Abstract
Recurrence of focal segmental glomerulosclerosis (rFSGS) after kidney transplantation is a cause of accelerated graft loss. To evaluate pathogenic antibodies (Abs) in rFSGS, we processed 141 serum samples from 64 patients with and without primary rFSGS and 34 non-FSGS control patients transplanted at four hospitals. We screened about 9000 antigens in pretransplant sera and selected 10 Abs targeting glomerular antigens for enzyme-linked immunosorbent assay (ELISA) validation. A panel of seven Abs (CD40, PTPRO, CGB5, FAS, P2RY11, SNRPB2, and APOL2) could predict posttransplant FSGS recurrence with 92% accuracy. Pretransplant elevation of anti-CD40 Ab alone had the best correlation (78% accuracy) with rFSGS risk after transplantation. Epitope mapping of CD40 with customized peptide arrays and rFSGS sera demonstrated altered immunogenicity of the extracellular CD40 domain in rFSGS. Immunohistochemistry of CD40 demonstrated a differential expression in FSGS compared to non-FSGS controls. Anti-CD40 Abs purified from rFSGS patients were particularly pathogenic in human podocyte cultures. Injection of anti-CD40/rFSGS Ab enhanced suPAR (soluble urokinase receptor)–mediated proteinuria in wild-type mice, yet no sensitizing effect was noted in mice deficient in CD40 or in wild-type mice that received blocking Ab to CD40. In conclusion, a panel of seven Abs can help identify primary FSGS patients at high risk of recurrence before transplantation. Intrarenal CD40 (and possibly other specific glomerular antigens) is an important contributor to FSGS disease pathogenesis. Human trials of anti-CD40 therapies are warranted to evaluate their efficacy for preventing rFSGS and improving graft survival.
INTRODUCTION
Primary focal segmental glomerulosclerosis (FSGS) is a proteinuric glomerular disease that affects podocyte function and survival and results in a typical pattern of histopathological injury, including glomerulosclerosis on kidney biopsy (1, 2). Renal transplant patients with primary FSGS face a high risk of disease recurrence in the allograft (20 to 40% after a first transplant and up to 80% for retransplantation) (3, 4). Recent clinical association studies (5, 6) detail the problem with FSGS recurrence. Detailed human sample, animal, and cell studies identified elevated pre- and posttransplantation serum levels of the soluble urokinase receptor (suPAR) (7, 8) as one of the potential factors causing native and recurrent FSGS (rFSGS) (7). However, not all molecular forms of suPAR are equally pathogenic to podocytes (9, 10), and thus, improvements in pretransplant risk stratification for rFSGS are still a major clinical challenge.
Circulating permeability factors and autoantibodies (autoAbs), such as anti-actin, anti–adenosine triphosphate synthase, anti-angiotensin II type 1 receptor, and anti-nephrin (11–14), have been implicated in the pathogenesis of rFSGS. It was suggested that autoAbs participate in the pathogenesis of rFSGS because autoAb directed against protein tyrosine phosphatase receptor type O (PTPRO), nephrin, or anti-Thy1 (15, 16) can cause an increase in glomerular permeability when injected into animal models, and rFSGS can be improved in some cases by manipulation of the humoral response with plasmapheresis and rituximab (17–20) . Although total suPAR serum levels sometimes correlate with FSGS recurrence (7, 8), they can also be elevated in other conditions such as sepsis, suggesting the presence of yet to be defined FSGS-specific suPAR forms. We have demonstrated that podocyte-specific expression of SMPDL3b in post-reperfusion kidney biopsies (18) may predict recurrent proteinuria, but alternative, noninvasive pretransplant biomarkers to predict rFSGS are still needed.
Here, we describe the identification of a panel of autoAbs to predict rFSGS before transplantation, by using an integrative bioinformatics approach on high-density protein array data (21–24), followed by an independent enzyme-linked immunosorbent assay (ELISA) validation. Furthermore, we elucidate a pathogenic role for patient-derived anti-CD40 Ab, which cooperate with circulating suPAR to elicit podocyte injury and proteinuria. We also present preclinical data that support CD40 as an additional therapeutic target for rFSGS.
RESULTS
Identification of Abs associated with rFSGS after renal transplantation
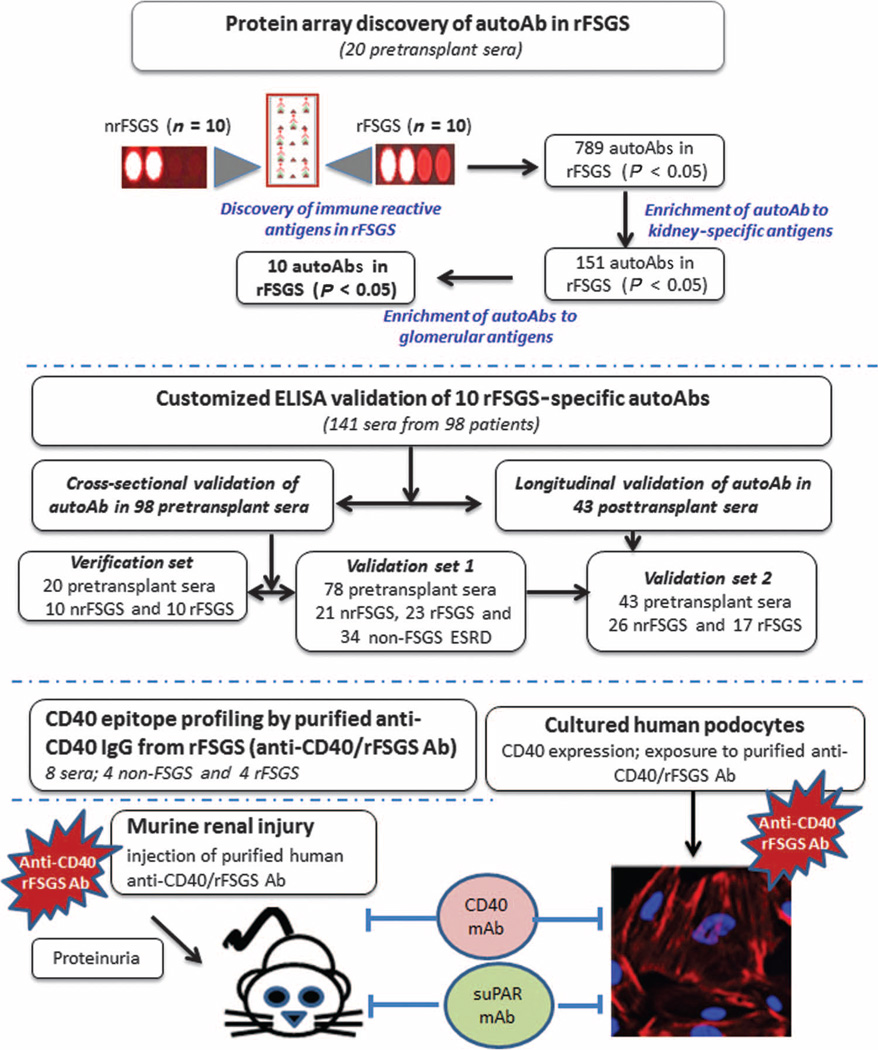
To identify potential autoAbs associated with rFSGS, we used a discovery set of pretransplant sera from 20 patients with biopsy-confirmed diagnosis of primary FSGS as the cause of their end-stage renal disease (ESRD), of whom 10 had progressed to rFSGS within the first year after transplant (mean time to recurrence, 36 days) and 10 had not had recurrence of proteinuria or histological disease after transplantation [nonrecurrent FSGS (nrFSGS)]. At transplant, these two groups of patients were indistinguishable by demographical, disease, or clinical parameters (Table 1). Recurrence was defined as heavy proteinuria with biopsy confirmation of FSGS, with glomerular sclerosis and podocyte fusion and injury, and without evidence of acute rejection, glomerulitis, or allograft glomerulopathy. The patients were monitored for 6 months before transplantation and followed up 12 months after transplantation. Twenty unique pretransplant serum samples were assayed on high-density protein microarrays. Figure 1 summarizes the study. Immunoglobulin G (IgG) Abs against a large panel (n = 789) of unique antigens were significantly increased (P value range, 0.0004 to 0.0433) in the recurrent group at transplant, with a smaller number of IgG Abs (n = 78) showing an increase in the nonrecurrent patients. These significantly different IgG profiles in the sera of rFSGS and nrFSGS patients demonstrated that rFSGS is associated with a signature of increased humoral responses to a variety of non–human leukocyte antigens (non-HLA) after kidney transplantation. The protein array data are available in the Gene Expression Omnibus (GEO) database (GEO ID: GSE57099). The normalized data are presented in table S1, and a volcano plot for the data is presented in fig. S1.
Table 1. Demographics of the patients with ESRD secondary to primary FSGS.
