Abstract
The survival rates of melanoma, like any type of cancer, become worse with advancing stage. Spectrum theory is most consistent with the progression of melanoma from the primary site to the in-transit locations, regional or sentinel lymph nodes and beyond to the distant sites. Therefore, early diagnosis and surgical treatment before its spread is the most effective treatment. Recently, new approaches have revolutionized the diagnosis and treatment of melanoma. Genomic profiling and sequencing will form the basis for molecular taxonomy for more accurate subgrouping of melanoma patients in the future. New insights of molecular mechanisms of metastasis are summarized in this review article. Sentinel lymph node biopsy has become a standard of care for staging primary melanoma without the need for a more morbid complete regional lymph node dissection. With recent developments in molecular biology and genomics, novel molecular targeted therapy is being developed through clinical trials.
Keywords: Melanoma, Biomarkers, Progression, Metastasis
Introduction
Stanley P. L. Leong
Recent advancements in molecular technologies have yielded a wide array of genetic, epigenetic, and protein biomarkers as prognostic and predictive markers in the management of melanoma patients [1, 2]. Multiple bio-markers have been found to be associated in melanoma progression, proliferation, immune response, oncogenesis, and others from the tissue microarray studies [2]. Exciting developments in metabolomics [3] and exon sequencing (http://www.ornl.gov/sci/techresources/Human_Genome/faq/seqfacts.shtml) will surely result in more detailed mutations of the genes, which may yield more relevant cancer biomarkers. The recent inclusion of melanoma in The Cancer Genome Atlas (TCGA) project (http://cancergenome.nih.gov/wwd/tumor_types.asp) is a major step to signify genomic profiles in their prognostic and therapeutic values in the treatment of melanoma. An important example is the recent FDA approval of BRAF inhibitor [4] in the treatment of metastatic melanoma. We are on the verge of combining the traditional TNM system [5] with the molecular profiles to a new TNM-Molecular staging system for cancer at large and for melanoma in particular so that staging of cancer can be more accurate and treatment more personalized.
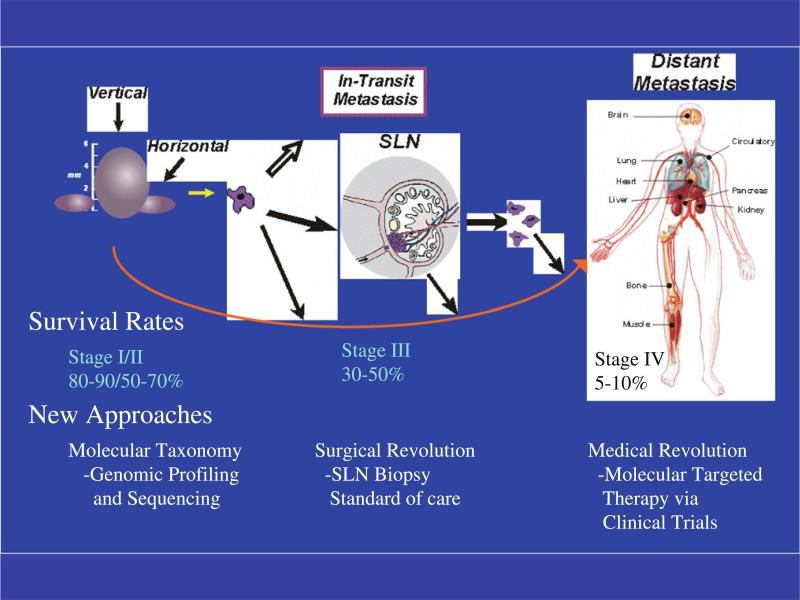
Cutaneous melanoma is an excellent model to study cancer metastasis [6]. In this review, we emphasize the proliferation of melanoma at its inception and progression through the lymphatics as in-transit metastases as well as sentinel lymph nodes (SLN) in the regional nodal basin to distant sites. The challenge is to define these steps of metastasis on a molecular basis and to ask the provocative question whether the initial aberrant cell of melanoma development is the same or a different cell that has metastasized to the regional nodes and distant sites. Exciting new developments have been made in the treatment of metastatic melanoma and will be emphasized in this review (Fig. 1).
Fig. 1.
The survival rates of melanoma become worse with advancing stage. Therefore, early diagnosis and surgical treatment before its spread is the most effective treatment. Recently, new approaches have revolutionized the diagnosis and treatment of melanoma. Genomic profiling and sequencing will form the pillar for molecular taxonomy for more accurate subgrouping of patients. Sentinel lymph node biopsy has become a standard of care for staging primary melanoma. Medical revolution is on its way to develop molecular targeted therapy through clinical trials
Progression from the primary site to the metastatic deposit: changes in the primary tumor
Martin C. Mihm Jr. and George F. Murphy
Introduction
In 1889, Dr. Stephen Paget proposed the “seed and soil” hypothesis to explain metastases. He wrote that for a malignant cell, the seed, to successfully spread and grow as a tumor in another organ, the soil, there must be an appropriate environment for the establishment of the successful metastasis. Over 30 years ago, Ras and Ben-Ze'ev described an experiment in which B16 melanoma cells changed their metastatic potential depending on their shape. They found that the cells plated on ordinary tissue culture plastic became spindled with little metastatic potential when injected into the tail vein of BALB/c C57B/6 mice. If the cells were plated on non-adherent medium, they became round [epithelioid] and metastasized widely after injection into the mice. Replanting aliquots of the metastatic round cells onto ordinary tissue culture plastic resulted in a resumption of the spindle shape and loss of metastatic potential. This wonderful, simple experiment demonstrated the importance of the medium—the stroma—the soil in the evolution of the metastatic process. This experiment also demonstrates the plasticity of the tumor cells, showing how the soil could modify the seed, thus establishing their potential interdependence [7].
The importance of the stroma—the soil—and the alterations induced by the tumor cell to insure its invasiveness will be reviewed with emphasis on protease activity and cytocrine production. Finally, the concept of the metastatic niche will be discussed and its relevance in understanding the site of metastasis and the issues of dormancy will be reviewed. Three excellent reviews describe the specific events that occur in the metastatic phenomenon [8–10]. All emphasize the loss of cellular adhesion in the primary tumor, followed by the acquisition of motility and the ability to invade. To be successful, the latter event must allow for the passage of viable tumor cells into lymphatic and/or blood vessels and the ability to maintain viability until they reach a place of exit through the vasculature to a suitable site that allows for proliferation [11]. In this presentation, the process will be explored by elucidation of the cellular changes involving the seed that are necessary for the metastatic process to begin. These changes include genetic and epigenetic phenomena. The manner in which these genetic changes are translated into cellular phenotypic changes with emphasis on pathways will be reviewed. The possible role of stem cells will be described. Phenotypic alterations of the cell that reflect biomolecular alterations at the cell surface, such as those involving cadherins, will be documented. As well, interactions involving inte-grins that allow for cell motility will be described. The advancing edge of the tumor cell will be considered in terms of changes the epithelial-mesenchymal transformation [12].
A very interesting therapeutic maneuver that serves as a proof of principle of the seed and soil hypothesis was described in 1984 by Tarin et al. where 29 patients, including those with ovarian and breast tumors, had peritoneovenous shunts to alleviate the symptoms of ascites. While literally millions of cells passed into the circulation, very few patients developed metastases in the lungs or elsewhere. Moreover, the metastases that developed were minimal and very small. This emphasizes in a most clinically- relevant way the importance of a pathophysiologic “match” between the wandering tumor cell and a truly habitable destination.
Melanoma progression as an aid to understanding metastases and the proto-oncogene B-Raf (BRAF) pathway
In order to understand the process of malignant transformation and evolution, a brief review of the concept of melanoma tumor progression will be helpful. Tumors that evolve through a radial growth to a vertical growth phase proliferate along the basement membrane zone that contains laminin. Meenhard Herlyn showed that laminin is a melanoma mitogen [13]. Once melanoma cells begin to migrate and invade into the papillary dermis and reticular dermis, they may become tumorigenic. In the tumorigenic phase, the cells are no longer dependent upon a physiologic laminin substrate, and proliferate to form roughly spherical micronodules replete with evidence of cell division (mitoses). At a given point in development, cells at the perimeter of these vertical growth phase micronodules again begin to migrate and appear to be non-tumorigenic as they insinuate among collagen bundles and interact with microvessels. These cells may more resemble mesenchymal elements, and with retention of certain stem cell characteristics that fuel their virulence, may be conceived as undergoing a form of epithelial-mesenchymal transition, as described by Weinberg [14, 15]. It is likely that only a small number of these cells which gain access to the lymphatic or blood vasculature will be successful in extravasating to a metastatic niche conducive to their viability [16]. Some who achieve this end may again proliferate as tumorigenic nodules, perhaps via reversion of the epithelial-mesenchymal transition that got them there, while others may remain dormant only to awaken at some distant time as a result of some as yet unknown stimulus in the metastatic niche.
To have insight into the changes that occur in the primary tumor, we must understand more about the pathways that lead to tumorigenicity. One of the most studied and important pathways is the mitogen-activated protein (MAP) kinase pathway and the BRAF mutation. 80 % of these mutations involve a single substitution of glutamate for valine (V600E). This mutation mimics phosphorylation on the regulatory domain of BRAF. Interestingly, this mutation is the most commonly involved in sun-exposed skin [17, 18]. The final step in this pathway involves phosphorylation of the ERK kinase resulting in a host of changes. In a recent study of vertical growth phase melanoma in 489 patients, it was shown that strong pERK expression in the nuclei of the tumor cells in the advancing edge of the tumor was significantly associated with early metastasis or death. This study emphasized the importance of changes in the advancing edge of the tumor. Features significant for our discussion include the increased expression of integrin (αvβ3), the depression of E-cadherin expression, and the production of metalloproteinases, especially 2 and 9. These changes reflect the transformation of a radial growth phase cell to a vertical growth phase cell. They result in the acquisition of the capacity for a tumor cell to leave the environment of the epidermis and to invade through the basement membrane zone into the dermis.
Changes in the melanoma cell that lead to metastasis
The suppression of E-cadherin with upregulation of MCAM frees the melanocyte from linkages with adjacent keratinocytes [12]. As the loss occurs, there is a distribution of actin binding protein filaments in the periphery of the cell where the cytoplasm forms an almost ameboid configuration as it invades through the basement membrane into the dermis [19]. Concomitant with this exit, the cell expresses αvβ3 integrin. A study from the University of Pennsylvania documented αvβ3 expression in the vertical growth phase tumor and found marked increase compared to the negative radial growth phase lesions [20]. The integrin facilitated the migration of the melanoma cells and also was found to interact with Thy-1 on activated endothelial cells with resultant intravasation of the tumor cells [21]. Furthermore, N-cadherin is expressed on the melanoma cells that allows for connection via gap junctions with fibroblasts and endothelial cells [6]. The production of metalloproteinases results in the digestion of matrix with resultant ease of invasion for the melanoma cells [22].
MMP-9 has been shown to be highly expressed in human melanoma cells in focal fashion implicating it in clonal selection and expansion [23]. Furthermore, a recent study has shown marked increase in MMP-9 and Heparanase in cutaneous melanoma cells, especially ones that have metastasized to regional lymph nodes, as compared to tumors with negative SLN [24]. In another study, it has been clearly shown that MDA-9/Syntenin has a critical role with regard to melanoma metastasis. This gene, melanoma differentiation associated gene-9, interacts with c-Src leading to the formation of c-Src/FAK (focal adhesion kinase). This combination produces a very complex signal that leads to the activation of NF-k B MMP [25]. Interestingly, Oroxylin A has been shown to inhibit both the production of MMP-2 and MMP-9 through repression of phosphorylation of ERK 1/2 among other effects that are upstream of the expression of the metalloproteinases [26]. Furthermore, focal adhesion kinase has been shown to be a crucial member of the metastasis complex. It is a critical component of the site of attachment to extra cellular matrix where integrins tend to cluster. Essentially, the FAK–CASCrk-DOCK180 complex is a molecular signal for invasion. Each of these factors have specific activating functions. For example, DOC180 through activation of RAC1 and JNK activity directly affects the actin cytoskeleton and formation of MMP [27].
Specific genes associated with metastases
Another gene that has been found to be associated with melanoma metastasis is the NEDD9 [27]. This gene was first found in genetically engineered mouse model of melanoma, but has now also been found in human melanomas. It is associated with gains in 6p in comparative genomic hybridization studies (CGH). Once again, this gene in overexpression has been shown to cause a striking increase in FAK activation implicating, again, the importance of this factor. The NEDD-9 overexpression also shows an increase in pERK and some increase in Stat2 activation. In fact, the gene product of NEDD9, a scaffolding protein, colocalizes with p-FAK and Crk that results in invasion. Thus, the molecular signal results from a variety of interrelated pathways. These pathways are all subjected to possible blockade by appropriate pharmacologic and immunologic factors.
Another very important observation is the role of SOX2 in melanoma. This embryonic neural crest stem cell transcription factor that is physiologically important in maintenance of pluripotency is concentrated within the nuclei of melanoma cells at the interface of tumor and stroma where they are intimately associated with peritumoral vessels. Of interest, cells that express SOX2 tend to be more fusiform in shape and tend to infiltrate peritumoral stroma, whereas those with low or absent expression patterns tend to form the more epithelioid, tumorigenic population. In vitro, SOX2 expression is associated with invasive function, and knockdown of the gene and protein inhibit invasion. Moreover, SOX2 colocalizes with and partially co-regulates MMP-3 expression, providing additional insight into how it may mediate invasive potential [28–30]. These changes represent only a few but several significant aspects of the events that occur in the primary melanoma that lead to metastatic behavior.
The importance of the stromal cells
These and undoubtedly many other molecular changes in the primary tumor definitely result in the interaction with stroma and the stromal cells. The production of certain cytokines is considered to be relegated to the fibroblasts, monocytes, and even the endothelial cells. Thus, for example, the melanoma cell produces factors such as IGF1 which leads to the stimulation of other growth factors such as PDGF by the fibroblasts. The melanoma cell also can produce VEGF, resulting in endothelial cell growth that is also stimulated by the monocytes [31]. Certainly, even the production of MMP13 [32] is in part derived from fibro-blasts/myofibroblast production as is the production of MMP2 [33].
A recent study by Navab et al. [34, 35] emphasizes the critical importance of the microenvironment of tumoral soil in cancer development, progression, and metastasis. Examining non-small cell lung cancer in a manner that may be generalized to malignancies of other organs, including skin, they demonstrated that unlike normal fibroblasts, cancer-associated fibroblasts (CAFs) enhance tumorigenicity of cancer cell lines via expression of cancer-associated genes, many of which encode for proteins regulated by the TGF-beta signaling pathway and that prominently induce the focal adhesion and MAPK pathways. This study breaks important ground in showing that cancer-associated stromal cells (fibroblasts) may interact with tumor cells in order to directly regulate their biologic behavior and in a manner that impacts on progression and prognosis. Moreover, knowledge that a major determinant of cancer cell function and virulence resides in the stroma provides novel insights into how therapeutic strategies might be devised to specifically target the stroma (in addition to the cancer cell itself)—an approach perhaps akin to devitalizing a weed by making the earth in which it grows more arid and devoid of all nutrients.
