Abstract
Men are less likely than women to suffer from anxiety disorders. Because gonadal hormones play a crucial role in many behavioral sex differences, they may underlie sex differences in human anxiety. In rodents, testosterone (T) exerts anxiolytic effects via the androgen receptor (AR): we found that male mice with a naturally-occurring mutation rendering the AR dysfunctional, referred to as spontaneous testicular feminization mutation (sTfm), showed more anxiety-like behaviors than wildtype (WT) males. Here, we used CreLox recombination technology to create another dysfunctional allele for AR. These induced Tfm (iTfm) animals also displayed more anxiety-like behaviors than WTs. We further found that ARmodulation of these behaviors interacts with circadian phase. When tested in the resting phase, iTfms appeared more anxious than WTs in the open field, novel object and elevated plus maze tests, but not the light/dark box. However, when tested during the active phase (lights off), iTfms showed more anxiety-related behavior than WTs in all four tests. Finally, we confirmed a role of T acting via AR in regulating HPA axis activity, as WT males with T showed a lower baseline and overall corticosterone response, and a faster return to baseline following mild stress than did WT males without T or iTfms. These findings demonstrate that this recombined AR allele is a valuable model for studying androgenic modulation of anxiety, that the anxiolytic effects of AR in mice are more prominent in the active phase, and that HPA axis modulation by T is AR dependent.
Keywords: Androgen receptor, Testosterone, Anxiety, Androgen insensitivity, CreLox technology, HPA axis, Corticosterone, Photoperiod
Anxiety disorders are the second most prevalent mood disorder. Each year, of approximately 40 million American adults, about 18 percent are diagnosed with an anxiety disorder (Kessler et al., 2005), resulting in more than $42 billion in annual costs (Greenberg et al., 1999). Interestingly, anxiety disorders are unevenly distributed among the sexes; subcategories such as agoraphobia, specific phobias, generalized anxiety disorder, panic disorder, and post-traumatic stress disorder are more prevalent in women than in men (McLean et al., 2011), a sex difference that emerges in adolescence (Zahn-Waxler et al., 2008). The onset at puberty and marked sex differences in prevalence suggest a role for gonadal hormones in anxiety disorders (Menger et al., 2010; Wu and Shah, 2011).
Clinical data indicate that gonadal hormones affect anxiety in humans. Women are more prone to episodes of anxiety during the premenstrual and postpartum periods, when estrogen (E) levels are low (Dean and Kendell, 1981; Douma et al., 2005; Freeman, 2002), and as they approach menopause, when E levels start to decline (Llaneza et al., 2012; Tangen and Mykletun, 2008). In addition, E treatment ameliorates anxiety in some postmenopausal women (Frye, 2009; Yazici et al., 2003), and in breast cancer survivors regardless of age (Decker et al., 2003). Like E, testosterone (T) has also been linked to anxiety levels in women, with low levels correlating with high anxiety levels (Giltay et al., 2012). Accordingly, treating women with T following ovariectomy decreases anxiety (Shifren et al., 2000). In aging men, anxiety levels increase with decreasing levels of T (Amore et al., 2009; Sternbach, 1998), and T treatment ameliorates anxiety in such men (Wang et al., 1996). Similarly, androgen blockade therapy for prostate cancer treatment has been reported to increase anxiety, which was alleviated when treatment ended (Almeida et al., 2004). Although the nature of the relationship between these steroid hormones and anxiety level is not always consistent (Demetrio et al., 2011; Kiesner, 2011; Thomson and Oswald, 1977), they appear to exert anxiolytic effects in a number of different circumstances.