Patients are subdivided into the test set (ProtoArray Discovery) and the validation set (ELISA validation).
| Test set |
Validation set |
||||||
|---|---|---|---|---|---|---|---|
| Parameters | Nonrecurrent (n = 10) |
Recurrent (n = 10) |
P | Control (n = 34) |
Nonrecurrent (n = 23) |
Recurrent (n = 27) |
|
| Age nephrotic syndrome began (year, mean ± SE) | 23.4 ± 2.9 | 28.2 ± 4.8 | 0.77 | 31 ± 2.5 | 26.6 ± 3.4 | 23.2 ± 2.8 | 0.33 |
| Race (% black) | 30 | 50 | 0.65 | 11 | 29 | 38 | 0.07 |
| Sex (% male) | 80 | 60 | 0.63 | 52 | 85 | 61 | 0.71 |
| Native kidney treated with steroids (% yes) | 70 | 50 | 0.65 | 35 | 73 | 72 | 0.0007 |
| Native kidney treated with cyclosporine (% yes) | 30 | 30 | 1.00 | 0 | 53 | 61 | 0.0001 |
| Nephrotic syndrome in native kidney (% yes) | 50 | 80 | 0.34 | 32 | 62 | 77 | 0.0007 |
| Previous transplantation (% yes) | 30 | 10 | 0.58 | 32 | 33 | 44 | 0.34 |
| Cumulative time on dialysis (year, mean ± SE) | 4.9 ± 1.1 | 3.3 ± 1.0 | 0.19 | 2.5 ± 1.8 | 5.2 ± 0.9 | 3.5 ± 0.7 | 0.09 |
| Weight day 0 (kg, mean ± SE) | 76.7 ± 9.5 | 69.5 ± 2.4 | 0.76 | 75 ± 2.3 | 68 ± 3 | 76 ± 5 | 0.45 |
| γ Globulin day 0 (g/liter, mean ± SE) | 11.0 ± 1.2 | 12.8 ± 1.4 | 0.23 | 10.85 ± 0.7 | 9.7 ± 1.2 | 12.8 ± 1.4 | 0.25 |
| Delayed graft function (% yes) | 10 | 40 | 0.30 | 27 | 19 | 16 | 0.25 |
| Number of dialysis treatments after transplant (n, mean ± SE) |
0.4 ± 0.4 | 1.2 ± 0.8 | 0.18 | 0.9 ± 0.4 | 0.7 ± 0.4 | 0.5 ± 0.3 | 0.77 |
| Rejection (% yes) | 20 | 10 | 1.00 | 41 | 33 | 33 | 0.82 |
| Serum creatinine at 3 months (mmol/liter, mean ± SE) | 122 ± 9 | 145 ± 30 | 0.08 | 132 ± 7.1 | 157 ± 14 | 130 ± 30 | 0.38 |
| Serum creatinine at last follow-up (mmol/liter, mean ± SE) | 146 ± 26 | 205 ± 38 | 0.27 | 137 ± 8.2 | 158 ± 15 | 172 ± 26 | 0.33 |
Fig. 1. Study work flow.
A schematic of the study outlining the independent patients and samples used in discovery and validation of the Ab panel for predicting rFSGS and the experimental evaluation of CD40 in the pathogenesis of rFSGS.
To select the best biomarkers for additional validation of rFSGS prediction from pretransplant sera, we filtered the ProtoArray data to maximize for fold increase (>2) and signal intensity of at least twice the background threshold (>1000), which resulted in a selection of 151 IgG Abs from the larger panel of 789 Abs (table S1). Because the hallmark of histological diagnosis in rFSGS is podocyte/glomerular injury, we additionally filtered the IgG Ab panel to select for antigens that are expressed in the renal glomerulus, using our previously published integrative antibiomic analysis (22), where we performed cross-mapping of autoAb targets with kidney compartment gene expression data (22). We used the kidney- and kidney compartment–specific antigens identified in a previous study by Li et al. (22) and found that there was an enrichment for Ab specifically targeting particular compartments of the kidney, mainly the glomerulus (P = 0.02), the renal outer cortex (P = 0.01), the renal pelvis (P = 0.02), and the renal papillary tips (P = 0.01). Pathway analysis for inferred mechanisms of injury (Ingenuity Pathway Analysis) showed that these Abs were involved in pathways related to antigen presentation (P = 5.97 × 10−6), inflammation (P = 5.97 × 10−6), and cell death (P value = 6.03 × 10−5). We used the filtering criteria described (fold change, signal intensity, inferred glomerular expression, and functional relevance in inflammation and kidney injury) to select a panel of 10 IgG Abs (CD40, SNRBP2, FAS, PTPRO, P2RY11, RXRA, CCL19, MYLK, APOL2, and CGB5) for further validation by ELISA.
ELISA validation of Abs that can predict rFSGS after renal transplantation
Customized ELISA assays were generated on the Meso Scale Discovery (MSD) platform as previously described (21) for the autoAbs shown in Table 2. One hundred forty-one sera collected at different time points from 98 patients were processed for customized ELISA assays for all 10 autoAbs in Table 2. ELISA analyses confirmed statistically significant elevation of all 10 Ab titers [CD40 (P = 0.0002), PTPRO (P = 0.015), FAS (P = 0.0036), CGB5 (P = 0.031), SNRBP2 (P = 0.0044), APOL2 (P = 0.024), P2RY11 (P = 0.019), RXRA (P = 0.01), CCL19 (P = 0.015), and MYLK (P = 0.016)] in pretransplant sera from patients who experienced recurrence of FSGS after transplantation (n = 19) (Table 2 and Fig. 2). The difference in titer of some of these Abs [CGB5 (P < 0.0001), FAS (P < 0.0001), CD40 (P = 0.0002), PTPRO (P = 0.0005), APOL2 (P = 0.0005), P2RY11 (P = 0.0004), and SNRBP2 (P = 0.0035)] remained significant at 1 year after transplantation in rFSGS sera compared to sera from nrFSGS patients (Fig. 2) (table S2). When we performed a subanalysis on changes in Ab titers in the rFSGS group from before transplant to 1 year after, segregating for treatment response (resolution of proteinuria secondary to rituximab and intravenous cyclosporin), the reduction in titers of anti-CD40 Ab approached significance (5.3 ± 1.2 versus 3.8 ± 0.7 arbitrary units; P = 0.087), suggesting that the CD40 axis may be pathogenically relevant in the evolution of rFSGS.
Table 2. Selected Abs elevated in the sera of rFSGS patients.
Significantly elevated Abs were determined by ProtoArray and independently validated by ELISA.
| Protein targets for 10 selected autoAbs elevated in the sera of patients with rFSGS |
Gene symbol |
P value from ProtoArray Discovery (pretransplant sera; rFSGS versus nrFSGS) |
P value from ELISA validation (pretransplant sera; rFSGS versus nrFSGS) |
|---|---|---|---|
| CD40 molecule–TNF receptor superfamily member 5 | CD40 | 0.0027 | 0.0002 |
| Protein tyrosine phosphatase receptor O | PTPRO | 0.043 | 0.015 |
| TNF receptor superfamily member 6 | FAS | 0.0015 | 0.0036 |
| Chorionic gonadotropin β | CGB5 | 0.00035 | 0.032 |
| Ribonucleoprotein B | SNRBP2 | 0.00035 | 0.0044 |
| Apoliprotein 2 | APOL2 | 0.043 | 0.024 |
| P2Y purinoceptor 11 | P2RY11 | 0.0054 | 0.019 |
| Small nuclear retinoid × receptor, α | RXRA | 0.0098 | 0.01 |
| Chemokine (C-C motif) ligand 19 | CCL19 | 0.028 | 0.015 |
| Myosin light kinase | MYLK | 0.034 | 0.016 |
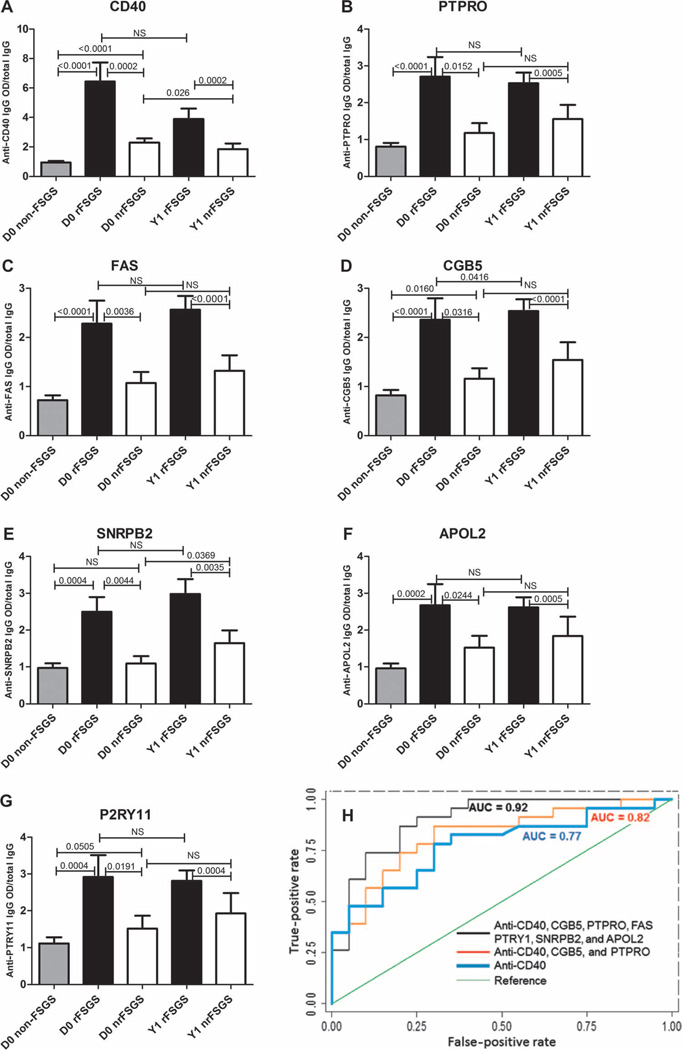
Fig. 2. Validation of the FAST Ab panel in rFSGS and the predictive accuracy of subsets of this panel.
(A to G) ELISA assays were developed and optimized to validate rFSGS-specific Ab. Black bars correspond to serum samples collected immediately before transplantation [day 0 (D0), n = 28] and at 1 year after transplantation [year 1 (Y1), n = 26] in patients who experienced rFSGS. White bars correspond to serum samples collected immediately before transplantation (day 0, n = 31) and at 1 year after transplantation (year 1, n = 17) in patients who did not experience rFSGS during the first year. Gray bars represent non-FSGS control samples (n = 34) and consist of patients with ESRD from causes other than FSGS (obstructive uropathy, reflux nephropathy, cystinosis, and glomerulonephritis) and six normal healthy controls. Y axis represents the ratio of the optical density (OD) from MSD ELISA assay over total IgG in mg/dl for each patient. ELISA results were analyzed with a Mann-Whitney test in GraphPad Prism. Respective P values are provided on the top of each bar. A P value of <0.05 is considered significant. NS, not significant. (H) ROC analysis for three fitted logistic regression models. The outcome is recurrence versus nonrecurrence of FSGS, and the independent predictors are the log-transformed relative fluorescent signal values of seven Abs: CD40, PTPRO, FAS, CGB5, SNRPB2, APOL2, and P2RY11. The three logistic regression models fitted are shown. Model 1 is the FAST (FSGS antibody serological test) panel with all seven Abs, giving an AUC = 0.9 (CI, 0.81 to 0.99). Model 2 used three Abs (CD40, PTPRO, and CGB5), and its ROC curve has an AUC of 0.82 (CI, 0.70 to 0.95). Model 3 used only CD40 Ab data for ROC analysis, resulting in an AUC of 0.77 (CI, 0.63 to 0.92).