Intravasation
As we have mentioned above, the intravasation is made possible through N cadherin interaction with endothelial cell receptors as well as further enhancement of invasion by the interaction of tumor cell alpha v, beta 3 with the Thy 1 that is expressed by activated endothelial cells. After intravasation, the cells then go on to find a friendly site for “soil” for their implantation and proliferation. The meta-static niche or the site where metastasis occurs has been rigorously studied over the last decade [30]. The most important aspect of the metastatic phenomenon and primary tumor development is that there must be the capacity of the tumor to self-renew and to avoid the changes of senescence. A transcriptional regulator in lung metastasis has been the ID 1 or the inhibitor of differentiation 1. This gene product has been found also in certain breast tumors. By suppressing this function, breast tumors and their metastases to lungs can be inhibited. In this way, the tumors can bypass senescence through inhibition of their differentiation [36–38].
Extravasation and the metastatic niche
Once the cells have survived the circulation [16], they then extravasate into the appropriate tissue. The metastatic niche has been identified as occurring very early in the evolution of the tumor as seen in experimental animals. The niche contains hematopoietic stems cells that express VEGFR1, CD133, and CD34. In the experimental animal with the arrival of the tumor cells, VEGF-R1 is suppressed and VEGFR2 is expressed with subsequent growth of the tumor [39]. It is clear that TGF-Beta has a role in preparing the niche. An interesting model of metastasis has been proposed with possible sites of blocking the metastatic phenomenon [40, 41]. Kaplan's work has shown that there are secretogogues from melanoma cells that are present in the tissue culture effluent that can form the metastatic niche in animals before tumor implantation. Treating such animals with VEGFR1 can block the metastatic process [39]. Thus, we are dealing with a process that has many aspects that can inhibit its success beginning with understanding the primary tumor changes as well as the other evolutionary events that occur in the metastatic process [36].
The metastatic niche, organ specificity, and chemokines
A variety of factors have been implicated to explain why tumor cells preferentially travel to favored sites, much as ocular melanoma extends to liver, as do other melanomas as well as spreading to brain. Breast carcinomas and prostate carcinomas have bone as sites of favored tumor growth. The difference in the structure of tumor blood vessels has also been invoked. For example, fenestrated blood vessels have been found in liver and lymph nodes and have been implicated as favoring passages of tumor cells into the parenchymal tissue. However, other organs, such as the brain, lack endothelial porosity yet are common sites for metastases such as melanoma. These observations make it clear that purely anatomic location cannot be responsible for the distribution of metastasis to selected sites [42].
More recent work has presented the endothelial cell's role in a given organ as presenting a surface receptor or ligand that becomes the address of the specific organ. These structures, chemokines and chemokine receptors, then interact and result in the tumor cell commencing to extravasate. Much work has been done on the role of these molecules in cell trafficking and migration. Early work exhibited definite migrational response of breast carcinoma cell lines [43]. It is clear that chemokines can influence not only the attraction of tumor cells to a given site but also affect survival and proliferation of the tumor. With regard to lymph node spread, it is possible that the structure of the lymph node sinusoid with its many gaps and poorly formed basement membrane may be at least responsible in part for the prevalence of metastases to this organ. In the case of melanoma, studies by Dadras et al. have shown that the extent of lymphatics in the primary tumor is related to the presence of metastases. The greater the area of lymphatic space in the primary tumor, the greater the frequency of metastatic deposits. SLN positivity has been shown to be associated with an immunosuppressed state of the lymph node probably induced by secretogogues of the primary tumor, possibly TGF-β, that induce plasmacytoid dendritic cell formation and leads to an increase in the production of IDO, indoleamine-2,3-deoxygenase, the inhibitor of T cell function [44].
Finally, a role for chemokines has been shown based on the presence of increased quantities of CCL21 produced by endothelial cells in the lymph node. The CCR7 receptor has been shown to be present on melanoma cells and has been clearly shown to result in melanoma cell migration when exposed to the receptor [42, 45, 46]. Furthermore, CXCL12, in addition to CCL21, is another ligand that interacts also with CCR4 found on melanoma cells. Another example of the importance of chemokines is the metastatic phenomenon to small bowel. The CCR9 receptor is highly expressed on melanomas metastatic to this part of the gastro-intestinal tract. The ligand CCL25 is produced by the cells of the small bowel (192). Various associations with other patterns of organ metastasis have been described [42].
Conclusion
The metastatic phenomenon is a highly complex event. There are many different hypotheses and theories concerning the determination of how a seed is formed and what is required for the soil. The great challenge is to learn how to use this information in a therapeutic manner such as to possibly interrupt or block the cascade of events and thus prevent metastatic disease. Another challenge lies in understanding of dormancy and its relationship to late metastatic disease. The understanding of these issues is necessary to effectively control cancer.
Dual epigenetic regulatory mechanisms influence: early stage melanoma metastasis
Dave S. B. Hoon
In discussing melanoma progression, we often consider genomics or transcriptome aberrations (see “Mohammed Kashani-Sabet's” section). Epigenomics is a rapidly growing field with significant development in the last decade in melanoma. Other cancer investigations such as colorectal and breast studies on epigenetics are more developed. Epigenomic aberrations can be used as bio-markers for prognosis and prediction in early and late stage melanomas [47–49]. These aberrations in cutaneous melanoma can cover several types of events such as CpG site methylation of the promoter region of genes, small non-coding RNA(microRNA), and chromatin histone protein methylation, phosphorylation, and acetylation. CpG site hypermethylation of gene promoter regions is one of the most significant mechanisms, whereby melanoma-related genes are turned on and off [48]. The same genes can in turn be turned on by hypomethylation of CpG sites. Both types of events on melanoma-related genes occur during tumor progression [47, 48]. Some known melanoma-related genes which are turned on and off in cutaneous melanoma progression include RASSF1A, WIF-1, estrogen receptor alpha, high molecular weight antigen, MAGE-A family members, SOCS1, RUNX3, AIM1, CXCR4, etc. [47–55]. There are many other melanoma-related genes that are turned on and off at various levels during melanoma growth, invasion, and metastasis. Currently, we do not still understand clearly what triggers these events during tumor progression. We have also observed that non-coding repeat genomic sequences are also regulated by CpG island methylation status such as MINTs and LINE-1 [48, 49]. Currently, we do not understand clearly the mechanisms which trigger these epigenomic events; however; recently, there have been clues that suggest that the genomic and transcriptome system biology of epigenomic events in cancer cells are well inter-connected. These events include methyltransferases, microRNA, genomic replication factors and methylation-related regulatory proteins, histones, genomic structural related factors, etc.
One important family of enzymes in CpG site methylation are DNMTs(1,3A,3B) which are DNA methyltransferases that use S-adenoxyl methionine (SAM) as a methyl donor. The DNMTs have been shown to be up- and downregulated during melanoma progression [56]. Recently, we have demonstrated how DNMT3 is upregulated during melanoma progression and regulates gene promoter methylation status of melanoma-related genes [56]. We have demonstrated that melanoma progression follows a phenomenon called CpG island methylated phenotype (CIMP) originally described in colorectal cancers. CIMP is well demonstrated in gastrointestinal cancers during tumor progression [57]. The events of CIMP involve global hypermethylation event changes in tumors that include both gene and non-gene coding promoter regions collectively. In general, patients’ tumors having the CIMP effect often are progressive and have a worse prognosis.
Another type of epigenomic event that has gotten recent attention is the microRNA pattern changes during melanoma progression. MicroRNA can turn on and off gene expression significantly in cancer progression. One of the inherent problems in microRNA is that they have more than one target gene. This has complicated the interpretation of these epigenetic regulatory functional studies in tumor cells. We recently determined that microRNA 29C could regulate DNMT3s and is related to melanoma progression [56]. MicroRNA 29C was shown to downregulate DNMT3 expression in primary melanomas, whereas it was downregulated in advance stage melanomas, thus allowing DNMT3 to be upregulated. This is an interesting dual epigenetic regulatory event regulating the gene expression through gene promoter methylation during melanoma progression. In this observation, low microRNA 29 and high DNMT3 were correlated with a poorer prognosis in stage III melanoma patients. In summary, we have shown that epigenomic events play a significant role in melanoma progression and are highly integrated together. These epigenomic events are also attractive as potential targets for new types of therapeutics.
Molecular markers for melanoma
Mohammed Kashani-Sabet
The last several years have been witness to an exciting transformation in the clinical approach to melanoma with the application of molecular techniques to the assessment and therapy of melanoma patients. Along with the advent of molecular therapies that are now part of the routine care of metastatic melanoma patients, the application of genome-wide approaches to melanoma biology have also resulted in an improved understanding of the molecular events governing melanoma progression, resulting in the development of novel biomarkers for melanoma. Here, we review recent work focused on the development of molecular diagnostic and prognostic markers for melanoma, derived from gene expression profiling analyses. This work took advantage of the classical model of melanoma progression, beginning with the melanocyte, with the potential to advance progressively through the stages of a benign nevus, primary radial and vertical growth phase melanoma, and culminating in metastatic melanoma. Until recently, few biomarkers were identified the differential expression of which corresponded to these important transitions in melanoma progression. Intriguingly, while mutations in the BRAF gene have emerged as a rational target for therapy of advanced melanoma, they are not able to successively distinguish between these various stages of melanoma progression. In 2005, our group utilized gene expression profiling using cDNA microarrays in an attempt to identify new melanoma biomarkers [58]. Using a small number of freshly acquired nevi, primary melanomas and metastatic melanomas, differentially expressed gene sets were identified to correspond to the key transitions in melanoma progression and were hypothesized to possess diagnostic and/or prognostic utility. More recent work has attempted to define the clinical context in which these biomarkers may be useful.
One application of these differentially expressed gene sets involved the development of a multi-marker diagnostic assay for melanoma [59]. This was accomplished using a dataset of 693 melanocytic neoplasms comprising a training set of 534 cases and four distinct validation sets more relevant to the differential diagnosis of nevus versus melanoma. Five markers were selected for validation from the original microarray analysis that was over-expressed in melanomas when compared with nevi: ARPC2, FN1, RGS1, SPP1, and WNT2. Marker intensity was scored by immunohistochemical analysis on a 0–3 scale both at the lesion junctional zone (termed the “top”) and the lesional base (termed the “bottom”), and a diagnostic algorithm was developed in the training set to diagnose each lesion as nevus or melanoma. Intriguingly, marker analysis indicated that, in the case of nevi, while the markers were expressed in the top of the lesion, there was a fairly uniform loss of expression at the bottom. In contrast, in the case of primary melanomas, there was a uniform marker expression from the top to the bottom without loss of expression. Application of the diagnostic algorithm to the training set yielded a specificity of 95 % and a sensitivity of 91 %, with an AUC of 0.93, in the diagnosis of melanoma. In the validation sets, the multi-marker assay was capable of correctly diagnosing a high percentage of melanomas arising in a nevus, Spitz nevi, and dysplastic nevi. Finally, application of the diagnostic assay to 24 misdiagnosed melanocytic neoplasms demonstrated that the markers were able to correctly identify 75 % of cases in which the routine initial pathologic evaluation failed to identify the nature of tumor. This suggested that the multi-marker assay could potentially prevent a high proportion of errors emanating from the routine histologic evaluation of melanocytic neoplasms. This multi-marker assay is currently the subject of additional validation studies before commercial application and availability.
Separately, these studies have resulted in the development of a multi-marker prognostic assay for melanoma [60], examining the expression of the RGS1, SPP1, and NCOA3 proteins by immunohistochemical analysis in two distinct cohorts: a 395-patient cohort from Northern California and a 141-patient cohort from Germany. Marker expression was scored for intensity on a 0–3 scale, by digital imaging analysis, and assessed against the following outcome parameters: disease-specific survival (DSS), the primary end point, and SLN metastasis. Marker overexpression was associated with significantly increased risk of SLN metastasis and death due to melanoma by univariate analysis. Each of these markers was previously shown to independently predict melanoma survival alone [61–63]. By multivariate logistic regression, the multi-marker score was significantly and independently predictive of SLN metastasis, and more significant than tumor thickness, the primary factor that is used to determine SLN eligibility. In the analysis of DSS, the multi-marker score was the most significant factor predicting death due to melanoma even with the incorporation of powerful factors such as tumor thickness, ulceration, mitotic rate, and SLN status. The significant impact of the multi-marker assay was also demonstrated by digital imaging analysis of marker expression. Finally, the multi-marker score remained significantly and independently predictive of DSS in the German cohort. More recent studies suggest that these prognostic markers may be useful in identifying high-risk patients within cohorts with low rates of SLN metastasis, such as patients with thin melanoma or patients with desmoplastic melanoma, to undergo SLN biopsy. Finally, these analyses suggest that marker expression may potentially identify a patient cohort to benefit from adjuvant interferon, which is currently the subject of additional validation using specimens from the Eastern Cooperative Oncology Group E1690 trial of observation versus interferon alpha.
In conclusion, molecular investigations of melanoma have demonstrated that melanoma progression can be understood as a series of distinct molecular events. These studies have begun to address some of the gaps in our understanding of markers the differential expression of which underlies the different stages in melanoma progression, such as in the transition between nevus and primary melanoma. Intriguingly, with respect to the prognostic markers, some of the markers identified have their greatest predictive impact on the development of lymph node metastasis, whereas others have their greatest prognostic impact through the prediction of distant metastasis, suggesting the potential to develop a suite of markers predictive of metastasis via the lymphatic versus hematogenous routes.
Potential for intralesional therapy for melanoma
Sanjiv S. Agarwala
While advanced melanoma is essentially a systemic disease and usually requires systemic therapy, there exists a significant fraction of patients who have predominantly locally advanced and/or regional disease as their main and sometimes, only presentation. Surgery is the usual option considered for such patients, but is often not curative or technically feasible. The toxicity and relative lack of efficacy of most systemic agents has been a deterrent to the use of aggressive systemic therapy in these patients. Various therapeutic modalities have been attempted for such clinical presentations including re-resection, radiation therapy, and regional perfusion with chemotherapeutic agents with limited success. The concept of direct intralesional injection of therapeutic agents into the tumor has always been an attractive and logical proposition for local regional disease that is clinically accessible. It is clear, however, that intralesional therapy without systemic efficacy is of limited value.
Intralesional therapy for metastases from melanoma was first systematically evaluated in the 1970s with Bacillus Calmette Guerin (BCG) which was first reported to produce remission in injected lesions and also distant metastases [64]. An immune-mediated systemic response was hypothesized to be the basis behind the occasional systemic response, but randomized trials of BCG have failed to confirm a significant clinical benefit [65].
There has been a recent resurgence of interest in intralesional therapy following the recent development of agents appear to not only have direct antitumor effects when injected intralesionally but also potential systemic effects that have been demonstrated both in animal models and clinical studies. Three such investigational agents that are in clinical development are Allovectin-7, OncoVEXGM-CSF and PV-10.
