Abstract
Recent evidence suggests that neutrophils play an important role in the pathogenesis of lupus. The goal of this study was to characterize the epigenetic architecture, by studying the DNA methylome, of neutrophils and low density granulocytes (LDGs) in lupus patients. We studied 15 lupus patients and 15 healthy age, sex, and ethnicity matched controls. Genome-wide DNA methylation was assessed using the Illumina HumanMethylation 450 BeadChip array, which includes over 485,000 methylation sites across the entire genome. Bisulfite DNA sequencing was used to validate the array results. Statistical and bioinformatic analysis was performed to identify and characterize differentially methylated loci and genes. We identified 293 differentially methylated CG sites in neutrophils between lupus patients and controls. The majority (68%) of differentially methylated CG sites were hypomethylated in lupus neutrophils compared to controls, suggesting overall hypomethylation. We found a robust and consistent demethylation of interferon signature genes in lupus neutrophils, and similar demethylation in the same genes in autologous LDGs. Indeed, the DNA methylome in lupus neutrophils and LDGs was almost identical, suggesting similar chromatin architecture in the two granulocyte subsets. A notable exception was the hypomethylation of a CG site in the promoter region of the cytoskeleton-regulating gene RAC1 in LDGs. Our findings demonstrate a pattern of robust demethylation of interferon signature genes in lupus patients supporting a pathogenic role for neutrophils in lupus. We suggest a model whereby DNA from lupus neutrophils and LDGs externalized by NETosis enhance type-I IFN production via TLR-9 stimulation by hypomethylated DNA.
Keywords: Lupus, Neutrophils, Methylome, LDG, Epigenetics
1. Introduction
Systemic lupus erythematosus is a chronic relapsing autoimmune disease characterized by autoantibody production and multiorgan involvement. The etiology of lupus is incompletely understood. However, a number of environmental triggers and epigenetic factors, in the setting of a genetic susceptibility background, are thought to contribute to the etiology of the disease [1]. Gene expression profiling in peripheral blood mononuclear cells (PBMCs) from patients with lupus demonstrates a robust and confirmed interferon signature [2]. In addition, a granulocyte expression signature has been observed [3], suggesting a role for neutrophils in the pathogenesis of lupus. Recent evidence indicates that PBMCs from lupus patients include a subset of “immature” or “abnormal” proinflammatory neutrophils with a lower density, which have been called low density granulocytes (LDGs) [4]. This neutrophil subset purifies with PBMCs upon density gradient centrifugation and explains the granulocyte signature observed in lupus PBMCs [3]. Subsequently, it was noted that this granulocyte subset is more prone to a recently described neutrophil cell death process by forming neutrophil extracellular traps (NETs) [5]. Indeed, NETs formation stimulates interferon alpha production from plasmacytoid dendritic cells, thereby contributing to the pathogenesis of lupus [6]. Evidence suggests that lupus neutrophils, and LDGs in particular, play an important role in tissue damage (including glomerulonephritis and lupus skin involvement), as well as endothelial damage in lupus patients [5]. Several studies have also suggested altered functional capacity of lupus neutrophils compared to healthy controls [6].
Despite the recent and important progress in recognizing the potential role of neutrophils in the pathogenesis of lupus, there remain significant knowledge gaps that require further investigation. DNA methylation changes play an important role in the pathogenesis of lupus. To understand the epigenetic accessibility that underlies the pathogenic role of neutrophils in lupus, we performed a genome-wide DNA methylation study in normal density lupus neutrophils (hereafter referred to as neutrophils) compared to age, sex, and ethnicity matched healthy controls, and in normal density lupus neutrophils compared to autologous LDGs.
2. Materials and methods
2.1. Lupus patients and controls
We included 15 female lupus patients and 15 age (±5 years), sex, and ethnicity matched healthy controls in this study (Table 1). The average age for patients and controls was 36.9yr ± 10.2 and 38.7yr ± 9.6, respectively (P = 0.62). All patients studied fulfilled the American College of Rheumatology (ACR) classification criteria for lupus, and were recruited from the University of Michigan rheumatology clinics or the Lupus Natural History Protocol at the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) of the National Institutes of Health (NIH). Lupus patients included in this study had a relatively inactive disease at the time of enrollment, as measured by Systemic Lupus Erythematosus Disease Activity Index (SLEDAI average = 1.7, range 0–5). The SLEDAI criteria present at the time of blood draw for our study, medications used, and the ACR classification criteria met in each patient are listed in Table 1. We excluded any patient who has received cyclophosphamide within a month of recruitment as this treatment can significantly affect hematopoietic cell production. Healthy controls were recruited by advertisement at the University of Michigan, or through approved protocols at the Clinical Center, NIH. All patients and controls signed an informed consent prior to participation in this study. This study was approved by the Institutional Review Boards at the University of Michigan and NIDDK.
Table 1.
Demographic and clinical information for the lupus patients and controls included in this study. All study participants were female.
