Abstract
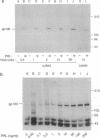
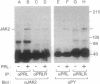
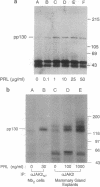
One of the earliest cellular responses to prolactin (PRL) binding in Nb2 cells, a rat pre-T lymphoma cell line, is an increase in tyrosine phosphorylation of cellular proteins. In this work, immunologic techniques have been used to demonstrate that in Nb2 cells and in mouse mammary gland explants, JAK2, a non-receptor tyrosine kinase, is activated following stimulation with PRL. PRL stimulated tyrosine phosphorylation of JAK2 at times as early as 30 sec and concentrations of PRL as low as 0.5 ng/ml (2.5 pM) in Nb2 cells and 100 ng/ml (5 nM) in mammary gland explants. When JAK2 was immunoprecipitated from solubilized Nb2 cells or mammary gland explants and incubated with [gamma-32P]ATP, 32P was incorporated into a protein migrating with an apparent molecular weight appropriate for JAK2 only when cells had been incubated with PRL, indicating that JAK2 tyrosine kinase activity is exquisitely sensitive to PRL. In Nb2 cells, JAK2 was found to associate with PRL receptor irrespective of whether or not the cells had been incubated with PRL. These results provide strong evidence that JAK2 is constitutively associated with the PRL receptor and that it is activated and tyrosine phosphorylated upon PRL binding to the PRL receptor. These results are consistent with JAK2 serving as an early, perhaps initial, signaling molecule for PRL.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ali S., Pellegrini I., Kelly P. A. A prolactin-dependent immune cell line (Nb2) expresses a mutant form of prolactin receptor. J Biol Chem. 1991 Oct 25;266(30):20110–20117. [PubMed] [Google Scholar]
- Argetsinger L. S., Campbell G. S., Yang X., Witthuhn B. A., Silvennoinen O., Ihle J. N., Carter-Su C. Identification of JAK2 as a growth hormone receptor-associated tyrosine kinase. Cell. 1993 Jul 30;74(2):237–244. doi: 10.1016/0092-8674(93)90415-m. [DOI] [PubMed] [Google Scholar]
- Bazan J. F. Structural design and molecular evolution of a cytokine receptor superfamily. Proc Natl Acad Sci U S A. 1990 Sep;87(18):6934–6938. doi: 10.1073/pnas.87.18.6934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron C. M., Kostyo J. L., Rillema J. A., Gennick S. E. Reduced and S-carboxymethylated human growth hormone: a probe for diabetogenic action. Am J Physiol. 1984 Nov;247(5 Pt 1):E639–E644. doi: 10.1152/ajpendo.1984.247.5.E639. [DOI] [PubMed] [Google Scholar]
- Campbell G. S., Christian L. J., Carter-Su C. Evidence for involvement of the growth hormone receptor-associated tyrosine kinase in actions of growth hormone. J Biol Chem. 1993 Apr 5;268(10):7427–7434. [PubMed] [Google Scholar]
- Cantley L. C., Auger K. R., Carpenter C., Duckworth B., Graziani A., Kapeller R., Soltoff S. Oncogenes and signal transduction. Cell. 1991 Jan 25;64(2):281–302. doi: 10.1016/0092-8674(91)90639-g. [DOI] [PubMed] [Google Scholar]
- Duronio V., Clark-Lewis I., Federsppiel B., Wieler J. S., Schrader J. W. Tyrosine phosphorylation of receptor beta subunits and common substrates in response to interleukin-3 and granulocyte-macrophage colony-stimulating factor. J Biol Chem. 1992 Oct 25;267(30):21856–21863. [PubMed] [Google Scholar]