Allovectin-7 is a plasmid/lipid complex with the DNA sequences encoding HLA-B7 and ß2 microglobulin, both components of major histocompatibility complex class I (MHC-I). It is known that lack of or reduced expression of MHC-I in melanoma cells is one mechanism by which these cells escape immune recognition. Allovectin-7 induces a fivefold increase in the frequency of HLA-B27 cytotoxic T cells, upregulates/restores MHC-I molecules, and induces a proinflammatory response. In a phase 2 trial of Allovectin-7 that included 133 patients with stage IIIB/C and IV M1a/b melanoma, the response rate (RR) was 12 %. Toxicity was tolerable [66]. A phase 3 trial of Allovectin-7 compared to chemotherapy with dacarbazine or temozolomide in recurrent stage III or IV melanoma has completed accrual and results are expected later in 2012.
OncoVEXGM-CSF is an oncolyticherpes simplex virus encoding GM-CSF. It is deleted for ICP-34.5 which gives it the ability to replicate selectively in tumor cells. This causes the tumor cells to undergo lysis and the lysed cells are then taken up by antigen presenting cells (APCs). Local expression of GM-CSF is thought to be synergistic. A phase 2 trial of OncoVEXGM-CSF in 50 patients with stage IIIC and IV melanoma was recently reported [67]. On overall RR of 26 % was noted with eight complete and five partial responders. Regression was observed in both injected and non-injected lesions and visceral responses were also noted. A phase 3 trial in 360 stage IIIB/IV melanoma patients randomized 2:1 to OncoVEXGM-CSF versus subcutaneous GMCSF alone has completed enrollment. The endpoints are durable response at 6 months and overall survival (OS).
PV-10 is a small molecule fluorescein derivative. It is a non-pyrogenic solution of Rose Bengal disodium (10 % RB) which is not metabolized, has about a 30 min circulatory half-life, and is excreted via the biliary system. PV-10 is selectively taken up by the plasmalemma of cancer cells and accumulates in the lysosomes, triggering lysosomal release leading to autolysis within 30–60 min. Antigenic tumor fragments taken up by APCs is believed to be the mechanism behind the systemic “bystander” effect in uninjected tumors.
Following promising phase 1 results [68], a multicenter, international phase 2 trial, in 80 patients with stage III–IV melanoma was conducted at multiple centers in the United States and Australia. Intralesional injections of PV-10 were administered to up to 10 target and up to 10 non-target cutaneous, subcutaneous or nodal lesions. The primary endpoint was objective RR for injected lesions. Twenty-four percent of patients had complete responses (CR) in target lesions and 25 % had partial responses (PR) for an overall response rate (ORR) of 49 %. The locoregional disease control [CR + PR + stable disease (SD)] rate was 71 %. Among subjects with bystander lesions, CR of their untreated lesions was reported in 24 %, ORR in 37 % and locoregional control in 55 %. Regression of bystander lesions strongly correlated with response in target lesions. A phase 3 trial of PV-10 is in development.
Major strides in the therapy of advanced melanoma have been made in the last 12 months. Two new and promising agents, ipilimumab, an anti-CTLA-4 antibody vemurafenib, a highly selective inhibitor of b-raf, a mutation found in approximately 50 % of melanomas, have both been approved by the FDA. The potential for them to be combined with a successful intralesional therapy with non-overlapping toxicity and mechanism of action is obvious.
An important aspect of these therapies is their relative ease of administration by local injection, low toxicity, and applicability to sicker patients who are not candidates for aggressive systemic therapy. However, only phase III randomized trials can truly establish their benefit and lead to regulatory approval. Results from the Allovectin-7 trial should be available later in 2012 and the phase III trial with Oncovex has been completed. If shown to be effective, they will add therapeutic tool to our melanoma war chest.
Regional intra-arterial chemotherapy, isolated limb: infusion and Chemosaturation-Percutaneous Hepatic Perfusion
Jonathan S. Zager
Regionally metastatic melanoma can be, and often is a challenging clinical scenario, with many therapeutic options. Isolated Limb Infusion (ILI) and Chemosaturation-Percutaneous Hepatic Perfusion (CS-PHP) are therapeutic options and provide an advantage in treating patients when metastatic disease is limited to a limb or the liver, respectively. The concept is the same with both modalities, ILI and CS-PHP, where the organ or limb is isolated from the systemic circulation and high dose chemotherapy is delivered right at the regional disease and then washed out so little to no chemotherapy is seen systemically limiting systemic toxicity.
Isolated Limb Infusion
Isolated Limb Infusion was initially described by John Thompson while at the Sydney Melanoma Unit (now the Melanoma Institute of Australia) in the mid 1990s as an alternative to the more complex and invasive hyperthermic isolated limb perfusion (HILP) as a procedure to treat in transit melanoma of the extremity [69–72]. In general, about 50 % of all melanomas (approximately 80,000 per year in the United States) occur on the extremity and about 2–10 % (1,500–8,000 cases per year in the United States) of these will recur in an intransit fashion [5, 73]. Although intransit melanoma presents a therapeutic challenge to the physician, however, there are many therapeutic options for the treatment of this form of recurrent disease including but not limited to surgical resection if fully resectable, radiation, local injections, described by Sanjiv Argarwala above, (BCG, OncoVex, PV-10, IL-2, interferon), systemic chemotherapy, ILI and HILP. ILI has been shown to be very efficacious in treating intransit melanoma of the extremities.
In brief, melphalan, the typical chemotherapeutic agent used in ILI and HILP, is dosed at 7.5 mg/L of tissue for the lower extremity and 10 mg/L for the upper extremity. Limb volume is calculated by a water displacement method or taking circumferential measurements of the extremity every 1.5–2 cm, encompassing the entire region to be infused. The dose of melphalan is now routinely corrected for ideal body weight which has been shown to lower regional toxicity while not affecting ORR [74, 75]. During an ILI, melphalan is infused rapidly into the arterial line (2–5 min) and then hand circulated for 30 min. In numerous single institution and multi-institutional series, ILI has shown ORR of 60–80 %, CR in up to 40, and PR in up to approximately 40 % [70–72]. Kroon et al. [72] from the Melanoma Institute of Australia reported on their 14-year experience with ILI showing an ORR of 84 % and a CR of 38 %. A multi-institutional retrospective review of ILI performed over an 8-year span in the United States claimed an ORR of 61 % in 166 ILIs with a CR of 33 %. The same US multi-institutional group described in a second publication the experience with toxicities from ILI both systemic and regional. In this report, Santillan et al. showed that correcting the dose of melphalan for ideal body weight lowered the risk of post-procedural regional toxicity (according to the Wieberdink toxicity scale) while not affecting the ORR and CR [74–76].
In a single institutional series at Moffitt Cancer Center we have seen a very good response to ILI with ORR of ~65 %, CR of 33 % and a PR 32 %. About 15 % of patients have stable disease with 25 % progressing in filed at first site and another 20 % progressing out of field as first site of progression. The median follow up after the diagnosis of melanoma in these patients was 40 months, with a median follow up after ILI of 14.5 months in this series. We have performed over 20 repeat ILIs after a previous regional therapy (HILP or ILI) and have shown ORR 61 %, CR 38 %, and PR 23 % after the repeat regional perfusion [77]. The recent publication by Chai et al. reviewed data from 44 patients, who underwent repeat regional perfusions (HILP or ILI) from 3 institutions beginning 1997 to 2010. The median followup was 21.4 (range 4–153) months. Of the repeat regional perfusions, there were 70 ILIs and 28 HILPs, the following groups were identified: group A, ILI → ILI (n = 25); group B, ILI → HILP (n = 10); group C, HILP → ILI (n = 12); and group D, HILP ? HILP (n = 3). The comparison of Wieberdink grade, serum creatine phosphokinase level, length of stay, and response rate between procedures (HILP vs. ILI) between sequence (initial vs. repeat) and among their interactions showed no statistically significant differences between groups. TTP after initial procedure did not differ between HILP and ILI (P = 0.08) and no survival difference was seen (P = 0.65) [77].
Recently completed ILI trials include a phase I ILI and systemic sorafenib trial and a phase II ILI and systemic n-cadherin inhibitor (ADH-1) trial [78, 79]. In a recent multi-center Phase II trial, 42 patients received systemic (intravenous) ADH-1 before (day 0) and after (day 8) a melphalan-based ILI. The combination was found to be a well-tolerated treatment for patients with advanced extremity melanoma; an ORR with the addition of ADH-1 was compared with a matched group that melphalan alone was and were not significantly different. In addition, there were no differences seen in the overall TTP of regional disease. Notably, we did not observe a correlation between tumor N-cadherin expression and response [78].
In a phase I dose escalation study of oral sorafenib and melphalan ILI, based on convincing pre-clinical data which showed enhanced responses of melanoma to the combination of melphalan and sorafenib, the investigators failed to show a improved clinical response when compared to historic controls and there appeared to be a higher than normal regional toxicity from the combination (data not published) [79].
Chemosaturation-Percutaneous Hepatic Perfusion (CS-PHP)
PHP is a minimally invasive technique of balloon occlusive and vascular isolation of the liver and regional intra-arterial therapy with veno bypass and chemofiltration. In contrast to open hepatic perfusion (IHP), in which an exploratory laparotomy is required to expose and isolate the inferior vena cava (IVC), porta hepatis, gastroduodenal artery, and perihepatic collaterals, CS-PHP utilizes catheters and balloon occlusion of the IVC to obtain the same type of vascular isolation seen in IHP.
At the heart of CS-PHP is a special double balloon catheter system created by Delcath System (Delcath Inc., New York, NY). This catheter permits occlusion of the IVC and allows for vascular isolation of the liver. High dose chemotherapeutic agent (melphalan) is then infused into the liver via a catheter in the hepatic artery. The procedure is performed under general anesthesia. After determining that the inflow catheter is properly positioned in the hepatic artery and any accessory arteries are embolized to prevent infusion of organs outside of the liver, the IVC catheter is positioned and balloons are inflated with a IVC venogram to insure appropriate isolation of the hepatic circulation with no leakage past the IVC balloons. The IVC catheter is attached to the extracorporeal circuit consisting of a centrifugal pump and drug filtration cartridges. The hepatic venous outflow is circulated into the pump and subsequently into two filtration cartridges that are connected in parallel. The filtered blood is then returned to the systemic circulation through an introducer catheter in the internal jugular vein [80–83].
In a phase I trial by Pingpank et al., 74 CS-PHP treatments with melphalan were performed on 28 patients of whom 10 had ocular melanoma. As a phase I trial, this study was also not established to primarily assess clinical response; however, in 27 of 28 patients, responses could be evaluated. Of the 10 patients with ocular melanoma, an objective tumor response was seen in 50 % and consisted of 3 patients with a PR and 2 patients with a CR. The duration of responses for the 2 patients with a CR was 10 and 12 months [81].
Based on these results, a phase III randomized multi-center trial was initiated to assess PHP in patients with either ocular or cutaneous melanoma metastatic to the liver. The trial involved randomization to either best alternative therapy or PHP. Up to 6 PHPs at 4–8 week intervals were performed provided that the patients showed no disease progression (in the liver or systemically) based on RECIST and that the patients did not have systemic or regional toxicities that precluded another CS-PHP. Each CS-PHP consisted of 30-min perfusions with melphalan followed by a washout of the liver for 30 min, while the balloons were still inflated. The primary endpoint assessment in this study was hepatic progression-free survival (hPFS). The preliminary results of this phase III trial were reported at the American Society of Clinical Oncology in 2010 and updated more recently and presented at the European Multidisciplinary Cancer Congress in Stockholm and the International Melanoma Centers Meeting in Tampa in 2011 [83–85]. A total of 93 patients were accrued in this study with 44 patients in the CS-PHP arm and 49 patients in the best alternative care (BAC) arm. Significant improvements in both hepatic progression-free survival (hPFS), overall PFS, and ORR were seen in patients treated with CS-PHP compared with patients treated with BAC. Median hepatic PFS in the CS-PHP arm was significantly higher (HR 0.34, P < 0.001) at 8.1 months compared with 1.6 months in the BAC arm Overall PFS was also improved in the CS-PHP arm at 6.4 vs. 16 months (HR 0.41, P < 0.001) [19, 20].
In the phase III trial, patients were allowed to cross over from BAC to CS-PHP upon 20 % or more progression in their liver. Cross over and successful CS-PHP treatment was seen in 51 % of the BAC patients in the phase III study [83]. The subset of patients treated (4 CS-PHP and 4 BAC) at Moffitt Cancer Center mirror imaged the results of the entire cohort in the phase III trial with a median hepatic PFS in the CS-PHP group of 310 days versus the BAC group of 48 days.
ILI and CS-PHP are minimally invasive techniques that do not require open surgical access to regional blood vessels and have been shown to be efficacious in obtaining regional control of melanoma (in the limb or liver) that often presents a therapeutic challenge to the clinician. Both ILI and CS-PHP are excellent methods that can be used to evaluate novel therapeutic agents. The ability to obtain real time PK analysis through the circuit and systemic circulation is unparalleled. In addition, the availability of tumor during ILI allows investigators to access biopsy tumor in the field of regional perfusion, before, during, and after the infusion for correlative biologic studies. Both modalities hold tremendous promise in the field of regional therapy for melanoma.
Impact of melanoma SLN micrometastasis on clinical outcomes
Stanley P. L. Leong, MD
SLN status in melanoma is a powerful predictor of clinical outcome [5, 86–109]. When a SLN is positive, about 20–30 % of the time, the regional nodes may be affected. Positive regional lymph nodes are associated with poorer outcomes [110–117]. Tumor burden in SLNs may be measured by aggregate diameter of metastatic foci [118], depth of nodal invasion [119, 120], the largest focus measurement [88, 105], and location within the lymph node [109]. By combining tumor burden (Rotterdam tumor load) and location in the lymph node by Dewar topography criteria, van der Ploeg et al. have found the combination to be a stronger predictor for positive nonsentinel lymph nodes and melanoma-specific survival [121]. Thus, micrometastasis in melanoma SLNs shows a spectrum of tumor burden [122] and may explain various or heterogeneous clinical outcomes for melanoma patients with positive SLNs. Therefore, it is important to quantitate the amount of tumor burden for stratification designs in clinical trials.
