Abstract
Cancer stem cells (CSCs) are tumor cells that have the principal properties of self-renewal, clonal tumor initiation capacity and clonal long-term repopulation potential. CSCs reside in niches, which are anatomically distinct regions within the tumor microenvironment. These niches maintain the principle properties of CSCs, preserve their phenotypic plasticity, protect them from the immune system and facilitate their metastatic potential. In this perspective, we focus on the CSC niche and discuss its contribution to tumor initiation and progression. Since CSCs survive many commonly employed cancer therapies, we examine the prospects of targeting the niche components as preferable therapeutic targets.
Keywords: Cancer stem cells, cancer stem cell niche, tumor microenvironment, metastasis, cancer therapy
Introduction
Cancer cells within individual tumors often exist in distinct phenotypic states that differ in functional attributes. Within this tumor heterogeneity, cancer stem cells (CSCs) are tumor cells that have the principal properties of self-renewal, clonal tumor initiation capacity and clonal long-term repopulation potential (Clarke et al., 2006; Nguyen et al., 2012). They also display plasticity by reversibly transitioning between stem and non-stem cell states. CSCs have the ability to evade cell death and metastasize, although they may stay dormant for long periods of time (Kreso et al., 2013). Both experimental models and clinical studies indicate that CSCs survive many commonly employed cancer therapeutics (Kreso and Dick, 2014).
As is the case for normal stem cells, CSCs are believed to reside in niches. Niches are specialized microenvironments that regulate adult stem cell fate by providing cues in the form of both cell-cell contacts and secreted factors. Niches have been identified for mammalian stem cells in various epithelial tissues, such as the intestine as well as in neural, epidermal, and hematopoietic systems (Voog and Jones, 2010). Normal niches are comprised of fibroblastic cells, immune cells, endothelial and perivascular cells or their progenitors, extracellular matrix (ECM) components and networks of cytokines and growth factors (Korkaya et al., 2011). The CSC niche itself is a part of the tumor microenvironment (TME), which is a collective term for the adjacent stroma along with the normal counterparts of the tumorigenic cells (Hanahan and Coussens, 2012). Non-CSC tumor cells are also part of the CSC niche. During the progression of tumors to a more malignant state, the CSC state in the primary tumor depends crucially on the TME and potentially on the CSC niches within it (Fessler et al., 2013). In this perspective, we focus on the emerging field of the CSC niche, which is yet to be fully elucidated. We critically discuss the contribution of the niche to tumor initiation and progression and examine the prospects of targeting the niche for cancer therapy. Although we focus on conceptual similarities between various niches, it is important to note that glioblastomas, melanomas and especially hematopoietic cancers may have a very different pattern of regulation than those in the more common carcinomas. One major difference is that hematopoietic cells are inherently mobile, whereas epithelial cells need to gain mobility de novo to metastasize.
Models of tumorigenesis, CSC plasticity and the role of the CSC niche
It has long been postulated that intratumoral heterogeneity contributes to disease progression, impacts therapeutic efficacy and therefore affects patient survival (Hanahan and Weinberg, 2011). The TME contributes to tumor heterogeneity along with genetic diversity and epigenetic modifications within tumor cells (Kreso and Dick, 2014). Two models, hierarchical and stochastic, have been used to understand tumor progression and heterogeneity. Although they differentially consider the weight that CSCs and their niche carry in driving a particular tumor, these two models are not mutually exclusive, and the concept of cellular plasticity unifies them into one model.
The hierarchical model
The hierarchical model designates malignant tumor-propagating cells as CSCs (Fig. 1). It relies on the paradigm that CSCs represent a biologically distinct subset within the total cancer cell population. According to this model, carcinogenesis occurs when a stem cell escapes regulation and gives rise to a stem cell-like counterpart, a CSC. CSCs represent a distinct population that can be isolated from the remainder of the tumor cells. They can self-renew their own population and have long-term clone-propagating capacity so they can generate short-lived progeny with self-limited proliferative capacity (Kreso and Dick, 2014). Due to the self-renewal capacity, CSCs represent the unit of selection in a tumor, while any of the other cells lead to clonal exhaustion (Greaves, 2013). The clinical implication from this model is that only complete eradication of all CSCs will eliminate the possibility of relapse. The hierarchical model was first demonstrated in acute myeloid leukemia, in which a subset of leukemia cells expressed stem cell markers and harbored the potential of self-renewal, propagation, and differentiation (Bonnet and Dick, 1997). In solid tumors CSCs were first shown in breast cancer, as they were particularly efficient in establishing tumors upon their isolation from the tumor bulk and their translation into mice (Al-Hajj et al., 2003). Since then, the existence of CSCs has been shown in various cancers including hematopoietic, head and neck, breast, prostate, lung, brain, colon, skin and pancreatic cancers as well as in sarcomas (reviewed in Kreso and Dick, 2014; Osakrsson et al., 2014).
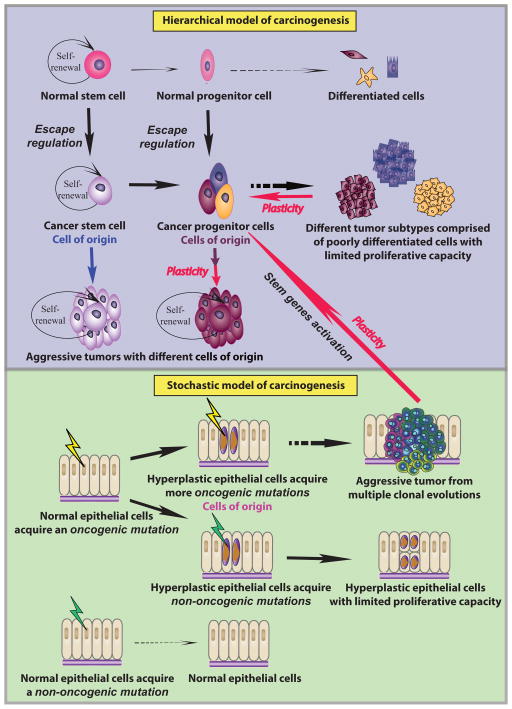
Fig. 1. Models of carcinogenesis.
Models are exemplified for an epithelial tissue.
Hierarchical model of carcinogenesis: Normal stem cells have limited proliferative capacity and give rise to progenitor cells that proliferate and differentiate into various types of cells. If a normal stem cell escapes regulation, it becomes a cancer stem cell, which can self-renew and produce cancer progenitor cells. If a normal progenitor cell escapes regulation, it becomes cancer progenitor cells, which can give rise to poorly differentiated cells. If those cells are generated from different types of cancer progenitor cells, they might form different subtypes of tumors with limited proliferative capacity. Due to plasticity (red arrows), the progenitor cells and some of the differentiated cells can de-differentiate to become CSCs again. Either CSCs from normal stem cells or from cancer progenitor cells initiate and sustain aggressive tumor growth, and the cells-of-origin for these two types of tumors are either CSCs (blue arrow) or cancer progenitor cells (purple arrow) respectively. Stochastic model of carcinogenesis: Healthy epithelial cells develop an oncogenic mutation (yellow strikes) that forms hyperplasia. Some of the hyperplastic cells can become the cells-of-origin developing additional oncogenic mutations and transform into tumor cells. Under multiple clonal evolutions (colonies shown with various colors), aggressive tumors can form. Some mutations can lead to a stem cell like permissive epigenome and thus create cancer progenitor cells. This process reconciles the stochastic model with the hierarchical model. However, if the hyperplastic cells develop non-oncogenic mutations (green strikes), they will not transform into tumor cells although they may continue to proliferate. If healthy epithelial cells initially undergo non-oncogenic mutations (green strikes), they can overcome such mutations and maintain a healthy tissue.
Given that cancer is characterized by proliferation and expansion yielding tissues that do not anatomically or functionally resemble the original organ, self-renewal, proliferation and differentiation are most likely deregulated in CSCs. Indeed, the majority of evidence indicates that CSCs in most solid tumors lack true multipotency and asymmetric cell division, and can only differentiate into a single type of descendant cancer cell that is unable to generate an entire array of lineages (Kreso and Dick, 2014). Consequently, some investigators have advocated the use of the term “tumor-initiating cell” (TIC) rather than CSC, to describe the subset of cells with tumorigenic potential (Hill and Perris, 2007). Although the TIC and the CSC have been used interchangeably, the TIC more appropriately denotes the cell-of-origin. Importantly, the hierarchical model assumes that the CSC is the cell-of-origin, i.e. the first abnormal cell that initiates the tumor. However and as explained later, due to cellular plasticity, the cell of origin is not necessarily the CSC, i.e. the cellular subset within the tumor that uniquely sustains primary and metastatic tumor growth.. Therefore, the phenotype and characteristic gene-expression patterns of the cell-of-origin may differ substantially from that of the CSC (Chaffer and Weinberg, 2015).
According to the hierarchical model, the same CSC or different sets of CSCs can give rise to different cancer subtypes within a certain organ or tissue (Visvader and Linderman, 2008), which results in the cellular heterogeneity of tumors. Those distinct sub-clones develop in a hierarchical fashion with their own CSCs. However, the major limitation of this model is that it conceptually precludes the interchange between differentiated and stem-like states within the same cell (Kreso et al., 2013). Nevertheless, it accommodates the possibility that CSCs, like their normal counterparts, may retain responsiveness to and even dependence on external cues to elicit their intrinsically determined potentialities for survival, growth and differentiation, irrespective of how perturbed the process of differentiation may be.
The stochastic model
The stochastic model states that every cell within a tumor is equally likely to be the cell-of-origin and facilitate tumor initiation and progression (Fig. 1). The variable activities of tumor cells are only partially determined by the environment in which the cells are found, but rather are determined by some stochastically varying intrinsic factors (Quail et al., 2012). The stochastic model relies on the premise that cancer is a disease defined by hyperproliferation and sequential acquisition of genetic mutations in cell cycle genes that contribute to subsequent clonal expansions in an otherwise relatively quiescent normal adult somatic cell. Indeed, advanced genome sequencing has demonstrated that cancer within a single patient is a heterogeneous mixture of genetically distinct sub-clones that arise through branching evolution (Greaves and Maley, 2012; Burrell et al., 2013) and seed different parts of a single tumor (Gerlinger et al., 2012). Although mutational burden is highly variable across tumor types (Lawrence et al., 2013), a typical tumor contains 2–8 driver mutations that regulate three core cellular processes: cell fate, cell survival, and genome maintenance (Vogelstein et al., 2013). Whole exome and whole genome sequencing of thousands of tumors show that in the same tumor type there is substantial variation in driver mutations and the same driver mutations can occur in different tumor types, suggesting that the same pathways can be active in different tumors (Alexandrov et al., 2013; Kandoth et al., 2013).
Several tumor types appear to adhere to the stochastic model; good examples are some colorectal cancers (Vogelstein et al., 1988) and B cell lymphoblastic leukemias (Williams et al., 2007). However, this model focuses on genetic heterogeneity without considering that individual cells within genetically homogeneous sub-clones might still exhibit phenotypic variations due to different microenvironmental cues and therefore may not account for the heterogeneity in tumor initiation capacity.
Cellular plasticity reconciles the hierarchical and stochastic theories into one model
Phenotypic plasticity characterizes a population of cancer cells that have the capacity to interconvert between differentiated and stem-like states, through a continuum of cell fate specifications (Quail et al., 2012). Based on this characteristic, the hierarchical vs. stochastic models is a false dichotomy, as hierarchically organized cell populations are more transitory between states than previously imagined and stochastic events are able to generate novel, hierarchically organized cell populations. Thus, depending on the genotype and the microenvironmental signals experienced by transit-amplifying/progenitor cells, at least in epithelial tissues, such cells may dedifferentiate and thereby enter back into the CSC pool to regain long-term tumor repopulation capacity (Chaffer and Weinberg, 2015).
This dedifferentiation capacity may be either inherited (hierarchical theory) or acquired via mutations that lead to a stem cell-like permissive epigenome (stochastic theory). Indeed, p53 inhibition and hTERT activation (Hahn et al., 1999; Stewart et al., 2002; Hong et al., 2009) or the aberrant acquisition of stem cell-associated factors such as NODAL, NOTCH and WNT proteins facilitates such phenotypic plasticity. Moreover, the concept of cellular plasticity suggests that symmetrical cell division may not be as necessary to enlarge the CSC pool and could be secondary to asymmetrical division as progenitor cells, asymmetrically divided from CSCs, are more proliferative and can convert back to CSCs. The fact that melanoma, breast, prostate, ovarian and lung cancer cells are all able to alter their gene expression to resemble cell types that are not part of their original lineage (Quail et al., 2012) exemplifies cancer cell plasticity which enables cancer cells to gain/lose stem cell properties (Shirakawa et al., 2002; Passalidou et al., 2002; Lim et al., 2009). Since regaining tumor-initiating capacity is potentially possible (Gupta et al., 2011), it is essential to understand how the TME and the CSC niche within it promote CSC phenotypes.
