Background: The utility of mesenchymal stem cells (MSCs) in the treatment of acute lung injury (ALI) is dependent on their ability to reach the sites of tissue damage.
Results: Transduction of CXCR4 conferred efficient mobilization of MSCs.
Conclusion: CXCR4 overexpression in MSCs facilitated treatment of ALI.
Significance: Overexpression of CXCR4 may improve the therapeutic potential of MSCs for the treatment of diseases with tissue damage.
Keywords: Cell Migration, Lung Injury, Mesenchymal Stem Cells (MSCs), Migration, Transplantation
Abstract
Novel therapeutic regimens for tissue renewal incorporate mesenchymal stem cells (MSCs) as they differentiate into a variety of cell types and are a stem cell type that is easy to harvest and to expand in vitro. However, surface chemokine receptors, such as CXCR4, which are involved in the mobilization of MSCs, are expressed only on the surface of a small proportion of MSCs, and the lack of CXCR4 expression may underlie the low efficiency of homing of MSCs toward tissue damage, which results in a poor curative effect. Here, a rat CXCR4 expressing lentiviral vector was constructed and introduced into MSCs freshly prepared from rat bone marrow. The influence of CXCR4 expression on migration, proliferation, differentiation, and paracrine effects of MSCs was examined in vitro. The in vivo properties of CXCR4-MSCs were also investigated in a model of acute lung injury in rats induced by lipopolysaccharide. Expression of CXCR4 in MSCs significantly enhanced the chemotactic and paracrine characteristics of the cells in vitro but did not affect self-renewal or differentiation into alveolar and vascular endothelial cells. In vivo, CXCR4 improved MSC homing and colonization of damaged lung tissue, and furthermore, the transplanted CXCR4-MSCs suppressed the development of acute lung injury in part by modulating levels of inflammatory molecules and the neutrophil count. These results indicated that efficient mobilization of MSCs to sites of tissue injury may be due to CXCR4, and therefore, increased expression of CXCR4 may improve their therapeutic potential in the treatment of diseases where tissue damage develops.
Introduction
Acute lung injury (ALI)3 and acute respiratory distress syndrome are critical diseases with a high mortality rate (40%) (1–3). Clinical manifestations of the diseases include acute progressive dyspnea, pulmonary edema, and refractory hypoxemia (1, 2). These diseases develop in response to a number of physiological insults, such as infection, trauma, and surgery. Although therapies such as mechanical ventilation, enzyme suppression, anti-oxidative stress, anti-media, and microcirculation improvement can decrease the mortality rate significantly (4–6), none prevent and/or revert the progression of disease and pulmonary injury. Treatment regimens that will reduce/prevent early damage and/or repair the tissue trauma-induced ALI are, therefore, necessary.
Recent efforts to prevent ALI have led investigators to focus on the potential of mesenchymal stem cells (MSCs) in the treatment of ALI and acute respiratory distress syndrome. MSCs are a type of adult stem cell that can be harvested from a wide variety of tissue types, including bone marrow, adipose tissue, skeletal muscle, synovium, and umbilical cord blood as well as other tissues (7, 8). MSCs are also capable of differentiating into multiple different cells types, such as osteoblasts, adipocytes, muscle cells, nerve cells, and other cell types (9–11). Bone marrow is a rich source of MSCs and, therefore, is a tissue often used for harvesting MSCs. Bone marrow-derived MSCs have previously been used in an ALI animal model and are shown to reduce the inflammation and lung tissue damage induced by lipopolysaccharide (LPS) as well as promote survival of these mice (12). In addition, MSCs can differentiate in vitro into lung-related cell types when co-cultured with lung tissue or conditioned medium from lung tissue. These results provide novel strategies for the clinical treatment of ALI from the perspective of both tissue regeneration and suppression of the inflammatory response (13, 14).
In the pathogenesis of ALI, inflammation and the development of lung tissue damage results in large part from the mobilization of inflammatory cells by chemokines. Chemokines are low molecular weight proteins that are often expressed abundantly in an inflammatory region and attract white blood cells to the site of infection. CXCL8, CXCL1, CXCL5, and CCL2 are all chemokines that have been detected in bronchoalveolar lavage (BAL) from damaged lung tissue caused by ALI (15, 16). In addition, high levels of SDF-1α (17) have been found within the inflamed tissue. SDF-1α was first identified in bone marrow and lymphoid tissue. This chemokine plays a vital role in the migration of hematopoietic stem cells and lymphocytes mediated by the receptor CXCR4 (18, 19). Expression of SDF-1α was subsequently more widely observed, but it was found to be especially high in alveoli affected by ALI and pulmonary fibrosis (20). The chemokines that promote inflammation are also the same molecules that attract MSCs to the site of tissue injury.
The utility of the MSC in the treatment of ALI is dependent on its ability to reach the sites of tissue damage and, thus, receptors such as CXCR4 that mediate migration. Although CXCR4 is expressed on the surface of a small proportion of MSCs, receptor expression is gradually decreased as cells are expanded in vitro, ultimately limiting their benefit in vivo (21, 22). To improve the therapeutic potential of MSCs in ALI, a construct containing CXCR4 was developed for high expression of the protein in MSCs. Migration, proliferation, and differentiation as well as the paracrine effects of the CXCR4 expressing MSCs (CXCR4-MSCs) were examined in vitro. The potential therapeutic efficacy of CXCR4-MSCs was subsequently investigated in the in vivo model of ALI induced by LPS and assessed on the ability of the cells to migrate to and colonize the damaged lung tissue.
EXPERIMENTAL PROCEDURES
Reagents
The expression construct for CXCR4 was developed by cloning the rat CXCR4 coding sequence into the GFP lentiviral vector pCDH-CMV-MCS-EF1-copGFP (System Biosciences; Mountain View, CA) at XbaI and EcoRI restriction sites (Invitrogen). Constructs were isolated from bacteria with the plasmid small kit without endotoxin (Omega Bio-tek; Norcross, GA) and transfected with packaging plasmids pLP1, pLP2, pLP/VSV-G (ViraPower Lentiviral Expression Systems; Invitrogen) with Lipofectamine 2000 into 293T cells (a gift from Professor Yan Yaping; Tianjin Medical University) in DMEM with glucose (Invitrogen).
Rat MSCs were cultured in SD rat bone marrow MSC dedicated complete medium (Cyagen Biosciences; Guangzhou, China). In vitro migration assays were performed in 8-μm hanging Transwell chambers (Corning China; Shanghai, China) with SDF-1α (PeproTech; Rocky Hill, NJ). Hematoxylin-eosin staining dye (Nanjing Jiancheng Bioengineering Institute; Nanjing City, China) was used to stain cells.
The following antibodies were used for immunocytochemistry: CXCR4 rabbit anti-rat antibody, VCAM-1 (vascular cell adhesion molecule-1), and ICAM-1 (intercellular adhesionmolecule-1) rabbit anti-rat antibodies (Santa Cruz Biotechnology, Inc.; Dallas, TX); vWF and SP-C rabbit anti-rat antibodies (Beijing Biosynthesis Biotechnology Co.; Beijing, China); Ki67 rabbit anti-rat antibody (Abcam; Cambridge, MA); Cy3-labeled donkey anti-rabbit fluorescence secondary antibody (Jackson ImmunoResearch Laboratories, Inc.; West Grove, PA). DAPI (Sigma) was used for staining of nuclei. IL-6, VEGF, IL-10, and TNF-α enzyme-linked immunosorbent assay (ELISA) kits (R&D Systems; Minneapolis, MN) were used for the detection of factors in cell supernatants or bronchoalveolar lavage (BAL) fluid.
Ethics Statement
All animal protocols were approved by the Animal Care Committee in Dalian Medical University (Dalian, China) and performed according to institutional guidelines.
Animals
Sprague-Dawley rats (age 4–6 weeks) were purchased from the experimental animal center of Dalian Medical University (SCXK (Liaoning) 2008-0002).
Primary Culture and Identification of MSCs
Male rats were anesthetized (10% urethane for 10 min), abdomens were disinfected with 75% alcohol, and long bones (femur and tibia) of the two hind limbs were prepared for the isolation of MSCs. Both ends of each long bone were cut off, and the marrow cavity was rinsed with low glucose DMEM repeatedly. The supernatant was centrifuged at 1200 rpm for 6 min, and the pelleted cells were collected as the rat MSCs. Cells were counted and plated (5 × l05) in 25-cm2 flasks and cultured at 37 °C, 5% CO2 in SD rat bone marrow MSC dedicated complete medium containing 10% fetal bovine serum (FBS). The medium was changed at 24 and 48 h, 50 and 100%, respectively, and the non-adherent cells were discarded. The attached cells were labeled P0, and the medium was changed once every 3 days. When cells became 90% confluent, cultures were dissociated with trypsin (1–1.5 ml 0.25% trypsin) at 37 °C 2–3 min, and serum was used to inactivate the enzyme. Cells were expanded, and at the third passage (P3), cells were characterized for cell surface markers by flow cytometry with the following fluorescently labeled antibodies: CD29-PE, CD34-FITC, CD44-FITC, CD45-FITC, and CD90-FITC (11). Quantitative flow cytometric analysis was also performed to determine the percentages of P0 through P3 cells positive for CXCR4 and CXCR7, a second receptor for SDF-1α on MSCs. Cell surface adhesion molecules VCAM-1 and ICAM-1 (23, 24) were examined by immunocytochemistry.
