Abstract
Background
Chronic hepatitis C virus (HCV) infection causes substantial health and economic burden in the United States (US). With the availability of direct-acting antiviral agents (DAAs), recently approved and other therapies under development and 1-time birth-cohort screening, the burden of HCV disease is expected to decrease.
Objective
To predict the impact of new therapies and screening on chronic HCV cases and associated disease outcomes.
Design
Individual-level state-transition model.
Setting
Existing and anticipated HCV therapies and screening in the US.
Patients
Total HCV-infected population in the US.
Measurements
Chronic HCV cases and advanced-stage HCV outcomes.
Results
The number of chronic HCV cases decreased from 3.2 million in 2001 to 2.3 million in 2013. One-time birth-cohort screening beginning in 2013 is expected to identify 487 000 HCV cases in the next 10 years. In contrast, 1-time universal screening could identify 933 700 HCV cases. With the availability of highly effective therapies, HCV could become a rare disease in the next 22 years. The adoption of recently approved HCV therapies and one-time birth-cohort screening can prevent approximately 124 200 cases of decompensated cirrhosis, 78 800 cases of hepatocellular carcinoma, 126 500 liver-related deaths and 9900 liver transplants by 2050. Increasing the treatment capacity would further reduce the burden of HCV-related disease.
Limitations
Empirical data on the effectiveness of the future HCV therapies, on the future annual incidence of HCV, and on HCV treatment capacity are lacking.
Conclusions
New HCV therapies along with widespread implementation of screening and treatment will play an important role in reducing the burden of HCV disease. More aggressive screening recommendations are needed to identify a large pool of infected patients.
Funding source
National Institutes of Health.
INTRODUCTION
Chronic hepatitis C virus (HCV) infection is a major health problem in the United States (US) affecting 3.2 million people (1). HCV is the leading cause of chronic liver disease and hepatocellular carcinoma (HCC) and is the leading indication for liver transplantation in the US (2). The number of deaths from HCV in the US surpassed those from human immunodeficiency virus infection in 2007 (3). In 2011, the economic burden associated with chronic HCV infection in the US was estimated at $6.5 billion (4).
HCV treatment has rapidly evolved over the past 2 decades. The launch of direct-acting antivirals (DAAs) in 2011 and recent availability of first all-oral HCV regimens, represent a significant shift in HCV treatment paradigm (5). The sustained virologic response (SVR) rates for certain patients increased to 97% (6). New treatments currently under investigation have shown potential to further increase response rates, decrease treatment duration, and improve side effect profiles. These therapies are being studied as combinations of DAAs, with and without ribavirin and interferon (7, 8).
In addition to advances in treatment, key changes in HCV screening recommendation have taken place. The Centers for Disease Control and Prevention (CDC) and the US Preventive Services Task Force (USPSTF) expanded their HCV screening recommendation to include 1-time screening for anyone born between 1945 and 1965 (9, 10). Modeling studies have shown that this screening strategy can be cost-effective and can reduce the burden of HCV disease (11–13). Finally, the Patient Protection and Affordable Care Act might facilitate the implementation of recommended HCV screening strategies and the link to care and treatment (14).
The launch of DAAs along with the combination of the new screening recommendations are collectively expected to substantially reduce the burden of HCV in the US; however, the effect of these changes has not yet been quantified. Previous studies did not project the burden of HCV infection under these changing dynamics but instead limited the studies’ analyses to the old standard of care (SOC)—peginterferon and ribavirin (PEG-RBV) without HCV screening (4, 15)—or evaluated only the cost-effectiveness of HCV screening without projecting the changing burden of HCV (11, 16, 17). Finally, the effect of limited treatment capacity on the burden of HCV disease also has not been studied. Our objective was thus to project the burden of HCV disease in the US by considering recent therapeutic advances, treatment capacity, and the implementation of a 1-time birth-cohort or universal screening.
METHODS
HCV-Infected Population Characterization
We developed an individual-level state-transition model (18) that simulated the HCV-infected population of the US from 2001 to 2050. We used a nationally representative distribution of patients’ age, gender, HCV awareness status, HCV genotype, stage of disease, and treatment history, using data from the National Health and Nutrition Examination Survey (NHANES, 1999–2002) and published clinical studies (Appendix Table 1) (11, 15, 19–22). We added new HCV infections in the model based on the annual new HCV infections reported by the CDC (Appendix Table 2) (23). Each newly infected patient was added as an acute case that could progress to the chronic phase (19). Patients could become aware of their HCV status in the course of disease progression (Appendix Table 3). At any given time, patients occupied one of the health states (Figure 1), and could transition to another state with a predefined probability depending on their current state (Appendix Table 4).
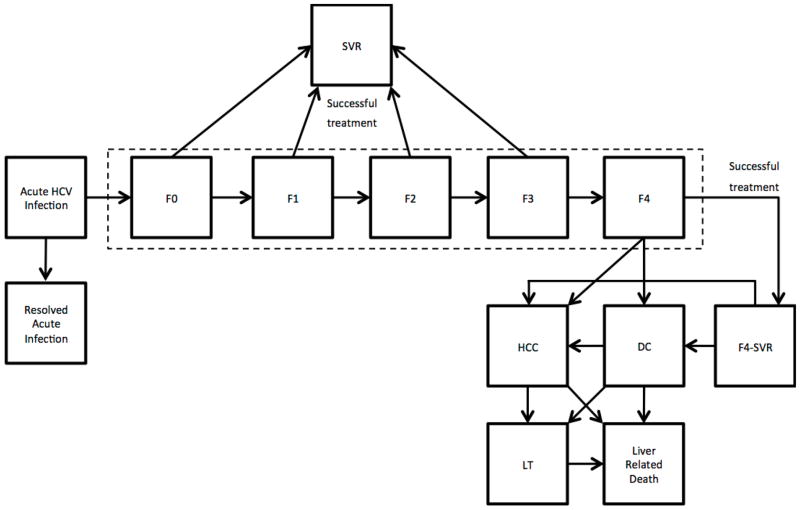
Figure 1.
State-transition diagram showing states of the hepatitis C disease-burden model. At any given time, a patient is represented by one of the health states, which are shown by squares. Arrows between states represent possible transitions based on annual probabilities (Appendix Table 1). Patients who are successfully treated transition to the “SVR” state. Patients who achieve SVR from F0–F3 states are assumed to be cured; however, F4 patients after a successful treatment transition to “F4-SVR” state and they could develop further complications. Patients in HCC, DC, and LT have a higher mortality than the general population, therefore can transition to “Liver-Related Death” state. All other patients have the same mortality risk as the general population. Abbreviations: HCV = hepatitis C virus; F0 = METAVIR stage for no liver fibrosis; F1 = METAVIR stage for portal fibrosis without septa; F2 = METAVIR stage for portal fibrosis with few septa; F3 = METAVIR stage for numerous septa without cirrhosis; F4 = METAVIR stage for cirrhosis; SVR = sustained virologic response; DC = decompensated cirrhosis; HCC = hepatocellular carcinoma; LT = liver transplant. Note: the probability of death from other causes exists in every state, but deaths from other causes are not shown in this figure.
Natural History of HCV
The chronic phase of the infection was defined using the METAVIR scoring system: no fibrosis of the liver (F0), portal fibrosis without septa (F1), portal fibrosis with few septa (F2), numerous septa without cirrhosis (F3), and cirrhosis (F4). Patients could further progress to decompensated cirrhosis (DC) or to HCC, receive a liver transplant, or die from liver-related complications (Figure 1). The model assumed a liver-transplantation age limit of 75 years (24). All disease progression probabilities are presented in Appendix Table 1. Patients who achieved SVR in F0–F3 states were assumed to be cured of HCV; however, those who achieved SVR in F4 state could further progress to DC and/or HCC, though at a slower rate than HCV-infected patients.
Simulation Scenario: Current Clinical Practice
We simulated the current clinical practice as our base case, i.e., 1-time birth-cohort HCV screening starting in 2013 and treatment with PEG-RBV or PI-based triple therapy before 2014, sofosbuvir- and simeprevir-based therapies starting in 2014, and future drugs as they become available.
