Abstract
Many implanted devices fail due to the formation of an avascular capsule surrounding the device. Additionally, fat has long been known to promote healing and vascularization. The goals of this study were to identify potential mechanisms of the provascular actions of adipose-derived stromal cells (ASCs) and to improve implant biocompatibility. First, adult ASCs and fibroblasts from rats were attached to polyurethane and polystyrene in vitro and their cytokine secretion profile was analyzed. Secretion of vascular endothelial growth factor (VEGF) from ASCs was 10 –70 times higher than fibroblasts after 3 and 6 days. Next, polyurethane, bare and with cellular coatings, was implanted subcutaneously in rats. The fibrous capsule surrounding bare polyurethane implants was 17%–32% thicker and the amount of collagen was 27% greater than the capsule surrounding ASC-coated implants. Finally, the microvessel density adjacent to ASC-coated polyurethane was approximately 50%–80% higher than bare polyurethane. In summary, ASCs attached to polyurethane have a dramatically increased VEGF production compared with fibroblasts in vitro, and these cells also produce an increased microvessel density in the surrounding tissue when implanted subcutaneously in rats.
Keywords: Adipose-derived stromal cells, Adipose stem cells, Foreign body response, Biomaterials, Cytokines
Introduction
Many chronically implanted devices fail as a result of the foreign body response. Chronically implanted devices as diverse as glucose sensors for diabetics, drainage devices for glaucoma patients, and breast implants following mastectomy all have fibrous capsule formation as their leading cause of failure [1–4]. The foreign body response that leads to the formation of a relatively avascular capsule varies significantly by tissue type [5–7]. For example, implants placed in lean tissues have very different healing responses than the same implants in fat [8–10]. Specifically, implants in rat subcutaneous lean tissue lead to the formation of extensive fibrous capsules as well as reduced vascularity in the tissue adjacent to the implants. In contrast, implants in the epididymal fat pad in rats exhibit greater microvessel density in the tissue surrounding the implant and less evidence of a fibrous capsule. The molecular mechanisms of the reduced severity of the foreign body response in adipose tissue are unclear.
Adipose-derived stromal cells (ASCs) are easy to isolate and are known to have exceptional regenerative abilities. For this reason, they are being intensively investigated by many groups for use in tissue engineering applications [11–18]. In these applications, rapid development of a microvascular network for good mass transport is a limiting factor in the size of the three-dimensional cell-material constructs. Thus, understanding the mechanism that adipose tissue uses to reduce the foreign body response and increase microvessel density is of keen interest for many implanted device applications.
The current study analyzed the cytokine secretion profile of ASCs attached to tissue culture polystyrene (TCPS) and polyurethane to identify molecular signals that could play a key role in altering the foreign body response. Fibroblasts were also used to determine whether results were cell specific. Other groups have suggested an important role for cytokines in the foreign body response [19, 20] and in neovascularization of tissue [5, 21], and it has been shown that ASCs secrete many pro-wound-healing and inflammatory cytokines [18, 22] that could have a direct impact on the foreign body response. Once it was determined that ASCs did have a different secretion profile of key wound healing cytokines, in vivo tests were performed to determine whether these cells, when attached to polyurethane ex vivo, ameliorate the response adjacent to the implanted biomaterial.
Materials and Methods
Cell Attachment and Isolation
Previous experiments focused on optimizing an attachment method for adipose-derived stromal cells onto polyurethane [23] and found fibronectin attachment to provide increased cell coverage compared with no treatment. For this experiment, medical-grade polyurethane (Pellethane 2363–80AE-050824, a soft segment polyurethane based on polyether and aromatic isocyanate acquired from Polyzen, Inc., Apex, NC, http://www.polyzen.com/) was cut into 1-cm × 1-cm square pieces of 0.8-mm thickness and sterilized with ethylene oxide, followed by at least 7 days of outgassing.
