Abstract
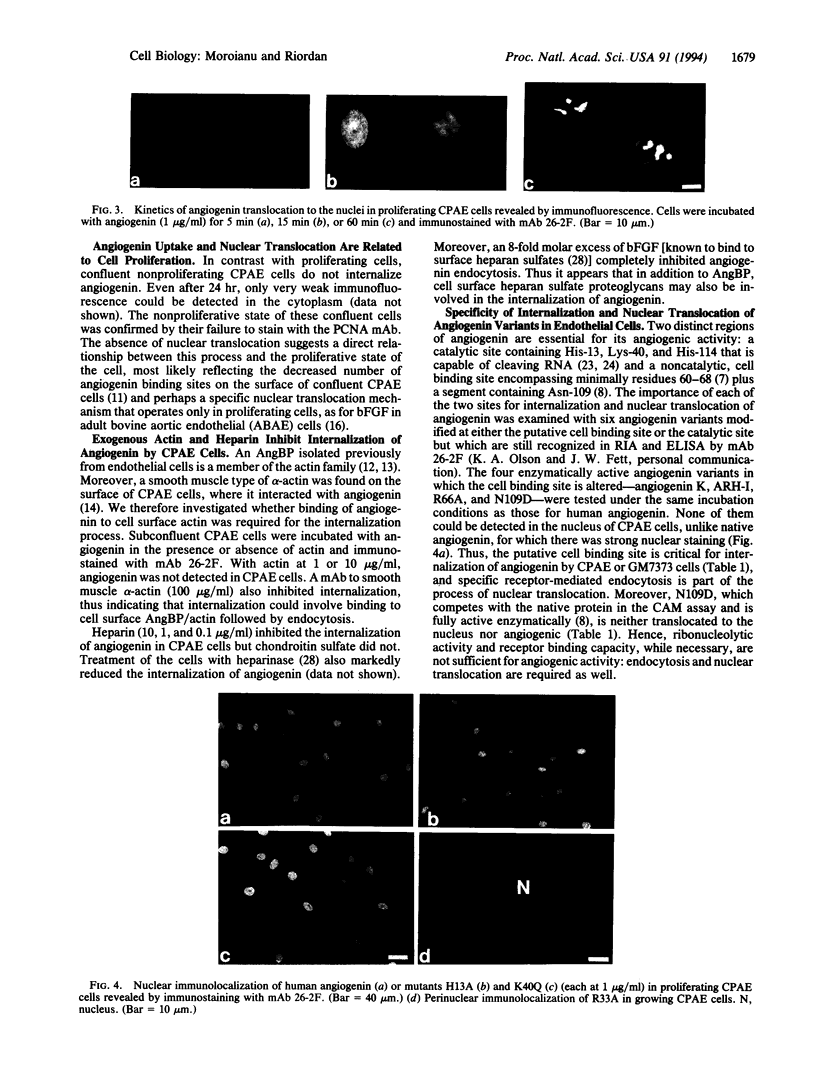
The intracellular pathway of human angiogenin in calf pulmonary artery endothelial (CPAE) cells has been studied by immunofluorescence microscopy. Proliferating CPAE cells specifically endocytose native angiogenin and translocate it to the nucleus, where it accumulates in the nucleoli. Nuclear translocation of angiogenin does not occur in nonproliferative, confluent CPAE cells. These cells were previously found to express an angiogenin-binding protein (AngBP) that was identified as smooth muscle alpha-actin. Exogenous actin, an anti-actin antibody, heparin, and heparinase treatment all inhibit the internalization of angiogenin, suggesting the involvement of cell surface AngBP/actin and heparan sulfate proteoglycans in this process. It has been established that two regions of angiogenin are essential for its angiogenic activity, one is its endothelial cell binding site and the other its catalytic site capable of cleaving RNA. CPAE cells do not internalize four enzymatically active angiogenin derivatives whose cell binding site is modified, but they do internalize two enzymatically inactive mutants whose cell binding site is intact. Thus, the putative cell binding site of angiogenin is necessary for both endocytosis and nuclear translocation, but the catalytic site is not. Three other angiogenic molecules are also translocated to the nucleus of growing CPAE cells. Overall, the results suggest that nuclear translocation of angiogenin and other angiogenic molecules is a critical step in the process of angiogenesis.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Badet J., Soncin F., Guitton J. D., Lamare O., Cartwright T., Barritault D. Specific binding of angiogenin to calf pulmonary artery endothelial cells. Proc Natl Acad Sci U S A. 1989 Nov;86(21):8427–8431. doi: 10.1073/pnas.86.21.8427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldin V., Roman A. M., Bosc-Bierne I., Amalric F., Bouche G. Translocation of bFGF to the nucleus is G1 phase cell cycle specific in bovine aortic endothelial cells. EMBO J. 1990 May;9(5):1511–1517. doi: 10.1002/j.1460-2075.1990.tb08269.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bicknell R., Vallee B. L. Angiogenin activates endothelial cell phospholipase C. Proc Natl Acad Sci U S A. 1988 Aug;85(16):5961–5965. doi: 10.1073/pnas.85.16.5961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bicknell R., Vallee B. L. Angiogenin stimulates endothelial cell prostacyclin secretion by activation of phospholipase A2. Proc Natl Acad Sci U S A. 1989 Mar;86(5):1573–1577. doi: 10.1073/pnas.86.5.1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouche G., Gas N., Prats H., Baldin V., Tauber J. P., Teissié J., Amalric F. Basic fibroblast growth factor enters the nucleolus and stimulates the transcription of ribosomal genes in ABAE cells undergoing G0----G1 transition. Proc Natl Acad Sci U S A. 1987 Oct;84(19):6770–6774. doi: 10.1073/pnas.84.19.6770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bugler B., Amalric F., Prats H. Alternative initiation of translation determines cytoplasmic or nuclear localization of basic fibroblast growth factor. Mol Cell Biol. 1991 Jan;11(1):573–577. doi: 10.1128/mcb.11.1.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fett J. W., Strydom D. J., Lobb R. R., Alderman E. M., Bethune J. L., Riordan J. F., Vallee B. L. Isolation and characterization of angiogenin, an angiogenic protein from human carcinoma cells. Biochemistry. 1985 Sep 24;24(20):5480–5486. doi: 10.1021/bi00341a030. [DOI] [PubMed] [Google Scholar]
