ABSTRACT
Viruses rely on host cellular metabolism to provide the energy and biosynthetic building blocks required for their replication. Dengue virus (DENV), a member of the Flaviviridae family, is one of the most important arthropod-borne human pathogens worldwide. We analyzed global intracellular metabolic changes associated with DENV infection of primary human cells. Our metabolic profiling data suggested that central carbon metabolism, particularly glycolysis, is strikingly altered during a time course of DENV infection. Glucose consumption is increased during DENV infection and depriving DENV-infected cells of exogenous glucose had a pronounced impact on viral replication. Furthermore, the expression of both glucose transporter 1 and hexokinase 2, the first enzyme of glycolysis, is upregulated in DENV-infected cells. Pharmacologically inhibiting the glycolytic pathway dramatically reduced DENV RNA synthesis and infectious virion production, revealing a requirement for glycolysis during DENV infection. Thus, these experiments suggest that DENV induces the glycolytic pathway to support efficient viral replication. This study raises the possibility that metabolic inhibitors, such as those that target glycolysis, could be used to treat DENV infection in the future.
IMPORTANCE Approximately 400 million people are infected with dengue virus (DENV) annually, and more than one-third of the global population is at risk of infection. As there are currently no effective vaccines or specific antiviral therapies for DENV, we investigated the impact DENV has on the host cellular metabolome to identify metabolic pathways that are critical for the virus life cycle. We report an essential role for glycolysis during DENV infection. DENV activates the glycolytic pathway, and inhibition of glycolysis significantly blocks infectious DENV production. This study provides further evidence that viral metabolomic analyses can lead to the discovery of novel therapeutic targets to block the replication of medically important human pathogens.
INTRODUCTION
Viruses are obligate intracellular parasites that depend on the metabolic machinery of the host cell to supply the energy and macromolecules necessary for successful replication. In recent years, research has focused on investigating how virus infection alters host metabolism with the hope that these studies will provide insight into the metabolic requirements of viral replication. Viral modulation of the host cell metabolic profile has been examined for several viruses, including human cytomegalovirus (HCMV), herpes simplex virus 1 (HSV-1), hepatitis C virus, influenza A virus, human immunodeficiency virus type 1 (HIV-1), Kaposi's sarcoma-associated herpesvirus (KSHV), vaccinia virus (VACV), and Epstein-Barr virus (1–11). These reports revealed that virus infection triggers dramatic changes in cellular metabolism, particularly in central carbon utilization pathways.
Glucose and glutamine represent the two main carbon sources used to support the energetic and biosynthetic needs of mammalian cells. In normal cells, the oxidation of glucose via glycolysis and the tricarboxylic acid (TCA) cycle is thought to be responsible for the bulk of ATP generation. However, in most cancer cells, glucose carbon is diverted away from the TCA cycle to be used biosynthetically and glutamine serves to anaplerotically replenish the TCA cycle (12–14). Extensive reprogramming of central carbon metabolism has also been observed during virus infection. For example, similar to tumor cells, HCMV-infected cells use glutamine to feed the TCA cycle so that glucose carbon can be utilized for fatty acid synthesis (FAS) (2, 15). Alternatively, VACV implements a unique carbon utilization program wherein glutamine is essential for maximal viral replication by maintaining the TCA cycle but glucose is completely dispensable for virus production (10, 16). These studies demonstrate that although viruses have metabolic requirements in common, they also induce distinct alterations in host cellular metabolism to complete their life cycles.
DENV is a positive-stranded RNA virus that belongs to the Flaviviridae family. DENV is transmitted to the human host via the Aedes genus mosquito vector and can cause several disease manifestations, ranging from dengue fever to the more severe dengue hemorrhagic fever and dengue shock syndrome. It is estimated that 390 million DENV infections occur annually worldwide (17), with approximately 2.5 billion people at risk of DENV transmission (17–20). There are currently no commercially available vaccines or specific antiviral therapies for DENV, making the virus a significant threat to global human health.
Previously, a multiplatform approach was developed to measure the levels of a limited number of extracellular metabolites following DENV infection of the human endothelial hybrid cell line EA.hy926 (21). However, a comprehensive examination of host cellular metabolism during DENV infection of primary human cells has yet to be conducted. To investigate alterations in the global metabolome following DENV infection, we performed intracellular metabolic profiling of mock- and DENV-infected primary human foreskin fibroblasts (HFFs) at multiple time points during the first 48 h of infection. Data from this analysis suggested that central carbon metabolism is significantly perturbed during DENV infection, particularly the glycolytic pathway of glucose utilization. We show that glycolysis is upregulated in DENV-infected cells and that inhibiting this metabolic pathway results in reduced DENV replication. Furthermore, the requirement for glycolysis to support production of infectious DENV is not a cell type-specific phenomenon. Therefore, DENV infection activates the glycolytic pathway of glucose metabolism to promote efficient viral replication.
MATERIALS AND METHODS
Cells and viruses.
Primary HFFs (ATCC PCS-201-010) were propagated and maintained in Dulbecco's modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 2 mM l-glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin. Human telomerase reverse transcriptase-immortalized microvascular endothelial (TIME) cells were maintained in EGM-2 medium (Lonza). For the glucose and glutamine depletion studies, DMEM lacking d-glucose, l-glutamine, sodium pyruvate, and phenol red (catalog number A14430-01; Invitrogen) was used. This medium was supplemented with 2% dialyzed fetal bovine serum (catalog number SH30079.03; HyClone), and for replete medium, 1 g/liter d-glucose and 2 mM l-glutamine were added. Dialyzed serum was thoroughly depleted of small molecules, including glucose and glutamine. The glucose concentration reported for the lot of dialyzed HyClone serum used was <20 mg/dl; therefore, when it is used at 2%, the glucose level in the medium at less than 0.004 g/liter.
Stocks of DENV type 2/NG-C (provided by Michael Gale, Jr., University of Washington) were propagated, and titers were determined by plaque assays on Vero cells. Experimental infection rates were determined by immunofluorescence assay. DENV-infected cells were fixed at 24 h postinfection (hpi) with 10% (wt/vol) formalin, permeabilized with 70% (vol/vol) ethanol, and blocked with 3% bovine serum albumin in Dulbecco's phosphate-buffered saline (DPBS). Cells were then immunostained with 1 μg/ml human monoclonal antibody (MAb) 1.6D (a generous gift from Sharon Isern and Scott Michael, Florida Gulf Coast University) and 2 μg/ml Alexa Fluor 488-conjugated goat anti-human antibody (Invitrogen) and counterstained with Vectashield mounting medium (Vector Laboratories).
Reagents.
Sodium oxamate (catalog number O2751), 2-deoxy-d-glucose (2DG; catalog number D8375), and deferoxamine mesylate salt (DFO; catalog number D9533) were obtained from Sigma-Aldrich. Sodium oxamate and 2DG were directly solubilized in cell culture medium and used at the specified concentrations. DFO was solubilized in deionized water and used at the final concentration indicated.
Virus infections for metabolic analysis.
