Abstract
Purpose
To uncover the genetic events leading to transformation of pediatric low-grade glioma (PLGG) to secondary high-grade glioma (sHGG).
Patients and Methods
We retrospectively identified patients with sHGG from a population-based cohort of 886 patients with PLGG with long clinical follow-up. Exome sequencing and array CGH were performed on available samples followed by detailed genetic analysis of the entire sHGG cohort. Clinical and outcome data of genetically distinct subgroups were obtained.
Results
sHGG was observed in 2.9% of PLGGs (26 of 886 patients). Patients with sHGG had a high frequency of nonsilent somatic mutations compared with patients with primary pediatric high-grade glioma (HGG; median, 25 mutations per exome; P = .0042). Alterations in chromatin-modifying genes and telomere-maintenance pathways were commonly observed, whereas no sHGG harbored the BRAF-KIAA1549 fusion. The most recurrent alterations were BRAF V600E and CDKN2A deletion in 39% and 57% of sHGGs, respectively. Importantly, all BRAF V600E and 80% of CDKN2A alterations could be traced back to their PLGG counterparts. BRAF V600E distinguished sHGG from primary HGG (P = .0023), whereas BRAF and CDKN2A alterations were less commonly observed in PLGG that did not transform (P < .001 and P < .001 respectively). PLGGs with BRAF mutations had longer latency to transformation than wild-type PLGG (median, 6.65 years [range, 3.5 to 20.3 years] v 1.59 years [range, 0.32 to 15.9 years], respectively; P = .0389). Furthermore, 5-year overall survival was 75% ± 15% and 29% ± 12% for children with BRAF mutant and wild-type tumors, respectively (P = .024).
Conclusion
BRAF V600E mutations and CDKN2A deletions constitute a clinically distinct subtype of sHGG. The prolonged course to transformation for BRAF V600E PLGGs provides an opportunity for surgical interventions, surveillance, and targeted therapies to mitigate the outcome of sHGG.
INTRODUCTION
Gliomas are the most frequent primary CNS neoplasms in adults and children.1 In contrast to adult low-grade gliomas, which invariably progress to secondary high-grade glioma (sHGG), pediatric low-grade glioma (PLGG) rarely exhibits malignant transformation.2–4 Only a handful of studies have addressed the clinical and molecular parameters leading to transformation of PLGG to sHGG. Radiation therapy is thought to play a role in malignant transformation of PLGG,5 particularly in the context of cancer predisposition.6,7 Before the genomic era, Broniscer et al3 were the first to describe several genetic events that occur in these cancers. Recent next-generation sequencing efforts have uncovered somatic mutations in TP53, ATRX, and IDH1/2, among other alterations that are present in adult low-grade gliomas and sHGGs.8 In addition, hTERT promoter mutations with resulting re-expression of telomerase and alternative lengthening of telomeres (ALT) are major telomere maintenance mechanisms observed in adult glioblastoma and sHGG, respectively.8,9 Similar efforts have characterized the genomic landscape of pediatric primary high-grade glioma (HGG) and PLGG, with the former containing somatic mutations in histone H3.3, ATRX, and TP53 genes, and others,10–12 and the latter almost universally exhibiting alterations causing activation of the MAPK/ERK pathway.13–15
Despite extensive characterization of primary HGG and PLGG, the incidence of childhood sHGG and the genetic determinants of PLGG transformation in children remain largely unknown. Because pediatric HGGs are invariably lethal,16 there is an urgent need to identify patients with PLGGs at high risk of malignant transformation and to initiate early aggressive surgical intervention, stratify these patients for existing targeted therapies, and implement tailored surveillance.
To address these needs, we performed a population-based long-term outcome study of all patients with PLGG treated in southern Ontario, Canada, from 1986 to 2013. We then analyzed genomic and genetic alterations observed in PLGG that subsequently transformed to sHGG and correlated these findings with multiple parameters including outcome. Here, we describe a new subgroup of sHGG for which early diagnosis and intervention may improve outcome.
PATIENTS AND METHODS
Patient Cohort
After institutional review board approval of the study, all patients treated at the Hospital for Sick Children (SickKids) in Toronto, Ontario, Canada, for PLGG between January 1, 1986, and December 31, 2013, were reviewed for malignant transformation. In Canada, patients are almost exclusively treated at their residential location. Because SickKids is the only reference center for children in a population of 10 million people, no selection bias is expected, and this qualifies as a population-based study. Furthermore, the Ontario health system tracks clinical outcome data on all residents, enabling us to collect long-term follow-up data including transformation events and survival for more than 97% of patients, including adults, until December 2013.17,18 Patients were considered as having transformation to sHGG if they exhibited one or more of the following: consecutive histologic diagnosis of low-grade gliomas and HGGs as per the WHO 2007 criteria19 (n = 20); histologic diagnosis of low-grade glioma, followed by at least 1 year of stable disease, followed by clinical and radiologic progression to HGG (n = 3; Data Supplement); or clinicoradiologic evidence of low-grade glioma, at least 1 year of stable disease, and subsequent histologic diagnosis of HGG (n = 3; Data Supplement). Demographic, treatment, and outcome data were retrieved from our PLGG database, as previously described.20,21 All PLGGs and corresponding sHGGs were rereviewed by the study pathologist (C.E.H.).