We implemented 1-time birth-cohort HCV screening of people born between 1945 and 1965 that detected unaware prevalent cases. We also included risk-based screening under this scenario. We assumed that 91% of these patients would accept screening and 90% of those who tested positive would receive those results (11). We assigned the uptake of screening such that the majority of these patients would receive screening gradually during 5 years beginning in 2013.
We estimated that 80% of the patients aware of their HCV status would initiate HCV treatment (11, 25–28). Treatment regimens were assigned based on patients’ prior treatment history, HCV genotype, contraindication to interferon, and the standard-of-care at the time of treatment.
For genotype 1 patients, we assigned PEG-RBV during 2001–2011, followed by a combination of a first-generation protease inhibitor (PI)—boceprevir or telaprevir, and PEG-RBV in 2012–2013. For non-genotype 1 patients, we assigned PEG-RBV during the entire period of 2001–2013. We assumed that the patients who failed PEG-RBV treatment could be retreated at most once with PEG-RBV or PI-based therapy. We also assumed that patients who failed PI-based therapy were not eligible for retreatment with the same drug class.
On the basis of recently published evidence, we expect higher treatment response rates in all patients after 2013 owing to the availability of new therapies, albeit at different intervals (29–42). Therefore, we assumed that these therapies could be divided into 2 major waves on the basis of therapy availability, cure rates and target populations (Table 1). We assumed that during 2011–2013, 75% of the eligible patients with mild fibrosis (F0–F2) and 25% of the eligible patients with bridging fibrosis (F3) waited for newer therapies (43).
Table 1.
Estimated Effectiveness of Treatment for Hepatitis C in the United States from 2001 to 2050
| Treatment history / Genotype | HCV state | PEG-RBV | BOC/TEL +PR | Wave 1 (2014) | Wave 2 (2017) | Reference |
|---|---|---|---|---|---|---|
| Naïve | ||||||
| Genotype 1 | (30, 35, 37, 38, 42, 59–66) | |||||
|
| ||||||
| F0–F2 | 0.54 | 0.75 | 0.90 | -- | ||
|
| ||||||
| F3 | 0.54 | 0.62 | 0.90 | -- | ||
|
| ||||||
| F4 | 0.36 | 0.62 | 0.80 | 0.90 | ||
|
| ||||||
| Genotype 2 | (60, 67–70) | |||||
|
| ||||||
| F0–F3 | 0.82 | -- | 0.90 | -- | ||
|
| ||||||
| F4 | 0.64 | -- | 0.80 | 0.90 | ||
|
| ||||||
| Genotype 3 | (60, 67, 68, 70, 71) | |||||
|
| ||||||
| F0–F3 | 0.70 | -- | 0.90 | -- | ||
|
| ||||||
| F4 | 0.49 | -- | 0.80 | 0.90 | ||
|
| ||||||
| Genotype 4/5/6 | (60, 64, 72) | |||||
|
| ||||||
| F0–F3 | 0.58 | -- | 0.90 | -- | ||
|
| ||||||
| F4 | 0.32 | -- | 0.80 | 0.90 | ||
|
| ||||||
| Relapser | ||||||
| Genotype 1 | (20, 29, 32, 36, 37, 63, 66, 69, 73, 74) | |||||
|
| ||||||
| F0–F2 | 0.27 | 0.87 | 0.90 | -- | ||
|
| ||||||
| F3 | 0.27 | 0.85 | 0.90 | -- | ||
|
| ||||||
| F4 | 0.13 | 0.84 | 0.80 | 0.90 | ||
|
| ||||||
| Genotype 2 | (21, 45, 69, 75) | |||||
|
| ||||||
| F0–F3 | 0.71 | -- | 0.90 | -- | ||
|
| ||||||
| F4 | 0.56 | -- | 0.70 | 0.90 | ||
|
| ||||||
| Genotype 3 | (21, 45, 71, 75) | |||||
|
| ||||||
| F0–F3 | 0.66 | -- | 0.85 | -- | ||
|
| ||||||
| F4 | 0.52 | -- | 0.60 | 0.90 | ||
|
| ||||||
| Genotype 4/5/6 | (21, 60, 64, 72) | |||||
|
| ||||||
| F0–F3 | 0.31 | -- | 0.90 | -- | ||
|
| ||||||
| F4 | 0.24 | -- | 0.75 | 0.90 | ||
|
| ||||||
| Partial responder | ||||||
| Genotype 1 | (20, 29, 32, 36, 37, 63, 66, 69, 73, 74) | |||||
|
| ||||||
| F0–F2 | 0.18 | 0.72 | 0.90 | -- | ||
|
| ||||||
| F3 | 0.18 | 0.56 | 0.90 | -- | ||
|
| ||||||
| F4 | 0.10 | 0.34 | 0.75 | 0.90 | ||
|
| ||||||
| Genotype 2 | (21, 45, 69, 75) | |||||
|
| ||||||
| F0–F3 | 0.69 | -- | 0.90 | -- | ||
|
| ||||||
| F4 | 0.55 | -- | 0.70 | 0.90 | ||
|
| ||||||
| Genotype 3 | (21, 45, 71, 75) | |||||
|
| ||||||
| F0–F3 | 0.64 | -- | 0.85 | -- | ||
|
| ||||||
| F4 | 0.51 | -- | 0.60 | 0.90 | ||
|
| ||||||
| Genotype 4/5/6 | (21, 60, 64, 72) | |||||
|
| ||||||
| F0–F3 | 0.31 | -- | 0.90 | -- | ||
|
| ||||||
| F4 | 0.24 | -- | 0.75 | 0.90 | ||
|
| ||||||
| Null responder | ||||||
| Genotype 1 | (20, 29, 32, 36, 37, 63, 66, 69, 73, 74) | |||||
|
| ||||||
| F0–F2 | 0.10 | 0.41 | 0.90 | -- | ||
|
| ||||||
| F3 | 0.10 | 0.39 | 0.90 | -- | ||
|
| ||||||
| F4 | 0.05 | 0.14 | 0.75 | 0.90 | ||
|
| ||||||
| Genotype 2 | (21, 45, 69, 75) | |||||
|
| ||||||
| F0–F3 | 0.54 | -- | 0.90 | -- | ||
|
| ||||||
| F4 | 0.42 | -- | 0.70 | 0.90 | ||
|
| ||||||
| Genotype 3 | (21, 45, 71, 75) | |||||
|
| ||||||
| F0–F3 | 0.50 | -- | 0.85 | -- | ||
|
| ||||||
| F4 | 0.39 | -- | 0.60 | 0.90 | ||
|
| ||||||
| Genotype 4/5/6 | (21, 60, 64, 72) | |||||
|
| ||||||
| F0–F3 | 0.31 | -- | 0.90 | -- | ||
|
| ||||||
| F4 | 0.24 | -- | 0.75 | 0.90 | ||
|
| ||||||
| Contraindicated with modifiable reasons | ||||||
| Genotype 1 | (20, 30, 38, 63, 69) - expert opinion | |||||
|
| ||||||
| F0–F2 | -- | -- | 0.90 | -- | ||
|
| ||||||
| F3 | 0.43 | 0.50 | 0.90 | -- | ||
|
| ||||||
| F4 | 0.28 | 0.36 | 0.70 | 0.90 | ||
|
| ||||||
| Genotype 2 | (45, 69) - expert opinion | |||||
|
| ||||||
| F0–F3 | 0.66 | -- | 0.90 | -- | ||
|
| ||||||
| F4 | 0.51 | -- | 0.70 | 0.90 | ||
|
| ||||||
| Genotype 3 | (45, 71, 75) - expert opinion | |||||
|
| ||||||
| F0–F3 | 0.56 | -- | 0.90 | -- | ||
|
| ||||||
| F4 | 0.40 | -- | 0.60 | 0.90 | ||
|
| ||||||
| Genotype 4/5/6 | (72) - expert opinion | |||||
|
| ||||||
| F0–F3 | 0.46 | -- | 0.90 | -- | ||
|
| ||||||
| F4 | 0.26 | -- | 0.70 | 0.90 | ||
|
| ||||||
| Contraindicated with non-modifiable reasons | ||||||
| Genotype 1/2/4/5/6 | (30, 38, 45, 69) - expert opinion | |||||
|
| ||||||
| F0–F3 | -- | -- | 0.90 | -- | ||
|
| ||||||
| F4 | -- | -- | 0.70 | 0.90 | ||
|
| ||||||
| Genotype 3 | (45, 71, 75) - expert opinion | |||||
|
| ||||||
| F0–F3 | -- | -- | 0.90 | -- | ||
|
| ||||||
| F4 | -- | -- | 0.60 | 0.90 | ||
|
| ||||||
| Failed triple therapy | ||||||
|
| ||||||
| Genotype 1 | (63) - expert opinion | |||||
| F0–F3 | -- | -- | 0.95 | -- | ||
| F4 | -- | -- | 0.75 | 0.90 | ||
Wave 1 = new therapies launched in 2014 for all patients that increased treatment response rates to 90% in non-cirrhotic patients and 60%–80% in cirrhotic patients; Wave 2 = future therapies that we assumed would be launched in 2017 and increase treatment response rates to 90% in cirrhotic patients; Relapser = a patient whose HCV RNA became undetectable during treatment with PEG-RBV, but reappeared after the end of treatment; Partial responder = a patient whose HCV RNA level decreased by 2 log IU/mL or more at week 12 of treatment with PEG-RBV, but was detectable at week 24; Null responder = a patient whose HCV RNA level decreased less than 2 log IU/mL at week 12 of treatment with PEG-RBV; Contraindicated with modifiable reasons = a patient who had contraindications to regiments that included pegylated interferon and ribavirin such as anemia, depression, and substance abuse, that were modifiable by medical or psychiatric interventions; Contraindicated with non-modifiable reasons = a patient who had contraindications to regiments that include pegylated interferon and ribavirin such as autoimmune disease, coronary artery disease, retinopathy, etc., that were not modifiable by medical or psychiatric interventions; Failed triple therapy = a patient whose HCV RNA level detectable after the treatment with boceprevir or telaprevir combined with a first-generation protease inhibitor.
