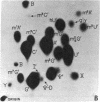
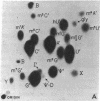
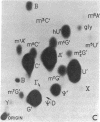
Abstract
Using a chemical isotope derivative method, we have determined the minor base composition of the “70S-associated” 4S RNA isolated from avian myeloblastosis virus. The minor base composition of this “70S-associated” 4S RNA is strikingly similar to that of the corresponding “free” 4S RNA of the virion. This minor base content, plus the capacity to esterify amino acids, establishes that both of these virion 4S RNA fractions contain transfer RNA. The percentage of minor bases in virion “70S-associated” 4S RNA is, however, much lower than in “free” 4S virion RNA and in myeloblast 4S RNA. The implication is that the “70S-associated” 4S RNA fraction as isolated herein also contains RNA that is not transfer RNA. This latter RNA may represent either degradation products of high molecular weight RNA or indigenous 4S RNA of undetermined function.
Keywords: tumor virus, isotope derivative method, tritium fluorography
Full text
PDF
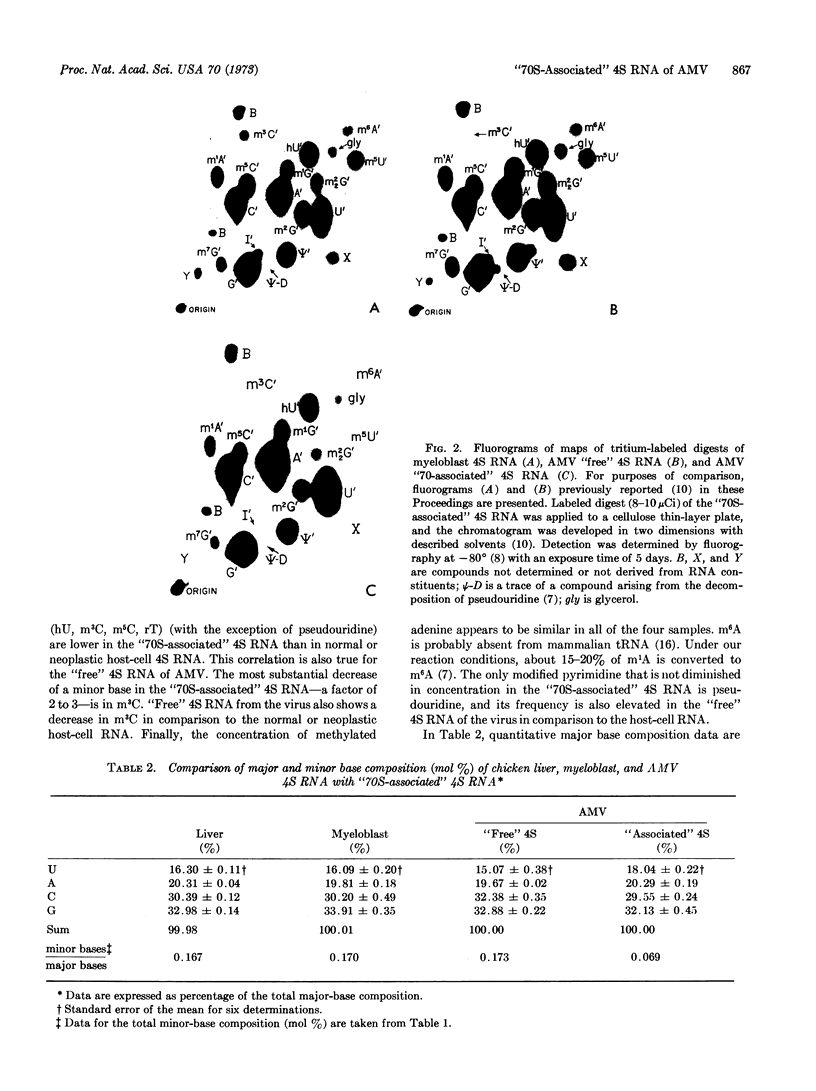


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen D. W. Actinomycin D inhibition of avian myeloblastosis virus production by chick-embryo fibroblasts. Biochim Biophys Acta. 1966 Mar 21;114(3):606–611. doi: 10.1016/0005-2787(66)90108-0. [DOI] [PubMed] [Google Scholar]
- Bishop J. M., Levinson W. E., Sullivan D., Fanshier L., Quintrell N., Jackson J. The low molecular weight RNAs of Rous sarcoma virus. II. The 7 S RNA. Virology. 1970 Dec;42(4):927–937. doi: 10.1016/0042-6822(70)90341-7. [DOI] [PubMed] [Google Scholar]
- Duesberg P. H., Robinson W. S. Isolation of the nucleic acid of Newcastle disease virus (NDV). Proc Natl Acad Sci U S A. 1965 Sep;54(3):794–800. doi: 10.1073/pnas.54.3.794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duesberg P. H. The RNA of influenza virus. Proc Natl Acad Sci U S A. 1968 Mar;59(3):930–937. doi: 10.1073/pnas.59.3.930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erikson E., Erikson R. L. Association of 4S ribonucleic acid with oncornavirus ribonucleic acids. J Virol. 1971 Aug;8(2):254–256. doi: 10.1128/jvi.8.2.254-256.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erikson E., Erikson R. L. Isolation of amino acid acceptor RNA from purified avian myeloblastosis virus. J Mol Biol. 1970 Sep 14;52(2):387–390. doi: 10.1016/0022-2836(70)90038-0. [DOI] [PubMed] [Google Scholar]
- Feldmann H., Falter H. Transfer ribonucleic acid from Mycoplasma laidlawii A. Eur J Biochem. 1971 Feb;18(4):573–581. doi: 10.1111/j.1432-1033.1971.tb01278.x. [DOI] [PubMed] [Google Scholar]
- Iwanami Y., Brown G. M. Methylated bases of transfer ribonucleic acid from HeLa and L cells. Arch Biochem Biophys. 1968 Mar 20;124(1):472–482. doi: 10.1016/0003-9861(68)90355-x. [DOI] [PubMed] [Google Scholar]