Genomic and Genetic Analysis of Tumors
Isolation of DNA from tumor and control tissues was carried out as previously described.22 Comprehensive analysis of mutations in protein-coding genes by whole-exome sequencing (WES), using the Illumina HiSeq 2000 platform (Illumina, San Diego, CA), and of copy number alterations (CNAs) by array comparative genomic hybridization were performed on available samples. Genotyping for candidate hotspot mutations was carried out as previously described.10,15,23,24 Specific CNAs were validated by real-time quantitative polymerase chain reaction. BRAF fusion status of tumors was obtained using interphase fluorescent in situ hybridization. Immunohistochemistry and genotyping also identified BRAF V600E mutations and determined p53 status. ALT was detected by the c-circle assay.25,26 Further information on methods used in this study is available in the Data Supplement.
Statistical Analysis
For all correlative studies, Fisher's exact test was used. The unpaired two-tailed t test was used to compare time to transformation, the number of somatic mutations, and p53 dysfunction between relevant groups. Survival analysis was performed using the Kaplan-Meier method. A log-rank test was used to compare groups, and curves were generated using Stata v12 (Stata, College Station, TX). P < .05 was considered significant.
RESULTS
Clinical Characteristics of Patients With Pediatric sHGG
Of 886 patients treated at our institution for PLGG from 1986 to 2013, 26 patients (2.9%) fulfilled the criteria for malignant transformation (Table 1; Data Supplement). Sixteen PLGGs that transformed (69%) were diagnosed histologically as either low-grade astrocytoma or pilocytic astrocytoma, whereas the remaining were pleomorphic xanthoastrocytomas (22%) and gangliogliomas (9%). Three sHGGs were disseminated on transformation, two of which were located in hemispheric regions.
Table 1.
Clinical and Pathologic Characteristics of Pediatric Secondary High-Grade Glioma
| Characteristic | No. of Patients |
|
|---|---|---|
| PLGG | sHGG | |
| Sex | ||
| Male | 14 | |
| Female | 12 | |
| Tumor location | ||
| Hemispheric | 11 | |
| Thalamic | 9 | |
| Brainstem | 5 | |
| Optic pathway | 1 | |
| Latency to MT, years | ||
| Median | 2.84 | |
| Range | 0.32-20.3 | |
| Outcome | ||
| Alive | 3 | |
| Deceased | 23 | |
| Disseminated at diagnosis | 0 | 3 |
| Histologic diagnosis | ||
| Low-grade astrocytoma | 9 | |
| Pilocytic astrocytoma | 7 | |
| Pleomorphic xanthoastrocytoma | 5 | |
| Ganglioglioma | 2 | |
| Anaplastic astrocytoma | 9 | |
| Glioblastoma | 7 | |
| Anaplastic pleomorphic xanthoastrocytoma | 5 | |
| Anaplastic ganglioglioma | 2 | |
| Extent of surgical resection | ||
| Gross total resection | 1 | 1 |
| Subtotal resection | 15 | 5 |
| Partial resection | 0 | 3 |
| Biopsy | 5 | 4 |
| Surgery done (extent unknown) | 0 | 5 |
| Treated with RT after diagnosis | 5 | 14 |
| Chemotherapy received | 9 | 17 |
| Age diagnosis, years | ||
| Median | 7.0 | 12.5 |
| Range | 0.39-15.6 | 0.57-33.7 |
| OS after diagnosis, years | ||
| Median | 3.75 | 0.8 |
| Range | 0.52-26.1 | 0.02-21.7 |
Abbreviations: MT, malignant transformation; OS, overall survival; PLGG, pediatric low-grade glioma; RT, radiotherapy; sHGG, secondary high-grade glioma.
The median age at PLGG diagnosis for all patients was 7.0 years (range, 0.39 to 15.6 years). The median age at sHGG diagnosis was 12.5 years (range, 0.57 to 33.7 years), respectively. Only five (19%) of 26 patients were treated with radiotherapy at PLGG diagnosis. Three patients (11%) had cancer predisposition syndromes. These included one patient with neurofibromatosis type 1, one with Li-Fraumeni syndrome, and one with biallelic mismatch repair deficiency syndrome. None of these patients received radiation at PLGG diagnosis.
Landscape of Point Mutations in sHGG
We performed WES on seven sHGGs and two matched PLGGs. Mean sequencing coverage of sHGG and matched control tissue (n = 7 pairs) was 94× and 114×, respectively. Interestingly, the patient with Li-Fraumeni syndrome (patient 7) harbored only 21 somatic mutations in their sHGG, whereas the patient with biallelic mismatch repair deficiency (patient 16) harbored 11,953 somatic mutations, representing a hypermutator phenotype. WES analysis identified a median somatic nonsilent mutation rate of 0.45/Mb in seven sHGGs (median total mutations, 25 mutations; range, six to 11,953 mutations). sHGGs exhibited a higher somatic mutation load than primary pediatric HGGs (median, 11 mutations; range, zero to 4,628 mutations; P = .0042; Data Supplement), available from previously published data.10 Importantly, none of these sHGGs were irradiated before surgical resection.
