synuclein, thus enhancing neuronal vulnerability. Recent observations have suggested a reciprocal relationship exists between GCase and α-synuclein.17,18 Moreover, a landmark finding supports this reciprocal relationship between GCase and α-synuclein by demonstrating that a partial loss of GCase activity can interfere with protein degradation in the lysosome, promote accumulation of α-synuclein, and enhance α-synuclein-mediated neurotoxicity.17 Therefore, accumulation of GluSph, and possibly GluCer, may contribute to the pathophysiology of PD by disrupting lysosomal function.
Acknowledgments
This work was supported by the Harvard Stem Cell Institute Translational Neuroscience Fund, the Harold and Ronna Cooper family, the Consolidated Anti-Aging Foundation, the Poul Hansen family, and the Canadian Institute of Health Research. We would like to thank the Harvard Brain Tissue Resource Center (Belmont, MA) for human tissue samples.
Conflict of Interest
None declared.
References
- Kasten M, Chade A, Tanner CM. Epidemiology of Parkinson's disease. Handb Clin Neurol. 2007;83:129–151. doi: 10.1016/S0072-9752(07)83006-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Vicente M, Sovak G, Cuervo AM. Protein degradation and aging. Exp Gerontol. 2005;40:622–633. doi: 10.1016/j.exger.2005.07.005. [DOI] [PubMed] [Google Scholar]
- Klionsky DJ. Autophagy: from phenomenology to molecular understanding in less than a decade. Nat Rev Mol Cell Biol. 2007;8:931–937. doi: 10.1038/nrm2245. [DOI] [PubMed] [Google Scholar]
- Chu CT, Zhu J, Dagda R. Beclin 1-independent pathway of damage-induced mitophagy and autophagic stress: implications for neurodegeneration and cell death. Autophagy. 2007;3:663–666. doi: 10.4161/auto.4625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asselta R, Rimoldi V, Siri C, et al. Glucocerebrosidase mutations in primary parkinsonism. Parkinsonism Relat Disord. 2014;20:1215–1220. doi: 10.1016/j.parkreldis.2014.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lees AJ, Hardy J, Revesz T. Parkinson's disease. Lancet. 2009;373:2055–2066. doi: 10.1016/S0140-6736(09)60492-X. [DOI] [PubMed] [Google Scholar]
- Neumann J, Bras J, Deas E, et al. Glucocerebrosidase mutations in clinical and pathologically proven Parkinson's disease. Brain. 2009;132(Pt 7):1783–1794. doi: 10.1093/brain/awp044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidransky E, Lopez G. The link between the GBA gene and parkinsonism. Lancet Neurol. 2012;11:986–998. doi: 10.1016/S1474-4422(12)70190-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark LN, Ross BM, Wang Y, et al. Mutations in the glucocerebrosidase gene are associated with early-onset Parkinson disease. Neurology. 2007;69:1270–1277. doi: 10.1212/01.wnl.0000276989.17578.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bras J, Singleton A, Cookson MR, Hardy J. Emerging pathways in genetic Parkinson's disease: potential role of ceramide metabolism in Lewy body disease. FEBS J. 2008;275:5767–5773. doi: 10.1111/j.1742-4658.2008.06709.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy KE, Gysbers AM, Abbott SK, et al. Reduced glucocerebrosidase is associated with increased alpha-synuclein in sporadic Parkinson's disease. Brain. 2014;137(Pt 3):834–848. doi: 10.1093/brain/awt367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidransky E, Nalls MA, Aasly JO, et al. Multicenter analysis of glucocerebrosidase mutations in Parkinson's disease. N Engl J Med. 2009;361:1651–1661. doi: 10.1056/NEJMoa0901281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gegg ME, Burke D, Heales SJ, et al. Glucocerebrosidase deficiency in substantia nigra of Parkinson disease brains. Ann Neurol. 2012;72:455–463. doi: 10.1002/ana.23614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvin JE, Lee VM, Trojanowski JQ. Synucleinopathies: clinical and pathological implications. Arch Neurol. 2001;58:186–190. doi: 10.1001/archneur.58.2.186. [DOI] [PubMed] [Google Scholar]
- Bras J, Paisan-Ruiz C, Guerreiro R, et al. Complete screening for glucocerebrosidase mutations in Parkinson disease patients from Portugal. Neurobiol Aging. 2009;30:1515–1517. doi: 10.1016/j.neurobiolaging.2007.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockmann K, Srulijes K, Pflederer S, et al. GBA-associated Parkinson's disease: reduced survival and more rapid progression in a prospective longitudinal study. Mov. 2014 doi: 10.1002/mds.26071. December [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Mazzulli JR, Xu YH, Sun Y, et al. Gaucher disease glucocerebrosidase and alpha-synuclein form a bidirectional pathogenic loop in synucleinopathies. Cell. 2011;146:37–52. doi: 10.1016/j.cell.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siebert M, Sidransky E, Westbroek W. Glucocerebrosidase is shaking up the synucleinopathies. Brain. 2014;137:1304–1322. doi: 10.1093/brain/awu002. (Pt 5): [DOI] [PMC free article] [PubMed] [Google Scholar]