Abstract
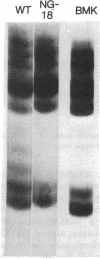
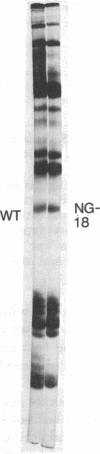
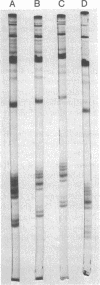
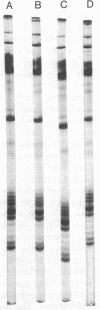
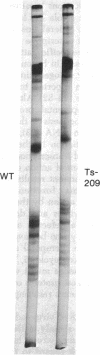
Histones H3 and H4 derived from transforming wild-type polyoma and simian virus 40 particles show extensive acetylation compared to the histones of the host cells. The same histone fractions derived from nontransforming host range mutants of polyoma virus fail to show this high degree of acetylation.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALLFREY V. G., FAULKNER R., MIRSKY A. E. ACETYLATION AND METHYLATION OF HISTONES AND THEIR POSSIBLE ROLE IN THE REGULATION OF RNA SYNTHESIS. Proc Natl Acad Sci U S A. 1964 May;51:786–794. doi: 10.1073/pnas.51.5.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamin T. L., Burger M. M. Absence of a cell membrane alteration function in non-transforming mutants of polyoma virus. Proc Natl Acad Sci U S A. 1970 Oct;67(2):929–934. doi: 10.1073/pnas.67.2.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamin T. L., Goldman E. Indirect complementation of a nontransforming mutant of polyoma virus. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 1):41–44. doi: 10.1101/sqb.1974.039.01.008. [DOI] [PubMed] [Google Scholar]
- Benjamin T. L. Host range mutants of polyoma virus. Proc Natl Acad Sci U S A. 1970 Sep;67(1):394–399. doi: 10.1073/pnas.67.1.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Candido E. P., Dixon G. H. Trout testis cells. 3. Acetylation of histones in different cell types from developing trout testis. J Biol Chem. 1972 Sep 10;247(17):5506–5510. [PubMed] [Google Scholar]
- Chou J. Y., Martin R. G. Complementation analysis of simian virus 40 mutants. J Virol. 1974 May;13(5):1101–1109. doi: 10.1128/jvi.13.5.1101-1109.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulbecco R., Eckhart W. Temperature-dependent properties of cells transformed by a thermosensitive mutant of polyoma virus. Proc Natl Acad Sci U S A. 1970 Dec;67(4):1775–1781. doi: 10.1073/pnas.67.4.1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckhart W. Complementation and transformation by temperature-sensitive mutants of polyoma virus. Virology. 1969 May;38(1):120–125. doi: 10.1016/0042-6822(69)90133-0. [DOI] [PubMed] [Google Scholar]
- Eckhart W., Dulbecco R., Burger M. M. Temperature-dependent surface changes in cells infected or transformed by a thermosensitive mutant of polyoma virus. Proc Natl Acad Sci U S A. 1971 Feb;68(2):283–286. doi: 10.1073/pnas.68.2.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckhart W., Dulbecco R. Properties of the ts3 mutant of polyoma virus during lytic infection. Virology. 1974 Aug;60(2):359–369. doi: 10.1016/0042-6822(74)90331-6. [DOI] [PubMed] [Google Scholar]
- Fey G., Hirt B. Fingerprints of polyoma virus proteins and mouse histones. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 1):235–241. doi: 10.1101/sqb.1974.039.01.030. [DOI] [PubMed] [Google Scholar]
- Fox T. O., Sheppard J. R., Burger M. M. Cyclic membrane changes in animal cells: transformed cells permanently display a surface architecture detected in normal cells only during mitosis. Proc Natl Acad Sci U S A. 1971 Jan;68(1):244–247. doi: 10.1073/pnas.68.1.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francke B., Eckhart W. Polyoma gene function required for viral DNA synthesis. Virology. 1973 Sep;55(1):127–135. doi: 10.1016/s0042-6822(73)81014-1. [DOI] [PubMed] [Google Scholar]
- Frearson P. M., Crawford L. V. Polyoma virus basic proteins. J Gen Virol. 1972 Feb;14(2):141–155. doi: 10.1099/0022-1317-14-2-141. [DOI] [PubMed] [Google Scholar]
- Gelb L. D., Kohne D. E., Martin M. A. Quantitation of Simian virus 40 sequences in African green monkey, mouse and virus-transformed cell genomes. J Mol Biol. 1971 Apr 14;57(1):129–145. doi: 10.1016/0022-2836(71)90123-9. [DOI] [PubMed] [Google Scholar]
