Abstract
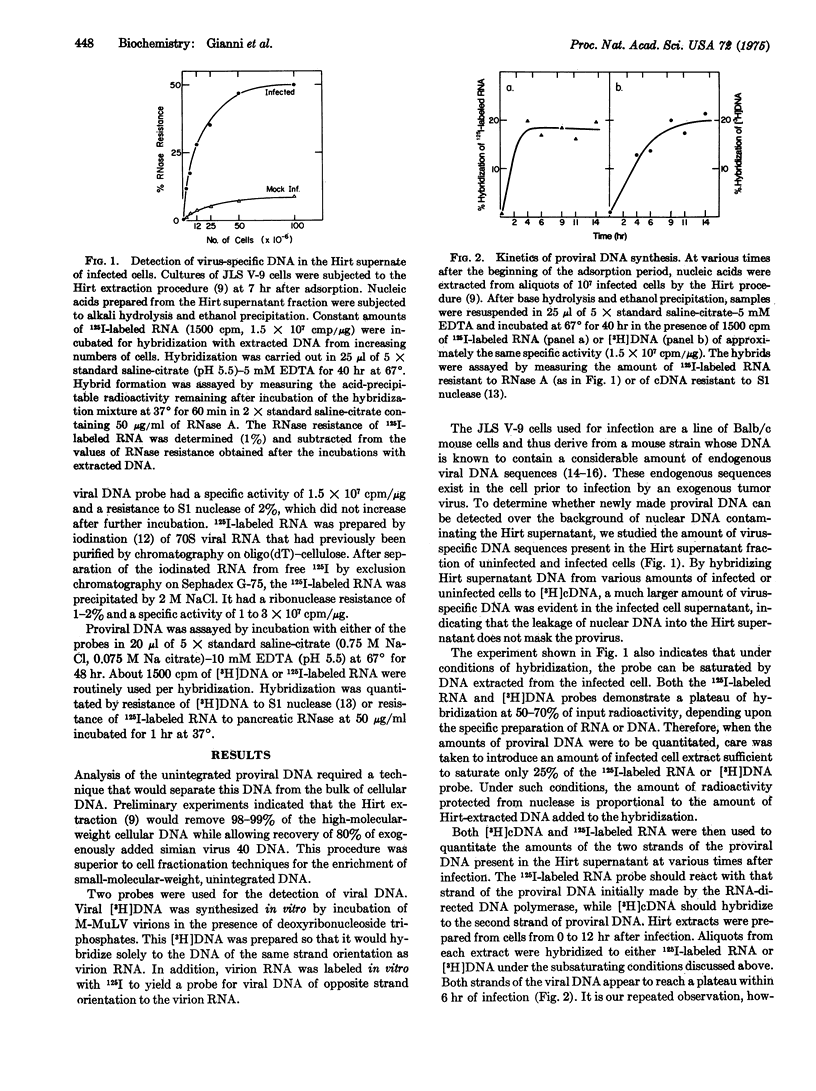
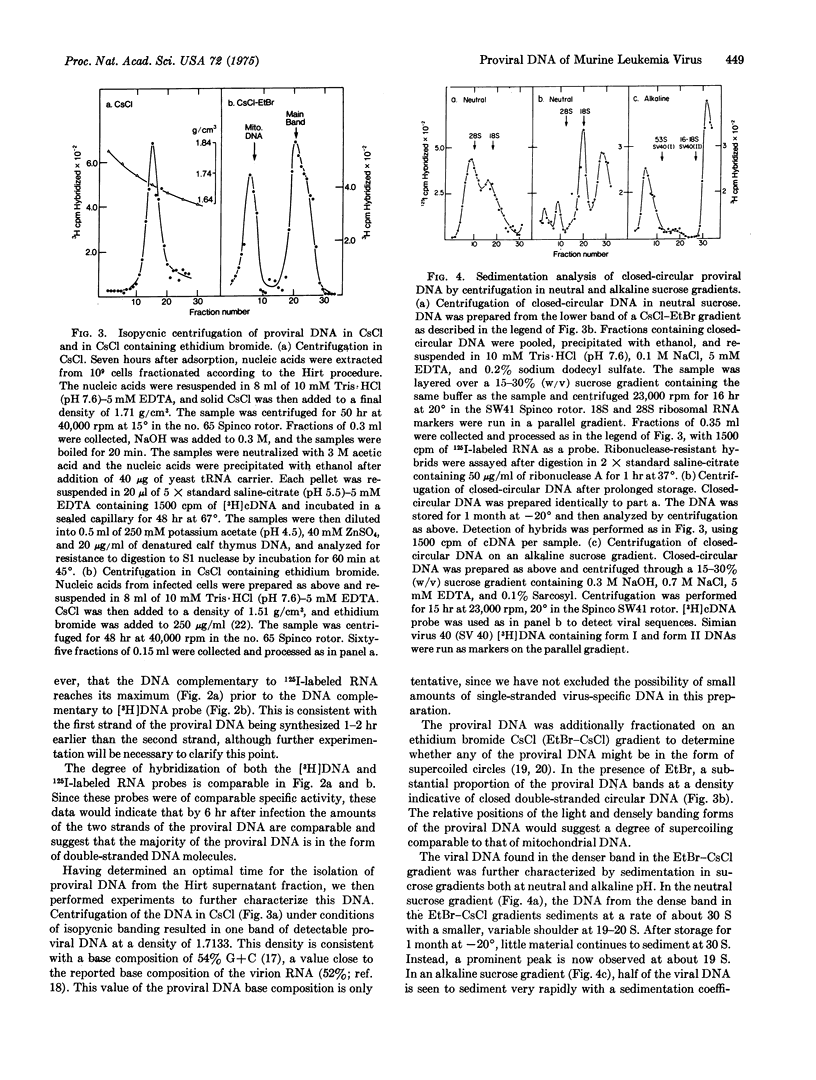
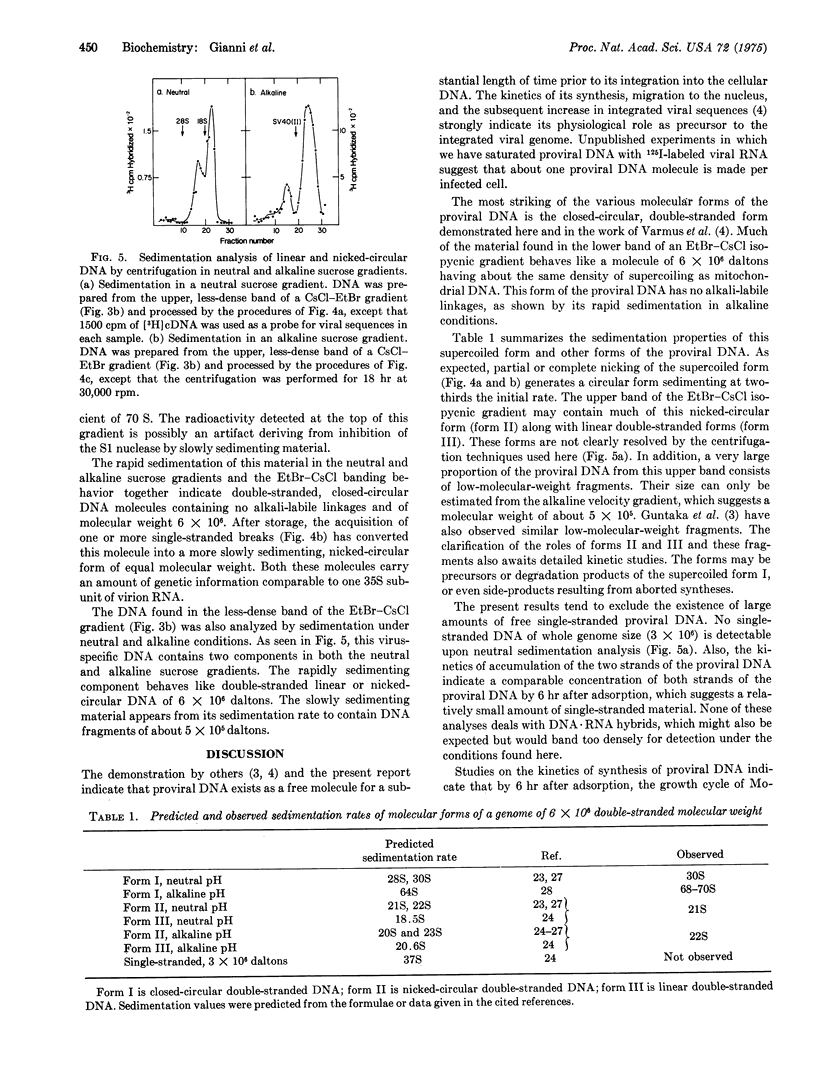
Infection of JLS V-9 (mouse bone marrow) cells with Moloney murine leukemia virus leads to appearance of virus-specific DNA in the low molecular weight DNA fraction from infected cells. Most of this DNA is double-stranded. Two discrete forms of double-stranded DNA, both of molecular weight 6 times 10-6, have been characterized. One of these forms is closed-circular, supercoiled DNA; the other form is a mixture of nicked-circular and linear DNA.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atherton K. T., Darby G. Extraction of RNA from mammalian tissue culture cells. Anal Biochem. 1974 Feb;57(2):403–412. doi: 10.1016/0003-2697(74)90095-5. [DOI] [PubMed] [Google Scholar]
- Birnstiel M. L., Sells B. H., Purdom I. F. Kinetic complexity of RNA molecules. J Mol Biol. 1972 Jan 14;63(1):21–39. doi: 10.1016/0022-2836(72)90519-0. [DOI] [PubMed] [Google Scholar]
- Boiron M., Lévy J. P., Périès J. In vitro investigations on murine leukemia viruses. Prog Med Virol. 1967;9:341–391. [PubMed] [Google Scholar]
- Böttger M., Bierwolf D., Wunderlich V., Graffi A. New calibration correlations for molecular weights of circular DNA: the molecular weight of the DNA of an oncogenic papova virus of the Syrian hamster. Biochim Biophys Acta. 1971 Feb 25;232(1):21–31. doi: 10.1016/0005-2787(71)90487-4. [DOI] [PubMed] [Google Scholar]
- Espejo R. T., Canelo E. S., Sinsheimer R. L. Replication of bacteriophage PM2 deoxyribonucleic acid: a closed circular double-stranded molecule. J Mol Biol. 1971 Mar 28;56(3):597–621. doi: 10.1016/0022-2836(71)90404-9. [DOI] [PubMed] [Google Scholar]