To build a best-fit prognostic Ab panel for the pretransplant selection of primary FSGS patients who have a high risk of recurrence, we conducted receiver operating characteristic (ROC) analysis on log-transformed concentrations of the 10 autoAbs measured by ELISA in an independent set of pretransplant sera and fitted these to logistic regression models. A panel of seven Abs, which included CD40, FAS, PTPRO, P2RY11, CGB5, SNRPB2, and APOL2, predicted rFSGS with an area under the curve (AUC) of 0.90 [confidence interval (CI), 0.81 to 0.99]. A second model was built on three Abs (CD40, PTPRO, and CGB5) and predicted rFSGS with a slightly lower AUC of 0.82 (CI, 0.70 to 0.95). Anti-CD40 Ab was found to exert the maximal impact of any one Ab on the prediction risk of rFSGS, and even when used alone as a predictive biomarker, it had an AUC of 0.77 (CI, 0.63 to 0.92) (Fig. 2H).
Characterizing CD40 immune reactive epitopes in FSGS
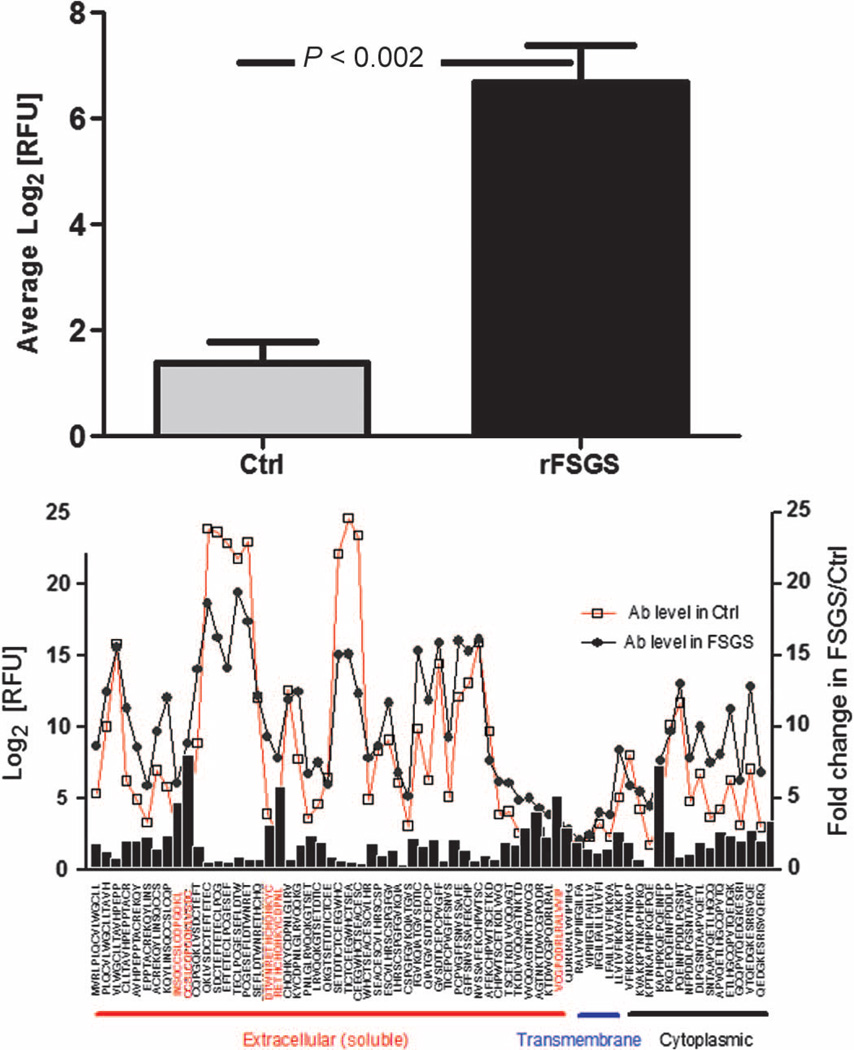
Because anti-CD40 Abs were found to be the most effective for predicting rFSGS, we evaluated possible mechanisms for the altered immunogenicity of the CD40 protein in rFSGS. We hypothesized that altered immune reactivity of the CD40 antigen may play a role in rFSGS and result in the increased concentration of anti-CD40 Ab seen in rFSGS both before and after transplantation. To test this hypothesis, we used a peptide array platform to synthesize peptides of 15 amino acids each, spanning the entire CD40 protein. This customized peptide array was hybridized with sera from four rFSGS patients and four nrFSGS control patients to measure the reactivity of different epitopes in the CD40 antigen (Fig. 3) in these two patient groups with similar causes of ESRD and different clinical outcomes. The peptide microarray scan revealed altered immunogenicity of the CD40 protein in the two β strand regions (NSQCC and ESEF) specifically in the rFSGS sera, suggesting that a perturbation in the conformation of the CD40 protein may cause rFSGS.
Fig. 3. Peptide array affinity mapping of the CD40 protein to anti-CD40/rFSGS Ab.
We used the peptide microarray platform to map differential binding of anti-CD40 Ab from rFSGS to CD40 epitopes. (A) Increased binding of anti-CD40/rFSGS Ab measured in terms of relative fluorescent intensity (RFU) to specific regions of CD40 (NSQCC and ESEF), compared to binding by the anti-CD40 Ab isolated from nrFSGS controls (Ctrl). (B) Ab binding against different CD40 epitopes, each spanning 15 amino acids, and covering the full-length CD40 protein. Anti-CD40 Ab was isolated from sera of patients with rFSGS (n = 4, black circles) and nrFSGS controls (n = 4, open squares). Black bars indicate the average fold increase in Ab responses against different CD40 antigenic epitopes in rFSGS sera relative to sera from nrFSGS. Areas of increased signal with more than two adjacent peptides in the extracellular region are boxed. Epitopes with unpaired t test P < 0.05 and ≥ 2-fold increase for rFSGS/nrFSGS controls (labeled in red) were considered significant.
Staining CD40 in kidney tissue by immunohistochemistry
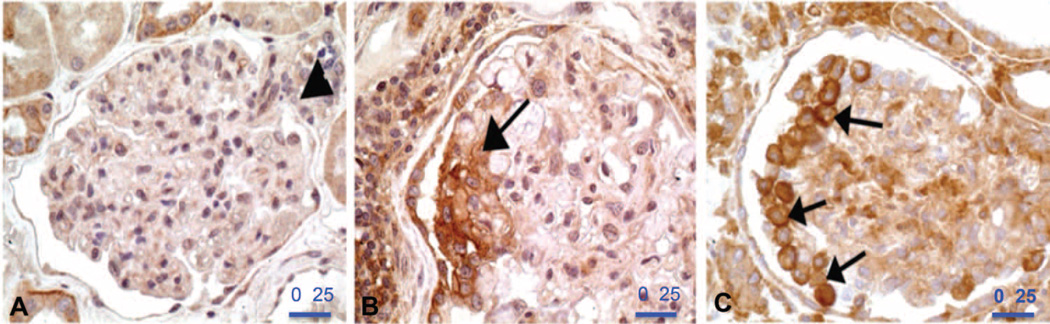
We used rabbit polyclonal primary Ab against CD40 to perform immunohistochemistry (IHC) on human kidney tissues. No signal was observed in the podocytes in a normal human kidney (samples obtained from the tumor-free part of a total nephrectomy for renal cell carcinoma) (Fig. 4A). However, strong focal staining for CD40 was observed in the podocytes from primary FSGS (n = 2) (Fig. 4B). Strong CD40 staining was also observed in the hyperplastic podocytes (arrows) covering an rFSGS lesion of the not otherwise specified variant (Fig. 4C).
Fig. 4. IHC of CD40 staining in kidney biopsies from patients with rFSGS.
(A) No CD40 staining is observed in the podocytes of this normal human glomerulus from the tumor-free part of a total nephrectomy for renal cell carcinoma. Vascular hilum is marked by an arrowhead. (B) Focal podocyte labeling for CD40 (arrow) in a case of rFSGS. (C) Strong CD40 signal is observed in the hyperplastic podocytes (arrows) covering an rFSGS lesion. Scale bars, 25 µm.
CD40 expression in human podocytes
To analyze the pathogenic relevance of CD40 in podocyte injury, the hallmark type of injury in rFSGS, we evaluated CD40 expression in cultured human podocytes and measured specific changes in CD40 expression after the podocytes were exposed to rFSGS sera. A human podocyte cell line was developed by transfection with the temperature-sensitive SV40-T gene. These cells proliferate at the “permissive” temperature (33°C) and are nondifferentiated. After transferring to the “nonpermissive” temperature (37°C), they enter growth arrest and express markers of differentiated in vivo podocytes, including podocyte proteins such as nephrin, podocin, CD2-associated protein (CD2AP), synaptopodin, and known molecules of the slit diaphragm ZO-1, α-, β-, and γ-catenin, and P-cadherin (25). We found that the CD40 protein is expressed in both nondifferentiated and differentiated cultured human podocytes (fig. S2A). Incubating podocytes with non-FSGS sera, nrFSGS sera, or rFSGS patient sera could not distinguish any changes in podocyte CD40 expression in culture (fig. S2B). Similarly, treatment of these cultured human podocytes with stimulators of podocyte injury, such as lipopolysaccharide (LPS) or tumor necrosis factor–α (TNF-α), did not alter podocyte CD40 expression (fig. S2, C and D).