| Patients | Controls | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Numbera | Age | Ethnicity | SLEDAI score |
SLEDAI criteria present |
ACR criteria met | Medications | Number | Age | Ethnicity |
| 1 | 39 | African–American | 4 | Alopecia, increased DNA binding |
Malar rash, arthritis, serositis (pleuritis), renal, immunologic, ANA |
Mycophenolate, prednisone, rituximab |
1 | 43 | African–American |
| 2 | 35 | European–American | 2 | Increased DNA binding |
Malar rash, immunologic, hematologic, ANA |
Hydroxychloroquine | 2 | 36 | European–American |
| 3 | 31 | European–American | 2 | Rash | Malar rash, oral ulcers, arthritis, renal, immunologic, ANA |
Prednisone | 3 | 34 | European–American |
| 4 | 36 | African–American | 5 | Alopecia, increased DNA binding, leukopenia |
Discoid rash, renal, neurologic, immunologic, ANA |
Hydroxychloroquine, Mycophenolate, Prednisone |
4 | 34 | African–American |
| 5 | 39 | European–American | 4 | Low complement, increased DNA binding |
Arthritis, serositis (pleuritis), immunologic, hematologic |
Hydroxychloroquine, mycophenolate, prednisone |
5 | 42 | European–American |
| 6 | 20 | European–American | 4 | Rash, pericarditis | Malar rash, photosensitivity, hematologic, immunologic, ANA |
Azathoprine, prednisone, hydroxychloroquine |
6 | 24 | European–American |
| 7 | 59 | European–American | 0 | – | Arthritis, hematologic, immunologic, ANA |
None | 7 | 60 | European–American |
| 8 | 45 | Asian | 0 | – | Oral ulcers, arthritis, serositis (pleuritis), renal, immunologic, ANA |
Mycophenolate, quinacrine |
8 | 41 | Asian |
| 9 | 50 | European–American | 0 | – | Malar rash, arthritis, hematologic, renal, ANA |
Hydroxychloroquine, prednisone |
9 | 45 | Hispanic/White |
| 10 | 35 | African–American | 0 | – | Serositis (pericarditis), neurologic, hematologic, immunologic, ANA |
Hydroxychloroquine, prednisone |
10 | 34 | African–American |
| 11 | 20 | African–American | 0 | – | Malar rash, arthritis, serositis (pericarditis), immunologic, ANA |
Hydroxychloroquine, prednisone |
11 | 22 | African–American |
| 12 | 40 | Asian | 0 | – | Arthritis, Renal, Immunologic, ANA |
None | 12 | 46 | Asian |
| 13 | 27 | Hispanic/White | 2 | Low complement | Oral ulcers, arthritis, hematologic, immunologic, ANA |
Hydroxychloroquine, prednisone, azathioprine |
13 | 31 | Hispanic/White |
| 14 | 37 | Hispanic/White | 2 | Low complement | Malar rash, arthritis, serositis (pleuritis), hematologic, immunologic, ANA |
Hydroxychloroquine, prednisone, mycophenolate, methotrexate |
14 | 42 | Hispanic/White |
| 15 | 40 | African–American | 0 | – | Arthritis, serositis (pericarditis), immunologic, ANA |
Mycophenolate, prednisone |
15 | 47 | African–American |
SLEDAI, Systemic lupus erythematosus disease activity index.
Neutrophils were extracted from all patients, and autologous LDGs were also simultaneously extracted from patients 1–8.
2.2. Neutrophil and LDG isolation and DNA extraction
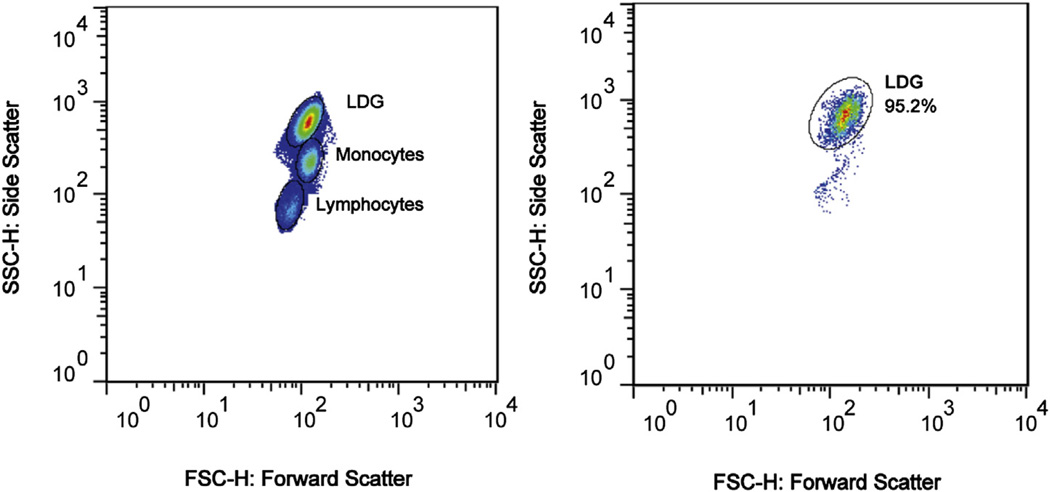
Fresh peripheral blood samples (25 ml) were collected and density gradient centrifugation (Ficoll) was used to collect PBMCs. LDGs were then isolated from PBMCs using indirect labeling and magnetic bead separation with the following antibodies: anti-CD3, anti-CD7, anti-CD19, anti-CD79b, anti-CD56, anti-MHCII, anti-CD86 and anti-CD235a as previously described [4]. LDG purity was confirmed by flow cytometry using forward and side scatter profiles developed and validated using surface expression of CD14 and CD15 as previously described [4], and was over 95% in all samples (Fig. 1). Neutrophils were extracted from the granulocyte layer after Ficoll density gradient centrifugation, following previously described protocols [5].
Figure 1.
Forward and side scatter flow cytometry plots demonstrating the LDG population in a representative sample before (left) and after (right) isolation.
DNA was extracted from each sample using the DNeasy Blood and Tissue Kit (Qiagen, Valencia, CA), then bisulfite-converted using the EZ DNA Methylation kit (Zymo Research, Irvine, CA) for DNA methylation studies.
2.3. DNA methylation profiling
Analysis of genome-wide DNA methylation in normal density neutrophil and LDG samples was performed using the Infinium HumanMethylation450 BeadChip Kit (Illumina), as previously described [7]. This array includes over 485,000 methylation sites (majority are CG dinucleotides) and covers over 99% of RefSeq genes and 96% of CG islands. An average of 17 CG sites per gene are included on the array to cover the promoter, 5′UTR, first exon, 3′UTR, and CG sites within the gene body. Other regions covered include ~3000 non-CG methylation sites and miRNA promoter regions.