- Fan H., Baltimore D. RNA metabolism of murine leukemia virus: detection of virus-specific RNA sequences in infected and uninfected cells and identification of virus-specific messenger RNA. J Mol Biol. 1973 Oct 15;80(1):93–117. doi: 10.1016/0022-2836(73)90235-0. [DOI] [PubMed] [Google Scholar]
- Fan H., Paskind M. Measurement of the sequence complexity of cloned Moloney murine leukemia virus 60 to 70S RNA: evidence for a haploid genome. J Virol. 1974 Sep;14(3):421–429. doi: 10.1128/jvi.14.3.421-429.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fareed G. C., McKerlie M. L., Salzman N. P. Characterization of Simian virus 40 DNA component II during viral DNA replication. J Mol Biol. 1973 Feb 25;74(2):95–111. doi: 10.1016/0022-2836(73)90101-0. [DOI] [PubMed] [Google Scholar]
- Gelb L. D., Milstien J. B., Martin M. A., Aaronson S. A. Characterization of murine leukaemia virus-specific DNA present in normal mouse cells. Nat New Biol. 1973 Jul 18;244(133):76–79. doi: 10.1038/newbio244076a0. [DOI] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Hudson B., Upholt W. B., Devinny J., Vinograd J. The use of an ethidium analogue in the dye-buoyant density procedure for the isolation of closed circular DNA: the variation of the superhelix density of mitochondrial DNA. Proc Natl Acad Sci U S A. 1969 Mar;62(3):813–820. doi: 10.1073/pnas.62.3.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manly K. F., Smoler D. F., Bromfeld E., Baltimore D. Forms of deoxyribonucleic acid produced by virions of the ribonucleic acid tumor viruses. J Virol. 1971 Jan;7(1):106–111. doi: 10.1128/jvi.7.1.106-111.