Disruption of podocyte actin cytoskeleton by purified anti-CD40 Abs from rFSGS
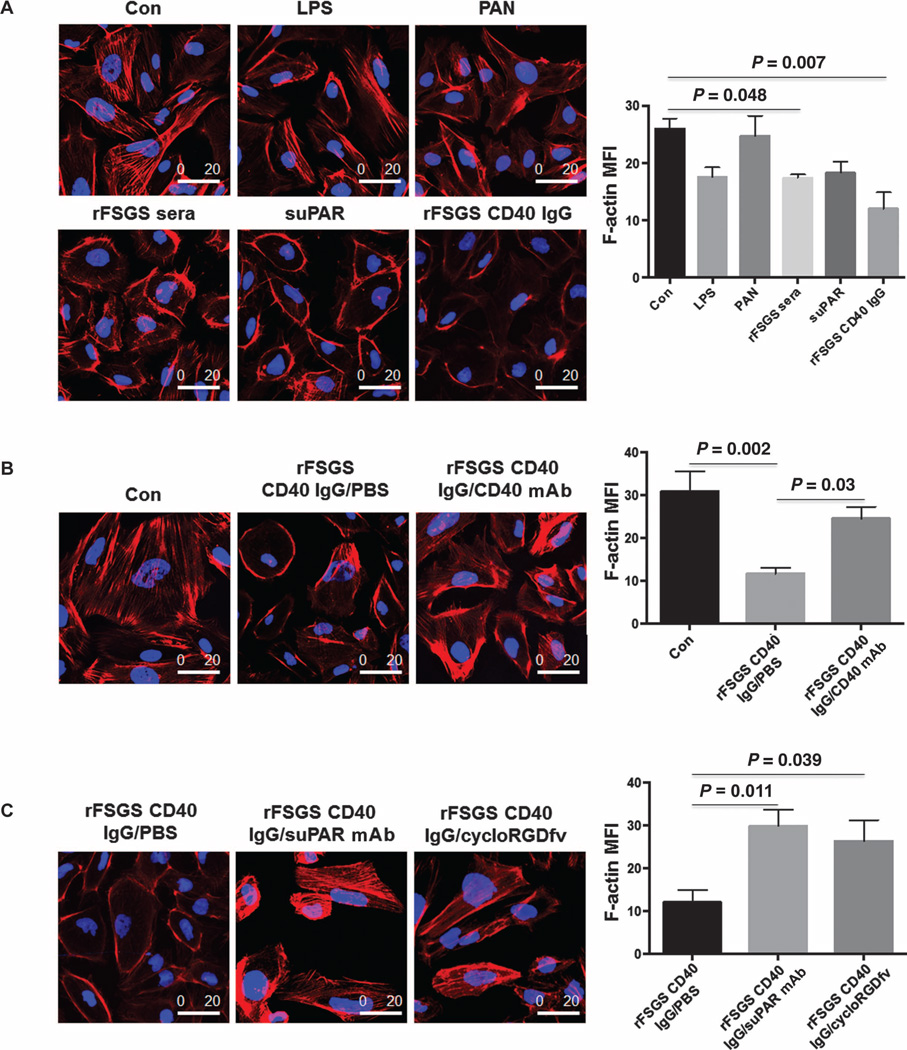
To determine whether anti-CD40 Abs from rFSGS patients have a pathogenic role, we examined the effect of purified anti-CD40 Ab isolated from rFSGS (anti-CD40/rFSGS Ab) and nrFSGS patients on podocyte architecture. We compared this effect to that of other known triggers of podocyte injury such as LPS, puromycin aminonucleoside (PAN), and suPAR, with phosphate-buffered saline (PBS) as the negative control. We found that rFSGS sera and purified anti-CD40 Ab from rFSGS patients caused podocyte depolarization and a reduction in overall cell size, with peripheral reorganization of F-actin as assessed by phalloidin staining (Fig. 5A). Quantification analysis confirmed an overall reduction in F-actin in podocytes treated with purified anti-CD40/rFSGS Ab (Fig. 5A). We next investigated whether the addition of a commercial monoclonal CD40-blocking Ab had any effect on the observed changes in podocyte architecture after previous treatment with the anti-CD40/rFSGS Ab. We observed partial reversal of anti-CD40/rFSGS Ab-induced podocyte depolarization (Fig. 5B) after coculture with the monoclonal CD40-blocking Ab, suggesting that inhibition of binding of anti-CD40/rFSGS Ab to human podocytes may be beneficial for recovery from anti-CD40/rFSGS Ab–induced podocyte injury. suPAR, a recently identified FSGS serological factor, also causes a disruption of podocyte F-actin filaments when used to treat podocytes in culture (Fig. 5A) (8), similar to the changes in podocyte architecture seen after cotreatment of cultured podocytes with anti-CD40/rFSGS Ab. We next evaluated whether the suPAR– β3 integrin signaling pathway was a mechanistic axis of the podocyte injury seen after treatment with suPAR and anti-CD40/rFSGS Ab. To this end, cultured human podocytes were cotreated with anti-CD40/rFSGS Ab and a monoclonal uPAR-blocking Ab, or with cycloRGDfV, a small molecule that blocks αvβ3 integrin activity. We observed amelioration of podocyte injury and reduced podocyte actin depolarization in both coculture experiments (Fig. 5C), suggesting that the suPAR–β3 integrin signaling pathway is involved in anti-CD40/rFSGS Ab–induced podo-cyte injury in vitro.
Fig. 5. Impact of CD40-blocking Ab on podocyte depolarization caused by human anti-CD40/rFSGS Ab.
(A) Completely differentiated human podo-cytes were treated with PBS (Con, control), LPS (50 µg/ml), PAN (50 µg/ml), 2% pretransplant sera from rFSGS patients, recombinant suPAR (1 µg/ml), or CD40 autoAb from FSGS patients. After treatment, the podocytes were stained with phalloidin for F-actin analysis. Treatment of podocytes with purified CD40 autoAb or FSGS sera, which contain a high concentration of CD40 autoAb, depolarized podocytes and caused peripheral aggregation and decreased central expression of F-actin assessed in terms of mean fluorescence intensity (MFI). suPAR treatment generated a similar phenotype. (B) Completely differentiated human podocytes were treated with a monoclonal CD40 Ab to examine its effect on CD40 autoAb–induced podocyte injury. Con, PBS control; rFSGS CD40 IgG, CD40 autoAb purified from rFSGS patient sera (2 µg/ml); CD40 mAb, a CD40 monoclonal Ab used at 1:1 ratio relative to CD40 autoAb. (C) Blocking suPAR–β3 integrin pathway ameliorates podocyte depolarization caused by human anti-CD40/rFSGS Ab. Completely differentiated human podocytes were cotreated with rFSGS CD40 IgG and suPAR-blocking monoclonal Ab (suPAR mAb, 1 µg/ml) or cycloRGDfV (1 µg/ml), a small molecule inhibiting αVβ3 integrin activity. Compared to rFSGS CD40 IgG alone, cotreatment with suPAR-blocking monoclonal Ab or cycloRGDfV increased podocyte polarity (left panel) and F-actin levels (right panel). One-way analysis of variance (ANOVA) was used to calculate P values (provided in the figure), and a P value <0.05 was considered significant. Scale bars, 20 µm.
Induction of proteinuria in C57BL/6 mice by human anti-CD40/rFSGS Ab
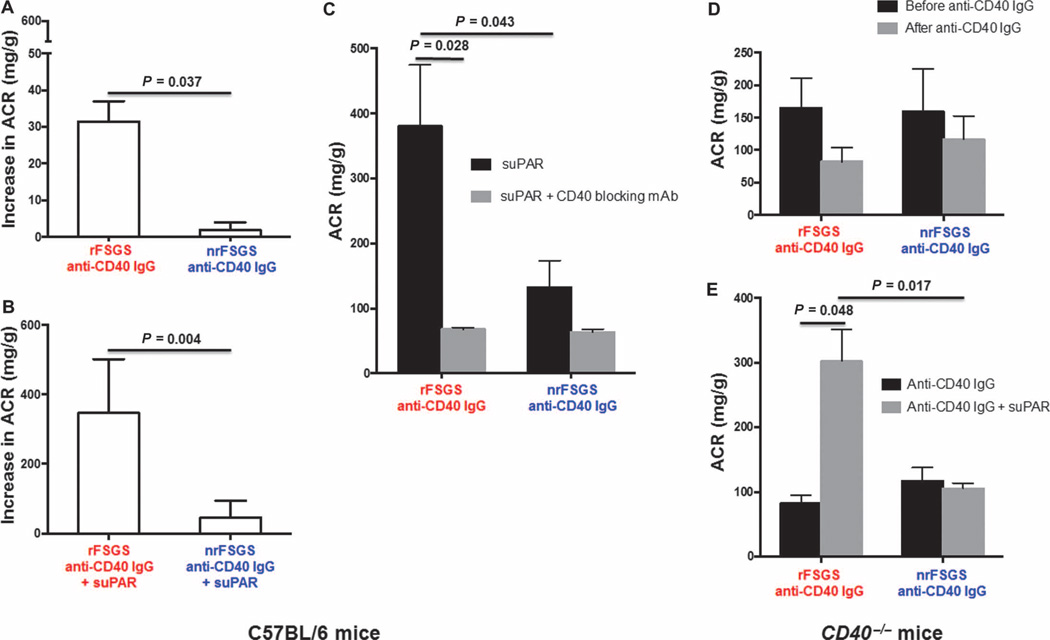
To evaluate the in vivo pathogenicity of human anti-CD40/rFSGS Ab, C57BL/6 mice were injected with either autoAb isolated from patients, such as anti-CD40/rFSGS Ab or anti-CD40/nrFSGS Ab to observe any changes in proteinuria within 8 days after injection. To control for the short half-life of injected human anti-CD40 IgG Ab (26), the Ab injection was repeated 48 hours after the first injection. Urine albumin-to-creatinine ratio (ACR) was calculated daily from day 1 to day 8 after first human anti-CD40/rFSGS Ab injections. A mild but significant increase in proteinuria over baseline was observed on day 8 with anti-CD40/rFSGS Ab injections (the increase of ACR from day 1 to day 8 with anti-CD40/rFSGS Ab injection was 31 ± 7.8 versus 4 ± 2.8 with injection of anti-CD40/nrFSGS Ab; P = 0.037) (Fig. 6A). In a separate experiment, we injected a single dose of full-length (three-domain) suPAR recombinant protein 6 hours after the second dose of the human anti-CD40/rFSGS Ab and observed a significant enhancement of proteinuria in C57BL/6 mice treated with the combination of anti-CD40/rFSGS and suPAR (increase in ACR of 346.8 ± 77.5 with anti-CD40/rFSGS Ab + suPAR versus increase in ACR of 46.3 ± 21.6 with anti-CD40/nrFSGS Ab + suPAR; P = 0.0043; baseline ACR, 102.9 ± 7) (Fig. 6B). This result shows that even three-domain suPAR, which reportedly does not cause proteinuria in wild-type mice (7, 27) when given alone, in contrast to alternative forms of suPAR (7), becomes pathogenic to podocytes in the presence of the autoAb and particularly so in the presence of anti-CD40/rFSGS Ab. Next, we injected a CD40-blocking Ab and found significant reduction in proteinuria in C57BL/6 mice that had received anti-CD40/rFSGS Ab (380.2 ± 94.3 before versus 67.8 ± 2.8 after blocking; P = 0.028) (Fig. 6C).