Three sHGGs harbored heterozygous missense mutations in chromatin-modifying genes including SETD1B, DOT1L, and DNMT1 (Data Supplement). The most recurrent mutation was BRAF V600E (c.1799T>A), identified in three (43%) of seven sHGGs. We then performed targeted genotyping of recurrent and common genes involved in primary HGG and adult sHGG on all childhood sHGGs. No mutations in H3F3A G34 or IDH1 were identified, and only one patient with a mutation in ATRX was observed. Nineteen percent of sHGGs harbored H3F3A K27M mutations, all of which were located in the brainstem or thalamus. BRAF V600E was identified to be the most recurrent somatic mutation, present in seven (39%) of 18 sHGGs (Fig 1A; Data Supplement), 71% of which (five of seven sHGGs) were hemispheric.
Fig 1.
Clinical, genetic and molecular characteristics in (A) secondary high-grade glioma and (B) pediatric low-grade glioma (PLGG) undergoing transformation. Frequency of genetic or molecular alterations indicated as percentage at the far right of each row. AA, anaplastic astrocytoma; AGG, anaplastic ganglioglioma; APXA, anaplastic pleomorphic xanthoastrocytoma; bMMRD, biallelic mismatch repair deficiency syndrome; CPS, cancer predisposition syndrome; Dx, diagnosis; GBM, glioblastoma; GG, ganglioglioma; HG, high grade; LFS, Li-Fraumeni syndrome; LG, low grade; LGA, low-grade astrocytoma; MT, malignant transformation; N/A, not applicable; PA, pilocytic astrocytoma; PXA, pleomorphic xanthoastrocytoma; RT, radiotherapy. p53 dysfunction is defined by more than 50% p53 immunopositive tumor cells and/or TP53 mutation.
CNAs in sHGG
We interrogated the same seven sHGGs for recurrent CNAs using array comparative genomic hybridization. Total CNA per sHGG genome was 100, and CNA was 56 in the two patient-matched PLGGs. The only recurrent CNA observed in the sHGGs was a focal deletion of chromosome 9 (p21.3), encompassing the CDKN2A gene. CDKN2A deletions were observed in 71% of patients from our initial cohort. To determine the frequency of the CDKN2A gene deletion in the entire sHGG cohort, we performed real-time quantitative polymerase chain reaction on 14 tumors. Overall, 57% of sHGGs (eight of 14 sHGGs) had heterozygous or homozygous deletions of CDKN2A (Fig 1A; Data Supplement).
BRAF V600E and CDKN2A Deletion Are Early Events in PLGG Undergoing Transformation
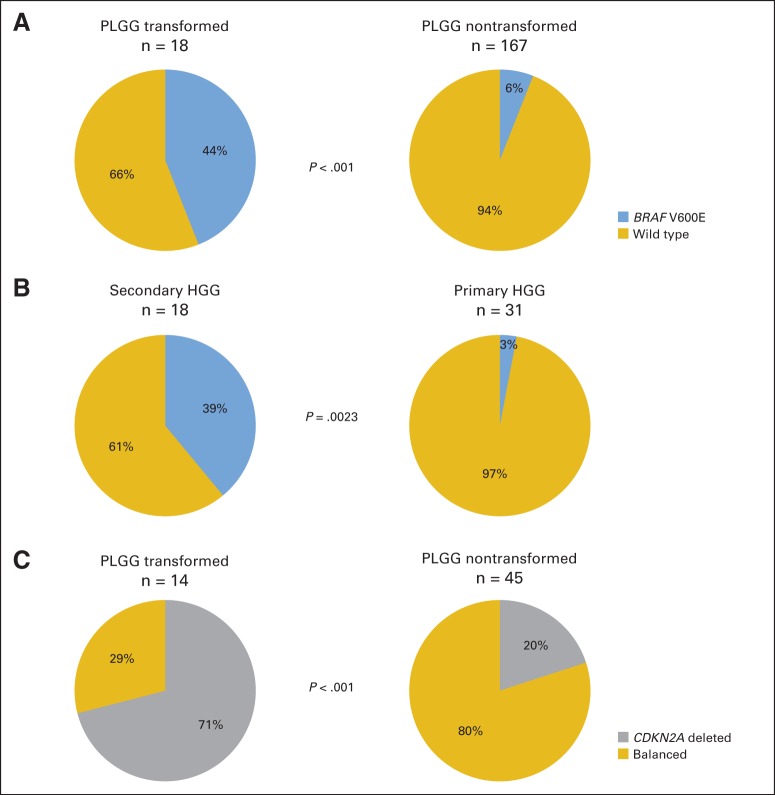
Analysis of patient-matched low- and high-grade samples revealed that BRAF mutations identified in the sHGG were also identified in 100% of the corresponding PLGGs and that 80% of sHGG CDKN2A deletions were also identified in the corresponding PLGGs (Fig 1). To determine whether these early events are unique to this subset of pediatric gliomas, we compared the frequency of these alterations to nontransformed PLGG and primary pediatric HGG. BRAF V600E was significantly enriched in PLGG that transformed, being present in 44% (eight of 18) of PLGGs that transformed compared with 6% (10 of 167) of PLGGs that did not transform (Fig 2A; P < .001; Data Supplement). The mutation was also highly enriched in sHGGs compared with only one of 31 primary pediatric HGGs (Fig 2B; P = .0023). Strikingly, this single patient had a 3-year history of headache and seizures before the diagnosis and is currently alive more than 15 years after treatment for their presumed primary HGG. Similar to BRAF mutations, CDKN2A deletions were significantly enriched in PLGG that transformed (71% v 20% of PLGGs that did not transform; Fig 2C; Data Supplement; P < .001). Interestingly, six (75%) of eight BRAF mutant PLGGs harbored concomitant CDKN2A deletions (Fig 1). Therefore, we conclude that BRAF V600E mutant sHGGs are a genetically distinct group.