HFFs were washed with DPBS and serum starved in DMEM for ∼16 h prior to infection. Cells were then mock or DENV infected (multiplicity of infection [MOI] of 9) in serum-free DMEM for 3 h, after which the inoculum was replaced with DMEM supplemented with 2% fetal bovine serum, 2 mM l-glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin. Cells were harvested with a cell scraper at the times indicated and washed once in cold DPBS, and pellets were snap-frozen in a dry ice-ethanol bath. Samples were stored at −80°C until shipment to Metabolon (Durham, NC).
A multitude of curation procedures were conducted by Metabolon to guarantee the quality of the data presented. Metabolon data analysts used proprietary visualization and interpretation software to confirm peak identification consistency and to limit system artifacts, misassignments, and background noise among the samples. Bradford assays were performed to normalize all samples by protein concentration, and each biochemical measured in the mock samples was rescaled to yield a median of 1. Following log transformation and imputation with minimum observed values for each compound, two-way analysis of variance (ANOVA) with contrast tests was used to determine which metabolites were significantly altered by DENV infection of HFFs at each time point across the four biological replicates.
Nutrient starvation and glycolytic inhibitor treatment studies.
HFFs were washed with DPBS and infected with DENV at an MOI of 3 in serum-free DMEM for 2 h. For nutrient starvation experiments, cells were washed with DPBS and fed replete medium, glucose-free medium, or glutamine-free medium. For 2DG treatment studies, DENV-infected HFFs were treated with 0, 10, or 50 mM 2DG. For oxamate treatment experiments, DENV-infected cells were treated with 0, 50, or 100 mM oxamate. TIME cells were infected with DENV at an MOI of 1 for 2 h and treated with 0 or 100 mM oxamate. Supernatant was collected from infected cells at 24 hpi and centrifuged to remove cellular debris, and titers were determined by focus-forming-unit reduction assays on Vero cells. Error bars reflect standard errors of the means from three separate experiments.
Focus-forming unit reduction assays.
Monolayers of Vero cells in 6- or 12-well plates were infected with supernatants collected from DENV-infected cells. Supernatants were diluted in serum-free DMEM and allowed to infect the cells for 1 h, after which the monolayers were overlaid with plugs consisting of a 1:1 ratio of 1.8% Agar, Noble (BD Biosciences) and 2× MEM (Lonza) supplemented with 20% FBS, 4 mM l-glutamine, 200 U/ml penicillin, and 200 μg/ml streptomycin. Four days postinfection, cells were fixed with 10% (wt/vol) formalin, permeabilized with 70% (vol/vol) ethanol, and subjected to immunostaining. Virus foci were detected with mouse anti-DENV MAb 3H5.1 (catalog number MAB8702; Millipore) or 4G2 (generous gift from Sharon Isern and Scott Michael, Florida Gulf Coast University), followed by horseradish peroxidase-conjugated goat anti-mouse immunoglobulin (catalog number 31410; Pierce), and developed with AEC chromogen substrate (Dako) or 3,3′-diaminobenzidine tetrahydrochloride (Sigma-Aldrich).
Glucose uptake assays.
HFFs in 12-well plates were serum starved for ∼16 h prior to infection. Cells were then mock or DENV infected at an MOI of 9 in serum-free DMEM for 2 h, after which the inoculum was replaced with DMEM supplemented with 2% fetal bovine serum, 2 mM l-glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin. Complete medium was replaced at 22 hpi with serum-free DMEM. At 24 hpi, cells were washed twice with DPBS (containing magnesium and calcium) and fed 0.5 μCi of deoxy-d-glucose, 2-[1,2-3H(N)] (catalog number NET549A250UC; PerkinElmer) in 500 μl of DPBS (containing magnesium and calcium) for 5 min at 37°C. Cells were then washed twice in ice-cold DPBS (without magnesium and calcium) and lysed in 300 μl of 1% SDS for 20 min at room temperature. Next, 150 μl of lysate was added to 4 ml of Biofluor Plus (PerkinElmer) and activity (disintegrations per minute) was measured by liquid scintillation counting. Cell-associated radioactivity was normalized to the protein concentration, which was determined with the BCA Protein Assay Reagent kit (Pierce). Error bars reflect standard errors of the means from three separate experiments.
Real-time RT-qPCR.
Total RNA was isolated from HFFs and TIME cells with the NucleoSpin RNA II kit (Macherey-Nagel). Two-step quantitative real-time reverse transcription-PCR (RT-qPCR; Bio-Rad) was used to measure transcript or DENV RNA levels. iScript Reverse Transcription Supermix for RT-qPCR (Bio-Rad) was used to synthesize 1 μg of cDNA from 1 μg of total RNA according to the manufacturer's instructions. One hundred nanograms of cDNA was used in SsoAdvanced SYBR green Supermix (Bio-Rad) according to the manufacturer's protocols. The primers used for hexokinase 2 (HK2) (22), glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (23), DENV RNA (24), and hypoxanthine-guanine phosphoribosyltransferase (HPRT) (10) have been described previously. Relative abundances of HK2 mRNA or DENV RNA were normalized by the delta threshold cycle method to the abundance of GAPDH or HPRT mRNA, with mock-infected cells set to 1 or DENV-infected cells fed replete medium set to 100. Error bars reflect standard errors of the means from three separate experiments for the HK2 expression studies. Error bars reflect standard errors of the means from at least two independent experiments for the DENV RNA quantitation studies.
Western blot analysis.
HFFs were mock or DENV infected at an MOI of 9 in serum-free DMEM for 2 h. Following infection, cells were treated with 0 or 150 μM DFO and harvested with a cell scraper at 24 hpi. Cytoplasmic and nuclear fractions were obtained with the NE-PER nuclear and cytoplasmic extraction kit (Thermo Scientific). Proteins were separated by SDS-PAGE and transferred to Immobilon-FL polyvinylidene difluoride membranes (Millipore). Blots were incubated with the indicated primary antibody (anti-HK2 [catalog number sc-6521; Santa Cruz Biotechnology], anti-GLUT1 [catalog number G3900-01J; US Biological], and anti-β-actin [catalog number A5441; Sigma-Aldrich]) and subsequently incubated with IRDye secondary antibody (LI-COR). Proteins were visualized and differences in band intensity were quantified with the Odyssey CLx Infrared Imaging System (LI-COR).
Statistical analysis.
Standard errors of the means are shown, and statistically significant differences between groups were analyzed with Student's t test (two-tailed) or one-way ANOVA. A P value of ≤0.05 was considered significant and is indicated by an asterisk in the figures. A P value of ≤0.01 is indicated by a double asterisk and a P value of <0.0001 is indicated by a triple asterisk in the figures.
RESULTS
DENV infection alters glucose metabolism.