The SVR rates were either derived directly from the references or were indirectly inferred on the basis of the mentioned references.
HCV = hepatitis C virus; F0 = METAVIR stage for no fibrosis; F1 = METAVIR stage for portal fibrosis without septa; F2 = METAVIR stage for portal fibrosis with few septa; F3 = METAVIR stage for numerous septa without cirrhosis; F4 = METAVIR stage for cirrhosis; PEG-RBV = peginterferon and ribavirin; BOC/TEL+PR = boceprevir or telaprevir plus peginterferon and ribavirin.
Wave 1 of new treatments was assumed to start in 2014; we also assumed that with Wave 1 the SVR rates would increase up to 90% in the groups of genotype 1–6 non-cirrhotic patients (Table 1). Though the reported SVR rates were as high as 97% in some patients, we used a conservative estimate of 90% in some patients to account for lower SVR rates in real-life (44). The first wave included therapies for genotype 1–6 cirrhotic patients as well, but we assumed that the response rates among these would still remain suboptimal (Appendix Figure 1) (45). We assumed that Wave 2 of treatment would begin in 2017 and increase the response rates up to 90% in all patients. We included the retreatment of patients who failed PEG-RBV or PI-based therapy before 2014 with Wave 1 or Wave 2 therapies. The SVR rates by treatment history, genotype, fibrosis stage, and interferon contraindication are presented in Table 1 and Appendix. Appendix Figure 1 illustrates the treatment used for each category of patients at different time intervals.
Since it is impracticable to treat all HCV-infected patients within a year, we introduced an annual constraint on the number of people who could access HCV treatment. Our rationale was to model the effect of limited treatment uptake as well as limited resources (budget, physicians, etc.) available to treat all eligible patients. For our base case, we used historic data to determine the national treatment uptake (46) and performed sensitivity analyses.
Simulation Scenario: Ideal Case
We simulated the effect of a hypothetically ideal scenario that represented an upper limit of the benefits that could be achieved by ongoing advancements in therapies and policy-level changes. We simulated best possible combination of 1-time universal screening in all adults, adoption of new drugs as they become available and unlimited treatment capacity. We distributed the uptake of screening proportionally over the period of 5 years beginning 2013.
Simulation Scenario: Pre-DAA and Natural-History
For the purpose of estimating the incremental benefits of therapeutic advancements and policy-level changes, we simulated two comparator scenarios: Pre-DAA scenario and natural-history scenario. The Pre-DAA scenario represented screening and treatment practice until the launch of DAAs. It simulated HCV treatment with PEG-RBV only, from 2001 onwards, with risk-based screening only. The natural-history scenario simulated the HCV disease burden under no screening and no treatment.
Model Outcomes
We projected the prevalence of HCV from 2001 to 2050. In addition, we projected the prevalence and incidence of early stages of HCV—fibrosis scores F0–F4, advanced stages of disease—DC, HCC, and the number of liver-transplants and liver-related deaths.
Model Validation
Using the model outcomes from 2001 to 2013, we validated our model with several published studies. First, we compared the predicted prevalence of HCV with a recently published NHANES 2003–2010 study (47). Second, we compared the predicted incidence and prevalence by stages of HCV disease with published studies and CDC reports (15, 22, 48–50). Third, we compared our model’s natural history of HCV with the results of a multicenter follow-up study of patients with advanced fibrosis (51). Finally, we cross-validated our model with earlier modeling studies (4, 15) by comparing the results of the natural-history and pre-DAA scenarios.
Sensitivity Analyses
We tested the effect of the SVR rates, the timing of the availability of future therapies, treatment capacity, patients’ decision to wait for new drugs, and changing annual HCV incidence on the burden of HCV disease. We performed deterministic sensitivity analyses on the natural history parameters of HCV and patient characteristics (Appendix Tables 4–5).
We also evaluated the effect of treatment capacity on HCV disease burden by simulating 4 scenarios: (1) increased treatment capacity by 10% after the launch of DAAs in 2012 and additional increased capacity by 50% after the launch of new therapies in 2014; (2) increased capacity by 10% in 2012 but decreased by 20% after the launch of new therapies in 2014 due to high drug cost; and (3) unlimited treatment capacity (Appendix Table 6).
Role of Funding Source
The study was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health (NIH) under award number KL2TR000146. The content is solely the responsibility of the authors and does not necessarily represent the views of the NIH.
RESULTS
Validation
Our model projected that the average number of chronic HCV cases in 2003–2010 were 2.7 million, which is equal to the reported values in NHANES 2003–2010 study (47) (Appendix Table 7). The projected average prevalence of HCC in 2001–2004 was within 3% of the reported values (48). The incidence of HCC and liver-related deaths in 2005 were within 1–15% of the reported values (22). The projected distribution of different stages of chronic HCV closely matched that of another modeling study (15). Finally, our model’s 10-year cumulative incidence rates of DC, HCC, and combined liver-related mortality and liver transplants closely matched the results of a recently published multicenter follow-up study (Appendix Table 8) (51).
HCV Disease Burden
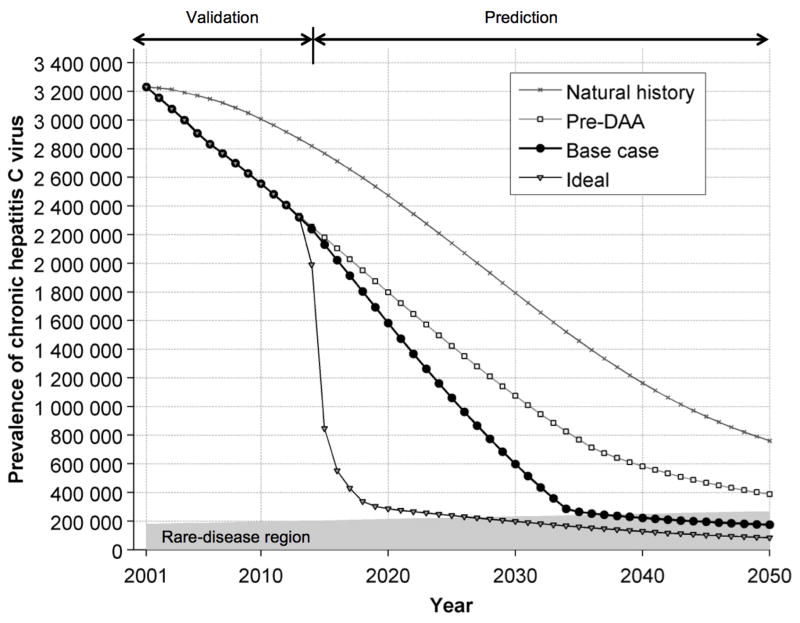
Our model projected that the chronic HCV cases in the US decreased from 3.2 million in 2001 to 2.3 million in 2013 (Figure 2). From 2001 to 2013, 157 300 HCV-infected people died because of liver-related complications, 415 000 died because of other reasons, and 589 100 achieved SVR. During the same period 251 000 new people got chronically infected with HCV. Considering the population growth in the US (52), we projected that HCV would become a rare disease by 2036, i.e. affecting about 1 in 1500 people (53). Under the ideal scenarios, HCV could become a rare disease by 2026.