Cloning of CXCR4 into Recombinant Lentiviral Vector
CXCR4 was amplified from rat mRNA by RT-PCR, gel-purified, and ligated with T4 DNA ligase into the pMD18-T vector. The ligation was transformed into competent Escherichia coli, and the transformation was plated onto an ampicillin plate. Colonies were picked 16 h later and inoculated into LB medium. Bacteria were grown in shaking culture at 37 °C for 18 h. Plasmid was extracted by the alkaline lysis method, digested with restriction endonucleases XbaI and EcoRI, and confirmed by PCR (pT-CXCR4). pT-CXCR4 was sequenced and ligated to the lentiviral vector pCDH-CMV-MCS-EF1-copGFP, which carries the green fluorescent protein (GFP) gene, to develop a construct co-expressing rat CXCR4 and GFP. pT-CXCR4 and the lentiviral vector were digested with XbaI and EcoRI, respectively. The targeting genes and the vector segment were recloned and ligated (T4 DNA ligase). Plasmid was isolated and digested to confirm that the CXCR4 gene fragment had inserted into the vector correctly. The final constructs were named L.v.-CXCR4 (co-expression CXCR4-GFP) and L.v.-GFP (expression GFP only) (25).
Lentiviral Infection of MSCs with Expression Constructs
L.v.-CXCR4-GFP and L.v.-GFP, along with packaging plasmids pLP1, pLP2, and pLP/VSV-G, were transfected into 293T packaging cells with Lipofectamine 2000 (Invitrogen). Green fluorescence was observed in both groups under a fluorescence microscope within 24 h. Supernatants containing lentivirus were collected from 293T cells at 36 and 72 h after transfection, centrifuged at 3000 rpm for 20 min to remove cellular debris, and filter-sterilized (0.22 μm). Supernatants were centrifuged at 5000 rpm for 1 h for a cut-off of 100 kDa to obtain concentrated viral titers. MSCs (P3) were infected with concentrated viral supernatant, and expression of CXCR4 was detected by immunocytochemistry 3 days after transfection.
In Vitro Migration Assay
Hanging transwell assay chambers (8 μm pore diameter) were set up in a 24-well plate. CXCR4- (5 day infection), GFP-, or Mock-infected MSCs were suspended in 100 μl (1 × 104 cells) and placed in the upper chamber. Varying concentrations of SDF-1α (50, 100 ng/ml in 600 μl) were added to the lower chamber. Transwell assays were incubated at 37 °C in 5% CO2 for 5 h, and the number of cells that crossed and adhered to the membrane was determined. The chamber was rinsed, and a cotton swab was used to remove cells, which did not migrate, from the plating surface of the membrane. Adherent cells were fixed with 4% paraformaldehyde for 15 min at room temperature, stained 10 min with 0.5% crystal violet, and counted. The number of transmembrane cells in 10 different fields under the microscope was averaged (26).
Growth Curves
CXCR4-, GFP (control MSCs)-, and Mock-infected MSCs were seeded on day 5 at 6 × 103/well in a 96-well plate, and the medium was changed once every 2 days. Cells from 6 wells were dissociated, counted, and averaged each day over 9 days. For the cell growth curve, the average cell numbers (vertical axis) were plotted as a function of time in days (horizontal axis).
Differentiation of MSCs into Lung Tissue
Conditioned medium (CM) for differentiation experiments was prepared from lung tissue inflammation induced by LPS in cells in vitro. Male rats (120–150 g) were anesthetized (10% urethane), the chest was opened, and the animals were perfused. The lungs were removed, and the fresh, perfused tissue was ground and pressed through a sieve (70 μm) for dissociation into single cells. Cells harvested from lung tissue were expanded and seeded into 6-well plates at a density of 5 × l04/ml in 2 ml per well of complete medium containing 10% FBS. After 24 h, fresh complete medium or complete medium plus LPS (10 μg/ml) for inducing inflammation was added to 6 wells each (27). The cell supernatant was collected 4 h later, centrifuged at 3000 rpm for 10 min to remove cellular debris, and stored at −80 °C for subsequent analysis. These supernatants were the control and LPS-induced lung injury CM.
For differentiation in CM, MSCs were seeded in 24-well plates at a density of 5×l04/well, and 50% CM was added 24 h later. The CM was changed once every 2 days for 8 days. At the end of 8 days, immunocytochemistry was used to determine expression of SP-C and vWF, markers for alveolar epithelial and endothelial cells (respectively), in the differentiated MSCs (28).
In Vivo Model of ALI
Rats (200–250 g) were injected intraperitoneally with LPS (10 mg/kg) (29–31) and sacrificed 72 h later. Success of the model was based on the following parameters: volume of the BAL fluid, the levels of inflammatory cytokines in serum, wet/dry weight ratio of lung tissue, and histological examination. Animals were divided into four groups of 10 animals per group: control (saline), intravenous injection of 0.2 ml PBS; ALI/model (LPS-induced acute lung injury), LPS intraperitoneal injection (10 mg/kg), 0.2 ml PBS through tail vein after 1 h; GFP-MSCs, LPS intraperitoneal injection (10 mg/kg), GFP-MSCs (1 × 106/rat) through tail vein after 1 h; CXCR4-MSCs, LPS intraperitoneal injection (10 mg/kg), CXCR4-MSCs (1 × 106/rat) through tail vein after 1 h. An additional 10 rats were prepared for harvesting fluids and lung tissue from animals transplanted with GFP- and CXCR4-MSCs to detect the degree of homing of MSCs after 2 weeks.
Collection of BAL Fluid and Lung Tissue Samples
BAL fluid preparation was performed as previously described (27, 29, 30). Briefly, rats were fixed in a supine position and anesthetized with ether cotton in a centrifuge tube set in the mouth and nose. The sternum was cut to expose the chest, the trachea, bronchus, and lung were dissociated, and both lungs were ligatured. The left bronchus was intubated, and three cycles of perfusion extraction were performed with 1.5 ml of saline. Lavage was performed for 30 s, and 1.0–1.2 ml BAL fluid was collected and centrifuged at 2000 rpm for 10 min. Protease inhibitor was added, and the BAL samples were stored at −80 °C for subsequent analysis. Neutrophils per ml were counted in PBS. The trachea was cannulated, and BAL was performed 6 times with 1 ml of 0.1 mm EDTA, phosphate-buffered saline. After counting the cells in the BAL fluid, cells were cytospun onto glass slides and stained with Hemacolor (EMD Chemicals; Gibbstown, NJ) for differential cell counting.
The right upper lung was cut, and the wet/dry weight ratio was determined. The right lower lung was immersed into ice-cold 4% paraformaldehyde (24 h), dehydrated, and embedded in paraffin. Paraffin blocks were sectioned (5 μm), and sections were slightly dried at room temperature followed by 40 °C constant temperature. Right middle lobes were embedded in OCT after freezing at −80 °C for frozen sections.
Detecting Cytokines by ELISA
ELISA was used to detect cytokines TNF-α, IL-6, and cytokines IL-10 and IL-6 and VEGF in BAL and supernatants, respectively, according to the manufacturer's protocols (R&D Systems).
Lung Histopathology
Sections (4 μm) from paraffin-embedded biopsies were H&E-stained and histologically examined. For each group, 10 biopsies from 5 rats were selected, and lung tissue injury was assessed based on the following histological changes (12, 31): alveolar congestion, alveolar hemorrhage, inflammatory infiltration, alveolar wall thickening, and hyaline membrane formation. The degree of injury was scored according to the following scale: no injury (0), 1–25% injury (1), 25–50% injury (2), 50–75% injury (3), 50–75% injury (4).
Lung Wet/Dry Weight Ratio
The right upper lobe of the lung was removed, and the wet weight was recorded. The lungs were placed in an incubator at 55 °C for 24 h to obtain the dry weight, and the wet-to-dry ratio was calculated (32, 33).
