Abstract
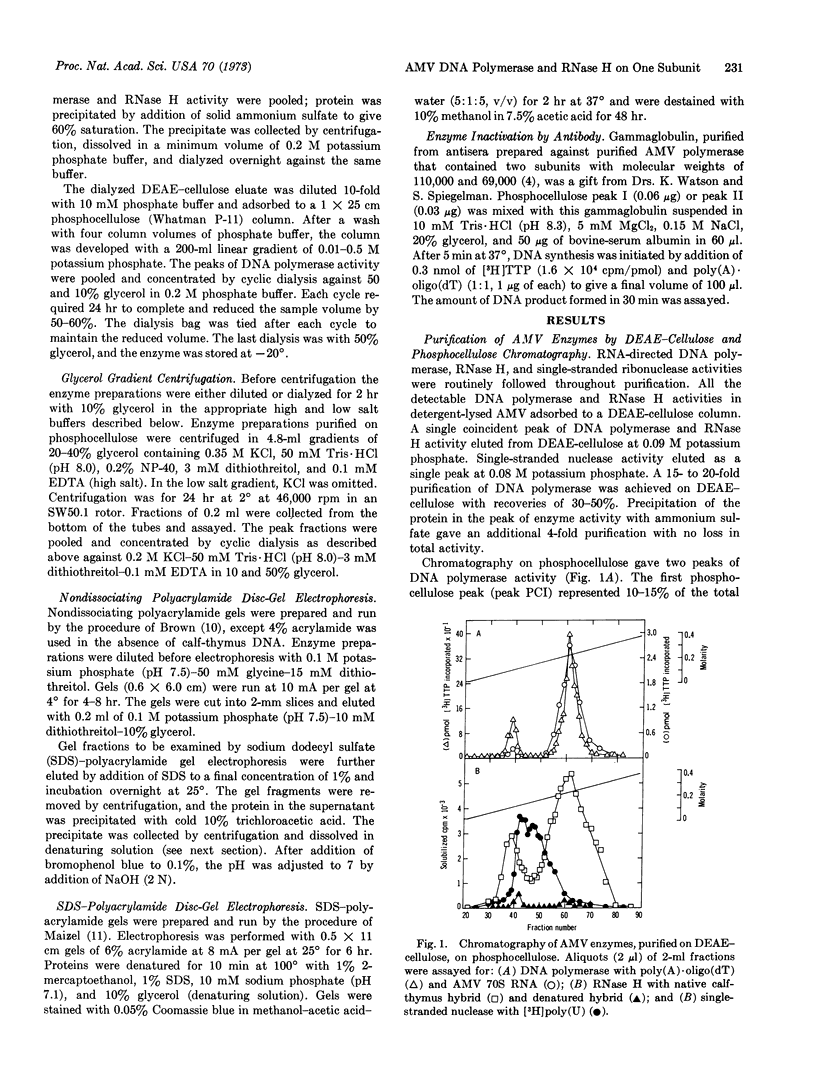
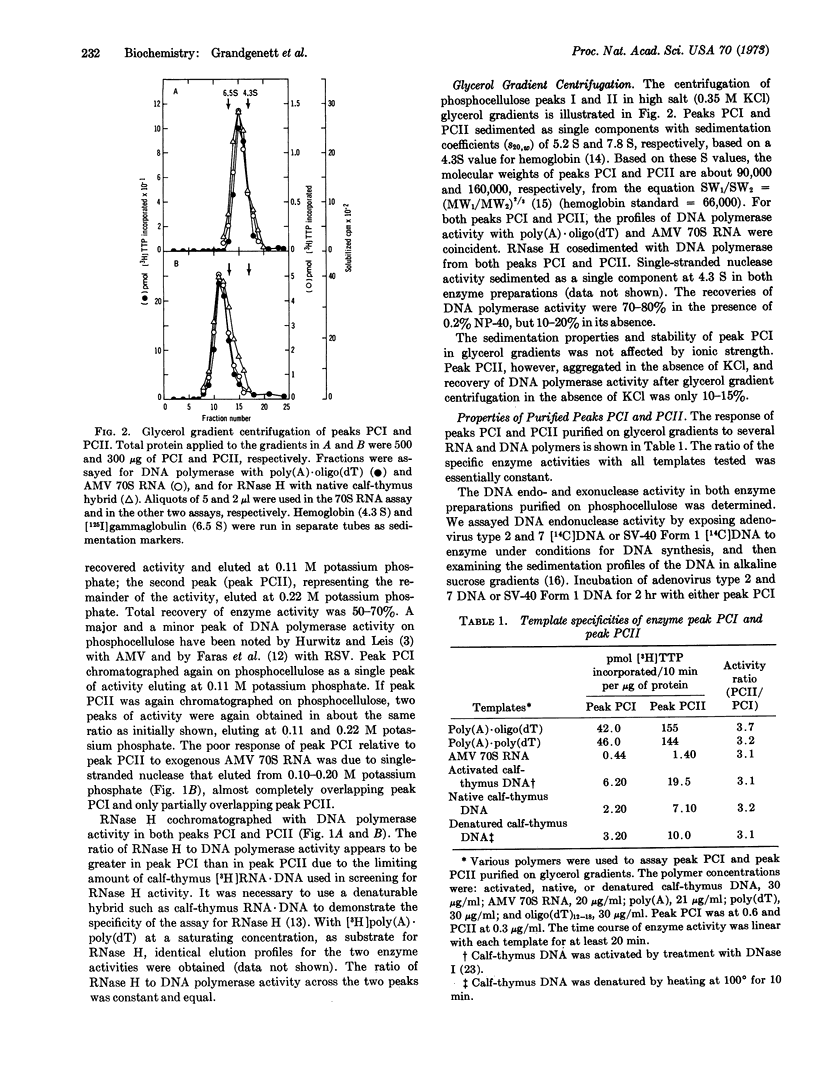
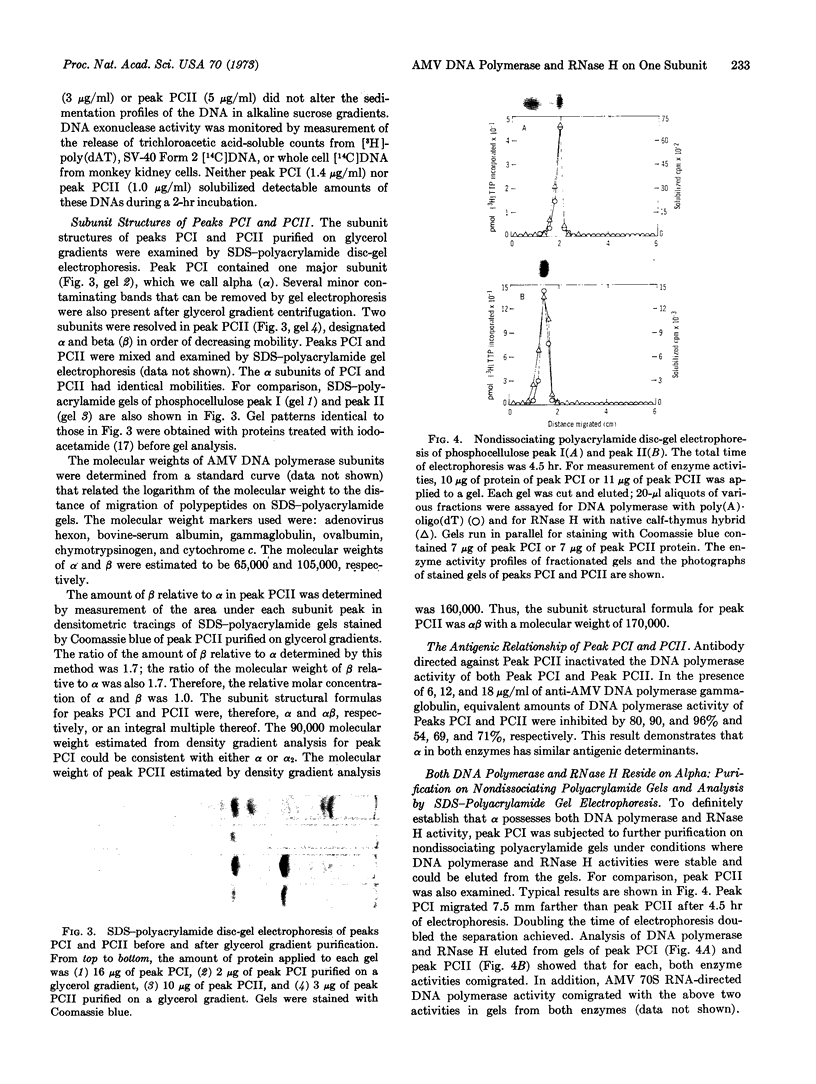
Two structurally distinct forms of RNA-directed DNA polymerase from avian myeloblastosis virus were resolved by chromatography on phosphocellulose and purified. In addition to RNA-directed DNA polymerase activity, both enzymes had ribonuclease H (RNase H) activity, which degraded the RNA moiety of RNA·DNA hybrids. As determined by sodium dodecyl sulfate-polyacrylamide disc-gel electrophoresis, one form had two subunits, alpha (α) and beta (β), with molecular weights of 65,000 and 105,000, respectively. The other had a single subunit, α, with a molecular weight of 65,000. The sedimentation coefficients of αβ and α, determined by glycerol gradient centrifugation in 0.35 M KCl, were 7.8 S and 5.2 S, respectively. Both enzymes had similar antigenic determinants and could not be distinguished by a differential response to several different RNA and DNA templates. We suggest that α, which contains both RNA-directed DNA polymerase and RNase H activity, is derived by dissociation of αβ; the function of the β subunit is unknown.