Fig. 6. Induction of proteinuria with human anti-CD40 Ab/rFSGS in mice.
(A to E) Wild-type C57BL/6 mice (A to C) or CD40−/− mice (D and E) were treated with anti-CD40 Ab isolated from rFSGS (red labels) or nrFSGS (blue labels) patients. The change in ACR was greater when C57BL/6 mice were injected with two doses of anti-CD40 Ab/rFSGS compared to anti-CD40 Ab/nrFSGS; the change in ACR between day 0 and day 8 is shown in (A). With co-injection of suPAR, the increase in ACR was again greater in C57BL/6 mice injected with anti-CD40 Ab/rFSGS, and proteinuria increased more than three-fold from a baseline of 102.9 ± 7 (B). Injection of CD40-blocking Ab into C57BL/6 mice cotreated with suPAR and anti-CD40 Ab/rFSGS significantly reduced proteinuria (C). Injection of anti-CD40 Ab/rFSGS into CD40−/− mice did not cause significant proteinuria (D). CD40−/− mice cotreated with anti-CD40 Ab/rFSGS and suPAR showed a significant increase in ACR, but this was not seen when these mice were cotreated with anti-CD40 Ab/nrFSGS and suPAR (E). P values (provided in the figure) were determined with unpaired t test calculated in GraphPad Prism. A P value of <0.05 was considered significant.
To further explore whether the effect of human anti-CD40 Ab in rFSGS patients was indeed mediated through CD40, we first examined the cross-species interaction of CD40 Ab and CD40 antigen and found that rFSGS patient-derived CD40 Ab interacted with mouse CD40 antigen, but anti-CD40 Abs/rFSGS or mouse anti-CD40 Abs do not bind to recombinant human CD40 antigen (fig. S3). Thereafter, we used CD40−/− mice and administered the same amount of anti-CD40 Ab as used in all previous experiments along with human suPAR. There was no significant change in the ACR with injection of anti-CD40 Ab/rFSGS alone (Fig. 6D), but cotreatment with the three-domain suPAR significantly increased ACR in CD40−/− mice (Fig. 6E; P = 0.048 for anti-CD40 Ab/rFSGS alone versus anti-CD40 Ab/rFSGS + suPAR). In contrast, cotreatment with suPAR and anti-CD40 Ab from nrFSGS patients did not increase ACR in CD40−/− mice (Fig. 6E). Thus, our data suggest that anti-CD40/rFSGS Ab and suPAR may cooperate in the development of proteinuria.
DISCUSSION
We describe an ELISA-based, seven-Ab composite biomarker panel (FAST) for the pretransplant identification of primary FSGS patients at high risk of posttransplant recurrence. In our study, the composite FAST panel was 92% accurate for detecting patients who had clinical and biopsy-confirmed rFSGS after transplantation. It should be able to identify primary FSGS patients at the highest risk of disease recurrence, allowing for customized management of FSGS patients with regard to transplant timing, organ source, preemptive therapeutic interventions such as plasmapheresis, and selection of induction immunosuppression. Because the longitudinal follow-up of the FAST panel shows that this predictive signature in rFSGS patients persists at 1 year after transplantation despite intensification of immunosuppressive therapy and plasma exchange, further studies are suggested to evaluate whether serial analysis of the FAST panel can help guide treatment choice and predict the treatment’s effects on proteinuria. Among the Abs included in the FAST panel, the strongest single impact on rFSGS prediction comes from the elevation of autoAb to the CD40 epitope in rFSGS. Our in vitro and in vivo pathogenicity studies on the human anti-CD40/rFSGS Ab provide insights into the pathophysiology of rFSGS.
A number of potential circulating factors have been suspected in FSGS (7, 28–31) including suPAR, a factor that has been reported to correlate with disease activity in rFSGS (7). Elevated plasma concentrations of suPAR have been seen in some cohorts of pediatric and adult rFSGS patients (8, 28), and altered renal tissue expression of suPAR has been noted in some rFSGS cases but not in others, suggesting that there may be different truncated forms of suPAR that need to be further evaluated for their pathogenic roles in rFSGS (29, 30). Although there was no significant difference in suPAR serum levels between the rFSGS and nrFSGS patients in this study, a major finding from this work is the cooperative effect of human anti-CD40/rFSGS Ab and suPAR on podocyte pathology. In this scenario, suPAR becomes pathogenic to podocytes in the presence of CD40 expression, possibly because the engagement of anti-CD40/rFSGS Ab primes suPAR to cause proteinuria. Its pathogenicity may be abrogated by blocking the binding of anti-CD40/rFSGS Ab to podocytes or by neutralization of suPAR. The in vivo experiments confirm that anti-CD40 Ab isolated from rFSGS patients is pathogenic to the kidney and that blocking CD40 interrupts the proteinuria. It is highly likely that anti-CD40 Ab/rFSGS and other members of the FAST Ab panel in rFSGS patients sensitize podocytes to other circulating factors that cause glomerular injury in rFSGS, such as suPAR. Thus, although blocking CD40 relieves proteinuria, blocking both CD40 and suPAR might be the best therapeutic option for rFSGS.
The finding that human anti-CD40 autoAb isolated from rFSGS patient sera caused increased proteinuria in the presence of recombinant suPAR also suggests that several injurious pathways may converge on the podocyte β3 integrin–actin pathway. Whereas suPAR alone can bind the αvβ3 integrin on the podocyte surface, causing its activation and pathological Rac-1 activation, we hypothesize that the anti-CD40/rFSGS Ab might further enhance the pathogenicity of suPAR by binding to CD40 on podocytes, facilitating or prolonging suPAR-mediated integrin activation, and/or potentiating the downstream signaling events allowing for enhanced podocyte motility and disruption of the slit diaphragm. In line with these data is a recent report that shows that activation of podocyte Rac-1 causes heavy proteinuria within a few hours (31). Podocyte β3 integrin signaling is coupled to Rac-1 activation (32) and can be altered by TNF-α or changes in local vascular endothelial growth factor production (33, 34). Although our current study shows that physiological, fully glycosylated suPAR can already achieve proteinuria in the presence of anti-CD40 autoAb, further testing will be required to determine whether truncated forms of suPAR or suPAR with glycosylation defects can lead to an aggravation of proteinuria and glomerular failure. Additional experimental work is also required to further test the pathogenicity of the additional autoAb types discovered in rFSGS.
One of the questions that will require further clarification is why human anti-CD40/rFSGS Abs are produced. Given the implication of suPAR in both primary and rFSGS, it might be possible that suPAR-mediated podocyte injury leads to unmasking of cryptic podocyte epitopes, allowing for the formation of anti-CD40 Ab in rFSGS. Epitope spreading could explain the increased number of IgG targeting glomerular proteins in the serum of rFSGS patients, as a result of glomerular injury by circulating FSGS factors. Epitope spreading can result from activation of autoreactive T cells by self epitopes released by the damaged tissue in rFSGS, or it may be caused by target antigens, such as CD40, being linked as a complex to self (glomerular) antigen in rFSGS disease. In line with this thinking, our data show that rFSGS is associated with an increased concentration of IgG against podocyte-specific epitopes and that there are specific differences in Ab-CD40-peptide binding between rFSGS and nrFSGS sera. FSGS has been associated with a T helper cell 2 (TH2) cytokine profile prone to Ab production (35, 36). The combination of antigen spreading and a TH2 cytokine profile could also explain the increased number of autoAb observed in the sera collected from rFSGS patients. This suggestion is supported by the enrichment of autoAb directed against glomerular antigens in rFSGS, as seen in the ProtoArray data. Furthermore, autoAb targeting glomerular proteins could also actively participate in the injury process, and Charba et al. have previously shown that Abs against PTPRO induce proteinuria in a glomerular cell culture model (15). Consequently, we assume that the efficacy of rituximab in preventing rFSGS could be due to its anti–B cell activity, in addition to its nonimmune podocyte effects, as suggested recently (18). The precise mechanism as well as the epitope that is being recognized by rFSGS-derived anti-CD40 and the potential complement-fixing property of the Ab will need to be the focus of major research in the future. rFSGS- and FSGS-derived anti-CD40 Ab does not recognize human CD40 (fig. S3), yet rFSGS-derived anti-CD40 has an effect (changes in F-actin organization) on human podocytes in culture. The cellular location of the CD40 antigen likely dictates this difference, because in the latter setting, CD40 is embedded in the plasma membrane and exposed to culture medium and serum. We also found that human renal allograft biopsies from patients with rFSGS show increased and selective CD40 staining in podocytes.