Figure 2.
The estimated prevalence of chronic hepatitis C virus cases in the United States from 2001 to 2050 under different simulation scenarios. Note: The rare-disease region is calculated based on the definition of a rare disease, and adjusted to the United States population. Based on the Rare Disease Act of 2002 (53), a rare disease affects about 1 in 1500 people. The rare-disease region is increasing with time because of population growth.
Natural history = simulation scenario with no screening and no treatment; Pre-DAA = simulation scenario with risk-based screening and peginterferon and ribavirin treatment; Base case = simulation scenario with risk-based and birth-cohort screening, treatment with peginterferon and ribavirin and/or DAAs before 2014, and newly approved and future therapies starting in 2014, and limited treatment capacity; Ideal = simulation scenario with 1-time universal screening, treatment with peginterferon and ribavirin and/or DAAs before 2014, and newly approved and future therapies starting in 2014, and unlimited treatment capacity; DAA = direct-acting antiviral agent.
In 2001, 682 400 people were chronically infected with HCV who were born between 1945 and 1965 and unaware of their disease. However, by 2013, only 531 200 HCV infected patients (24% of the total HCV infection in the US) were eligible for birth-cohort screening, i.e., unaware of their disease status and still between fibrosis scores F0–F4. The implementation of 1-time birth-cohort screening beginning 2013 is expected to identify 487 000 additional HCV cases in this cohort in the next 10 years.
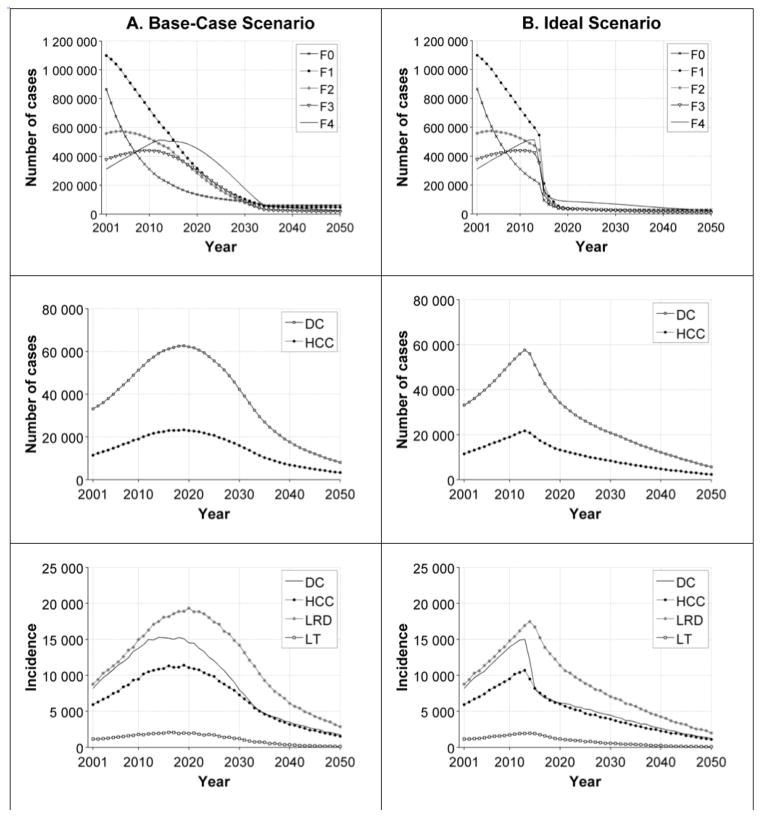
Under the base-case scenario, our model projected that the prevalence of DC and HCC, and liver-related deaths will reach their peak values during 2019–2020 and start declining afterwards (Figure 3).
Figure 3.
Model results according to the base-case scenario (column A) and the ideal scenario (column B) of hepatitis C disease burden in the United States from 2001 to 2050. Row 1: the prevalence of fibrosis stages; Row 2: the prevalence of DC and HCC; Row 3: the incidence of DC, DCC, LRD, and LT. Note: The results of the natural-history and pre-DAA scenarios are presented in Appendix Figure 2.
Natural history = simulation scenario with no screening and no treatment; Pre-DAA = simulation scenario with risk-based screening and peginterferon and ribavirin treatment; Base case = simulation scenario with risk-based and birth-cohort screening, treatment with peginterferon and ribavirin and/or DAAs before 2014, and newly approved and future therapies starting in 2014, and limited treatment capacity; Ideal = simulation scenario with universal screening, treatment with peginterferon and ribavirin and/or DAAs before 2014, and newly approved and future therapies starting in 2014, and unlimited treatment capacity.
Abbreviations: DC = decompensated cirrhosis; HCC = hepatocellular carcinoma; LRD = liver-related deaths; LT = liver transplants; DAA = direct-acting antiviral agent.
Ideal Scenario
Under the ideal scenario, HCV can become a rare disease by 2026, i.e. 10 years earlier than that with the base case (Figure 2). The implementation of 1-time universal screening could identify 933 700 HCV cases in the next 10 years. Compared with the base case (current clinical practice), ideal scenario could reduce the total number of DC cases, HCC cases, liver-related deaths, and liver-transplants by 135 800 (46%), 96 300 (40%), 161 500 (37%), and 13 900 (37%), respectively during 2014–2050 (Table 3).
Table 3.
Estimated Effect of Each Scenario on the Outcomes of Advanced-Stage Hepatitis C Outcomes According to Our Model of Hepatitis C Disease Burden in the United States from 2014 to 2050
| Scenario | ||||
|---|---|---|---|---|
| Advanced-stage disease outcomes | Natural history | Pre-DAA | Base case | Ideal |
| Decompensated cirrhosis | ||||
| Cumulative incidence (2014–2050) | 647 000 | 418 100 | 293 900 | 158 100 |
| Peak annual prevalence | 90 700 | 68 000 | 62 700 | 56 000 |
| Year of peak annual prevalence | 2025 | 2022 | 2019 | 2014 |
| Peak annual incidence | 22 800 | 16 800 | 15 300 | 12 000 |
| Year of peak annual incidence | 2023 | 2020 | 2014 | 2014 |
| Hepatocellular carcinoma | ||||
| Cumulative incidence (2014–2050) | 473 000 | 318 900 | 240 200 | 143 900 |
| Peak annual prevalence | 33 200 | 25 000 | 23 200 | 20 800 |
| Year of peak annual prevalence | 2025 | 2021 | 2019 | 2014 |
| Peak annual incidence | 16 300 | 12 200 | 11 400 | 9 500 |
| Year of peak annual incidence | 2025 | 2021 | 2019 | 2014 |
| Liver-related deaths | ||||
| Total deaths (2014–2050) | 811 600 | 560 100 | 433 600 | 272 100 |
| Peak annual deaths | 27 500 | 20 600 | 19 300 | 17 500 |
| Year of peak annual deaths | 2025 | 2023 | 2020 | 2014 |
| Liver transplants | ||||
| Total transplants (2014–2050) | 67 100 | 47 800 | 37 900 | 24 000 |
| Peak annual liver transplants | 2700 | 2100 | 2100 | 2000 |
| Year of peak annual liver transplants | 2024 | 2021 | 2016 | 2014 |
Natural history = simulation scenario with no screening and no treatment; Pre-DAA = simulation scenario with risk-based screening and peginterferon and ribavirin treatment; Base case = simulation scenario with risk-based and birth-cohort screening, treatment with peginterferon and ribavirin and/or DAAs before 2014, and newly approved and future therapies starting in 2014, and limited treatment capacity; Ideal = simulation scenario with universal screening, treatment with peginterferon and ribavirin and/or DAAs before 2014, and newly approved and future therapies starting in 2014, and unlimited treatment capacity; DAA = direct-acting antiviral agent.