To identify DENV-induced alterations in host cellular metabolism, we utilized an intracellular metabolic profiling approach. Primary cells were used for these experiments, as transformed cells exhibit extensively altered metabolism and are therefore not ideal for investigation of virus-induced metabolic alterations (reviewed in references 13, 25, and 26). HFFs were the most permissive primary cell type tested for DENV infection and thus were chosen for these studies. HFFs were serum starved for approximately 16 h prior to infection to synchronize the cells in the G0/G1 phase. Cells were then mock or DENV infected at an MOI of 9 and harvested at 10, 24, and 48 hpi for metabolic analysis. When infected at an MOI of 9 (determined as the number of PFU/Vero cell), ∼50% of the HFFs expressed detectable levels of DENV envelope (E) glycoprotein by 24 hpi, as quantified by immunofluorescence. Each sample consisted of approximately 4 × 106 cells, and samples from four independent experiments were analyzed on both gas chromatography-mass spectrometry (MS) and liquid chromatography-tandem MS platforms. DENV induced dynamic perturbations in the host cellular metabolome following infection of HFFs (see Data Set S1 in the supplemental material). Table 1 summarizes the changes observed in the superpathways of amino acid, carbohydrate, lipid, and nucleotide metabolism during DENV infection. Of particular interest, we noted striking alterations in glucose and glutamine utilization, with glycolysis representing one of the most dramatically perturbed metabolic pathways in DENV-infected cells. As shown in Fig. 1A, the levels of all of the glycolytic metabolites detected were significantly different in DENV-infected cells from those in mock-infected cells during the first 48 h of infection. Levels of several intermediates of glycolysis were significantly increased early during DENV infection, whereas glycolytic metabolites were measured at significantly decreased levels only at the latest time point examined postinfection. Interestingly, we observed two main trends in metabolite levels throughout the course of DENV infection. Early glycolytic intermediates, such as glucose 6-phosphate and fructose 6-phosphate, increased over time, whereas late glycolytic intermediates, including 3-phosphoglycerate and phosphoenolpyruvate, were initially higher at 10 hpi but decreased over time (Fig. 1B). It is important to note that elevated levels of a given intracellular metabolite may reflect either an increase in its production or a decrease in its consumption. Although an upregulation in glycolytic flux cannot be inferred from our global metabolomic screen, these data indicate that DENV infection modulates glucose utilization in HFFs.
TABLE 1.
Specific metabolic superpathways that are perturbed by DENV infection
| Superpathway | No. of metabolites changed in DENV-infected vs mock-infected cellsa |
||
|---|---|---|---|
| 10 hpi | 24 hpi | 48 hpi | |
| Amino acids | ↑12, ↓0 | ↑3, ↓2 | ↑3, ↓15 |
| Carbohydrates | ↑13, ↓0 | ↑5, ↓0 | ↑6, ↓5 |
| Lipids | ↑6, ↓0 | ↑6, ↓0 | ↑12, ↓13 |
| Nucleotides | ↑6, ↓0 | ↑2, ↓0 | ↑3, ↓0 |
Number of metabolites significantly (P ≤ 0.05) elevated or decreased within a given superpathway in DENV-infected cells versus mock-infected cells.
FIG 1.
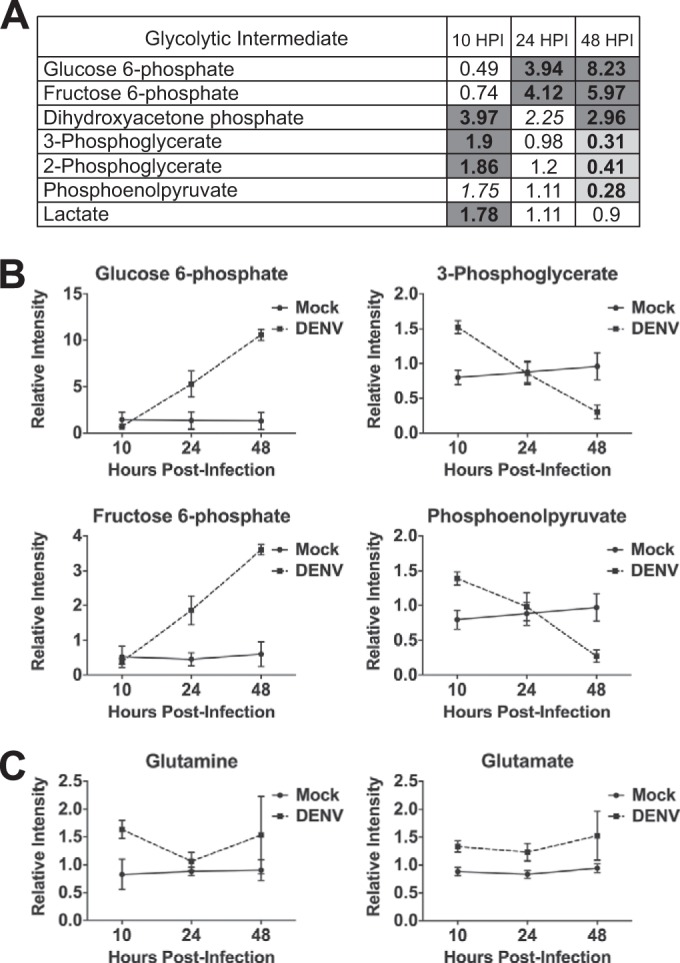
Carbon metabolism is altered during DENV infection. HFFs were mock or DENV infected (MOI of 9) and harvested at 10, 24, and 48 hpi for intracellular metabolic analysis by Metabolon. (A) Heat map visualization of fold changes in levels of glycolytic intermediates profiled during a time course of DENV infection. For paired comparisons, shaded cells indicate P ≤ 0.05 (dark gray indicates that the mean values are significantly higher in DENV-infected cells for that comparison, and light gray indicates that the values are significantly lower). Italicized values indicate 0.05 < P < 0.1. (B and C) Line plots of selected glycolytic intermediate levels (B) or glutaminolytic intermediate levels (C) in mock- and DENV-infected cells measured at the time points indicated.
In addition, we observed a considerable perturbation of glutamine metabolism during the course of DENV infection. Glutamine is catabolized via glutaminolysis, a pathway consisting of two deamination steps. Glutamine is first deaminated to glutamate and subsequently deaminated to the TCA cycle intermediate α-ketoglutarate. Figure 1C shows that both glutamine and glutamate levels were higher in DENV-infected cells than in their mock-infected counterparts. Glutamine levels were significantly increased at 10 hpi, whereas glutamate levels were significantly elevated at all three time points examined. Taken together, these data reveal that DENV implements a distinct carbon utilization program during infection.
Exogenous glucose is required during DENV infection.
Since our metabolomic screen identified DENV-induced perturbations in central carbon metabolism, we investigated the impact of glucose and glutamine deprivation on DENV production. HFFs were infected with DENV at an MOI of 3 and subsequently fed replete medium containing both glucose and glutamine or medium lacking either glucose or glutamine. At 24 hpi, the release of infectious extracellular virus was quantified by focus-forming unit reduction assays. As shown in Fig. 2A, depriving DENV-infected cells of exogenous glucose reduced the production of infectious virus by greater than 2 logs. We have previously shown that 24 h of glucose or glutamine starvation has only a modest impact on HFF proliferation and no significant impact on cell viability (10). Furthermore, depriving HFFs of exogenous glucose does not inhibit VACV replication, indicating that the block in DENV production was not due to the effects of glucose deprivation on cellular integrity. Interestingly, depriving DENV-infected cells of exogenous glutamine, the other primary carbon source for mammalian cells, reduced infectious virus production by only approximately 60% (Fig. 2A). To determine if exogenous glucose starvation has an impact on DENV genome replication, we measured viral RNA levels in DENV-infected cells fed replete medium or medium lacking glucose. Figure 2B shows that DENV RNA accumulation is significantly reduced under conditions of glucose deprivation. These results indicate that DENV demonstrates a selective dependence on exogenous glucose during infection.