The CD40 axis is important in immunity and inflammation; the CD40 ligand activates endothelium and leads to increased expression of metalloproteases, chemokines, urokinase, and increased suPAR synthesis by endothelial cells (37). Glomerular mesangial cells stimulated by CD40 ligand have been shown to produce CCL2 (38), and importantly, blocking CD40-CD40 ligand interaction has been seen to protect against FSGS induction in murine models (39). Anti-CD40 Ab isolated from the sera of patients with rFSGS is activating and pathogenic in vitro and in vivo and cooperates with suPAR, whereas commercial monoclonal CD40-blocking Ab inhibits this effect. The therapeutic use of anti-CD40 Abs is being tested in cancer and immune-mediated diseases (40), and the data from this study suggest that further evaluation of the potential anti-proteinuric effect of therapeutic anti-CD40 Ab is warranted in human FSGS.
In conclusion, we have discovered the FAST Ab signature for prediction of rFSGS before renal transplantation and demonstrated an etiologic role for perturbations of the CD40 antigen in rFSGS. The podocytopathic effects of anti-CD40 suggest its involvement in the pathogenesis of rFSGS, possibly in a suPAR-dependent manner. This study highlights the convergence of pathological signals in FSGS with the regulatory pathways that maintain podocyte cytoskeletal integrity and function and defines an example of cooperation between an autoAb and circulating proteinuria factor suPAR. The limitations of this study include the small sample size, although collection of additional samples is difficult because of the low incidence of rFSGS and the difficulty of obtaining a well-defined clinical cohort of rFSGS patients worldwide. Thus, additional validation of the FAST panel in other rFSGS and glomerular disease cohorts is warranted. Serial monitoring with the FAST panel may pave the way for the development of preemptive therapeutic approaches, such as Ab depletion with targeted Ab immunoadsorption and pretransplantation plasmapheresis, B cell manipulation with bortezomib or rituximab, or disruption of the CD40 axis with commercially available CD40-blocking Abs such as ASKP1240, a fully human anti-CD40 monoclonal Ab [Astellas (41)], and lucatumumab, a fully humanized anti-CD40 antagonist monoclonal Ab [Novartis (42)]. The clinical and economic impact of changing the course of FSGS recurrence has far-reaching implications through improved graft survival, reduced patient morbidity, reduced costs of salvage therapies including dialysis, and the preservation and increased availability of transplant organs.
MATERIALS AND METHODS
Study design
A total of 141 available sera from 98 renal transplantation patients were chosen for this study to evaluate IgG Abs specific to rFSGS. All patients with rFSGS had primary FSGS as the cause of ESRD and demonstrated heavy proteinuria (nephrotic range) without evidence of acute rejection, glomerulitis, allograft glomerulopathy, or recurrence of any other primary renal disease. All FSGS cases were biopsy-proven. A panel of seven IgG Abs specific to rFSGS were validated as a biomarker panel for the pretransplant detection of FSGS recurrence. Anti-CD40 Abs isolated from rFSGS and nrFSGS were evaluated for differential antigen binding, Ab pathogenicity in vitro in a human podocyte cell culture model, and Ab pathogenicity in vivo in murine models (C57BL/6 mice and CD40-null mice). The addition of CD40-blocking Ab was evaluated for its ability to abrogate pathogenicity for the above models, in vitro and in vivo. For the ELISA validation, the samples were blinded for the person in charge of conducting the experiment and initial data processing.
Patients and samples
We processed 141 serum samples, obtained from 98 renal transplant patients (64 of whom had FSGS) before renal transplantation and 1 year after. The patients were enrolled at four international transplant centers: Stanford University Medical Center (Stanford, CA, USA); Transplantation Renale Adulte, Hôpital Necker-Enfants Malades (Paris, France); The Johns Hopkins Hospital (Baltimore, MD, USA); and Nephrology and Renal Transplantation, University Hospitals Leuven (Leuven, Belgium). The study was approved by the Institutional Review Boards of the respective institutions. Among the 98 patients, 64 had FSGS as the cause for ESRD requiring transplantation, whereas 34 had other causes of ESRD, such as obstructive uropathy, IgA nephropthy, diabetic nephropathy, hypertensive nephropathy, and glomerulonephritis. Among the 64 FSGS patients, 33 had recurrence of FSGS after renal transplantation (rFSGS), and mean time to biopsy-confirmed recurrence was 2 months ± 16 days. Serum samples were also collected at 12 months after transplantation from 43 patients with FSGS as the cause of ESRD. The detailed demographics of the nrFSGS and rFSGS groups are shown in Table 1.
Immune response biomarker profiling by protein microarrays and ELISA validation
The ProtoArray Human Protein Microarray was used for profiling serum IgG autoAb in 20 pretransplant sera from 10 patients with and without rFSGS (22). Kidney-specific Ab responses in rFSGS were mapped as previously published by our group (22, 43). The MSD technology was used for customized ELISA validation of elevated rFSGS Ab titers against FAS, CD40, CCL19, MYLK, CGB5, SNRBP2, RXRA, P2RY11, PTPRO, and APOL2. One hundred forty-one sera from 98 patients were processed for customized ELISA assays. Sera were obtained from 64 patients with FSGS as a cause of ESRD before transplantation; 33 of these patients had rFSGS within the first year after transplant, and 31 did not. Patients were demographically matched (Table 1) (Fig. 1). In a subset of these patients, follow-up sera samples were available at 1 year after transplantation (17 with rFSGS in the first posttransplant year and 26 patients without recurrence); the customized ELISA assays for all 10 autoAbs were also run on these samples to obtain a longitudinal analysis of Ab titers in the first posttransplant year.
IHC analysis of CD40 expression in human biopsies
Human kidney biopsy tissues were fixed in formalin or AFA (alcohol–formalin–acetic acid) solution and embedded in paraffin. For CD40, antigen retrieval was performed by proteinase K digestion and then hydrolysis by heating in a microwave oven with citrate buffer. The rabbit polyclonal primary anti-CD40 Ab (Anti-CD40 Ab 11E9, Abcam) was diluted at 1:150 and incubated overnight at +4°C. A three-step technique was used with biotinylated anti-rabbit secondary Ab, avidin-biotin-peroxidase complex as the vector, and 3,3′-diaminobenzidine (DAB) as the substrate.
Podocyte cell cultures
Normal human podocytes were cultured in the presence of patients’ sera and lysates for Western blotting (25), using mouse monoclonal Abs against CD40 and GAPDH (glyceraldehyde-3-phosphate dehydrogenase) (7). Cultured human podocytes were treated for 24 hours with rFSGS sera, which contained high concentrations of CD40 autoAb and separately with CD40 Ab from rFSGS patients (n = 4). Cellular F-actin expression and distribution were evaluated by phalloidin staining. For assessment of CD40 expression, some cells were lysed for SDS–polyacrylamide gel electrophoresis and Western blotting with a rabbit polyclonal CD40 Ab (Santa Cruz). Some cells were fixed with 4% para-formaldehyde (PFA) and immunostained with the same primary Ab followed by Alexa Fluor 488–conjugated anti-rabbit IgG (Life Technologies). For FSGS serum treatment, the medium of the cultured podocytes was removed, and pooled recurrent or nonrecurrent pretransplant FSGS serum was added at 4% and incubated for 24 hours. Recombinant suPAR protein (7) and a small molecule that blocks β3 integrin activity, cycloRGDfV, were each used at 1 µg/ml for additional treatment of cultured podocytes. Monoclonal CD40 Ab (Novus Biologicals) was given at different ratios (1:1 and 3:1) relative to CD40 autoAb to assess for competition between the monoclonal CD40 and the autoAb isolated from rFSGS sera. Twenty-four hours after treatment, the cells were fixed with 4% PFA and immunostained with phalloidin (Life Technologies) and 4′,6-diamidino-2-phenylindole (DAPI). Imaging was performed by Leica deconvolution microscopy.
Examination of the pathogenicity of anti-CD40 Ab in vivo
We investigated the effect of anti-CD40 Ab (CD40 IgG) isolated from patents with rFSGS and nrFSGS in vivo by injecting these Abs into C57BL/6J mice and after the development of proteinuria. We injected anti-CD40 Ab (5 µg/ml) isolated from sera of patients with and without rFSGS into C57BL/6J mice (n = 5 in each group). To control for the short half-life of injected IgG Ab (26), anti-CD40 Ab was injected twice, 48 hours apart. ACR was calculated daily. To examine any additive effect of suPAR on renal injury, on the background of treatment with human anti-CD40 Ab injections from rFSGS and nrFSGS patients, we used female C57BL/6 mice, aged 10 weeks, with body weight ranging from 18 to 20 g. Eight mice were randomly chosen to receive intravenous injections of anti-CD40 Ab isolated from rFSGS patients, and seven were to receive human anti-CD40 Ab isolated from nrFSGS. The dose was inferred from the ratio of CD40 autoAb in rFSGS patients to that in nrFSGS patients, which is about 4:1. The estimated final concentration was 8 µg/ml for anti-CD40 Ab from rFSGS and 2 µg/ml for nrFSGS. Anti-CD40 Ab injection was given every other day, for a total of six doses. Six hours after the last dose of anti-CD40 Ab, 10 µg of recombinant human suPAR (R&D) protein was given intravenously to all mice to analyze the effect of suPAR. Twenty-four hours after the last dose of anti-CD40 Ab, blocking mouse monoclonal CD40 Ab (Santa Cruz Biotechnology Inc., cat. no. sc-59047) was administered intraperitoneally at a dose of 3 µg per mouse. Urine was collected before and every day after the first injection of anti-CD40 Ab to analyze urinary albumin with a mouse albumin ELISA kit (Bethyl Laboratories Inc.) and creatinine concentration with a urine creatinine assay kit (Cayman Chemical Company). Proteinuria is expressed as albumin (mg)/creatinine (g) ratio.
The evaluation of human anti-CD40 Ab and suPAR in CD40−/− mice (Jackson Labs, stock no. 002928, female, 15 to 20 g, 8 to 10 weeks; n = 3 in each group) was conducted in the same way as detailed above for wild-type C57BL/6J mice.
Epitope profiling of anti-CD40 IgG
The PepStar human peptide microarray (JPT Peptide Technologies), consisting of 15-mer peptides with overlapping by four amino acids, was used to map reactive epitopes of CD40 by probing with serum samples from patients with (n = 4) and without (n = 4) rFSGS. See Supplementary Materials and Methods for additional details.