Pre-DAA Scenario
Under the Pre-DAA scenario, HCV did not become a rare disease. Compared with the base-case, Pre-DAA scenario would have increased the number of DC cases, HCC cases, liver-related deaths, and liver-transplants by 124 200 (30%), 78 700 (25%), 126 500 (23%), and 9900 (21%), respectively, during 2014–2050 (Table 3).
Sensitivity analyses
We evaluated the effect of increased treatment capacity on the burden of disease (Appendix Table 6). Compared to the base case, 10% increase in treatment capacity in 2012 and 50% increase beyond 2014 (Scenario 1) would reduce the number of DC, HCC, liver-related deaths and liver transplants by 9–14%. Whereas, 20% decrease in treatment capacity beyond 2014 (Scenario 2) would increase the corresponding adverse outcomes by 16–22%. Compared to the base case, unlimited treatment capacity from 2014 onwards (Scenario 3) would prevent 128 800 DC, 91 000 HCC, and 153 200 liver-related deaths and 13 400 liver transplants.
When the SVR rates of the available and future drugs were reduced by 10%, the cumulative incidence of DC and HCC, and liver-related deaths and liver transplants increased by 4% to 23%, depending on the simulation scenario (Appendix Table 9). Delayed or early launch of Wave 2 of HCV therapies did not substantially change the disease burden (Appendix Table 10). In addition, we found that the results were not sensitive to the percentages of patients in F0–F3 who might choose to wait for future therapies instead of initiating treatment with PI-based therapies (Appendix Table 11). Among the natural-history parameters, we found that the probability of developing DC and HCC in cirrhotic patients had the greatest effect on the disease burden.
We also performed a sensitivity analysis on the prevalence of HCV. Assuming 4.9 million people were infected with HCV in 2001 which was the 95% CI upper limit NHANES 1999–2002 estimate (19), the cumulative incidence of DC, HCC, and liver-related mortality increased by 23–25% in comparison with the base-case scenario (Appendix Tables 12–13). Finally, we evaluated the impact of decreasing and increasing annual HCV incidence and found no substantial effect on the outcomes (Appendix Table 14).
DISCUSSION
Our model estimated that 2.3 million people were chronically infected in the beginning of 2013, as compared with 3.2 million people in 2001. With the implementation of birth-cohort screening and the availability of highly effective new therapies, HCV could become a rare disease by 2036. In addition, these changes could substantially decrease the overall clinical burden associated with HCV in the US.
Our study also identified trends in the HCV disease burden that have not been previously reported. As corroborated by recently published NHANES 2003–2010 data (47), we estimated that the current number of chronic HCV cases in the US is actually lower than the commonly reported 3.2 million estimate. The HCV prevalence decreased mainly because of deaths and successful treatments in this cohort. Also, our model projected that fewer patients are eligible for birth-cohort screening than estimated in a previously published study (11). Our results differed because we accounted for the possibility that birth-cohort patients progressed beyond cirrhosis or became aware of their disease before the implementation of screening in 2013.
Our study underscores the need for more aggressive screening strategies and higher treatment capacity to further reduce the burden of HCV. Birth-cohort screening, though impactful, would fail to identify a large pool of existing HCV patients who could advance to severe disease stages without treatment. In addition, the number of patients who are able to receive treatment greatly affects the potential disease burden. This number is dependent on the treatment capacity, availability of new drugs, treatment cost, and insurance coverage. With the launch of all-oral drugs that can simplify treatment, primary care physicians or infectious disease specialists also may take on the role of treating HCV patients, thus alleviating the burden on specialists (54). In addition, programs like the Extension for Community Healthcare Outcomes can further help to increase the treatment capacity by improving access to care for underserved populations (55). However, the high price of new therapies could become a barrier to timely HCV treatment, thus inhibit the full potential of therapeutic advances and screening recommendations (56).
Our study has several limitations. The historic number of HCV cases in the model is based on NHANES 1999–2002 data that underestimate the prevalence of HCV in the US by excluding the institutionalized population. However, we tested its effect on the future HCV burden in a sensitivity analysis. Second, we estimated the total patients who received treatment from drug prescription data reported by insurance companies (46), which may underestimate the number of patients who got treated. Third, our model does not account for co-infections and other risk factors, such as alcohol consumption, that affect disease progression (57, 58). These limitations may have resulted in an underestimation of the projected burden of HCV disease. Fourth, we do not consider the potential effect of treatment on disease transmission. Although improved treatment would be expected to decrease HCV transmission, new cases are a very small proportion of the existing HCV cases.
Information about SVR rates and the launch time of new therapies is limited. Our SVR rates were based on results from several phase 2 and 3 clinical studies, but the real-life SVR rates may be different. Our assumptions about the launch time of new therapies were based on the end dates of clinical trials. Finally, due to the lack of knowledge in the retreatment of patients who will fail recently approved and future therapies, the analysis of the retreatment of these patients is beyond the scope of our analysis.
In conclusion, we evaluated the effect of the launch of DAAs, recently approved and other potential future therapies, and changes in HCV-screening recommendations on the future burden of HCV disease in the US. We found that with ongoing therapeutic advancements and screening policy changes, HCV could become a rare disease within the next 22 years. We also found that the current screening recommendations are helpful in decreasing the future burden of HCV, but more aggressive recommendations should be proposed in conjunction with an increase in HCV treatment capacity.
Supplementary Material
Table 2.
Default Characteristics of the Scenarios in Our Model of Hepatitis C Disease Burden in the United States, from 2001 to 2050
| Characteristics | |||
|---|---|---|---|
| Scenario | HCV treatment (time period) | Screening | Treatment capacity |
| Natural history | No treatment | No screening | N/A |
| Pre-DAA | PEG-RBV (2001–2050) | Risk-based | Variant based on historic data (2001–2007) Constant at 83 270 (2008–2050) |
| Base case | PEG-RBV (2001–2011) BOC/TEL+PR (2012–2013) Wave 1 (2014–2016) Wave 2 (2017–2050) |
Risk-based and Birth-cohort | Variant based on historic data (2001–2007) Constant at 83 270 (2008–2050) |
| Ideal | PEG-RBV (2001–2011) BOC/TEL+PR (2012–2013) Wave 1 (2014–2016) Wave 2 (2017–2050) |
Universal | Unlimited treatment capacity |
Natural history = simulation scenario with no screening and no treatment; Pre-DAA = simulation scenario with risk-based screening and peginterferon and ribavirin treatment; Base case = simulation scenario with risk-based and birth-cohort screening, treatment with peginterferon and ribavirin and/or DAAs before 2014, and newly approved and future therapies starting in 2014, and limited treatment capacity; Ideal = simulation scenario with universal screening, treatment with peginterferon and ribavirin and/or DAAs before 2014, and newly approved and future therapies starting in 2014, and unlimited treatment capacity.
HCV = hepatitis C virus; PEG-RBV = peginterferon and ribavirin; BOC/TEL+PR = boceprevir or telaprevir plus peginterferon and ribavirin; DAA = direct-acting antiviral agent; Wave 1 = new therapies launched in 2014 for all patients that increased treatment response rates to 90% in non-cirrhotic patients and 60%–80% in cirrhotic patients; Wave 2 = future therapies that we assumed would be launched in 2017 and increase treatment response rates to 90% in cirrhotic patients.
Acknowledgments
Financial Support:
This study was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health (NIH) under Award Number KL2TR000146.
We thank Elamin Elbasha, PhD and Katherine Bornschlegel, MPH for their constructive comments that improved the quality of the manuscript; John Grefenstette, PhD for technical support on simulation runs; and Jill Delsigne, PhD and Diane Hackett for editing the manuscript.