Recently, we have completed a study to correlate the extent of microscopic tumor burden in melanoma SLNs with PFS and OS [123]. Further, we have explored the independent contributions of extent of SLN tumor burden and primary melanoma thickness (PMT) with respect to PFS and OS. Sixty-three patients (41 male and 22 female) with one or more positive SLNs were evaluated with median followup of 6.8 years. PMT was measured with a micrometer and SLN metastases were determined for size, as maximum metastasis size (MMS) in mm. Kaplan–Meier estimates of PFS and OS differed significantly by MMS (log-rank P = 0.031 for PFS and P = 0.016 for OS) and PMT (log-rank P = 0.036 for PFS and P < 0.001 for OS). Following adjustment for age and gender, the hazard ratio (HR) associated with MMS was 1.09 per mm increase (P = 0.05) for PFS, and 6.30 (P = 0.014) and 5.41 (P = 0.048) for OS in patients, respectively, with MMS of 0.6–5.5 mm and MMS ≥ 5.5 mm compared with those with MMS < 0.6 mm. When patients were stratified by their PMT, the risk for disease progression and OS was substantially worse for the group with PMT ≥ 4.5 mm (HR = 13.10 and P = 0.022 for PFS; HR = 17.26 and P < 0.001 for OS) relative to the baseline group with PMT < 1.6 mm. PMT and MMS were independently prognostic of PFS and OS in melanoma patients. Except for four patients, all patients had complete lymph node dissection (CLND). Patients with positive CLND (14, 22.2 %) showed significant worse PFS (P = 0.002) and OS (P = 0.0003) than the negative CLND group (45, 71.4 %). Thus, the progression of melanoma from the primary tumor site to the SLN and from the SLN to distant sites is most consistent with the spectrum theory [122, 124]; in that, tumor growth is progressive and disease outcome depends on the spectrum of tumor burden within the primary site or in the SLN. From the Multicenter Selective Lymphadenectomy Trial I, Morton et al. have concluded that the staging of intermediate thickness (1.2–3.5 mm) primary melanomas provides important prognostic information. Among patients with nodal metastases, the 5-year survival rate was higher among those who underwent immediate lymphadenectomy with a positive SLN biopsy than among those in whom lymphadenectomy was delayed until the lymph node became palpable (72.3 ± 4.6 vs. 52.4 ± 5.9 %; HR for death, 0.51; 95 % CI, 0.32–0.81; P = 0.004) [125]. CLND versus observation for SLN-positive patients is currently being evaluated in a randomized Multicenter Selective Lymphadenectomy Trial II [126].
Thus, the clinical outcomes of melanoma patients may be affected by factors other than SLN status as shown in the above-mentioned study. In fact, in recent phase III adjuvant Interferon European Organisation for Research and Treatment of Cancer (EORTC) trials 18952 and 18991, ulceration of the primary melanoma and stage are predictive of interferon efficacy [127]. Further analysis of EORTC trials 18952 [intermediate doses of interferon a-2b (IFN) versus observation in stage IIb–III patients] and 18991 [pegylated (PEG)-IFN versus observation in stage III patients], the predictive benefit of ulceration on the efficacy of IFN/PEG-IFN with regard to relapse-free survival (RFS), distant metastasis-free survival (DMFS), and OS was assessed in the overall population and in subgroups stratified by stage [IIb and III-N1 (microscopic nodal disease) and III-N2 (macroscopic nodal disease)]. In the entire population, the comparison of IFN/PEG-IFN versus observation for RFS, DMFS and OS resulted estimated HR of 0.85 (P = 0.004), 0.89 (P = 0.04) and 0.94 (P = 0.36), respectively. The ulceration group (n = 849) had a greater benefit from treatment as compared with the non-ulceration group (n = 1,336) for RFS (test for interaction: P = 0.02), DMFS (P < 0.001) and OS (P < 0.001). The greatest risk reductions were observed in patients with ulceration and stage IIb/III-N1 with estimated HR for RFS, DMFS, and OS of 0.69 (P = 0.003), 0.59 (P < 0.0001) and 0.58 (P < 0.0001). The efficacy of IFN/PEG-IFN was lower in stage III-N2 patients with ulceration and was uniformly absent in patients without ulceration. There was a consistency between the data of both trials. Interpretation: This meta-analysis of the EORTC 18952 and 18991 trials indicated that both tumor stage and ulceration were predictive factors for the efficacy of adjuvant IFN/PEGIFN therapy. Based on this pivotal trial FDA has approved pegylated interfere or Sylatron for stage III melanoma.
Clinical trials on targeted therapies for melanoma
Axel Hauschild and Vernon K. Sondak
A variety of different treatment approaches have been used for unresectable metastatic melanoma in the past. Chemo-therapy is known to lead to an overall response rate of only 10–15 % and few durable responses. Dacarbazine (DTIC) chemotherapy is an approved and widely used single agent. While it was never established to improve survival compared to supportive care, it has been widely used as a comparator to new drugs in randomized clinical trials [128].
The findings of Curtin and co-workers of melanomas carrying distinct “driver” mutations based on etiologic factors paved the way for a new molecular understanding of melanoma. Activating BRAF mutations have been observed in approximately 40–50 % of all melanomas, particularly in cutaneous melanomas arising on intermittently sun-exposed skin. The majority of BRAF mutations (over 85 %) are located in codon 600, mostly V600E mutations but with a minority of V600K, V600D and V600R mutations, as well as a small number in other codons. Among melanomas that do not have BRAF mutations, 15–20 % harbor NRAS mutations, predominantly Q61R or Q61K mutations. Simultaneous mutations of BRAF and NRAS are highly unusual, [129] presumably because either mutation alone is sufficient to drive the malignant phenotype.
Acral lentiginous melanomas and mucosal melanomas are more likely than other types to harbor activating c-KIT mutations (8–17 % of such tumors). Some of the activating c-KIT mutations are sensitive to treatment with imatinib mesylate, [18] but many are either insensitive to that drug or more sensitive to other kinase inhibitors such as sorafenib or dasatanib.
Current trials on BRAF inhibitors
The oral BRAF inhibitor vemurafenib (Zelboraf®, Genentech, South San Francisco, CA) is relatively specific for the V600E mutant form of the BRAF protein. Vemurafenib was approved for treatment of unresectable, BRAF-mutated metastatic melanoma by the FDA in 2011 and by the EMA in Europe in 2012. Phase I and phase II trials [130, 131] demonstrated remarkably high objective response rates, with progression-free survival of about 6 to 7 months and overall survival times of approximately 16 months for vemurafenib-treated patients in first- and second-line settings. In the pivotal phase III trial (BRIM-3), there was a striking difference between vemurafenib- and dacarbazine-treated patients not only for response rate and duration of response, but also for progression-free survival and overall survival. A phase II trial is evaluating vemurafenib in patients suffering from brain metastases with and without previous brain-directed therapies, and other phase II trials are planned for patients with mutations in codon V600 that are not V600E and in children with V600E BRAF mutated melanoma.
At ASCO 2012, the results of the Early Access Program (EAP) forvemurafenib,conducted invarious countries, willbe presented. The EAP will report particularly on the tolerability of vemurafenib in the setting of the patient's daily routine.
Furthermore, vemurafenib is currently being tested in combination with the anti-CTLA-4 antibody ipilimumab, which was also approved in 2011 for unresectable or metastatic melanoma (regardless of mutation status). It will be interesting to see whether this combination of a rapidly acting drug with a high incidence of eventual resistance developing (vemurafenib) plus a drug ordinarily slow to achieve responses that are nonetheless often durable, can offer a high rate of durable responses in patients with BRAF mutated melanomas. Other approaches to the question of overcoming mechanisms of resistance to BRAF inhibitors are currently being addressed in some study settings. These other early phase clinical trials of vemurafenib are listed on www.clinicaltrials.gov.
Another selective BRAF inhibitor, dabrafenib (GSK 2218436), also showed promising results in phase I/II trials in BRAF-mutated patients. A phase III trial comparing dabrafenib to dacarbazine (BREAK-3) is currently underway. The primary endpoint of this clinical trial is the improvement of progression-free survival (PFS). This allows patients in the chemotherapy arm to cross over after progression during DTIC treatment, something that was not allowed in the vemurafenib phase III trial (BRIM-3). The results of this multinational trial will also be presented at ASCO 2012.
Exciting data on the use of a combination of inhibitors were presented at ASCO 2011. The combination of the BRAF inhibitor dabrafenib and a MEK inhibitor, trametinib (GSK1120212), showed a clinical benefit for almost all treated patients. The response rate was as high as 80 %. It appeared that time to progression is also prolonged compared to single-agent treatment with the BRAF inhibitor, but—while exciting—the data presented at ASCO 2011 were immature. An updated analysis will be presented in 2012. A randomized phase III trial evaluating this promising combination is expected [132]. It is likely that the selective BRAF inhibitors will additionally be tested in adjuvant therapy trials in the near future. Whether single-agent BRAF inhibitors or a combination of a BRAF and MEK inhibitor will be chosen as the appropriate test regimen for a phase III trial is still under discussion.
Current trials on NRAS inhibitors
A MEK inhibitor from Novartis (MEK 162) is currently being tested in patients whose tumors harbor either NRAS or BRAF V600 mutations. MEK162 is a selective inhibitor of the kinases MEK1 and MEK2 and has shown preclinical activity in BRAF- and NRAS-mutant melanoma models. An open-label phase II trial in locally advanced and unresectable or metastatic cutaneous melanoma has been performed. The results will be presented at ASCO 2012.
c-KIT inhibitors
As mentioned previously, some acral lentiginous and mucosal melanomas carry activating c-KIT mutations. Several small reports and phase II trials have shown that imatinib mesylate, an inhibitor of c-KIT, is able to demonstrate objective responses in around 20 % of these patients [133]. An international trial, the TEAM trial (Tasigna® Efficacy in Advanced Melanoma) uses a second generation c-KIT inhibitor, nilotinib. The trial was originally designed as randomized phase III trial comparing nilotinib to dacarbazine. However, due to the significantly lower numbers of c-KIT-mutated melanoma patients compared to the initial assumptions and to extremely slow trial recruitment, the study design was changed to a phase II trial using nilotinib only in 2011.
Other drugs in development
Several other drugs, particularly more or less specific inhibitors of key molecules on the various signal transduction pathways of greatest known importance in melanoma, but also multikinase inhibitors and anti-angiogenetic agents, are currently being tested as single agents or in combinational trias. Some multicenter trials are evaluating chemotherapy combined with temsirolimus or everolimus, inhibitors of mTOR. Recently the results of a trial testing the combination of carboplatin and paclitaxel with or without bevacizumab have been released. The BEAM trial, while far from definitive, suggested there may have been some benefit for the patients who got bevacizumab in addition to chemotherapy 134].
Summary
The discovery of specific mutations in different types of melanomas led to the development and testing of clinically useful inhibitors in a very short time frame. In less than 5 years, selective BRAF inhibitors like vemurafenib were brought from preclinical data via animal models to phase III clinical trials, and now vemurafenib has received approval for treatment of metastatic melanoma. However, this is not considered to be the end, but more the beginning of a new era of melanoma treatment. It is a whole new world for melanoma patients and clinical researchers [135]. Melanoma now serves as a model for targeted therapies and the future of melanoma treatment almost surely lies in treatment combinations. The combination of inhibitors of one signal transduction pathway or of various molecules targeting different signal transduction pathways appears to be particularly attractive. But also the combination of targeted therapies with effective immunotherapeutic agents like the CTLA-4 antibody ipilimumab is an area for active exploration in the near future. The approval of new drugs for unresectable or metastatic melanoma will necessarily lead to new clinical trial endpoints in subsequent studies. There is no question that, despite the availability of newly approved drugs for metastatic melanoma patients, clinical trials will still be mandatory in order to address the goal of cure [135].
New paradigms in treatment: the patient perspective
Valerie Guild
From the patient advocacy point of view and on behalf of the AIM at Melanoma Foundation (http://www.aimatmelanoma.org/en/index.html), I am tremendously delighted on the progress of melanoma both in diagnosis and treatment. Although we have not found a cure for melanoma, we may have turned it into a chronic disease. But, if we look at where we have come from, and even I in the 7 years remember all of those meetings which were just a succession of failed trials followed by failed agents, I think the fact that we have come as far as we have come is almost miraculous. We understand that it is only through the perseverance of melanoma researchers that we have gotten towhere wehave. And,for melanomapatients and their families, and myself included, who have sat on the other side of the desk, the fact that finally after all of these years because of all of your work we actually now—and I never thought this day would come—have the ability to hope.
Discussant summary and comment
John M. Kirkwood
Kirkwood: Thanks very much for inviting me to speak here. It's really an honor to be in front of what is, I think, the Who's Who of melanoma. I remember meeting Donald Morton at Lloyd Olds’ laboratory where I worked 1967–1969, just as his paper on BCG intralesional therapy had come out [136] and being thrilled that there was really going to be something that we could utilize as therapy for this refractory neoplasm. The revolution of the past couple of years of progress has transformed our field—and will make this the year of melanoma—instead of one drug, one biologic and no targeted therapies, we now have the prospect of means to modulate chemotherapy resistance that I'll come back to, which grow naturally out of what David Hoon has presented in terms of epigenetics. We have a host of immunotherapies approved with CTLA-4 in the wings. This finally makes sense, as we are critically in need of ways to down-regulate the immunosuppression that is the central confounding problem in melanoma, as Martin Mihm spoke about in terms of the soil, where the seeds wind up. It all starts with asking what our patients want. Valerie Guild's talk is important in that regard. The new journal Health Outcomes and Research in Medicine, is one that focuses upon the patient reported outcomes that we too often pass over...and we ask patients for patient reported outcomes, but it is high time for this. The dilemma is that we have a couple of old and now some new agents that can cure a very small number, and targeted agents that can clearly remit disease, as Axel Hauschild presented in terms of symptoms mitigated hours after the initiation of BRAF inhibitors or MEK inhibitors, and RR, PFS and OS improvement, but still at 2 years uncertain durability of these effects. Is it response, relapse interval prolongation, or OS that are the key benchmarks. With the new adjuvant therapy PEG Intron it isn't OS but relapse free survival that therapy can improve. However, the final end point that I think we've been laboring for to improve is OS. Martin Mihm presented this aptly and I would just add that new checkpoints beyond CTLA-4/PD1 such as TIM3 [137] are the basis of hope that we will continue to pursue with the hope to ultimately have more effective and less toxic therapies to cure melanoma. These may eclipse both PD1 and CTLA-4, and our decade old therapy IL-2, but it's the tumor infiltration that may be a biomarker of very great relevance to all of these outcomes. This whole conference on nodal disease began with Stanley Leong's clairvoyant realization that the node is the turnstile through which this tumor travels in the majority of patients [138]. We have ignored the lymph node—(not the surgeons here, but many outside of the surgical community) have ignored the node despite performing sentinel node biopsy upon hundreds of patients each year. We need to turn to more in-depth profiling studies of those sentinel nodes, to ask what the signatures of those patients with differing prognosis, and possibly differing sensitivity to new immunotherapies, and molecular interventions. As medical oncologists, we are always swimming in blood and we have blood studies to really show us perhaps the first hints that pro inflammatory cytokines, IL-6, TNF, IL-1 alpha, MIP1 alpha and beta predict the benefits of one of the oldest of our agents—interferon alpha. As Jeff Weber has presented, IL-17 is very interesting in a similar fashion in predicting the potential toxicity and benefit of the CTLA-4 blocking antibodies, but we will come back and talk about the tumor. As I mentioned already, David Hoon's talk has suggested that individual gene promoter methylation, global methylation, and micro-RNA changes in melanoma may be related to its refractoriness, rather than simply the genetic aberrations Axel Hauschild has just discussed. These may predict progression and also may predict cytotoxic drug responsiveness. Hussein Tawbi presented to ASCO 2 years ago [139] that methylation patterns combined with gene expression profiling may predict who will respond to temozolomide or dacarbazine. These methylation and genomic analyses of tumor tissue identified a half dozen genes that are up-regulated, another few that are down regulated and predictive of the patients who have the capacity to respond to temozolomide. So it is high time to approach the disease in a combined modality program with decitabine and temozolomide, as Dr. Tawbi and his colleagues have done, to augment the response and quality of responses to temozolomide. Further combinations with PARP inhibitors through base excision repair may improve responsiveness further, and chemotherapy may then return to the therapeutic armamentarium in this disease.