CSC assays should consider niche contributions
In general, stem cell markers (Supplementary Table 1) and transcriptional signatures specific to CSCs functionally correlate with aggressive behavior and are highly predictive of overall patient survival. These clinical data suggest that CSCs may be critical therapeutic targets (Suva et al., 2009; Karnoub et al., 2007). However, it became increasingly clear that the frequency of CSCs could vary dramatically between tumor types and also between tumors of the same origin (Visvader and Lindeman, 2008). A related problem is that the variability in the frequency and identity of tumorigenic cells between patients shows that markers identified in one tumor cannot be assumed to distinguish CSCs in other tumors or in other contexts (Ricardo et al., 2011; Lopez et al., 2005; Rocco et al., 2012).
Many theoretical and experimental caveats to the CSC model have remained unexplored, largely due to technological challenges. The gold standard measure of a stem cell is maintenance of long-term clonal growth in functional repopulation assays, originally used for studies of the hematopoietic system. Until recently, most CSC studies utilized the transplantation assay to prove the existence of CSCs for a particular tumor. The markers for CSCs are primarily chosen as robust and heterogeneously expressed cell surface markers that allow the faithful flow cytometric sorting of marker-positive and -negative subsets in a certain tumor type. These subsets are transplanted into immunodeficient mice by limiting dilution, after which tumor growth is scored within several weeks or months. Different tumor initiation capacities between cell subsets are then interpreted as evidence for the presence of CSCs in the primary tumor (Clevers, 2011). Self-renewal is further demonstrated by the ability to establish or maintain the tumor clone in serial transplantation assays at clonal cell doses, and give rise to daughter cells that possess limited proliferative capacity (Clarke et al., 2006). Often no clear morphological or cell cycle distinction is obvious between the tumorigenic and non-tumorigenic cancer cells (Al Hajj et al., 2003), and yet the tumors seem to be organized hierarchically when tested functionally.
There are several problems with the transplantation assays commonly used to identify CSC activity. The sorted and transplanted human cancer cells are challenged by various experimental manipulations and subsequently end up in a context that is dramatically different from the original tumor niche. The new recipient microenvironments can then differentially influence the transplanted cells based on time, species barrier, host strain, developmental stages, and even gender (LaBarge, 2010). Thus, the frequent need for the inoculation of 105 cells in transplantation experiments to allow efficient tumor engraftment may not be indicative of a rare tumor-initiating cell but rather represent the inability to create the proper niche. On the other hand, extremely immunodeficient models can support tumor initiation from the majority of tumor cells, even those not associated with stem cell markers, as shown for patient-derived melanoma cells (Quintana et al., 2008). It is worth noting that melanoma may represent a unique cell type that is particularly poised to enter into the CSC state since melanocytes may be naturally inclined to stem cell states that enhance a migratory phenotype (Quintana et al., 2012). Furthermore, transplantation assays provide only a snapshot of the state of cancer cells at the time of tumor removal, and basically ignore CSC plasticity (Kreso and Dick, 2014). Therefore, the host microenvironments in those assays may distort the original tumorigenic potential and frequently select for the most robust TICs that can grow due to multiple long treatments and loss of their native TME (Kreso and Dick, 2014). Conversely, some cells with tumorigenic potential do not contribute to tumor growth because they are in a non-permissive environment or eliminated by immune effector cells, but will do so upon transplantation.
To date, most CSC markers are not selected based on a deep understanding of the underlying stem cell biology of the relevant tissue from which the cancer originates, since developmental hierarchy is still poorly characterized in most tissues that develop solid cancers. Moreover, only very few CSC markers are currently available for various solid tumors (Clevers, 2011). In some cases, the markers used to rigorously demonstrate the existence of CSCs in a particular cancer subtype were very specific for that cancer, as was shown for breast cancer cells (Clarke et al., 2006). The fact that the markers used are not widely applicable to other types of cancers does not weaken the conclusion of such studies. Nevertheless, these CSC markers only strongly enrich (even by two orders of magnitude) for CSCs within bulk populations of cancer cells but there is no evidence that in such enriched populations, the CSCs exist in a pure state rather than constituting a subset of the cells with a greatly heightened ability to initiate tumors. Moreover, at the time of transplantation, these cells may not necessarily possess CSC capabilities, but rather gain them upon transplantation, which may not have happened within their native niches.
To separate between the inherent plasticity of CSCs and/or the plasticity induced or inferred by the experimental limitations discussed above, it will be crucial to continue and optimize transplantation assays potentially by development of more immune-deficient recipient mice and humanizing these with human TME and/or growth factors (Rongvaux et al., 2013), to estimate as accurately as possible the spectrum of cancer cells that retain the potential to contribute to tumor growth (Meacham and Morrison, 2013). It was recently shown that the growth of dormant cancer sub-clones could be solely induced by microenvironmental changes caused by a sub-population of cancer cells that does not display the higher fitness commonly associated with CSCs (Marusyk et al., 2014). In addition, co-transplantation with stromal cells from myeloproliferative neoplasms enabled engraftment and expansion of neoplastic cells that was otherwise not as successful (Medyouf et al., 2014).
Given that the major limitation of transplantation assays is that they cannot reveal the actual fate of the transplanted cell in its original tissue or tumor (Shackleton et al., 2009), it is of central importance to develop assays that can visualize and localize CSCs and their function within the primary tumor in situ. Live imaging methodologies could bring us closer to unraveling whether, in a particular niche and at a particular point in time, the cell visualized is indeed a CSC rather than a representative of a cell population that is only enriched in CSCs. It would allow us to examine whether, under a specific microenvironment, a cell is able to proliferate and produce progeny/various clones. Integration of genomic and functional properties of CSCs that have yet to be extensively utilized could further facilitate the identification of single, definitive marker genes for CSCs of a particular cancer. Based on such markers, knock-in mouse models or viral-tagging strategies may facilitate genetic lineage tracing (Kreso and Dick, 2014). Lineage tracing or fate-mapping assays are indeed a complementary measure for the long-term clonal growth of stem cells. These assess the actual fate of tumor cells in a particular context, frequently the native tumor environment rather than the potential of what these cells can do under permissive conditions. Yet, lineage-tracing experiments may also provide only limited support for the CSC model. Although intestinal adenomas were shown to be hierarchically organized by Lgr5+ CSCs, both Lgr5− cells and Lgr5+ cells can act as the cell-of-origin via WNT-pathway activation, as exhibited by fate-mapping (Schwitalla et al., 2013). This raises the question of whether adenomas that exhibit hierarchical organization lose it after they progress to malignancies. Although brain tumors may be different from carcinomas, similar concerns have been shown for markers such as CD133 in brain tumors (Meacham and Morrison, 2013). Ultimately, it will be necessary to integrate the data from both transplantation studies and fate-mapping studies of significant numbers of human and mouse tumors to understand the biological diversity. Additionally, the selective ablation of genetically defined subsets of cells (Plaks et al., 2013) can test which tumor cells are fated to contribute to tumor growth or progression in the native tumor environment. Collectively, combining in vivo models and ex vivo systems discussed should prove useful in systematically characterizing the intricate molecular language of cell-cell communication in the CSC niche.
Cross talk between CSCs and their niches
Niches are anatomically distinct microenvironments within the overall TME. Cells within the CSC niche produce factors which stimulate CSC self-renewal, induce angiogenesis, and recruit immune and other stromal cells which secrete additional factors to promote tumor cell invasion and metastasis as reviewed in (Oskarsson et al., 2014; Ye et al., 2014) and summarized below (Figs. 2, 3).
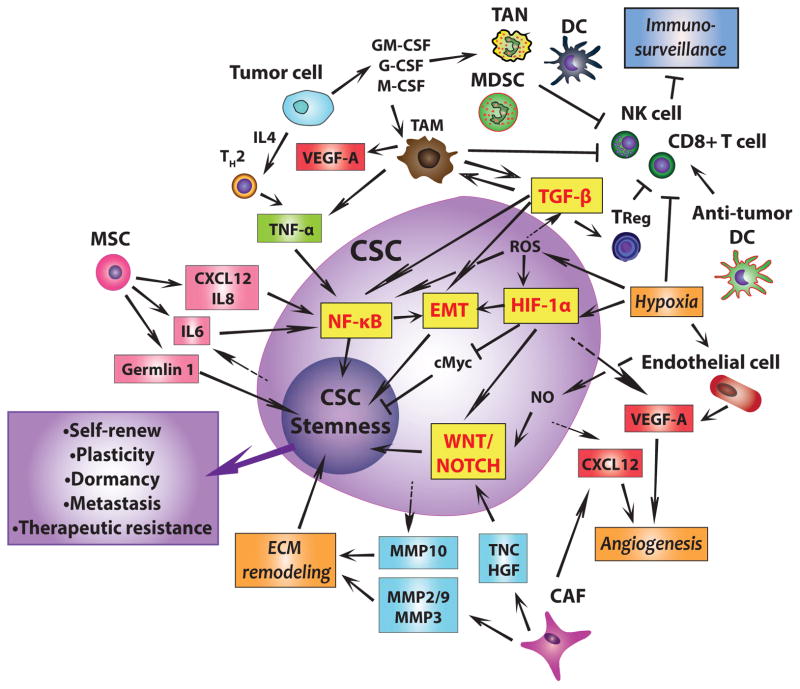
Fig. 2. The molecular and cellular basis of the cross talk between CSCs and their niches.
Cancer stem cell (CSCs) are metastatic cancer cells that can self-renew. Their plasticity and dormancy correlates with their therapeutic resistance. By secreting CXCL12, IL6, and IL8, mesenchymal stem cells (MSCs) promote cancer cell stemness through upregulating NF-κB while CSCs secrete IL6 to attract more MSCs. They also produce the antagonist, Gremlin 1, to promote the undifferentiated state. Surrounding tumor cells produce IL4 to accumulate TH2, which produces TNFα to upregulate the NF-κB signaling pathway and facilitates a pro-tumor microenvironment. In such a microenvironment, tumor cells produce M-CSF, GM-CSF and G-CSF to induce expansion of tumor-associated macrophages (TAMs), myeloid-derived suppressor cells (MDSCs), tumor-associated neutrophils (TANs) and dendritic cells (DCs). TAM produces TNFα and TGF-β to promote NF-κB dependent or TGF-β dependent EMT and thus enhance CSC plasticity. TGF-β can also directly interact with NF-κB signaling pathways to further enhance cancer cell stemness. In addition, TGF-β produced by TAMs accumulates Treg cells. In addition, TAM, TReg and the hypoxic environment inhibit immunosurveillance by inhibiting CD8+ T cell and NK cells cytotoxicity and macrophage phagocytosis. A subset of anti-tumor stimulatory DCs necessary for T-cell-mediated tumor rejection is kept away from the niche. Furthermore, hypoxia increases ROS, which promotes cell survival and induces EMT through the TGF-β signaling pathway. Both hypoxia and ROS induce CSCs to express HIF-1α, directly promote EMT. Moreover, hypoxia also inhibits cell proliferation by downregulating c-Myc expression, enhancing stemness. Hypoxia further promotes cancer cell stemness by promoting an undifferentiated state through TGF-β and WNT signaling pathways. Under hypoxia, endothelial cells produce TF to promote angiogenesis. CSCs and cancer-associated fibroblasts (CAFs) produce CXCL12 to promote angiogenesis, and hypoxia causes both CSCs and endothelial cells to produce VEGF, which further induces angiogenesis. CAFs produce TNC and HGF to enhance WNT and NOTCH signaling for CSC maintenance. CAFs also produce MMP2, 3, and 9. Along with the MMP10 produced by CSCs, those MMPs promote ECM degradation and remodeling which enhances EMT and CSC state. Of note, this figure does not provide spatial information as to the exact localization of CSCs and niche cells.
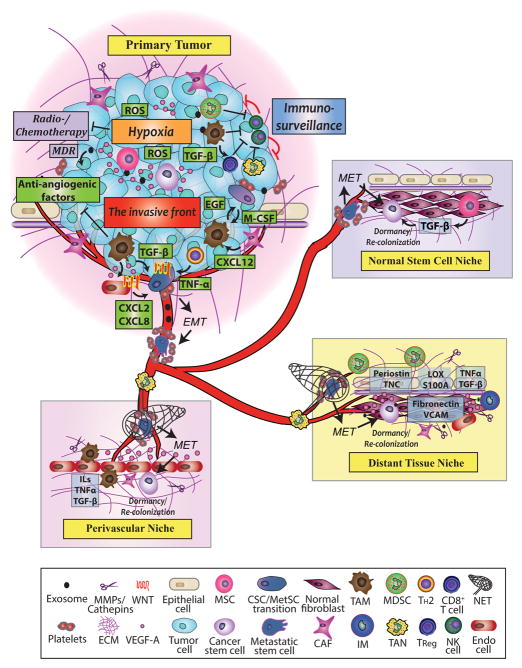
Fig. 3. CSC niches in the primary tumor and metastasis.