Fig 2.
Correlative studies identifying that (A) BRAF V600E is enriched in pediatric low-grade gliomas (PLGGs) that later transform versus PLGGs that do not transform (P < .001); (B) BRAF V600E distinguishes secondary from primary high-grade gliomas (HGGs) in children (P = .0023); and (C) CDKN2A deletions are enriched in PLGGs that later transform versus PLGGs that do not transform (P < .001). Statistical significance was evaluated using Fisher's exact test.
Integrative Genetic and Molecular Analysis of Childhood sHGG
Several important biologic features emerged from our analysis of PLGGs and their sHGG counterparts. First, no sHGG harbored the 7q34 duplication, which is indicative of the oncogenic BRAF-KIAA549 gene fusion.27,28 Notably, only one of the PLGGs that later transformed harbored that aberration. For this single patient, the 7q34 duplication was identified in 10% of tumor cells examined and was not observed at all in the corresponding sHGG (Fig 1).
Second, additional evidence of clonal evolution was observed in the TP53 data. Specifically, patient 16 harbored a deleterious TP53 (p.R273C) missense mutation in the PLGG at 7% allele frequency. The matched sHGG had a 30% allele frequency of the same mutation. Similarly, the PLGG had less than 25% immunopositivity for p53, whereas the matched sHGG tumor had more than 90% of tumor cells staining positive for p53 (Data Supplement). Eighty percent of patients (eight of 10 patients) demonstrated increases in p53 tumor cell immunopositivity between matched low- and high-grade tumor samples. Overall, there was a higher proportion of p53 immunopositive sHGG (72%) versus PLGG (27%; Fig 1; P = .0149; Data Supplement).
Third, telomere maintenance abnormalities were observed in 54% of sHGGs. In contrast to adult low-grade gliomas, no PLGG exhibited ALT (zero of 12 PLGGs), whereas hTERT promoter mutations were seen in 15% of PLGGs (two of 13 PLGGs), both of which were conserved in their high-grade counterparts. All ALT and hTERT promoter mutation cases were mutually exclusive (Fig 1).
Finally, alterations of the p53 and/or RB tumor suppressor pathways were identified in 14 (93%) of 15 sHGGs. Surprisingly, a high proportion of PLGGs (12 of 14 PLGGs; 86%) also harbored overexpression of p53 or CDKN2A deletions, suggesting early ablation of cell cycle control in PLGGs that later transform (Fig 1; Data Supplement).
BRAF V600E Defines a Clinically Distinct Subset of sHGG
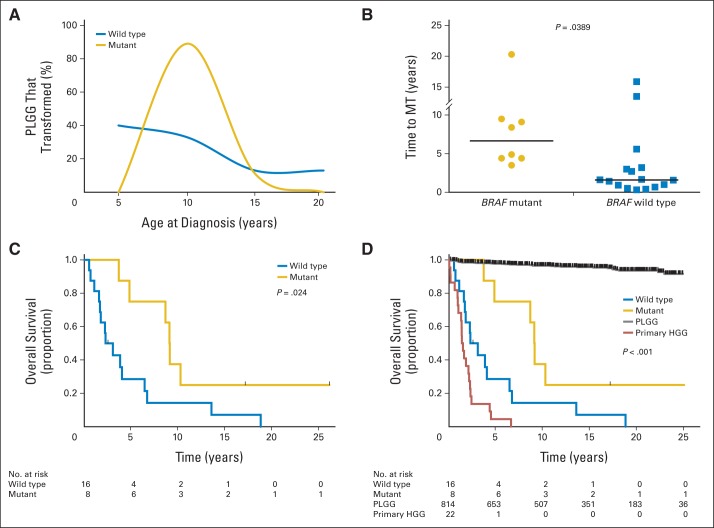
To understand the clinical consequences of the identified BRAF V600E–driven sHGG subset, we compared onset and outcome of this subgroup to BRAF wild-type sHGG and other pediatric gliomas. Patients with BRAF mutant PLGGs that later transformed were all older than age 5 years at diagnosis (range, 5.1 to 13.4 years), and 89% of these patients were between 5 and 10 years of age, in contrast to all other patients with PLGGs that later transformed (Fig 3A).
Fig 3.
(A) Age distribution for BRAF mutant and BRAF wild-type patients at initial diagnosis of pediatric low-grade glioma (PLGG). Eighty-nine percent of patients who were BRAF positive were diagnosed between 5 and 10 years of age. (B) BRAF mutant PLGGs have a prolonged latency to malignant transformation (MT) compared with BRAF wild-type PLGGs (P = .0389). Horizontal lines indicate medians. Statistical significance was evaluated using an unpaired t test. (C) Kaplan-Meier survival estimates showing improved overall survival after initial diagnosis for BRAF mutant versus wild-type PLGGs that later transform. (D) Primary high-grade gliomas (HGGs) have a worse overall survival after initial diagnosis than BRAF wild-type secondary HGG (P = .0163). Nontransformed PLGGs have a favorable overall survival compared with all other groups, at 98% ± 0.5%.
Most notably, BRAF V600E mutant PLGGs had significantly prolonged latency periods to transformation (median, 6.65 years; range, 3.50 to 20.3 years) compared with BRAF wild-type PLGGs that also experienced transformation (median, 1.59 years; range, 0.32 to 15.9 years; Fig 3B; P = .0389). As a result, all BRAF mutant sHGGs were diagnosed at age greater than 9 years (Fig 1A).