What is the problem in metastatic melanoma? Clearly by the time it gets to the red zone we have many things wrong. We have so many things wrong that it's hard to imagine that any single intervention will have the capacity to alter the outcome of durable disease control. If we simply schematize this in advanced disease, the multitude of things gone wrong at both the tumor level, and at the level of the host, suggest that single agents have an impossible challenge to achieve durable impact. I would like to suggest that the adjuvant approaches with new agents will be far more important, and far more informative. Neoadjuvant approaches that I will come back to discuss have been both clinically and mechanistically rewarding. Work from our program, and the laboratory of Walt Storkus has shown that patients who have active bulky tumor have Th2 biased T cell responses. Patients without measurable disease, have by contrast, a consistent Th1 biased T cell response pattern when evaluated in relation to a half dozen peptide vaccine antigens, in vitro using Elispot T cell assays. A scatter plot for six different antigens thus demonstrate IL-4, IL-5, and IL-10 responses from peripheral blood T cells, which you know comprise the signature of tolerance; patients who have no measurable disease have blood T cell responses yielding TNF, interferon gamma, and a Th1 cytokine pattern of effector T cells. This says again that pushing harder on the broken immune response of patients with advanced disease may only get you deeper in the hole, not ahead of the game. The role of ulceration—an established prognosticator for localized primary melanoma, has been added to the AJCC synoptic for microstaging of melanoma. But Mohammed Kashani-Sabet has shown us a multi-marker profile that may eclipse ulceration in predicting disease specific survival in his patients studied with Richard Sagebiel at UCSF. These factors will be very interesting to look at in relation to the progression of melanoma, both to regional disease, and to systemic metastasis. We now have the opportunity to do this in relation to the E1690 intergroup trial cohort, from whom we have banked tissue samples that Mohammed Kashani-Sabet is studying. In fact, Martin Mihm and Uma Rao, pathologists of the ECOG Melanoma Committee, have gone through and scored TIL infiltrates in these patients, showing once again the importance of TILs. However molecular profiling has yet to be done, and the Pleckstrin homology domain (PHIP) is a very interesting new molecule that may add to our prediction of the biology of this disease. If, in fact, high PHIP expression predicts worse prognosis, and reduces the likelihood of interferon benefit, this would comprise both a prognostic and a predictive marker. Mohammed Kashani-Sabet's new algorithm for the flow of this disease demonstrates that we really need to know much more of the molecular basis of progression, and the sequential cascade that may predict, on the one side, nodal metastasis, and on the other hematogenous visceral metastasis. The question really is whether the process is stochastic or parallel? Does the disease spread through the lymphatics and through the bloodstream at the same time, such that the nodal station is a weathervane but not a point of useful intervention, or in a stochastic manner which may be interrupted at the nodal station? We have prognostic blood markers such as S100 that we have recently published [140] where this biomarker added significantly to our ability to predict mortality in the E1694 trial. Helen Gogas has reported upon the value of clinical and serological manifestations of autoimmunity, and the germline genetic biomarkers as predictive bio-markers or surrogates of therapeutic benefit [141, 142]; these are potentially relevant to multiple immunotherapeutic modalities, beyond interferon alpha. These are currently planned to be evaluated in relation to trials of the CTLA-4 blocking antibodies.
Cytotoxic T-lymphocyte-associated antigen-4 (CTLA-4) has been found to block the signaling of the MHC/antigen peptide, T cell receiptor, B7 and CD 28 complex, resulting the attenuation of this signal. Thus, immune response against a target is blunted. Since, CTLA-4 plays a primary role in down-regulating the immune response, it has become an important therapeutic target for cancer immunotherapy in that fully developed human monoclonal antibodies against CTLA-4 have been used in clinical trials [143]. Enhancement of the immune response against cancer using monoclonal antibody blockade of CTLA-4 has resulted in a significant survival benefit for patients with metastatic melanoma [144].
For more than 25 years, we have known that autoimmunity has been correlated with the clinical benefits of IL-2 therapy, since patients who benefit from immunotherapy with IL-2 were found to have developed autoimmunity—and in particular, thyroiditis and vitiligo-like leukoderma of the skin [142, 145]. The demonstration of a 50-fold improvement in the likelihood of relapse-free and OS among patients found to have serological or clinical signs of autoimmunity is something we are now corroborating in relation to the adjuvant trials of the ECOG and intergroup, to see whether in our hands the serological antibody mediated autoimmune responses predict the patients who don't progress, and who have the prospect of improved survival [140]. This coincidence of autoimmunity and benefit from several immunotherapies is important to think about, because we don't understand what predicts the autoimmunity in some but not other patients, at this time. Data from our multiplex luminex assays in the context of the ECOG 1690 and 1694 trials has shown evidence that corroborates the suggestion that patients who benefit (and are <5 years disease-free) demonstrated elevations of baseline blood levels of six pro inflammatory cytokines [146]. In this same trial, the vaccine (GMK) group may be taken as a control group, and the same pro inflammatory cytokines made no difference whatsoever in relation to long-term RFS and OS. The question of how long to treat has been answered clearly, in our E1697 trial: there is no durable benefit of treatment for 1 month, compared to observation—where our several trials of HDI for 1 year have shown consistent durable prevention of relapse, and improved mortality in two of these trials. We have tested the impact of the HDI regimen using the neoadjuvant setting—where careful studies in 20 patients taught us more about mechanism of action than thousands of patients in randomized controlled trials, because we could harvest tumor tissue before, and after therapy for 1 month—to ask what was going on in the tumor tissue. Prior to therapy, there were few T cells in tumor tissue, where after 1 month of HDI there were dense infiltrates of T cells. Similarly, before treatment there were no DCs, and after treatment there were droves of DCs [147]. Why would the interferon-alpha therapy for a month daily by the IV route at 20 MU/M2 drive these cells into the tumor? Our studies of the signaling molecules in the tumor tissue answered this question, demonstrating copious STAT3 richly expressed before treatment that was wiped out after treatment for 1 month. This has been shown repeatedly and not just for melanoma, but also for head and neck cancer, and other tumors to be discussed at this congress. The suppression of STAT3 and pSTAT3 in tumor tissue is something that may explain the immunomodulatory action of HDI [148]. The clinical response is much greater in the earlier disease, where neoadjuvant application has been most informative. The immunologic effects we have observed in these studies proves to be the only impact we can show with interferon alpha—the basis of therapeutic benefit in the adjuvant setting is thus not anti-angiogenesis, nor pro-apoptotic effects, or other direct effects of the IFN. Rather, it is immunologic, like a vaccine. Certainly daily therapy for a month, and thrice weekly therapy for a year, is major hurdle for patients to endure, in treatment following the 1684 regimen. The FDA's approval of PegIFN adds this to the list of adjuvant therapeutic options for patients with melanoma. There was a significant impact upon RFS, but in this study, no significant impact upon OS—and when analyzed by burden of disease the impact appears to have occurred solely in the stage IIIA or N1 patients, as designated in the EORTC. Specifically, patients with gross nodal disease do not benefit from this therapy. With these caveats, this regimen will provide an alternative therapeutic strategy for patients who have microscopic nodal disease (only), and cannot entertain the established and confirmed regimen of high-dose IFN after the E1684 design—with its more mature evidence for durable benefit in both microscopic and macroscopic nodal disease, as well as stage IIB deep primary disease. The question that remains as to how this therapy works? Biomarkers of angiogenesis planned in the context of the EORTC trial have yet to be reported. Of interest, ulceration appears to be associated with benefit from the pegylated and other regimens of low-dose or intermediate-dose IFN. These have not been evident in our analyses of the several ECOG Phase III trials of HDI. So, there may be alternative mechanisms of action for the different regimens of therapy with this pleiotropic agent.
For the first time, we have positive phase III trials of IFNs, CTLA-4 blocking antibodies, and BRAF inhibitors. We have new formulations of IFN, with an adjuvant role of Peg IFN in microscopic stage III melanoma, for patients who are unable or not willing to consider the standard HDI regimen. We also have the MAGE A3 vaccine, Allovectin B7 vaccine, and Biovex pending final analysis.
The hope is palpable that our 30 years of blight with only a couple of regimens of marginal efficacy in meta-static disease may give rise to new more rational and less toxic therapeutic options, with durable and, dare we say it, curative effects. You've seen these discussed at this symposium already, and I think the fact that the BRAF mutation status is a pivot for therapeutic decision-making for patients with metastatic symptomatic disease may give rise to analyses of BRAF mutation status, PTEN, c-Kit and NRAS status, as the basis for more personalized therapy of melanoma. Analysis of the basis of therapy resistance will increasingly become our focus, since while primary resistance is seen in only 10 or 15 %, acquired resistance is nearly universal after 6–12 months of therapy. Brain disease is a further major obstacle that we are approaching systematically using new BRAF inhibitors from GSK, where we thought we would never be able to hope to remit disease in patients with predictable regularity, but now see more than 50 % of patients showing antitumor benefit in brain metastases [149]. Abrogation of resistance using combinations such as combinations that impact parallel pathways, or that may be collimated in the MAP/MEK pathway, have early suggestions of added benefit and perhaps reduced toxicity. The use of immunomodulators in combination with BRAF inhibitors is also of keen interest, and will be pursued in the US National cooperative groups as well as international Melanoma Working Group, as well as the Melanoma Breakthrough Consortium of the Melanoma Research Foundation, as well as at individual institutions such as the University of Pittsburgh. The transformational results of 2011 suggest that immunotherapy cures patients with metastatic disease, and that immunotherapy with anti-CTLA4 blocking antibodies adds at least 10% to the survival fraction in metastatic melanoma patient populations past 2 years in patients receiving ipilimumab at the 3 mg/kg dose. Autoimmune toxicity is a new wrinkle that we have to deal with, and hopefully biomarkers will predict which patients are the beneficiaries, as well as those who are at increased risk of toxicity, so that we're not treating a hundred people to get 10 who have benefit down the road.
Future perspectives
Where to go from here? I think that the adjuvant application of these modalities, singly at first and then in combination, is clearly where greater impact is likely to be seen. How to combine these agents with vaccines may be the biggest hurdle, because while mouse models suggested that vaccines plus CTLA-4 blocking antibodies gave dramatically improved outcome, the experience to date in human melanoma is that the vaccine combination gives no added benefit, and if anything degrades the benefit with ipilimumab. Why? There is no answer presently known. EORTC will complete its trial EORTC 18071 this year, testing Ipilimumab at high (10 mg/kg) dosage given every 3 weeks × 4, then every 3 months for 3 years versus placebo in node metastatic stage III melanoma. The intergroup E1609 activates in May 2011, offering a head-to-head comparison of Ipilimumab versus HDI in resectable stage IIIB and IV melanoma, where 500 patients will be enrolled for treatment for 1 year with either ipilimumab at 10 mg/kg or at 3 mg/kg every 3 weeks 9 4 then every 3 months × 3, versus standard IFN alfa-2b at high dosage (20 MU/M2/day × 5 for 4 weeks and 10 MU/M2/day TIW for 11 months. Neoadjuvant approaches are the logical sequel and offer an avenue to explore for anti-CTLA4 blocking antibodies alone, and in combination with IFN alfa-2b. At the UPCI and UPMC, we have conducted trial 08-144, a neoadjuvant evaluation of ipilimumab in which we have now treated 29 patients. Mature data were presented in ASCO 2011 [150], testing Ipilimumab at the 10 mg/kg dose, with biopsy done before therapy, and ipilimumab given twice before and twice after definitive surgery at intervals of 3 weeks. What will we get out of it? Well, first hope is for early signals that patient may benefit and, in fact, several of these patients have already demonstrated clinical responses. We also get information from the tissue biopsies. Tissue here will inform our decision matrix more than anything we've had before. What dosage is optimal? Steve O'Day has shown some very interesting data that I think we all took at face value, suggesting that dosages of Ipilimumab of 0.3 mg/kg gave benefits that were less than for 3 mg/kg, and in turn less than for 10 mg/ kg. But we have an approved dose of 3 mg/kg, and many trials including the EORTC 18071 and ECOG E1609 that are looking at 10 mg/kg. For the moment it is not certain what the optimal dosage may be. What is the optimal duration of therapy? The approved regimen is four cycles of therapy. Twelve weeks has been prolonged to 12 months, for our adjuvant E1609 trial, and even longer for the EORTC 18071 trial. Combinations are obviously the place to go. Anti-CTLA-4 blocking antibodies given together with interferon alpha has been a combination of interest to us at the University of Pittsburgh for several years, and we've completed a trial of Pfizer's Tremelimumab combined with IFN alfa-2b [151]. The UPCI trial 05-125 trial recently published suggests additive benefit of tremelimumab at full dose 15 mg/kg, with high-dose interferon alpha. Toxicity was no greater than with either agent given alone. Anti-CTLA-4 combined with GM-CSF is being pursued by Steve Hodi in trial ECOG 1608, which is one of the most rapidly accrued trials in ECOG Melanoma Committee history, with 240 patients in less than 6 months. The combination of anti-GD3 antibody and interferon alpha has been pursued in UPCI Trial 07-023, testing whether ADCC and other mechanisms of potential synergy may achieve improved therapeutic goals; this study has been conducted between UPCI and U Chicago, with support of the Ludwig Institute. Vaccines and inter-feron alpha are being pursued by Lisa Butterfield in the context of our SPORE project 2, which will reach the clinic in the next year. Vaccines and CTLA-4 have been pursued in the phase III trial 020, without evident added benefits. But Hassane Zarour has proposed to evaluate the combination of anti-CTLA4 blocking antibody and the protein NY-ESO-1 vaccine. The combination of BRAF inhibitors and anti-CLTA-4 blocking antibodies is an obvious opportunity, although the fundamental rationale in terms of mechanism has not been well worked out. Our evaluation of anti-CTLA4 blocking antibody plus interferon alfa-2 has been gratified by a number of CR and PR, with an overall RR approaching 30 %, with many durable. Disease that is remitted and well controlled for long intervals by this therapy may be an attractive goal beyond the obvious goal of CR. Biomarkers of response with this combination include autoimmunity as I mentioned already, and CRP levels in the blood. High CRP levels predicted non-response to therapy, whereas low CRP levels predicted benefit. This has been seen with anti-CTLA4 blocking antibody alone in the phase III trial, so appears to be real. Absolute lymphocyte counts, and the change in ALC between baseline and 7 weeks of therapy have been shown to correspond to dosage, and to therapeutic outcome, but we really need to mine these trials further for biomarkers, both in the blood, and in the tumor tissues—since responses are seen in a minority of treated patients at this time, and a tool that would allow the selection of the patients who benefit would immediately improve the therapeutic index by several fold. One of the most interesting trials we've planned is the neoadjuvant trial of ipilimumab with interferon (UPCI 11-063). This will be high-dose IFN alfa-2b administered with either ipilimumab at 3 or 10 mg/kg in a neoadjuvant setting. The trial will select treatment between the two dosages in a randomized manner, and accrue 30 patients: tissue biopsies taken before and after two cycles of ipilimumab will allow us to ask what's going on at ground zero, in the tumor tissue. This will be an important mechanistic assessment conducted in parallel with the phase III trial of IFN versus Ipilimumab, E1609, already discussed and a study planned in the multicenter setting through ECOG, E3611. With the new check points PD-1 and TIM-3, I think we have reagents now to assail that really deserve to be tested [137]. In the end, it is hoped that our immunotherapy demonstrates the survival and progression-free benefit in advanced and adjuvant setting. Interferon is the standard, but IL-2 we've had before this, and anti-CTLA-4 blocking antibodies, all three of these induce autoimmunity. All three of these have very similar signatures, a pro inflammatory cytokine predictive of benefit. And so I think the paradigm shifts are that we have now an understanding of the role of driver mutations, BRAF for sure we need to assail. Whether c-Kit really stays on the map I think will depend upon what we see in several trials that are currently ongoing. Dasatinib, tested in the ECOG trial 2607, is moving from enrollment of patients with acral and mucosal melanomas, to enrollment only of patients with specifically proven c-Kit mutations that appear to be more sensitive to the molecular inhibitors.