In the primary tumor, hypoxia develops within the tumor mass due to impaired vascularization, and ROS is increased. Both hypoxia and ROS upregulate the CSC stress signaling pathways to enhance cancer cell survival and maintain cancer cell stemness. At the same time, MSCs and CSCs produce angiogenic factors to stimulate angiogenesis. In the primary tumor, various chemokines and cytokines are secreted to recruit MDSCs, TAMs, and TANs. These pro-tumorigenic and pro-metastatic cells suppress the cytotoxic functions of NK cells and CD8+ T cells and inhibit immunosurveillance. Treg cells are accumulated by TAMs to further down-regulate T cell cytotoxicity. TAMs, CAFs, newly generated blood vessels, and other stromal cells accumulate at the invasive front CAFs secrete M-CSF to turn on TAMs’ pro-angiogenic switch. TAMs suppress anti-angiogenic factor expression and secrete VEGF-A and WNT to promote angiogenesis. CAF derived CXCL12 triggers the EGF-M-CSF loop in which cancer cells stimulate TAMs to produce EGF by secreting M-CSF while the activated EGF receptor on CSCs increases their invasiveness. By physically contacting with the platelets, CSCs undergo epithelial-to-mesenchymal (EMT) and become MetSC. At the invasive front, WNT, NOTCH, TNF-α, TGF-β, and other cytokines secreted by tumor stroma support the survival of MetSCs. Meanwhile, TAMs and CSCs release exosomes to prepare the potential metastatic sites for the survival of arriving tumor cells, and various stromal cells and released factors help establish metastatic niches at distant sites. Exosomes also facilitate multidrug resistance (MDR) in tumor cells. In the blood vessels, platelets surround and prevent MetSCs from dying in the harsh and foreign environment. Clusters of tumor cells in the blood vessels secrete M-CSF and EGF family members to direct macrophage and MetSCs to the sites of metastasis. After successful extravasation and seeding of metastatic niches, MetSCs potentially undergo mesenchymal-to-epithelial transition (MET) to become CSCs, which can become dormant or grow metastases in three types of metastatic niche sites.
The CSCs can hijack normal stem cell niches established by MSCs. The normal stem cell niche has various factors like TGF-β and various cells to maintain the stemness of CSCs and support their survival. In the niche, CSCs can upregulate EMT pathways in the surrounding nontumorigenic cells and transform them into CSCs to further support the CSCs to colonize the new niche.
Primary CSCs can also manipulate distant tissue niches to create a metastatic niche for their future arrival. The primary tumor sends off VEGF-A, TGF-β, TNF-α and LOX, which induce chemotactic protein S100A expression and extracellular matrix remodeling in the metastatic sites, which creates the pre-metastatic niche. Newly formed blood vessels express fibronectin and VCAM to attract inflammatory monocytes (IM) to secrete MMPs for metastatic growth. In the niche, integrins facilitate the migration of arriving CSCs, which is maintained by periostin and TNC upregulate while LOX and S100A actively recruit MDSCs to promote metastatic growth.
CSCs initiate their metastatic outgrowth around blood capillaries created by perivascular niches enriched in angiocrine factors like VEGF-A. Surrounding TANs also potentially enhance MetSCs settlement by producing neutrophil extracellular traps (NETs). As the niche is established, CSCs recruit TAMs, CAFs, and other stromal cells to establish the paracrine loops to supply CSCs with TNF-α, TGF-β, and ILs for CSC maintenance. At the meantime, the surrounding stromal cells secrete MMPs and cathepsins to further break down the ECM, which in turn releases TGF-β and various growth factors like VEGF-A, to allow tumor expansion.
Cancer-associated fibroblasts
There is evidence pointing to factors produced by CSCs and endothelial cells (ECs) in the TME that can transform normal fibroblasts into cancer-associated fibroblasts (CAFs) (reviewed in Kalluri and Zeisberg, 2006). Compared with normal tissue fibroblasts, CAFs have increased proliferation, enhanced extracellular matrix production and unique cytokine secretion as CXCL12, vascular endothelial growth factor (VEGF), platelet-derived growth factor (PDGF) and hepatocyte growth factor (HGF) (Juntilla and de Sauvage, 2013). CAFs (as well as other cells within the niche) stimulate stemenss via activation of the WNT and NOTCH pathways. Canonical WNT is a major pathway that regulates CSCs and induces stemness in colon and other cancers (Vermeulen et al., 2010; He et al., 2004). Alternatively, epithelial non-stem cells can re-express stem cell markers upon WNT activation and can “dedifferentiate” to TICs (Schwitalla et al., 2013). NOTCH signaling has also been implicated in stem cell maintenance and cell-fate decisions (Quail et al., 2012). NOTCH prevents cells from responding to differentiation cues coming from their immediate environment (Milner et al., 1999). In breast and prostate cancers, NOTCH receptors tend to be overexpressed, and their ligand expression correlates with aggressive phenotypes (Weijzen et al., 2002; Liu et al., 2006). The interplay of the WNT and NOTCH signaling with other pathways like bone morphogenic protein (BMP) (see below) and Hedgehog signaling pathways determines the differentiation state of cells (Fessler et al., 2013).
Mesenchymal stem cells
Mesenchymal stem cells (MSCs) are multipotent stromal cells that have been implicated in multiple mechanisms promoting cancer cell proliferation and metastasis, fostering angiogenesis and generating an immunosuppressive microenvironment (Cuiffo and Karnoub, 2012; Nishimura et al., 2012). They provide an advantageous TME for the restoration of CSCs as they secrete a variety of cytokines that have both paracrine and autocrine functions in the tumor milieu. MSCs can promote cancer stemness through NF- B pathway by secreting CXCL12, interleukin (IL) 6, and IL8 (Cabarcas et al., 2011). Moreover, MSCs can stimulate tumor progression by producing the BMP antagonist, Gremlin 1, to promote the undifferentiated state (Davis et al., 2015). Furthermore, MSCs can cause elevated miR-199a expression in breast cancer cells, which leads to aberrant expression of a set of interrelated microRNAs and suppressed FOXP2 expression, providing tumor cells with CSC properties (Cuiffo et al., 2014).
Inflammatory cells
Currently, one of the areas of greatest interest is the role of the CSC niche in modulating the level of tumor immunity. The TME is characterized by chronic inflammation, which stimulates tumor cell proliferation and metastasis (Cabarcas et al., 2011). To evade immune surveillance and thus enable tumor progression, the niche must immunosuppress the cytotoxic function and infiltration of natural killer cells (NKs) and CD8+ T cells (Kitamura et al., 2015; Casbon et al., 2015). For example, it was recently shown that a rare sub-population of anti-tumor CD103+ dendritic cells (DCs), which can efficiently stimulate CD8+ T cells, is masked from tumor antigens by other tolerizing antigen-presenting myeloid cell populations (Broz et al., 2014). Numerous cell types recruited by chemokines and cytokines that are secreted by cancer cells contribute to this immunosuppression, which include tumor-associated macrophages (TAM), tumor-associated neutrophils (TAN), and a population functionally identified as myeloid-derived suppressor cells (MDSCs). TAMs secrete TGF-β, which recruits T regulatory cells (Tregs) that also participate in immunosuppression (Chanmee et al., 2014). MDSCs are a heterogeneous population of cells from monocytic and granulocytic origins, which secrete IL6, TGF-β and other cytokines and, among other functions, also recruit T helper 17 cells to promote their immunosuppressive function (Kitamura et al., 2015).
TAMs and TANs are derived from polarized macrophages and neutrophils respectively, which results in their pro-tumor phenotypes that facilitate tumor growth and stimulate angiogenesis (Lohela et al., 2014; Casbon et al., 2015). In addition, TAMs promote ECM breakdown, invasion, and metastasis (reviewed in Noy and Pollard, 2014; Kitamura et al., 2015). TAMs (and MSCs) can produce exosomes, enabling ingress of mRNAs and microRNAs (miRNAs) into various cell types (Ratajczak et al., 2006; Jing et al., 2012) for cancer cell growth and metastasis (Fabbri, 2012). Exosomes also facilitate multidrug resistance (MDR) in tumor cells via the transfer of efflux transporters (Jaiswal et al., 2013). Transformed epithelial cells often undergo epithelial-to-mesenchymal transition (EMT)-like alterations during which they lose their cellular polarity and cell-cell adhesion, and become mesenchymal-like or stem cell-like, gaining migratory and invasive properties (Kalluri and Weinberg, 2009; Karreth and Tuveson, 2004). In the inflammatory TME, TAMs and CD4+ T cells secrete TNFα which upregulates NF-κB signaling pathways to induce Slug, Snail and Twist and increase the crosstalk with the TGF-β signaling pathway which stimulates self-renewal (Smith et al., 2012; Cabarcas et al., 2011); thus, they can induce EMT and ultimately promote migration and invasion of CSCs. The correlation between stemness and EMT implies that non-CSCs can convert into CSCs through EMT induced plasticity.
Hypoxia and angiogenesis
Perturbed accessibility to vasculature results in hypoxia within various tumors. This advances stemness through activation of stem genes and dedifferentiation (Bennewith and Durand, 2004; Brurberg et al., 2006). Hypoxic CSCs impede CD8+ T cell proliferation and activation and inhibit immunosurveillance (Wei et al., 2011). Hypoxia also protects CSCs from chemo- and radiotherapy. Hypoxia further promotes CSC survival and EMT through reactive oxygen species (ROS)-activated stress response pathways (Liu et al., 2008) and through ROS-induced TGF-β and TNF-α signaling pathways (Pavlides et al., 2010). Activation of TGF-β as well as WNT signaling pathways by hypoxia induces stemness by promoting an undifferentiated state in tumor cells (Anido et al, 2010; Scheel et al., 2011). In various solid cancers, ECs promote self-renewal of CSCs by direct cell–cell contact or by nitric oxide (NO) production via the NOTCH signaling pathway (Charles et al., 2010). HIF-1α also can directly increase NOTCH signaling (Quail et al., 2012). HIF-1α antagonizes c-Myc activation, thus slowing down cell cycle progression to protect CSCs from DNA damage and enhance stemness (Koshiji et al., 2004).
Hypoxia induces CSCs to express hypoxia-inducible factors (HIFs), which are regulated and stabilized by TGF-β (Cabarcas et al., 2011). The HIF genes are the primary factors for driving angiogenesis via induction of VEGF. Under hypoxia, both ECs and CSCs produce VEGF to stimulate tumor angiogenesis. In the hypoxic regions of the tumor, VEGF-A can recruit monocytes and macrophages (Kitamura et al., 2015). A positive correlation between TAM infiltration and angiogenesis was found in many human cancers. TAMs become pro-angiogenic through their response to M-CSF (Lohela et al., 2014), secreted by tumor cells, which induces VEGF-A production and suppresses anti-angiogenic factor expression.
Extracellular matrix-cell interactions and cell-cell contact
The extracellular matrix (ECM) is an essential noncellular component of the adult stem cell niche. In solid tumors, increased ECM stiffness can be a physical barrier blocking therapeutics and thus protect CSCs from chemotherapeutic agents (Wong and Rustgi, 2013; Ye et al., 2014). Matrix metalloproteinases (MMPs) that degrade components of ECM in tumors, release cytokines, growth factors, and other molecules from the ECM and cell surface (Noel et al., 2012) and facilitate angiogenesis, tumor cell invasion, and metastasis (Siefert and Sarker, 2012; Kessenbrock et al., 2010). CAFs produce MMP2, 3, and 9 for ECM remodeling, which promotes EMT, enhances CSC-related marker expression and exacerbates therapeutic resistance (Cabarcas et al., 2011). Interestingly, MMPs can increase canonical WNT signaling and stemness (Kessenbrock et al., 2013). Increased MMP3 expression facilitates genomic instability, EMT and tumor formation as shown in a mouse model of breast cancer (Derek et al., 2005).
In normal stem cell niches, anchoring stem cells to the niche through cell-cell contacts is critical to keep them far from differentiation stimuli and physically adjacent to niche factors that specify self-renewal (Sneddon and Werb, 2007; Borovski et al., 2011). CSCs also utilize cell-cell contact to preserve their phenotype and exert their functions. For example, direct cell contact is necessary for MSCs to exert their maximal effect on CSCs (Roorda et al., 2010). Hedgehog and NOTCH signaling pathways (Gilbertson and Rich, 2007) require cell-cell contact. Notch ligands are mostly transmembrane proteins, particularly Jagged and Delta (Gilbertson and Rich, 2007). Glial cells in the brain may act as a cell–cell adhesion unit to tether glioma cells (Lin et al., 2002; Riquelme et al., 2008). In addition, to protect themselves from shear forces and NK cell-mediated lysis, and to improve their adhesion to endothelium, disseminated cancer cells surround themselves with platelets, forming a physical shield (Fessler et al., 2013). Lastly, although there is yet little evidence to support this, the development of cancer might suggest an enlargement or growth in the size of the niche to accommodate numerous CSCs (Shiozawa et al., 2011).