- Goldman E., Benjamin T. L. Analysis of host range of nontransforming polyoma virus mutants. Virology. 1975 Aug;66(2):372–384. doi: 10.1016/0042-6822(75)90210-x. [DOI] [PubMed] [Google Scholar]
- Krause M. O., Stein G. S. Properties of the genome in normal and SV40 transformed WI38 human diploid fibroblasts. II. Metabolism and binding of histones. Exp Cell Res. 1975 Apr;92(1):175–190. doi: 10.1016/0014-4827(75)90651-5. [DOI] [PubMed] [Google Scholar]
- Ledinko N. Transient stimulation of deoxyribonucleic acid-dependent ribonucleic acid polymerase and histone acetylation in human embryonic kidney cultures infected with adenovirus 2 or 12: apparent induction of host ribonucleic acid synthesis. J Virol. 1970 Jul;6(1):58–68. doi: 10.1128/jvi.6.1.58-68.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libby P. R. Histone acetylation and hormone action. Early effects of aldosterone on histone acetylation in rat kidney. Biochem J. 1973 Aug;134(4):907–912. doi: 10.1042/bj1340907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libby P. R. Histone acetylation and hormone action. Early effects of oestradiol-17beta on histone acetylation in rat uterus. Biochem J. 1972 Dec;130(3):663–669. doi: 10.1042/bj1300663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami W. T., Fine R., Harrington M. R., Sassan Z. B. Properties and amino acid composition of polyoma virus purified by zonal ultracentrifugation. J Mol Biol. 1968 Aug 28;36(1):153–166. doi: 10.1016/0022-2836(68)90226-x. [DOI] [PubMed] [Google Scholar]
- Oxman M. N., Takemoto K. K., Eckhart W. Polyoma T antigen synthesis by temperature-sensitive mutants of polyoma virus. Virology. 1972 Sep;49(3):675–682. doi: 10.1016/0042-6822(72)90524-7. [DOI] [PubMed] [Google Scholar]
- Panyim S., Chalkley R. High resolution acrylamide gel electrophoresis of histones. Arch Biochem Biophys. 1969 Mar;130(1):337–346. doi: 10.1016/0003-9861(69)90042-3. [DOI] [PubMed] [Google Scholar]
- Pogo B. G., Allfrey V. G., Mirsky A. E. RNA synthesis and histone acetylation during the course of gene activation in lymphocytes. Proc Natl Acad Sci U S A. 1966 Apr;55(4):805–812. doi: 10.1073/pnas.55.4.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pogo B. G., Pogo A. O., Allfrey V. G., Mirsky A. E. Changing patterns of histone acetylation and RNA synthesis in regeneration of the liver. Proc Natl Acad Sci U S A. 1968 Apr;59(4):1337–1344. doi: 10.1073/pnas.59.4.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Carrillo A., Wangh L. J., Littau V. C., Allfrey V. G. Changes in histone acetyl content and in nuclear non-histone protein composition of avian erythroid cells at different stages of maturation. J Biol Chem. 1974 Nov 25;249(22):7358–7368. [PubMed] [Google Scholar]
- Shepherd G. R., Hardin J. M., Noland B. J. Methylation of lysine residues of histone fractions in synchronized mammalian cells. Arch Biochem Biophys. 1971 Mar;143(1):1–5. doi: 10.1016/0003-9861(71)90180-9. [DOI] [PubMed] [Google Scholar]
- Shepherd G. R., Noland B. J., Hardin J. M. Histone acetylation in synchronized mammalian cell cultures. Biochim Biophys Acta. 1971 Jan 28;228(2):544–549. doi: 10.1016/0005-2787(71)90060-8. [DOI] [PubMed] [Google Scholar]
- Shepherd G. R., Noland B. J., Hardin J. M. Histone phosphorylation in synchronized mammalian cell cultures. Arch Biochem Biophys. 1971 Jan;142(1):299–302. doi: 10.1016/0003-9861(71)90287-6. [DOI] [PubMed] [Google Scholar]
- Wangh L., Ruiz-Carrillo A., Allfrey V. G. Separation and analysis of histone subfractions differing in their degree of acetylation: some correlations with genetic activity in development. Arch Biochem Biophys. 1972 May;150(1):44–56. doi: 10.1016/0003-9861(72)90008-2. [DOI] [PubMed] [Google Scholar]
- Yoshiike K. Studies on DNA from low-density particles of SV40. I. Heterogeneous defective virions produced by successive undiluted passages. Virology. 1968 Mar;34(3):391–401. doi: 10.1016/0042-6822(68)90059-7. [DOI] [PubMed] [Google Scholar]