Adult ASCs were isolated from the inguinal fat pad of syngeneic male Lewis rats (Charles River Laboratories, Wilmington, MA, http://www.criver.com/) as described previously [23]. Briefly, the fat was rinsed, minced, incubated in collagenase A, separated by centrifugation, and treated for 1 minute with red cell lysis buffer. The isolated cells were plated at a density of 1 × 106 cells per T75 flask and then frozen at a density of 5 × 105 cells per milliliter of freezing media (α minimum essential medium [α-MEM], 20% fetal bovine serum [FBS], and 10% dimethyl sulfoxide). Cells were thawed 2 days prior to attachment at a density of 7.5 × 105 viable cells per T75 flask. The cells were frozen to allow the same isolation to provide cells for both the in vitro and in vivo experiments. The differentiation potential of both fresh isolates and frozen cells was confirmed to ensure the cells retained their multipotent potential after freezing. Briefly, cells were differentiated into adipocytes and osteoblasts to confirm that an ASC population was truly isolated. Adipogenic media contained Dulbecco’s modified Eagle’s medium (DMEM), 10% FBS, 0.5 mM isobutylmethylxanthine, 10 μM insulin, 0.5 μM dexamethasone, and 1% penicillin/streptomycin [13]. Osteogenic media contained DMEM, 10% FBS, 10 mM b-glycerol phosphate, 50 μg/ml ascorbate 2-phosphate, 10 nM dexamethasone, 100 U/ml penicillin, and 0.1 mg/ml streptomycin [24]. Oil red O staining after 28 days in culture was used to confirm adipose differentiation, and von Kossa staining after 28 days was used to confirm bone differentiation.
For the following experiments, two cell types were studied, ASCs and terminally differentiated fibroblasts. Both cell types were isolated as described above. The ASCs were frozen after passage 1 and thawed as needed. Additionally, prior to freezing, a group of the isolated cells was grown to passage 12, at which time the cells were terminally differentiated into fibroblasts. To confirm a fibroblast population, the cells were found to have fibroblast morphology, to not stain for smooth muscle actin, and to secrete collagen. The fibroblasts were used as a control cell type in both the in vitro and in vivo experiments.
In Vitro
For the in vitro experiments, fibroblasts were used as a control cell type since they are known to be involved in the wound healing response. ASCs and fibroblasts were seeded onto TCPS and polyurethane using fibronectin adsorption. For all fibronectin samples, 25 μg/ml fibronectin was adsorbed for 2 hours at 37°C. All cells were seeded at 1.6 × 105 cells/well in 24-well plates for 1 hour before a media change.
Supernatant media were collected at day 3 during media replacement and also at day 6. Samples were transferred to a −80°C freezer prior to cytokine analysis. Cytokine analysis was done using the Bioplex (Bio-Rad, Hercules, CA, http://www.bio-rad.com) and a Linco kit (Linco, St. Charles, MO, http://www.millipore.com) to determine the concentration of the following cytokines in the samples: interleukin 1β (IL-1β), IL-2, IL-4, IL-6, IL-10, leptin, tumor necrosis factor α (TNF-α), and vascular endothelial growth factor (VEGF).
In Vivo
Cell Attachment
One day prior to implantation, the materials were assigned to one of the following four treatment groups: (a) bare: materials were rinsed in phosphate-buffered saline (PBS) and then incubated in media; (b) fibronectin: materials were rinsed, incubated with 25 μg/ml fibronectin, rinsed, and then incubated in media; (c) ASC-coated materials: materials were rinsed, incubated with 25 μg/ml fibronectin, rinsed, and then incubated with a cell suspension of 1.6 × 105 ASCs; (d) fibroblast-coated materials: materials were rinsed, incubated with 25 μg/ml fibronectin, rinsed, and then incubated with a cell suspension of 1.6 × 105 fibroblasts. Medium used for all experiments was α-MEM with 10% FBS. On the day of implantation, cells were rinsed three times with PBS directly prior to surgery.
The animal protocol was approved by the Institutional Animal Care and Use Committee at Duke University prior to initiation of any studies. Samples were implanted for 1, 4, and 8 weeks into 15 rats (5 rats per time point; n = 5 for each time point; n = 15 total). Rats were anesthetized with isoflurane (2.5%–3%), shaved, and prepared with chlorhexidine and alcohol. For each of the implantations, a lateral incision was made and a small subcutaneous pocket was formed. All four groups were implanted into each animal and the implant position for each animal was rotated so that the implants at each time point occupied all possible positions. Each implant was inserted with the treated side toward the skin, and the incision was closed with wound clips. The wound clips were removed 7 days after surgery.
