Abstract
Both short- (1 wk) and long-term (2–12 mo) high-fat diet (HFD) studies reveal enhanced β-cell mass due to increased β-cell proliferation. β-Cell proliferation following HFD has been postulated to occur in response to insulin resistance; however, whether HFD can induce β-cell proliferation independent of insulin resistance has been controversial. To examine the kinetics of HFD-induced β-cell proliferation and its correlation with insulin resistance, we placed 8-wk-old male C57Bl/6J mice on HFD for different lengths of time and assayed the following: glucose tolerance, insulin secretion in response to glucose, insulin tolerance, β-cell mass, and β-cell proliferation. We found that β-cell proliferation was significantly increased after only 3 days of HFD feeding, weeks before an increase in β-cell mass or peripheral insulin resistance was detected. These results were confirmed by hyperinsulinemic euglycemic clamps and measurements of α-hydroxybutyrate, a plasma biomarker of insulin resistance in humans. An increase in expression of key islet-proliferative genes was found in isolated islets from 1-wk HFD-fed mice compared with chow diet (CD)-fed mice. These data indicate that short-term HFD feeding enhances β-cell proliferation before insulin resistance becomes apparent.
Keywords: high-fat diet, mouse models, insulin resistance, β-cell proliferation, β-cell mass
β-cell compensation in response to obesity is observed in both humans and rodent models. Human autopsy studies have revealed that nondiabetic obese individuals have 50% greater β-cell mass compared with lean individuals, and pancreata from type 2 diabetes patients have diminished β-cell mass compared with nondiabetic BMI-matched individuals (7). In mice, high-fat diet (HFD) feeding leads to increased body weight and a corresponding expansion in β-cell mass via increased β-cell proliferation (19, 35, 43, 46). The β-cell response to HFD feeding in mice could enhance our understanding of the β-cell response to energy excess in humans and help us develop new strategies for augmenting β-cell mass in type 2 diabetes patients, but a key point is to discern early responses vs. compensatory responses that might occur after prolonged HFD feeding.
The β-cell response to HFD feeding has been studied extensively; however, the data are difficult to interpret due to varying model systems and experimental designs. Studies vary in the β-cell characteristics measured, the composition and timing of diet, and mouse genotype, age, and, sex. Only by combining parallel measurements can a relationship between β-cell adaptations and the progression of diet-induced obesity be defined. The majority of studies utilize long-term HFD consumption, and the β-cell response is assessed after rodents have been on a HFD for anywhere from 2 to 12 mo. These studies demonstrated that long-term HFD consumption induces glucose intolerance, insulin resistance, and enhanced β-cell mass and proliferation (2, 6, 17, 26, 27, 32, 40, 44). Some studies have explored the acute consequences of overnutrition; for example, significant weight gain and hyperglycemia have been reported in mice after as little as 1 wk after beginning a diet high in fat (2, 34, 46). β-Cell mass expansion due to increased β-cell proliferation has been reported in response to only 1 wk of HFD feeding, and this was suggested to occur in the absence of insulin resistance (35). However, the value of the intraperitoneal insulin tolerance test (IPITT), which was used in that study, to assess insulin resistance in mice is limited by the potentially confounding effect of counterregulatory responses (5, 26) and the very short half-life of insulin in mice (8). Therefore, in the current study, after the primary screen using IPITTs, we performed hyperinsulinemic euglycemic clamps to assess insulin sensitivity with greater sensitivity as recommended by the Mouse Metabolic Phenotyping Center Consortium (5).
The current study was designed to assess both the onset and the magnitude of functional and morphological adaptations of β-cells in response to HFD feeding. To this end, starting at 8 wk of age, male C57Bl/6J mice were fed either a normal chow diet (CD) or a HFD for a duration of 11 wk and periodically examined for indications of insulin resistance, glucose intolerance, β-cell mass, and β-cell proliferation. We determined not only when but also the degree to which the parameters change in response to overnutrition. Here, we report that HFD induces β-cell proliferation as early as 3 days after diet initiation, the earliest this has been shown to occur. Furthermore, we found that enhanced β-cell proliferation induced by HFD feeding occurs in response to factors not initiated by HFD-induced insulin resistance (as determined by hyperinsulinemic euglycemic clamp). A precise characterization of the natural progression of β-cell proliferation and insulin resistance is key to understanding the pathogenesis of type 2 diabetes and the regulation of compensatory β-cell proliferation in response to metabolic and nutritional cues.
RESEARCH DESIGN AND METHODS
Experimental animals.
With the exception of hyperinsulinemic euglycemic clamp studies, all experiments were performed in the Vanderbilt University facility. Male C57Bl/6J mice (Jackson Laboratory, Bar Harbor, ME) were delivered to the Association for Assessment and Accreditation of Laboratory Animal Care International-accredited Division of Animal Care at Vanderbilt University at 7 wk of age. After 1 wk of acclimatization, mice were weighed and randomly assigned to one of two groups: 1) CD (11% kcal from fat, Lab Diet 5LJ5; Purina, St. Louis, MO) and 2) HFD (60% kcal from fat; BioServ F3282, Frenchtown, NJ). Mice were housed on a 12:12-h light-dark cycle, receiving water and food ad libitum. Body weight was obtained prior to starting the diets and prior to each metabolic measurement. Euthanasia was performed at time of pancreatic dissection, using isoflurane until the mice were unconscious, followed by cervical dislocation. These methods are consistent with the Panel on Euthanasia of the American Veterinary Medical Association. All procedures were conducted in accordance with Vanderbilt University Division of Animal Care and Use Committee-approved protocols and under the supervision of the Division of Animal Care. For hyperinsulinemic euglycemic clamps, male C57Bl/6J mice (Jackson Laboratory, Bar Harbor, ME) were delivered to the Animal Facility of the Research Center of the University of Montreal Hospital Cente (CRCHUM) at 7 wk of age and the clamps performed at 8 wk of age. All procedures were approved by the Institutional Committee for the Protection of Animals at the CRCHUM.
Metabolic measurements.
Intraperitoneal glucose tolerance tests (IPGTT) and IPITT were performed as described previously (18). For IPGTT, animals were fasted for 16 h. Fasting blood glucose was measured from 2 μl of tail vein blood with an Accuchek glucometer and glucose test strips (Abbott Diabetes Care). Animals received an intraperitoneal (ip) injection of filter-sterilized glucose (2 mg dextrose/g body wt), and blood glucose was measured at 15-, 30-, 60-, 90-, and 120-min intervals following injection; n = 3 (weeks 3, 7, and 9) or 6 (weeks 1, 5, and 11). For IPITT, animals were fasted for 6 h. Fasting blood glucose was measured as described above, and animals received an ip injection of 0.075 U/ml insulin (recombinant human insulin, no. I9278; Sigma-Aldrich) in filter-sterilized 1× PBS at 0.1 ml/10 g body wt. Subsequent changes in blood glucose were measured at 15-, 30-, 60-, 90-, and 120-min intervals following injection; n = 3 (weeks 3, 7, and 9) or 6 (weeks 1, 5, and 11). Plasma insulin assays were performed as described in (31), with the modification that blood was harvested from the saphenous veins of 16-h-fasted animals using heparinized Natelson blood-collecting tubes (Kimble Chase, Rockwood, TN).
Hyperinsulinemic euglycemic clamps.
Two-hour hyperinsulinemic-euglycemic clamps were performed in 4-h-fasted mice (n = 6 for CD and 7 for HFD), as described previously (4). Briefly, following a 1-min bolus insulin infusion (85 mU/kg; Humulin R), insulin was infused at 8 mU·kg−1·min−1. Twenty percent dextrose was infused beginning 5 min after the insulin infusion to clamp glycemia at ∼120 mg/dl. Insulin levels during the steady state were measured at 90 and 120 min using the AlphaLISA kit. The insulin sensitivity index (M/I) was calculated as the glucose infusion rate (GIR) divided by the average insulinemia during the last 30 min of the clamp (I); n = 6 CD and 7 HFD.