I think a signature of chemotherapy resistance, as I showed in relation to Dave Hoon's presentation, would be most interesting because this is where we started 30 years ago. No matter what it was in the past, melanoma is no longer one disease. We had the morphotypes of superficial, nodular lentiginous, and acral melanoma that others have articulated previously—and hoped that these might guide the personalization of therapy. Now we have cutaneous melanoma—and multiple pathway driven distinctions of BRAF mutant vs. wild type, and clinical variables that are likely to be important in driving the decision of whether to use BRAF inhibitors—such as whether the patient is symptomatic? I still think it's important whether the patient is symptomatic: If they are, I think it's obvious the BRAF inhibitors play a new and dynamic role. If they are asymptomatic, BRAF mutation or not, I think the immunotherapies still take top drawer. For those patients treated with IL-2, or ipilimumab in the advanced disease setting, or interferon in the adjuvant setting there are durable effects that extend past 5–10 years now. Acral mucosal melanomas represent a different biology that we've discussed. Uveal melanoma represents a nominally related disease, where very different mutations in GNAQ and GNA11 are identified, albeit not yet targeted by therapeutic agents. For unknown primary melanomas, it is very much the same as for cutaneous melanoma. This new algorithm of therapy will doubtless change, but offers the prospect of personalized therapy with greater gain, and less toxicity, than our prior therapies.
Acknowledgments
John Kirkwood The project described was supported by Award Number P50CA121973 from the National Cancer Institute. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health.
Contributor Information
Stanley P. L. Leong, Center for Melanoma Research and Treatment and Department of Surgery, California Pacific Medical Center, San Francisco, CA, USA Center for Melanoma Research and Treatment, California Pacific Medical Center, Sutter Pacific Medical Foundation, San Francisco, CA, USA; California Pacific Medical Center, 2340 Clay Street, 2nd Floor, San Francisco, CA 94115, USA.
Martin C. Mihm, Jr., Center for Melanoma Oncology, Dana Farber/Brigham and Women's Cancer Center, Boston, MA, USA
George F. Murphy, Center for Melanoma Oncology, Dana Farber/Brigham and Women's Cancer Center, Boston, MA, USA
Dave S. B. Hoon, John Wayne Cancer Institute, Santa Monica, CA, USA
Mohammed Kashani-Sabet, Center for Melanoma Research and Treatment, California Pacific Medical Center, Sutter Pacific Medical Foundation, San Francisco, CA, USA.
Sanjiv S. Agarwala, Department of Hematology/Oncology, St. Luke's University Health Network, Bethlehem, PA, USA
Jonathan S. Zager, Department of Cutaneous Oncology, Moffitt Cancer Center, Tampa, FL, USA
Axel Hauschild, Department of Dermatology, University of Kiel, Kiel, Germany.
Vernon K. Sondak, Department of Cutaneous Oncology, Moffitt Cancer Center, Tampa, FL, USA
Valerie Guild, AIM at Melanoma Foundation, Richmond, CA, USA.
John M. Kirkwood, Department of Medicine, University of Pittsburgh, Pittsburgh, PA, USA
References
- 1.Murphy MJ. Diagnostic and prognostic biomarkers and therapeutic targets in melanoma. Springer; New York: 2012. [Google Scholar]
- 2.Becker D, Mihm MC, Hewitt SM, et al. Markers and tissue resources for melanoma: meeting report. Cancer Res. 2006;66:10652–10657. doi: 10.1158/0008-5472.CAN-06-0921. [DOI] [PubMed] [Google Scholar]
- 3.Beecher C. The human metabolome. In: Harrigan G, Goodacre R, editors. Metabolic profilings: its role in biomarker discovery and gene function analysis. Kluwer Academic Publishers; Boston: 2003. pp. 311–319. [Google Scholar]
- 4.Flaherty KT. BRAF inhibitors and melanoma. Cancer J. 2011;17:505–511. doi: 10.1097/PPO.0b013e31823e5357. [DOI] [PubMed] [Google Scholar]
- 5.Balch CM, Gershenwald JE, Soong SJ, et al. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol. 2009;27:6199–6206. doi: 10.1200/JCO.2009.23.4799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leong SP, Gershenwald JE, Soong SJ, et al. Cutaneous melanoma: a model to study cancer metastasis. J Surg Oncol. 2011;103:538–549. doi: 10.1002/jso.21816. [DOI] [PubMed] [Google Scholar]
- 7.Raz A, Ben-Ze'ev A. Modulation of the metastatic capability in B16 melanoma by cell shape. Science. 1983;221:1307–1310. doi: 10.1126/science.6612347. [DOI] [PubMed] [Google Scholar]
- 8.Gupta GP, Massague J. Cancer metastasis: building a framework. Cell. 2006;127:679–695. doi: 10.1016/j.cell.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 9.Valastyan S, Weinberg RA. Tumor metastasis: molecular insights and evolving paradigms. Cell. 2011;147:275–292. doi: 10.1016/j.cell.2011.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Damsky WE, Curley DP, Santhanakrishnan M, et al. beta-catenin signaling controls metastasis in Braf-activated Ptendeficient melanomas. Cancer Cell. 2011;20:741–754. doi: 10.1016/j.ccr.2011.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fidler IJ. The pathogenesis of cancer metastasis: the ‘seed and soil’ hypothesis revisited. Nat Rev Cancer. 2003;3:453–458. doi: 10.1038/nrc1098. [DOI] [PubMed] [Google Scholar]
- 12.Bogenrieder T, Herlyn M. Axis of evil: molecular mechanisms of cancer metastasis. Oncogene. 2003;22:6524–6536. doi: 10.1038/sj.onc.1206757. [DOI] [PubMed] [Google Scholar]
- 13.Mortarini R, Gismondi A, Maggioni A, et al. Mitogenic activity of laminin on human melanoma and melanocytes: different signal requirements and role of beta 1 integrins. Cancer Res. 1995;55:4702–4710. [PubMed] [Google Scholar]
- 14.Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119:1420–1428. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Scheel C, Weinberg RA. Phenotypic plasticity and epithelial-mesenchymal transitions in cancer and normal stem cells? Int J Cancer. 2011;129:2310–2314. doi: 10.1002/ijc.26311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ma J, Lin JY, Alloo A, et al. Isolation of tumorigenic circulating melanoma cells. Biochem Biophys Res Commun. 2010;402:711–717. doi: 10.1016/j.bbrc.2010.10.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maldonado JL, Fridlyand J, Patel H, et al. Determinants of BRAF mutations in primary melanomas. J Natl Cancer Inst. 2003;95:1878–1890. doi: 10.1093/jnci/djg123. [DOI] [PubMed] [Google Scholar]
- 18.Curtin JA, Busam K, Pinkel D, et al. Somatic activation of KIT in distinct subtypes of melanoma. J Clin Oncol. 2006;24:4340–4346. doi: 10.1200/JCO.2006.06.2984. [DOI] [PubMed] [Google Scholar]
- 19.Bouffard D, Duncan LM, Howard CA, et al. Actin-binding protein expression in benign and malignant melanocytic proliferations. Hum Pathol. 1994;25:709–714. doi: 10.1016/0046-8177(94)90305-0. [DOI] [PubMed] [Google Scholar]
- 20.Van Belle PA, Elenitsas R, Satyamoorthy K, et al. Progression-related expression of beta3 integrin in melanomas and nevi. Hum Pathol. 1999;30:562–567. doi: 10.1016/s0046-8177(99)90202-2. [DOI] [PubMed] [Google Scholar]
- 21.Saalbach A, Wetzel A, Haustein UF, et al. Interaction of human Thy-1 (CD 90) with the integrin alphavbeta3 (CD51/CD61): an important mechanism mediating melanoma cell adhesion to activated endothelium. Oncogene. 2005;24:4710–4720. doi: 10.1038/sj.onc.1208559. [DOI] [PubMed] [Google Scholar]
- 22.Hofmann UB, Westphal JR, Waas ET, et al. Matrix metalloproteinases in human melanoma cell lines and xenografts: increased expression of activated matrix metalloproteinase-2 (MMP-2) correlates with melanoma progression. Br J Cancer. 1999;81:774–782. doi: 10.1038/sj.bjc.6690763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bodey B, Bodey B, Jr, Siegel SE, et al. Matrix metalloproteinase expression in malignant melanomas: tumor-extra-cellular matrix interactions in invasion and metastasis. In Vivo (Athens, Greece) 2001;15:57–64. [PubMed] [Google Scholar]
- 24.Chen Y, Huang L, Yu J. Evaluation of heparanase and matrix metalloproteinase-9 in patients with cutaneous malignant melanoma. J Dermatol. 2012;39:339–343. doi: 10.1111/j.1346-8138.2011.01441.x. [DOI] [PubMed] [Google Scholar]
- 25.Das SK, Bhutia SK, Kegelman TP, et al. MDA-9/syntenin: a positive gatekeeper of melanoma metastasis. Front Biosci. 2012;17:1–15. doi: 10.2741/3911. [DOI] [PubMed] [Google Scholar]
- 26.Lu Z, Lu N, Li C, et al. Oroxylin A inhibits matrix metalloproteinase-2/9 expression and activation by up-regulating tissue inhibitor of metalloproteinase-2 and suppressing the ERK1/2 signaling pathway. Toxicol Lett. 2012;209:211–220. doi: 10.1016/j.toxlet.2011.12.022. [DOI] [PubMed] [Google Scholar]
- 27.Kim M, Gans JD, Nogueira C, et al. Comparative oncogenomics identifies NEDD9 as a melanoma metastasis gene. Cell. 2006;125:1269–1281. doi: 10.1016/j.cell.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 28.Girouard SD, Laga AC, Mihm MC, et al. SOX2 contributes to melanoma cell invasion. Lab Investig. 2012;92:362–370. doi: 10.1038/labinvest.2011.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Laga AC, Zhan Q, Weishaupt C, et al. SOX2 and nestin expression in human melanoma: an immunohistochemical and experimental study. Exp Dermatol. 2011;20:339–345. doi: 10.1111/j.1600-0625.2011.01247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Laga AC, Lai CY, Zhan Q, et al. Expression of the embryonic stem cell transcription factor SOX2 in human skin: relevance to melanocyte and merkel cell biology. Am J Pathol. 2010;176:903–913. doi: 10.2353/ajpath.2010.090495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ruiter D, Bogenrieder T, Elder D, et al. Melanoma-stroma interactions: structural and functional aspects. Lancet Oncol. 2002;3:35–43. doi: 10.1016/s1470-2045(01)00620-9. [DOI] [PubMed] [Google Scholar]
- 32.Zigrino P, Kuhn I, Bauerle T, et al. Stromal expression of MMP-13 is required for melanoma invasion and metastasis. J Investig Dermatol. 2009;129:2686–2693. doi: 10.1038/jid.2009.130. [DOI] [PubMed] [Google Scholar]
- 33.Hoffmann C, Horst HA, Weichenthal M, et al. Malignant melanoma and HIV infection—aggressive course despite immune reconstitution. Onkologie. 2005;28:35–37. doi: 10.1159/000082291. [DOI] [PubMed] [Google Scholar]
- 34.Navab R, Strumpf D, Bandarchi B, et al. Prognostic gene-expression signature of carcinoma-associated fibroblasts in non-small cell lung cancer. Proc Natl Acad Sci USA. 2011;108:7160–7165. doi: 10.1073/pnas.1014506108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Navab R, Liu J, Seiden-Long I, et al. Co-overexpression of Met and hepatocyte growth factor promotes systemic metastasis in NCI-H460 non-small cell lung carcinoma cells. Neoplasia. 2009;11:1292–1300. doi: 10.1593/neo.09622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chiang AC, Massague J. Molecular basis of metastasis. N Engl J Med. 2008;359:2814–2823. doi: 10.1056/NEJMra0805239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gupta GP, Perk J, Acharyya S, et al. ID genes mediate tumor reinitiation during breast cancer lung metastasis. Proc Natl Acad Sci USA. 2007;104:19506–19511. doi: 10.1073/pnas.0709185104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Swarbrick A, Roy E, Allen T, et al. Id1 cooperates with oncogenic Ras to induce metastatic mammary carcinoma by subversion of the cellular senescence response. Proc Natl Acad Sci USA. 2008;105:5402–5407. doi: 10.1073/pnas.0801505105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kaplan RN, Riba RD, Zacharoulis S, et al. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature. 2005;438:820–827. doi: 10.1038/nature04186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mihm MC, Jr, Nelson JS. Hypothesis: the metastatic niche theory can elucidate infantile hemangioma development. J Cutan Pathol. 2010;37(Suppl 1):83–87. doi: 10.1111/j.1600-0560.2010.01521.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li F, Tiede B, Massague J, et al. Beyond tumorigenesis: cancer stem cells in metastasis. Cell Res. 2007;17:3–14. doi: 10.1038/sj.cr.7310118. [DOI] [PubMed] [Google Scholar]
- 42.Damsky WE, Rosenbaum LE, Bosenberg M. Decoding melanoma metastasis. Cancers. 2011;3(1):126–163. doi: 10.3390/cancers3010126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Youngs SJ, Ali SA, Taub DD, et al. Chemokines induce migrational responses in human breast carcinoma cell lines. Int J Cancer. 1997;71:257–266. doi: 10.1002/(sici)1097-0215(19970410)71:2<257::aid-ijc22>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 44.Shu S, Cochran AJ, Huang RR, et al. Immune responses in the draining lymph nodes against cancer: implications for immunotherapy. Cancer Metastasis Rev. 2006;25:233–242. doi: 10.1007/s10555-006-8503-7. [DOI] [PubMed] [Google Scholar]
- 45.Hoon DS, Kitago M, Kim J, et al. Molecular mechanisms of metastasis. Cancer Metastasis Rev. 2006;25:203–220. doi: 10.1007/s10555-006-8500-x. [DOI] [PubMed] [Google Scholar]
- 46.Kawada K, Sonoshita M, Sakashita H, et al. Pivotal role of CXCR3 in melanoma cell metastasis to lymph nodes. Cancer Res. 2004;64:4010–4017. doi: 10.1158/0008-5472.CAN-03-1757. [DOI] [PubMed] [Google Scholar]