Statistical analysis
Original data for composite figures are provided in table S2. Statistical analysis of demographic data was performed with Mann-Whitney, one-way ANOVA, and Fisher’s exact tests. ELISA validation results were analyzed with a Mann-Whitney test using GraphPad Prism. Nominal logistic regression modeling was performed to compare expression of Abs between rFSGS and nrFSGS groups analyzed by ELISA. Abs with significant P value (<0.05) were selected by stepwise methods for each Ab. ROC analysis was conducted on the entire panel of autoAb to find the best autoAbs to predict rFSGS. For this purpose, we used three fitted logistic regression models with log-transformed concentrations of Abs; the model selected a panel of 7 of the 10 Abs (against CD40, FAS, PTPRO, P2RY11, CGB5, SNRPB2, and APOL2) for the best discrimination of rFSGS and a minimal set of 3 Abs (CD40, CGB5, and PTPRO) for optimal discrimination of rFSGS. The panel of seven Abs is referred to as FAST.
Supplementary Material
Acknowledgments
We thank C. Suberbielle and M. A. Alianakian for their help in collecting patient serum samples. We also thank M.-t. Vu, S.-C.H., and L. Nawbat for their help with manuscript preparation.
Funding: M.M.S. was supported by R01 and U01 grants DK083447 and U01AI113362. M.D. received financial support for this study from Novartis. J.R. was supported by R01 grants DK089394, DK073495, and DK101350. A.F. was supported by the NIH and National Institute of Diabetes and Digestive Kidney Diseases grant nos. DK090316, 5U24DX076169, 1UL1TR000460, and 12GHSU176. C.W. was supported by Terumo BCT Plasma Exchange Innovation Award.
Footnotes
SUPPLEMENTARY MATERIALS
www.sciencetranslationalmedicine.org/cgi/content/full/6/256/256ra136/DC1
Materials and Methods
Fig. S1. Serological responses mapped in rFSGS by protein arrays.
Fig. S2. Expression of CD40 in human podocytes.
Fig. S3. Cross-reactivity of human and mouse CD40 Ab with human CD40 and mouse CD40.
Table S1. Data for protein array experiments (provided as an Excel file).
Table S2. Original data (provided as an Excel file).
Author contributions: M.M.S., M.D., T.K.S., and C.W. designed and performed experiments, manuscript preparation, and revision; J.L., S.-C.H., A.F., P.B., and G.W.B. helped with experiments and data generation used in the manuscript; M.N., N.A., and A.J. provided study samples and clinical information. G.C. provided clinical information on Necker study samples. C.L., D.A., J.R., and M.M.S. provided funding and helped with data analysis and manuscript preparation and revision.
Competing interests: M.M.S. is on the Scientific Advisory Boards for Organ-i Inc. and Immucor and is a consultant for Bristol-Myers Squibb, Immucor, and Genentech. A.F. serves as a consultant for Hoffmann–La Roche and Mesoblast and has received honoraria from Novo Nordisk on subject matters that are unrelated to the current manuscript. J.R. has pending or issued patents on antiproteinuric therapies, has served as an adviser for the Abbott Renal Scientific Advisory Council, Genzyme Renal Innovations Program, Johnson & Johnson Renal Program, Genentech, and Questcor, and is or was a paid consultant for Johnson & Johnson, Abbott, Amgen, AstraZeneca, Genentech, Genzyme, Merck, Pfizer, Questcor, Roche, ViroGates, and Trisaq. All other authors declare that they have no competing interests.
Data and materials availability: The high-density protein array data for this study have been deposited in the GEO database (GEO ID:GSE57099).
REFERENCES AND NOTES
- 1.D′Agati VD, Kaskel FJ, Falk RJ. Focal segmental glomerulosclerosis. N. Engl. J. Med. 2011;365:2398–2411. doi: 10.1056/NEJMra1106556. [DOI] [PubMed] [Google Scholar]
- 2.Gbadegesin R, Lavin P, Foreman J, Winn M. Pathogenesis and therapy of focal segmental glomerulosclerosis: An update. Pediatr. Nephrol. 2011;26:1001–1015. doi: 10.1007/s00467-010-1692-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Canaud G, Dion D, Zuber J, Gubler MC, Sberro R, Thervet E, Snanoudj R, Charbit M, Salomon R, Martinez F, Legendre C, Noel LH, Niaudet P. Recurrence of nephrotic syndrome after transplantation in a mixed population of children and adults: Course of glomerular lesions and value of the Columbia classification of histological variants of focal and segmental glomerulosclerosis (FSGS) Nephrol. Dial. Transplant. 2010;25:1321–1328. doi: 10.1093/ndt/gfp500. [DOI] [PubMed] [Google Scholar]
- 4.Pardon A, Audard V, Caillard S, Moulin B, Desvaux D, Bentaarit B, Remy P, Sahali D, Roudot-Thoraval F, Lang P, Grimbert P. Risk factors and outcome of focal and segmental glomerulosclerosis recurrence in adult renal transplant recipients. Nephrol. Dial. Transplant. 2006;21:1053–1059. doi: 10.1093/ndt/gfk005. [DOI] [PubMed] [Google Scholar]
- 5.Vinai M, Waber P, Seikaly MG. Recurrence of focal segmental glomerulosclerosis in renal allograft: An in-depth review. Pediatr. Transplant. 2010;14:314–325. doi: 10.1111/j.1399-3046.2009.01261.x. [DOI] [PubMed] [Google Scholar]
- 6.Senggutuvan P, Cameron JS, Hartley RB, Rigden S, Chantler C, Haycock G, Williams DG, Ogg C, Koffman G. Recurrence of focal segmental glomerulosclerosis in transplanted kidneys: Analysis of incidence and risk factors in 59 allografts. Pediatr. Nephrol. 1990;4:21–28. doi: 10.1007/BF00858431. [DOI] [PubMed] [Google Scholar]
- 7.Wei C, El Hindi S, Li J, Fornoni A, Goes N, Sageshima J, Maiguel D, Karumanchi SA, Yap HK, Saleem M, Zhang Q, Nikolic B, Chaudhuri A, Daftarian P, Salido E, Torres A, Salifu M, Sarwal MM, Schaefer F, Morath C, Schwenger V, Zeier M, Gupta V, Roth D, Rastaldi MP, Burke G, Ruiz P, Reiser J. Circulating urokinase receptor as a cause of focal segmental glomerulosclerosis. Nat. Med. 2011;17:952–960. doi: 10.1038/nm.2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wei C, Trachtman H, Li J, Dong C, Friedman AL, Gassman JJ, McMahan JL, Radeva M, Heil KM, Trautmann A, Anarat A, Emre S, Ghiggeri GM, Ozaltin F, Haffner D, Gipson DS, Kaskel F, Fischer DC, Schaefer F, Reiser J. PodoNet and FSGS CT Study Consortia Circulating suPAR in two cohorts of primary FSGS. J. Am. Soc. Nephrol. 2012;23:2051–2059. doi: 10.1681/ASN.2012030302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang J, Liu G, Zhang YM, Cui Z, Wang F, Liu XJ, Chu R, Zhao MH. Urinary soluble urokinase receptor levels are elevated and pathogenic in patients with primary focal seg-mental glomerulosclerosis. BMC Med. 2014;12:81. doi: 10.1186/1741-7015-12-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Trachtman H. suPAR and Team Nephrology. BMC Med. 2014;12:82. doi: 10.1186/1741-7015-12-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pescovitz MD, Book BK, Sidner RA. Resolution of recurrent focal segmental glomerulo-sclerosis proteinuria after rituximab treatment. N. Engl. J. Med. 2006;354:1961–1963. doi: 10.1056/NEJMc055495. [DOI] [PubMed] [Google Scholar]
- 12.Dantal J, Godfrin Y, Koll R, Perretto S, Naulet J, Bouhours JF, Soulillou JP. Antihuman immunoglobulin affinity immunoadsorption strongly decreases proteinuria in patients with relapsing nephrotic syndrome. J. Am. Soc. Nephrol. 1998;9:1709–1715. doi: 10.1681/ASN.V991709. [DOI] [PubMed] [Google Scholar]
- 13.Musante L, Candiano G, Bruschi M, Santucci L, Carnemolla B, Orecchia P, Giampuzzi M, Zennaro C, Sanna-Cherchi S, Carraro M, Oleggini R, Camussi G, Perfumo F, Ghiggeri GM. Circulating anti-actin and anti-ATP synthase antibodies identify a sub-set of patients with idiopathic nephrotic syndrome. Clin. Exp. Immunol. 2005;141:491–499. doi: 10.1111/j.1365-2249.2005.02862.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alachkar N, Gupta G, Montgomery RA. Angiotensin antibodies and focal segmental glomer-ulosclerosis. N. Engl. J. Med. 2013;368:971–973. doi: 10.1056/NEJMc1207233. [DOI] [PubMed] [Google Scholar]
- 15.Charba DS, Wiggins RC, Goyal M, Wharram BL, Wiggins JE, McCarthy ET, Sharma R, Sharma M, Savin VJ. Antibodies to protein tyrosine phosphatase receptor type O (PTPro) increase glomerular albumin permeability (Palb) Am. J. Physiol. Renal Physiol. 2009;297:F138–F144. doi: 10.1152/ajprenal.00122.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Topham PS, Kawachi H, Haydar SA, Chugh S, Addona TA, Charron KB, Holzman LB, Shia M, Shimizu F, Salant DJ. Nephritogenic mAb 5–1–6 is directed at the extracellular domain of rat nephrin. J. Clin. Invest. 1999;104:1559–1566. doi: 10.1172/JCI7728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Savin VJ, Sharma R, Sharma M, McCarthy ET, Swan SK, Ellis E, Lovell H, Warady B, Gunwar S, Chonko AM, Artero M, Vincenti F. Circulating factor associated with increased glomerular permeability to albumin in recurrent focal segmental glomerulosclerosis. N. Engl. J. Med. 1996;334:878–883. doi: 10.1056/NEJM199604043341402. [DOI] [PubMed] [Google Scholar]