CSCs and Non-CSCs
As inferred above, also CSCs secrete a variety of factors that help recruit, activate and even create specific cell types to control the regulation of their differentiation states. Breast CSCs can produce IL6, which attracts and activates MSCs to produce the CSC-supportive cytokine CXCL7 (Liu et al., 2011). CSCs play an important role in TAM recruitment by secreting macrophage chemoattractants (Yi et al., 2012). CSCs promote angiogenesis through HIF-1α and the release of VEGF-A and CXCL12 (Ricci-Vitiani et al., 2010; Borovski et al., 2011). They help prevent ECs from undergoing hypoxia- or irradiation-induced apoptosis resulting in resistance to vascular disrupting agents (Takakura, 2012). CSCs can produce factors, such as TGF-β, to help transform fibroblasts to CAFs (Kalluri and Zeisberg, 2006). MMP10 is highly expressed in CSCs, correlating with metastasis in many human tumor types (Jaiswal et al., 2013). Its repression leads to a loss of stem cell-related gene expression. Tumor cells, which may not have CSC characteristics, also take part in the niche and secrete cytokines and exosomes (Fessler et al., 2013; Ye et al., 2014).
A bidirectional conversion between CSCs and non-CSCs can be triggered by an inflammatory stroma, which is characterized by elevated NF-κB signaling, enhancing Wnt activation and inducing dedifferentiation of non-CSCs that acquire tumor-initiating capacity (Schwitalla et al., 2013). Interestingly, it has been shown that tumors can be driven by a sub-population of non-CSCs. These cells that do not have higher fitness, but instead they stimulate growth of other tumor cells by inducing tumor-promoting microenvironmental changes. Conversely, the clonal expansion of this non-cell autonomous driver does not necessarily translate into increased tumor growth rates. This driver sub-clone can be outcompeted by a sub-clone with a higher proliferative yield, thus collapsing the tumor (Marusyk et al., 2014).
CSCs and metastasis: the primary tumor microenvironment and the metastatic niche
As summarized below, interactions of CSCs with their niches are also critical throughout metastatic progression.
CSCs and Metastatic CSCs
Although CSCs may not be the only cells instigating or maintaining metastasis, the CSC-generated hierarchy of stem-like and differentiated tumor cells is able to initiate metastatic growth and is also seen in late-stage cancers and at metastatic sites (Dalerba et al., 2011; Merlos-Suárezet al., 2011; Vermeulen et al., 2008). Large-scale genome sequencing studies suggest that primary tumors accumulate most of the mutations vital to metastasis, showing a predominance of similarity between metastatic stem cells (MetSCs) and primary CSCs (Yachida et al., 2010). Gene expression signatures have identified mediators of metastatic mutations in primary tumors (as stem cell markers) that correlate with poor prognosis and relapse (Oskarsson et al., 2014). Cancer cells expressing stem cell markers have been detected in the blood of breast cancer patients; when inoculated into immunodeficient mice these cells can generate bone, liver, and lung metastases (Baccelli et al., 2013). In addition, analysis of human colorectal cancer samples using clonal lentiviral marking demonstrates that metastases arise from primary tumor cells that display long-term self-renewal capacity and are quiescent and resistant to chemotherapy (Dieter et al., 2011; Kreso et al., 2013). Even cancers, such as melanoma, that do not appear to rely on a hierarchical organization, still contain MetSCs (Meacham and Morrison, 2013). Although there is some evidence suggesting that primary tumors and metastases may arise from different cells (LaBarge, 2010), it could be that MetSCs simply develop from the original CSCs that evolved throughout tumor progression due to tumor cell plasticity or generation of MetSCs. MetSCs may be generated de novo as a result of de novo niche formation due to competition between cancer and normal stem cells for niche occupancy (Shiozawa et al., 2011). If MetSCs or disseminated tumor cells (DTCs) are primary CSCs, many of the CSC niche considerations will also apply to MetSCs.
The TME supports cancer cell dissemination
Beyond the passive role of circulation patterns, cancer cell dissemination is actively influenced by cancer cell autonomous functions such as invadopodia formation, paracrine factors as VEGF and EGF family members, proteases as MMPs and cathepsins and recruitment of stromal components and immunosuppressive cells as TAMs (Oskarsson et al., 2014). The tumor invasive front is a likely site for selection of metastatic traits (Cheung et al., 2013). This site is rich with blood vessels as well as niche cells and factors that support the survival and fitness of CSCs (Joyce and Pollard, 2009; Takebe et al., 2011) (Fig. 2). Primary tumor stroma also select for organ-specific seeding traits by releasing exosomes that alter niche content. In the circulation, transient contact between platelets and DTCs induces EMT and a CSC-like state (Fessler, 2013). TIE2+ macrophages lining the vasculature direct cancer cell migration along collagen fibers toward higher concentrations of metastasized cells. Clusters of tumor cells in blood vessels secrete EGF family members, further directing cancer cells and macrophages to sites of metastasis (Noy and Pollard, 2014) (Fig. 3).
The metastatic niche supports seeding and growth of metastasis
Circulating tumor cells need the right “soil” in which to seed and survive, since most metastatic sites are less hospitable than the origin (Fig. 3). The survival and fitness of metastasis-initiating DTCs depends on specific components of the host environment that play the part of a niche for these cells, as inferred by massive CSC loss/apoptosis and failure to form macrometastases in colorectal and breast CSCs. Although no foreign tissue may be welcoming to metastatic seeds, certain tissues may be less hostile than others. Similar to the CSC niche, the metastatic niche designates the specific locations, stromal cell types, diffusible signals, and ECM proteins that bear consequences for the metastasis of DTCs (Oskarsson et al., 2014). Beyond cell-autonomous failures, the inability to metastasize results from scarcity of survival signals in the host parenchyma, lack of a supportive stroma, and overexposure to innate immunity (Chambers et al., 2002; Fidler, 2003; Nguyen et al., 2009; Schreiber et al., 2011).
Interestingly, the traits required for metastatic dissemination are distinct from those that mediate overt metastatic colonization months or years later. Dormancy is a critical issue for tumor recurrence and metastatic spread after long lag periods in many cancers, including breast, melanoma, and leukemia (Pece et al. 2010; Roesch et al., 2010; Saito et al., 2010). Since dormant cells are proliferatively quiescent, they survive chemotherapy and contribute to tumor regrowth, irrespective of genetic differences. Therefore, understanding the role of the microenvironment in regulating exit from dormancy is of crucial importance. The mechanisms of tumor dormancy and the ability of CSCs to remain quiescent are intertwined with angiogenic dormancy (Cabarcas, 2011). Restricted supplies of nutrients and oxygen due to poor vascularization cause an arrest in growth (Almog, 2010), which can also potentially result from the absence of necessary factors required by CSCs to reinitiate tumor formation or metastasis. Although angiogenic stimulators such as Myc, VEGF, and FGF-2 (Shachaf et al., 2004; Naumov et al., 2006) may play a role in mediating tumor exit from dormancy, still little is known about entering and exiting dormancy and it remains an overarching challenge for successfully combating many cancers.
Although DTCs in bone marrow appear dormant, the overall DTC population is not static (Muller et al., 2005; Pantel et al., 1993). DTCs may constantly transition between dormant and active states during metastatic latency, being further selected for colonization functionality. Circulating metastatic cells co-express EMT and stem markers (Plaks et al., 2013). Although EMT enables migration, it interferes with proliferation and metastatic growth (Ocana et al., 2012; Stankic et al., 2013). Thus, MetSCs that have undergone EMT may need to reacquire an epithelial phenotype to seed and resume growth at the metastatic site. This reverse process is called mesenchymal-to-epithelial transition (MET) (Tsai et al., 2012; Ocana et al., 2012; Gupta et al., 2007). TGF-β causes EMT before extravasation, but MET after extravasation by a yet unknown mechanism. Despite the clinical importance of metastatic latency, mouse models lack a prolonged dormancy of MetSCs and xenograft assays may either restrict CSC detection to only the most robustly proliferating cells (Quintana et al., 2008), since they are read within months after transplantation, or activate dormant cells by serial transplantation. Therefore, little is known about entering and exiting dormancy, forms of dormancy and signaling during dormancy, so better models are needed (Kreso et al., 2013).
Metastatic seeding occurs in a variety of niches
DTCs may occupy normal stem cell niches in the host tissues (Fig. 3). MSCs produce TGF-β family molecules, CXCL12 and Hedgehog signals in the bone marrow for hematopoietic stem cell maintenance while metastatic cancer cells from other sites occupy this niche to benefit from cues that enhance stem cell properties and deter differentiation (Shiozawa et al., 2011). The cognate chemokine receptor CXCR4 is frequently overexpressed in bone metastatic cells and provides CSCs with chemotaxis and PI3K-mediated survival signals that mediate oncogenic transformation (Muller et al., 2001; Zlotnik et al., 2011).
DTCs initiate metastatic outgrowth around blood capillaries, in perivascular niches (Fig. 3). These may support MetSCs by supplying attachment, oxygen, nutrients and paracrine factors from the activated endothelium (Butler et al., 2010; Fessler et al., 2013). The perivascular niche is a preferred residence for glioma CSCs that supplies them with Hedgehog-, NOTCH-, and PI3K-activating signals. Breast cancer, lung cancer, and melanoma cells that infiltrate the brain surround capillaries and some stretch themselves over the perivascular basal lamina (Charles and Holland, 2010; Hambardzumyan et al., 2008).
DTCs seed metastasis in distant tissue niches (Fig. 3). In mouse models, breast, lung, and gastrointestinal tumors establish premetastatic niches by secreting systemic factors such as VEGF-A, TGF-β, G-CSF, tumor necrosis factor (TNF) and lysyl oxidase (LOX) that induce expression of chemotactic proteins (S100A8, S100A9 and serum amyloid A3 (SAA3)), ECM-remodeling enzymes and exosomes into the circulation and directs various cells to induce pro-metastatic changes in the lung parenchyma microenvironment before DTCs arrive (Oskarsson, 2014; Kaplan et al., 2005; Hiratsuka et al., 2006; Casbon et al., 2015). Primary tumors induce recruitment and mobilization of VEGFR1+ bone marrow–derived hematopoietic progenitor cells (HPCs) before the arrival of tumor cells (Kaplan et al., 2005). Pre-existing fibroblasts increase fibronectin deposition in these sites, which binds and clusters HPCs, and fibroblasts induce remodeling of stroma (Olaso et al., 1997). Macrophages, activated neutrophils and Tregs are also recruited to the niche to promote future metastasis. Neutrophils also potentially enhance MetSC settlement by producing neutrophil extracellular traps (NETs) (Casbon et al., 2015; Cools-Lartigue et al., 2013; Kitamura et al., 2015). The metastatic niches are populated by Gr1+ CD11b+ myeloid cells recruited by LOX and S100A proteins (Erler, 2009; Psaila and Lyden, 2009; Yan et al., 2010). However, direct evidence showing a pro-metastatic role for these myeloid cells through immunosuppression is lacking, even though CD11b+Gr1+ and CD11b+Ly6G+ cells promote metastatic processes (Yang et al., 2010; Casbon et al., 2015). The ECM component tenascin C (TNC) is found in stem cell niches, frequently supplied by CAFs and associated with increased risk of metastasis (Oskarsson et al., 2011). TNC regulates Musashi and other factors to enhance NOTCH and WNT signaling to support CSCs.
Once metastatic cells arrive, they continue to remodel their microenvironment. Breast CSCs induce the expression of the ECM molecule periostin in lung fibroblasts that binds WNT ligands to help maintain stemness of arriving CSCs. As metastatic lesions grow, the cancer cells recruit TAMs, myeloid precursors, and mesenchymal cells that establish paracrine loops feeding back to the cancer cells with various survival and self-renewal factors (Kitamura et al., 2015). In osteolytic bone metastasis of breast cancer, osteoclasts resorb bone matrix, to make room for the metastatic growth and release TGF-β and other growth factors. These factors stimulate cancer cells in a feed-forward cycle of tissue destruction and metastatic expansion (Ell and Kang, 2012; Weilbaecher et al., 2011). The metastatic cells also trigger angiogenesis, and the newly forming blood vessels attract more MetSCs by expressing fibronectin and VCAM (Fessler et al., 2013). These MetSCs produce CCL2 and attract CCR2+ inflammatory moncytes that become metastatic associated macrophages and support metastatic growth (Kitamura et al., 2015).
Interestingly, in models of brain metastasis from breast and lung cancers, brain stroma takes an active role in killing the infiltrating cancer cells (Valiente et al., 2014). However, little is known about what kills the majority of DTCs. More information on how the reactive stroma repels DTCs could yield clues for how to leverage these mechanisms for therapeutic benefit.
The stem cell niche as a target for cancer therapy
Generally, CSCs appear to be resistant to conventional cancer therapies such as ionizing radiation and conventional anti-proliferative chemotherapy due to their quiescence (Bao et al., 2006; Li et al., 2008). On the other hand, CSCs can be more sensitive to some therapies as compared to non-tumorigenic cells. Rapamycin treatment in a mouse model of leukemia induced by conditional Pten deletion in haematopoietic cells causes the depletion of leukemia-initiating cells and restores normal hematopoietic stem cell function. Although the histological evidence of leukemia persisted, the mice were overtly healthy (Yilmaz et al., 2006). Radiation or cisplatin therapy may preferably target the undifferentiated cells that drive testicular germ cell tumors (Clevers, 2011). Differentiation therapies that specifically target CSCs by exploiting their capacity to differentiate can be effective in some cases (Meacham and Morrison, 2013). This strategy is successful in inducing cell cycle progression in acute myeloid leukemia stem cells by supplying G-CSF to promote sensitivity to chemotherapy (Saito et al., 2010). Similarly, mouse glioblastoma stem cells can be induced to differentiate into glia by treatment with the protein BMP4, resulting in reduced proliferation, tumor growth and tumor-initiation capability of CSCs upon transplantation (Lombardo et al., 2011; Piccirillo et al., 2006).