FIG 2.
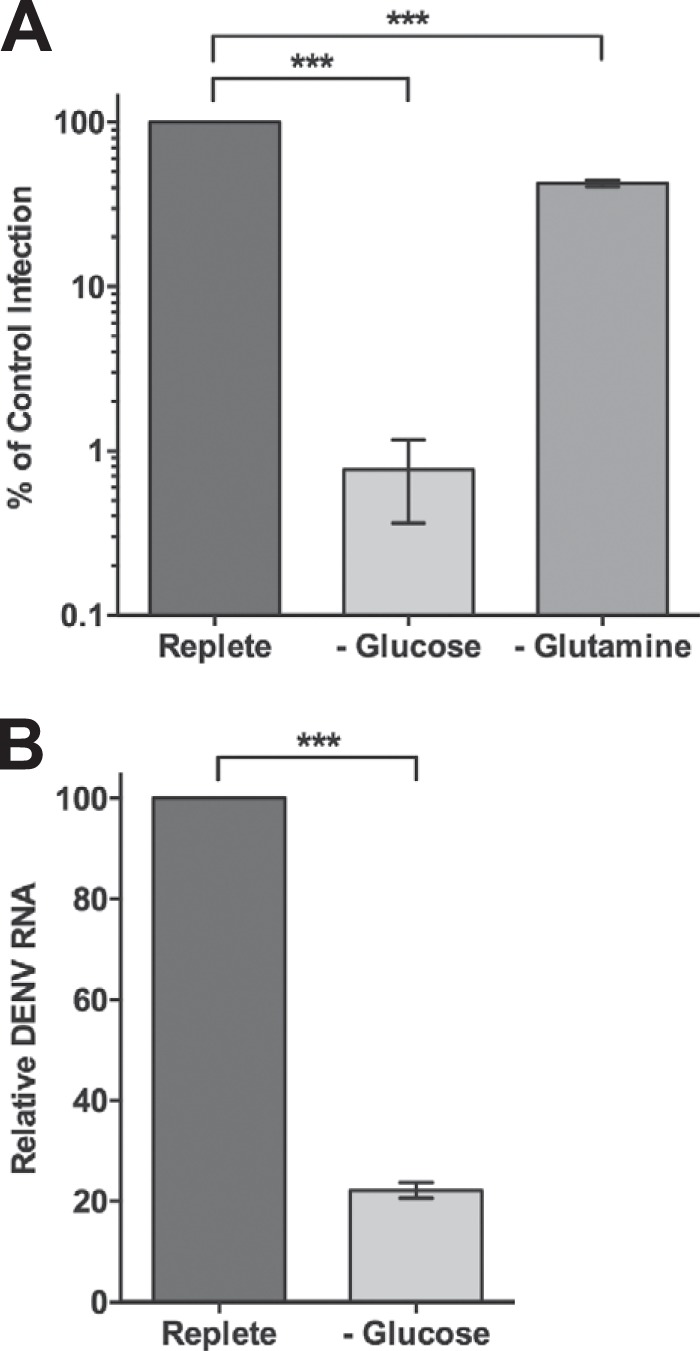
Exogenous glucose is necessary for efficient infectious DENV production. HFFs were infected with DENV at an MOI of 3 and fed replete, glucose-free, or glutamine-free medium at 2 hpi. At 24 hpi, released infectious virus was quantified by focus-forming-unit reduction assays on Vero cells (A) or intracellular viral RNA from DENV-infected cells fed replete or glucose-free medium was measured by real-time RT-qPCR (B). ***, P < 0.0001.
Glycolysis is activated during DENV infection.
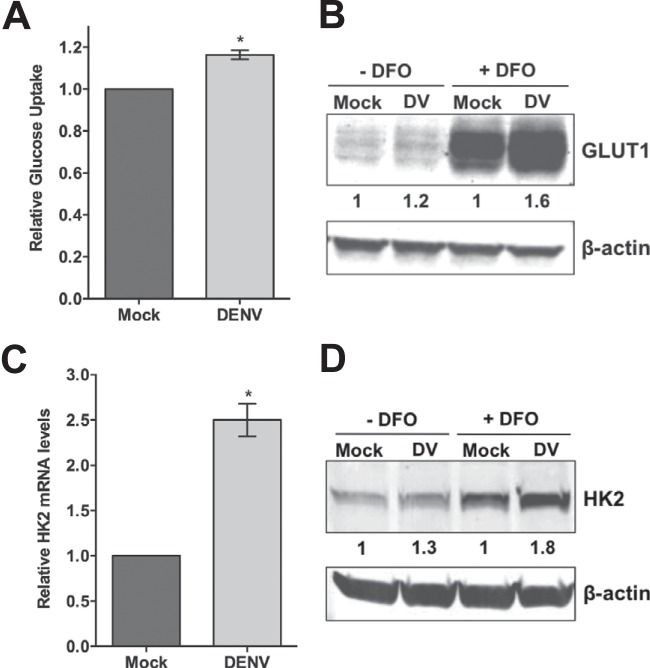
The metabolic profiling data show that the glycolytic pathway of glucose metabolism is perturbed in DENV-infected cells, and we found that DENV requires exogenous glucose for optimal replication. To determine if DENV activates glycolysis, we first examined glucose consumption following DENV infection. HFFs were mock or DENV infected at an MOI of 9, and uptake of the radiolabeled glucose analog 2-deoxy-d-[3H]glucose was measured at 24 hpi. As shown in Fig. 3A, DENV-infected cells exhibited approximately 16% greater glucose uptake than their mock-infected counterparts. Importantly, an average infection rate of 50% was achieved in these experiments. Therefore, the population of uninfected cells in the culture masked the full impact DENV infection has on glucose consumption.
FIG 3.
Glycolysis is induced during DENV infection. (A) Glucose uptake is increased during DENV infection. At 24 hpi, mock- and DENV-infected (MOI of 9) HFFs were exposed to a radiolabeled glucose analog for 5 min and then intracellular radioactivity was quantified. (B, C, and D) Expression levels of GLUT1 and HK2 are elevated in DENV-infected cells. HFFs were mock or DENV (DV) infected (MOI of 9) and treated with 0 or 150 μM DFO at 2 hpi. Cell lysates harvested at 24 hpi were subjected to immunoblot analysis with the antibodies indicated. The values below the lanes are the relative intensities of the major bands, and β-actin served as a loading control. (C) Real-time RT-qPCR analysis of HK2 transcript levels in mock- and DENV-infected cells (MOI of 5) harvested at 24 hpi. Relative abundance of HK2 mRNA was normalized to the abundance of GAPDH mRNA by the delta threshold cycle method. *, P ≤ 0.05.