Furthermore, 5-year overall survival rates of children with BRAF mutant and wild-type PLGGs that transformed were 75% ± 15% and 29% ± 12%, respectively (P = .024;Fig 3C). To further confirm that these two groups of sHGG are distinct, we compared the outcomes of these patients with those of patients with PLGG and primary HGG in our institution. Children with nontransformed PLGG had a 5-year overall survival rate of 98% ± 0.5%. Importantly, children with primary HGG had a 5-year overall survival rate of 5% ± 4%, a significantly worse outcome than any subgroup of sHGG, including BRAF wild-type PLGGs that transform (Fig 3D; P = .0163). After malignant transformation, 23 of 26 patients died, and no significant difference in survival between subgroups of sHGG was observed (P = .49; Data Supplement).
DISCUSSION
In this population-based study, we define the risk of PLGG transformation to sHGG (2.9%) and uncover clinically applicable genetic subgroups of these cancers. The absolute risk of PLGG transformation is lower than previously estimated.3 Broniscer et al3 estimated that approximately 10% of PLGGs will transform to sHGG; however, that study represents a referral center experience that may be biased to more aggressive cancers (Data Supplement). The provincial health care system in Ontario and follow-up data allowed us to track patients for more than 30 years and detect even late transformation events in survivors of PLGG. Indeed, survivors of childhood BRAF mutant PLGG may experience malignant transformation up to 20 years after their initial diagnosis when they are well into adulthood.
Our study reveals additional molecular and genetic differences between childhood and adult gliomas. Common alterations found in adult low-grade gliomas that transform such as ATRX, IDH1, and ALT were extremely rare in our cohort, whereas BRAF V600E, H3.3, and hTERT promoter mutations, which are uncommon in adult sHGG, were highly enriched in our cohort. Nevertheless, genomic analyses of both adult and pediatric sHGG reveal common aberrations in p53 and RB cancer pathways, which are key to avoiding cellular senescence and growth arrest. An important clinical implication of this observation is the high incidence of both p53-positive immunostaining and CDKN2A deletion observed in our PLGG cohort that subsequently experienced transformation. Because these two markers are extremely uncommon in PLGGs that do not transform, integration of these tests to the panel used at diagnosis may enable us to detect a high-risk patient population for follow-up.
The complete lack of the BRAF-KIAA1549 gene fusion observed in sHGG represents another clinically important parameter for risk assessment. In our PLGG database of 886 patients, only one patient with chromosome 7q34 duplication went on to experience transformation. This patient had only 10% of cells harboring this duplication, and this clone disappeared in the corresponding sHGG, suggesting that minor subclones harboring the BRAF duplication do not portend the same good clinical outcome. Because more than half of PLGG are expected to harbor this alteration, inclusion of this test will add important negative information on the risk of transformation.21 BRAF V600E mutations have been described in a variety of pediatric gliomas, most frequently in gangliogliomas and pleomorphic xanthoastrocytomas.25–27 The latter also manifest concomitant CDKN2A deletions.14 Interestingly, pleomorphic xanthoastrocytomas have the tendency to transform to high-grade tumors.29,30 Nevertheless, 69% of PLGGs in our cohort were initially diagnosed as grade 1 astrocytomas, demonstrating that transformation is not restricted to these pathologic subtypes. The frequency of BRAF mutations in pediatric HGG is controversial. Different studies reveal rates between 10% and 25%.11,31–33 Our observations resolve some of these discrepancies, because the mutation may be specific to the subgroup of secondary pediatric HGG rather than primary pediatric HGG.
Perhaps the most important observation of this study is that specific alterations identified in sHGG could be traced back to their PLGG counterparts. Both BRAF V600E and CDKN2A-deleted tumors may be eligible for targeted therapies. If so, preventive measures, including a more aggressive surgical approach and consideration of medical therapies34 even when a stable tumor is observed, may be able to mitigate the devastating transformation event. This concept is further highlighted because the BRAF V600E mutation and CDKN2A deletion were observed in 63% and 100% of tumors in peripheral locations, respectively (Fig 1B), suggesting enrichment of these alterations in tumors that are more amenable to surgical resection.
Wild-type BRAF sHGG could be divided into two groups. One includes the brainstem lesions that are enriched for the H3F3A K27M mutations. This mutation can differentiate them from other brainstem PLGGs that have excellent survival.20 The second group of sHGG includes patients with cancer predisposition syndromes. These patients may also benefit from surveillance and early intervention to prevent tumor transformation.35,36
Together, we observed known alterations in 21 (88%) of 24 patients with PLGG that later transformed and no BRAF fusions in any secondary HGG. These genetic alterations could stratify PLGG into the following risk groups (Data Supplement): tumors that have the BRAF fusion but lack the rest of the previously mentioned alterations (excellent long-term outcome and extremely low risk for transformation); PLGGs harboring the BRAF V600E mutations and alterations in CDKN2A or TP53 (higher risk for transformation and should be managed with care); PLGGs originating with cancer predisposition syndromes associated with HGG (these tumors will eventually transform); and finally, midline PLGGs harboring H3.3 K27M mutations that will behave as primary HGGs. Because all of these tests could be performed in most clinical laboratories, it may be worthwhile to add them to the current battery of tests used for PLGG.