Tissue preparation and histology.
At euthanization, pancreata were processed as described previously (14). Antibodies were guinea pig anti-insulin (1:500; Dako, Carpinteria, CA), rabbit anti-Ki67 (1:500; AbCam, Cambridge, MA), Cy2-conjugated anti-guinea pig IgG (1:300; Jackson Laboratories, Bar Harbor, ME), Cy3-conjugated anti-rabbit IgG (1:300, Jackson Laboratories), and horseradish peroxidase-conjugated anti-guinea pig IgG (1:300, Jackson Laboratories).
β-Cell mass, β-cell proliferation, and β-cell death.
Analysis and quantification of β-cell mass was performed as described in (18). For β-cell mass assessment, ∼2% of each pancreas was immunolabeled and analyzed (5–10 sections/animal, each separated by 250 μm). Slides were scanned at ×20 magnification (Scan Bright field Scope System; Aperio, Vista, CA), and an algorithm developed from a Genie macro within Spectrum (Aperio) was used to identify β-cells and other tissue (15); n = 3 (weeks 1, 3, 7, and 9 for CD and HFD), 5 (week 11 for CD), or 6 (week 5 for CD and HFD and week 11 for HFD). β-Cell proliferation was determined by immunolabeling sections ∼400 μm apart (∼5 slides/animal) for insulin and Ki-67 (Abcam; 1:500) or insulin and phosphorylated histone H3 (pHH3, 1:200; Cell Signaling Technology). For Ki-67 labeling, antigen retrieval consisted of microwaving slides for 14 min in 10 mM sodium citrate buffer; n = 3 (weeks 3, 7, and 9 for CD and HFD), 4 (3 days for CD), 5 (3 days for HFD), or 6 (weeks 1, 5, and 11 for CD and HFD). For pHH3 labeling, antigen retrieval was placed in TEG buffer at pH 9.0 and microwaved on high power for 1 min and then 10% power for 7.5 min. Nuclei were labeled with 1 μg/ml 4′,6′-diamidino-2-phenylindole (DAPI, Molecular Probes, Grand Island, NY) and mounted with Aqua-Mount (Thermo Scientific, Kalamazoo, MI); n = 3 for all time points and diets. Slides were scanned as above. At least 5,000 insulin-positive cells/mouse were counted using MetaMorph software. Calculations were made by dividing the number of insulin/Ki-67 or insulin/pHH3 colabeled cells by the total number of insulin-positive cells. To assess β-cell death, terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL) was performed using the ApoAlert Kit (Clontech) according to the manufacturer's instructions (16). For TUNEL assay, pancreata (3 sections/animal) from three CD- and three HFD-fed animals were analyzed at each of three time points (3 days, 1 wk, and 11 wk).
Quantitative RT-PCR.
Islet RNA from 1-wk-treated mice was isolated, and quantitative RT-PCR (qRT-PCR) was performed as described previously (1). Primer sequences are listed in Table 1. Data are shown as 2−ΔΔCT (24); n = 6.
Table 1.
qRT-PCR sequences
| Gene | Forward | Reverse |
|---|---|---|
| Ki-67 | AGCTTCTGTGCTGACCCTGATG | TGCAGAAAGGCCCTTGGCATAC |
| FoxM1 | CACTTGGATTGGGACCACTT | GTCGTTTCTGCTGTGATTCC |
| Cyclin D1 | CTGACACCAATCTCCTCAACGAC | GCGGCCAGGTTCCACTTHAGC |
| Cyclin D2 | CACCGACAACTCTGTGAAGC | TCCACTTCAGCTTACCCAACA |
| Cyclin A2 | CTTGGCTGCACCAACAGTAA | CAAACTCAGTTCTCCCAAAAACA |
| Cyclin B1 | TCTTGACAACGGTGAATGGA | TCTTAGCCAGGTGCTGCATA |
| P16 | CCGTCGTACCCCGATTCAG | GCACCGTAGTTGAGCAGAAGAG |
qRT-PCR, quantitative RT-PCR.
Metabolomic analysis.
Whole liver, epididymal fat, skeletal muscle (gastrocnemius, soleus, and plantaris muscles), bone (fibula and tibia), and plasma were collected from 9-wk-old C57Bl/6J mice either fed a HFD (n = 8) or maintained on a CD (n = 8) for 1 wk. Tissues were dissected, flash-frozen, and kept at −80°C before being shipped to Metabolon (Durham, NC) for metabolite analysis. Sample preparation, instrument analysis, and data processing analysis were performed by Metabolon, as detailed in previous publications (9, 27).
Statistical analysis and calculations.
Data are shown as means ± SE (12). P values were calculated with either the two-tailed unpaired Student's t-test or the two-way ANOVA with the Sidek correction for multiple comparisons as indicated. P values ≤0.05 were considered significant, and P values >0.05 were not reported. For metabolomic data shown in Fig. 5B, any missing values were assumed to be below the detection limit and were imputed with the minimum observed value. Following imputation and log transformation, P values were calculated using the Welsh two-sample t-test, and estimates of the false discovery rate (q value) were calculated to account for multiple comparisons, as described previously (36).
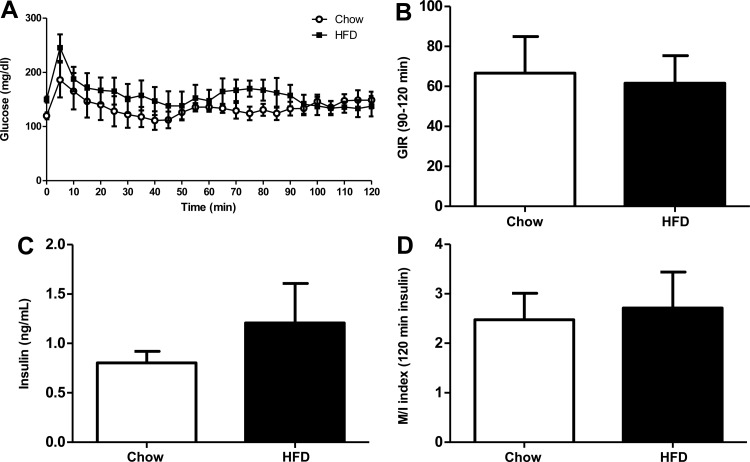
Fig. 5.
Insulin sensitivity is not modified after 1 wk of HFD. A: blood glucose during hyperinsulinemic euglycemic clamp. B: the average glucose infusion rate is calculated during the 90- to 120-min period where blood glucose levels are identical between CD- and HFD-fed mice. C: plasma insulin at 120 min trends higher for HFD mice but is not significantly different from CD mice. D: insulin sensitivity between CD and HFD is not changed, as indicated by similar insulin sensitivity index (M/I) values. Data are shown as means ± SE. Open bars, CD (n = 6); black bars, HFD (n = 7).
RESULTS
Weight gain and impaired glucose tolerance in HFD-fed mice.