Exogenous glucose uptake into mammalian cells is mediated by a family of at least 14 isoforms of facilitative transporter membrane proteins known as the glucose transporters (GLUTs). GLUT1 is ubiquitously expressed in many different cell types of various tissues and is responsible for basal glucose transport (reviewed in reference 27). To determine if the increase in glucose consumption observed in DENV-infected cells is due to upregulation of GLUT expression, we measured GLUT1 protein levels via Western blot analysis. Because GLUT1 expression is enhanced by hypoxia, we treated mock- and DENV-infected cells with DFO, a hypoxia-mimetic agent, to better visualize the relative levels of GLUT1. HFFs were mock or DENV infected at an MOI of 9, and cytoplasmic extracts were harvested from normoxic and DFO-treated cells at 24 hpi. As shown in Fig. 3B, GLUT1 protein levels were elevated in DENV-infected cells, with the greatest increase in GLUT1 expression detected under conditions of DFO treatment. These results suggest that GLUT1 induction is likely responsible, at least in part, for enhanced glucose consumption during DENV infection.
To further examine if DENV activates glycolysis, we also measured hexokinase expression in mock- and DENV-infected cells. Hexokinase is the first rate-limiting enzyme of the glycolytic pathway and the HK2 isoform, in particular, has been shown to be a key mediator of aerobic glycolysis (22, 28). We utilized real-time RT-qPCR to measure HK2 mRNA levels during DENV infection. HFFs were mock and DENV infected at an MOI of 5, and total RNA was isolated from cells harvested at 24 hpi. Figure 3C shows that HK2 expression was significantly higher in DENV-infected cells than in mock-infected cells. Levels of the three isoforms of phosphofructokinase 1, the second rate-limiting enzyme of glycolysis, were not significantly increased during DENV infection (data not shown). We next confirmed HK2 upregulation via Western blot analysis. As HK2 levels are low in normal cells, we again utilized DFO to enhance HK2 protein expression for a comparison of mock- and DENV-infected cells at 24 hpi. Figure 3D shows that HK2 protein levels were increased in DENV-infected cells, which, similar to GLUT1 levels, were enhanced following DFO treatment. Again, the GLUT1 and HK2 protein expression increases detected are diminished by the lower infection rates obtained with DENV infection of HFFs. Combined with the metabolic profiling data, these results reveal that glycolysis is induced during DENV infection.
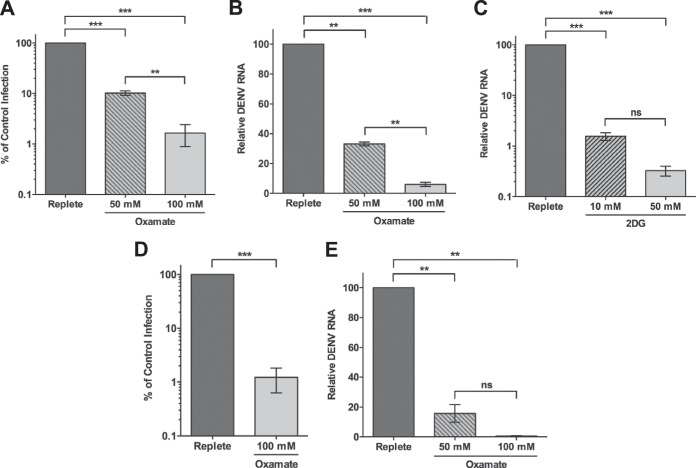
Glycolysis is required for optimal infectious DENV production.
Because glycolysis is activated during DENV infection and exogenous glucose is essential for DENV production, we hypothesized that glycolysis is necessary for efficient viral replication. Thus, we next examined DENV replication following treatment with oxamate, a glycolytic inhibitor. Oxamate is a pyruvate analog that specifically inhibits lactate dehydrogenase, the enzyme that catalyzes the conversion of pyruvate to lactate (29–31). Figure 4A shows that DENV-infected HFFs treated with increasing concentrations of oxamate exhibited a dose-dependent decrease in the release of extracellular infectious virus. Moreover, viral RNA levels were similarly reduced following oxamate treatment (Fig. 4B), indicating that glycolysis is required at an early stage in the DENV life cycle. We confirmed these results with 2DG, a glucose analog that inhibits hexokinase, the first enzyme in the glycolytic pathway. As shown in Fig. 4C, treatment with 2DG significantly impaired DENV RNA synthesis. To determine if glycolysis is necessary for DENV replication in cell types other than HFFs, we measured the impact of glycolytic inhibition on infectious virus production and DENV genome synthesis in TIME cells. Although it is immortal, this cell line has been shown to exhibit metabolism similar to primary endothelial cells (9, 32). DENV-infected endothelial cells have been found in dengue patients and murine models of DENV disease and may contribute to DENV pathogenesis (33–40). Infected TIME cells treated with oxamate showed a block in both production of infectious DENV progeny and viral RNA levels (Fig. 4D and E), revealing that the requirement for glycolysis is not HFF specific. Together, these data indicate that glycolysis is a critical metabolic pathway for optimal DENV replication.
FIG 4.
Glycolysis is required for maximal DENV replication. (A, B, and C) HFFs were infected with DENV at an MOI of 3 and fed replete medium (0 mM) or replete medium supplemented with oxamate (50 or 100 mM) or 2DG (10 or 50 mM) at 2 hpi. At 24 hpi, released infectious virus (A) and viral RNA (B and C) were quantified by focus-forming unit reduction assays or real-time RT-qPCR, respectively. (D and E) TIME cells were infected with DENV at an MOI of 1 and fed replete medium supplemented with 0, 50, or 100 mM oxamate at 2 hpi. Infectious extracellular virus (D) and viral RNA (E) were quantified at 24 hpi as described above. **, P ≤ 0.01; ***, P < 0.0001; ns, not significant.
DISCUSSION
Viruses require host cellular metabolism to provide the energy and molecular building blocks needed for successful viral replication. In this study, we utilized a metabolomic approach to identify intracellular metabolic alterations that occur during a time course of DENV infection. Among other changes, this screen detected striking DENV-induced perturbations in glucose and glutamine utilization. Dynamic changes in the levels of numerous glycolytic intermediates are observed throughout the first 48 h of DENV infection. As elevated metabolite concentrations may reflect either an increase in the production of intermediates or a decrease in their consumption, we sought to determine if glucose metabolism is required during DENV infection. In support of our metabolomic data, we show that infectious DENV production is drastically decreased when DENV-infected cells are deprived of exogenous glucose. Furthermore, depriving DENV-infected cells of exogenous glutamine has only a modest impact on viral yield, though this effect is statistically significant. We previously found that glutamine metabolism is considerably altered during VACV infection of HFFs and is necessary to anaplerotically replenish the TCA cycle (10). Interestingly, the changes in glutamine and glutamate levels throughout the course of infection are similar in VACV-infected and DENV-infected cells, raising the possibility that glutamine also serves as an anaplerotic substrate for the TCA cycle during DENV infection. However, glutamine is consumed in multiple metabolic pathways, such as providing the nitrogen for nucleotide biosynthesis. The observed block in DENV production under conditions of glutamine deprivation could also be due to the requirement for increased intracellular nucleotide pools during DENV replication. Consistent with this notion, levels of several biochemicals involved in purine and pyrimidine metabolism are significantly elevated in DENV-infected cells (see Data Set S1 in the supplemental material). Therefore, it may be that DENV requires glutamine as both a carbon and a nitrogen source to support its replicative needs. Metabolic carbon and nitrogen flux analysis is warranted to gain further insight into global glucose and glutamine usage during DENV infection. However, the results presented here reveal that glucose is the more critical carbon source for DENV replication.