Keywords: RNA virus, polyacrylamide gel electrophoresis
Full text
PDF
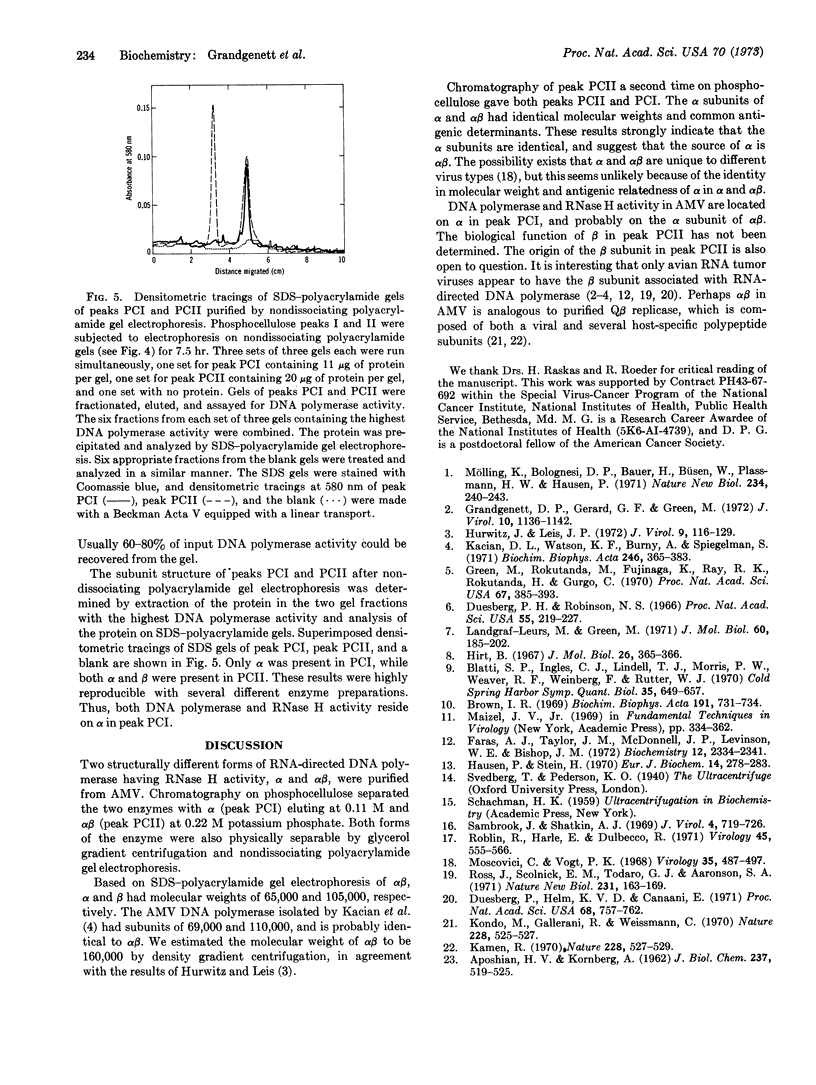
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- APOSHIAN H. V., KORNBERG A. Enzymatic synthesis of deoxyribonucleic acid. IX. The polymerase formed after T2 bacteriophage infection of Escherichia coli: a new enzyme. J Biol Chem. 1962 Feb;237:519–525. [PubMed] [Google Scholar]
- Brown I. R. Polyacrylamide gel electrophoresis of DNA polymerase from Ehrlich ascites tumor cells and recovery of active enzyme. Biochim Biophys Acta. 1969;191(3):731–734. doi: 10.1016/0005-2744(69)90372-6. [DOI] [PubMed] [Google Scholar]
- Duesberg P. H., Robinson W. S. Nucleic acid and proteins isolated from the Rauscher mouse leukemia virus (MLV). Proc Natl Acad Sci U S A. 1966 Jan;55(1):219–227. doi: 10.1073/pnas.55.1.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faras A. J., Taylor J. M., McDonnell J. P., Levinson W. E., Bishop J. M. Purification and characterization of the deoxyribonucleic acid polymerase associated with Rous sarcoma virus. Biochemistry. 1972 Jun 6;11(12):2334–2342. doi: 10.1021/bi00762a020. [DOI] [PubMed] [Google Scholar]
- Grandgenett D. P., Gerard G. F., Green M. Ribonuclease H: a ubiquitous activity in virions of ribonucleic acid tumor viruses. J Virol. 1972 Dec;10(6):1136–1142. doi: 10.1128/jvi.10.6.1136-1142.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green M., Rokutanda M., Fujinaga K., Ray R. K., Rokutanda H., Gurgo C. Mechanism of carcinogenesis by RNA tumor viruses. I. An RNA-dependent DNA polymerase in murine sarcoma viruses. Proc Natl Acad Sci U S A. 1970 Sep;67(1):385–393. doi: 10.1073/pnas.67.1.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausen P., Stein H. Ribonuclease H. An enzyme degrading the RNA moiety of DNA-RNA hybrids. Eur J Biochem. 1970 Jun;14(2):278–283. doi: 10.1111/j.1432-1033.1970.tb00287.x. [DOI] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Hurwitz J., Leis J. P. RNA-dependent DNA polymerase activity of RNA tumor viruses. I. Directing influence of DNA in the reaction. J Virol. 1972 Jan;9(1):116–129. doi: 10.1128/jvi.9.1.116-129.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kacian D. L., Watson K. F., Burny A., Spiegelman S. Purification of the DNA polymerase of avian myeloblastosis virus. Biochim Biophys Acta. 1971 Sep 24;246(3):365–383. doi: 10.1016/0005-2787(71)90773-8. [DOI] [PubMed] [Google Scholar]