2.4. Data processing and statistical and bioinformatics analysis
Data processing and data analysis were performed as previously described by our group [7–11]. Data pre-processing was performed as described by Illumina. Briefly, normalization of Infinium HumanMethylation450 probes was accomplished using over 90 pairs of normalization control probes designed to target the same region within housekeeping genes. These probes contain no underlying CG sites. One probe in each pair will incorporate a base in the green (CG bases) or red channel (AT bases) and normalization values from each channel are calculated and applied separately. A constant normalization factor is calculated as the average of AT and CG normalization controls in the first sample in the sample list (which is an arbitrary choice). This normalization constant is multiplied by the control probe intensity values in each individual sample and the product is divided by the average of the control probe intensities in that channel in the given sample. In addition to normalizing red and green channel intensities, background fluorescence is subtracted during sample preprocessing which minimizes the variation in the background signals between arrays. The background signal is the 5th percentile of negative control probes calculated in the red and green channels separately. The channel-specific background intensity is subtracted from the probe intensities and if the intensity becomes negative it is set to 0. The background intensity is also used in probe detection calculations.
Controls that monitor the different steps of staining, extension and hybridization of samples, target sequence removal, specificity, bisulfite conversion, non-polymorphic and negative controls are also included. The staining control monitors staining efficiency in the green and red channels. The sample-independent extension controls monitor extension efficiency of the A, C, G and T nucleotides. The hybridization and target removal controls are also sample independent and rely on synthetic targets included in the hybridization buffer added with the samples when hybridizing them to the array. The hybridization control tests the overall performance of the Infinium Assay at different concentrations of target synthetic sequences that bind perfectly to their probes. The target removal control monitors the stripping of target sequences from probes after extension. The bisulfite conversion and specificity controls monitor the Infinium I and Infinium II-type beads independently and are sample dependent. The bisulfite conversion controls report extension of probes that target converted or unconverted sites. Specificity controls monitor non-specific primer extension of probes and are designed against non-polymorphic T sites. The negative controls consist of 600 randomly permutated probe sequences that target bisulfite-converted sequences that lack CG sites and should not bind to DNA template. The mean signals of the probes define the system background value. Non-polymorphic controls query a particular base in a non-polymorphic region of the genome to test the overall performance of the assay and compare it across the samples.
All of our samples passed the detailed quality control measures discussed above. Probe intensity values were normalized and used to determine the average methylation level on each methylation site in each sample (beta value or β). Probes that include a genetic variant within the first 10 bp region of the 3′ end of the probe, and probes that had a detection P value (detection above background) of ≥0.05 were excluded from the analysis. Differentially methylated sites between groups were determined, and defined as methylation sites with an absolute difference in beta value (delta beta or Δβ) of at least 0.1 and that remained significant (P < 0.01) after correction for multiple testing using a Benjamini and Hochberg false discovery rate of 0.05.
The variation in the estimate of β is a function of β, and was estimated by Illumina for all values of β by repeatedly measuring loci with known methylation fractions ranging from 0 to 1, and then fitting a parabola to the standard deviation as a function of β (GenomeStudio Methylation Module User Guide, Illumina, USA). The standard deviation estimate is calculated by s = Aβ2 + Bβ + C, where A = −0.1511; B = 0.1444; and C = 0.01646
P values for differential DNA methylation were calculated using the formula:
where z is the two-sided tail probability of the standard normal distribution.
Differentially methylated loci were mapped to genes or gene regions, and then subjected to bioinformatic analyses using Ingenuity Pathway Analysis (IPA), IRIDESCENT [12], and the Database for Annotation, Visualization, and Integrated Discovery (DAVID) [13].
2.5. Co-regulation clustering analysis
Co-expression correlation analysis was performed by downloading and processing 3900 human 2-color microarrays from NCBI's GEO database as previously described [14]. Two color microarrays were chosen so that co-regulation tendencies could be identified (i.e., gene pairs that change in expression together when comparing two conditions). Pearson's correlation coefficients were calculated, quantifying the global co-expression correlations, for each gene–gene pair.
2.6. Bisulfite sequencing
Bisulfite DNA sequencing on selected differentially methylated CG sites was performed to validate the results obtained from the Infinium HumanMethylation450 BeadChip arrays. One differentially methylated CG site in MX1 was selected for validation. Primers were designed using Sequenom's EpiDesigner online tool to include this CG site and an additional 20 CG sites in its vicinity. Primer quality was verified using Premier Biosoft's Netprimer online tool (Sequenom, San Diego, CA). The following primers were used: forward 5′-TTTAGTGGATGTTATGTTTGGGGT-3′, reverse 5′-CACTACTACCTACCAAAACCCCTAAA-3’. PCR was performed on a Bio-Rad T100 (Bio-Rad, Hercules, CA) using ZymoTaq one-step master mix (Zymo, Orange, CA). The cycling conditions were as follows: 95 °C for 10 min followed by 40 cycles of 95 °C for 30 s, then 58.3 °C for 40 s, then 72 °C for 1 min, followed by 72 °C for 7 min. Each PCR product was confirmed with a small aliquot by 2% agarose gel electrophoresis. The remainder of the PCR product was then purified and sequenced using an Applied Biosystems 3730 XL sequencer. Sequencing trace files were then used to calculate the percentage of cytosine methylation on each CG site using ESME software package (Epigenomics AG, Berlin).
3. Results
We observed significant and consistent DNA methylation differences between neutrophils in lupus patients when compared to age, sex, and ethnicity matched healthy controls. There were a total of 293 differentially methylated CG sites, with 199 (68%) hypomethylated and 94 (32%) hypermethylated in lupus neutrophils (Supplementary Table S1). The top hypomethylated and hypermethylated sites are listed in Table 2. Raw and normalized DNA methylation data were deposited in Gene Expression Omnibus (GEO accession: GSE65097).
Table 2.