- Folkman J., Klagsbrun M. Angiogenic factors. Science. 1987 Jan 23;235(4787):442–447. doi: 10.1126/science.2432664. [DOI] [PubMed] [Google Scholar]
- Garcia-Bustos J., Heitman J., Hall M. N. Nuclear protein localization. Biochim Biophys Acta. 1991 Mar 7;1071(1):83–101. doi: 10.1016/0304-4157(91)90013-m. [DOI] [PubMed] [Google Scholar]
- Hallahan T. W., Shapiro R., Strydom D. J., Vallee B. L. Importance of asparagine-61 and asparagine-109 to the angiogenic activity of human angiogenin. Biochemistry. 1992 Sep 1;31(34):8022–8029. doi: 10.1021/bi00149a036. [DOI] [PubMed] [Google Scholar]
- Hallahan T. W., Shapiro R., Vallee B. L. Dual site model for the organogenic activity of angiogenin. Proc Natl Acad Sci U S A. 1991 Mar 15;88(6):2222–2226. doi: 10.1073/pnas.88.6.2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper J. W., Vallee B. L. A covalent angiogenin/ribonuclease hybrid with a fourth disulfide bond generated by regional mutagenesis. Biochemistry. 1989 Feb 21;28(4):1875–1884. doi: 10.1021/bi00430a067. [DOI] [PubMed] [Google Scholar]
- Hu G. F., Chang S. I., Riordan J. F., Vallee B. L. An angiogenin-binding protein from endothelial cells. Proc Natl Acad Sci U S A. 1991 Mar 15;88(6):2227–2231. doi: 10.1073/pnas.88.6.2227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu G. F., Strydom D. J., Fett J. W., Riordan J. F., Vallee B. L. Actin is a binding protein for angiogenin. Proc Natl Acad Sci U S A. 1993 Feb 15;90(4):1217–1221. doi: 10.1073/pnas.90.4.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imamura T., Engleka K., Zhan X., Tokita Y., Forough R., Roeder D., Jackson A., Maier J. A., Hla T., Maciag T. Recovery of mitogenic activity of a growth factor mutant with a nuclear translocation sequence. Science. 1990 Sep 28;249(4976):1567–1570. doi: 10.1126/science.1699274. [DOI] [PubMed] [Google Scholar]
- Isacchi A., Statuto M., Chiesa R., Bergonzoni L., Rusnati M., Sarmientos P., Ragnotti G., Presta M. A six-amino acid deletion in basic fibroblast growth factor dissociates its mitogenic activity from its plasminogen activator-inducing capacity. Proc Natl Acad Sci U S A. 1991 Apr 1;88(7):2628–2632. doi: 10.1073/pnas.88.7.2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine S. P., Wohl H. Human platelet factor 4: Purification and characterization by affinity chromatography. Purification of human platelet factor 4. J Biol Chem. 1976 Jan 25;251(2):324–328. [PubMed] [Google Scholar]
- Maes P., Damart D., Rommens C., Montreuil J., Spik G., Tartar A. The complete amino acid sequence of bovine milk angiogenin. FEBS Lett. 1988 Dec 5;241(1-2):41–45. doi: 10.1016/0014-5793(88)81027-5. [DOI] [PubMed] [Google Scholar]
- Maher D. W., Lee B. A., Donoghue D. J. The alternatively spliced exon of the platelet-derived growth factor A chain encodes a nuclear targeting signal. Mol Cell Biol. 1989 May;9(5):2251–2253. doi: 10.1128/mcb.9.5.2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maione T. E., Gray G. S., Petro J., Hunt A. J., Donner A. L., Bauer S. I., Carson H. F., Sharpe R. J. Inhibition of angiogenesis by recombinant human platelet factor-4 and related peptides. Science. 1990 Jan 5;247(4938):77–79. doi: 10.1126/science.1688470. [DOI] [PubMed] [Google Scholar]
- Moroianu J., Fett J. W., Riordan J. F., Vallee B. L. Actin is a surface component of calf pulmonary artery endothelial cells in culture. Proc Natl Acad Sci U S A. 1993 May 1;90(9):3815–3819. doi: 10.1073/pnas.90.9.3815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscatelli D., Presta M., Joseph-Silverstein J., Rifkin D. B. Both normal and tumor cells produce basic fibroblast growth factor. J Cell Physiol. 1986 Nov;129(2):273–276. doi: 10.1002/jcp.1041290220. [DOI] [PubMed] [Google Scholar]