- 47.Greenberg ES, Chong KK, Huynh KT, Tanaka R, Hoon DS. Epigenetic biomarkers in skin cancer. Cancer Lett. 2012 doi: 10.1016/j.canlet.2012.01.020. doi: 10.1016\j.canlet.2010.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hoshimoto S, Kuo CT, Chong KK, et al. AIM1 and LINE-1 epigenetic aberrations in tumor and serum relate to melanoma progression and disease outcome. J Investig Dermatol. 2012;132:1689–1697. doi: 10.1038/jid.2012.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tanemura A, Terando AM, Sim MS, et al. CpG island methylator phenotype predicts progression of malignant melanoma. Clin Cancer Res. 2009;15:1801–1807. doi: 10.1158/1078-0432.CCR-08-1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mori T, Kim J, Yamano T, et al. Epigenetic up-regulation of C-C chemokine receptor 7 and C-X-C chemokine receptor 4 expression in melanoma cells. Cancer Res. 2005;65:1800–1807. doi: 10.1158/0008-5472.CAN-04-3531. [DOI] [PubMed] [Google Scholar]
- 51.Mori T, Martinez SR, O'Day SJ, et al. Estrogen receptor-alpha methylation predicts melanoma progression. Cancer Res. 2006;66:6692–6698. doi: 10.1158/0008-5472.CAN-06-0801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Goto Y, Arigami T, Murali R, et al. High molecular weight-melanoma-associated antigen as a biomarker of desmoplastic melanoma. Pigment Cell Melanoma Res. 2010;23:137–140. doi: 10.1111/j.1755-148X.2009.00660.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Goto Y, Ferrone S, Arigami T, et al. Human high molecular weight-melanoma-associated antigen: utility for detection of metastatic melanoma in sentinel lymph nodes. Clin Cancer Res. 2008;14:3401–3407. doi: 10.1158/1078-0432.CCR-07-1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hoon DS, Spugnardi M, Kuo C, et al. Profiling epigenetic inactivation of tumor suppressor genes in tumors and plasma from cutaneous melanoma patients. Oncogene. 2004;23:4014–4022. doi: 10.1038/sj.onc.1207505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kitago M, Martinez SR, Nakamura T, et al. Regulation of RUNX3 tumor suppressor gene expression in cutaneous melanoma. Clin Cancer Res. 2009;15:2988–2994. doi: 10.1158/1078-0432.CCR-08-3172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nguyen T, Kuo C, Nicholl MB, et al. Downregulation of microRNA-29c is associated with hypermethylation of tumor-related genes and disease outcome in cutaneous melanoma. Epigenetics. 2011;6:388–394. doi: 10.4161/epi.6.3.14056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.de Maat MF, van de Velde CJ, van der Werff MP, et al. Quantitative analysis of methylation of genomic loci in early-stage rectal cancer predicts distant recurrence. J Clin Oncol. 2008;26:2327–2335. doi: 10.1200/JCO.2007.14.0723. [DOI] [PubMed] [Google Scholar]
- 58.Haqq C, Nosrati M, Sudilovsky D, et al. The gene expression signatures of melanoma progression. Proc Natl Acad Sci USA. 2005;102:6092–6097. doi: 10.1073/pnas.0501564102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kashani-Sabet M, Rangel J, Torabian S, et al. A multi-marker assay to distinguish malignant melanomas from benign nevi. Proc Natl Acad Sci USA. 2009;106:6268–6272. doi: 10.1073/pnas.0901185106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kashani-Sabet M, Venna S, Nosrati M, et al. A multi-marker prognostic assay for primary cutaneous melanoma. Clin Cancer Res. 2009;15:6987–6992. doi: 10.1158/1078-0432.CCR-09-1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rangel J, Torabian S, Shaikh L, et al. Prognostic significance of nuclear receptor coactivator-3 overexpression in primary cutaneous melanoma. J Clin Oncol. 2006;24:4565–4569. doi: 10.1200/JCO.2006.07.3833. [DOI] [PubMed] [Google Scholar]
- 62.Rangel J, Nosrati M, Torabian S, et al. Osteopontin as a molecular prognostic marker for melanoma. Cancer. 2008;112:144–150. doi: 10.1002/cncr.23147. [DOI] [PubMed] [Google Scholar]
- 63.Rangel J, Nosrati M, Leong SP, et al. Novel role for RGS1 in melanoma progression. Am J Surg Pathol. 2008;32:1207–1212. doi: 10.1097/PAS.0b013e31816fd53c. [DOI] [PubMed] [Google Scholar]
- 64.Mastrangelo MJ, Bellet RE, Berkelhammer J, et al. Regression of pulmonary metastatic disease associated with intralesional BCG therapy of intracutaneous melanoma metastases. Cancer. 1975;36:1305–1308. doi: 10.1002/1097-0142(197510)36:4<1305::aid-cncr2820360417>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 65.Agarwala SS, Neuberg D, Park Y, et al. Mature results of a phase III randomized trial of bacillus Calmette-Guerin (BCG) versus observation and BCG plus dacarbazine versus BCG in the adjuvant therapy of American Joint Committee on Cancer Stage I-III melanoma (E1673): a trial of the Eastern Oncology Group. Cancer. 2004;100:1692–1698. doi: 10.1002/cncr.20166. [DOI] [PubMed] [Google Scholar]
- 66.Bedikian AY, Richards J, Kharkevitch D, et al. A phase 2 study of high-dose Allovectin-7 in patients with advanced metastatic melanoma. Melanoma Res. 2010;20:218–226. doi: 10.1097/CMR.0b013e3283390711. [DOI] [PubMed] [Google Scholar]
- 67.Senzer NN, Kaufman HL, Amatruda T, et al. Phase II clinical trial of a granulocyte-macrophage colony-stimulating factor-encoding, second-generation oncolytic herpesvirus in patients with unresectable metastatic melanoma. J Clin Oncol. 2009;27:5763–5771. doi: 10.1200/JCO.2009.24.3675. [DOI] [PubMed] [Google Scholar]
- 68.Thompson JF, Hersey P, Wachter E. Chemoablation of metastatic melanoma using intralesional Rose Bengal. Melanoma Res. 2008;18:405–411. doi: 10.1097/CMR.0b013e32831328c7. [DOI] [PubMed] [Google Scholar]
- 69.Gimbel MI, Delman KA, Zager JS. Therapy for unresectable recurrent and in-transit extremity melanoma. Cancer Control. 2008;15:225–232. doi: 10.1177/107327480801500305. [DOI] [PubMed] [Google Scholar]
- 70.Beasley GM, Petersen RP, Yoo J, et al. Isolated limb infusion for in-transit malignant melanoma of the extremity: a well-tolerated but less effective alternative to hyperthermic isolated limb perfusion. Ann Surg Oncol. 2008;15:2195–2205. doi: 10.1245/s10434-008-9988-9. [DOI] [PubMed] [Google Scholar]
- 71.Lindner P, Doubrovsky A, Kam PC, et al. Prognostic factors after isolated limb infusion with cytotoxic agents for melanoma. Ann Surg Oncol. 2002;9:127–136. doi: 10.1007/BF02557363. [DOI] [PubMed] [Google Scholar]
- 72.Kroon HM, Moncrieff M, Kam PC, et al. Outcomes following isolated limb infusion for melanoma. A 14-year experience. Ann Surg Oncol. 2008;15:3003–3013. doi: 10.1245/s10434-008-9954-6. [DOI] [PubMed] [Google Scholar]
- 73.Fraker DL. Hyperthermic regional perfusion for melanoma and sarcoma of the limbs. Curr Probl Surg. 1999;36:841–907. doi: 10.1016/s0011-3840(99)80008-2. [DOI] [PubMed] [Google Scholar]
- 74.Beasley GM, Caudle A, Petersen RP, et al. A multi-institutional experience of isolated limb infusion: defining response and toxicity in the US. J Am Coll Surg. 2009;208:706–715. doi: 10.1016/j.jamcollsurg.2008.12.019. discussion 715–717. [DOI] [PubMed] [Google Scholar]
- 75.Santillan AA, Delman KA, Beasley GM, et al. Predictive factors of regional toxicity and serum creatine phosphokinase levels after isolated limb infusion for melanoma: a multi-institutional analysis. Ann Surg Oncol. 2009;16:2570–2578. doi: 10.1245/s10434-009-0563-9. [DOI] [PubMed] [Google Scholar]
- 76.Wieberdink J, Benckhuysen C, Braat RP, et al. Dosimetry in isolation perfusion of the limbs by assessment of perfused tissue volume and grading of toxic tissue reactions. Eur J Cancer Clin Oncol. 1982;18:905–910. doi: 10.1016/0277-5379(82)90235-8. [DOI] [PubMed] [Google Scholar]
- 77.Chai CY, Deneve JL, Beasley GM, et al. A multi-institutional experience of repeat regional chemotherapy for recurrent melanoma of extremities. Ann Surg Oncol. 2012;19:1637–1643. doi: 10.1245/s10434-011-2151-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Beasley GM, Riboh JC, Augustine CK, et al. Prospective multicenter phase II trial of systemic ADH-1 in combination with melphalan via isolated limb infusion in patients with advanced extremity melanoma. J Clin Oncol. 2011;29:1210–1215. doi: 10.1200/JCO.2010.32.1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Augustine CK, Toshimitsu H, Jung SH, et al. Sorafenib, a multikinase inhibitor, enhances the response of melanoma to regional chemotherapy. Mol Cancer Ther. 2010;9:2090–2101. doi: 10.1158/1535-7163.MCT-10-0073. [DOI] [PubMed] [Google Scholar]
- 80.Curley SA, Newman RA, Dougherty TB, et al. Complete hepatic venous isolation and extracorporeal chemofiltration as treatment for human hepatocellular carcinoma: a phase I study. Ann Surg Oncol. 1994;1:389–399. doi: 10.1007/BF02303811. [DOI] [PubMed] [Google Scholar]
- 81.Pingpank JF, Libutti SK, Chang R, et al. Phase I study of hepatic arterial melphalan infusion and hepatic venous hemofiltration using percutaneously placed catheters in patients with unresectable hepatic malignancies. J Clin Oncol. 2005;23:3465–3474. doi: 10.1200/JCO.2005.00.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Alexander HR, Jr, Libutti SK, Pingpank JF, et al. Hyperthermic isolated hepatic perfusion using melphalan for patients with ocular melanoma metastatic to liver. Clin Cancer Res. 2003;9:6343–6349. [PubMed] [Google Scholar]
- 83.Pingpank JF, Hughes MS, Alexander HR, et al. A phase III random assignment trial comparing percutaneous hepatic perfusion with melphalan (PHP-mel) to standard of care for patients with hepatic metastases from metastatic ocular or cutaneous melanoma.. Presented at the American Society of Clinical Oncology; June 2010.2010. [Google Scholar]
- 84.Pingpank JF, Hughes M, Alexander HR, et al. Percutaneous hepatic perfusion (PHP) vs. best alternative care (BAC) for patients with melanoma liver metastases—efficacy update of the phase 3 trial ( NCT00324727).. European Multidisciplinary Cancer Congress; Stockholm, Sweden. Sept 2011.2011. [Google Scholar]
- 85.Zager JS, JF P. Percutaneous hepatic perfusion (PHP) vs. best alternative care (BAC) for patients (pts) with melanoma liver metastases: updated data from the phase III trial.. Pigment Cell Melanoma Res; International Melanoma Centers Meeting; Tampa, Florida. 2011. pp. 1010–1011. [Google Scholar]
- 86.Balch CM, Soong SJ, Gershenwald JE, et al. Prognostic factors analysis of 17,600 melanoma patients: validation of the American Joint Committee on Cancer melanoma staging system. J Clin Oncol. 2001;19:3622–3634. doi: 10.1200/JCO.2001.19.16.3622. [DOI] [PubMed] [Google Scholar]
- 87.van Akkooi AC, de Wilt JH, Verhoef C, et al. Clinical relevance of melanoma micrometastases (<0.1 mm) in sentinel nodes: are these nodes to be considered negative? Ann Oncol. 2006;17:1578–1585. doi: 10.1093/annonc/mdl176. [DOI] [PubMed] [Google Scholar]
- 88.van Akkooi AC, Nowecki ZI, Voit C, et al. Sentinel node tumor burden according to the Rotterdam criteria is the most important prognostic factor for survival in melanoma patients: a multicenter study in 388 patients with positive sentinel nodes. Ann Surg. 2008;248:949–955. doi: 10.1097/SLA.0b013e31818fefe0. [DOI] [PubMed] [Google Scholar]
- 89.Leong SP, Steinmetz I, Habib FA, et al. Optimal selective sentinel lymph node dissection in primary malignant melanoma. Arch Surg. 1997;132:666–672. doi: 10.1001/archsurg.1997.01430300108021. discussion 673. [DOI] [PubMed] [Google Scholar]
- 90.Leong SP. Sentinel lymph node mapping and selective lymphadenectomy: the standard of care for melanoma. Curr Treat Options Oncol. 2004;5:185–194. doi: 10.1007/s11864-004-0010-x. [DOI] [PubMed] [Google Scholar]
- 91.Ranieri JM, Wagner JD, Azuaje R, et al. Prognostic importance of lymph node tumor burden in melanoma patients staged by sentinel node biopsy. Ann Surg Oncol. 2002;9:975–981. doi: 10.1007/BF02574515. [DOI] [PubMed] [Google Scholar]
- 92.Carlson GW, Murray DR, Lyles RH, et al. The amount of metastatic melanoma in a sentinel lymph node: does it have prognostic significance? Ann Surg Oncol. 2003;10:575–581. doi: 10.1245/aso.2003.03.054. [DOI] [PubMed] [Google Scholar]
- 93.Reeves ME, Delgado R, Busam KJ, et al. Prediction of nonsentinel lymph node status in melanoma. Ann Surg Oncol. 2003;10:27–31. doi: 10.1245/aso.2003.03.020. [DOI] [PubMed] [Google Scholar]
- 94.Lee JH, Essner R, Torisu-Itakura H, et al. Factors predictive of tumor-positive nonsentinel lymph nodes after tumor-positive sentinel lymph node dissection for melanoma. J Clin Oncol. 2004;22:3677–3684. doi: 10.1200/JCO.2004.01.012. [DOI] [PubMed] [Google Scholar]
- 95.Scolyer RA, Li LX, McCarthy SW, et al. Micromorphometric features of positive sentinel lymph nodes predict involvement of nonsentinel nodes in patients with melanoma. Am J Clin Pathol. 2004;122:532–539. doi: 10.1309/TDWJ-TR15-TDM1-TG7Q. [DOI] [PubMed] [Google Scholar]
- 96.Sabel MS, Griffith K, Sondak VK, et al. Predictors of nonsentinel lymph node positivity in patients with a positive sentinel node for melanoma. J Am Coll Surg. 2005;201:37–47. doi: 10.1016/j.jamcollsurg.2005.03.029. [DOI] [PubMed] [Google Scholar]
- 97.Debarbieux S, Duru G, Dalle S, et al. Sentinel lymph node biopsy in melanoma: a micromorphometric study relating to prognosis and completion lymph node dissection. Br J Dermatol. 2007;157:58–67. doi: 10.1111/j.1365-2133.2007.07937.x. [DOI] [PubMed] [Google Scholar]