Top hypomethylated and hypermethylated (delta beta < −0.20 or >0.20) CG sites in lupus neutrophils compared to age, sex, and ethnicity matched healthy control neutrophils. Significant and consistent hypomethylation in interferon signature genes is noted.
| CG ID | Chr | Position (HG19) | Gene | Methylation fraction (β) | Delta β | Fold change (patients/controls) |
P value | |
|---|---|---|---|---|---|---|---|---|
| Patients | Controls | |||||||
| cg21549285 | 21 | 42799141 | MX1 | 0.250 | 0.739 | −0.489 | 0.339 | 1.69E-34 |
| cg05696877 | 1 | 79088769 | IFI44L | 0.249 | 0.594 | −0.346 | 0.418 | 1.69E-34 |
| cg23570810 | 11 | 315102 | IFITM1 | 0.382 | 0.692 | −0.310 | 0.551 | 1.69E-34 |
| cg22862003 | 21 | 42797588 | MX1 | 0.287 | 0.595 | −0.309 | 0.482 | 1.69E-34 |
| cg22930808 | 3 | 122281881 | PARP9;DTX3L | 0.223 | 0.529 | −0.306 | 0.421 | 1.69E-34 |
| cg14864167 | 8 | 66751182 | PDE7A | 0.336 | 0.641 | −0.305 | 0.524 | 1.69E-34 |
| cg08122652 | 3 | 122281939 | PARP9;DTX3L | 0.437 | 0.740 | −0.303 | 0.591 | 1.69E-34 |
| cg07839457 | 16 | 57023022 | NLRC5 | 0.223 | 0.520 | −0.297 | 0.430 | 1.69E-34 |
| cg05552874 | 10 | 91153143 | IFIT1 | 0.205 | 0.500 | −0.295 | 0.411 | 1.69E-34 |
| cg01079652 | 1 | 79118191 | IFI44 | 0.472 | 0.751 | −0.279 | 0.628 | 1.69E-34 |
| cg20045320 | 11 | 319555 | – | 0.288 | 0.565 | −0.277 | 0.510 | 1.69E-34 |
| cg06872964 | 1 | 79085250 | IFI44L | 0.169 | 0.431 | −0.262 | 0.392 | 1.69E-34 |
| cg03038262 | 11 | 315262 | IFITM1 | 0.403 | 0.658 | −0.255 | 0.613 | 1.69E-34 |
| cg13304609 | 1 | 79085162 | IFI44L | 0.521 | 0.775 | −0.254 | 0.672 | 1.69E-34 |
| cg01028142 | 2 | 7004578 | CMPK2 | 0.528 | 0.781 | −0.253 | 0.676 | 1.69E-34 |
| cg06559318 | 6 | 32526260 | HLA-DRB6 | 0.334 | 0.583 | −0.250 | 0.572 | 1.69E-34 |
| cg03607951 | 1 | 79085586 | IFI44L | 0.146 | 0.390 | −0.244 | 0.375 | 1.69E-34 |
| cg05883128 | 4 | 169239131 | DDX60 | 0.297 | 0.540 | −0.243 | 0.550 | 1.69E-34 |
| cg17052170 | 8 | 144099482 | LY6E;LOC100133669 | 0.448 | 0.669 | −0.221 | 0.669 | 8.83E-33 |
| cg08993878 | 12 | 98151379 | – | 0.368 | 0.587 | −0.219 | 0.627 | 8.30E-31 |
| cg11888470 | 6 | 32526021 | HLA-DRB6 | 0.479 | 0.696 | −0.217 | 0.688 | 1.26E-32 |
| cg21442271 | 22 | 18738315 | – | 0.334 | 0.548 | −0.214 | 0.610 | 1.37E-29 |
| cg06188083 | 10 | 91093005 | IFIT3 | 0.333 | 0.546 | −0.213 | 0.610 | 2.65E-29 |
| cg20098015 | 22 | 50971140 | ODF3B | 0.205 | 0.415 | −0.210 | 0.494 | 1.67E-33 |
| cg05030953 | 6 | 31241000 | HLA-C | 0.313 | 0.522 | −0.209 | 0.599 | 1.71E-28 |
| cg26312951 | 21 | 42797847 | MX1 | 0.209 | 0.417 | −0.209 | 0.500 | 9.08E-33 |
| cg14293575 | 22 | 18635460 | USP18 | 0.633 | 0.838 | −0.205 | 0.755 | 1.69E-34 |
| cg26536949 | 17 | 57053 | – | 0.701 | 0.500 | 0.201 | 1.403 | 1.04E-34 |
| cg27473997 | 4 | 9355351 | USP17 | 0.689 | 0.452 | 0.237 | 1.524 | 1.04E-34 |