Data from our metabolic screen suggest that the glycolytic pathway of glucose utilization is specifically altered during DENV infection. As our laboratory and others have previously reported, many human viruses activate glycolysis (1, 3, 5, 8, 32, 41). Moreover, inhibition of the glycolytic pathway has been shown to restrict HCMV and HSV-1 replication and induce apoptosis in cells latently infected with KSHV (32, 41–43). Here, we report the activation of glycolysis by DENV infection. We show that GLUT1 and HK2 expression is upregulated in DENV-infected cells, with a concurrent increase in glucose uptake. Several viruses have been found to upregulate the expression and/or activity of GLUT1 and HK2 (11, 32, 44–46), thus revealing common strategies viruses use to reprogram carbon metabolism. As many viruses induce glycolysis, it is possible that this metabolic alteration is simply a cellular response to infection. Viral replication may lead to the depletion of critical metabolite pools that could trigger a metabolic switch to increased aerobic glycolysis. However, a number of viral genes have been shown to specifically activate the glycolytic pathway in the absence of replication (11, 44, 47). Additionally, we recently found that VACV does not appear to induce glycolysis in the same cell type used in the present study (10), indicating that not all viruses activate glucose catabolism. Therefore, rather than increased glycolysis representing a general host response to viral infection, it is more likely that certain viruses have evolved to activate this pathway to support their life cycles.
The data presented here demonstrate that inhibition of the glycolytic pathway via oxamate or 2DG treatment results in a significant reduction in DENV replication. While the requirement for increased glycolysis during DENV infection is clear, how glycolysis is utilized for viral replication remains to be determined. HCMV induces enhanced flux through the glycolytic pathway to direct glucose carbon toward the TCA cycle and away from the mitochondrion in the form of citrate to fuel FAS (2, 3). DENV may also activate glycolysis to support lipid biogenesis. DENV infection upregulates FAS (24), and inhibition of fatty acid synthase (FASN), a major enzyme in the metabolic pathway, significantly impairs DENV replication in both human (24, 48) and mosquito (49) cells. Furthermore, FASN is relocalized to sites of viral replication by the DENV nonstructural protein 3 (24). Thus, it has been proposed that DENV modulates fatty acid metabolism to generate the lipid material needed for DENV replication complex (RC) formation (24), as these structures are assembled on invaginated cytoplasmic membranes (50, 51). Therefore, DENV might induce glycolysis to ultimately promote the establishment of DENV RCs. However, fatty acids also play a role in ATP synthesis during DENV infection (52). Autophagy-dependent processing of lipid droplets in DENV-infected cells releases free fatty acids, which undergo β-oxidation to produce ATP. Importantly, these processes are necessary for optimal DENV replication (52). Hence, lipid metabolism may be required at multiple stages during the DENV life cycle. Although enhanced glycolytic flux has been connected to increased FAS during virus infection, we cannot exclude the possibility that DENV requires the activation of glycolysis to directly support other replicative needs, such as the generation of ATP and NADH. Metabolic carbon flux analysis will provide further insight into global glucose usage during DENV infection.
This study and others have identified glycolysis as an essential metabolic pathway for the life cycle of several important human viruses. Although glycolysis plays a central role in cellular metabolism and function, it is possible that its short-term inhibition could be used to treat acute infections with DENV and other viruses that require the glycolytic pathway for replication. Pharmacological inhibitors of the glycolytic pathway show promising anticancer activity since a wide variety of human cancers exhibit increased aerobic glycolysis (reviewed in references 53 to 56). As several of these glycolytic inhibitors are currently in preclinical and clinical development, it is intriguing to consider the potential for these compounds to also be used as novel broad-spectrum antiviral therapies.
Supplementary Material
ACKNOWLEDGMENTS
We thank Sharon Isern and Scott Michael (Florida Gulf Coast University) for reagents and helpful discussions and Michael Gale, Jr. (University of Washington), for reagents.
M.L. was supported by grants from the NIAID, the NCI, and NIDCR of the NIH under awards U54AI081680, RO1CA097934, and PO1DE021954. K.A.F. was supported in part by Public Health Service National Research Service award T32 GM007270 from NIGMS.
The content of this report is solely our responsibility and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JVI.02309-14.
REFERENCES
- 1.Munger J, Bajad SU, Coller HA, Shenk T, Rabinowitz JD. 2006. Dynamics of the cellular metabolome during human cytomegalovirus infection. PLoS Pathog 2:e132. doi: 10.1371/journal.ppat.0020132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Munger J, Bennett BD, Parikh A, Feng XJ, McArdle J, Rabitz HA, Shenk T, Rabinowitz JD. 2008. Systems-level metabolic flux profiling identifies fatty acid synthesis as a target for antiviral therapy. Nat Biotechnol 26:1179–1186. doi: 10.1038/nbt.1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vastag L, Koyuncu E, Grady SL, Shenk TE, Rabinowitz JD. 2011. Divergent effects of human cytomegalovirus and herpes simplex virus-1 on cellular metabolism. PLoS Pathog 7:e1002124. doi: 10.1371/journal.ppat.1002124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rabinowitz JD, Purdy JG, Vastag L, Shenk T, Koyuncu E. 2011. Metabolomics in drug target discovery. Cold Spring Harb Symp Quant Biol 76:235–246. doi: 10.1101/sqb.2011.76.010694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Diamond DL, Syder AJ, Jacobs JM, Sorensen CM, Walters KA, Proll SC, McDermott JE, Gritsenko MA, Zhang Q, Zhao R, Metz TO, Camp DG II, Waters KM, Smith RD, Rice CM, Katze MG. 2010. Temporal proteome and lipidome profiles reveal hepatitis C virus-associated reprogramming of hepatocellular metabolism and bioenergetics. PLoS Pathog 6:e1000719. doi: 10.1371/journal.ppat.1000719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roe B, Kensicki E, Mohney R, Hall WW. 2011. Metabolomic profile of hepatitis C virus-infected hepatocytes. PLoS One 6:e23641. doi: 10.1371/journal.pone.0023641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ritter JB, Wahl AS, Freund S, Genzel Y, Reichl U. 2010. Metabolic effects of influenza virus infection in cultured animal cells: intra- and extracellular metabolite profiling. BMC Syst Biol 4:61. doi: 10.1186/1752-0509-4-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hollenbaugh JA, Munger J, Kim B. 2011. Metabolite profiles of human immunodeficiency virus infected CD4+ T cells and macrophages using LC-MS/MS analysis. Virology 415:153–159. doi: 10.1016/j.virol.2011.