Detection of MSCs Homing to Lung Tissue
Frozen sections (10 μm) were prepared from right middle lung specimens. Sections were fixed in 4% paraformaldehyde (30 min), permeabilized with 0.1% Triton X-100 (20 min), and blocked with 3% BSA (1 h at room temperature). Primary antibodies (rabbit anti-rat CXCR4 and Ki67, 1:100) were added and incubated overnight at 4 °C. For detection, Cy3-labeled secondary antibody (1:150) was added, and slides were incubated in the dark (1 h). Sections were subsequently stained with DAPI (5 min in the dark), mounted with anti-fluorescence quenching mounting medium, and viewed under fluorescence. Slides were rinsed three times with PBS after each incubation. Five rats per group were analyzed, 5 sections per rat were selected, and 10 fields were photographed in each section to determine the number of CXCR4-MSCs (CXCR4+/GFP+/DAPI+) homing to the lungs/site of injury (34, 35). Cells triple-labeled with Ki67+/GFP+/DAPI+ were identified as MSCs with proliferation potential. Cells were counted with Image J software (free download available at rsb.info.nih.gov).
Statistical Analysis
Statistical analysis was performed with SPSS version 16.0 (2007; Chicago, IL). Data are expressed as the mean ± S.D., and one-way analysis of variance with Bonferroni correction for multiple comparisons was used to analyze the differences between the groups.
RESULTS
Confirmation of Rat MSCs with Cell Surface Markers
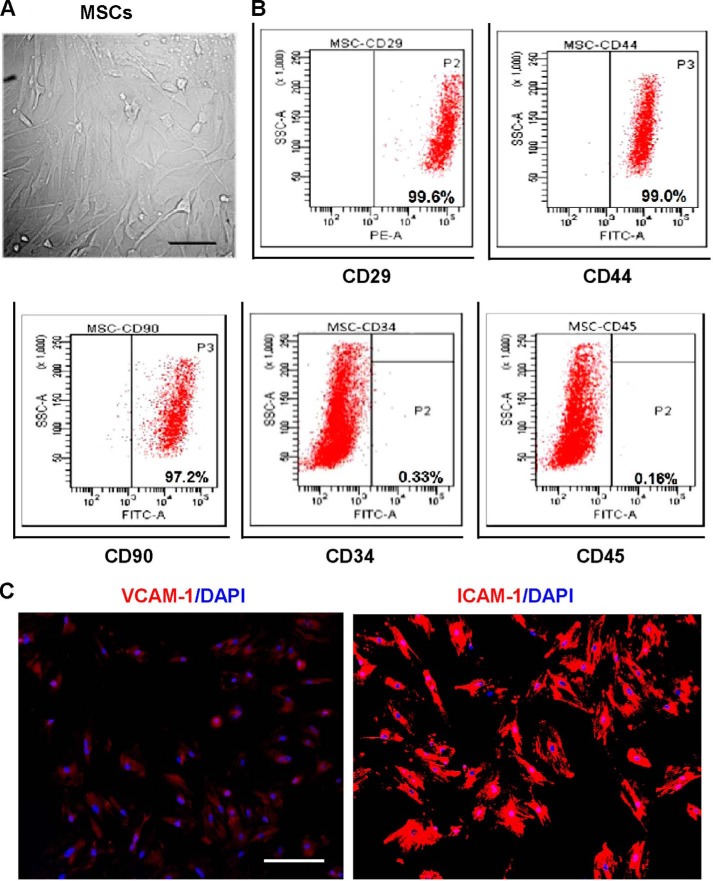
The cells obtained from rat bone marrow were characterized phenotypically and molecularly at P3. At P3, cells were adherent in serum-containing medium and had the long, spindle-shaped morphology typical of an MSC (Fig. 1A). Flow cytometry was used to determine expression of cell surface markers that typically characterize MSCs. The majority of cells were positive for CD29, CD44, and CD90 and negative for CD45 and CD34, indicating that the isolated cells were MSCs and could be used in subsequent experiments (Fig. 1B). Immunocytochemistry was subsequently used to detect the expression of stromal cell markers VCAM-1 and ICAM-1. The MSCs were positive for both surface adhesion molecules; the staining was uniformly relatively weak for VCAM-1 but strong for ICAM-1 (Fig. 1C).
FIGURE 1.
Generation and molecular characterization of rat MSCs from bone marrow. A, morphological characteristics of MSCs at P3 under phase-contrast microscopy. B, immunophenotype analysis of MSCs at P3 by flow cytometry. Adherent MSCs are positive to CD29, CD44, and CD90 but negative for CD34 and CD45. Data were compared with isotype-matched controls. C, immunocytochemistry to detect stromal cell markers VCAM-1 and ICAM-1 (red) on MSCs. One representative experiment of three performed is shown. DAPI+ nuclei (blue). Scale bar: 50 μm in A and C.
Expression of CXCR4 in MSCs
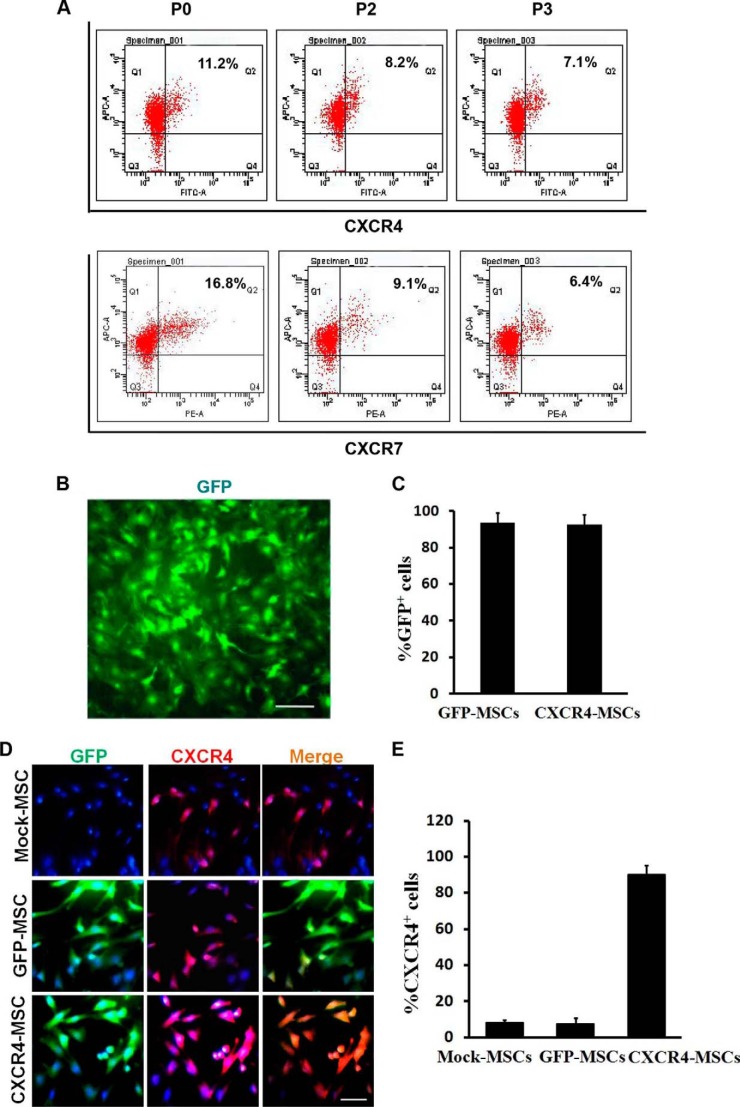
The expression of CXCR4 and CXCR7 was examined first in MSCs at P0 by flow cytometry. Results indicated that only a small proportion of MSCs expressed CXCR4 (11.2%) and CXCR7 (16.8%) and that the expression of both receptors was gradually diminished as cells were expanded in vitro (7.1% and 6.4% in P3 cells, respectively; Fig. 2A).
FIGURE 2.
CXCR4 is highly expressed in CXCR4-transduced MSCs. A, quantitative flow cytometry for CXCR4 and CXCR7 expression in MSCs expanded in culture from P0 to P3. A small proportion of MSCs expressed CXCR4 and CXCR7, and the percentages of positive cells gradually decreased as cells were expanded in vitro. One representative experiment of three performed is shown. B, L.v.-GFP transduced MSCs in monolayer culture under fluorescence microscopy. C, quantitative analysis of the percentages of GFP+ cells for the total number of transduced MSCs (DAPI+) by image J. Symbols represent the mean ± S.D. of 4 independent experiments. D, immunocytochemistry to detect CXCR4 expression in Mock-MSCs, GFP-MSCs, and CXCR4-MSCs. All transduced MSCs expressed GFP and were visible under fluorescence microscopy within 3 days after infection, whereas CXCR4 was highly expressed only in CXCR4-transduced MSCs. One representative experiment of three performed is shown. E, comparison of percentages of CXCR4+ cells among three groups of MSCs by flow cytometric analysis. Symbols represent the mean ± S.D. (n = 3). green, GFP+; red, CXCR4+; blue, DAPI+ nuclei. Scale bar: 50 μm in B, 25 μm in D.