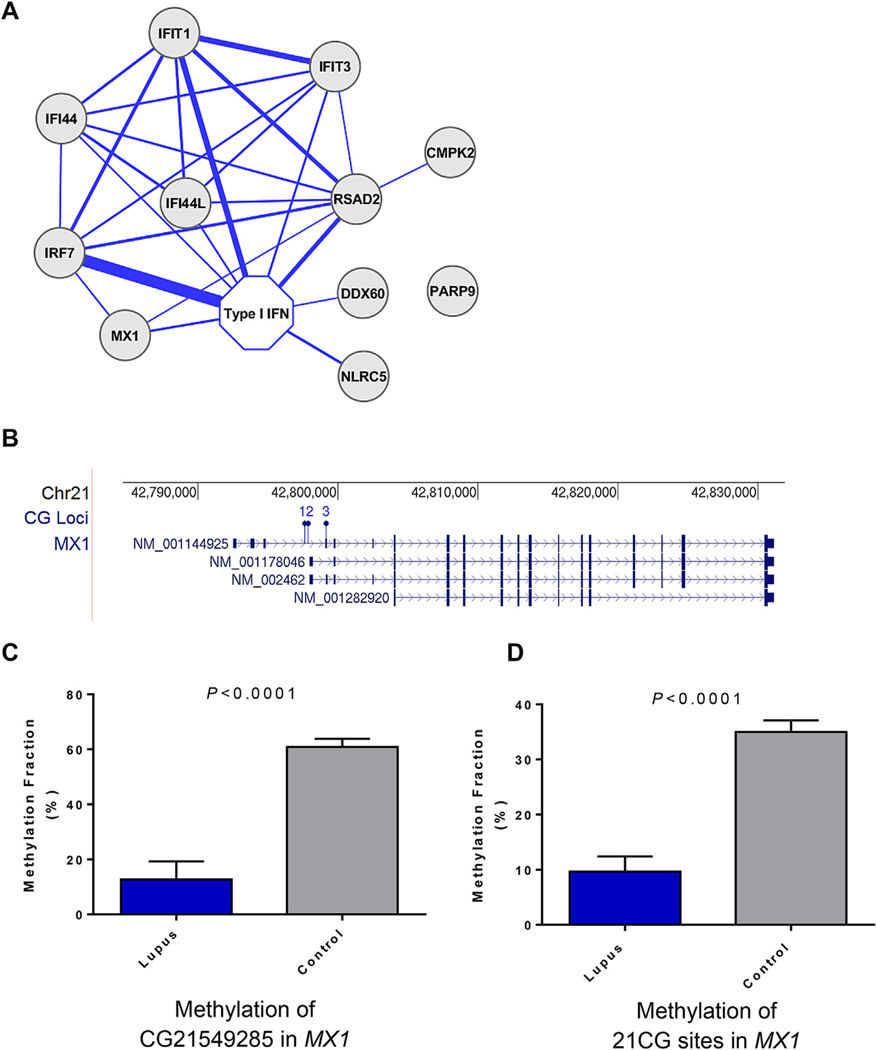
The most significant hypomethylated genes in lupus neutrophils include multiple interferon-regulated genes, such as MX1, IFI44L, IFITM1, PARP9, IFIT3, DDX60, LY6E, ISG15 and others (Fig. 2A). Indeed, the most hypomethylated CG site in lupus neutrophils compared to healthy matched controls is in MX1 (cg21549285, βpatients = 0.25, βcontrols = 0.74, P = 1.69 × 10−34) and in IFI44L (cg05696877, βpatients = 0.25, βcontrols = 0.59, P = 1.69 × 10−34). Gene Ontology and pathway analysis of hypomethylated genes identified “Interferon Signaling” as the top unifying canonical pathway among these genes (P = 8.56 × 10−7). Bisulfite sequencing was used to validate and confirm the results from the methylation array in lupus and control neutrophils (Fig. 2BC,D).
Figure 2.
A) An overview of how the differentially methylated genes within the interferon signature group are connected in the literature to each other and to type I interferon (IFN). Thickness of the lines is proportional to how many publications mention each of the connected genes (minimum = 3 publications, maximum = 232 publications). B) The location of the 3 CG sites included on the array that were differentially methylated in MX1 between lupus and control neutrophils are depicted (1 = CG22862003, 2 = CG26312951, 3 = CG21549285). Bisulfite DNA sequencing was used to confirm and extend the results obtained from the methylation array in MX1. Bisulfite DNA sequencing primers were designed to include CG21549285, and additional 20 CG sites in its vicinity. Panel (C) shows the methylation fraction obtained from bisulfite DNA sequencing in CG21549285, and (D) shows the average methylation fraction across all 21 CG sites in lupus and control neutrophils. P values were calculated using the Mann–Whitney non-parametric test.
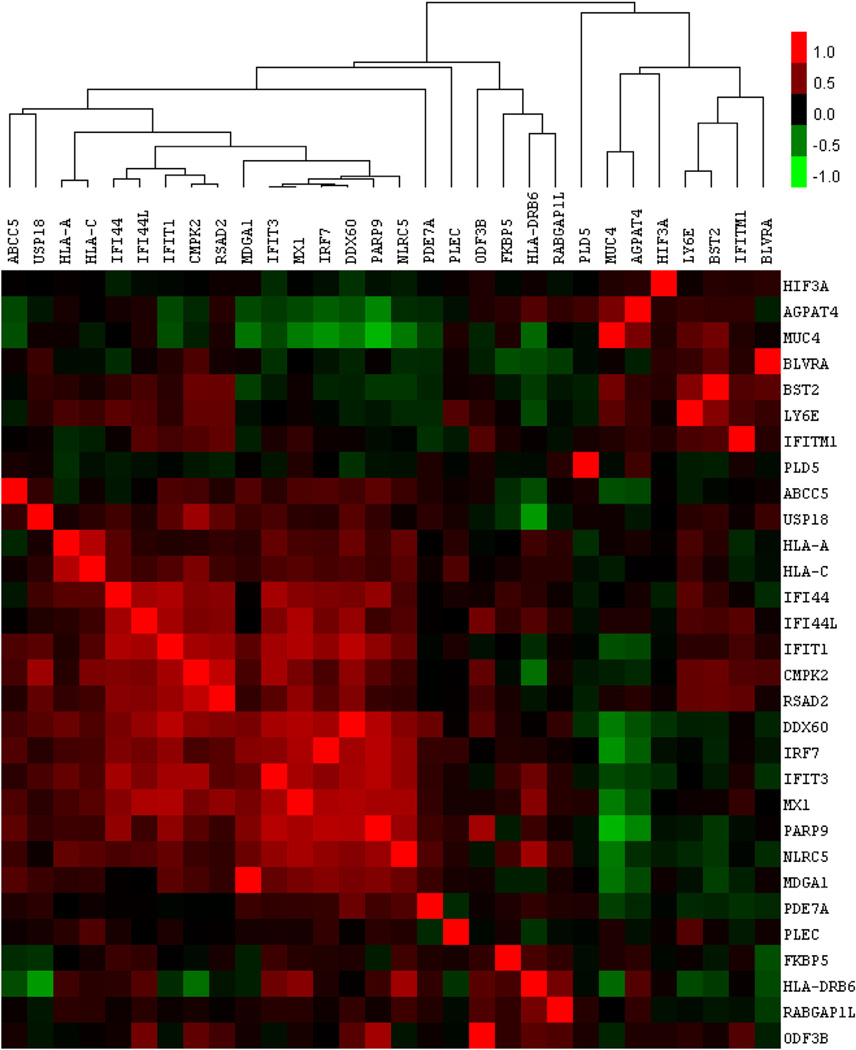
Next, we examined transcriptional co-regulation among the top 30 most significantly hypomethylated genes in lupus neutrophils. Their transcriptional correlations were clustered, using gene expression data obtained from 3900 two-color expression microarray experiments in human. We observed that the interferon-regulated genes hypomethylated in lupus neutrophils are also highly correlated in their expression levels across various cell types and experimental conditions (Fig. 3). This is evidence of the functional relevance of at least a portion of these demethylated genes, because not only are they correlated in their loss of methylation in these experiments, they have been repeatedly observed as correlated in their transcriptional changes across a large sample of other experiments. Consequently, their demethylation, like their co-expression, is likely induced by a single pathogenic process.