A potential pitfall of all studies investigating transformation of PLGG to sHGG involves instances where tumors, according to the biopsy, are PLGGs, but nevertheless behave as aggressively as primary HGGs. This can occur in two scenarios. One is where a small biopsy may have missed malignant regions as a result of tumor heterogeneity. This is particularly the case for deep-seated tumors. Indeed, our study (three patients) and previous reports3 have identified low-grade lesions on initial biopsy followed by a second biopsy revealing HGG in a short time span. The second scenario is where tumors with low-grade histology nevertheless behave as high grade. This has been demonstrated for low-grade astrocytomas of the brainstem that harbor H3.3 K27M mutations.37,38 In both scenarios, the addition of molecular markers as described in this study may help to establish (eg, BRAF-KIAA1549 fusion) or refute (eg, H3F3A mutation) the PLGG diagnosis. PLGGs that harbor H3.3 K27M, TP53 mutations, or mismatch repair deficiency may in fact represent high-grade–behaving gliomas (Data Supplement), whereas cancers harboring BRAF V600E mutations, if they transform, will have significant lag before transformation even if they are deeply seated midline tumors. This study has the classical limitations of a retrospective analysis over several decades and should be interpreted accordingly. Although the importance of BRAF mutations together with CDKN2A as risk factors for transformation is clear, the level of risk requires further study. It will be important to test in the future how many incompletely resected tumors that harbor both alterations will not transform using long clinical follow-up.
In summary, this study defines molecular and genetic subgroups of pediatric sHGG. Most of these alterations are early events that can guide the management of patients with PLGG at risk of malignant transformation. Further population-based research is required to better define the role of BRAF and CDKN2A alterations in childhood gliomas.
Supplementary Material
Glossary Terms
- BRAF:
an isoform of RAF.
Footnotes
See accompanying editorial on page 978
Supported by b.r.a.i.n.child Canada, the JPA Foundation, and the Canadian Institute of Health Research Grant No. MOP-123268. M.M. received funding from The Hospital for Sick Children RESTRACOMP fund and Alex's Lemonade Stand Foundation. R.K. was funded in part by the Society of Neuro-Oncology (International Research Development Fellowship Program). K.L.L. received support from National Institutes of Health Grant No. P01CA142536 and the Pediatric Low-Grade Astrocytoma Foundation.
Terms in blue are defined in the glossary, found at the end of this article and online at www.jco.org.
Authors' disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Disclosures provided by the authors are available with this article at www.jco.org.
AUTHOR CONTRIBUTIONS
Conception and design: Matthew Mistry, Cynthia E. Hawkins, Uri Tabori
Financial support: Cynthia E. Hawkins, Uri Tabori
Provision of study materials or patients: Jennifer Chan, Keith L. Ligon, James T. Rutka, Peter B. Dirks, David Malkin, Cynthia E. Hawkins, Uri Tabori
Collection and assembly of data: Matthew Mistry, Nataliya Zhukova, Patricia Rakopoulos, Rahul Krishnatry, Jason D. Pole, Marc Remke, Joshua Mangerel, Ana Guerreiro Stucklin, Cindy Zhang, Doua Bakry, Suzanne Laughlin, Jennifer Chan, Keith L. Ligon, James T. Rutka, Peter B. Dirks, Michael D. Taylor, Mark Greenberg, David Malkin, Eric Bouffet, Cynthia E. Hawkins, Uri Tabori
Data analysis and interpretation: Matthew Mistry, Nataliya Zhukova, Daniele Merico, Patricia Rakopoulos, Rahul Krishnatry, Mary Shago, James Stavropoulos, Noa Alon, Jason D. Pole, Peter N. Ray, Vilma Navickiene, Marc Remke, Pawel Buczkowicz, Vijay Ramaswamy, Joshua Mangerel, Ana Guerreiro Stucklin, Martin Li, Edwin J. Young, Pedro Castelo-Branco, Adam Shlien, James T. Rutka, Peter B. Dirks, Michael D. Taylor, Mark Greenberg, David Malkin, Annie Huang, Eric Bouffet, Cynthia E. Hawkins, Uri Tabori
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
BRAF Mutation and CDKN2A Deletion Define a Clinically Distinct Subgroup of Childhood Secondary High-Grade Glioma
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jco.ascopubs.org/site/ifc.