- Dusanter-Fourt I., Casadevall N., Lacombe C., Muller O., Billat C., Fischer S., Mayeux P. Erythropoietin induces the tyrosine phosphorylation of its own receptor in human erythropoietin-responsive cells. J Biol Chem. 1992 May 25;267(15):10670–10675. [PubMed] [Google Scholar]
- Foster C. M., Shafer J. A., Rozsa F. W., Wang X. Y., Lewis S. D., Renken D. A., Natale J. E., Schwartz J., Carter-Su C. Growth hormone promoted tyrosyl phosphorylation of growth hormone receptors in murine 3T3-F442A fibroblasts and adipocytes. Biochemistry. 1988 Jan 12;27(1):326–334. doi: 10.1021/bi00401a049. [DOI] [PubMed] [Google Scholar]
- Gala R. R. Prolactin and growth hormone in the regulation of the immune system. Proc Soc Exp Biol Med. 1991 Oct;198(1):513–527. doi: 10.3181/00379727-198-43286b. [DOI] [PubMed] [Google Scholar]
- Hunter T., Sefton B. M. Transforming gene product of Rous sarcoma virus phosphorylates tyrosine. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1311–1315. doi: 10.1073/pnas.77.3.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly P. A., Djiane J., Postel-Vinay M. C., Edery M. The prolactin/growth hormone receptor family. Endocr Rev. 1991 Aug;12(3):235–251. doi: 10.1210/edrv-12-3-235. [DOI] [PubMed] [Google Scholar]
- Lesueur L., Edery M., Ali S., Paly J., Kelly P. A., Djiane J. Comparison of long and short forms of the prolactin receptor on prolactin-induced milk protein gene transcription. Proc Natl Acad Sci U S A. 1991 Feb 1;88(3):824–828. doi: 10.1073/pnas.88.3.824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mena F., Enjalbert A., Carbonell L., Priam M., Kordan C. Effect of suckling on plasma prolactin and hypothalamic monoamine levels in the rat. Endocrinology. 1976 Aug;99(2):445–451. doi: 10.1210/endo-99-2-445. [DOI] [PubMed] [Google Scholar]
- Meyer D. J., Campbell G. S., Cochran B. H., Argetsinger L. S., Larner A. C., Finbloom D. S., Carter-Su C., Schwartz J. Growth hormone induces a DNA binding factor related to the interferon-stimulated 91-kDa transcription factor. J Biol Chem. 1994 Feb 18;269(7):4701–4704. [PubMed] [Google Scholar]
- Miura O., D'Andrea A., Kabat D., Ihle J. N. Induction of tyrosine phosphorylation by the erythropoietin receptor correlates with mitogenesis. Mol Cell Biol. 1991 Oct;11(10):4895–4902. doi: 10.1128/mcb.11.10.4895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller M., Briscoe J., Laxton C., Guschin D., Ziemiecki A., Silvennoinen O., Harpur A. G., Barbieri G., Witthuhn B. A., Schindler C. The protein tyrosine kinase JAK1 complements defects in interferon-alpha/beta and -gamma signal transduction. Nature. 1993 Nov 11;366(6451):129–135. doi: 10.1038/366129a0. [DOI] [PubMed] [Google Scholar]
- Okamura H., Raguet S., Bell A., Gagnon J., Kelly P. A. Purification and protein sequence analysis of rat liver prolactin receptor. J Biol Chem. 1989 Apr 5;264(10):5904–5911. [PubMed] [Google Scholar]
- Rillema J. A., Campbell G. S., Lawson D. M., Carter-Su C. Evidence for a rapid stimulation of tyrosine kinase activity by prolactin in Nb2 rat lymphoma cells. Endocrinology. 1992 Aug;131(2):973–975. doi: 10.1210/endo.131.2.1639035. [DOI] [PubMed] [Google Scholar]
- Rillema J. A. Early actions of prolactin on uridine metabolism in mammary gland explants. Endocrinology. 1973 Jun;92(6):1673–1679. doi: 10.1210/endo-92-6-1673. [DOI] [PubMed] [Google Scholar]
- Rui H., Djeu J. Y., Evans G. A., Kelly P. A., Farrar W. L. Prolactin receptor triggering. Evidence for rapid tyrosine kinase activation. J Biol Chem. 1992 Nov 25;267(33):24076–24081. [PubMed] [Google Scholar]