At the time of explantation, rats were anesthetized with isoflurane (2.5%–3%) and shaved. For each sample, the implant and tissue surrounding the implant were removed, embedded in optimal cutting temperature compound using aluminum base molds, and immediately snap frozen in a liquid nitrogen and dry ice slurry. The tissue was mounted and sectioned at 12 μm and placed on charged slides.
Histology and Data Analysis
Tissue samples for histological analysis were stained with Gomori’s trichrome and hematoxylin & eosin (H&E), as well as von Willebrand factor (vWF) immunohistochemical stain (a fluorescein-conjugated antibody specific for vWF that is a large multimeric glycoprotein present in blood plasma and produced constitutively in endothelium, megakaryocytes [α-granules of platelets], and subendothelial connective tissue). Images of the trichrome and H&E samples were collected using ×10 and ×20 objectives (×100 or ×200 magnification) with a Zeiss Axioskop 2 Plus optical microscope (Carl Zeiss, Chester, VA, http://www.zeiss.com) equipped with a Micropublisher 3.3 RTV digital color camera (QImaging, Surrey, BC, Canada, http://www.qimaging.com/). Tissue samples treated with the vWF immunohistochemical stain were examined using the same microscope. Images were captured at ×200 magnification with a CE CCD digital camera (QImaging).
Capsule thickness was measured from trichrome-stained tissue samples. The foreign body capsule was defined as the region of dense collagen oriented parallel to the implant. Capsule thickness was determined using ImageJ (NIH software; Bethesda, MD, http://www.nih.gov/) and measuring the distance from the implant to the edge of the capsule. For all implant types, three images taken at ×10 magnification were analyzed from each of five rats. A semiquantitative collagen index was calculated by applying a digital threshold with MATLAB (The MathWorks, Inc., Novi, MI, http://www.mathworks.com/) to cropped images of the previously determined capsule. The threshold was applied such that all pixels resulting from green/blue collagen tissue were converted to black pixels and background tissue was converted to white pixels. The number of black pixels was then normalized as a percentage of the total number of pixels in the image and reported as the collagen index. At each time point, the collagen index was calculated from three images analyzed from each of five rats.
The number of microvessels in proximity to each implant was determined by capturing ×200-magnification images of the vWF-stained samples at predetermined locations around each implant. A blinded observer counted the number of vWF-stained blood microvessels in the 200-μm area adjacent to the implant for each image. Each vessel counted had to be of sufficient size to have a red blood cell pass through it. The average number of blood microvessels for each sample was calculated from four images taken from five rats at each time point.
Statistics
All data presented represent an average value ± standard error of the mean, and were analyzed for significance (p < .05) with a repeated measure analysis of variance followed by a Fisher’s protected least significance difference test or a t test (p < .05).
Results
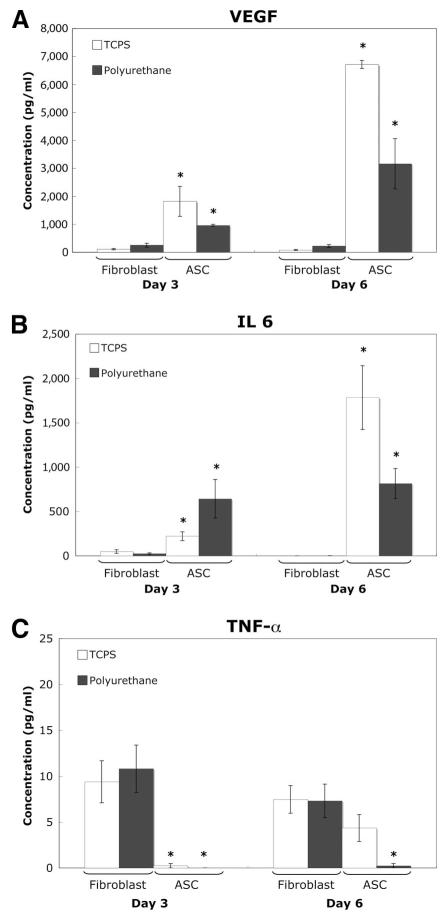
The in vitro experiments, displayed in Figure 1, show that the secretion profile of cytokines differs for ASCs and fibroblasts on both TCPS and on polyurethane. VEGF secretion was significantly higher than control fibroblasts at both day 3, 10–20 times higher, and at day 6, 40–70 times higher. VEGF is an anti-inflammatory, pro-wound-healing and provascular cytokine that is known to work in vivo in the picogram per milliliter concentration range to both induce and stabilize vessel formation. In addition, although less severe, IL-6 and TNF-α secretion were also statistically different.