Matthew Mistry
No relationship to disclose
Nataliya Zhukova
No relationship to disclose
Daniele Merico
No relationship to disclose
Patricia Rakopoulos
No relationship to disclose
Rahul Krishnatry
No relationship to disclose
Mary Shago
No relationship to disclose
James Stavropoulos
No relationship to disclose
Noa Alon
No relationship to disclose
Jason D. Pole
No relationship to disclose
Peter N. Ray
No relationship to disclose
Vilma Navickiene
No relationship to disclose
Joshua Mangerel
No relationship to disclose
Marc Remke
No relationship to disclose
Pawel Buczkowicz
No relationship to disclose
Vijay Ramaswamy
No relationship to disclose
Ana Guerreiro Stucklin
No relationship to disclose
Martin Li
No relationship to disclose
Edwin J. Young
No relationship to disclose
Cindy Zhang
No relationship to disclose
Pedro Castelo-Branco
No relationship to disclose
Doua Bakry
No relationship to disclose
Suzanne Laughlin
No relationship to disclose
Adam Shlien
No relationship to disclose
Jennifer Chan
No relationship to disclose
Keith L. Ligon
Consulting or Advisory Role: EMD Serono, Midatech
Research Funding: GlaxoSmithKline (Inst), Bristol-Myers Squibb (Inst)
Patents, Royalties, Other Intellectual Property: Patent pending on diagnostic assay (Inst)
James T. Rutka
No relationship to disclose
Peter B. Dirks
No relationship to disclose
Michael D. Taylor
No relationship to disclose
Mark Greenberg
No relationship to disclose
David Malkin
No relationship to disclose
Annie Huang
No relationship to disclose
Eric Bouffet
No relationship to disclose
Cynthia E. Hawkins
Travel, Accommodations, Expenses: NanoString Technologies
Uri Tabori
No relationship to disclose
REFERENCES
- 1.Ostrom QT, Gittleman H, Farah P, et al. CBTRUS statistical report: Primary brain and central nervous system tumors diagnosed in the United States in 2006-2010. Neuro Oncol. 2013;15(suppl 2):ii1–ii56. doi: 10.1093/neuonc/not151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kleihues P, Ohgaki H. Primary and secondary glioblastomas: From concept to clinical diagnosis. Neuro Oncol. 1999;1:44–51. doi: 10.1093/neuonc/1.1.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Broniscer A, Baker SJ, West AN, et al. Clinical and molecular characteristics of malignant transformation of low-grade glioma in children. J Clin Oncol. 2007;25:682–689. doi: 10.1200/JCO.2006.06.8213. [DOI] [PubMed] [Google Scholar]
- 4.Krieger MD, Gonzalez-Gomez I, Levy ML, et al. Recurrence patterns and anaplastic change in a long-term study of pilocytic astrocytomas. Pediatr Neurosurg. 1997;27:1–11. doi: 10.1159/000121218. [DOI] [PubMed] [Google Scholar]
- 5.Dirks PB, Jay V, Becker LE, et al. Development of anaplastic changes in low-grade astrocytomas of childhood. Neurosurgery. 1994;34:68–78. [PubMed] [Google Scholar]
- 6.Sharif S, Ferner R, Birch JM, et al. Second primary tumors in neurofibromatosis 1 patients treated for optic glioma: Substantial risks after radiotherapy. J Clin Oncol. 2006;24:2570–2575. doi: 10.1200/JCO.2005.03.8349. [DOI] [PubMed] [Google Scholar]
- 7.Merchant TE, Rose SR, Bosley C, et al. Growth hormone secretion after conformal radiation therapy in pediatric patients with localized brain tumors. J Clin Oncol. 2011;29:4776–4780. doi: 10.1200/JCO.2011.37.9453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kannan K, Inagaki A, Silber J, et al. Whole-exome sequencing identifies ATRX mutation as a key molecular determinant in lower-grade glioma. Oncotarget. 2012;3:1194–1203. doi: 10.18632/oncotarget.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Killela PJ, Reitman ZJ, Jiao Y, et al. TERT promoter mutations occur frequently in gliomas and a subset of tumors derived from cells with low rates of self-renewal. Proc Natl Acad Sci U S A. 2013;110:6021–6026. doi: 10.1073/pnas.1303607110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu G, Diaz AK, Paugh BS, et al. The genomic landscape of diffuse intrinsic pontine glioma and pediatric non-brainstem high-grade glioma. Nat Genet. 2014;46:444–450. doi: 10.1038/ng.2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schwartzentruber J, Korshunov A, Liu XY, et al. Driver mutations in histone H3.3 and chromatin remodelling genes in paediatric glioblastoma. Nature. 2012;482:226–231. doi: 10.1038/nature10833. [DOI] [PubMed] [Google Scholar]
- 12.Sturm D, Witt H, Hovestadt V, et al. Hotspot mutations in H3F3A and IDH1 define distinct epigenetic and biological subgroups of glioblastoma. Cancer Cell. 2012;22:425–437. doi: 10.1016/j.ccr.2012.08.024. [DOI] [PubMed] [Google Scholar]
- 13.Jones DT, Hutter B, Jäger N, et al. Recurrent somatic alterations of FGFR1 and NTRK2 in pilocytic astrocytoma. Nat Genet. 2013;45:927–932. doi: 10.1038/ng.2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang J, Wu G, Miller CP, et al. Whole-genome sequencing identifies genetic alterations in pediatric low-grade gliomas. Nat Genet. 2013;45:602–612. doi: 10.1038/ng.2611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pfister S, Janzarik WG, Remke M, et al. BRAF gene duplication constitutes a mechanism of MAPK pathway activation in low-grade astrocytomas. J Clin Invest. 2008;118:1739–1749. doi: 10.1172/JCI33656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Broniscer A, Gajjar A. Supratentorial high-grade astrocytoma and diffuse brainstem glioma: Two challenges for the pediatric oncologist. Oncologist. 2004;9:197–206. doi: 10.1634/theoncologist.9-2-197. [DOI] [PubMed] [Google Scholar]