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radloff R., Bauer W., Vinograd J. A dye-buoyant-density method for the detection and isolation of closed circular duplex DNA: the closed circular DNA in HeLa cells. Proc Natl Acad Sci U S A. 1967 May;57(5):1514–1521. doi: 10.1073/pnas.57.5.1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe W. P., Pugh W. E., Hartley J. W. Plaque assay techniques for murine leukemia viruses. Virology. 1970 Dec;42(4):1136–1139. doi: 10.1016/0042-6822(70)90362-4. [DOI] [PubMed] [Google Scholar]
- SCHILDKRAUT C. L., MARMUR J., DOTY P. Determination of the base composition of deoxyribonucleic acid from its buoyant density in CsCl. J Mol Biol. 1962 Jun;4:430–443. doi: 10.1016/s0022-2836(62)80100-4. [DOI] [PubMed] [Google Scholar]
- STUDIER F. W. SEDIMENTATION STUDIES OF THE SIZE AND SHAPE OF DNA. J Mol Biol. 1965 Feb;11:373–390. doi: 10.1016/s0022-2836(65)80064-x. [DOI] [PubMed] [Google Scholar]
- Sakakibara Y., Tomizawa J. Replication of colicin E1 plasmid DNA in cell extracts. II. Selective synthesis of early replicative intermediates. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1403–1407. doi: 10.1073/pnas.71.4.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmir M., Révet B. M., Vinograd J. Dependence of the sedimentation coefficient of denatured closed circular DNA in alkali on the degree of strand interwinding. The absolute sense of supercoils. J Mol Biol. 1974 Feb 15;83(1):35–45. doi: 10.1016/0022-2836(74)90422-7. [DOI] [PubMed] [Google Scholar]
- Sweet R. W., Goodman N. C., Cho J. R., Ruprecht R. M., Redfield R. R., Spiegelman S. The presence of unique DNA sequences after viral induction of leukemia in mice. (RNA tumor virus-nucleic acid hybridization-insertion of viral DNA). Proc Natl Acad Sci U S A. 1974 May;71(5):1705–1709. doi: 10.1073/pnas.71.5.1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tereba A., McCarthy B. J. Hybridization of 125I-labeled ribonucleic acid. Biochemistry. 1973 Nov 6;12(23):4675–4679. doi: 10.1021/bi00747a020. [DOI] [PubMed] [Google Scholar]