- Nakanishi Y., Kihara K., Mizuno K., Masamune Y., Yoshitake Y., Nishikawa K. Direct effect of basic fibroblast growth factor on gene transcription in a cell-free system. Proc Natl Acad Sci U S A. 1992 Jun 15;89(12):5216–5220. doi: 10.1073/pnas.89.12.5216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neufeld G., Gospodarowicz D. Protamine sulfate inhibits mitogenic activities of the extracellular matrix and fibroblast growth factor, but potentiates that of epidermal growth factor. J Cell Physiol. 1987 Aug;132(2):287–294. doi: 10.1002/jcp.1041320213. [DOI] [PubMed] [Google Scholar]
- Newmeyer D. D. The nuclear pore complex and nucleocytoplasmic transport. Curr Opin Cell Biol. 1993 Jun;5(3):395–407. doi: 10.1016/0955-0674(93)90003-9. [DOI] [PubMed] [Google Scholar]
- Quarto N., Finger F. P., Rifkin D. B. The NH2-terminal extension of high molecular weight bFGF is a nuclear targeting signal. J Cell Physiol. 1991 May;147(2):311–318. doi: 10.1002/jcp.1041470217. [DOI] [PubMed] [Google Scholar]
- Rakowicz-Szulczynska E. M., Otwiaska D., Rodeck U., Koprowski H. Epidermal growth factor (EGF) and monoclonal antibody to cell surface EGF receptor bind to the same chromatin receptor. Arch Biochem Biophys. 1989 Feb 1;268(2):456–464. doi: 10.1016/0003-9861(89)90313-5. [DOI] [PubMed] [Google Scholar]
- Rakowicz-Szulczynska E. M., Rodeck U., Herlyn M., Koprowski H. Chromatin binding of epidermal growth factor, nerve growth factor, and platelet-derived growth factor in cells bearing the appropriate surface receptors. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3728–3732. doi: 10.1073/pnas.83.11.3728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renko M., Quarto N., Morimoto T., Rifkin D. B. Nuclear and cytoplasmic localization of different basic fibroblast growth factor species. J Cell Physiol. 1990 Jul;144(1):108–114. doi: 10.1002/jcp.1041440114. [DOI] [PubMed] [Google Scholar]
- Roghani M., Moscatelli D. Basic fibroblast growth factor is internalized through both receptor-mediated and heparan sulfate-mediated mechanisms. J Biol Chem. 1992 Nov 5;267(31):22156–22162. [PubMed] [Google Scholar]
- Sano H., Forough R., Maier J. A., Case J. P., Jackson A., Engleka K., Maciag T., Wilder R. L. Detection of high levels of heparin binding growth factor-1 (acidic fibroblast growth factor) in inflammatory arthritic joints. J Cell Biol. 1990 Apr;110(4):1417–1426. doi: 10.1083/jcb.110.4.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savion N., Vlodavsky I., Gospodarowicz D. Nuclear accumulation of epidermal growth factor in cultured bovine corneal endothelial and granulosa cells. J Biol Chem. 1981 Feb 10;256(3):1149–1154. [PubMed] [Google Scholar]
- Schweigerer L., Neufeld G., Friedman J., Abraham J. A., Fiddes J. C., Gospodarowicz D. Capillary endothelial cells express basic fibroblast growth factor, a mitogen that promotes their own growth. Nature. 1987 Jan 15;325(6101):257–259. doi: 10.1038/325257a0. [DOI] [PubMed] [Google Scholar]
- Shapiro R., Fox E. A., Riordan J. F. Role of lysines in human angiogenin: chemical modification and site-directed mutagenesis. Biochemistry. 1989 Feb 21;28(4):1726–1732. doi: 10.1021/bi00430a045. [DOI] [PubMed] [Google Scholar]