- Randerath E., Broeke J. W.T., Randerath K. Analysis of nucleic acid derivatives at the subnanomolar level. (IV) Analysis of polyribonucleotides by conversion to tritiated nucleoside derivatives. FEBS Lett. 1968 Nov;2(1):10–12. doi: 10.1016/0014-5793(68)80086-9. [DOI] [PubMed] [Google Scholar]
- Randerath E., Yu C. T., Randerath K. Base analysis of ribopolynucleotides by chemical tritium labeling: a methodological study with model nucleosides and purified tRNA species. Anal Biochem. 1972 Jul;48(1):172–198. doi: 10.1016/0003-2697(72)90181-9. [DOI] [PubMed] [Google Scholar]
- Randerath K. An evaluation of film detection methods for weak beta-emitters, particularly tritium. Anal Biochem. 1970 Mar;34:188–205. doi: 10.1016/0003-2697(70)90100-4. [DOI] [PubMed] [Google Scholar]
- Randerath K., Flood K. M., Randerath E. Analysis of nucleic acid derivatives at the subnanomole level. (V) High resolution mapping of tritium labelled RNA derivatives. FEBS Lett. 1969 Sep;5(1):31–33. doi: 10.1016/0014-5793(69)80285-1. [DOI] [PubMed] [Google Scholar]
- Randerath K., Randerath E. Analysis of nucleic acid derivatives at the subnanomole level. 3. A tritium labeling procedure for quantitative analysis of ribose derivatives. Anal Biochem. 1969 Apr 4;28(1):110–118. doi: 10.1016/0003-2697(69)90162-6. [DOI] [PubMed] [Google Scholar]
- Randerath K., Rosenthal L. J., Zamecnik P. C. Base composition differences between avian myeloblastosis virus transfer RNA and transfer RNA isolated from host cells. Proc Natl Acad Sci U S A. 1971 Dec;68(12):3233–3237. doi: 10.1073/pnas.68.12.3233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson M. L., Wirthlin L. S., Scott J. F., Zamecnik P. C. The 3'-terminal nucleosides of the high molecular weight RNA of avian myeloblastosis virus. Proc Natl Acad Sci U S A. 1972 May;69(5):1176–1180. doi: 10.1073/pnas.69.5.1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart T. S., Roberts R. J., Strominger J. L. Novel species of tRNA. Nature. 1971 Mar 5;230(5288):36–38. doi: 10.1038/230036a0. [DOI] [PubMed] [Google Scholar]
- Strauss J. H., Jr, Kelly R. B., Sinsheimer R. L. Denaturation of RNA with dimethyl sulfoxide. Biopolymers. 1968 Jun;6(6):793–807. doi: 10.1002/bip.1968.360060604. [DOI] [PubMed] [Google Scholar]
- Trávnícek M. RNA with amino acid-acceptor activity isolated from an oncogenic virus. Biochim Biophys Acta. 1968 Oct 29;166(3):757–759. [PubMed] [Google Scholar]
- Trávnícek M. Some properties of amino acid-acceptor RNA isolated from avian tumour virus BAI strain A (avian myeloblastosis). Biochim Biophys Acta. 1969 Jun 17;182(2):427–439. [PubMed] [Google Scholar]
- Verma I. M., Meuth N. L., Bromfeld E., Manly K. F., Baltimore D. Covalently linked RNA-DNA molecule as initial product of RNA tumour virus DNA polymerase. Nat New Biol. 1971 Sep 29;233(39):131–134. doi: 10.1038/newbio233131a0. [DOI] [PubMed] [Google Scholar]