The MSCs at P3 were infected with the two different lentiviral constructs. The total number of cells infected was determined based on expression of the GFP gene, which was also contained in the constructs. Within 3 days after infection, GFP (green) expression was first observed in MSCs under fluorescence microscopy (Fig. 2B). The infection with either construct was extremely efficient as the proportion of GFP expressing MSCs was 93.7% and 92.8% for CXCR4-GFP and GFP, respectively (Fig. 2C). Immunocytochemistry and flow cytometric analysis was subsequently used to determine the expression of CXCR4 in each cell population. Although the chemokine receptor was expressed in the three cell populations, CXCR4 (red) was highly expressed only in CXCR4-MSCs (Fig. 2D). The percentage of CXCR4-positive cells was as high as 90.2% in CXCR4-MSCs in contrast to 7.6% in GFP-MSCs and 7.5% in mock-MSCs (Fig. 2E).
Overexpression of CXCR4 Enhances Migration of MSCs in Vitro
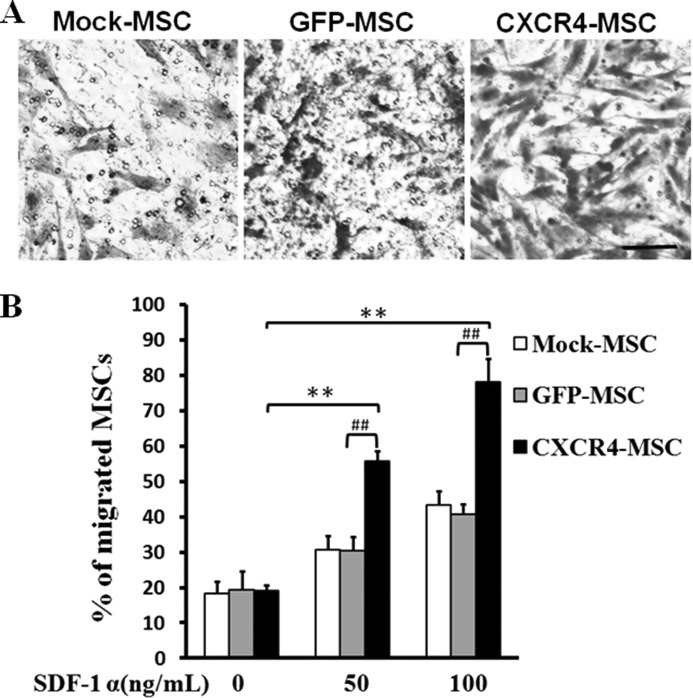
CXCR4 is a receptor for the chemokine SDF-1α, and the chemokine is chemotactic for cells expressing the receptor. In these experiments the hanging Transwell chamber was used to determine whether CXCR4 expression influenced the migration of the rat MSCs in response to the chemokine SDF-1α in vitro. Cell counts revealed that proportionally more of the CXCR4-MSCs than the GFP-MSCs migrated to SDF-1α (Fig. 3, A and B). The results indicated that CXCR4 expression significantly enhanced migration of MSCs in response to the chemokine SDF-1α.
FIGURE 3.
SDF-1α chemotaxis assay for CXCR4-transduced MSCs. A, MSCs were transduced with CXCR4/GFP or GFP. On day 5 cells were plated into the upper well of a 24-well Transwell plate, and different concentrations of SDF-1α were added to the lower well (50, 100 ng/ml). Assays were incubated for 5 h, and cells that migrated into the lower well were fixed, stained with crystal violet, and counted. Scale bar = 25 μm. One representative experiment of three is shown. B, quantitative analysis of percentage of migrated cells in total number of plated cells. Data represent the mean values ± S.D. of three wells from three independent experiments. The migration of CXCR4-MSCs and GFP-MSCs is compared with non-transduced cells in response to SDF-1α (50, 100 ng/ml). **, p < 0.01; CXCR4- versus GFP-MSCs at either concentration. ##, p < 0.01.
CXCR4 Does Not Affect the Proliferative Capacity of MSCs
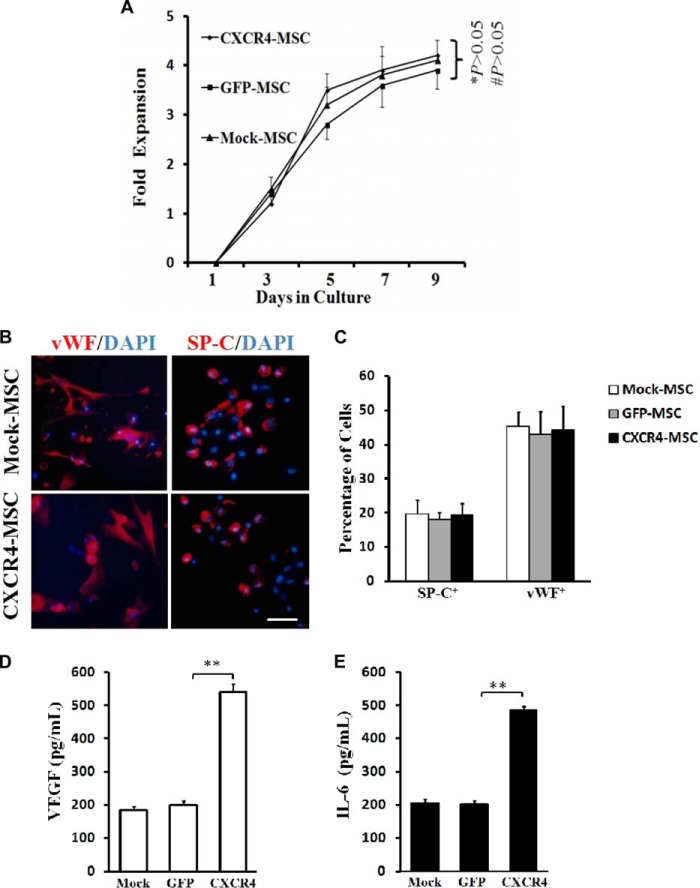
Proliferation is tightly linked to self-renewal capabilities of stem cells. To determine whether CXCR4 altered the proliferation of MSCs, growth curves were derived for CXCR4-, GFP-, and Mock-MSCs to detect any differences in the rates of proliferation in vitro. At 5 days after infection, cells were replated and counted every second day over 9 days. The curves displayed no significant differences in proliferation among three cell types (p > 0.05; Fig. 4A). These results indicated that gene transfer and/or expression of CXCR4 does not affect the proliferative capacity of MSCs.
FIGURE 4.
CXCR4 overexpression does not alter self-renewal and differentiation potential of MSCs but stimulates the cells to secrete VEGF and IL-6 in vitro. A, growth curves of MSCs. CXCR4-, GFP-, or Mock-MSCs were seeded at the same density in 24-well plates 5 days post gene-transduction. At days 1, 3, 5, 7, and 9, MSCs in each well (n = 6) were dissociated into single cells, and numbers of cells were counted with a hemocytometer and plotted as a function of time in days to reveal growth curves. B, differentiation of MSCs into lung cell types. After culture in CM for 8 days, three groups of MSCs differentiated into alveolar epithelial cells (SP-C+) and vascular endothelial cells (vWF+) as verified by immunocytochemistry. DAPI+ nuclei (blue). Scale bar: 25 μm. C, quantitative analysis of percentages of differentiated MSCs. Symbols represent the mean ± S.D. of four independent experiments. No significant differences were observed among the three groups of MSCs. E and F, levels of VEGF and IL-6 in supernatants of the three cell types were assayed by ELISA. Data represent the mean ± S.D. of three independent experiments (n = 9). Comparison between GFP-MSCs and Mock-MSCs, p > 0.05; comparison between CXCR4-MSCs and GFP-MSCs, ** p < 0.01.
CXCR4 Gene Transfer Does Not Affect the Differentiation Potential of MSCs
To test whether CXCR4 gene transfer influenced differentiation, MSCs were incubated with CM made from lung cells cultured in vitro and treated with LPS. MSCs were evaluated after 8 days for expression of marker proteins SP-C and vWF for alveolar epithelial and vascular endothelial cells, respectively. By immunofluorescence, the number of cells expressing each of the markers was determined for CXCR4-, GFP-, and Mock-MSCs (Fig. 4B). Quantitative analysis showed that all cell populations differentiated with equal capability into lung and endothelial cell types. These results indicated that CXCR4 gene transfer did not affect the ability of MSCs to differentiate (p > 0.05; Fig. 4C).