- Shuai K., Schindler C., Prezioso V. R., Darnell J. E., Jr Activation of transcription by IFN-gamma: tyrosine phosphorylation of a 91-kD DNA binding protein. Science. 1992 Dec 11;258(5089):1808–1812. doi: 10.1126/science.1281555. [DOI] [PubMed] [Google Scholar]
- Silvennoinen O., Witthuhn B. A., Quelle F. W., Cleveland J. L., Yi T., Ihle J. N. Structure of the murine Jak2 protein-tyrosine kinase and its role in interleukin 3 signal transduction. Proc Natl Acad Sci U S A. 1993 Sep 15;90(18):8429–8433. doi: 10.1073/pnas.90.18.8429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha Y. N., Salocks C. B., Lewis U. J., VanderLaan W. P. Influence of nursing on the release of prolactin and GH in mice with high and low incidence of mammary tumors. Endocrinology. 1974 Oct;95(4):947–954. doi: 10.1210/endo-95-4-947. [DOI] [PubMed] [Google Scholar]
- Tai P. K., Liao J. F., Chen E. H., Dietz J., Schwartz J., Carter-Su C. Differential regulation of two glucose transporters by chronic growth hormone treatment of cultured 3T3-F442A adipose cells. J Biol Chem. 1990 Dec 15;265(35):21828–21834. [PubMed] [Google Scholar]
- Tanaka T., Shiu R. P., Gout P. W., Beer C. T., Noble R. L., Friesen H. G. A new sensitive and specific bioassay for lactogenic hormones: measurement of prolactin and growth hormone in human serum. J Clin Endocrinol Metab. 1980 Nov;51(5):1058–1063. doi: 10.1210/jcem-51-5-1058. [DOI] [PubMed] [Google Scholar]
- Velazquez L., Fellous M., Stark G. R., Pellegrini S. A protein tyrosine kinase in the interferon alpha/beta signaling pathway. Cell. 1992 Jul 24;70(2):313–322. doi: 10.1016/0092-8674(92)90105-l. [DOI] [PubMed] [Google Scholar]
- Wang X., Uhler M. D., Billestrup N., Norstedt G., Talamantes F., Nielsen J. H., Carter-Su C. Evidence for association of the cloned liver growth hormone receptor with a tyrosine kinase. J Biol Chem. 1992 Aug 25;267(24):17390–17396. [PubMed] [Google Scholar]
- Watling D., Guschin D., Müller M., Silvennoinen O., Witthuhn B. A., Quelle F. W., Rogers N. C., Schindler C., Stark G. R., Ihle J. N. Complementation by the protein tyrosine kinase JAK2 of a mutant cell line defective in the interferon-gamma signal transduction pathway. Nature. 1993 Nov 11;366(6451):166–170. doi: 10.1038/366166a0. [DOI] [PubMed] [Google Scholar]
- Witthuhn B. A., Quelle F. W., Silvennoinen O., Yi T., Tang B., Miura O., Ihle J. N. JAK2 associates with the erythropoietin receptor and is tyrosine phosphorylated and activated following stimulation with erythropoietin. Cell. 1993 Jul 30;74(2):227–236. doi: 10.1016/0092-8674(93)90414-l. [DOI] [PubMed] [Google Scholar]
- Yamada K., Donner D. B. Structures of the somatotropin receptor and prolactin receptor on rat hepatocytes characterized by affinity labelling. Biochem J. 1984 Jun 1;220(2):361–369. doi: 10.1042/bj2200361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura A., Lodish H. F. In vitro phosphorylation of the erythropoietin receptor and an associated protein, pp130. Mol Cell Biol. 1992 Feb;12(2):706–715. doi: 10.1128/mcb.12.2.706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vos A. M., Ultsch M., Kossiakoff A. A. Human growth hormone and extracellular domain of its receptor: crystal structure of the complex. Science. 1992 Jan 17;255(5042):306–312. doi: 10.1126/science.1549776. [DOI] [PubMed] [Google Scholar]