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Delgado T, Sanchez EL, Camarda R, Lagunoff M. 2012. Global metabolic profiling of infection by an oncogenic virus: KSHV induces and requires lipogenesis for survival of latent infection. PLoS Pathog 8:e1002866. doi: 10.1371/journal.ppat.1002866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fontaine KA, Camarda R, Lagunoff M. 2014. Vaccinia virus requires glutamine but not glucose for efficient replication. J Virol 88:4366–4374. doi: 10.1128/JVI.03134-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xiao L, Hu ZY, Dong X, Tan Z, Li W, Tang M, Chen L, Yang L, Tao Y, Jiang Y, Li J, Yi B, Li B, Fan S, You S, Deng X, Hu F, Feng L, Bode AM, Dong Z, Sun LQ, Cao Y. 2014. Targeting Epstein-Barr virus oncoprotein LMP1-mediated glycolysis sensitizes nasopharyngeal carcinoma to radiation therapy. Oncogene 33:4568–4578. doi: 10.1038/onc.2014.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DeBerardinis RJ, Mancuso A, Daikhin E, Nissim I, Yudkoff M, Wehrli S, Thompson CB. 2007. Beyond aerobic glycolysis: transformed cells can engage in glutamine metabolism that exceeds the requirement for protein and nucleotide synthesis. Proc Natl Acad Sci U S A 104:19345–19350. doi: 10.1073/pnas.0709747104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deberardinis RJ, Sayed N, Ditsworth D, Thompson CB. 2008. Brick by brick: metabolism and tumor cell growth. Curr Opin Genet Dev 18:54–61. doi: 10.1016/j.gde.2008.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wise DR, DeBerardinis RJ, Mancuso A, Sayed N, Zhang XY, Pfeiffer HK, Nissim I, Daikhin E, Yudkoff M, McMahon SB, Thompson CB. 2008. Myc regulates a transcriptional program that stimulates mitochondrial glutaminolysis and leads to glutamine addiction. Proc Natl Acad Sci U S A 105:18782–18787. doi: 10.1073/pnas.0810199105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chambers JW, Maguire TG, Alwine JC. 2010. Glutamine metabolism is essential for human cytomegalovirus infection. J Virol 84:1867–1873. doi: 10.1128/JVI.02123-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Greseth MD, Traktman P. 2014. De novo fatty acid biosynthesis contributes significantly to establishment of a bioenergetically favorable environment for vaccinia virus infection. PLoS Pathog 10:e1004021. doi: 10.1371/journal.ppat.1004021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, Moyes CL, Drake JM, Brownstein JS, Hoen AG, Sankoh O, Myers MF, George DB, Jaenisch T, Wint GR, Simmons CP, Scott TW, Farrar JJ, Hay SI. 2013. The global distribution and burden of dengue. Nature 496:504–507. doi: 10.1038/nature12060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Halstead SB. 2007. Dengue. Lancet 370:1644–1652. doi: 10.1016/S0140-6736(07)61687-0. [DOI] [PubMed] [Google Scholar]
- 19.Guzman MG, Halstead SB, Artsob H, Buchy P, Farrar J, Gubler DJ, Hunsperger E, Kroeger A, Margolis HS, Martinez E, Nathan MB, Pelegrino JL, Simmons C, Yoksan S, Peeling RW. 2010. Dengue: a continuing global threat. Nat Rev Microbiol 8(12 Suppl):S7–S16. doi: 10.1038/nrmicro2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beatty ME, Stone A, Fitzsimons DW, Hanna JN, Lam SK, Vong S, Guzman MG, Mendez-Galvan JF, Halstead SB, Letson GW, Kuritsky J, Mahoney R, Margolis HS. 2010. Best practices in dengue surveillance: a report from the Asia-Pacific and Americas Dengue Prevention Boards. PLoS Negl Trop Dis 4:e890. doi: 10.1371/journal.pntd.0000890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Birungi G, Chen SM, Loy BP, Ng ML, Li SF. 2010. Metabolomics approach for investigation of effects of dengue virus infection using the EA.hy926 cell line. J Proteome Res 9:6523–6534. doi: 10.1021/pr100727m. [DOI] [PubMed] [Google Scholar]
- 22.Wolf A, Agnihotri S, Micallef J, Mukherjee J, Sabha N, Cairns R, Hawkins C, Guha A. 2011. Hexokinase 2 is a key mediator of aerobic glycolysis and promotes tumor growth in human glioblastoma multiforme. J Exp Med 208:313–326. doi: 10.1084/jem.20101470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carroll PA, Brazeau E, Lagunoff M. 2004. Kaposi's sarcoma-associated herpesvirus infection of blood endothelial cells induces lymphatic differentiation. Virology 328:7–18. doi: 10.1016/j.virol.2004.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heaton NS, Perera R, Berger KL, Khadka S, Lacount DJ, Kuhn RJ, Randall G. 2010. Dengue virus nonstructural protein 3 redistributes fatty acid synthase to sites of viral replication and increases cellular fatty acid synthesis. Proc Natl Acad Sci U S A 107:17345–17350. doi: 10.1073/pnas.1010811107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vander Heiden MG, Cantley LC, Thompson CB. 2009. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science 324:1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Muñoz-Pinedo C, El Mjiyad N, Ricci JE. 2012. Cancer metabolism: current perspectives and future directions. Cell Death Dis 3:e248. doi: 10.1038/cddis.2011.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Augustin R. 2010. The protein family of glucose transport facilitators: it's not only about glucose after all. IUBMB Life 62:315–333. doi: 10.1002/iub.315. [DOI] [PubMed] [Google Scholar]
- 28.Gershon TR, Crowther AJ, Tikunov A, Garcia I, Annis R, Yuan H, Miller CR, Macdonald J, Olson J, Deshmukh M. 2013. Hexokinase-2-mediated aerobic glycolysis is integral to cerebellar neurogenesis and pathogenesis of medulloblastoma. Cancer Metab 1:2. doi: 10.1186/2049-3002-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Papaconstantinou J, Colowick SP. 1961. The role of glycolysis in the growth of tumor cells. I. Effects of oxamic acid on the metabolism of Ehrlich ascites tumor cells in vitro. J Biol Chem 236:278–284. [PubMed] [Google Scholar]
- 30.Papaconstantinou J, Colowick SP. 1961. The role of glycolysis in the growth of tumor cells. II. The effect of oxamic acid on the growth of HeLa cells in tissue culture. J Biol Chem 236:285–288. [PubMed] [Google Scholar]
- 31.Elwood JC. 1968. Effect of oxamate on glycolysis and respiration in sarcoma 37 ascites cells. Cancer Res 28:2056–2060. [PubMed] [Google Scholar]