Tumor cell plasticity presents a huge challenge to the development of targeted cancer therapies, as tumor cell populations are continually evolving and therapeutic eradication of existing CSC populations might be followed by their regeneration from non-CSCs within the tumor under treatment (Chaffer and Weinberg, 2015). In addition, most stem cell markers used to date are not good targets for antibody therapy. Moreover, many of these markers, especially in solid tumors, fail to distinguish normal stem cells from CSCs. High-throughput screening could be an unbiased approach to uncover known or new compounds that specifically target CSCs (Clevers, 2011).
An alternative strategy: targeting the unique aberrant microenvironment of CSCs
Since the TME has the potential to support and initiate stem cell-like programs in cancer cells, targeting CSC niche factors that regulate plasticity may prove to be a more powerful modality for the treatment and prevention of tumor cell plasticity and progression than targeting the CSCs directly. However, it should equally be taken into account that in a particular cancer type/stage, CSCs may evolve to escape niche constraints and become independent of niches. Therefore, targeting the niche may be a critical aspect of effective cancer therapy in systems where the aberrant activation of the pathway that is about to be targeted is regulating CSCs at the cell surface level rather than a cell-autonomous mutation, which provides independence from growth factors or abolishes an apoptotic response to drive clonal expansions (Clevers, 2011). In cases where tumor progression is limited by microenvironmental constraints that cannot be overcome by a cell-autonomous increase in proliferation rates, it is possible that these secreted factors not only preferentially benefit the CSCs, enabling their clonal dominance but also actually mediate inter-clonal interactions that could also be drivers of the tumor (Marusyk et al., 2012). Overall, it seems that the niche has a differential importance depending on the cancer type and even on the specific stage of that particular cancer. Experimental analysis and clinical diagnostics still need to take place in order to elucidate such mechanisms in various cancers.
Some attempts to target the niche has already show promise. Antibodies that abrogate the activation of c-Met by HGF significantly inhibit xenograft growth of colon tumors (Hoey et al., 2009). Fibronectin and hyaluronic acid facilitate a quiescent state in some cancer cells when they are under siege from chemotherapy. Indeed, antibodies against the fibronectin receptor α4β1 integrin prevent association of tumor cells with metastatic niches (Kaplan et al., 2005). Targeting MMPs is likely to be more effective in early-stage tumors that are more dependent on their activity than late-stage, established tumors and the effect on CSCs should be investigated (Kessenbrock et al., 2010). Targeting hypoxia is another attempt to manipulate a niche of quiescent, drug resistant cells. HIF-1α and HIF-2α, which promote cell cycle via c-Myc represent a promising target for therapy for glioma patients (Gordan et al., 2007; Li et al., 2009). Various angiogenic inhibitors have shown positive results in various cancers. Anti-angiogenic therapy targeting VEGF can deplete the tumor vasculature and ablate self-renewing CSCs (Ye et al., 2014) thus inhibiting tumor growth. Interfering with tumor EC growth and survival could inhibit not only angiogenesis but also the self-replication of CSCs (Gu et al., 2012).
A successful approach in combating tumors is targeting immune checkpoints by either blocking immunosuppressive mechanisms to restore T-cell function (such as PD1 and its ligand PDL1) or enhance immune function by engaging co-stimulatory receptors such as OX40 with agonist antibodies. Most successful is the use of a monoclonal antibody targeting the negative immune checkpoint protein CTLA-4 (Juntilla and de Sauvage, 2013). Other technologies that are currently in clinical development attempt to directly engage T-cell-mediated killing. Adoptive cell-transfer (ACT) therapy, which involves the ex vivo expansion and reinfusion of tumor-reactive T cells, is emerging as a potential curative treatment for patients with advanced-stage cancer (Klebanoff et al., 2012). Overall, immunotherapy is an emerging field and the exact mechanism by which these therapies may abrogate the ability of CSCs to reinitiate tumors is still under investigation.
Combinatorial treatment with conventional cancer therapies may be an effective strategy. IFN-γ shows synergistic effects with the conventional anticancer drug oxaliplatin to eliminate both CSCs and differentiated cancer cells in colorectal cancer (Ni and Huang, 2013). Depletion of TAMs or inflammatory monocytes by inhibiting either CCR2 or M-CSF receptor resulted in decreased CSCs in pancreatic tumors, improved chemotherapeutic efficacy, inhibited metastasis, and increased antitumor T-cell responses (Mitchem et al., 2013). Targeting components of the innate immune system along with conventional therapy is also under clinical evaluation. For example, the anti-CD40 agonist antibody and gemcitabine combination therapy has shown early clinical promise in treating pancreatic cancer (Juntilla and de Sauvage, 2013). Targeting the bulk of the tumor with standard cancer therapy could help remodel the CSCs niche, exposing crucial niche component(s) and making it more receptive to niche-targeted therapeutics. For example, using conventional cancer therapeutics to expose anti-tumor DCs to antigens that are otherwise inaccessible to them (Broz et al., 2014) with a combination of immunotherapy using engineered DCs with enhance ability to stimulate T-cell-mediated tumor rejection could potentially be a successful strategy to eradicate CSCs.
Concluding remarks
It is now accepted that most cancers originate from cells that gained tumor-initiating capacity and that these cells are plastic in nature. The tumor-initiating capacity or cancer stemness of these cells could therefore be influenced by extrinsic factors. It is also postulated that in many cancers the TME and especially the closely related niches have detrimental effects on the ability of these cells to initiate a tumor and/or metastasize. Due to their plasticity and given that CSCs need to be eradicated to prevent malignancy and metastasis, targeting specific niche components relevant to that particular cancer type in addition to standard cancer therapy which tackles the bulk of the tumor bears therapeutic promise. A better understanding of CSC biology and niche factors of each cancer subtype as well as their modulation using various therapeutic designs is paramount for this paradigm to be fully applicable in the clinic.
Supplementary Material
Acknowledgments
We would like to thank Renske van Den Bijgaart for help with designing the figures. This study was supported by funds from the National Cancer Institute to ZW (CA057621 and CA180039) and by a Department of Defense postdoctoral fellowship to VP (W81XWH-11-01-0139). Given the length constraints, we were not able to cite all the papers that are relevant to this topic. We apologize to the scientists whose papers were left out.
Footnotes
Author Contributions
V.P. conceived the ideas and figures and wrote the manuscript. N.K. summarized the relevant literature, and designed and produced figures. Z.W. guided the overall approach and trajectory of this piece and edited the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci USA. 2003;100:3983–3988. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexandrov LB, Nik-Zainal S, Wedge DC, Aparicio SA, Behjati S, Biankin AV, Bignell GR, Bolli N, Borg A, Børresen-Dale AL, et al. Signatures of mutational processes in human cancer. Nature. 2013;500:415–421. doi: 10.1038/nature12477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almog N. Molecular mechanisms underlying tumor dormancy. Cancer Lett. 2010;294:139–46. doi: 10.1016/j.canlet.2010.03.004. [DOI] [PubMed] [Google Scholar]
- Anido J, Saez-Borderias A, Gonzalez-Junca A, Rodon L, Folch G, Carmona MA, Prieto-Sanchez RM, Barba I, Martinez-Saez E, Prudkin L, et al. TGF-β Receptor Inhibitors Target the CD44high/Id1high Glioma-Initiating Cell Population in Human Glioblastoma. Cancer Cell. 2010;18:655–668. doi: 10.1016/j.ccr.2010.10.023. [DOI] [PubMed] [Google Scholar]
- Baccelli I, Schneeweiss A, Riethdorf S, Stenzinger A, Schillert A, Vogel V, Klein C, Saini M, Bauerle T, Wallwiener M, et al. Identification of a population of blood circulating tumor cells from breast cancer patients that initiates metastasis in a xenograft assay. Nat Biotechnol. 2013;31:539–544. doi: 10.1038/nbt.2576. [DOI] [PubMed] [Google Scholar]
- Bao S, Wu Q, McLendon RE, Hao Y, Shi Q, Hjelmeland AB, Dewhirst MW, Bigner DD, Rich JN. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444:756–760. doi: 10.1038/nature05236. [DOI] [PubMed] [Google Scholar]
- Bennewith KL, Durand RE. Quantifying transient hypoxia in human tumor xenografts by flow cytometry. Cancer Res. 2004;64:6183–6189. doi: 10.1158/0008-5472.CAN-04-0289. [DOI] [PubMed] [Google Scholar]
- Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nature Medicine. 1997;3:730–737. doi: 10.1038/nm0797-730. [DOI] [PubMed] [Google Scholar]
- Borovski T, Sousa, De Sousa E, Melo F, Vermeulen L, Hanahan D, Medema JP. Cancer stem cell niche: the place to be. Cancer Res. 2011;71:634–639. doi: 10.1158/0008-5472.CAN-10-3220. [DOI] [PubMed] [Google Scholar]
- Broz ML, Binnewies M, Boldajipour B, Nelson AE, Pollack JL, Erle DJ, Barczak A, Rosenblum MD, Daud A, Barber DL, et al. Dissecting the tumor myeloid compartment reveals rare activating antigen-presenting cells critical for T cell immunity. Cancer Cell. 2014;26:638–652. doi: 10.1016/j.ccell.2014.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brurberg KG, Thuen M, Ruud EB, Rofstad EK. Fluctuations in pO 2 in irradiated human melanoma xenografts. Radiat Res. 2006;165:16–25. doi: 10.1667/rr3491.1. [DOI] [PubMed] [Google Scholar]
- Burrell RA, McGranahan N, Bartek J, Swanton C. The causes and consequences of genetic heterogeneity in cancer evolution. Nature. 2013;501:338–345. doi: 10.1038/nature12625. [DOI] [PubMed] [Google Scholar]
- Butler JM, Kobayashi H, Rafii S. Instructive role of the vascular niche in promoting tumour growth and tissue repair by angiocrine factors. Nat Rev Cancer. 2010;10:138–146. doi: 10.1038/nrc2791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabarcas SM, Mathews LA, Farrar WL. The cancer stem cell niche—there goes the neighborhood? Int J Cancer. 2011;129:2315–2327. doi: 10.1002/ijc.26312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casbon A-J, Reynaud D, Park C, Khuc E, Gan DD, Schepers K, Passegué E, Werb Z. Tumors reprogram early myeloid differentiation in the bone marrow to generate immunosuppresive neutrophils. Proc Natl Acad Sci USA. 2015;112:E566–E575. doi: 10.1073/pnas.1424927112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaffer CL, Weinberg RA. How Does Multistep Tumorigenesis Really Proceed? Cancer Discov. 2015;5:22–24. doi: 10.1158/2159-8290.CD-14-0788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers AF, Groom AC, MacDonald IC. Dissemination and growth of cancer cells in metastatic sites. Nat Rev Cancer. 2002;2:563–572. doi: 10.1038/nrc865. [DOI] [PubMed] [Google Scholar]