References
- 1.Ghany MG, Strader DB, Thomas DL, Seeff LB. Diagnosis, management, and treatment of hepatitis C: an update. Hepatology. 2009;49(4):1335–74. doi: 10.1002/hep.22759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rosen HR. Chronic hepatitis C infection. N Engl J Med. 2011;364(25):2429–38. doi: 10.1056/NEJMcp1006613. [DOI] [PubMed] [Google Scholar]
- 3.Ly KN, Xing J, Klevens RM, Jiles RB, Ward JW, Holmberg SD. The increasing burden of mortality from viral hepatitis in the United States between 1999 and 2007. Ann Intern Med. 2012;156(4):271–8. doi: 10.7326/0003-4819-156-4-201202210-00004. [DOI] [PubMed] [Google Scholar]
- 4.Razavi H, El Khoury A, Elbasha E, Estes C, Pasini K, Poynard T, et al. Chronic hepatitis C virus (HCV) disease burden and cost in the United States. Hepatology. 2013;57(6):2164–70. doi: 10.1002/hep.26218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Drenth JP. HCV Treatment—No More Room for Interferonologists? N Engl J Med. 2013;368(20):1931–2. doi: 10.1056/NEJMe1303818. [DOI] [PubMed] [Google Scholar]
- 6. [February 27, 2014];American Association for the Study of Liver Diseases and Infectious Diseases Society of America Recommendations for Testing, Managing, and Treating Hepatitis C. 2014 doi: 10.1002/hep.31060. Accessed at http://www.hcvguidelines.org/full-report-view on. [DOI] [PMC free article] [PubMed]
- 7.Dieterich D. The end of the beginning for hepatitis C treatment. Hepatology. 2012;55(3):664–5. doi: 10.1002/hep.25528. [DOI] [PubMed] [Google Scholar]
- 8.Liang TJ, Ghany MG. Current and future therapies for hepatitis C virus infection. N Engl J Med. 2013;368(20):1907–17. doi: 10.1056/NEJMra1213651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moyer VA. Screening for hepatitis C virus infection in adults: US preventive services task force recommendation statement. Ann Intern Med. 2013;159(5):349–57. doi: 10.7326/0003-4819-159-5-201309030-00672. [DOI] [PubMed] [Google Scholar]
- 10.Smith BD, Morgan RL, Beckett G, Falck-Ytter Y, Holtzman D, Teo C, et al. Recommendations for the identification of chronic hepatitis C virus infection among persons born during 1945–1965. MMWR Recomm Rep. 2012;61:1–32. [PubMed] [Google Scholar]
- 11.Rein DB, Smith BD, Wittenborn JS, Lesesne SB, Wagner LD, Roblin DW, et al. The cost-effectiveness of birth-cohort screening for hepatitis C antibody in U.S. primary care settings. Ann Intern Med. 2012;156(4):263–70. doi: 10.7326/0003-4819-156-4-201202210-00378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hagan LM, Schinazi RF. Best strategies for global HCV eradication. Liver International. 2013;33(s1):68–79. doi: 10.1111/liv.12063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gonzalez SA, Davis GL. Demographics of hepatitis C virus today. Clinical Liver Disease. 2012;1(1):2–5. doi: 10.1002/cld.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ngo-Metzger Q, Ward JW, Valdiserri RO. Expanded hepatitis C virus screening recommendations promote opportunities for care and cure. Ann Intern Med. 2013;159(5):364–5. doi: 10.7326/0003-4819-159-5-201309030-00675. [DOI] [PubMed] [Google Scholar]
- 15.Davis G, Alter M, El-Serag H, Poynard T, Jennings L. Aging of hepatitis C virus (HCV)-infected persons in the United States: a multiple cohort model of HCV prevalence and disease progression. Gastroenterology. 2010;138(2):513–21. doi: 10.1053/j.gastro.2009.09.067. [DOI] [PubMed] [Google Scholar]
- 16.Coffin PO, Scott JD, Golden MR, Sullivan SD. Cost-effectiveness and population outcomes of general population screening for hepatitis C. Clin Infect Dis. 2012;54(9):1259–71. doi: 10.1093/cid/cis011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McGarry LJ, Pawar VS, Panchmatia HR, Rubin JL, Davis GL, Younossi ZM, et al. Economic model of a birth cohort screening program for hepatitis C virus. Hepatology. 2012;55(5):1344–55. doi: 10.1002/hep.25510. [DOI] [PubMed] [Google Scholar]
- 18.Siebert U, Alagoz O, Bayoumi AM, Jahn B, Owens DK, Cohen DJ, et al. State-transition modeling: a report of the ISPOR-SMDM modeling good research practices task force-3. Value Health. 2012;15(6):812–20. doi: 10.1016/j.jval.2012.06.014. [DOI] [PubMed] [Google Scholar]
- 19.Armstrong GL, Wasley A, Simard EP, McQuillan GM, Kuhnert WL, Alter MJ. The prevalence of hepatitis C virus infection in the United States, 1999 through 2002. Ann Intern Med. 2006;144(10):705. doi: 10.7326/0003-4819-144-10-200605160-00004. [DOI] [PubMed] [Google Scholar]
- 20.Zeuzem S, Andreone P, Pol S, Lawitz E, Diago M, Roberts S, et al. Telaprevir for retreatment of HCV infection. N Engl J Med. 2011;364(25):2417–28. doi: 10.1056/NEJMoa1013086. [DOI] [PubMed] [Google Scholar]
- 21.Poynard T, Colombo M, Bruix J, Schiff E, Terg R, Flamm S, et al. Peginterferon alfa-2b and ribavirin: effective in patients with hepatitis C who failed interferon alfa/ribavirin therapy. Gastroenterology. 2009;136(5):1618–28. e2. doi: 10.1053/j.gastro.2009.01.039. [DOI] [PubMed] [Google Scholar]
- 22.Surveillance for Viral Hepatitis – United States. Center for Disease Control and Prevention; 2011. [20 November 2013]. Accessed at. at http://www.cdc.gov/HEPATITIS/Statistics/index.htm on. [Google Scholar]
- 23.Blatt LM, Mutchnick MG, Tong MJ, Klion FM, Lebovics E, Freilich B, et al. Assessment of hepatitis C virus RNA and genotype from 6807 patients with chronic hepatitis C in the United States. J Viral Hepat. 2000;7(3):196–202. doi: 10.1046/j.1365-2893.2000.00221.x. [DOI] [PubMed] [Google Scholar]
- 24.Kim WR, Stock PG, Smith JM, Heimbach JK, Skeans MA, Edwards EB, et al. OPTN/SRTR 2011 Annual Data Report: liver. Am J Transplant. 2013;13(Suppl 1)(s1):73–102. doi: 10.1111/ajt.12021. [DOI] [PubMed] [Google Scholar]
- 25.Falck-Ytter Y, Kale H, Mullen KD, Sarbah SA, Sorescu L, McCullough AJ. Surprisingly small effect of antiviral treatment in patients with hepatitis C. Ann Intern Med. 2002;136(4):288–92. doi: 10.7326/0003-4819-136-4-200202190-00008. [DOI] [PubMed] [Google Scholar]
- 26.National Health and Nutrition Examination Survey Data. Department of Health and Human Services, Center for Disease Control and Prevention; 2001–2006. [20 November 2013]. Accessed at the. at http://www.cdc.gov/nchs/nhanes.htm on. [Google Scholar]
- 27.Zeuzem S, Feinman SV, Rasenack J, Heathcote EJ, Lai MY, Gane E, et al. Peginterferon alfa-2a in patients with chronic hepatitis C. N Engl J Med. 2000;343(23):1666–72. doi: 10.1056/NEJM200012073432301. [DOI] [PubMed] [Google Scholar]