Figure 1.
In vitro cytokine secretion. (A): Vascular endothelial growth factor (VEGF). (B): Interleukin-6 (IL-6). (C): Tumor necrosis factor α (TNF-α). Cells were attached using fibronectin adsorption and supernatant samples were taken at day 3 and day 6 after attachment for cytokine analysis. Mean ± SEM. * indicates significance (p < .05) versus fibroblasts with the same material treatments on the same day. Abbreviations: ASC, adipose-derived stromal cell; TCPS, tissue culture polystyrene.
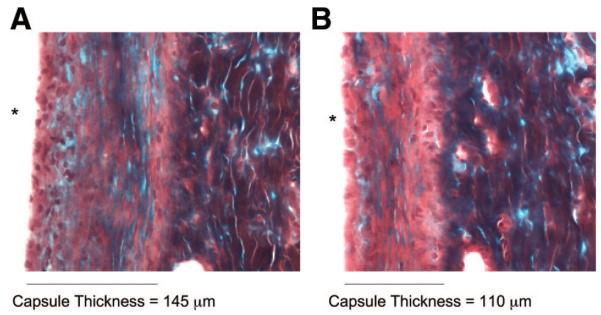
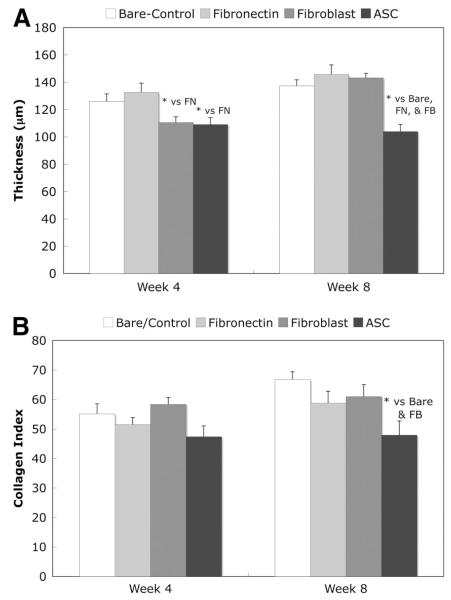
For the in vivo experiments, Gomori’s trichrome-stained sections, examples shown in Figure 2, were used to calculate capsule thickness as well as the collagen index in the capsule. The capsule thickness results are shown in Figure 3A. At week 1, the capsule was poorly defined and therefore no results were calculated for this time point. At week 4, the fibroblast- and ASC-coated samples had significantly thinner capsules than the other implants. However, by week 8, the capsule around the fibroblast-coated implant had thickened and the ASC-coated sample had a significantly thinner capsule than all of the other implants.
Figure 2.
Gomori’s trichrome histology of frozen tissue at week 8. (A): Bare implant. (B): Adipose-derived stromal cell-coated implant. * signifies location of implant.
Figure 3.
Capsule thickness and capsule collagen index at 4 weeks and 8 weeks after implantation. (A): Thickness of capsule surrounding polyurethane implants. (B): Collagen index of capsule. The semiquantitative collagen index was calculated by applying a digital threshold to cropped images of the trichrome-stained capsule images and then calculating the percentage of blue/green pixels. n = 5 for each implant type at each time (total n of 15 rats). Normal rat subcutis tissue has a collagen index of approximately 40. Mean ± SEM, *, t test (p < .05). Abbreviations: ASC, adipose-derived stromal cell; FB, fibroblast; FN, fibronectin.