- Chanmee T, Ontong P, Konno K, Itano N. Tumor-associated macrophages as major players in the tumor microenvironment. Cancers (Basel) 2014;6:1670–90. doi: 10.3390/cancers6031670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charles N, Holland EC. The perivascular niche microenvironment in brain tumor progression. Cell Cycle. 2010;9:3012–3021. doi: 10.4161/cc.9.15.12710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charles N, Ozawa T, Squatrito M, Bleau AM, Brennan CW, Hambardzumyan D, Holland EC. Perivascular Nitric Oxide Activates Notch Signaling and Promotes Stem-like Character in PDGF-Induced Glioma Cells. Cell Stem Cell. 2010;6:141–152. doi: 10.1016/j.stem.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung KJ, Gabrielson E, Werb Z, Ewald AJ. Collective invasion in breast cancer requires a conserved basal epithelial program. Cell. 2013;155:1639–1651. doi: 10.1016/j.cell.2013.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke MF, Dick JE, Dirks PB, Eaves CJ, Jamieson CH, Jones DL, Visvader J, Weissman IL, Wahl GM. Cancer stem cells—perpectives on current status and future directions: AACR Workshop on cancer stem cells. Cancer Res. 2006;66:9339–9344. doi: 10.1158/0008-5472.CAN-06-3126. [DOI] [PubMed] [Google Scholar]
- Clevers H. The cancer stem cell: premises, promises, and challenges. Nat Med. 2011;17:313–319. doi: 10.1038/nm.2304. [DOI] [PubMed] [Google Scholar]
- Cools-Lartigue J, Spicer J, McDonald B, Gowing S, Chow S, Giannias B, Bourdeau F, Kubes P, Ferri L. Neutrophil extracellular traps sequester circulating tumor cells and promote metastasis. J Clin Invest. 2013;123:3446–3458. doi: 10.1172/JCI67484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuiffo BG, Campagne A, Bell GW, Lembo A, Orso F, Lien EC, Bhasin MK, Raimo M, Hanson SE, Marusyk A, et al. MSC-Regulated MicroRNAs Converge on the Transcription Factor FOXP2 and Promote Breast Cancer Metastasis. Cell Stem Cell. 2014;15:762–774. doi: 10.1016/j.stem.2014.10.001. [DOI] [PubMed] [Google Scholar]
- Cuiffo BG, Karnoub AE. Mesenchymal stem cells in tumor development: emerging roles and concepts. Cell Adhes Migr. 2012;6:220–230. doi: 10.4161/cam.20875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalerba P, Kalisky T, Sahoo D, Rajendran PS, Rothenberg ME, Leyrat AA, Sim S, Okamoto J, Johnston DM, Qian D, et al. Single-cell dissection of transcriptional heterogeneity in human colon tumors. Nat Biotechnol. 2011;29:1120–1127. doi: 10.1038/nbt.2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis H, Irshad S, Bansal M, Rafferty H, Boitsova T, Bardella C, Jaeger E, Lewis A, Freeman-Mills L, Giner FC, et al. Aberrant epithelial GREM1 expression initiates colonic tumorigenesis from cells outside the stem cell niche. Nat Med. 2015;21:62–70. doi: 10.1038/nm.3750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieter SM, Ball CR, Hoffmann CM, Nowrouzi A, Herbst F, Zavidij O, Abel U, Arens A, Weichert W, Brand K, et al. Distinct types of tumor-initiating cells form human colon cancer tumors and metastases. Cell Stem Cell. 2011;9:357–365. doi: 10.1016/j.stem.2011.08.010. [DOI] [PubMed] [Google Scholar]
- Ell B, Kang Y. SnapShot: Bone metastasis. Cell. 2012;151:690–690.e1. doi: 10.1016/j.cell.2012.10.005. [DOI] [PubMed] [Google Scholar]
- Erler JT, Bennewith KL, Cox TR, Lang G, Bird D, Koong A, Le QT, Giaccia AJ. Hypoxia-induced lysyl oxidase is a critical mediator of bone marrow cell recruitment to form the premetastatic niche. Cancer Cell. 2009;15:35–44. doi: 10.1016/j.ccr.2008.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabbri M. TLRs as miRNA receptors. Cancer Res. 2012;72:6333–6337. doi: 10.1158/0008-5472.CAN-12-3229. [DOI] [PubMed] [Google Scholar]
- Fessler E, Dijkgraaf FE, Melo EFS, Medema JP. Cancer stem cell dynamics in tumor progression and metastasis: Is the microenvironment to blame? Cancer Lett. 2013;341:97–104. doi: 10.1016/j.canlet.2012.10.015. [DOI] [PubMed] [Google Scholar]
- Fidler IJ. The pathogenesis of cancer metastasis: the ‘seed and soil’ hypothesis revisited. Nat Rev Cancer. 2003;3:453–458. doi: 10.1038/nrc1098. [DOI] [PubMed] [Google Scholar]
- Gerlinger M, Rowan AJ, Horswell S, Larkin J, Endesfelder D, Gronroos E, Martinez P, Matthews N, Stewart A, Tarpey P, et al. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N Engl J Med. 2012;366:883–892. doi: 10.1056/NEJMoa1113205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbertson RJ, Rich JN. Making a tumour’s bed: glioblastoma stem cells and the vascular niche. Nat Rev Cancer. 2007;7:733–736. doi: 10.1038/nrc2246. [DOI] [PubMed] [Google Scholar]
- Goldman JM, Green AR, Holyoake T, Jamieson C, Mesa R, Mughal T, Pellicano F, Perrotti D, Skoda R, Vannucchi AM. Chronic myeloproliferative diseases with and without the Ph chromosome: some unresolved issues. Leukemia. 2009;23:1708–1715. doi: 10.1038/leu.2009.142. [DOI] [PubMed] [Google Scholar]
- Goldstein A, Huang J, Guo C, Garraway IP, Witte ON. Identification of a cell-of-origin for human prostate cancer. Science. 2010;329:568–571. doi: 10.1126/science.1189992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordan JD, Bertout JA, Hu CJ, Diehl JA, Simon MC. HIF-2alpha promotes hypoxic cell proliferation by enhancing c-myc transcriptional activity. Cancer Cell. 2007;11:335–347. doi: 10.1016/j.ccr.2007.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greaves M. Cancer stem cells as ‘units of selection’. Evol Appl. 2013;6:102–108. doi: 10.1111/eva.12017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greaves M, Maley CC. Clonal evolution in cancer. Nature. 2012;481:306–313. doi: 10.1038/nature10762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu JW, Rizzo P, Pannuti A, Golde T, Osborne B, Miele L. Notch signals in the endothelium and cancer “stem-like” cells: opportunities for cancer therapy. Vasc Cell. 2012;4:7. doi: 10.1186/2045-824X-4-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta PB, Fillmore CM, Jiang G, Shapira SD, Tao K, Kuperwasser C, Lander ES. Stochastic state transitions give rise to phenotypic equilibrium in populations of cancer cells. Cell. 2011;146:633–644. doi: 10.1016/j.cell.2011.07.026. [DOI] [PubMed] [Google Scholar]
- Hahn WC, Counter CM, Lundberg AS, Beijersbergen RL, Brooks MW, Weinberg RA. Creation of human tumour cells with defined genetic elements. Nature. 1999;400:464–468. doi: 10.1038/22780. [DOI] [PubMed] [Google Scholar]
- Hambardzumyan D, Becher OJ, Holland EC. Cancer stem cells and survival pathways. Cell Cycle. 2008;7:1371–1378. doi: 10.4161/cc.7.10.5954. [DOI] [PubMed] [Google Scholar]
- Hanahan D, Coussens LM. Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell. 2012;21:309–322. doi: 10.1016/j.ccr.2012.02.022. [DOI] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- He XC, Zhang J, Tong WG, Tawfik O, Ross J, Scoville DH, Tian Q, Zeng X, He X, Wiedemann LM, et al. BMP signaling inhibits intestinal stem cell self-renewal through suppression of Wnt-beta-catenin signaling. Nat Genet. 2004;36:1117–1121. doi: 10.1038/ng1430. [DOI] [PubMed] [Google Scholar]
- Hill RP, Perris R. Destemming” cancer stem cells. J Natl Cancer Inst. 2007;99:1435–1440. doi: 10.1093/jnci/djm136. [DOI] [PubMed] [Google Scholar]
- Hiratsuka S, Watanabe A, Aburatani H, Maru Y. Tumour-mediated upregulation of chemoattractants and recruitment of myeloid cells predetermines lung metastasis. Nat Cell Biol. 2006;8:1369–1375. doi: 10.1038/ncb1507. [DOI] [PubMed] [Google Scholar]
- Hoey T, Yen WC, Axelrod F, Basi J, Donigian L, Dylla S, Fitch-Bruhns M, Lazetic S, Park IK, Sato A, et al. DLL4 blockade inhibits tumor growth and reduces tumor-initiating cell frequency. Cell Stem Cell. 2009;5:168–177. doi: 10.1016/j.stem.2009.05.019. [DOI] [PubMed] [Google Scholar]
- Hong H, Takahashi K, Ichisaka T, Aoi T, Kanagawa O, Nakagawa M, Okita K, Yamanaka S. Suppression of induced pluripotent stem cell generation by the p53-p21 pathway. Nature. 2009;460:1132–1135. doi: 10.1038/nature08235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaiswal R, Luk F, Dalla PV, Grau GE, Bebawy M. Breast cancer-derived microparticles display tissue selectivity in the transfer of resistance proteins to cells. PLoS One. 2013;8:e61515. doi: 10.1371/journal.pone.0061515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing Y, Han Z, Liu Y, Sun K, Zhang S, Jiang G, Li R, Gao L, Zhao X, Wu D, et al. Mesenchymal stem cells in inflammation microenvironment accelerates hepatocellular carcinoma metastasis by inducing epithelial–mesenchymal transition. PLoS One. 2012;7:e43272. doi: 10.1371/journal.pone.0043272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyce JA, Pollard JW. Microenvironmental regulation of metastasis. Nat Rev Cancer. 2009;9:239–252. doi: 10.1038/nrc2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junttila MR, de Sauvage FJ. Influence of tumour micro-environment heterogeneity on therapeutic response. Nature. 2013;501:346–354. doi: 10.1038/nature12626. [DOI] [PubMed] [Google Scholar]
- Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119:1420–1428. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalluri R, Zeisberg M. Fibroblasts in cancer. Nat Rev Cancer. 2006;6:392–401. doi: 10.1038/nrc1877. [DOI] [PubMed] [Google Scholar]
- Kandoth C, McLellan MD, Vandin F, Ye K, Niu B, Lu C, Xie M, Zhang Q, McMichael JF, Wyczalkowski MA, et al. Mutational landscape and significance across 12 major cancer types. Nature. 2013;502:333–339. doi: 10.1038/nature12634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan RN, Riba RD, Zacharoulis S, Bramley AH, Vincent L, Costa C, MacDonald DD, Jin DK, Shido K, Kerns SA, et al. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature. 2005;438:820–827. doi: 10.1038/nature04186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karnoub AE, Dash AB, Vo AP, Sullivan A, Brooks MW, Bell GW, Richardson AL, Polyak K, Tubo R, Weinberg RA. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature. 2007;449:557–563. doi: 10.1038/nature06188. [DOI] [PubMed] [Google Scholar]
- Karreth F, Tuveson DA. Twist induces an epithelial-mesenchymal transition to facilitate tumor metastasis. Cancer Biol Ther. 2004;3:1058–1059. doi: 10.4161/cbt.3.11.1302. [DOI] [PubMed] [Google Scholar]
- Kerkar SP, Restifo NP. Cellular Constituents of Immune Escape within the Tumor Microenvironment. Cancer Res. 2012;72:3125. doi: 10.1158/0008-5472.CAN-11-4094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessenbrock K, Dijkgraaf GJP, Lawson DA, Littlepage LE, Shahi P, Pieper U, Werb Z. A role for matrix metalloproteinases in regulating mammary stem cell function via the Wnt signaling pathway. Cell Stem Cell. 2013;13:300–313. doi: 10.1016/j.stem.2013.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessenbrock K, Plaks V, Werb Z. Matrix metalloproteinases: Regulators of the tumor microenvironment. Cell. 2010;141:52–67. doi: 10.1016/j.cell.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura T, Qian B-Z, Pollard JW. Immune cell promotion of metastasis. Nat Rev Immunol. 2015;15:73–86. doi: 10.1038/nri3789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klebanoff CA, Gattinoni L, Restifo NP. Sorting through subsets: which T-cell populations mediate highly effective adoptive immunotherapy? J Immunother. 2012;35:651–660. doi: 10.1097/CJI.0b013e31827806e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korkaya H, Liu S, Wicha MS. Breast cancer stem cells, cytokine networks, and the tumor microenvironment. J Clin Invest. 2011;121:3804–3809. doi: 10.1172/JCI57099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshiji M, Kageyama Y, Pete EA, Horikawa I, Barrett JC, Huang LE. HIF-1alpha induces cell cycle arrest by functionally counter-acting Myc. EMBO J. 2004;23:1949–56. doi: 10.1038/sj.emboj.7600196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreso A, Dick JE. Evolution of the cancer stem cell model. Cell Stem Cell. 2014;14:275–291. doi: 10.1016/j.stem.2014.02.006. [DOI] [PubMed] [Google Scholar]