- Kamen R. Characterization of the subunits of Q-beta replicase. Nature. 1970 Nov 7;228(5271):527–533. doi: 10.1038/228527a0. [DOI] [PubMed] [Google Scholar]
- Kondo M., Gallerani R., Weissmann C. Subunit structure of Q-beta replicase. Nature. 1970 Nov 7;228(5271):525–527. doi: 10.1038/228525a0. [DOI] [PubMed] [Google Scholar]
- Landgraf-Leurs M., Green M. Adenovirus DNA. 3. Separation of the complementary strands of adenovirus types 2, 7 and 12 DNA molecules. J Mol Biol. 1971 Aug 28;60(1):185–202. doi: 10.1016/0022-2836(71)90457-8. [DOI] [PubMed] [Google Scholar]
- Moran D. T., Varela F. G. Microtubules and sensory transduction. Proc Natl Acad Sci U S A. 1971 Apr;68(4):757–760. doi: 10.1073/pnas.68.4.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscovici C., Vogt P. K. Effects of genetic cellular resistance on cell transformation and virus replication in chicken hematopoietic cell cultures infected with avian myeloblastosis virus (BAI-A). Virology. 1968 Aug;35(4):487–497. doi: 10.1016/0042-6822(68)90278-x. [DOI] [PubMed] [Google Scholar]
- Mölling K., Bolognesi D. P., Bauer H., Büsen W., Plassmann H. W., Hausen P. Association of viral reverse transcriptase with an enzyme degrading the RNA moiety of RNA-DNA hybrids. Nat New Biol. 1971 Dec 22;234(51):240–243. doi: 10.1038/newbio234240a0. [DOI] [PubMed] [Google Scholar]
- Roblin R., Härle E., Dulbecco R. Polyoma virus proteins. 1. Multiple virion components. Virology. 1971 Sep;45(3):555–566. doi: 10.1016/0042-6822(71)90171-1. [DOI] [PubMed] [Google Scholar]
- Ross J., Scolnick E. M., Todaro G. J., Aaronson S. A. Separation of murine cellular and murine leukaemia virus DNA polymerases. Nat New Biol. 1971 Jun 9;231(23):163–167. doi: 10.1038/newbio231163a0. [DOI] [PubMed] [Google Scholar]
- Sambrook J., Shatkin A. J. Polynucleotide ligase activity in cells infected with simian virus 40, polyoma virus, or vaccinia virus. J Virol. 1969 Nov;4(5):719–726. doi: 10.1128/jvi.4.5.719-726.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]