Overexpression of CXCR4 Promotes MSCs to Secrete Cytokines
An important property of MSCs in the process may be the cytokines secreted when attracted to the site of injury. To detect the cytokines secreted by MSCs after lentiviral infection, ELISA was used to detect the level of VEGF and IL-6 in the supernatants of CXCR4-, GFP-, and Mock-MSCs in vitro. MSCs with expression of CXCR4 secreted high levels of VEGF and IL-6 relative to GFP-MSCs (p < 0.01; Fig. 4, D and E). These results indicated that CXCR4 expressing MSCs might ultimately display enhanced paracrine effects at a site of injury.
Overexpression of CXCR4 Enhances the Inhibition of Lung Tissue Inflammation in ALI
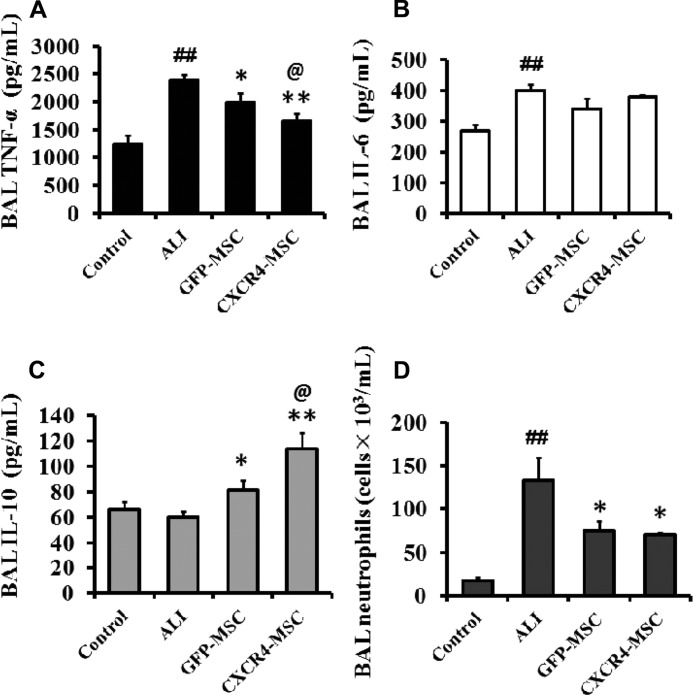
To study the role of CXCR4-MSCs in the treatment of ALI in rats, CXCR4- or GFP-MSCs (1 × 106/rat) were transplanted via tail vein 1 h after intraperitoneal injection of LPS (10 mg/kg) into rats. Lavage fluid from bronchial alveoli was removed 72 h later. As a measure of the inflammatory response to ALI among the different treatment groups, the levels of TNF-α, IL-6, IL-10, and the neutrophil count were determined.
The levels of each of the molecules and the neutrophil count followed the expected profiles for inflammation in the ALI model. TNF-α, IL-6, and the neutrophil count were significantly increased in the untreated ALI animals relative to controls, whereas IL-10, an anti-inflammatory molecule, remained unchanged (p < 0.01; p < 0.01; p < 0.05; Fig. 5).
FIGURE 5.
CXCR4 overexpression enhances the ability of MSCs to modulate the inflammatory response to ALI. Rats were injected with CXCR4- or GFP-MSCs 1 h post LPS-injection, and lavage fluid from bronchial alveoli was removed 72 h later. A–C, levels of TNF-α, IL-6, and IL-10 in BAL fluid were assayed by ELISA. D, numbers of neutrophils in BAL fluid were counted. Symbols represent the mean ± S.D. of 3 independent experiments (n = 9). ALI versus control group; ##, p < 0.01. GFP- and CXCR4-MSCs versus ALI; *, p < 0.05, and **, p < 0.01, respectively. CXCR4-MSCs versus GFP-MSCs, @, p < 0.05.
The injection of MSCs, either GFP- or CXCR4-MSCs, altered the inflammation profile of induced ALI in the LPS-treated animals. In both GFP- and CXCR4-MSCs treated animals, the inflammatory molecule TNF-α was reduced relative to the levels in the untreated ALI animals (p < 0.05; p < 0.01; Fig. 5), but CXCR4-MSCs had a greater effect (p < 0.05; Fig. 5A). However, there was no statistically significant difference in IL-6 levels in treated relative to untreated ALI animals (Fig. 5B). In contrast, the anti-inflammatory molecule IL-10 increased significantly when GFP- and CXCR4-MSCs were injected compared with the untreated ALI animals. Again, the change with CXCR4-MSCs was more significant (p < 0.05; Fig. 5C). Finally, the neutrophil count was reduced in ALI animals treated with GFP- or CXCR4-MSCs. However, there was no significant difference between GFP- and CXCR4-MSCs, indicating that CXCR4 had no apparent advantage in suppressing the neutrophil count (Fig. 5D). The results indicated that MSCs in general influenced inflammation, but compared with GFP-MSCs, CXCR4-MSCs had a more significant effect on the expression of TNF-α and IL-10 in the target site of tissue damage.
Overexpression of CXCR4 Enhances MSC Inhibition of Lung Injury
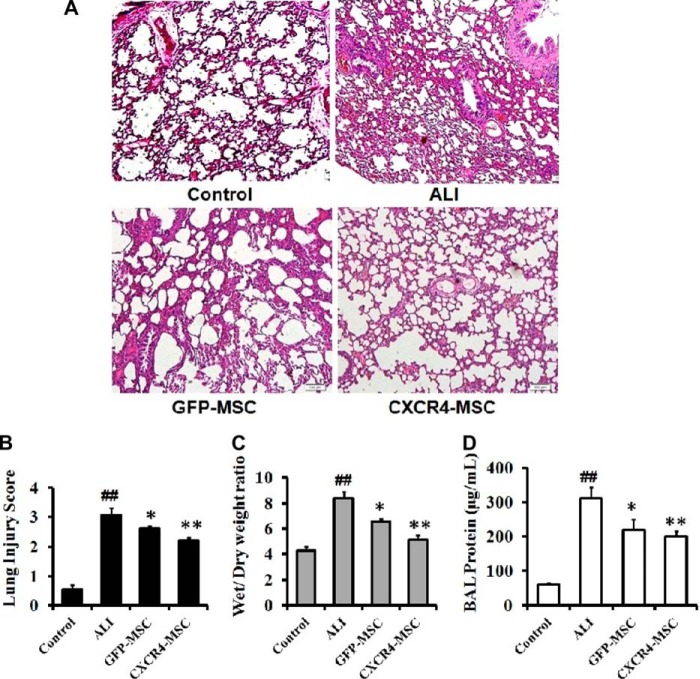
At a molecular level, the MSCs appeared to suppress inflammation, but the final test was whether pathological differences were still evident. To assess at a pathological level events in treated ALI animals, lung tissue was histologically examined 72 h after LPS and cell injection and compared with tissue from untreated animals. Pathological lesions were scored on H&E staining. Protein liquid exudation mainly caused by pulmonary vascular hemorrhage was apparent as well as hyperemia and neutrophil infiltration of lung tissue in ALI animals. In addition, pathological changes such as alveolar wall thickening and fracture had occurred to some degree (Fig. 6A). The score of pulmonary tissue injury was significantly increased compared with the control animals (3.06 ± 0.25; p < 0.01; Fig. 6B).
FIGURE 6.
CXCR4 overexpression enhances suppression of ALI. Lung tissue of ALI rats was histologically examined 72 h after injection of cells. A, H&E staining of lung. B, pathological score. C, lung wet/dry weight ratios. D, BAL protein concentrations. Data represent the mean ± S.D.; for each group, 10 biopsies from 5 rats were evaluated. ALI versus control animals; ##, p < 0.01. GFP- and CXCR4-MSCs versus ALI; *, p < 0.05, and **, p < 0.01 respectively. CXCR4- versus GFP-MSCs; @, p < 0.05. Scale bar: 100 μm in A.
Tissue specimens from GFP-MSC-treated animals exhibited reduced bleeding and protein liquid exudation relative to untreated ALI animals, but the pathological changes of alveolar wall thickening were still observed. Overall, pathological lesions were less severe when compared with ALI animals (p < 0.05). CXCR4-MSCs improved pulmonary vascular hemorrhage, pulmonary congestion, and tissue protein liquid exudation as well as the thickening of the alveolar wall in alveolar fracture. The pathological score was 2.20 ± 0.13 (versus untreated ALI animals; p < 0.01). CXCR4-MSCs appeared to suppress pathological damage more effectively than GFP-MSCs. The pathological differences between these two groups reached statistical significance (p < 0.01).