- 28.Honeycutt AA, Harris JL, Khavjou O, Buffington J, Jones TS, Rein DB. The costs and impacts of testing for hepatitis C virus antibody in public STD clinics. Public Health Rep. 2007;122(Suppl 2)(Suppl 2):55–62. doi: 10.1177/00333549071220S211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Feld J, Jacobson I, Jensen D, Foster GR, Pol S, Tam E, et al. Up to 100% SVR4 rates with ritonavir-boosted danoprevir (DNVr), mericitabine (MCB), and ribavirin (R) ± peginterferon alfa-2a (40KD) (P) in HCV genotype 1-infected partial and null responders: results from the MATTERHORN study. Hepatology. 2012;56(Suppl):231A–2A. [Google Scholar]
- 30.Gane EJ, Pockros P, Zeuzem S, Marcellin P, Shikhman A, Bernaards C, et al. Interferon-Free Treatment with a Combination of Mericitabine and Danoprevir/R with or without Ribavirin in Treatment-Naive Hcv Genotype 1-Infected Patients. J Hepatol. 2012;56(56):S555–S6. [Google Scholar]
- 31.Lok AS, Gardiner DF, Lawitz E, Martorell C, Everson GT, Ghalib R, et al. Preliminary study of two antiviral agents for hepatitis C genotype 1. N Engl J Med. 2012;366(3):216–24. doi: 10.1056/NEJMoa1104430. [DOI] [PubMed] [Google Scholar]
- 32.Kowdley K, Lawitz E, Poordad F. A 12-week interferon-free treatment regimen with ABT-450/r, ABT-267, ABT-333 and Ribavirin achieves SVR12 rates (observed data) of 99% in treatment-naive patients and 93% in prior null responders with HCV genotype 1 infection. Hepatology. 2012;56(Suppl):LB1. [Google Scholar]
- 33.Gane EJ, Stedman CA, Hyland RH, Sorensen RD, Symonds WT, Hindes R, et al. Once Daily Sofosbuvir (GS-7977) Plus Ribavirin in Patients with HCV Genotypes 1, 2, and 3: The ELECTRON Trial. Hepatology. 2012;56:306A–7A. [Google Scholar]
- 34.Zeuzem S, Soriano V, Asselah T, Bronowicki JP, Lohse AW, Mullhaupt B, et al. Interferon (IFN)-free combination treatment with the HCV NS3/4A protease inhibitor BI 201335 and the nonnucleoside NS5B inhibitor BI 207127±ribavirin (R): Final results of SOUND-C2 and predictors of response. Hepatology. 2012;56(Suppl):308–9A. [Google Scholar]
- 35.Soriano V, Gane E, Angus P, Stickel F, Bronowicki JP, Roberts SK, et al. Efficacy and safety of the interferon (IFN)-free combination of BI 201335+ BI 207127±ribavirin (RBV) in treatment-naïve patients with HCV genotype (GT) 1 infection and compensated liver cirrhosis: Results from the SOUND-C2 study. Hepatology. 2012;56(Suppl):234A. [Google Scholar]
- 36.Jacobson IM, Jensen DM, Pol S, Foster GR, Feld JJ, Yoshida EM, et al. Safety and efficacy of ritonavir-boosted danoprevir (DNVr), peginterferon alpha-2a (40KD) (P) and ribavirin (R) with or without mericitabine in HCV genotype (G)1-infected treatment-experienced patients with advanced hepatic fibrosis. Hepatology. 2012;56:232A–3A. [Google Scholar]
- 37.Poordad F, Fried M, Zeuzem S, Ferenci P, Lenz O, Sinha R, et al. Efficacy and tolerability of TMC435 150 mg once daily with peginterferon α-2a and ribavirin for treatment of HCV genotype 1 infection in patients with Metavir score F3 and F4 (PILLAR and ASPIRE trials) Hepatology. 2012;56(Suppl):233A. [Google Scholar]
- 38.Jacobson IM, Sulkowski MS, Gane EJ, Koziel MJ, De Souza C, Kieffer TL, et al. VX-222, Telaprevir and Ribavirin in Treatment-Naive Patients with Genotype 1 Chronic Hepatitis C: Results of the ZENITH Study Interferon-Free Regimen. Hepatology. 2012;56:308A-A. [Google Scholar]
- 39.Pawlotsky JM, Sarin SK, Foster GR, Peng CY, Rasenack J, Flisiak R, et al. Alisporivir plus Ribavirin achieves high rates of sustained HCV clearance (SVR24) as interferon (IFN)-free or IFN-add-on regimen in treatment-naive patients with HCV GT2 or GT3: Final results from VITAL-1 study. Hepatology. 2012;56:309A–10A. [Google Scholar]
- 40.Osinusi A, Heytens L, Lee YJ, Bon D, Shivakumar B, Nelson A, et al. High Efficacy Of GS-7977 In Combination With Low or Full dose Ribavirin for 24 weeks In Difficult To Treat HCV Infected Genotype 1 Patients : Interim Analysis From The SPARE Trial. Hepatology. 2012;56(6):1518. [Google Scholar]
- 41.Everson G, Sims K, Rodriguez-Torres M. An interferon-free, ribavirin-free 12-week regimen of daclatasvir (DCV), asunaprevir (ASV), and BMS-791325 yielded SVR4 of 94% in treatment-naive patients with genotype (GT) 1 chronic hepatitis C virus (HCV) infection. Hepatology. 2012;56(Suppl):LB3. [Google Scholar]
- 42.Doyle JS, Aspinall E, Liew D, Thompson AJ, Hellard ME. Current and emerging antiviral treatments for hepatitis C infection. Br J Clin Pharmacol. 2012;75(4):931–43. doi: 10.1111/j.1365-2125.2012.04419.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Aronsohn A, Jensen D. Informed deferral: a moral requirement for entry into the hepatitis C virus treatment warehouse. Hepatology. 2012;56(5):1591–2. doi: 10.1002/hep.25957. [DOI] [PubMed] [Google Scholar]
- 44.Kanwal F, El-Serag HB. HCV Treatment: The Unyielding Chasm between Efficacy and Effectiveness. Clin Gastroenterol Hepatol. 2014 doi: 10.1016/j.cgh.2014.02.031. (Available online 4 March 2014) [DOI] [PubMed] [Google Scholar]
- 45.Jacobson IM, Gordon SC, Kowdley KV, Yoshida EM, Rodriguez-Torres M, Sulkowski MS, et al. Sofosbuvir for hepatitis C genotype 2 or 3 in patients without treatment options. N Engl J Med. 2013;368(20):1867–77. doi: 10.1056/NEJMoa1214854. [DOI] [PubMed] [Google Scholar]
- 46.Volk ML, Tocco R, Saini S, Lok AS. Public health impact of antiviral therapy for hepatitis C in the United States. Hepatology. 2009;50(6):1750–5. doi: 10.1002/hep.23220. [DOI] [PubMed] [Google Scholar]
- 47.Denniston MM, Jiles RB, Drobeniuc J, Klevens RM, Ward JW, McQuillan GM, et al. Chronic Hepatitis C Virus Infection in the United States, National Health and Nutrition Examination Survey 2003 to 2010. Ann Intern Med. 2014;160(5):293–300. doi: 10.7326/M13-1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rein DB, Wittenborn JS, Weinbaum CM, Sabin M, Smith BD, Lesesne SB. Forecasting the morbidity and mortality associated with prevalent cases of pre-cirrhotic chronic hepatitis C in the United States. Dig Liver Dis. 2011;43(1):66–72. doi: 10.1016/j.dld.2010.05.006. [DOI] [PubMed] [Google Scholar]
- 49.El-Serag HB. Hepatocellular carcinoma: recent trends in the United States. Gastroenterology. 2004;127(5):S27–S34. doi: 10.1053/j.gastro.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 50.Lang K, Danchenko N, Gondek K, Shah S, Thompson D. The burden of illness associated with hepatocellular carcinoma in the United States. J Hepatol. 2009;50(1):89–99. doi: 10.1016/j.jhep.2008.07.029. [DOI] [PubMed] [Google Scholar]