- Kreso A, O’Brien CA, van Galen P, Gan OI, Notta F, Brown AM, Ng K, Ma J, Wienholds E, Dunant C, et al. Variable clonal repopulation dynamics influence chemotherapy response in colorectal cancer. Science. 2013;339:543–548. doi: 10.1126/science.1227670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaBarge MA. The difficulty of targeting cancer stem cell niches. Clin Cancer Res. 2010;16:3121–3129. doi: 10.1158/1078-0432.CCR-09-2933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence MS, Stojanov P, Polak P, Kryukov GV, Cibulskis K, Sivachenko A, Carter SL, Stewart C, Mermel CH, Roberts SA, et al. Mutational heterogeneity in cancer and the search for new cancer-associated genes. Nature. 2013;499:214–218. doi: 10.1038/nature12213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Lewis MT, Huang J, Gutierrez C, Osborne CK, Wu M-F, Hilsenbeck SG, Pavlick A, Zhang X, Chamness GC, et al. Intrinsic resistance of tumorigenic breast cancer cells to chemotherapy. J Natl Cancer Inst. 2008;100:672–679. doi: 10.1093/jnci/djn123. [DOI] [PubMed] [Google Scholar]
- Li Z, Bao S, Wu Q, Wang H, Eyler C, Sathornsumetee S, Shi Q, Cao Y, Lathia J, McLendon RE, et al. Hypoxia-inducible factors regulate tumorigenic capacity of glioma stem cells. Cancer Cell. 2009;15:501–513. doi: 10.1016/j.ccr.2009.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim E, Vaillant F, Wu D, Forrest NC, Pal B, Hart AH, Asselin-Labat ML, Gyorki DE, Ward T, Partanen A, et al. Aberrant luminal progenitors as the candidate target population for basal tumor development in BRCA1 mutation carriers. Nat Med. 2009;15:907–913. doi: 10.1038/nm.2000. [DOI] [PubMed] [Google Scholar]
- Lin JH, Takano T, Cotrina ML, Arcuino G, Kang J, Liu S, Gao Q, Jiang L, Li F, Lichtenberg-Frate H. Connexin 43 Enhances the Adhesivity and Mediates the Invasion of Malignant Glioma Cells. J Neurosci. 2002;22:4302–4311. doi: 10.1523/JNEUROSCI.22-11-04302.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Wise DR, Diehl JA, Simon MC. Hypoxic reactive oxygen species regulate the integrated stress response and cell survival. J Biol Chem. 2008;283:31153–31162. doi: 10.1074/jbc.M805056200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Ginestier C, Ou SJ, Clouthier SG, Patel SH, Monville F, Korkaya H, Heath A, Dutcher J, Kleer C, et al. Breast cancer stem cells are regulated by mesenchymal stem cells through cytokine networks. Cancer Res. 2011;71:614–24. doi: 10.1158/0008-5472.CAN-10-0538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu ZJ, Xiao M, Balint K, Smalley KS, Brafford P, Qiu R, Pinnix CC, Li X, Herlyn M. Notch1 signaling promotes primary melanoma progression by activating mitogen-activated protein kinase/phosphatidylinositol 3-kinase-Akt pathways and up-regulating N-cadherin expression. Cancer Res. 2006;66:4182–4190. doi: 10.1158/0008-5472.CAN-05-3589. [DOI] [PubMed] [Google Scholar]
- Lohela M, Casbon A-J, Olowa A, Bonham L, Branstetter D, Weng N, Smith J, Werb Z. Intravital imaging reveals distinct responses of depleting dynamic tumor-associated macrophage and dendritic cell subpopulations. Proc Natl Acad Sci USA. 2014;111:E5086–5095. doi: 10.1073/pnas.1419899111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombardo Y, Scopelliti A, Cammareri P, Todaro M, Iovino F, Ricci-Vitiani L, Gulotta G, Dieli F, de Maria R, Stassi G. Bone morphogenetic protein 4 induces differentiation of colorectal cancer stem cells and increases their response to chemotherapy in mice. Gastroenterology. 2011;140:297–309. doi: 10.1053/j.gastro.2010.10.005. [DOI] [PubMed] [Google Scholar]
- Lopez JI, Camenisch TD, Stevens MV, Sands BJ, McDonald J, Schroeder JA. CD44 attenuates metastatic invasion during breast cancer progression. Cancer Res. 2005;65:6755–6763. doi: 10.1158/0008-5472.CAN-05-0863. [DOI] [PubMed] [Google Scholar]
- Marusyk A, Almendro V, Polyak K. Intra-tumour heterogeneity: a looking glass for cancer? Nat Rev Cancer. 2012;12:323–334. doi: 10.1038/nrc3261. [DOI] [PubMed] [Google Scholar]
- Marusyk A, Tabassum DP, Altrock PM, Almendro V, Michor F, Polyak K. Non-cell-autonomous driving of tumour growth supports sub-clonal heterogeneity. Nature. 2014;514:54–58. doi: 10.1038/nature13556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meacham CE, Morrison SJ. Tumour heterogeneity and cancer cell plasticity. Nature. 2013;501:328–337. doi: 10.1038/nature12624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medyouf H, Mossner M, Jann JC, Nolte F, Raffel S, Herrmann C, Lier A, Eisen C, Nowak V, Zens B, et al. Myelodysplastic cells in patients reprogram mesenchymal stromal cells to establish a transplantable stem cell niche disease unit. Cell Stem Cell. 2014;14:824–837. doi: 10.1016/j.stem.2014.02.014. [DOI] [PubMed] [Google Scholar]
- Merlos-Suarez A, Barriga FM, Jung P, Iglesias M, Cespedes MV, Rossell D, Sevillano M, Hernando-Momblona X, da Silva-Diz V, Munoz P, et al. The intestinal stem cell signature identifies colorectal cancer stem cells and predicts disease relapse. Cell Stem Cell. 2011;8:511–524. doi: 10.1016/j.stem.2011.02.020. [DOI] [PubMed] [Google Scholar]
- Milner LA, Bigas A. Notch as a mediator of cell fate determination in hematopoiesis: evidence and speculation. Blood. 1999;93:2431–2448. [PubMed] [Google Scholar]
- Mishalian I, Bayuh R, Eruslanov E, Michaeli J, Levy L, Zolotarov L, Singhal S, Albelda SM, Granot Z, Fridlender ZG. Neutrophils recruit regulatory T-cells into tumors via secretion of CCL17--a new mechanism of impaired antitumor immunity. Int J Cancer. 2014;135:1178–1186. doi: 10.1002/ijc.28770. [DOI] [PubMed] [Google Scholar]
- Mitchem JB, Brennan DJ, Knolhoff BL, Belt BA, Zhu Y, Sanford DE, Belaygorod L, Carpenter D, Collins L, Piwnica-Worms D, et al. Targeting tumor-infiltrating macrophages decreases tumor-initiating cells, relieves immunosuppression, and improves chemotherapeutic responses. Cancer Res. 2013;73:1128–1141. doi: 10.1158/0008-5472.CAN-12-2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller A, Homey B, Soto H, Ge N, Catron D, Buchanan ME, McClanahan T, Murphy E, Yuan W, Wagner SN, et al. Involvement of chemokine receptors in breast cancer metastasis. Nature. 2001;410:50–56. doi: 10.1038/35065016. [DOI] [PubMed] [Google Scholar]
- Muller V, Stahmann N, Riethdorf S, Rau T, Zabel T, Goetz A, Janicke F, Pantel K. Circulating tumor cells in breast cancer: correlation to bone marrow micrometastases, heterogeneous response to systemic therapy and low proliferative activity. Clin Cancer Res. 2005;11:3678–3685. doi: 10.1158/1078-0432.CCR-04-2469. [DOI] [PubMed] [Google Scholar]
- Naumov GN, Akslen LA, Folkman J. Role of angiogenesis in human tumor dormancy: animal models of the angiogenic switch. Cell Cycle. 2006;5:1779–1787. doi: 10.4161/cc.5.16.3018. [DOI] [PubMed] [Google Scholar]
- Nguyen DX, Bos PD, Massague J. Metastasis: from dissemination to organ-specific colonization. Nat Rev Cancer. 2009;9:274–284. doi: 10.1038/nrc2622. [DOI] [PubMed] [Google Scholar]
- Nguyen LV, Vanner R, Dirks P, Eaves CJ. Cancer stem cells: an evolving concept. Nat Rev Cancer. 2012;12:133–143. doi: 10.1038/nrc3184. [DOI] [PubMed] [Google Scholar]
- Ni C, Huang J. Dynamic regulation of cancer stem cells and clinical challenges. Clin Transl Oncol. 2013;15:253–258. doi: 10.1007/s12094-012-0927-7. [DOI] [PubMed] [Google Scholar]
- Nishimura K, Semba S, Aoyagi K, Sasaki H, Yokozaki H. Mesenchymal stem cells provide an advantageous tumor microenvironment for the restoration of cancer stem cells. Pathobiol. 2012;79:290–306. doi: 10.1159/000337296. [DOI] [PubMed] [Google Scholar]
- Noel A, Gutierrez-Fernandez A, Sounni NE, Behrendt N, Maquoi E, Lund IK, Cal S, Hoyer-Hansen G, López-Otín C. New and paradoxical roles of matrix metalloproteinases in the tumor microenvironment. Front Pharmacol. 2012;3:140. doi: 10.3389/fphar.2012.00140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noy R, Pollard JW. Tumor-associated macrophages: from mechanisms to therapy. Immunity. 2014;41:49–61. doi: 10.1016/j.immuni.2014.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ocana OH, Corcoles R, Fabra A, Moreno-Bueno G, Acloque H, Vega S, Barrallo-Gimeno A, Cano A, Nieto MA. Metastatic colonization requires the repression of the epithelial-mesenchymal transition inducer Prrx1. Cancer Cell. 2012;22:709–724. doi: 10.1016/j.ccr.2012.10.012. [DOI] [PubMed] [Google Scholar]
- Olaso E, Santisteban A, Bidaurrazaga J, Gressner AM, Rosenbaum J, Vidal-Vanaclocha F. Tumor-dependent activation of rodent hepatic stellate cells during experimental melanoma metastasis. Hepatology. 1997;26:634–642. doi: 10.1002/hep.510260315. [DOI] [PubMed] [Google Scholar]
- Oskarsson T, Acharyya S, Zhang XH, Vanharanta S, Tavazoie SF, Morris PG, Downey RJ, Manova-Todorova K, Brogi E, Massague J. Breast cancer cells produce tenascin C as a metastatic niche component to colonize the lungs. Nat Med. 2011;7:867–874. doi: 10.1038/nm.2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oskarsson T, Batlle E, Massague J. Metastatic Stem Cells: Sources, Niches, and Vital Pathways. Cell Stem Cell. 2014;14:306–321. doi: 10.1016/j.stem.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantel K, Schlimok G, Braun S, Kutter D, Lindemann F, Schaller G, Funke I, Izbicki JR, Riethmuller G. Differential expression of proliferation-associated molecules in individual micrometastatic carcinoma cells. J Natl Cancer Inst. 1993;85:1419–1424. doi: 10.1093/jnci/85.17.1419. [DOI] [PubMed] [Google Scholar]
- Passalidou E, Trivella M, Singh N, Ferguson M, Hu J, Cesario A, Granone P, Nicholson G, Goldstraw P, Ratcliffe C, et al. Vascular phenotype in angiogenic and non-angiogenic lung non-small cell carcinomas. Br J Cancer. 2002;86:244–249. doi: 10.1038/sj.bjc.6600015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlides S, Tsirigos A, Vera I, Flomenberg N, Frank PG, Casimiro MC, Wang C, Pestell RG, Martinez-Outschoorn UE, Howell A, et al. Transcriptional evidence for the “Reverse Warburg Effect” in human breast cancer tumor stroma and metastasis: similarities with oxidative stress, inflammation, Alzheimer’s disease, and “Neuron-Glia Metabolic Coupling”. Aging. 2010;2:185–199. doi: 10.18632/aging.100134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pece S, Tosoni D, Confalonieri S, Mazzarol G, Vecchi M, Ronzoni S, Bernard L, Viale G, Pelicci PG, Di Fiore PP. Biological and molecular heterogeneity of breast cancers correlates with their cancer stem cell content. Cell. 2010;140:62–73. doi: 10.1016/j.cell.2009.12.007. [DOI] [PubMed] [Google Scholar]
- Piccirillo SG, Reynolds BA, Zanetti N, Lamorte G, Binda E, Broggi G, Brem H, Olivi A, Dimeco F, Vescovi AL. Bone morphogenetic proteins inhibit the tumorigenic potential of human brain tumour-initiating cells. Nature. 2006;444:761–765. doi: 10.1038/nature05349. [DOI] [PubMed] [Google Scholar]
- Plaks V, Brenot A, Lawson DA, Linnemann JR, Van Kappel EC, Wong KC, De Sauvage F, Klein OD, Werb Z. Lgr5-expressing cells are sufficient and necessary for postnatal mammary gland organogenesis. Cell Rep. 2012;3:70–78. doi: 10.1016/j.celrep.2012.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plaks V, Koopman CD, Werb Z. Circulating Tumor Cells. Science. 2013;341:1186–1188. doi: 10.1126/science.1235226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Psaila B, Lyden D. The metastatic niche: adapting the foreign soil. Nat Rev Cancer. 2009;9:285–293. doi: 10.1038/nrc2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quail DF, Taylor MJ, Postovit LM. Microenvironmental regulation of cancer stem cell phenotypes. Curr Stem Cell Res Ther. 2012;7:197–216. doi: 10.2174/157488812799859838. [DOI] [PubMed] [Google Scholar]