To investigate the underlying mechanisms of CXCR4-MSCs in the treatment of invasive injury by ALI in rats, the wet/dry weight ratio and total protein content in BAL were also examined. The wet/dry ratio increased significantly compared with the normal group 72 h after intraperitoneal injection of LPS (Fig. 6C; p < 0.01). When treatment with GFP- and CXCR4-MSCs by injection via tail vein was performed, the ratio decreased significantly compared with untreated ALI animals (p < 0.05, p < 0.01). Furthermore, the change with CXCR4-MSCs was significantly greater than with GFP-MSCs (5.13 ± 0.37 versus 6.54 ± 0.25; p < 0.05).
The results of the determination of the total protein content in BAL demonstrated that protein in BAL from untreated ALI animals was significantly higher than in controls (p < 0.01) and that GFP- and CXCR4-MSCs significantly reduced the concentration of protein in BAL (Fig. 6D; p < 0.05, p < 0.01). These results taken together demonstrated that treatment with CXCR4-MSCs was more effective in reducing the lung pathology score, the wet/dry ratio, and the total protein content in BAL.
Overexpression of CXCR4 Facilitates Homing of MSCs to Damaged Lung Tissue
One of the putative mechanisms for suppression of inflammation by the CXCR4-MSCs is that they simply have greater facility to migrate to the injured tissue. Tissues were, therefore, examined for differences in the number of cells that had reached the damaged lung tissue 2 weeks after transplantation of CXCR4- and GFP-MSCs by immunocytochemistry and quantitative analysis. GFP-positive cells were found in lung tissue in both cases, and the vast majority of transplanted GFP+ MSCs were Ki67-positive (Ki67+/GFP+/DAPI+, Fig. 7A), a marker for cell proliferation (36). This result indicated that the two cell types in vivo had a high proliferation potential (87.05 ± 9.39% and 85.89 ± 11.08% of CXCR4- and GFP-MSCs, respectively, Fig. 7C). High expression of CXCR4 was observed only in the GFP+ cells in lung tissue where CXCR4-MSCs had been transplanted (CXCR4+/GFP+/DAPI+, Fig. 7B). Moreover, the number of GFP+ cells in the CXCR4-MSCs group was significantly higher than in rats injected with GFP-MSCs (10.83 ± 1.85/mm2 versus 3.07 ± 0.98 cells/mm2, p < 0.01, Fig. 7D). These results indicated that high expression of CXCR4 facilitated the homing of MSCs to damaged lung tissue. The homing of MSCs in vivo may thus be an important mechanism influencing the efficacy of CXCR4-MSCs in this model of ALI.
FIGURE 7.
Localization and migration of transplanted CXCR4-MSCs in the lungs. Lung tissue of ALI rats was harvested for immunocytochemistry with anti-CXCR4 and anti-Ki67 antibodies 2 weeks post MSC injection. A, GFP+ MSCs (green) were observed in the lung tissue in both cases, and the majority of GFP+ MSCs were Ki67-positive (red), corresponding to the proliferation potential of the cells. B, CXCR4 was strongly expressed (red) only in the lung of rats treated with CXCR4-MSCs. The white arrows highlight cells that are double-positive to CXCR4 and GFP. Nuclei were stained with DAPI (blue). Scale bar: 100 in A, 50 μm in B. C, quantitative analysis of percentages of Ki67+/GFP+/DAPI+ cells in the total number of GFP+/DAPI+ cells in lungs of ALI rats. D, quantitative analysis of the number of GFP+ MSCs (cells/mm2) in lungs of ALI rats. Symbols represent the mean ± S.D.; n = 5 rats per group, 5 sections per rat were selected, and 10 fields were photographed in each section. Cells were counted by Image J software. Comparison between groups of CXCR4-MSCs and GFP-MSCs. **, p < 0.01.
DISCUSSION
Pathogenesis of ALI includes the following steps (37, 38): apoptosis of alveolar epithelial cells, damage of endothelial cells, which leads to release of inflammatory cells, exudation of proteins, release of inflammatory cytokines such as IL-1β, IL-6, IL-8, and TNF-α from macrophages of alveoli, promotion of chemotaxis and activation of neutrophils, and stimulation of fibroblasts to produce extracellular matrix. Activated neutrophils secrete superoxide, proteases, and platelet-activating factors, which destroy alveolar and endothelial cells. Exudate fluid rich in protein hinders the effect of surface-active substances, aggravates impairment of lung function, and thus, initiates a vicious cycle. Treatment programs effective in the prevention or the progression of ALI are still deficient. Our goal was, therefore, to investigate the utility of a novel therapeutic strategy, injection of genetically manipulated MSCs, which overexpress CXCR4, in the treatment of ALI. Properties of the altered cells, such as proliferation, differentiation, and migration were extensively characterized first in vitro and second in vivo in an animal model of ALI. Results demonstrated that although the fundamental characteristics of MSCs remained unchanged, CXCR4 enhanced various activities of MSCs, most importantly, mobilization of the cell type to the site of tissue injury.
MSCs have distinct advantages for therapy because of their multi-lineage differentiation potential, their immunomodulatory properties, and the ease of isolation and ex vivo expansion (39, 40). MSCs are considered to be immunoprivileged because of their low immunogenicity as they express very low levels of MHC class I and no MHC class II and, furthermore, do not induce activation of allogeneic lymphocytes (41, 42), which enables allogeneic MSCs to evade the allogeneic immune system and allows for their usage across MHC barriers. Previous studies have shown that MSCs can improve the symptoms of autoimmune diseases, such as ALI, by executing immunomodulatory effects through paracrine mechanisms, reducing systemic inflammation, mitigating tissue damage, and achieving tissue regeneration through colonization and differentiation after migration to the lesion (12, 13, 43, 44). The migration of MSCs in vivo after transplantation is inefficient, which limits their efficacy. Therefore, to facilitate migration through genetic, or possibly chemical, manipulation may enhance the efficacy of MSCs in the treatment of ALI.
SDF-1α is the main chemokine that is secreted by damaged lung tissue and attracts CXCR4 expressing cells to the lesions (45, 46). MSCs normally express some level of CXCR4, but the receptor decreases over time in culture, which ultimately affects the efficiency of their migration after transplantation (21, 22). A reasonable strategy is to exploit increased expression of CXCR4 to enhance the migration of transplanted MSCs. Therefore, we hypothesized that to transfer a gene construct containing CXCR4 into MSCs may enable mobilization to damaged lung tissue and would thereby enhance the efficacy of MSCs.
To verify the above hypothesis, MSCs from bone marrow were first expanded to >90% purity in vitro, as determined by flow cytometry. CXCR4 under a constitutive promoter in a lentiviral vector was subsequently transferred into MSCs. GFP-positive cells were found in lung tissue in both cases, and the majority of transplanted GFP+ MSCs in the lung were Ki67-positive (Ki67+/GFP+/DAPI+), indicating a high proliferation potential. Ki67 is a cell marker for proliferation (36). It is strictly associated with and may be necessary for cell proliferation (47, 48). High expression of CXCR4 did not affect proliferation or differentiation of MSCs into lung cell types and promoted migration in vitro. Furthermore, high expression of CXCR4 significantly stimulated MSCs to secrete VEGF and IL-6, two factors mediating paracrine effects of MSCs. IL-6, a factor secreted by MSCs under inflammatory conditions, is critically required for MSC migration (49, 50). MSCs inhibit inflammation and preserve vascular endothelial integrity in lungs (51). VEGF, a common factor associated with vascular growth, is involved in the rebuilding of vascular endothelial integrity (52) and increases the proliferation of MSCs (53). Increases in the two factors contribute to stem cell homing and neovascularization. However, experiments performed with a CXCR4 antagonist or miRNA-CXCR4 will determine whether CXCR4 signaling underlies the induction of cytokine.