- Varmus H. E., Bishop J. M., Vogt P. K. Appearance of virus-specific DNA in mammalian cells following transformation by Rous sarcoma virus. J Mol Biol. 1973 Mar 15;74(4):613–626. doi: 10.1016/0022-2836(73)90052-1. [DOI] [PubMed] [Google Scholar]
- Varmus H. E., Guntaka R. V., Fan W. J., Heasley S., Bishop J. M. Synthesis of viral DNA in the cytoplasm of duck embryo fibroblasts and in enucleated cells after infection by avian sarcoma virus. Proc Natl Acad Sci U S A. 1974 Oct;71(10):3874–3878. doi: 10.1073/pnas.71.10.3874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varmus H. E., Vogt P. K., Bishop J. M. Integration of deoxyribonucleic acid specific for Rous sarcoma virus after infection of permissive and nonpermissive hosts. Proc Natl Acad Sci U S A. 1973 Nov;70(11):3067–3071. doi: 10.1073/pnas.70.11.3067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viola M. V., White L. R. Differences in murine leukaemia virus-specific DNA sequences in normal and malignant cells. Nature. 1973 Dec 21;246(5434):485–487. doi: 10.1038/246485a0. [DOI] [PubMed] [Google Scholar]
- Wong P. K., Russ L. J., McCarter J. A. Rapid, selective procedure for isolation of spontaneous temperature-sensitive mutants of Moloney leukemia virus. Virology. 1973 Feb;51(2):424–431. doi: 10.1016/0042-6822(73)90441-8. [DOI] [PubMed] [Google Scholar]
- Wright B. S., O'Brien P. A., Shibley G. P., Mayyasi S. A., Lasfargues J. C. Infection of an established mouse bone marrow cell line (JLS-V9) with Rauscher and Moloney murine leukemia viruses. Cancer Res. 1967 Sep;27(9):1672–1677. [PubMed] [Google Scholar]