- Quintana E, Shackleton M, Sabel MS, Fullen DR, Johnson TM, Morrison SJ. Efficient tumour formation by single human melanoma cells. Nature. 2008;456:593–598. doi: 10.1038/nature07567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintana E, Piskounova E, Shackleton M, Weinberg D, Eskiocak U, Fullen DR, Johnson TM, Morrison SJ. Human melanoma metastasis in NSG mice correlates with clinical outcome in patients. Sci Transl Med. 2012;4:159ra149. doi: 10.1126/scitranslmed.3004599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radisky DC, Levy DD, Littlepage LE, Liu H, Nelson CM, Fata JE, Leake D, Godden EL, Albertson DG, Nieto MA, et al. Rac1b and reactive oxygen species mediate MMP-3-induced EMT and genomic instability. Nature. 2005;436:123–127. doi: 10.1038/nature03688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratajczak J, Wysoczynski M, Hayek F, Janowska-Wieczorek A, Ratajczak MZ. Membrane-derived microvesicles: important and underappreciated mediators of cell-to-cell communication. Leukemia. 2006;20:1487–1495. doi: 10.1038/sj.leu.2404296. [DOI] [PubMed] [Google Scholar]
- Ricardo S, Vieira AF, Gerhard R, Leitão D, Pinto RP, Cameselle-Teijeiro JF, Milanezi F, Schmitt F, Paredes J. Breast cancer stem cell markers CD44, CD24 and ALDH1: expression distribution within intrinsic molecular subtype. Journal of Clinical Pathology. 2011;64:937–946. doi: 10.1136/jcp.2011.090456. [DOI] [PubMed] [Google Scholar]
- Ricci-Vitiani L, Pallini R, Biffoni M, Todaro M, Invernici G, Cenci T, Maira G, Parati EA, Stassi G, Larocca LM, Ruggero DM. Tumour vascularization via endothelial differentiation of glioblastoma stem-like cells. Nature. 2010;468:824–828. doi: 10.1038/nature09557. [DOI] [PubMed] [Google Scholar]
- Riquelme PA, Drapeau E, Doetsch F. Brain micro-ecologies: neural stem cell niches in the adult mammalian brain. Phil Trans R Soc B Biol Sci. 2008;363:123–137. doi: 10.1098/rstb.2006.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocco A, Liguori E, Pirozzi G, Tirino V, Compare D, Franco R, Tatangelo F, Palaia R, D’Armiento FP, Pollastrone G, et al. CD133 and CD44 cell surface markers do not identify cancer stem cells in primary human gastric tumors. J Cell Physiol. 2012;227:2686–2693. doi: 10.1002/jcp.23013. [DOI] [PubMed] [Google Scholar]
- Roesch A, Fukunaga-Kalabis M, Schmidt EC, Zabierowski SE, Brafford PA, Vultur A, Basu D, Gimotty P, Vogt T, Herlyn M. Atemporarily distinct subpopulation of slow-cycling melanoma cells is required forcontinuous tumor growth. Cell. 2010;141:583–594. doi: 10.1016/j.cell.2010.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roorda BD, ter Elst A, Meeuwsen-de Boer TGJ, Kamps WA, de Bont ESJM. Mesenchymal Stem Cells Contribute to Tumor Cell Proliferation by Direct Cell–Cell Contact Interactions. Cancer Invest. 2010;28:526–534. doi: 10.3109/07357900903179625. [DOI] [PubMed] [Google Scholar]
- Rongvaux A, Takizawa H, Strowig T, Willinger T, Eynon EE, Flavell RA, Manz MG. Human hemato-lymphoid system mice: current use and future potential for medicine. Annu Rev Immunol. 2013;31:635–674. doi: 10.1146/annurev-immunol-032712-095921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito Y, Uchida N, Tanaka S, Suzuki N, Tomizawa-Murasawa M, Sone A, Najima Y, Takagi S, Aoki Y, Wake A, et al. Induction of cell cycle entry eliminates human leukemia stem cells in a mouse model of AML. Nat Biotechnol. 2010;28:275–280. doi: 10.1038/nbt.1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheel C, Eaton EN, Li SH, Chaffer CL, Reinhardt F, Kah KJ, Bell G, Guo W, Rubin J, Richardson AL, Weinberg RA. Paracrine and Autocrine signals induce and maintain mesenchymal and stem cell states in the breast. Cell. 2011;145:926–940. doi: 10.1016/j.cell.2011.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science. 2011;331:1565–1570. doi: 10.1126/science.1203486. [DOI] [PubMed] [Google Scholar]
- Schwitalla S, Fingerle AA, Cammareri P, Nebelsiek T, Göktuna SI, Ziegler PK, Canli O, Heijmans J, Huels DJ, Moreaux G, et al. Intestinal tumorigenesis initiated by dedifferentiation and acquisition of stem-cell-like properties. Cell. 2013;152:25–38. doi: 10.1016/j.cell.2012.12.012. [DOI] [PubMed] [Google Scholar]
- Shachaf CM, Kopelman AM, Arvanitis C, Karlsson A, Beer S, Mandl S, Bachmann MH, Borowsky AD, Ruebner B, Cardiff RD, et al. MYC inactivation uncovers pluripotent differentiation and tumour dormancy in hepatocellular cancer. Nature. 2004;431:1112–1117. doi: 10.1038/nature03043. [DOI] [PubMed] [Google Scholar]
- Shackleton M, Quintana E, Fearon ER, Morrison SJ. Heterogeneity in cancer: cancer stem cells versus clonal evolution. Cell. 2009;138:822–829. doi: 10.1016/j.cell.2009.08.017. [DOI] [PubMed] [Google Scholar]
- Shiozawa Y, Pedersen EA, Havens AM, Jung Y, Mishra A, Joseph J, Kim JK, Patel LR, Ying C, Ziegler AM, et al. Human prostate cancer metastases target the hematopoietic stem cell niche to establish footholds in mouse bone marrow. J Clin Invest. 2011;121:1298–1312. doi: 10.1172/JCI43414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirakawa K, Kobayashi H, Heike Y, Kawamoto S, Brechbiel MW, Kasumi F, Iwanaga T, Konishi F, Terada M, Wakasugi H. Hemodynamics in vasculogenic mimicry and angiogenesis of inflammatory breast cancer xenograft. Cancer Res. 2002;62:560–566. [PubMed] [Google Scholar]
- Siefert SA, Sarkar R. Matrix metalloproteinases in vascular physiology and disease. Vascular. 2012;20:210–206. doi: 10.1258/vasc.2011.201202. [DOI] [PubMed] [Google Scholar]
- Smith AL, Robin TP, Ford HL. Molecular pathways: targeting the TGF-β pathway for cancer therapy. Clin Cancer Res. 2012;18:4514–4521. doi: 10.1158/1078-0432.CCR-11-3224. [DOI] [PubMed] [Google Scholar]
- Sneddon JB, Werb Z. Location, location, location: The cancer stem cell niche. Cell Stem Cell. 2007;1:607–611. doi: 10.1016/j.stem.2007.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stankic M, Pavlovic S, Chin Y, Brogi E, Padua D, Norton L, Massague J, Benezra R. TGF-b-Id1 signaling opposes Twist1 and promotes metastatic colonizationviaamesenchymal-to-epithelial transition. Cell Rep. 2013;5:1228–1242. doi: 10.1016/j.celrep.2013.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart SA, Hahn WC, O’Connor BF, Banner EN, Lundberg AS, Modha P, Mizuno H, Brooks MW, Fleming M, Zimonjic DB, et al. Telomerase contributes totumorigenesis by a telomere length-independent mechanism. Proc Natl Acad Sci USA. 2002;99:12606–12611. doi: 10.1073/pnas.182407599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suva ML, Riggi N, Janiszewska M, Radovanovic I, Provero P, Stehle J-C, Baumer K, Le Bitoux M-A, Marino D, Cironi L, et al. EZH2 is essential for glioblastoma cancer stem cell maintenance. Cancer Res. 2009;69:9211–9218. doi: 10.1158/0008-5472.CAN-09-1622. [DOI] [PubMed] [Google Scholar]
- Takebe N, Harris PJ, Warren RQ, Ivy SP. Targeting cancer stem cells by inhibiting Wnt, Notch, and Hedgehog pathways. Nat Rev Clin Oncol. 2011;8:97–106. doi: 10.1038/nrclinonc.2010.196. [DOI] [PubMed] [Google Scholar]
- Tsai JH, Donaher JL, Murphy DA, Chau S, Yang J. Spatiotemporal regulation of epithelial-mesenchymal transition is essential for squamous cell carcinoma metastasis. Cancer Cell. 2012;22:725–736. doi: 10.1016/j.ccr.2012.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valiente M, Obenauf AC, Jin X, Chen Q, Zhang XHF, Lee DJ, Chaft JE, Kris MG, Huse JT, Brogi E, Massague J. Serpins promote cancer cell survival and vascular cooption in brain metastasis. Cell. 2014;156:1002–1016. doi: 10.1016/j.cell.2014.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermeulen L, de Sousa E, Melo F, Van Der Heijden M, Cameron K, de Jong JH, Borovski T, Tuynman JB, Todaro M, Merz C, et al. Wnt activity defines colon cancer stem cells and is regulated by the microenvironment. Nat Cell Biol. 2010;12:468–476. doi: 10.1038/ncb2048. [DOI] [PubMed] [Google Scholar]
- Vermeulen L, Todaro M, deSousaMello F, Sprick MR, Kemper K, Perez Alea M, Richel DJ, Stassi G, Medema JP. Single-cell cloning of colon cancer stem cells reveals a multi-lineage differentiation capacity. Proc Natl Acad Sci USA. 2008;105:13427–13432. doi: 10.1073/pnas.0805706105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visvader JE, Lindeman GJ. Cancer stem cells in solid tumours: accumulating evidence and unresolved questions. Nat Rev Cancer. 2008;8:755–768. doi: 10.1038/nrc2499. [DOI] [PubMed] [Google Scholar]
- Vogelstein B, Fearon ER, Hamilton SR, Kern SE, Preisinger AC, Leppert M, Nakamura Y, White R, Smits AM, Bos JL. Genetic alterations during colorectal-tumor development. N Engl J Med. 1988;319:525–532. doi: 10.1056/NEJM198809013190901. [DOI] [PubMed] [Google Scholar]
- Vogelstein B, Papadopoulos N, Velculescu V, Zhou S, Diaz LA, Jr, Kinzler KW. Cancer genome landscapes. Science. 2013;339:1546–1558. doi: 10.1126/science.1235122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voog J, Jones DL. Stem cells and the niche: a dynamic duo. Cell Stem Cell. 2010;6:103–115. doi: 10.1016/j.stem.2010.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei J, Wu A, Kong LY, Wang Y, Fuller G, Fokt I, Melillo G, Priebe W, Heimberger AB. Hypoxia potentiates glioma-mediated immunosuppression. PLoS One. 2011;6:e16195. doi: 10.1371/journal.pone.0016195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weijzen S, Rizzo P, Braid M, Vaishnav R, Jonkheer SM, Zlobin A, Osborne BA, Gottipati S, Aster JC, Hahn WC, et al. Activation of Notch-1 signaling maintains the neoplastic phenotype in human Ras-transformed cells. Nat Med. 2002;8:979–986. doi: 10.1038/nm754. [DOI] [PubMed] [Google Scholar]
- Weilbaecher KN, Guise TA, McCauley LK. Cancer to bone: a fatal attraction. Nat Rev Cancer. 2011;11:411–425. doi: 10.1038/nrc3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams R, Besten W, Sherr CJ. Cytokine-dependent imatinib resistance in mouse BCR-ABL+, Arf-null lymphoblastic leukemia. Genes Dev. 2007;21:2283–2287. doi: 10.1101/gad.1588607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong GS, Rustgi AK. Matricellular proteins: priming the tumour microenvironment for cancer development and metastasis. Br J Cancer. 2013;108:755–761. doi: 10.1038/bjc.2012.592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yachida S, Jones S, Bozic I, Antal T, Leary R, Fu B, Kamiyama M, Hruban RH, Eshleman JR, Nowak MA, et al. Distant metastasis occurs late during the genetic evolution of pancreatic cancer. Nature. 2010;467:1114–1117. doi: 10.1038/nature09515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan HH, Pickup M, Pang Y, Gorska AE, Li Z, Chytil A, Geng Y, Gray JW, Moses HL, Yang L. Gr-1+CD11b+ myeloid cells tip the balance of immune protection to tumor promotion in the premetastatic lung. Cancer Res. 2010;70:6139–6149. doi: 10.1158/0008-5472.CAN-10-0706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang MH, Hsu DSS, Wang HW, Wang HJ, Lan HY, Yang WH, Huang CH, Kao SY, Tzeng CH, Tai SK, et al. Bmi1 is essential in Twist1-induced epithelial-mesenchymal transition. Nat Cell Biol. 2010;12:982–992. doi: 10.1038/ncb2099. [DOI] [PubMed] [Google Scholar]
- Ye J, Wu D, Wu P, Chen Z, Huang J. The cancer stem cell niche: cross talk between cancer stemcells and their microenvironment. Tumor Biol. 2014;35:3945–3951. doi: 10.1007/s13277-013-1561-x. [DOI] [PubMed] [Google Scholar]
- Yi SY, Hao YB, Nan KJ, Fan TL. Cancer stem cells niche: a target for novel cancer therapeutics. Cancer Treat Rev. 2012;39:290–296. doi: 10.1016/j.ctrv.2012.10.004. [DOI] [PubMed] [Google Scholar]
- Yilmaz OH, Valdez R, Theisen BK, Guo W, Ferguson DO, Wu H, Morrison SJ. Pten dependence distinguishes haematopoietic stem cells from leukaemia-initiating cells. Nature. 2006;441:475–482. doi: 10.1038/nature04703. [DOI] [PubMed] [Google Scholar]
- Zlotnik A, Burkhardt AM, Homey B. Homeostatic chemokine receptors and organ-specific metastasis. Nat Rev Immunol. 2011;11:597–606. doi: 10.1038/nri3049. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.