CXCR4-MSCs were directly compared with GFP-MSCs after transplantation via tail vein into rats with ALI. In the in vivo assay, although both manipulated cell types inhibited the inflammation of lung tissue in ALI and decreased the extent of damage, CXCR4-MSCs had a significantly greater effect on reducing TNF-α and elevating IL-10 in lung tissue than GFP-MSCs. These results indicated that high CXCR4 expression promoted immunomodulatory functions of MSCs. In addition, pathological damage was less severe when CXCR4-MSCs rather than GFP-MSCs were transplanted. The lung pathology score as well as the reduction of the wet/dry weight ratio was significant in comparison to GFP-MSCs (p < 0.01 and p < 0.05, respectively). The greatest difference was evident in the colonization of the two cell types in damaged lung tissue biopsies. The number of GFP-positive cells at the site of tissue injury in animals injected with CXCR4-MSCs was significantly greater than in GFP-MSC control animals (10.83 ± 1.85 cells/mm2 versus 3.07 ± 0.98 cells/mm2). These results indicated that high expression of CXCR4 in more cells (90.2% of CXCR4 transduced cells in contrast to 7.6% of control cells) facilitated the homing of MSCs to lung tissue damage, which is a mechanism fundamental to the efficacy of MSCs in ALI. Alternatively, improved migration may also reflect an increase in the number of CXCR4 molecules per MSC after viral transduction. However, the CXCR4 antagonist AMD3100 will help to further establish a role for CXCR4 in the causal signaling and functional response of MSCs to the disease state. Additional studies also need to be performed to compare the therapeutic potential of different delivery routes of cells, such as intraperitoneal, intrapulmonary delivery, or intrabone marrow cavity injection and thereby select the appropriate method of delivery.
In summary, increased expression of CXCR4 significantly enhanced migration and the paracrine properties of MSCs in vitro but did not affect their capacity to proliferate and differentiate. Transplantation of CXCR4-MSCs in vivo demonstrated that CXCR4 expression could suppress inflammatory processes involved in the damage of lung tissue. Furthermore, increased expression of CXCR4 significantly enhanced the colonization of MSCs in damaged lung tissue, which is perhaps the fundamental mechanism underlying their efficacy.
This study was supported by the Natural Science Fund of China (no. 81273923).
- ALI
- acute lung injury
- MSC
- mesenchymal stem cell
- BAL
- bronchoalveolar lavage
- VCAM-1
- vascular cell adhesion molecule-1
- ICAM-1
- intercellular adhesionmolecule-1
- CM
- conditioned medium.
REFERENCES
- 1. Dushianthan A., Grocott M. P., Postle A. D., Cusack R. (2011) Acute respiratory distress syndrome and acute lung injury. Postgrad. Med. J. 87, 612–622 [DOI] [PubMed] [Google Scholar]
- 2. Matthay M. A., Zemans R. L. (2011) The acute respiratory distress syndrome: pathogenesis and treatment. Annu. Rev. Pathol. 6, 147–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Villar J., Sulemanji D., Kacmarek R. M. (2014) The acute respiratory distress syndrome: incidence and mortality, has it changed? Curr. Opin. Crit. Care 20, 3–9 [DOI] [PubMed] [Google Scholar]
- 4. Spieth P. M., Zhang H. (2014) Pharmacological therapies for acute respiratory distress syndrome. Curr. Opin. Crit. Care 20, 113–121 [DOI] [PubMed] [Google Scholar]
- 5. Bosma K. J., Taneja R., Lewis J. F. (2010) Pharmacotherapy for prevention and treatment of acute respiratory distress syndrome: current and experimental approaches. Drugs 70, 1255–1282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.(2000) Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The Acute Respiratory Distress Syndrome Network. N. Engl. J. Med. 342, 1301–1308 [DOI] [PubMed] [Google Scholar]
- 7. Keating A. (2012) Mesenchymal stromal cells: new directions. Cell Stem. Cell 10, 709–716 [DOI] [PubMed] [Google Scholar]
- 8. Hayes M., Curley G., Laffey J. G. (2012) Mesenchymal stem cells: a promising therapy for acute respiratory distress syndrome. F1000 Med. Rep. 4, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sensebé L., Krampera M., Schrezenmeier H., Bourin P., Giordano R. (2010) Mesenchymal stem cells for clinical application. Vox. Sang 98, 93–107 [DOI] [PubMed] [Google Scholar]
- 10. Griffin M., Iqbal S. A., Bayat A. (2011) Exploring the application of mesenchymal stem cells in bone repair and regeneration. J. Bone Joint Surg. Br. 93, 427–434 [DOI] [PubMed] [Google Scholar]
- 11. Dominici M., Le Blanc K., Mueller I., Slaper-Cortenbach I., Marini F., Krause D., Deans R., Keating A., Prockop D. j., Horwitz E. (2006) Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 8, 315–317 [DOI] [PubMed] [Google Scholar]
- 12. Gupta N., Su X., Popov B., Lee J. W., Serikov V., Matthay M. A. (2007) Intrapulmonary delivery of bone marrow-derived mesenchymal stem cells improves survival and attenuates endotoxin-induced acute lung injury in mice. J. Immunol. 179, 1855–1863 [DOI] [PubMed] [Google Scholar]
- 13. Kotton D. N., Ma B. Y., Cardoso W. V., Sanderson E. A., Summer R. S., Williams M. C., Fine A. (2001) Bone marrow-derived cells as progenitors of lung alveolar epithelium. Development 128, 5181–5188 [DOI] [PubMed] [Google Scholar]
- 14. Rojas M., Xu J., Woods C. R., Mora A. L., Spears W., Roman J., Brigham K. L. (2005) Bone marrow-derived mesenchymal stem cells in repair of the injured lung. Am. J. Respir. Cell Mol. Biol. 33, 145–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Goodman R. B., Strieter R. M., Martin D. P., Steinberg K. P., Milberg J. A., Maunder R. J., Kunkel S. L., Walz A., Hudson L. D., Martin T. R. (1996) Inflammatory cytokines in patients with persistence of the acute respiratory distress syndrome. Am. J. Respir. Crit. Care Med. 154, 602–611 [DOI] [PubMed] [Google Scholar]
- 16. Donnelly S. C., Strieter R. M., Kunkel S. L., Walz A., Robertson C. R., Carter D. C., Grant I. S., Pollok A. J., Haslett C. (1993) Interleukin-8 and development of adult respiratory distress syndrome in at-risk patient groups. Lancet 341, 643–647 [DOI] [PubMed] [Google Scholar]
- 17. Petty J. M., Sueblinvong V., Lenox C. C., Jones C. C., Cosgrove G. P., Cool C. D., Rai P. R., Brown K. K., Weiss D. J., Poynter M. E., Suratt B. T. (2007) Pulmonary stromal-derived factor-1 expression and effect on neutrophil recruitment during acute lung injury. J. Immunol. 178, 8148–8157 [DOI] [PubMed] [Google Scholar]
- 18. Bleul C. C., Farzan M., Choe H., Parolin C., Clark-Lewis I., Sodroski J., Springer T. A. (1996) The lymphocyte chemoattractant SDF-1 is a ligand for LESTR/fusin and blocks HIV-1 entry. Nature 382, 829–833 [DOI] [PubMed] [Google Scholar]
- 19. Oberlin E., Amara A., Bachelerie F., Bessia C., Virelizier J. L., Arenzana-Seisdedos F., Schwartz O., Heard J. M., Clark-Lewis I., Legler D. F., Loetscher M., Baggiolini M., Moser B. (1996) The CXC chemokine SDF-1 is the ligand for LESTR/fusin and prevents infection by T-cell-line-adapted HIV-1. Nature 382, 833–835 [DOI] [PubMed] [Google Scholar]
- 20. Phillips R. J., Burdick M. D., Hong K., Lutz M. A., Murray L. A., Xue Y. Y., Belperio J. A., Keane M. P., Strieter R. M. (2004) Circulating fibrocytes traffic to the lungs in response to CXCL12 and mediate fibrosis. J. Clin. Invest. 114, 438–446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Honczarenko M., Le Y., Swierkowski M., Ghiran I., Glodek A. M., Silberstein L. E. (2006) Human bone marrow stromal cells express a distinct set of biologically functional chemokine receptors. Stem Cells 24, 1030–1041 [DOI] [PubMed] [Google Scholar]
- 22. Wynn R. F., Hart C. A., Corradi-Perini C., O'Neill L., Evans C. A., Wraith J. E., Fairbairn L. J., Bellantuono I. (2004) A small proportion of mesenchymal stem cells strongly expresses functionally active CXCR4 receptor capable of promoting migration to bone marrow. Blood 104, 2643–2645 [DOI] [PubMed] [Google Scholar]