Figure 3.
Transcriptional correlation analysis of the top 30 genes demethylated in lupus neutrophils (relative to control neutrophils). Pearson's correlation coefficients of the reported transcriptional fold changes were calculated between each of these 30 genes across 3900 public human 2-color microarray data sets. Positive correlations are shown in red and range from 1.0 (perfect positive correlation) to a minimum of −1.0. A large group of interferon-related genes can be seen here tightly clustered, indicating they are transcriptionally co-regulated.
In a subset of the lupus patients included in this study (8 out of 15 patients), we also collected LDGs with a purity of >95%.We then compared genome-wide DNA methylation patterns between neutrophils and autologous LDGs extracted from the same blood sample at the same time. The DNA methylome in lupus LDGs was virtually identical to autologous neutrophils from the same patients. Differential DNA methylation was only detected in 3 out of over 485,000 methylation sites examined, two being hypomethylated and one hypermethylated in LDGs compared to neutrophils (Table 3). The most significant methylation change in LDGs is hypomethylation in a CG site in the promoter region of RAC1 (2.46 fold, P = 2.68 × 10−9). To determine if hypomethylation of RAC1 in LDGs is associated with increased RAC1 expression, we examined mRNA expression data from lupus LDGs compared to neutrophils (GEO accession: GSE26975). We found no difference in mRNA expression between LDGs and neutrophils in lupus patients (n = 10, P = 0.35).
Table 3.
Differentially methylated CG sites between neutrophils and autologous LDGs from lupus patients. Only 3 CG sites were differentially methylated.
| CG ID | Chr | Position (HG19) | Gene | Methylation fraction (β) | Delta β | Fold change (LDGs/neutrophils) | P value | |
|---|---|---|---|---|---|---|---|---|
| Neutrophils | LDGs | |||||||
| cg00885365 | 2 | 215702120 | – | 0.720 | 0.592 | −0.129 | 0.821 | 0.0022 |
| cg18404925 | 7 | 6413861 | RAC1 | 0.235 | 0.096 | −0.139 | 0.407 | 2.68E-09 |
| cg26783481 | 5 | 93125895 | FAM172A | 0.733 | 0.837 | 0.103 | 1.141 | 0.0022 |
4. Discussion
The role of components of the innate immune response in the pathogenesis of systemic autoimmune diseases, such as lupus, has been increasingly recognized [15,16]. Lupus is characterized by activation of plasmacytoid dendritic cells, a robust type-I interferon expression signature, and hyper-responsiveness of PBMCs to type-I interferon [17,18]. Indeed, T cells in lupus patients are characterized by a permissive epigenetic architecture in interferon-regulated genes, which precedes active transcription [7]. Furthermore, monocytes from lupus patients show evidence of increased histone acetylation in interferon-regulated genes [19].
Recent evidence supports a role for neutrophils in the pathogenesis of lupus. There is ample evidence to suggest that neutrophils in lupus patients aare activated, and produce enhanced amounts of type-I interferon that may drive abnormal B cell differentiation in the bone marrow [6,20]. In addition, PBMCs from lupus patients include a subset of pro-inflammatory LDGs that are more prone to NETosis and have been reported to have a distinct gene expression profile from autologous normal density neutrophils [4,5]. In this study, we aimed to infer the epigenetic transcriptional accessibility of neutrophils in lupus, by comparing DNA methylation levels across the genome between lupus patients and healthy matched controls. Our data showed robust demethylation of interferon-regulated gene in lupus neutrophils compared to controls. This is consistent with our previous findings in lupus T cells, and suggests that demethylation of interferon-regulated genes in lupus is triggered by an event that crosses the boundaries between distinct hematopoietic cell lineages. It is possible that exposure to type-I interferon during the course of the disease primes cells for type-I interferon hyper-responsiveness by directly or indirectly inducing chromatin accessibility in type-I interferon regulated genes. As neutrophils themselves have been demonstrated to produce increased amounts of type-I interferons in lupus, especially interferon alpha [20], it is possible that an autocrine feedback loop exists whereby interferon alpha production increases interferon responsiveness in the same cells by inducing epigenetic changes.
LDGs were shown to produce higher quantities of type-I interferon, interferon-gamma, and TNF-alpha, and are more prone to NETosis compared to normal density neutrophils in lupus [4,5]. Therefore, we tested the hypothesis that epigenetic differences could provide an explanation for these observations. Further, we suspected that the DNA methylome signature is unique in functionally distinct cell types. Surprisingly, our data suggest that interferon-regulated genes in LDGs are equally demethylated compared to autologous neutrophils in lupus. Indeed, the DNA methylome in neutrophils and LDGs is almost identical, suggesting that any functional differences between neutrophils and LDGs would have to be explained by factors unrelated to chromatic accessibility or structure. Factors such as mRNA stability, differences in microRNA expression, or availability and activation status of transcription factors might be involved.