- 18.Fornoni A, Sageshima J, Wei C, Merscher-Gomez S, Aguillon-Prada R, Jauregui AN, Li J, Mattiazzi A, Ciancio G, Chen L, Zilleruelo G, Abitbol C, Chandar J, Seeherunvong W, Ricordi C, Ikehata M, Rastaldi MP, Reiser J, Burke III GW. Rituximab targets podocytes in recurrent focal segmental glomerulosclerosis. Sci. Transl. Med. 2011;3:85ra46. doi: 10.1126/scitranslmed.3002231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chikamoto H, Hattori M, Kuroda N, Kajiho Y, Matsumura H, Fujii H, Ishizuka K, Hisano M, Akioka Y, Nozu K, Kaito H, Shimizu M. Pretransplantation combined therapy with plas-mapheresis and rituximab in a second living-related kidney transplant pediatric recipient with a very high risk for focal segmental glomerulosclerosis recurrence. Pediatr. Transplant. 2012;16:E286–E290. doi: 10.1111/j.1399-3046.2011.01610.x. [DOI] [PubMed] [Google Scholar]
- 20.Assmann KJ, van Son JP, Dïjkman HB, Mentzel S, Wetzels JF. Antibody-induced albuminuria and accelerated focal glomerulosclerosis in the Thy-1.1 transgenic mouse. Kidney Int. 2002;62:116–126. doi: 10.1046/j.1523-1755.2002.00428.x. [DOI] [PubMed] [Google Scholar]
- 21.Sigdel TK, Li L, Tran TQ, Khatri P, Naesens M, Sansanwal P, Dai H, Hsieh SC, Sarwal MM. Non-HLA antibodies to immunogenic epitopes predict the evolution of chronic renal allograft injury. J. Am. Soc. Nephrol. 2012;23:750–763. doi: 10.1681/ASN.2011060596. [DOI] [PubMed] [Google Scholar]
- 22.Li L, Wadia P, Chen R, Kambham N, Naesens M, Sigdel TK, Miklos DB, Sarwal MM, Butte AJ. Identifying compartment-specific non-HLA targets after renal transplantation by integrating transcriptome and ″antibodyome″ measures. Proc. Natl. Acad. Sci. U.S.A. 2009;106:4148–4153. doi: 10.1073/pnas.0900563106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Butte A, Sigdel TK, Wadia PP, Miklos DB, Sarwal MM. Protein microarrays discover angiotensinogen and PRKRIP1 as novel targets for autoantibodies in chronic renal disease. Mol. Cell. Proteomics. 2011;10 doi: 10.1074/mcp.M110.000497. M110.000497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sutherland SM, Li L, Sigdel TK, Wadia PP, Miklos DB, Butte AJ, Sarwal MM. Protein microarrays identify antibodies to protein kinase Cζ that are associated with a greater risk of allograft loss in pediatric renal transplant recipients. Kidney Int. 2009;76:1277–1283. doi: 10.1038/ki.2009.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saleem MA, O′Hare MJ, Reiser J, Coward RJ, Inward CD, Farren T, Xing CY, Ni L, Mathieson PW, Mundel P. A conditionally immortalized human podocyte cell line demonstrating nephrin and podocin expression. J. Am. Soc. Nephrol. 2002;13:630–638. doi: 10.1681/ASN.V133630. [DOI] [PubMed] [Google Scholar]
- 26.Peppard JV, Orlans E. The biological half-lives of four rat immunoglobulin isotypes. Immunology. 1980;40:683–686. [PMC free article] [PubMed] [Google Scholar]
- 27.Cathelin D, Placier S, Ploug M, Verpont MC, Vandermeersch S, Luque Y, Hertig A, Rondeau E, Mesnard L. Administration of recombinant soluble urokinase receptor per se is not sufficient to induce podocyte alterations and proteinuria in mice. J. Am. Soc. Nephrol. 2014;25:1662–1668. doi: 10.1681/ASN.2013040425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang J, Liu G, Zhang YM, Cui Z, Wang F, Liu XJ, Chu R, Chen Y, Zhao MH. Plasma soluble urokinase receptor levels are increased but do not distinguish primary from secondary focal segmental glomerulosclerosis. Kidney Int. 2013;84:366–372. doi: 10.1038/ki.2013.55. [DOI] [PubMed] [Google Scholar]
- 29.Alachkar N, Wei C, Arend LJ, Jackson AM, Racusen LC, Fornoni A, Burke G, Rabb H, Kakkad K, Reiser J, Estrella MM. Podocyte effacement closely links to suPAR levels at time of posttransplantation focal segmental glomerulosclerosis occurrence and improves with therapy. Transplantation. 2013;96:649–656. doi: 10.1097/TP.0b013e31829eda4f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Trachtman H, Wei C, Reiser J. Circulating factor in FSGS: A black sheep in the suPAR family? Pediatr. Nephrol. 2013;28:1151–1152. doi: 10.1007/s00467-013-2463-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yu H, Suleiman H, Kim AH, Miner JH, Dani A, Shaw AS, Akilesh S. Rac1 activation in podocytes induces rapid foot process effacement and proteinuria. Mol. Cell. Biol. 2013;33:4755–4764. doi: 10.1128/MCB.00730-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wei C, Möller CC, Altintas MM, Li J, Schwarz K, Zacchigna S, Xie L, Henger A, Schmid H, Rastaldi MP, Cowan P, Kretzler M, Parrilla R, Bendayan M, Gupta V, Nikolic B, Kalluri R, Carmeliet P, Mundel P, Reiser J. Modification of kidney barrier function by the urokinase receptor. Nat. Med. 2008;14:55–63. doi: 10.1038/nm1696. [DOI] [PubMed] [Google Scholar]
- 33.Veron D, Villegas G, Aggarwal PK, Bertuccio C, Jimenez J, Velazquez H, Reidy K, Abrahamson DR, Moeckel G, Kashgarian M, Tufro A. Acute podocyte vascular endothelial growth factor (VEGF-A) knockdown disrupts alphaVbeta3 integrin signaling in the glomerulus. PLOS One. 2012;7:e40589. doi: 10.1371/journal.pone.0040589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bitzan M, Babayeva S, Vasudevan A, Goodyer P, Torban E. TNFα pathway blockade ameliorates toxic effects of FSGS plasma on podocyte cytoskeleton and β3 integrin activation. Pediatr. Nephrol. 2012;27:2217–2226. doi: 10.1007/s00467-012-2163-3. [DOI] [PubMed] [Google Scholar]
- 35.Le Berre L, Hervé C, Buzelin F, Usal C, Soulillou JP, Dantal J. Renal macrophage activation and Th2 polarization precedes the development of nephrotic syndrome in Buffalo/Mna rats. Kidney Int. 2005;68:2079–2090. doi: 10.1111/j.1523-1755.2005.00664.x. [DOI] [PubMed] [Google Scholar]
- 36.Grimbert P, Valanciute A, Audard V, Pawlak A, Le gouvelo S, Lang P, Niaudet P, Bensman A, Guellaën G, Sahali D. Truncation of C-mip (Tc-mip), a new proximal signaling protein, induces c-maf Th2 transcription factor and cytoskeleton reorganization. J. Exp. Med. 2003;198:797–807. doi: 10.1084/jem.20030566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van Kooten C, Gaillard C, Galizzi JP, Hermann P, Fossiez F, Banchereau J, Blanchard D. B cells regulate expression of CD40 ligand on activated T cells by lowering the mRNA level and through the release of soluble CD40. Eur. J. Immunol. 1994;24:787–792. doi: 10.1002/eji.1830240402. [DOI] [PubMed] [Google Scholar]
- 38.Tanaka T, Kuroiwa T, Ikeuchi H, Ota F, Kaneko Y, Ueki K, Tsukada Y, McInnes IB, Boumpas DT, Nojima Y. Human platelets stimulate mesangial cells to produce monocyte chemoattractant protein-1 via the CD40/CD40 ligand pathway and may amplify glomerular injury. J. Am. Soc. Nephrol. 2002;13:2488–2496. doi: 10.1097/01.asn.0000029588.07166.20. [DOI] [PubMed] [Google Scholar]
- 39.Kairaitis L, Wang Y, Zheng L, Tay YC, Wang Y, Harris DC. Blockade of CD40-CD40 ligand protects against renal injury in chronic proteinuric renal disease. Kidney Int. 2003;64:1265–1272. doi: 10.1046/j.1523-1755.2003.00223.x. [DOI] [PubMed] [Google Scholar]
- 40.Law CL, Grewal IS. Therapeutic interventions targeting CD40L (CD154) and CD40: The opportunities and challenges. Adv. Exp. Med. Biol. 2009;647:8–36. doi: 10.1007/978-0-387-89520-8_2. [DOI] [PubMed] [Google Scholar]
- 41.Bensinger W, Maziarz RT, Jagannath S, Spencer A, Durrant S, Becker PS, Ewald B, Bilic S, Rediske J, Baeck J, Stadtmauer EA. A phase 1 study of lucatumumab a fully human anti-CD40 antagonist monoclonal antibody administered intravenously to patients with relapsed or refractory multiple myeloma. Br. J. Haematol. 2012;159:58–66. doi: 10.1111/j.1365-2141.2012.09251.x. [DOI] [PubMed] [Google Scholar]
- 42.Watanabe M, Yamashita K, Suzuki T, Kamachi H, Kuraya D, Koshizuka Y, Ogura M, Yoshida T, Aoyagi T, Fukumori D, Shimamura T, Okimura K, Maeta K, Miura T, Sakai F, Todo S. ASKP1240, a fully human anti-CD40 monoclonal antibody prolongs pancreatic islet allograft survival in nonhuman primates. Am. J. Transplant. 2013;13:1976–1988. doi: 10.1111/ajt.12330. [DOI] [PubMed] [Google Scholar]
- 43.Higgins JP, Wang L, Kambham N, Montgomery K, Mason V, Vogelmann SU, Lemley KV, Brown PO, Brooks JD, van de Rijn M. Gene expression in the normal adult human kidney assessed by complementary DNA microarray. Mol. Biol. Cell. 2004;15:649–656. doi: 10.1091/mbc.E03-06-0432. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.