Figure 3B displays the collagen index, which is a semiquantitative indication of the amount of collagen in the capsule. At week 8, the collagen index was significantly lower in the ASC-coated versus the bare and fibroblast-coated samples.
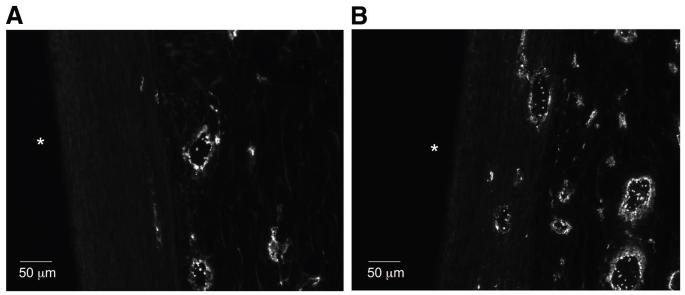
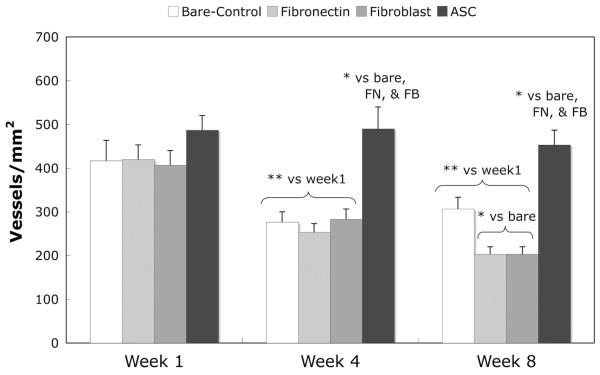
Von Willebrand factor immunostaining was used to image microvessels in the tissue surrounding the implant, and sample images are displayed in Figure 4. The number of microvessels per square millimeter within 200 μm of the implant was calculated and displayed in Figure 5. At both weeks 4 and 8, the number of microvessels in the tissue surrounding the bare control, fibronectin, and fibroblast samples decreased from week 1. However, the number of microvessels in the tissue surrounding the adipose-derived stromal cell implants remained constant and was therefore significantly greater than all other samples at both weeks 4 and 8.
Figure 4.
Von Willebrand factor staining for microvessels at week 8. (A): Bare implant. (B): Adipose-derived stromal cell-coated implant. * signifies location of implant.
Figure 5.
Vascularity in tissue surrounding implants. The number of microvessels within 200 μm of the implant surface was calculated using images of von Willebrand factor-stained slides. n = 5 for each implant type at each time (total n of 15 rats). Normal rat subcutis tissue has approximately 300 microvessels/mm2. Mean ± SEM; *, t test (p < .05). Abbreviations: ASC, adipose-derived stromal cell; FB, fibroblast; FN, fibronectin.
Discussion
The in vitro tests provided clear evidence, as shown in Figure 1, that the secretion profile of cytokines differs for ASCs and fibroblasts on both TCPS and on polyurethane. When ASCs were attached via fibronectin to both TCPS and polyurethane, VEGF secretion was significantly higher than control fibroblasts. At day 3, VEGF secretion was 10–20 times higher and at day 6, VEGF secretion was 40–70 times higher. VEGF is an anti-inflammatory, pro-wound-healing, and provascular cytokine that is known to work in vivo in the picogram per milliliter concentration range to both induce and stabilize vessel formation. Baseline expression of VEGF from fibroblasts is typically less than 10–100 pg/ml, which was the level observed in these experiments [25, 26]. It was therefore hypothesized that such high secretion would have a noticeable effect on the microvessel density in vivo. Additionally, although less severe, the cytokine secretion profile of IL-6 and TNF-α was significantly different for ASCs. ASCs produced higher levels of IL-6 than fibroblast, but lower levels of TNF-α. Both IL-6 and TNF-α are proinflammatory cytokines. The main sources for IL-6 in vivo are stimulated monocytes, fibroblasts, and endothelial cells. In addition to being proinflammatory, IL-6 is also known to be synergistic with other cytokines in vitro to promote the proliferation of multipotent hematopoietic progenitor cells. TNF-α is secreted by macrophages, monocytes, neutrophils, and T cells. It is also a growth factor for normal human fibroblasts, which if autocrine, would explain secretion by the fibroblasts in vitro. TNF-α in a similar range has been shown to lead to the destruction of small blood vessels within malignant tumors. Therefore secretion in vivo would likely have a negative impact on the foreign body response.