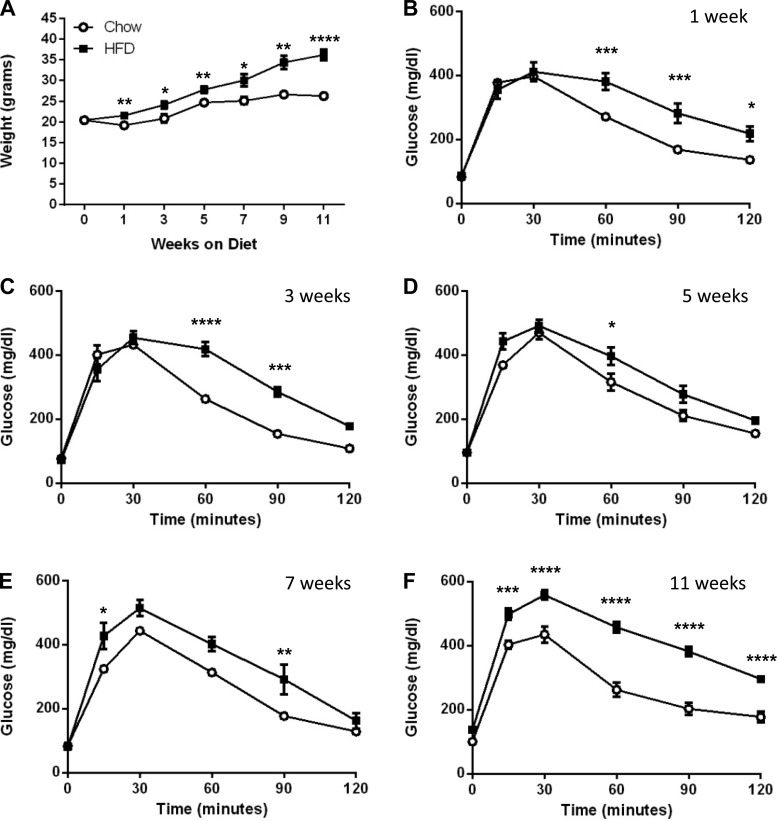
To correlate the consumption of HFD with changes in body weight, glucose homeostasis, and β-cell growth, 8-wk-old male mice were either maintained on CD or switched to HFD. The average initial body weight for the CD group was identical to the HFD group (20.4 ± 2.1 vs. 20.5 ± 1.5 g, respectively). Mice from each diet were examined at 1, 3, 5, 7, 9, and 11 wk after the start of the study for body weight, glucose homeostasis, β-cell mass, and β-cell proliferation. HFD-fed mice weighed significantly more than CD-fed mice throughout the study, starting with an initial weight gain of 1.1 ± 0.9 g (P = 0.006) in the 1st wk compared with the static weight of the CD group (−0.7 ± 1.5 g, P = not significant) (Fig. 1A). The largest weight increase for both diets occurred between weeks 3 and 5, where CD-fed mice gained 3.9 ± 1.0 g (P = 0.001) and HFD-fed mice gained a similar 3.7 ± 1.1 g (P = 0.008). After week 5, the HFD group continued to gain weight, showing an 8.4 ± 1.5-g (P = 0.0001) increase over the next 6 wk, whereas body weights of CD-fed mice plateaued during this time frame with an insignificant increase in body weight of 1.6 ± 0.8 g.
Fig. 1.
Impaired glucose tolerance occurs within 1 wk of HFD feeding and persists throughout the study. A: high-fat diet (HFD)-fed mice (■) gain weight progressively over an 11-wk period. Weight of chow diet (CD)-fed mice (○) plateaus after week 5. B–F: glucose tolerance tests are shown for weeks 1 (B), 3 (C), 5 (D), 7 (E), and 11 (F). Data are shown as means ± SE [n = 6–9 for body weight and 3–6 for intraperitoneal insulin tolerance test (IPGTT)]. P values were calculated using the unpaired t-test, comparing the difference in weight between CD and HFD within the weekly time points. Significance for IPGTTs was calculated using the 2-way ANOVA with Sidek correction for multiple comparisons. *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001; ****P ≤ 0.0001.
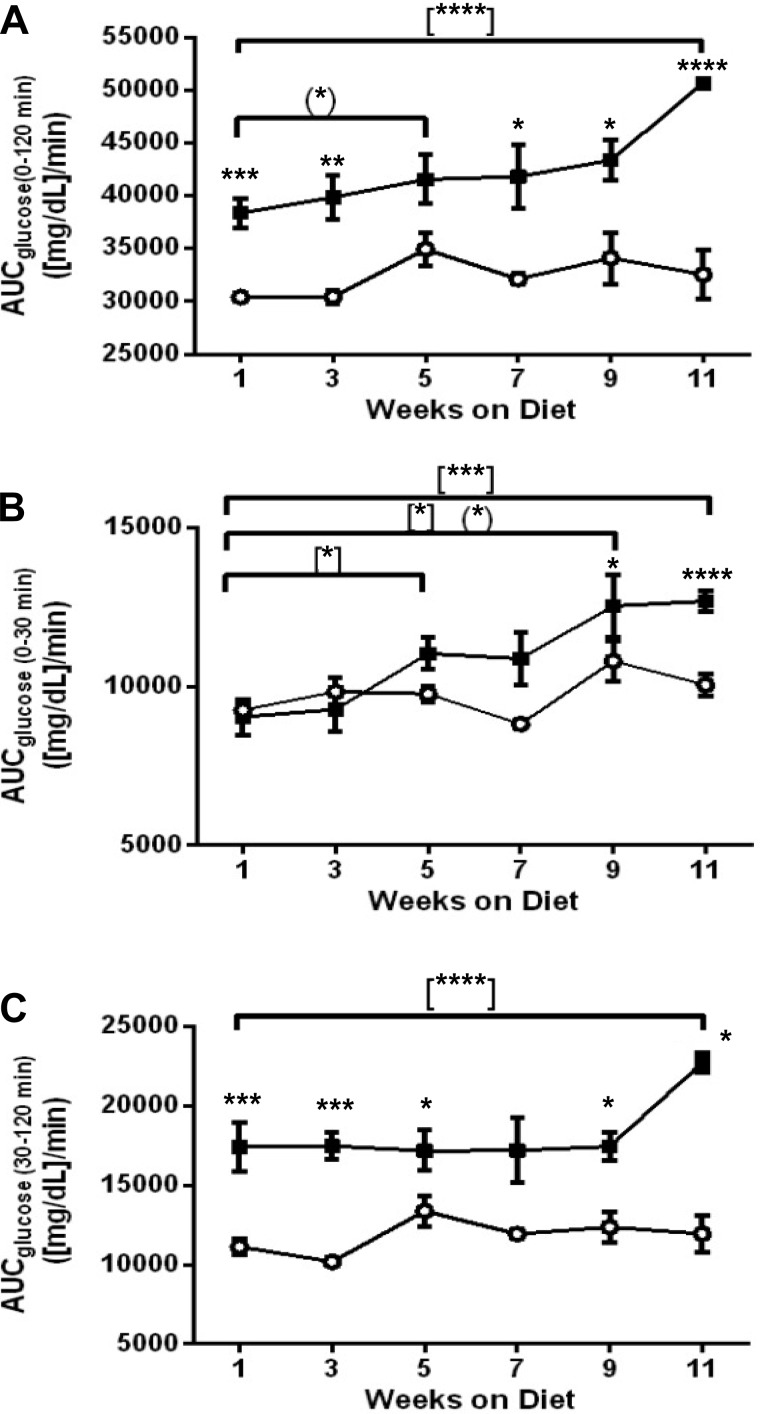
Changes in glucose tolerance in response to both acute and long-term HFD consumption were tracked by performing an IPGTT every other week. As expected from previously published studies, glucose tolerance was already impaired after 1 wk of HFD and was consistently impaired throughout the study despite unchanged fasting blood glucose (Fig. 1, B–F). To depict subtle but significant changes in glucose tolerance as mice were maintained on HFD, we calculated the area under the curve for glucose (AUCglc) both between diets and between intradiet time points (Fig. 2, A–C). The AUCglc for HFD-fed mice increased gradually between weeks 1 and 9 and culminated with a highly significant increment in AUCglc from weeks 9 to 11 (P = 0.001); the AUCglc for CD-fed mice was relatively unchanged (Fig. 2A). The gradual increase in HFD-fed AUCglc between weeks 1 and 9 was due to an elevation in glucose during the first 30 min of the IPGTTs (Fig. 2B). In fact, the last 90 min of the IPGTTs remained relatively consistent between intradiet time points, with the exception of week 11 in the HFD-fed group, when a large increase in AUCglc occurs (Fig. 2C).