Our data indicate that DNA in lupus neutrophils and LDGs is hypomethylated. Further, hypomethylation of LINE-1 repeats has been recently reported in lupus neutrophils [21]. It is therefore conceivable that this DNA demethylation also plays a role in the pathogenesis of lupus in a non-gene specific manner. We suggest that increased NETosis in lupus neutrophils and LDGs could potentially be more pathogenic than NETosis of healthy neutrophils, in part due to exposing hypomethylated DNA during NETs formation, thereby stimulating further type-I IFN production through TLR-9 stimulation[22,23]. In turn, a vicious cycle of more NETosis induced by more type-I IFN production and more type-I IFN production induced by demethylated DNA secretion from lupus neutrophils and LDGs propagates the disease process.
The most significant methylation difference between lupus neutrophils and LDGs is in a CG site located the promoter region of the cytoskeleton related gene RAC1. This CG site was hypomethylated in LDGs compared to neutrophils in lupus. RAC1 is a Rho GTPase involved in regulating actin polymerization required during cell motility, migration, adhesion, and tissue invasion [24]. Mice with conditional Rac1 deficiency in myeloid cells demonstrate that Rac1-deficient neutrophils exhibit a broad functional defect at multiple levels, including profound reduction of neutrophil recruitment to inflammatory sites in vivo, and defective chemotaxis- mediated cell migration and actin assembly in vitro [25]. Of interest, LDG infiltration has been observed in the skin and kidneys of lupus patients, and whether RAC1 hypomethylation plays a pathogenic role allowing heightened migratory capacity of these cells is an intriguing possibility that deserves further examination. It should be noted that we do not find it very surprising that no expression difference in RAC1 was observed between lupus LDGs and neutrophils, as DNA methylation changes are more stable and generally predate mRNA expression. Further, it is very possible that upon appropriate transcription factor availability and active expression of RAC1, LDGs would be already recruited into tissue and unavailable for sampling using peripheral blood. We have previously observed this disconnect between DNA methylation changes and active gene expression in naïve CD4+ T cells in lupus for example [7]. Importantly, LDGs are more prone to NETosis, a process that involves drastic changes and remodeling in the cytoskeleton, and a potentially important role for Rac small GTPases in the formation of NETs was recently described [26]. It remains to be determined if hypomethylation of RAC1 plays an important role in promoting the enhanced NETs formation observed in LDGs. In addition, LDGs have increased phagocytic capacity, and Rho GTPases have been also shown to play a role in phagocytosis [27,28].
5. Conclusion
We characterized the DNA methylome of lupus neutrophils for the first time. We showed evidence for robust but identical level of DNA demethylation in interferon-regulated genes in lupus neutrophils and LDGs. Our data suggest that neutrophils and LDGs in lupus patients are highly identical at the epigenetic level, with the notable exception of the cytoskeleton regulating gene RAC1, which is demethylated in at least 1 CG site in lupus LDGs compared to autologous neutrophils. Importantly, we suggest that the pathogenicity of neutrophils and LDGs in lupus can be explained in part by exposing more demethylated DNA, sensed by TLR-9, during NETosis.
Supplementary Material
Acknowledgments
funding
Research reported in this publication was supported by the Lupus Research Institute, the Intramural Research Program, NIAMS, NIH, and the National Institute of Allergy and Infectious Diseases, NIH under award number R01AI097134.
Appendix A. Supplementary data
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.jaut.2015.01.004.
Footnotes
Competing interests
None declared.
References
- 1.Costa-Reis P, Sullivan KE. Genetics and epigenetics of systemic lupus erythematosus. Curr Rheumatol Rep. 2013;15:369. doi: 10.1007/s11926-013-0369-4. [DOI] [PubMed] [Google Scholar]
- 2.Crow MK. Type I interferon in the pathogenesis of lupus. J Immunol. 2014;192:5459–5468. doi: 10.4049/jimmunol.1002795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bennett L, Palucka AK, Arce E, Cantrell V, Borvak J, Banchereau J, et al. Interferon and granulopoiesis signatures in systemic lupus erythematosus blood. J Exp Med. 2003;197:711–723. doi: 10.1084/jem.20021553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Denny MF, Yalavarthi S, Zhao W, Thacker SG, Anderson M, Sandy AR, et al. A distinct subset of proinflammatory neutrophils isolated from patients with systemic lupus erythematosus induces vascular damage and synthesizes type I IFNs. J Immunol. 2010;184:3284–3297. doi: 10.4049/jimmunol.0902199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Villanueva E, Yalavarthi S, Berthier CC, Hodgin JB, Khandpur R, Lin AM, et al. Netting neutrophils induce endothelial damage, infiltrate tissues, and expose immunostimulatory molecules in systemic lupus erythematosus. J Immunol. 2011;187:538–552. doi: 10.4049/jimmunol.1100450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Knight JS, Kaplan MJ. Lupus neutrophils: 'NET' gain in understanding lupus pathogenesis. Curr Opin Rheumatol. 2012;24:441–450. doi: 10.1097/BOR.0b013e3283546703. [DOI] [PubMed] [Google Scholar]