Additional cytokines that were not available for analysis are also very important in the foreign body response and angiogenesis. Specifically, fibroblast growth factor (FGF) and transforming growth factor β (TGF-β) are of interest. FGFs, especially FGF-1, are known to promote angiogenesis in vivo through the promotion of endothelial cell proliferation and the physical organization of endothelial cells into tube-like structures. In addition, FGF stimulates the proliferation of fibroblasts that give rise to granulation tissue during the wound-healing response. TGF-β controls proliferation and cellular differentiation in most cell types and can act as a negative autocrine growth factor. For these reasons, FGF and TGF-β are of interest in these experiments. However, these cytokines were not commercially available for the Bioplex at the time of these experiments and therefore were not included.
The final set of experiments was performed to determine whether ASC attachment to polyurethane, a commonly used biomaterial, would alter the in vivo foreign body response, and the results are shown in Figures 3 and 5. Histological analyses of materials explanted at 1, 4, and 8 weeks were used to characterize the tissue response. Trichrome-stained histology was used to determine the collagen capsule thickness surrounding the implant and to calculate the collagen index within the capsule, and sample images are shown in Figure 2. At week 1, the capsule was poorly defined, and therefore no results were calculated at this time point. As shown in Figure 3, at week 8, the capsule surrounding the ASC-coated implants was approximately 25% thinner than the bare polyurethane. Additionally, at week 8, the amount of collagen in the capsule surrounding the ASC-coated samples, as characterized by the collagen index, was 25% less than the collagen in the capsule surrounding the bare material. As reported by Sharkawy et al. [27], the fibrous capsule that forms around implants retards the diffusion of low-molecular-weight analytes such as glucose, and this encapsulation tissue can consequently lead to the failure of many in vivo devices. However, a thinner capsule with less collagen, such as the one observed around the ASC-coated samples, would be extremely beneficial in increasing diffusion from the tissue to the implant surface.
In addition to the dense collagen capsule that impedes the diffusion of small analytes, the typical avascular nature of the collagen capsule leads to longer diffusion distances, which impair the flux of analytes. Immunostaining for von Willebrand factor was used to identify microvessels in the tissue surrounding the implants and sample images are shown in Figure 4. As shown in Figure 5, the microvessel density profile for the ASC-coated implants was significantly different from the other three implants. At week 1, all of the implants had a similar number of microvessels. As the wound-healing process continued to weeks 4 and 8, many of the microvessels surrounding the bare, fibronectin-, and fibroblast-coated samples regressed. However the number of microvessels in the tissue surrounding the ASC-coated implants remained constant. At weeks 4 and 8, there were 50%–80% more microvessels in the tissue surrounding the ASC-coated implants than in the tissue surrounding the bare polyurethane.
The specific choice of ASCs for this experiment was based on the healing response that occurs within fat and also the in vitro cytokine secretion experiments that were performed. Fat has long been used to promote wound healing [28] and has also been shown to stimulate an intense neovascularization of the cornea [28]. As discussed earlier, the more microvessels in the tissue surrounding the implant indicate better the mass transport of small analytes and typically healthier tissue. From the in vitro experiments, it can be inferred that the secretion profile of several cytokines, including VEGF, could possibly be the mechanism by which the ASC attachment increased the number of vessels in the tissue surrounding the implants.
Finally, the fibronectin- and fibroblast-coated samples were minimally different from bare polyurethane. Fibroblasts were used as a control cell type to determine whether attachment of any cells to the material would have an effect. Since the differences seen in the response to the ASC-coated samples were not seen with the fibroblast samples, it is assumed that these differences are specific to the cell type used, not simply due to the presence of cells.