- 23. Hu Y., Cheng P., Xue Y. X., Liu Y. H. (2012) Glioma cells promote the expression of vascular cell adhesion molecule-1 on bone marrow-derived mesenchymal stem cells: a possible mechanism for their tropism toward gliomas. J. Mol. Neurosci. 48, 127–135 [DOI] [PubMed] [Google Scholar]
- 24. Rüster B., Göttig S., Ludwig R. J., Bistrian R., Müller S., Seifried E., Gille J., Henschler R. (2006) Mesenchymal stem cells display coordinated rolling and adhesion behavior on endothelial cells. Blood 108, 3938–3944 [DOI] [PubMed] [Google Scholar]
- 25. Yan Y., Ding X., Li K., Ciric B., Wu S., Xu H., Gran B., Rostami A., Zhang G. X. (2012) CNS-specific therapy for ongoing EAE by silencing IL-17 pathway in astrocytes. Mol. Ther. 20, 1338–1348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Du Z., Wei C., Yan J., Han B., Zhang M., Peng C., Liu Y. (2013) Mesenchymal stem cells overexpressing C-X-C chemokine receptor type 4 improve early liver regeneration of small-for-size liver grafts. Liver Transpl. 19, 215–225 [DOI] [PubMed] [Google Scholar]
- 27. Zhao J., He D., Su Y., Berdyshev E., Chun J., Natarajan V., Zhao Y. (2011) Lysophosphatidic acid receptor 1 modulates lipopolysaccharide-induced inflammation in alveolar epithelial cells and murine lungs. Am. J. Physiol. Lung Cell Mol. Physiol. 301, L547–L556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yang J., Jiang Z., Fitzgerald D. C., Ma C., Yu S., Li H., Zhao Z., Li Y., Ciric B., Curtis M., Rostami A., Zhang G. X. (2009) Adult neural stem cells expressing IL-10 confer potent immunomodulation and remyelination in experimental autoimmune encephalitis. J. Clin. Invest. 119, 3678–3691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lee J. W., Fang X., Gupta N., Serikov V., Matthay M. A. (2009) Allogeneic human mesenchymal stem cells for treatment of E. coli endotoxin-induced acute lung injury in the ex vivo perfused human lung. Proc. Natl. Acad. Sci. U.S.A. 106, 16357–16362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Liang Z. X., Sun J. P., Wang P., Tian Q., Yang Z., Chen L. A. (2011) Bone marrow-derived mesenchymal stem cells protect rats from endotoxin-induced acute lung injury. Chin. Med. J. 124, 2715–2722 [PubMed] [Google Scholar]
- 31. Li J., Li D., Liu X., Tang S., Wei F. (2012) Human umbilical cord mesenchymal stem cells reduce systemic inflammation and attenuate LPS-induced acute lung injury in rats. J. Inflamm. (Lond) 9, 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tai W. L., Dong Z. X., Zhang D. D., Wang D. H. (2012) Therapeutic effect of intravenous bone marrow-derived mesenchymal stem cell transplantation on early-stage LPS-induced acute lung injury in mice. Nan. Fang Yi Ke Da Xue Xue Bao. 32, 283–290 [PubMed] [Google Scholar]
- 33. Curley G. F., Hayes M., Ansari B., Shaw G., Ryan A., Barry F., O'Brien T., O'Toole D., Laffey J. G. (2012) Mesenchymal stem cells enhance recovery and repair following ventilator-induced lung injury in the rat. Thorax 67, 496–501 [DOI] [PubMed] [Google Scholar]
- 34. Yang J., Yan Y., Ciric B., Yu S., Guan Y., Xu H., Rostami A., Zhang G. X. (2010) Evaluation of bone marrow- and brain-derived neural stem cells in therapy of central nervous system autoimmunity. Am. J. Pathol. 177, 1989–2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Yang J., Yan Y., Ma C. G., Kang T., Zhang N., Gran B., Xu H., Li K., Ciric B., Zangaladze A., Curtis M., Rostami A., Zhang G. X. (2012) Accelerated and enhanced effect of CCR5-transduced bone marrow neural stem cells on autoimmune encephalomyelitis. Acta Neuropathol. 124, 491–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Scholzen T., Gerdes J. (2000) The Ki-67 protein: from the known and the unknown. J. Cell. Physiol. 182, 311–322 [DOI] [PubMed] [Google Scholar]
- 37. Hariprashad A., Rizzolo D. (2013) Acute respiratory distress syndrome: an overview for physician assistants. JAAPA 26, 23–28 [DOI] [PubMed] [Google Scholar]
- 38. Miller A. C., Elamin E. M., Suffredini A. F. (2014) Inhaled anticoagulation regimens for the treatment of smoke inhalation-associated acute lung injury: a systematic review. Crit. Care Med. 42, 413–419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Aggarwal S., Pittenger M. F. (2005) Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood 105, 1815–1822 [DOI] [PubMed] [Google Scholar]
- 40. Bernardo M. E., Locatelli F., Fibbe W. E. (2009) Mesenchymal stromal cells. Ann. N.Y. Acad. Sci. 1176, 101–117 [DOI] [PubMed] [Google Scholar]
- 41. Schu S., Nosov M., O'Flynn L., Shaw G., Treacy O., Barry F., Murphy M., O'Brien T., Ritter T. (2012) Immunogenicity of allogeneic mesenchymal stem cells. J. Cell Mol. Med. 16, 2094–2103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Le Blanc K., Tammik C., Rosendahl K., Zetterberg E., Ringdén O. (2003) HLA expression and immunologic properties of differentiated and undifferentiated mesenchymal stem cells. Exp. Hematol. 31, 890–896 [DOI] [PubMed] [Google Scholar]
- 43. Zhu Y. G., Feng X. M., Abbott J., Fang X. H., Hao Q., Monsel A., Qu J. M., Matthay M. A., Lee J. W. (2014) Human mesenchymal stem cell microvesicles for treatment of Escherichia coli endotoxin-induced acute lung injury in mice. Stem. Cells 32, 116–125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Inamdar A. C., Inamdar A. A. (2013) Mesenchymal stem cell therapy in lung disorders: pathogenesis of lung diseases and mechanism of action of mesenchymal stem cell. Exp. Lung Res. 39, 315–327 [DOI] [PubMed] [Google Scholar]
- 45. Sordi V., Malosio M. L., Marchesi F., Mercalli A., Melzi R., Giordano T., Belmonte N., Ferrari G., Leone B. E., Bertuzzi F., Zerbini G., Allavena P., Bonifacio E., Piemonti L. (2005) Bone marrow mesenchymal stem cells express a restricted set of functionally active chemokine receptors capable of promoting migration to pancreatic islets. Blood 106, 419–427 [DOI] [PubMed] [Google Scholar]
- 46. Lapidot T., Dar A., Kollet O. (2005) How do stem cells find their way home? Blood 106, 1901–1910 [DOI] [PubMed] [Google Scholar]
- 47. Bullwinkel J., Baron-Lühr B., Lüdemann A., Wohlenberg C., Gerdes J., Scholzen T. (2006) Ki-67 protein is associated with ribosomal RNA transcription in quiescent and proliferating cells. J. Cell. Physiol. 206, 624–635 [DOI] [PubMed] [Google Scholar]
- 48. Rahmanzadeh R., Hüttmann G., Gerdes J., Scholzen T. (2007) Chromophore-assisted light inactivation of pKi-67 leads to inhibition of ribosomal RNA synthesis. Cell Prolif. 40, 422–430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ke F., Zhang L., Liu Z., Liu J., Yan S., Xu Z., Bai J., Zhu H., Lou F., Wang H., Shi Y., Jiang Y., Su B., Wang H. (2014) Autocrine interleukin-6 drives skin-derived mesenchymal stem cell trafficking via regulating voltage-gated Ca2+ channels. Stem. Cells 32, 2799–2810 [DOI] [PubMed] [Google Scholar]
- 50. Sung S. Y., Liao C. H., Wu H. P., Hsiao W. C., Wu I. H., Jinpu, Yu, Lin S. H., Hsieh C. L. (2013) Loss of let-7 microRNA upregulates IL-6 in bone marrow-derived mesenchymal stem cells triggering a reactive stromal response to prostate cancer. PLoS ONE 8, e71637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Pati S., Gerber M. H., Menge T. D., Wataha K. A., Zhao Y., Baumgartner J. A., Zhao J., Letourneau P. A., Huby M. P., Baer L. A., Salsbury J. R., Kozar R. A., Wade C. E., Walker P. A., Dash P. K., Cox C. S., Jr., Doursout M. F., Holcomb J. B. (2011) Bone marrow derived mesenchymal stem cells inhibit inflammation and preserve vascular endothelial integrity in the lungs after hemorrhagic shock. PLoS ONE 6, e25171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Clark-Raymond A., Meresh E., Hoppensteadt D., Fareed J., Sinacore J., Halaris A. (2014) Vascular endothelial growth factor: a potential diagnostic biomarker for major depression. J. Psychiatr. Res. 59, 22–27 [DOI] [PubMed] [Google Scholar]
- 53. Zhang P., Dong L., Yan K., Long H., Yang T. T., Dong M. Q., Zhou Y., Fan Q. Y., Ma B. A. (2013) CXCR4-mediated osteosarcoma growth and pulmonary metastasis is promoted by mesenchymal stem cells through VEGF. Oncol. Rep. 30, 1753–1761 [DOI] [PubMed] [Google Scholar]