- 7.Coit P, Jeffries M, Altorok N, Dozmorov MG, Koelsch KA, Wren JD, et al. Genome-wide DNA methylation study suggests epigenetic accessibility and transcriptional poising of interferon-regulated genes in naive CD4+ T cells from lupus patients. J Autoimmun. 2013;43:78–84. doi: 10.1016/j.jaut.2013.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hughes T, Ture-Ozdemir F, Alibaz-Oner F, Coit P, Direskeneli H, Sawalha AH. Epigenome-wide scan identifies a treatment-responsive pattern of altered DNA methylation among cytoskeletal remodeling genes in monocytes and CD4+ T cells in Behcet's disease. Arthritis Rheum. 2014;66:1648–1658. doi: 10.1002/art.38409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Altorok N, Coit P, Hughes T, Koelsch KA, Stone DU, Rasmussen A, et al. Genome-wide DNA methylation patterns in naive CD4+ T cells from patients with primary sjogren's syndrome. Arthritis Rheum. 2014;66:731–739. doi: 10.1002/art.38264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Altorok N, Tsou PS, Coit P, Khanna D, Sawalha AH. Genome-wide DNA methylation analysis in dermal fibroblasts from patients with diffuse and limited systemic sclerosis reveals common and subset-specific DNA methylation aberrancies. Ann Rheum Dis. 2014 May 8; doi: 10.1136/annrheumdis-2014-205303. http://dx.doi.org/10.1136/annrheumdis-2014-205303. pii: annrheumdis-2014-205303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jeffries MA, Donica M, Baker L, Stevenson M, Annan AC, Humphrey MB, et al. Genome-wide DNA methylation study identifies significant epigenomic changes in osteoarthritic cartilage. Arthritis Rheum. 2014;66:2804–2815. doi: 10.1002/art.38762. [DOI] [PubMed] [Google Scholar]
- 12.Wren JD, Garner HR. Shared relationship analysis: ranking set cohesion and commonalities within a literature-derived relationship network. Bioinformatics. 2004;20:191–198. doi: 10.1093/bioinformatics/btg390. [DOI] [PubMed] [Google Scholar]
- 13.Da Wei Huang BTS, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2008;4:44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- 14.Wren JD. A global meta-analysis of microarray expression data to predict unknown gene functions and estimate the literature-data divide. Bioinformatics. 2009;25:1694–1701. doi: 10.1093/bioinformatics/btp290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shrivastav M, Niewold TB. Nucleic acid sensors and type I interferon production in systemic lupus erythematosus. Front Immunol. 2013;4:319. doi: 10.3389/fimmu.2013.00319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kahlenberg JM, Kaplan MJ. The inflammasome and lupus: another innate immune mechanism contributing to disease pathogenesis? Curr Opin Rheumatol. 2014;26:475–481. doi: 10.1097/BOR.0000000000000088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ronnblom L, Pascual V. The innate immune system in SLE: type I interferons and dendritic cells. Lupus. 2008;17:394–399. doi: 10.1177/0961203308090020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kirou KA, Lee C, George S, Louca K, Papagiannis IG, Peterson MG, et al. Co-ordinate overexpression of interferon-alpha-induced genes in systemic lupus erythematosus. Arthritis Rheum. 2004;50:3958–3967. doi: 10.1002/art.20798. [DOI] [PubMed] [Google Scholar]
- 19.Zhang Z, Song L, Maurer K, Petri MA, Sullivan KE. Global H4 acetylation analysis by ChIP-chip in systemic lupus erythematosus monocytes. Genes Immun. 2010;11:124–133. doi: 10.1038/gene.2009.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Palanichamy A, Bauer JW, Yalavarthi S, Meednu N, Barnard J, Owen T, et al. Neutrophil-mediated IFN activation in the bone marrow alters B cell development in human and murine systemic lupus erythematosus. J Immunol. 2014;192:906–918. doi: 10.4049/jimmunol.1302112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sukapan P, Promnarate P, Avihingsanon Y, Mutirangura A, Hirankarn N. Types of DNA methylation status of the interspersed repetitive sequences for LINE-1, Alu, HERV-E and HERV-K in the neutrophils from systemic lupus erythematosus patients and healthy controls. J Hum Genet. 2014;59:178–188. doi: 10.1038/jhg.2013.140. [DOI] [PubMed] [Google Scholar]
- 22.Garcia-Romo GS, Caielli S, Vega B, Connolly J, Allantaz F, Xu Z, et al. Netting neutrophils are major inducers of type I IFN production in pediatric systemic lupus erythematosus. Science translational medicine. 2011 Mar 9;3(73):73–20. doi: 10.1126/scitranslmed.3001201. PubMed PMID: 21389264. Pubmed Central PMCID: 3143837.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lande R, Ganguly D, Facchinetti V, Frasca L, Conrad C, Gregorio J, et al. Neutrophils activate plasmacytoid dendritic cells by releasing self-DNA-peptide complexes in systemic lupus erythematosus. Science translational medicine. 2011 Mar 9;3(73):73–19. doi: 10.1126/scitranslmed.3001180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Donnelly SK, Bravo-Cordero JJ, Hodgson L. Rho GTPase isoforms in cell motility: don't fret, we have FRET. Cell Adh Migr. 2014:8. doi: 10.4161/cam.29712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Glogauer M, Marchal CC, Zhu F, Worku A, Clausen BE, Foerster I, et al. Rac1 deletion in mouse neutrophils has selective effects on neutrophil functions. J Immunol. 2003;170:5652–5657. doi: 10.4049/jimmunol.170.11.5652. [DOI] [PubMed] [Google Scholar]
- 26.Lim MB, Kuiper JW, Katchky A, Goldberg H, Glogauer M. Rac2 is required for the formation of neutrophil extracellular traps. J Leukoc Biol. 2011;90:771–776. doi: 10.1189/jlb.1010549. [DOI] [PubMed] [Google Scholar]
- 27.Niedergang F, Chavrier P. Regulation of phagocytosis by rho GTPases. Curr Top Microbiol Immunol. 2005;291:43–60. doi: 10.1007/3-540-27511-8_4. [DOI] [PubMed] [Google Scholar]
- 28.Bokoch GM. Regulation of innate immunity by rho GTPases. Trends Cell Biol. 2005;15:163–171. doi: 10.1016/j.tcb.2005.01.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.