Conclusions
In conclusion, when ASCs were attached via fibronectin to tissue culture polystyrene and polyurethane, VEGF secretion was 10–70 times higher than control fibroblasts. Additionally, although less pronounced, the cytokine secretion profiles of IL-6 and TNF-α were significantly different for ASCs. Finally, the in vivo analysis showed that the tissue surrounding the ASC-coated samples had thinner capsules with less collagen and significantly more microvessels than the other samples. There were 50%–80% more microvessels adjacent to the ASC-coated implants at weeks 4 and 8 than the bare polyurethane. These findings indicate that coating devices with ASCs prior to implantation can reduce the foreign body response.
Acknowledgments
These studies were supported by NIH grants T32 GM08555 and DK54932 and by the Robert Jones Fund. The authors gratefully acknowledge the technical assistance and image analysis of Danubia Hester and Nancy Iheanacho.
Footnotes
Disclosure of Potential Conflicts of Interest
The authors indicate no potential conflicts of interest.
References
- 1.Gerritsen M. Problems associated with subcutaneously implanted glucose sensors. Diabetes Care. 2000;23:143–145. doi: 10.2337/diacare.23.2.143. [DOI] [PubMed] [Google Scholar]
- 2.Pickup JC, Hussain F, Evans ND, et al. In vivo glucose monitoring: the clinical reality and the promise. Biosens Bioelectron. 2005;20:1897–1902. doi: 10.1016/j.bios.2004.08.016. [DOI] [PubMed] [Google Scholar]
- 3.Chang L, Crowston JG, Cordeiro MF, et al. The role of the immune system in conjunctival wound healing after glaucoma surgery. Surv Ophthalmol. 2000;45:49–68. doi: 10.1016/s0039-6257(00)00135-1. [DOI] [PubMed] [Google Scholar]
- 4.Burkhardt BR, Dempsey PD, Schnur PL, et al. Capsular contracture: A prospective-study of the effect of local antibacterial agents. Plast Reconstr Surg. 1986;77:919–930. [PubMed] [Google Scholar]
- 5.Anderson JM. Inflammation and the foreign body response. Problems Gen Surg. 1994;11:147–160. [Google Scholar]
- 6.Anderson JM. Biological responses to materials. Ann Rev Materials Res. 2001;31:81–110. [Google Scholar]
- 7.Castner DG, Ratner BD. Biomedical surface science: Foundations to frontiers. Surface Sci. 2002;500:28–60. [Google Scholar]
- 8.Williams SK, Berman SS, Kleinert LB. Differential healing and neovascularization of ePTFE implants in subcutaneous versus adipose tissue. J Biomed Materials Res. 1997;35:473–481. doi: 10.1002/(sici)1097-4636(19970615)35:4<473::aid-jbm7>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 9.Kellar RS, Kleinert LB, Williams SK. Characterization of angiogenesis and inflammation surrounding ePTFE implanted on the epicardium. J Biomed Materials Res. 2002;61:226–233. doi: 10.1002/jbm.10021. [DOI] [PubMed] [Google Scholar]
- 10.Wisniewski N, Rajamand N, Adamsson U, et al. Analyte flux through chronically implanted subcutaneous polyamide membranes differs in humans and rats. Am J Physiol Endocrinol Metab. 2002;282:E1316–E1323. doi: 10.1152/ajpendo.00259.2001. [DOI] [PubMed] [Google Scholar]
- 11.Rodriguez AM, Elabd C, Amri EZ, et al. The human adipose tissue is a source of multipotent stem cells. Biochimie. 2005;87:125–128. doi: 10.1016/j.biochi.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 12.Zuk PA, Zhu M, Ashjian P, et al. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13:4279–4295. doi: 10.1091/mbc.E02-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zuk PA, Zhu M, Mizuno H, et al. Multilineage cells from human adipose tissue: Implications for cell-based therapies. Tissue Eng. 2001;7:211–228. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