- 17.Cheng S, Pole JD, Sung L. Early deaths in pediatric acute leukemia: A population-based study. Leuk Lymphoma. 2014;55:1518–1522. doi: 10.3109/10428194.2013.850685. [DOI] [PubMed] [Google Scholar]
- 18.Schechter T, Pole JD, Darmawikarta D, et al. Late mortality after hematopoietic SCT for a childhood malignancy. Bone Marrow Transplant. 2013;48:1291–1295. doi: 10.1038/bmt.2013.64. [DOI] [PubMed] [Google Scholar]
- 19.Louis DN, Ohgaki H, Wiestler OD, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114:97–109. doi: 10.1007/s00401-007-0243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fried I, Hawkins C, Scheinemann K, et al. Favorable outcome with conservative treatment for children with low grade brainstem tumors. Pediatr Blood Cancer. 2012;58:556–560. doi: 10.1002/pbc.23200. [DOI] [PubMed] [Google Scholar]
- 21.Hawkins C, Walker E, Mohamed N, et al. BRAF-KIAA1549 fusion predicts better clinical outcome in pediatric low-grade astrocytoma. Clin Cancer Res. 2011;17:4790–4798. doi: 10.1158/1078-0432.CCR-11-0034. [DOI] [PubMed] [Google Scholar]
- 22.Torchia EC, Boyd K, Rehg JE, et al. EWS/FLI-1 induces rapid onset of myeloid/erythroid leukemia in mice. Mol Cell Biol. 2007;27:7918–7934. doi: 10.1128/MCB.00099-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Parsons DW, Jones S, Zhang X, et al. An integrated genomic analysis of human glioblastoma multiforme. Science. 2008;321:1807–1812. doi: 10.1126/science.1164382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Remke M, Ramaswamy V, Peacock J, et al. TERT promoter mutations are highly recurrent in SHH subgroup medulloblastoma. Acta Neuropathol. 2013;126:917–929. doi: 10.1007/s00401-013-1198-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Henson JD, Cao Y, Huschtscha LI, et al. DNA C-circles are specific and quantifiable markers of alternative-lengthening-of-telomeres activity. Nat Biotechnol. 2009;27:1181–1185. doi: 10.1038/nbt.1587. [DOI] [PubMed] [Google Scholar]
- 26.Lau LM, Dagg RA, Henson JD, et al. Detection of alternative lengthening of telomeres by telomere quantitative PCR. Nucleic Acids Res. 2013;41:e34. doi: 10.1093/nar/gks781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sievert AJ, Jackson EM, Gai X, et al. Duplication of 7q34 in pediatric low-grade astrocytomas detected by high-density single-nucleotide polymorphism-based genotype arrays results in a novel BRAF fusion gene. Brain Pathol. 2009;19:449–458. doi: 10.1111/j.1750-3639.2008.00225.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jones DT, Kocialkowski S, Liu L, et al. Tandem duplication producing a novel oncogenic BRAF fusion gene defines the majority of pilocytic astrocytomas. Cancer Res. 2008;68:8673–8677. doi: 10.1158/0008-5472.CAN-08-2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Binesh F, Akhavan A, Navabii H. Pleomorphic xanthoastrocytoma with malignant transformation and multiple recurrences in an Iranian girl. BMJ Case Rep. 2012;20:2012. doi: 10.1136/bcr.12.2011.5372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rodriguez-Mena R, Joanes-Alepuz V, Barbella-Aponte R, et al. Pleomorphic xanthoastrocytoma with intraventricular extension and anaplastic transformation in an adult patient: Case report. Neurocirugia (Astur) 2012;23:203–210. doi: 10.1016/j.neucir.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 31.Nicolaides TP, Li H, Solomon DA, et al. Targeted therapy for BRAFV600E malignant astrocytoma. Clin Cancer Res. 2011;17:7595–7604. doi: 10.1158/1078-0432.CCR-11-1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schiffman JD, Hodgson JG, VandenBerg SR, et al. Oncogenic BRAF mutation with CDKN2A inactivation is characteristic of a subset of pediatric malignant astrocytomas. Cancer Res. 2010;70:512–519. doi: 10.1158/0008-5472.CAN-09-1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brennan CW, Verhaak RG, McKenna A, et al. The somatic genomic landscape of glioblastoma. Cell. 2013;155:462–477. doi: 10.1016/j.cell.2013.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rush S, Foreman N, Liu A. Brainstem ganglioglioma successfully treated with vemurafenib. J Clin Oncol. 2013;31:e159–e160. doi: 10.1200/JCO.2012.44.1568. [DOI] [PubMed] [Google Scholar]
- 35.Bakry D, Aronson M, Durno C, et al. Genetic and clinical determinants of constitutional mismatch repair deficiency syndrome: Report from the constitutional mismatch repair deficiency consortium. Eur J Cancer. 2014;50:987–996. doi: 10.1016/j.ejca.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 36.Villani A, Malkin D, Tabori U. Syndromes predisposing to pediatric central nervous system tumors: Lessons learned and new promises. Curr Neurol Neurosci Rep. 2012;12:153–164. doi: 10.1007/s11910-011-0244-5. [DOI] [PubMed] [Google Scholar]
- 37.Khuong-Quang DA, Buczkowicz P, Rakopoulos P, et al. K27M mutation in histone H3.3 defines clinically and biologically distinct subgroups of pediatric diffuse intrinsic pontine gliomas. Acta Neuropathol. 2012;124:439–447. doi: 10.1007/s00401-012-0998-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Buczkowicz P, Bartels U, Bouffet E, et al. Histopathological spectrum of paediatric diffuse intrinsic pontine glioma: Diagnostic and therapeutic implications. Acta Neuropathol. 2014;128:573–581. doi: 10.1007/s00401-014-1319-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.