Fig. 2.
Area under the curve (AUC) reveals that HFD causes the greatest changes in late time points of IPGTTs. AUC was determined from 0 to 120 min (A), 0 to 30 min (B), and 30 to 120 min (C). ○, CD; ■, HFD. Data are shown as means ± SE (n = 3–6). The unpaired t-test was used for comparing CD and HFD areas under the curve. *P ≤ 0.05, comparisons between diets {(*)comparison between CD weekly time points; [*]comparison between HFD weekly time points}. **P ≤ 0.01; ***P ≤ 0.001; ****P ≤ 0.0001.
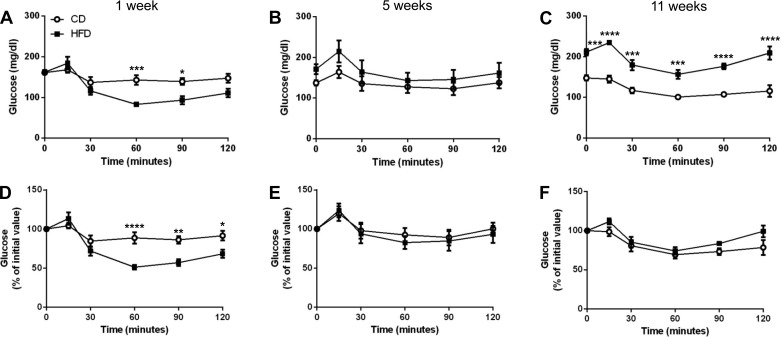
Insulin resistance is not apparent with short term HFD feeding, but impaired insulin tolerance develops with prolonged HFD feeding.
The progression of impaired glucose tolerance in response to both acute and long-term HFD suggests impairments in insulin secretion and/or insulin sensitivity. First, we determined the effects of short- and long-term HFD feeding on insulin tolerance. Since injection of insulin can dramatically lower blood glucose levels, mice were fasted for only 6 h prior to insulin injection. Fasting blood glucose concentrations were not altered in response to short-term HFD feeding but were elevated with long-term HFD feeding (Fig. 3C). Interestingly, mice fed HFD for 1 wk exhibited a significantly enhanced decline in blood glucose during the latter time points of the IPITT (Fig. 3A), and this was still apparent after normalization to fasting blood glucose (Fig. 3D). In week 5, the blood glucose concentrations of the HFD-fed group were indistinguishable from controls (Fig. 3, B and E). After 11 wk of HFD feeding, we observed elevated blood glucose throughout the test relative to CD-fed mice (Fig. 3C). These data show that insulin tolerance in HFD-fed mice gradually diminished with duration of HFD, whereas the CD-fed mice maintained a consistent response throughout the study. However, after normalization to basal glucose concentrations, insulin tolerance in the long-term HFD-fed mice was only slightly compromised at the later time points (Fig. 3F). Similar to week 5, there was no significant difference between the diets at weeks 3, 7, and 9 (data not shown).
Fig. 3.
Insulin tolerance was enhanced in HFD-fed mice in week 1 but worsened gradually over time. Insulin tolerance tests are shown for weeks 1 (A and D), 5 (B and E), and 11 (C and F). Results for each time point normalized to fasting blood glucose levels are shown in D–F. ○, CD; ■, HFD. Data are shown as means ± SE (n = 3–6); significance was calculated using 2-way ANOVA with Sidek correction for multiple comparisons. *P ≤ 0.05; **P < 0.01; ***P ≤ 0.001; ****P ≤ 0.0001.
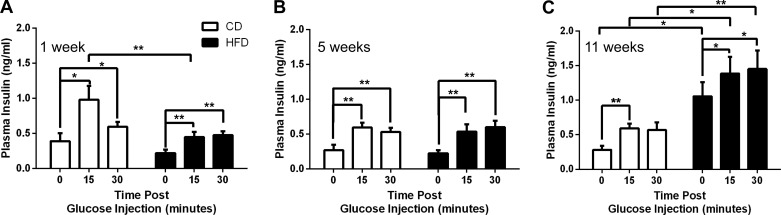
Plasma was assayed every other week for circulating insulin in response to glucose. Although there was a significant increase in plasma insulin in response to the glucose challenge in animals fed HFD for 1 wk, this response was reduced by 50% compared with the response of the CD-fed group (Fig. 4A). The CD-fed plasma insulin response remained consistent through week 11 (Fig. 4, B and C). Plasma insulin values for HFD-fed mice were virtually unchanged for weeks 1–7 (Fig. 4, A and B, and data not shown). As predicted, hyperinsulinemia was apparent in long-term HFD-fed mice (Fig. 4C). Fasting plasma insulin was elevated in HFD-fed mice by week 9 (data not shown) and exacerbated further in week 11 (Fig. 4C), at which point it was nearly fourfold higher in the HFD-fed group compared with the CD-fed group (P = 0.02). Despite elevated fasting plasma insulin in HFD-fed mice at week 11, they still presented with a moderate but significant response to the initial glucose challenge at 15 min (P = 0.03).
Fig. 4.
Hyperinsulinemia became evident after 11 wk of HFD feeding. Fasting and glucose-induced plasma insulin secretion were assayed at weeks 1 (A), 5 (B), and 11 (C). Open bars, CD; black bars, HFD. Data are shown as means ± SE (n = 5–6); significance was calculated with the paired t-test and 2-way ANOVA with Sidek correction for calculation of multiple comparisons. *P ≤ 0.05; **P ≤ 0.01.
Our data at later time points agree with a wealth of previously published studies demonstrating insulin resistance after prolonged HFD feeding. The more provocative finding of the present study is a lack of apparent insulin resistance 1 wk after initiation of HFD. To more rigorously investigate whether insulin resistance is present after 1 wk of HFD feeding, we performed hyperinsulinemic euglycemic clamps (Fig. 5). The GIR required to maintain blood glucose levels (Fig. 5A) at the target level was not different between the two groups (Fig. 5B). Although circulating insulin levels at the end of the clamp were slightly higher in the HFD-fed group (Fig. 5C), this difference was not statistically significant, and the M/I index was not different between both groups (Fig. 5D). These results suggest that insulin sensitivity was unaltered after 1 wk of HFD.
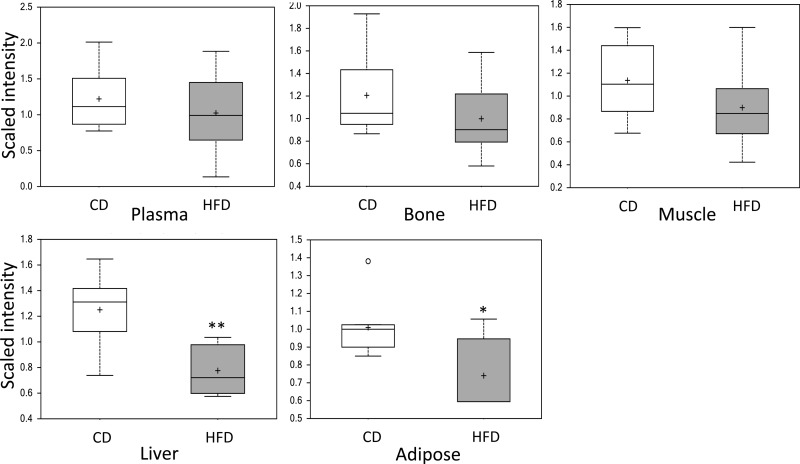
In humans, elevations of the metabolite α-hydroxybutyrate (α-HB) in plasma are indicative of insulin resistance (11, 13). Given the results from the IPITT and hyperinsulinemic euglycemic clamps suggesting lack of insulin resistance at 1 wk of HFD feeding, we queried results from metabolomic analyses of metabolically relevant tissues (including plasma) to determine whether levels of α-HB were increased in response to HFD at this time point. Metabolomic analysis of liver, skeletal muscle, epididymal fat, bone, and plasma collected from 1-wk-HFD- and -CD-fed mice revealed decreased α-HB in the liver (0.62-fold lower, P = 0.002, q = 0.012) and adipose tissue (0.73-fold lower, P = 0.011, q = 0.002) in HFD-fed mice compared with CD-fed mice; α-HB was unchanged in other tissues, including plasma, at this time point (Fig. 6).