- 14.Guilak F, Lott KE, Awad HA, et al. Clonal analysis of the differentiation potential of human adipose-derived adult stem cells. J Cell Physiol. 2006;206:229–237. doi: 10.1002/jcp.20463. [DOI] [PubMed] [Google Scholar]
- 15.Ogawa R, Mizuno H, Watanabe A, et al. Osteogenic and chondrogenic differentiation by adipose-derived stem cells harvested from GFP transgenic mice. Biochem Biophys Res Comm. 2004;313:871–877. doi: 10.1016/j.bbrc.2003.12.017. [DOI] [PubMed] [Google Scholar]
- 16.Safford KM, Hicok KC, Safford SD, et al. Neurogenic differentiation of murine and human adipose-derived stromal cells. Biochem Biophys Res Comm. 2002;294:371–379. doi: 10.1016/S0006-291X(02)00469-2. [DOI] [PubMed] [Google Scholar]
- 17.Awad HA, Wickham MQ, Leddy HA, et al. Chondrogenic differentiation of adipose-derived adult stem cells in agarose, alginate, and gelatin scaffolds. Biomaterials. 2004;25:3211–3222. doi: 10.1016/j.biomaterials.2003.10.045. [DOI] [PubMed] [Google Scholar]
- 18.Gimble JM, Katz AJ, Bunnell BA. Adipose-derived stem cells for regenerative medicine. Circ Res. 2007;100:1249–1260. doi: 10.1161/01.RES.0000265074.83288.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Anderson JM, Jones JA. Phenotypic dichotomies in the foreign body reaction. Biomaterials. 2007;28:5114–5120. doi: 10.1016/j.biomaterials.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brodbeck WG, Nakayama Y, Matsuda T, et al. Biomaterial surface chemistry dictates adherent monocyte/macrophage cytokine expression in vitro. Cytokine. 2002;18:311–319. doi: 10.1006/cyto.2002.1048. [DOI] [PubMed] [Google Scholar]
- 21.Chen RR, Mooney DJ. Polymeric growth factor delivery strategies for tissue engineering. Pharm Res. 2003;20:1103–1112. doi: 10.1023/a:1025034925152. [DOI] [PubMed] [Google Scholar]
- 22.Kim WS, Park BS, Sung JH, et al. Wound heating effect of adipose-derived stem cells: A critical role of secretory factors on human dermal fibroblasts. J Dermatol Sci. 2007;48:15–24. doi: 10.1016/j.jdermsci.2007.05.018. [DOI] [PubMed] [Google Scholar]
- 23.Prichard HL, Reichert MW, Klitzman B. Adult adipose-derived stem cell attachment to biomaterials. Biomaterials. 2007;28:936–946. doi: 10.1016/j.biomaterials.2006.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee JA, Parrett BM, Conejero JA, et al. Biological alchemy: Engineering bone and fat from fat-derived stem cells. Ann Plast Surg. 2003;50:610–617. doi: 10.1097/01.SAP.0000069069.23266.35. [DOI] [PubMed] [Google Scholar]
- 25.Ollivier V, Chabbat J, Herbert JM, et al. Vascular endothelial growth factor production by fibroblasts in response to factor VIIa binding to tissue factor involves thrombin and factor Xa. Arterioscl Thromb Vasc Biol. 2000;20:1374–1381. doi: 10.1161/01.atv.20.5.1374. [DOI] [PubMed] [Google Scholar]
- 26.Berse B, Hunt JA, Diegel RJ, et al. Hypoxia augments cytokine (transforming growth factor-beta (TGF-beta) and IL-1)-induced vascular endothelial growth factor secretion by human synovial fibroblasts. Clin Exp Immunol. 1999;115:176–182. doi: 10.1046/j.1365-2249.1999.00775.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sharkawy AA, Klitzman B, Truskey GA, et al. Engineering the tissue which encapsulates subcutaneous implants. 1. Diffusion properties. J Biomed Materials Res. 1997;37:401–412. doi: 10.1002/(sici)1097-4636(19971205)37:3<401::aid-jbm11>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 28.Silverman KJ, Lund DP, Zetter BR, et al. Angiogenic activity of adiposetissue. Biochem Biophys Res Comm. 1988;153:347–352. doi: 10.1016/s0006-291x(88)81229-4. [DOI] [PubMed] [Google Scholar]