- 98.Scheri RP, Essner R, Turner RR, et al. Isolated tumor cells in the sentinel node affect long-term prognosis of patients with melanoma. Ann Surg Oncol. 2007;14:2861–2866. doi: 10.1245/s10434-007-9472-y. [DOI] [PubMed] [Google Scholar]
- 99.Page AJ, Carlson GW, Delman KA, et al. Prediction of nonsentinel lymph node involvement in patients with a positive sentinel lymph node in malignant melanoma. Am Surg. 2007;73:674–678. discussion 678–679. [PubMed] [Google Scholar]
- 100.Glumac N, Hocevar M, Zadnik V, et al. Sentinel lymph node micrometastasis may predict non-sentinel involvement in cutaneous melanoma patients. J Surg Oncol. 2008;98:46–48. doi: 10.1002/jso.21066. [DOI] [PubMed] [Google Scholar]
- 101.Rossi CR, De Salvo GL, Bonandini E, et al. Factors predictive of nonsentinel lymph node involvement and clinical outcome in melanoma patients with metastatic sentinel lymph node. Ann Surg Oncol. 2008;15:1202–1210. doi: 10.1245/s10434-007-9734-8. [DOI] [PubMed] [Google Scholar]
- 102.Frankel TL, Griffith KA, Lowe L, et al. Do micromorphometric features of metastatic deposits within sentinel nodes predict nonsentinel lymph node involvement in melanoma? Ann Surg Oncol. 2008;15:2403–2411. doi: 10.1245/s10434-008-0024-x. [DOI] [PubMed] [Google Scholar]
- 103.Guggenheim M, Dummer R, Jung FJ, et al. The influence of sentinel lymph node tumour burden on additional lymph node involvement and disease-free survival in cutaneous melanoma–a retrospective analysis of 392 cases. Br J Cancer. 2008;98:1922–1928. doi: 10.1038/sj.bjc.6604407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Gershenwald JE, Andtbacka RH, Prieto VG, et al. Microscopic tumor burden in sentinel lymph nodes predicts synchronous nonsentinel lymph node involvement in patients with melanoma. J Clin Oncol. 2008;26:4296–4303. doi: 10.1200/JCO.2007.15.4179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.van der Ploeg IM, Kroon BB, Antonini N, et al. Comparison of three micromorphometric pathology classifications of melanoma metastases in the sentinel node. Ann Surg. 2009;250:301–304. doi: 10.1097/SLA.0b013e3181b1735b. [DOI] [PubMed] [Google Scholar]
- 106.Francischetto T, Spector N, Neto Rezende JF, et al. Influence of sentinel lymph node tumor burden on survival in melanoma. Ann Surg Oncol. 2010;17:1152–1158. doi: 10.1245/s10434-009-0884-8. [DOI] [PubMed] [Google Scholar]
- 107.Satzger I, Volker B, Meier A, et al. Prognostic significance of isolated HMB45 or Melan A positive cells in melanoma sentinel lymph nodes. Am J Surg Pathol. 2007;31:1175–1180. doi: 10.1097/PAS.0b013e3180341ebc. [DOI] [PubMed] [Google Scholar]
- 108.White RL, Jr, Ayers GD, Stell VH, et al. Factors predictive of the status of sentinel lymph nodes in melanoma patients from a large multicenter database. Ann Surg Oncol. 2011;18:3593–3600. doi: 10.1245/s10434-011-1826-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Dewar DJ, Newell B, Green MA, et al. The microanatomic location of metastatic melanoma in sentinel lymph nodes predicts nonsentinel lymph node involvement. J Clin Oncol. 2004;22:3345–3349. doi: 10.1200/JCO.2004.12.177. [DOI] [PubMed] [Google Scholar]
- 110.Bachter D, Balda BR, Vogt H, et al. Primary therapy of malignant melanomas: sentinel lymphadenectomy. Int J Dermatol. 1998;37:278–282. doi: 10.1046/j.1365-4362.1998.00358.x. [DOI] [PubMed] [Google Scholar]
- 111.Pijpers R, Borgstein PJ, Meijer S, et al. Sentinel node biopsy in melanoma patients: dynamic lymphoscintigraphy followed by intraoperative gamma probe and vital dye guidance. World J Surg. 1997;21:788–792. doi: 10.1007/s002689900307. discussion 793. [DOI] [PubMed] [Google Scholar]
- 112.Brady MS, Coit DG. Sentinel lymph node evaluation in melanoma. Arch Dermatol. 1997;133:1014–1020. [PubMed] [Google Scholar]
- 113.Reintgen D, Cruse CW, Wells K, et al. The orderly progression of melanoma nodal metastases. Ann Surg. 1994;220:759–767. doi: 10.1097/00000658-199412000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Glass LF, Fenske NA, Messina JL, et al. The role of selective lymphadenectomy in the management of patients with malignant melanoma. Dermatol Surg. 1995;21:979–983. doi: 10.1111/j.1524-4725.1995.tb00537.x. [DOI] [PubMed] [Google Scholar]
- 115.Krag DN, Meijer SJ, Weaver DL, et al. Minimal-access surgery for staging of malignant melanoma. Arch Surg. 1995;130:654–658. doi: 10.1001/archsurg.1995.01430060092018. discussion 659–660. [DOI] [PubMed] [Google Scholar]
- 116.Thompson JF, McCarthy WH, Bosch CM, et al. Sentinel lymph node status as an indicator of the presence of metastatic melanoma in regional lymph nodes. Melanoma Res. 1995;5:255–260. doi: 10.1097/00008390-199508000-00008. [DOI] [PubMed] [Google Scholar]
- 117.Lenisa L, Santinami M, Belli F, et al. Sentinel node biopsy and selective lymph node dissection in cutaneous melanoma patients. J Exp Clin Cancer Res. 1999;18:69–74. [PubMed] [Google Scholar]
- 118.Wagner JD, Gordon MS, Chuang TY, et al. Predicting sentinel and residual lymph node basin disease after sentinel lymph node biopsy for melanoma. Cancer. 2000;89:453–462. doi: 10.1002/1097-0142(20000715)89:2<453::aid-cncr34>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 119.Starz H, Balda BR, Kramer KU, et al. A micromorphometry-based concept for routine classification of sentinel lymph node metastases and its clinical relevance for patients with melanoma. Cancer. 2001;91:2110–2121. [PubMed] [Google Scholar]
- 120.Starz H, Siedlecki K, Balda BR. Sentinel lymphonodectomy and s-classification: a successful strategy for better prediction and improvement of outcome of melanoma. Ann Surg Oncol. 2004;11:162S–168S. doi: 10.1007/BF02523622. [DOI] [PubMed] [Google Scholar]
- 121.van der Ploeg AP, van Akkooi AC, Rutkowski P, et al. Prognosis in patients with sentinel node-positive melanoma is accurately defined by the combined Rotterdam tumor load and Dewar topography criteria. J Clin Oncol. 2011;29:2206–2214. doi: 10.1200/JCO.2010.31.6760. [DOI] [PubMed] [Google Scholar]
- 122.Leong SP. Paradigm of metastasis for melanoma and breast cancer based on the sentinel lymph node experience. Ann Surg Oncol. 2004;11:192S–197S. doi: 10.1007/BF02523627. [DOI] [PubMed] [Google Scholar]
- 123.Baehner FL, Li R, Jenkins T, et al. The impact of primary melanoma thickness and microscopic tumor burden in sentinel lymph nodes on melanoma patient survival. Ann Surg Oncol. 2012;19:1034–1042. doi: 10.1245/s10434-011-2095-3. [DOI] [PubMed] [Google Scholar]
- 124.Hellman S. Karnofsky Memorial Lecture. Natural history of small breast cancers. J Clin Oncol. 1994;12:2229–2234. doi: 10.1200/JCO.1994.12.10.2229. [DOI] [PubMed] [Google Scholar]
- 125.Morton DL, Thompson JF, Cochran AJ, et al. Sentinelnode biopsy or nodal observation in melanoma. N Engl J Med. 2006;355:1307–1317. doi: 10.1056/NEJMoa060992. [DOI] [PubMed] [Google Scholar]
- 126.Amersi F, Morton DL. The role of sentinel lymph node biopsy in the management of melanoma. Adv Surg. 2007;41:241–256. doi: 10.1016/j.yasu.2007.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Eggermont AM, Suciu S, Testori A, et al. Ulceration and stage are predictive of interferon efficacy in melanoma: results of the phase III adjuvant trials EORTC 18952 and EORTC 18991. Eur J Cancer. 2012;48:218–225. doi: 10.1016/j.ejca.2011.09.028. [DOI] [PubMed] [Google Scholar]
- 128.Eigentler TK, Caroli UM, Radny P, et al. Palliative therapy of disseminated malignant melanoma: a systematic review of 41 randomised clinical trials. Lancet Oncol. 2003;4:748–759. doi: 10.1016/s1470-2045(03)01280-4. [DOI] [PubMed] [Google Scholar]
- 129.Curtin JA, Fridlyand J, Kageshita T, et al. Distinct sets of genetic alterations in melanoma. N Engl J Med. 2005;353:2135–2147. doi: 10.1056/NEJMoa050092. [DOI] [PubMed] [Google Scholar]
- 130.Flaherty KT, Puzanov I, Kim KB, et al. Inhibition of mutated, activated BRAF in metastatic melanoma. N Engl J Med. 2010;363:809–819. doi: 10.1056/NEJMoa1002011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Sosman JA, Kim KB, Schuchter L, et al. Survival in BRAF V600-mutant advanced melanoma treated with vemurafenib. N Engl J Med. 2012;366:707–714. doi: 10.1056/NEJMoa1112302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Greger JG, Eastman SD, Zhang V, et al. Combinations of BRAF, MEK, and PI3K/mTOR inhibitors overcome acquired resistance to the BRAF inhibitor GSK2118436 dabrafenib, mediated by NRAS or MEK mutations. Mol Cancer Ther. 2012;11:909–920. doi: 10.1158/1535-7163.MCT-11-0989. [DOI] [PubMed] [Google Scholar]
- 133.Guo J, Si L, Kong Y, et al. Phase II, open-label, single-arm trial of imatinib mesylate in patients with metastatic melanoma harboring c-Kit mutation or amplification. J Clin Oncol. 2011;29:2904–2909. doi: 10.1200/JCO.2010.33.9275. [DOI] [PubMed] [Google Scholar]
- 134.Kim KB, Sosman JA, Fruehauf JP, et al. BEAM: a randomized phase II study evaluating the activity of bevacizumab in combination with carboplatin plus paclitaxel in patients with previously untreated advanced melanoma. J Clin Oncol. 2012;30:34–41. doi: 10.1200/JCO.2011.34.6270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Eggermont AM, Robert C. New drugs in melanoma: it's a whole new world. Eur J Cancer. 2011;47:2150–2157. doi: 10.1016/j.ejca.2011.06.052. [DOI] [PubMed] [Google Scholar]
- 136.Morton DL, Eilber FR, Holmes EC, et al. BCG immunotherapy of malignant melanoma: summary of a seven-year experience. Ann Surg. 1974;180:635–643. doi: 10.1097/00000658-197410000-00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Fourcade J, Sun Z, Benallaoua M, et al. Upregulation of Tim-3 and PD-1 expression is associated with tumor antigen-specific CD8? T cell dysfunction in melanoma patients. J Exp Med. 2010;207:2175–2186. doi: 10.1084/jem.20100637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Leong SP, Zuber M, Ferris RL, et al. Impact of nodal status and tumor burden in sentinel lymph nodes on the clinical outcomes of cancer patients. J Surg Oncol. 2011;103:518–530. doi: 10.1002/jso.21815. [DOI] [PubMed] [Google Scholar]
- 139.Tawbi HA, Buch SC. Chemotherapy resistance abrogation in metastatic melanoma. Clin Adv Hematol Oncol. 2010;8:259–266. [PubMed] [Google Scholar]
- 140.Tarhini AA, Stuckert J, Lee S, et al. Prognostic significance of serum S100B protein in high-risk surgically resected melanoma patients participating in Intergroup Trial ECOG 1694. J Clin Oncol. 2009;27:38–44. doi: 10.1200/JCO.2008.17.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Gogas H, Kirkwood JM, Falk CS, et al. Correlation of molecular human leukocyte antigen typing and outcome in high-risk melanoma patients receiving adjuvant interferon. Cancer. 2010;116:4326–4333. doi: 10.1002/cncr.25211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Gogas H, Ioannovich J, Dafni U, et al. Prognostic significance of autoimmunity during treatment of melanoma with interferon. N Engl J Med. 2006;354:709–718. doi: 10.1056/NEJMoa053007. [DOI] [PubMed] [Google Scholar]
- 143.Hodi FS, O'Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Salama AK, Hodi FS. Cytotoxic T-lymphocyte-associated antigen-4. Clin Cancer Res. 2011;17:4622–4628. doi: 10.1158/1078-0432.CCR-10-2232. [DOI] [PubMed] [Google Scholar]
- 145.Atkins MB, Mier JW, Parkinson DR, et al. Hypothyroidism after treatment with interleukin-2 and lymphokine-activated killer cells. N Engl J Med. 1988;318:1557–1563. doi: 10.1056/NEJM198806163182401. [DOI] [PubMed] [Google Scholar]
- 146.Yurkovetsky ZR, Kirkwood JM, Edington HD, et al. Multiplex analysis of serum cytokines in melanoma patients treated with interferon-alpha2b. Clin Cancer Res. 2007;13:2422–2428. doi: 10.1158/1078-0432.CCR-06-1805. [DOI] [PubMed] [Google Scholar]
- 147.Moschos SJ, Edington HD, Land SR, et al. Neoadjuvant treatment of regional stage IIIB melanoma with high-dose interferon alfa-2b induces objective tumor regression in association with modulation of tumor infiltrating host cellular immune responses. J Clin Oncol. 2006;24:3164–3171. doi: 10.1200/JCO.2005.05.2498. [DOI] [PubMed] [Google Scholar]
- 148.Wang W, Edington HD, Rao UN, et al. Modulation of signal transducers and activators of transcription 1 and 3 signaling in melanoma by high-dose IFNalpha2b. Clin Cancer Res. 2007;13:1523–1531. doi: 10.1158/1078-0432.CCR-06-1387. [DOI] [PubMed] [Google Scholar]
- 149.Falchook GS, Long GV, Kurzrock R, et al. Dabrafenib in patients with melanoma, untreated brain metastases, and other solid tumours: a phase 1 dose-escalation trial. Lancet. 2012;379:1893–1901. doi: 10.1016/S0140-6736(12)60398-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Tarhini AA, Edington H, Butterfield LH, et al. Neoadjuvant ipilimumab in locally/regionally advanced melanoma: clinical outcome and immune monitoring. Proceedings. J Clin Oncol. 2012;30 Abstr 8533. [Google Scholar]
- 151.Tarhini AA, Cherian J, Moschos SJ, et al. Safety and efficacy of combination immunotherapy with interferon alfa-2b and tremelimumab in patients with stage IV melanoma. J Clin Oncol. 2012;30:322–328. doi: 10.1200/JCO.2011.37.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]