Fig. 6.
Assessment of α-hydroxybutyrate (α-HB) in plasma, bone, muscle, liver, and adipose tissue collected from 1-wk-HFD- (gray boxes) and CD-fed mice (white boxes) revealed significantly decreased α-HB in the liver and adipose tissue of HFD-fed mice. Data are shown as means ± SE; P values were calculated using the unpaired t-test (n = 8). *P ≤ 0.05; **P ≤ 0.01.
β-Cell mass expansion begins after 3 wk of HFD consumption.
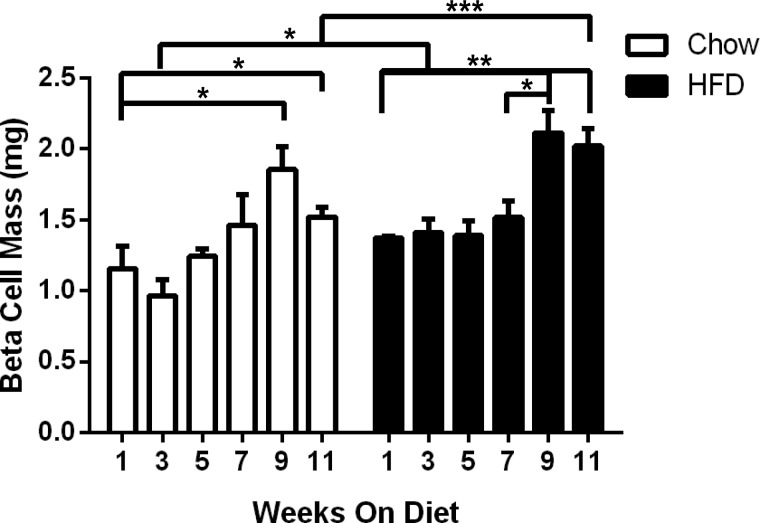
β-Cell mass expansion is well established in long-term HFD feeding studies; however, the timing of this expansion is less clear. To address this, β-cell mass was determined every other week between 1 and 11 wk (Fig. 7). A significant increase in β-cell mass was observed after 3 wk of HFD feeding; however, this increase was not sustained throughout the treatment period. The β-cell mass within each diet group remained relatively constant until week 9, when a significant increase was observed in both groups. Compared with the chow-fed animals, a further increase in β-cell mass was observed between weeks 9 and 11 in the HFD-fed group. Comparison between the two diets at the 11-wk time point revealed a 1.3-fold increase in β-cell mass for HFD-fed mice over CD-fed mice (P = 0.001).
Fig. 7.
β-Cell mass expansion in response to HFD first becomes evident in week 3. β-Cell mass in HFD-fed mice compared with CD-fed mice was slightly elevated in week 3, becoming significantly elevated during weeks 9–11. Open bars, CD; black bars, HFD. Data are shown as means ± SE (n = 3–6); P values were calculated using the unpaired t-test. *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001.
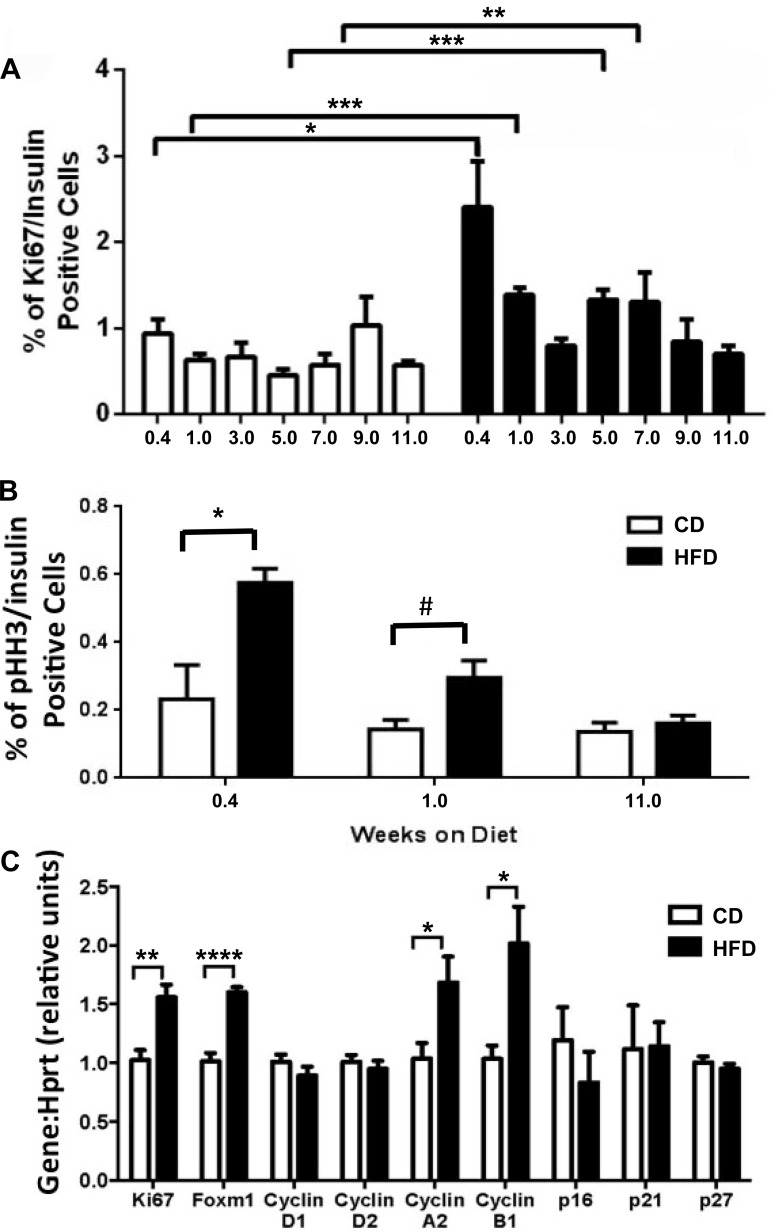
β-Cell proliferation was induced with short-term HFD feeding.
To ascertain the kinetics of the β-cell-proliferative response to HFD feeding, pancreata were assayed at different durations of HFD for β-cell proliferation using Ki-67 and pHH3 immunolabeling. Whereas CD-fed mice exhibited a constant low percentage of Ki-67/insulin double-positive cells throughout the study, β-cell proliferation in the HFD-fed group was dynamic and enhanced after just 3 days (Fig. 8A). Compared with CD, β-cell proliferation was enhanced 2.6-, 2.2-, 2.9-, and 2.3-fold for the HFD-fed mice at 3 days (P = 0.05), 1 wk (P = 0.00005), 5 wk (P = 0.0001), and 7 wk (P = 0.003), respectively. Similar results were obtained using pHH3 as a proliferative marker; the greatest enhancement in β-cell proliferation was observed after only 3 days of HFD, and an increase in β-cell proliferation was maintained at 1 wk of HFD but was no longer apparent at 11 wk of HFD (Fig. 8B).
Fig. 8.
β-Cell proliferation was elevated substantially after 3 days (0.4 wk) of HFD feeding. β-Cell proliferation in CD-fed mice did not significantly change throughout the study, as assessed by immunolabeling for either Ki-67 (A) or phosphorylated histone H3 (B); β-cell proliferation was acutely affected by HFD feeding after only 3 days. C: quantitative RT-PCR for genes positively and negatively associated with β-cell proliferation was performed after 1 wk of diet. Open bars, CD; black bars, HFD. Data are shown as means ± SE (n = 3–6); significance was calculated with the paired t-test and 2-way ANOVA with Sidek correction for calculation of multiple comparisons. *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001; #P < 0.0597.