- Shapiro R., Harper J. W., Fox E. A., Jansen H. W., Hein F., Uhlmann E. Expression of Met-(-1) angiogenin in Escherichia coli: conversion to the authentic less than Glu-1 protein. Anal Biochem. 1988 Dec;175(2):450–461. doi: 10.1016/0003-2697(88)90569-6. [DOI] [PubMed] [Google Scholar]
- Shapiro R., Riordan J. F., Vallee B. L. Characteristic ribonucleolytic activity of human angiogenin. Biochemistry. 1986 Jun 17;25(12):3527–3532. doi: 10.1021/bi00360a008. [DOI] [PubMed] [Google Scholar]
- Shapiro R., Strydom D. J., Olson K. A., Vallee B. L. Isolation of angiogenin from normal human plasma. Biochemistry. 1987 Aug 11;26(16):5141–5146. doi: 10.1021/bi00390a037. [DOI] [PubMed] [Google Scholar]
- Shapiro R., Vallee B. L. Identification of functional arginines in human angiogenin by site-directed mutagenesis. Biochemistry. 1992 Dec 15;31(49):12477–12485. doi: 10.1021/bi00164a026. [DOI] [PubMed] [Google Scholar]
- Shapiro R., Vallee B. L. Site-directed mutagenesis of histidine-13 and histidine-114 of human angiogenin. Alanine derivatives inhibit angiogenin-induced angiogenesis. Biochemistry. 1989 Sep 5;28(18):7401–7408. doi: 10.1021/bi00444a038. [DOI] [PubMed] [Google Scholar]
- Silver P. A. How proteins enter the nucleus. Cell. 1991 Feb 8;64(3):489–497. doi: 10.1016/0092-8674(91)90233-o. [DOI] [PubMed] [Google Scholar]
- Soncin F. Angiogenin supports endothelial and fibroblast cell adhesion. Proc Natl Acad Sci U S A. 1992 Mar 15;89(6):2232–2236. doi: 10.1073/pnas.89.6.2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Clair D. K., Rybak S. M., Riordan J. F., Vallee B. L. Angiogenin abolishes cell-free protein synthesis by specific ribonucleolytic inactivation of ribosomes. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8330–8334. doi: 10.1073/pnas.84.23.8330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strydom D. J., Fett J. W., Lobb R. R., Alderman E. M., Bethune J. L., Riordan J. F., Vallee B. L. Amino acid sequence of human tumor derived angiogenin. Biochemistry. 1985 Sep 24;24(20):5486–5494. doi: 10.1021/bi00341a031. [DOI] [PubMed] [Google Scholar]
- Taylor S., Folkman J. Protamine is an inhibitor of angiogenesis. Nature. 1982 May 27;297(5864):307–312. doi: 10.1038/297307a0. [DOI] [PubMed] [Google Scholar]
- Tessler S., Neufeld G. Basic fibroblast growth factor accumulates in the nuclei of various bFGF-producing cell types. J Cell Physiol. 1990 Nov;145(2):310–317. doi: 10.1002/jcp.1041450216. [DOI] [PubMed] [Google Scholar]
- Vlodavsky I., Folkman J., Sullivan R., Fridman R., Ishai-Michaeli R., Sasse J., Klagsbrun M. Endothelial cell-derived basic fibroblast growth factor: synthesis and deposition into subendothelial extracellular matrix. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2292–2296. doi: 10.1073/pnas.84.8.2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waseem N. H., Lane D. P. Monoclonal antibody analysis of the proliferating cell nuclear antigen (PCNA). Structural conservation and the detection of a nucleolar form. J Cell Sci. 1990 May;96(Pt 1):121–129. doi: 10.1242/jcs.96.1.121. [DOI] [PubMed] [Google Scholar]
- Wu Y., Mikulski S. M., Ardelt W., Rybak S. M., Youle R. J. A cytotoxic ribonuclease. Study of the mechanism of onconase cytotoxicity. J Biol Chem. 1993 May 15;268(14):10686–10693. [PubMed] [Google Scholar]
- Yeh H. J., Pierce G. F., Deuel T. F. Ultrastructural localization of a platelet-derived growth factor/v-sis-related protein(s) in cytoplasm and nucleus of simian sarcoma virus-transformed cells. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2317–2321. doi: 10.1073/pnas.84.8.2317. [DOI] [PMC free article] [PubMed] [Google Scholar]