- 51.van der Meer AJ, Veldt BJ, Feld JJ, Wedemeyer H, Dufour J-F, Lammert F, et al. Association between sustained virological response and all-cause mortality among patients with chronic hepatitis C and advanced hepatic fibrosis. JAMA. 2012;308(24):2584–93. doi: 10.1001/jama.2012.144878. [DOI] [PubMed] [Google Scholar]
- 52.National Population Projections. United States Census Bureau; 2012. [30 November 2013; 2012]. Accessed at the. at http://www.census.gov/population/projections/data/national/2012.html on. [Google Scholar]
- 53.Public Law 107–280, Rare Diseases Act of 2002. US Government Printing Office (GPO); [30 November 2013]. Accessed at the. at http://www.gpo.gov/fdsys/pkg/PLAW-107publ280/content-detail.html on. [Google Scholar]
- 54.McGovern BH. Hepatitis C virus and the infectious disease physician: a perfect match [Editorial] Clin Infect Dis. 2012;55(3):414–7. doi: 10.1093/cid/cis378. [DOI] [PubMed] [Google Scholar]
- 55.Arora S, Thornton K, Murata G, Deming P, Kalishman S, Dion D, et al. Outcomes of treatment for hepatitis C virus infection by primary care providers. N Engl J Med. 2011;364(23):2199–207. doi: 10.1056/NEJMoa1009370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hoofnagle JH, Sherker AH. Therapy for hepatitis C--the costs of success. N Engl J Med. 2014;370(16):1552–3. doi: 10.1056/NEJMe1401508. [DOI] [PubMed] [Google Scholar]
- 57.Poynard T, Ratziu V, Charlotte F, Goodman Z, McHutchison J, Albrecht J. Rates and risk factors of liver fibrosis progression in patients with chronic hepatitis C. J Hepatol. 2001;34(5):730–9. doi: 10.1016/s0168-8278(00)00097-0. [DOI] [PubMed] [Google Scholar]
- 58.Benhamou Y, Bochet M, Di Martino V, Charlotte F, Azria F, Coutellier A, et al. Liver fibrosis progression in human immunodeficiency virus and hepatitis C virus coinfected patients. Hepatology. 1999;30(4):1054–8. doi: 10.1002/hep.510300409. [DOI] [PubMed] [Google Scholar]
- 59.Bourlière M, Khaloun A, Wartelle-Bladou C, Oules V, Portal I, Benali S, et al. Future treatment of patients with HCV cirrhosis. Liver International. 2012;32(s1):113–9. doi: 10.1111/j.1478-3231.2011.02702.x. [DOI] [PubMed] [Google Scholar]
- 60.Lawitz E, Mangia A, Wyles D, Rodriguez-Torres M, Hassanein T, Gordon SC, et al. Sofosbuvir for Previously Untreated Chronic Hepatitis C Infection. N Engl J Med. 2013;368(20):1878–87. doi: 10.1056/NEJMoa1214853. [DOI] [PubMed] [Google Scholar]
- 61.Jacobson IM, McHutchison JG, Dusheiko G, Di Bisceglie AM, Reddy KR, Bzowej NH, et al. Telaprevir for previously untreated chronic hepatitis C virus infection. N Engl J Med. 2011;364(25):2405–16. doi: 10.1056/NEJMoa1012912. [DOI] [PubMed] [Google Scholar]
- 62.Poordad F, McCone J, Jr, Bacon BR, Bruno S, Manns MP, Sulkowski MS, et al. Boceprevir for untreated chronic HCV genotype 1 infection. N Engl J Med. 2011;364(13):1195–206. doi: 10.1056/NEJMoa1010494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jacobson IM, Ghalib R, Rodriguez-Torres M. SVR results of a once-daily regimen of simeprevir (TMC435) plus sofosbuvir (GS-7977) with or without ribavirin in cirrhotic and non-cirrhotic HCV genotype 1 treatment-naive and prior null responder patients: the COSMOS study. American Association for the Study of Liver Diseases (AASLD); Washington, DC: 2013. p. Abstract LB-3. [Google Scholar]
- 64.Nguyen MH, Keeffe EB. Prevalence and treatment of hepatitis C virus genotypes 4, 5, and 6. Clin Gastroenterol Hepatol. 2005;3:S97–S101. doi: 10.1016/s1542-3565(05)00711-1. [DOI] [PubMed] [Google Scholar]
- 65.Lawitz E, Poordad FF, Pang PS, Hyland RH, Ding X, Mo H, et al. Sofosbuvir and ledipasvir fixed-dose combination with and without ribavirin in treatment-naive and previously treated patients with genotype 1 hepatitis C virus infection (LONESTAR): an open-label, randomised, phase 2 trial. 2013. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 66.Poordad F, Hezode C, Trinh R, Kowdley KV, Zeuzem S, Agarwal K, et al. ABT-450/r-Ombitasvir and Dasabuvir with Ribavirin for Hepatitis C with Cirrhosis. N Engl J Med. 2014 doi: 10.1056/NEJMoa1402869. [DOI] [PubMed] [Google Scholar]
- 67.Shiffman ML, Suter F, Bacon BR, Nelson D, Harley H, Solá R, et al. Peginterferon alfa-2a and ribavirin for 16 or 24 weeks in HCV genotype 2 or 3. N Engl J Med. 2007;357(2):124–34. doi: 10.1056/NEJMoa066403. [DOI] [PubMed] [Google Scholar]
- 68.Dore G, Lawitz E, H’ezode C, Shafran S, Ramji A, Tatum H, et al. DACLATASVIR COMBINED WITH PEGINTERFERON ALFA-2A AND RIBAVIRIN FOR 12 OR 16 WEEKS IN PATIENTS WITH HCV GENOTYPE 2 OR 3 INFECTION: COMMAND GT2/3 STUDY. J Hepatol. 2013;58:S570–S1. [Google Scholar]
- 69.Gane EJ, Stedman CA, Hyland RH, Ding X, Svarovskaia E, Symonds WT, et al. Nucleotide Polymerase Inhibitor Sofosbuvir plus Ribavirin for Hepatitis C. N Engl J Med. 2013;368(1):34–44. doi: 10.1056/NEJMoa1208953. [DOI] [PubMed] [Google Scholar]
- 70.Lawitz E, Lalezari JP, Hassanein T, Kowdley KV, Poordad FF, Sheikh AM, et al. Sofosbuvir in combination with peginterferon alfa-2a and ribavirin for non-cirrhotic, treatment-naive patients with genotypes 1, 2, and 3 hepatitis C infection: a randomised, double-blind, phase 2 trial. Lancet Infect Dis. 2013;13(5):401–8. doi: 10.1016/S1473-3099(13)70033-1. [DOI] [PubMed] [Google Scholar]
- 71.Zeuzem S, Dusheiko G, Salupere R, Mangia A, Flisiak R, Hyland R. Sofosbuvir + Ribavirin for 12 or 24 Weeks for Patients with HCV Genotype 2 or 3: the VALENCE trial. Hepatology. 2013;58(S1) [Google Scholar]
- 72.Ruane P, Ain D, Raid J, Meshrekey R, Stryker R, Wolfe P. Sofosbuvir plus Ribavirin in the Treatment of Chronic HCV Genotype 4 Infection in Patients of Egyptian Ancestry. Hepatology. 2013;58(S1) doi: 10.1016/j.jhep.2014.10.044. [DOI] [PubMed] [Google Scholar]
- 73.Bacon BR, Gordon SC, Lawitz E, Marcellin P, Vierling JM, Zeuzem S, et al. Boceprevir for previously treated chronic HCV genotype 1 infection. N Engl J Med. 2011;364(13):1207–17. doi: 10.1056/NEJMoa1009482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bronowicki J, Davis M, Flamm S, Gordon S, Lawitz E, Yoshida E, et al. 11 SUSTAINED VIROLOGIC RESPONSE (SVR) IN PRIOR PEGINTERFERON/RIBAVIRIN (PR) TREATMENT FAILURES AFTER RETREATMENT WITH BOCEPREVIR (BOC)-+-PR: THE PROVIDE STUDY INTERIM RESULTS. J Hepatol. 2012;56:S6. [Google Scholar]
- 75.Lawitz E, Poordad F, Brainard D. Sofosbuvir in combination with pegIFN and ribavirin for 12 weeks provides high SVR rates in HCV-infected genotype 2 or 3 treatment experienced patients with and without compensated cirrhosis: results from the LONESTAR-2 study. American Association for the Study of Liver Diseases (AASLD); Washington, DC: 2013. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.