Since a significant increase in β-cell proliferation was detected within 1 wk of HFD treatment, qRT-PCR was conducted on RNA from isolated islets at 1 wk to analyze changes in gene expression for key proliferative genes. Expression of Ki67, Foxm1, cyclin A2, and cyclin B1 was upregulated 1.6-, 1.6-, 1.7-, and twofold (P = 0.003, 0.00004, 0.03, and 0.01), respectively, in 1-wk-HFD-fed mouse islets compared with CD islets. Expression of cyclins D1 and D2 was unchanged. Expression of the cell cycle inhibitors cdkn2A (p16), cdkn1A (p21), and cdkn1B (p27) was not changed significantly in HFD-fed mice (Fig. 8C).
DISCUSSION
Long-term HFD feeding can result in insulin resistance, glucose intolerance, and fasting hyperglycemia and can impact the β-cell, resulting in hyperinsulinemia, increased β-cell proliferation, and increased β-cell mass. However, little is known about the initiation, extent, and duration of β-cell proliferation in response to HFD feeding or the relationship between β-cell proliferation and insulin resistance. Understanding how and when the β-cell adapts to increased consumption of fat could lead to advances in diabetes therapy. In the current study, we found that overt insulin resistance does not precede proliferation; quite the contrary, β-cell proliferation begins within 3 days of a HFD being started, weeks before insulin resistance is detected. The results from our analyses of HFD duration are summarized in Table 2.
Table 2.
Summary of results
| Duration of HFD |
|||||||
|---|---|---|---|---|---|---|---|
| Parameters | 3 Days | 1 Wk | 3 Wk | 5 Wk | 7 Wk | 9 Wk | 11 Wk |
| Glucose tolerance | ND | Impaired | Impaired | Impaired | Impaired | Impaired | Impaired |
| Insulin tolerance | ND | Improved | ND | No change | No change | No change | Impaired |
| Fasting insulin | ND | No change | No change | No change | No change | Increased | Increased |
| Glucose-stimulated insulin secretion | ND | Impaired | ND | No change | ND | ND | Impaired |
| α-Hydroxylbutyrate | ND | Decreased | ND | ND | ND | ND | ND |
| β-Cell mass | ND | No change | Increased | No change | No change | No change | Increased |
| β-Cell proliferation | Increased | Increased | No change | Increased | Increased | No change | No change |
| β-Cell death | No change | No change | ND | ND | ND | ND | No change |
HFD, high-fat diet; ND, not determined.
All caparisons are with animals on control diet for the same duration.
Genetic background, age, and sex are three confounding variables in previous mouse studies that make interpreting the β-cell response to HFD consumption difficult (10, 20, 42). We chose C57Bl/6J mice because of the significant and well-documented HFD-induced effects on β-cell adaptation in this strain (19, 37, 38, 43). Age is another variable that has a large impact on β-cell proliferation. β-Cell proliferation in response to HFD declines sharply after 7 mo of age, becoming nearly nonexistent after 12 mo (0.07%/day) (39, 43). Conversely, examining very young mice could be problematic due to changing hormone levels associated with normal growth and puberty. Therefore, we chose 8-wk-old mice since these mice have reached sexual maturity and are still young enough to exhibit robust β-cell proliferation (33). The sex of the mice was another factor taken into consideration. We chose male mice because, although both male and female mice experience similar weight gain on HFD, glucose tolerance and insulin secretion are more severely impaired in males, whereas β-cell mass in females is resistant to the effects of HFD (14, 29).
The current study clearly defines how glucose tolerance, insulin sensitivity, and hyperinsulinemia change with progressive consumption of a HFD and, more importantly, how these relate to β-cell proliferation. Previous studies reported impaired glucose tolerance in as little as 3 days to 1 wk in HFD-fed mice but did not examine the progressive changes that occur in glucose tolerance with increased duration on HFD (2, 24, 28, 29, 39, 42). Our study agrees with a previous report that impaired glucose tolerance occurs after 3 days of HFD and is maintained through 12 wk of HFD feeding (44). However, this previous study utilized only AUC to report the IPGTT results. The present study clearly indicates that the impaired glucose tolerance observed 1 wk after HFD feeding is confined to the 30-min time point, suggesting an intact early insulin response after short-term HFD feeding and a diminished second-phase response. Whereas IPGTTs for CD-fed animals remained relatively steady from week to week throughout the study, the HFD-fed group showed worsening glucose tolerance with increased diet duration, particularly during the first 30 min. This effect may be attributable to a number of factors, including adiposity-related changes in the rate of glucose absorption and impaired first-phase insulin secretion. Interestingly, the latter 90 min of the HFD-fed IPGTTs did not deviate until week 11. A significant increase in circulating insulin in response to glucose was observed for both diets at week 1; however, the insulin levels in HFD-fed mice were not sufficient to maintain proper glucose homeostasis. Although the elevated fasting plasma insulin in week 11 HFD-fed mice suggested insulin resistance, there was still an intact response to glucose injection.
Mice fed HFD for 1 wk showed a more significant decline in blood glucose in response to exogenous insulin than CD-fed mice. These data are supported by a previous report where nonfasted 1-wk-HFD-fed mice exhibited increased insulin sensitivity, which becomes apparent when results in that study are normalized for fasting blood glucose (35). On the surface, it appears that HFD-fed mice are more insulin sensitive; however, further scrutiny suggests otherwise. Insulin has an estimated circulating half-life of 10 min in mice (8); therefore, after the first 30 min of the IPITT, all exogenous insulin should be cleared. The blood glucose measurements between 30 and 60 min are more indicative of secondary effects (e.g., counterregulatory hormone responses). Interestingly, a study done in humans more than four decades ago reported a similar phenomenon where “mild” diabetic test subjects were more insulin tolerant during the latter time points of the test (40). These authors suggested that this may be due to increasing endogenous insulin levels in the “mild” diabetic patients; contrary to this, our plasma insulin data imply that the lowering of blood glucose during the latter part of the IPITT is not due to an increase in endogenous insulin. Thus, at present it is unclear why 1 wk HFD feeding appears to improve insulin tolerance, but these results warrant further investigation.
β-Cell proliferation was increased as early as 3 days after HFD feeding was initiated, a finding supported by the upregulation of key β-cell-proliferative genes at 1 wk. FoxM1, a transcription factor required for postnatal β-cell proliferation and elevated in islets from HFD-fed mice (14, 47), is activated by the G2 cyclin, cyclin A2 (21). Furthermore, cyclin B1 is a downstream target of FoxM1 during the G2/M transition, whereas cyclins D1 and D2, predominant during G1, are unaffected by Foxm1 overexpression (23). These data are further supported by a recent study that found similar changes in proliferative gene expression in islets from mice fed HFD for 1 wk (35). The impaired glucose tolerance and diminished plasma insulin concentration after 1 wk of HFD feeding may be due in part to this observed increase in β-cell proliferation. Oncology studies have shown that proliferating cells effectively switch to a less efficient metabolism (45), and although controversial, some studies suggest that proliferating β-cells are partially dedifferentiated (reviewed in Ref. 17). Therefore, actively proliferating β-cells may temporarily experience decreased functionality. Although maximal β-cell proliferation is observed after 3 days or 1 wk of HFD, a detectable increase in β-cell mass is not observed until 3 wk. A delay between increased β-cell proliferation and a detectable increase in β-cell mass was not unexpected, as this also occurs in maternal islets during pregnancy, where productive cell divisions must accumulate to translate into measurable increases in β-cell mass. We also assessed whether there was an increase in β-cell death in response to HFD feeding that might explain the delay in β-cell mass increase but were unable to detect any increase in TUNEL-labeled insulin+ cells in the HFD-fed animals compared with controls at 3 days, 1 wk, or 11 wk of HFD (data not shown).