- 32.Delgado T, Carroll PA, Punjabi AS, Margineantu D, Hockenbery DM, Lagunoff M. 2010. Induction of the Warburg effect by Kaposi's sarcoma herpesvirus is required for the maintenance of latently infected endothelial cells. Proc Natl Acad Sci U S A 107:10696–10701. doi: 10.1073/pnas.1004882107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jessie K, Fong MY, Devi S, Lam SK, Wong KT. 2004. Localization of dengue virus in naturally infected human tissues, by immunohistochemistry and in situ hybridization. J Infect Dis 189:1411–1418. doi: 10.1086/383043. [DOI] [PubMed] [Google Scholar]
- 34.Bhoopat L, Bhamarapravati N, Attasiri C, Yoksarn S, Chaiwun B, Khunamornpong S, Sirisanthana V. 1996. Immunohistochemical characterization of a new monoclonal antibody reactive with dengue virus-infected cells in frozen tissue using immunoperoxidase technique. Asian Pac J Allergy Immunol 14:107–113. [PubMed] [Google Scholar]
- 35.Hall WC, Crowell TP, Watts DM, Barros VL, Kruger H, Pinheiro F, Peters CJ. 1991. Demonstration of yellow fever and dengue antigens in formalin-fixed paraffin-embedded human liver by immunohistochemical analysis. Am J Trop Med Hyg 45:408–417. [DOI] [PubMed] [Google Scholar]
- 36.Balsitis SJ, Coloma J, Castro G, Alava A, Flores D, McKerrow JH, Beatty PR, Harris E. 2009. Tropism of dengue virus in mice and humans defined by viral nonstructural protein 3-specific immunostaining. Am J Trop Med Hyg 80:416–424. [PubMed] [Google Scholar]
- 37.Avirutnan P, Malasit P, Seliger B, Bhakdi S, Husmann M. 1998. Dengue virus infection of human endothelial cells leads to chemokine production, complement activation, and apoptosis. J Immunol 161:6338–6346. [PubMed] [Google Scholar]
- 38.Huang YH, Lei HY, Liu HS, Lin YS, Chen SH, Liu CC, Yeh TM. 2003. Tissue plasminogen activator induced by dengue virus infection of human endothelial cells. J Med Virol 70:610–616. doi: 10.1002/jmv.10438. [DOI] [PubMed] [Google Scholar]
- 39.Huang YH, Lei HY, Liu HS, Lin YS, Liu CC, Yeh TM. 2000. Dengue virus infects human endothelial cells and induces IL-6 and IL-8 production. Am J Trop Med Hyg 63:71–75. [DOI] [PubMed] [Google Scholar]
- 40.Warke RV, Xhaja K, Martin KJ, Fournier MF, Shaw SK, Brizuela N, de Bosch N, Lapointe D, Ennis FA, Rothman AL, Bosch I. 2003. Dengue virus induces novel changes in gene expression of human umbilical vein endothelial cells. J Virol 77:11822–11832. doi: 10.1128/JVI.77.21.11822-11832.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McArdle J, Schafer XL, Munger J. 2011. Inhibition of calmodulin-dependent kinase kinase blocks human cytomegalovirus-induced glycolytic activation and severely attenuates production of viral progeny. J Virol 85:705–714. doi: 10.1128/JVI.01557-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Radsak KD, Weder D. 1981. Effect of 2-deoxy-d-glucose on cytomegalovirus-induced DNA synthesis in human fibroblasts. J Gen Virol 57:33–42. doi: 10.1099/0022-1317-57-1-33. [DOI] [PubMed] [Google Scholar]
- 43.Courtney RJ, Steiner SM, Benyesh-Melnick M. 1973. Effects of 2-deoxy-d-glucose on herpes simplex virus replication. Virology 52:447–455. doi: 10.1016/0042-6822(73)90340-1. [DOI] [PubMed] [Google Scholar]
- 44.Ramière C, Rodriguez J, Enache LS, Lotteau V, Andre P, Diaz O. 2014. Activity of hexokinase is increased by its interaction with hepatitis C virus protein NS5A. J Virol 88:3246–3254. doi: 10.1128/JVI.02862-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Loisel-Meyer S, Swainson L, Craveiro M, Oburoglu L, Mongellaz C, Costa C, Martinez M, Cosset FL, Battini JL, Herzenberg LA, Atkuri KR, Sitbon M, Kinet S, Verhoeyen E, Taylor N. 2012. Glut1-mediated glucose transport regulates HIV infection. Proc Natl Acad Sci U S A 109:2549–2554. doi: 10.1073/pnas.1121427109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gonnella R, Santarelli R, Farina A, Granato M, D'Orazi G, Faggioni A, Cirone M. 2013. Kaposi sarcoma associated herpesvirus (KSHV) induces AKT hyperphosphorylation, bortezomib-resistance and GLUT-1 plasma membrane exposure in THP-1 monocytic cell line. J Exp Clin Cancer Res 32:79. doi: 10.1186/1756-9966-32-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yogev O, Lagos D, Enver T, Boshoff C. 2014. Kaposi's sarcoma herpesvirus microRNAs induce metabolic transformation of infected cells. PLoS Pathog 10:e1004400. doi: 10.1371/journal.ppat.1004400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Samsa MM, Mondotte JA, Iglesias NG, Assuncao-Miranda I, Barbosa-Lima G, Da Poian AT, Bozza PT, Gamarnik AV. 2009. Dengue virus capsid protein usurps lipid droplets for viral particle formation. PLoS Pathog 5:e1000632. doi: 10.1371/journal.ppat.1000632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Perera R, Riley C, Isaac G, Hopf-Jannasch AS, Moore RJ, Weitz KW, Pasa-Tolic L, Metz TO, Adamec J, Kuhn RJ. 2012. Dengue virus infection perturbs lipid homeostasis in infected mosquito cells. PLoS Pathog 8:e1002584. doi: 10.1371/journal.ppat.1002584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.den Boon JA, Diaz A, Ahlquist P. 2010. Cytoplasmic viral replication complexes. Cell Host Microbe 8:77–85. doi: 10.1016/j.chom.2010.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Welsch S, Miller S, Romero-Brey I, Merz A, Bleck CK, Walther P, Fuller SD, Antony C, Krijnse-Locker J, Bartenschlager R. 2009. Composition and three-dimensional architecture of the dengue virus replication and assembly sites. Cell Host Microbe 5:365–375. doi: 10.1016/j.chom.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Heaton NS, Randall G. 2010. Dengue virus-induced autophagy regulates lipid metabolism. Cell Host Microbe 8:422–432. doi: 10.1016/j.chom.2010.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pelicano H, Martin DS, Xu RH, Huang P. 2006. Glycolysis inhibition for anticancer treatment. Oncogene 25:4633–4646. doi: 10.1038/sj.onc.1209597. [DOI] [PubMed] [Google Scholar]
- 54.Scatena R, Bottoni P, Pontoglio A, Mastrototaro L, Giardina B. 2008. Glycolytic enzyme inhibitors in cancer treatment. Expert Opin Invest Drugs 17:1533–1545. doi: 10.1517/13543784.17.10.1533. [DOI] [PubMed] [Google Scholar]
- 55.Ganapathy-Kanniappan S, Geschwind JF. 2013. Tumor glycolysis as a target for cancer therapy: progress and prospects. Mol Cancer 12:152. doi: 10.1186/1476-4598-12-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tennant DA, Duran RV, Gottlieb E. 2010. Targeting metabolic transformation for cancer therapy. Nat Rev Cancer 10:267–277. doi: 10.1038/nrc2817. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.