Based on previous studies that reported HFD-induced insulin resistance in as few as 3–7 days after initiation of HFD feeding (2, 22, 32, 44), we expected to detect symptoms of insulin resistance in 1-wk-HFD-fed mice. Instead, the present studies suggest that insulin resistance occurs much later, after at least 5 wk of HFD-feeding. The later time points are concurrent with an increase in insulin resistance, as evidenced by the growing impairment in glucose tolerance, hyperinsulinemia, and the worsening insulin tolerance in weeks 9 and 11. The absence of insulin resistance after 1 wk of HFD was supported by similar M/I indices in hyperinsulinemic euglycemic clamps. Metabolomics assessment of α-HB, a plasma biomarker of insulin resistance in humans (11), confirmed the absence of insulin resistance in multiple tissues of 1-wk-CD- and -HFD-fed mice, thus validating this biomarker in mouse models. Our results are supported by two studies; one study failed to detect insulin resistance in 1-wk-HFD-fed mice, and another measured insulin resistance in skeletal muscle, adipose tissue, and liver and found that insulin resistance was detectable after 3 wk of HFD feeding (28, 35). Together, these results suggest that β-cells initially proliferate in response to a change in diet or an unknown HFD-induced factor before they respond to insulin resistance. As HFD consumption continues, insulin resistance increases, and hyperinsulinemia and enhanced β-cell mass ensue. However, at later stages of HFD feeding, β-cell proliferation declines and glucose tolerance becomes more impaired.
In conclusion, our data suggest that β-cell proliferation in response to HFD feeding is highly dynamic, occurring within 3 days of starting a diet high in fat. This is exciting evidence showing that adult pancreatic β-cells can indeed respond robustly to proliferative cues without the complications of insulin resistance. To our knowledge, this is the first study to definitively indicate that HFD-induced β-cell proliferation precedes overt insulin resistance, although this was suggested by the study from Stamateris et al (35). These studies lay the groundwork for defining the proliferative signals that are present in response to HFD.
GRANTS
This work was funded by grants from the Veterans Administration (1BX000990-01A1 to M. Gannon), Juvenile Diabetes Research Foundation (17-2012-26 to M. Gannon and V. Poitout), National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK; R01-DK-58096 to V. Poitour), and a ADA/TAKEDA Mentor-Based Postdoctoral Fellowship Award (7-10-BETA-03 to M.G.). V. Poitout holds the Canada Research Chair in Diabetes and Pancreatic β-Cell Function. Islet isolations and slide scanning were performed in the Islet Procurement and Analysis Core of the Vanderbilt Diabetes Research and Training Center supported by NIDDK Grant DK-20593.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
R.E.M., V.P., and M.G. conception and design of research; R.E.M., M.F.M., V.S.M., J.C.D., B.A.C., K.A., and K.P. performed experiments; R.E.M., V.S.M., J.C.D., B.A.C., K.P., V.P., and M.G. analyzed data; R.E.M., K.P., V.P., and M.G. interpreted results of experiments; R.E.M., B.A.C., K.P., and M.G. prepared figures; R.E.M. drafted manuscript; R.E.M., K.A., K.P., V.P., and M.G. edited and revised manuscript; B.A.C. and M.G. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Anastasia Coldren (Vanderbilt) for help with islet isolations and Wade M. Calcutt (Vanderbilt) for assistance with gas chromatography-mass spectrometry. We thank Dr. Thierry Alquier (University of Montreal), Grace Fergusson (CRCHUM), and Mélanie Ethier (CRCHUM) of the Rodent Metabolic Phenotyping Core Facility of the CRCHUM for help with hyperinsulinemic euglycemic clamps, Dr. David Wasserman (Vanderbilt) for critical reading and helpful discussions of the manuscript, and members of the Gannon Laboratory for helpful discussions about the project.
REFERENCES
- 1.Ackermann Misfeldt A, Costa RH, Gannon M. Beta-cell proliferation, but not neogenesis, following 60% partial pancreatectomy is impaired in the absence of FoxM1. Diabetes 57: 3069–3077, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahrén B, Pacini G. Insufficient islet compensation to insulin resistance vs. reduced glucose effectiveness in glucose-intolerant mice. Am J Physiol Endocrinol Metab 283: E738–E744, 2002. [DOI] [PubMed] [Google Scholar]
- 4.Alquier T, Peyot ML, Latour MG, Kebede M, Sorensen CM, Gesta S, Ronald Kahn C, Smith RD, Jetton TL, Metz TO, Prentki M, Poitout V. Deletion of GPR40 impairs glucose-induced insulin secretion in vivo in mice without affecting intracellular fuel metabolism in islets. Diabetes 58: 2607–2615, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ayala JE, Samuel VT, Morton GJ, Obici S, Croniger CM, Shulman GI, Wasserman DH, McGuinness OP. Standard operating procedures for describing and performing metabolic tests of glucose homeostasis in mice. Dis Model Mech 3: 525–534, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berglund ED, Li CY, Poffenberger G, Ayala JE, Fueger PT, Willis SE, Jewell MM, Powers AC, Wasserman DH. Glucose metabolism in vivo in four commonly used inbred mouse strains. Diabetes 57: 1790–1799, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Butler AE, Janson J, Bonner-Weir S, Ritzel R, Rizza RA, Butler PC. Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes 52: 102–110, 2003. [DOI] [PubMed] [Google Scholar]
- 8.Cresto JC, Lavine RL, Buchly ML, Penhos JC, Bhathena SJ, Recant L. Half life of injected 125I-insulin in control and ob/ob mice. Acta Physiol Lat Am 27: 7–15, 1977. [PubMed] [Google Scholar]
- 9.Evans AM, DeHaven CD, Barrett T, Mitchell M, Milgram E. Integrated, nontargeted ultrahigh performance liquid chromatography/electrospray ionization tandem mass spectrometry platform for the identification and relative quantification of the small-molecule complement of biological systems. Anal Chem 81: 6656–6667, 2009. [DOI] [PubMed] [Google Scholar]
- 10.Fearnside JF, Dumas ME, Rothwell AR, Wilder SP, Cloarec O, Toye A, Blancher C, Holmes E, Tatoud R, Barton RH, Scott J, Nicholson JK, Gauguier D. Phylometabonomic patterns of adaptation to high fat diet feeding in inbred mice. PLoS One 3: e1668, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ferrannini E, Natali A, Camastra S, Nannipieri M, Mari A, Adam KP, Milburn MV, Kastenmuller G, Adamski J, Tuomi T, Lyssenko V, Groop L, Gall WE. Early metabolic markers of the development of dysglycemia and type 2 diabetes and their physiological significance. Diabetes 62: 1730–1737, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fontes G, Zarrouki B, Hagman DK, Latour MG, Semache M, Roskens V, Moore PC, Prentki M, Rhodes CJ, Jetton TL, Poitout V. Glucolipotoxicity age-dependently impairs beta cell function in rats despite a marked increase in beta cell mass. Diabetologia 53: 2369–2379, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gall WE, Beebe K, Lawton KA, Adam KP, Mitchell MW, Nakhle PJ, Ryals JA, Milburn MV, Nannipieri M, Camastra S, Natali A, Ferrannini E; RISC Study Group. alpha-hydroxybutyrate is an early biomarker of insulin resistance and glucose intolerance in a nondiabetic population. PLoS One 5: e10883, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Golson ML, Misfeldt AA, Kopsombut UG, Petersen CP, Gannon M. High Fat Diet Regulation of β-Cell Proliferation and β-Cell Mass. Open Endocrinol J 4: 68–79, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Golson ML, Bush WS, Brissova M. Automated quantification of pancreatic β-cell mass. Am J Physiol Endocrinol Metab 306: E1460–E1467, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Golson ML, Maulis MF, Dunn JC, Poffenberger G, Schug J, Kaestner KH, Gannon MA. Activated FoxM1 attenuates streptozotocin-mediated β-cell death. Mol Endocrinol 28: 1435–1447, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Granger A, Kushner JA. Cellular origins of beta-cell regeneration: a legacy view of historical controversies. J Intern Med 266: 325–338, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Henley KD, Gooding KA, Economides AN, Gannon M. Inactivation of the dual Bmp/Wnt inhibitor Sostdc1 enhances pancreatic islet function. Am J Physiol Endocrinol Metab 303: E752–E761, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hull RL, Kodama K, Utzschneider KM, Carr DB, Prigeon RL, Kahn SE. Dietary-fat-induced obesity in mice results in beta cell hyperplasia but not increased insulin release: evidence for specificity of impaired beta cell adaptation. Diabetologia 48: 1350–1358, 2005. [DOI] [PubMed] [Google Scholar]
- 20.Kooptiwut S, Kebede M, Zraika S, Visinoni S, Aston-Mourney K, Favaloro J, Tikellis C, Thomas MC, Forbes JM, Cooper ME, Dunlop M, Proietto J, Andrikopoulos S. High glucose-induced impairment in insulin secretion is associated with reduction in islet glucokinase in a mouse model of susceptibility to islet dysfunction. J Mol Endocrinol 35: 39–48, 2005. [DOI] [PubMed] [Google Scholar]
- 21.Laoukili J, Alvarez M, Meijer LA, Stahl M, Mohammed S, Kleij L, Heck AJ, Medema RH. Activation of FoxM1 during G2 requires cyclin A/Cdk-dependent relief of autorepression by the FoxM1 N-terminal domain. Mol Cell Biol 28: 3076–3087, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee YS, Li P, Huh JY, Hwang IJ, Lu M, Kim JI, Ham M, Talukdar S, Chen A, Lu WJ, Bandyopadhyay GK, Schwendener R, Olefsky J, Kim JB. Inflammation is necessary for long-term but not short-term high-fat diet-induced insulin resistance. Diabetes 60: 2474–2483, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leung TW, Lin SS, Tsang AC, Tong CS, Ching JC, Leung WY, Gimlich R, Wong GG, Yao KM. Over-expression of FoxM1 stimulates cyclin B1 expression. FEBS Lett 507: 59–66, 2001. [DOI] [PubMed] [Google Scholar]
- 24.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods 25: 402–408, 2001. [DOI] [PubMed] [Google Scholar]
- 26.McGuinness OP, Ayala JE, Laughlin MR, Wasserman DH. NIH experiment in centralized mouse phenotyping: the Vanderbilt experience and recommendations for evaluating glucose homeostasis in the mouse. Am J Physiol Endocrinol Metab 297: E849–E855, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nieman DC, Shanely RA, Gillitt ND, Pappan KL, Lila MA. Serum metabolic signatures induced by a three-day intensified exercise period persist after 14 h of recovery in runners. J Proteome Res 12: 4577–4584, 2013. [DOI] [PubMed] [Google Scholar]
- 28.Park SY, Cho YR, Kim HJ, Higashimori T, Danton C, Lee MK, Dey A, Rothermel B, Kim YB, Kalinowski A, Russell KS, Kim JK. Unraveling the temporal pattern of diet-induced insulin resistance in individual organs and cardiac dysfunction in C57BL/6 mice. Diabetes 54: 3530–3540, 2005. [DOI] [PubMed] [Google Scholar]
- 29.Pettersson US, Waldén TB, Carlsson PO, Jansson L, Phillipson M. Female mice are protected against high-fat diet induced metabolic syndrome and increase the regulatory T cell population in adipose tissue. PLoS One 7: e46057, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Plank JL, Frist AY, LeGrone AW, Magnuson MA, Labosky PA. Loss of Foxd3 results in decreased beta-cell proliferation and glucose intolerance during pregnancy. Endocrinology 152: 4589–4600, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Posey KA, Clegg DJ, Printz RL, Byun J, Morton GJ, Vivekanandan-Giri A, Pennathur S, Baskin DG, Heinecke JW, Woods SC, Schwartz MW, Niswender KD. Hypothalamic proinflammatory lipid accumulation, inflammation, and insulin resistance in rats fed a high-fat diet. Am J Physiol Endocrinol Metab 296: E1003–E1012, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rankin MM, Kushner JA. Adaptive beta-cell proliferation is severely restricted with advanced age. Diabetes 58: 1365–1372, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reimer MK, Ahren B. Altered beta-cell distribution of pdx-1 and GLUT-2 after a short-term challenge with a high-fat diet in C57BL/6J mice. Diabetes 51, Suppl 1: S138–S143, 2002. [DOI] [PubMed] [Google Scholar]
- 35.Stamateris RE, Sharma RB, Hollern DA, Alonso LC. Adaptive β-cell proliferation increases early in high-fat feeding in mice, concurrent with metabolic changes, with induction of islet cyclin D2 expression. Am J Physiol Endocrinol Metab 305: E149–E159, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Storey JD, Tibshirani R. Statistical significance for genomewide studies. Proc Natl Acad Sci USA 100: 9440–9445, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Surwit RS, Kuhn CM, Cochrane C, McCubbin JA, Feinglos MN. Diet-induced type II diabetes in C57BL/6J mice. Diabetes 37: 1163–1167, 1988. [DOI] [PubMed] [Google Scholar]
- 38.Surwit RS, Seldin MF, Kuhn CM, Cochrane C, Feinglos MN. Control of expression of insulin resistance and hyperglycemia by different genetic factors in diabetic C57BL/6J mice. Diabetes 40: 82–87, 1991. [DOI] [PubMed] [Google Scholar]
- 39.Teta M, Long SY, Wartschow LM, Rankin MM, Kushner JA. Very slow turnover of beta-cells in aged adult mice. Diabetes 54: 2557–2567, 2005. [DOI] [PubMed] [Google Scholar]
- 40.Tomasi T, Sledz D, Wales JK, Recant L. Insulin half-life in normal and diabetic subjects. Rev Neuropsychiatr Infant 14: 315–317, 1966. [DOI] [PubMed] [Google Scholar]
- 42.Toye AA, Lippiat JD, Proks P, Shimomura K, Bentley L, Hugill A, Mijat V, Goldsworthy M, Moir L, Haynes A, Quarterman J, Freeman HC, Ashcroft FM, Cox RD. A genetic and physiological study of impaired glucose homeostasis control in C57BL/6J mice. Diabetologia 48: 675–686, 2005. [DOI] [PubMed] [Google Scholar]
- 43.Tschen SI, Dhawan S, Gurlo T, Bhushan A. Age-dependent decline in beta-cell proliferation restricts the capacity of beta-cell regeneration in mice. Diabetes 58: 1312–1320, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Turner N, Kowalski GM, Leslie SJ, Risis S, Yang C, Lee-Young RS, Babb JR, Meikle PJ, Lancaster GI, Henstridge DC, White PJ, Kraegen EW, Marette A, Cooney GJ, Febbraio MA, Bruce CR. Distinct patterns of tissue-specific lipid accumulation during the induction of insulin resistance in mice by high-fat feeding. Diabetologia 56: 1638–1648, 2013. [DOI] [PubMed] [Google Scholar]
- 45.Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science 324: 1029–1033, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Winzell MS, Ahrén B. The high-fat diet-fed mouse: a model for studying mechanisms and treatment of impaired glucose tolerance and type 2 diabetes. Diabetes 53, Suppl 3: S215–S219, 2004. [DOI] [PubMed] [Google Scholar]
- 47.Zhang H, Ackermann AM, Gusarova GA, Lowe D, Feng X, Kopsombut UG, Costa RH, Gannon M. The FoxM1 transcription factor is required to maintain pancreatic beta-cell mass. Mol Endocrinol 20: 1853–1866, 2006. [DOI] [PubMed] [Google Scholar]