Sex differences in anxiety-related behavior are also present in other mammals, including laboratory rodents. Although the direction of these differences varies by species and strain, gonadal hormones also have anxiolytic effects in rodents. Laboratory rodents are nocturnal prey species that are averse to open and brightly lit areas; therefore, behavioral tests like the open field (OF), novel object (NO), elevated plus maze (EPM) and light/dark (L/D) box, which subject animals to open or lit spaces, are used to infer anxiety levels. Results from these tests suggest that E acts as an anxiolytic. Specifically, when circulating E levels are high during proestrous, female rodents spend more time in the open arms of the EPM than when E levels are low during diestrous (Marcondes et al., 2001; Walf et al., 2009; Zuloaga et al., 2011a). Likewise, E-treated females enter the center area of an OF more often and spend more time in the open arms of an EPM than do untreated females (Walf et al., 2008), while females given inhibitors of estrogen biosynthesis show more anxiety-related behavior in the OF and EPM (Meng et al., 2011; however, Morgan and Pfaff, 2002). Similarly, aged females enter the center area of an OF more often and spend more time in the light side of the L/D box when treated with E than do oil treated females (Walf and Frye, 2010). These effects of E appear to be mediated through ERβ (Hughes et al., 2008; Imwalle et al., 2005; Krezel et al., 2001; Rocha et al., 2005; Walf et al., 2009; Walf et al., 2008) and not ERα (Krezel et al., 2001; Lund et al., 2005; Walf and Frye, 2005).
Androgen treatment can also have anxiolytic effects in rodents. T treatment reduces anxiety-like behaviors in gonadectomized male mice in the OF, EPM and L/D box tests (Aikey et al., 2002; Frye et al., 2008). T also has anxiolytic effects on females, as measured by their increased number of entries into the center area of an OF and more time spent in the open arms of an EPM (Frye and Lacey, 2001). DHT, a 5α-reduced form of testosterone, also acts as an anxiolytic in male and female rats on measures of the OF and EPM tests (Aikey et al., 2002; Edinger and Frye, 2004, 2006; Frye and Lacey, 2001). However, it is not clear from these studies whether these androgens are acting on AR or ER. T and DHT both activate AR, but they are also capable of activating ER via their by-products. T can be aromatized into E, and DHT can be reduced to 3β-diol (Handa et al., 2008; Oliveira et al., 2007), which has an affinity for ERβ, the estrogen receptor that has been implicated in anxiolysis. While the role of ER has been widely studied, a role of androgen receptors (AR) has been neglected until recently.
Our laboratory recently found a role for ARs in anxiety-related behavior in rats (Zuloaga et al., 2011b) and in mice (Zuloaga et al., 2008a). Such studies were conducted during the resting phase (lights on) of mice with a naturally-occurring mutation rendering the AR dysfunctional, which we refer to as spontaneous testicular feminization mutation (sTfm). Specifically, T treatment had anxiolytic effects on measures of anxiety-related behavior in wild type males, but not sTfm males. Here, we replicate the anxiolytic effects of T mediated through AR using an allele for AR that has been altered by recombination using CreLox technology, thus demonstrating that this recombination indeed interferes with AR’s anxiolytic action. We also expand these findings and demonstrate an effect of the circadian phase on AR’s anxiolytic influence and confirm a role of AR in regulating the HPA axis response to mild stress.
Materials and Methods
Experimental Animals
We used the CreLox system in mice in an attempt to recapitulate the effect of a spontaneous mutation in the AR gene, namely the sTfm. Mice carrying a conserved lox sequence of 34 base pairs at the two ends of exon 2 of the AR gene (“floxed” AR, a generous gift from De Gendt et al., 2004) were crossed with transgenic animals carrying the cyclization recombination (Cre) transgene under the control of the universal adenovirus Ella promoter (“deleter” mice, Jax stock 003724). The presence of both genotypes in the same cell should result in the excision of the targeted sequence in AR exon 2, which encodes the first zinc finger of the DNA-binding domain, essential for the recognition of androgen response elements. This deletion should cause a frame-shift mutation resulting in the premature termination of AR transcription, rendering AR dysfunctional (De Gendt et al., 2004). Female offspring carrying such a recombined AR allele were identified by PCR, as described below, then bred to wildtype (WT) C57BL/6 (Jax) males; the recombined AR allele was transmitted to some female progeny without the cre transgene, as confirmed by PCR. These females were used to found a colony of mice perpetuating the recombined AR allele. We confirmed that breeding these females with WT males produces genetic male offspring that recapitulate the phenotype of XY mice carrying the sTfm mutant of AR: small, abdominal testes, short anogenital distance, feminine external genitalia, and nipples (Figure 1). Hence, we refer to these mice as induced Tfm, or iTfm mice, to distinguish them from the sTfm mice. As with our sTfm colony, these females also produced male offspring carrying the WT AR allele to serve as controls. AR immunocytochemistry (methods described below) confirmed full knock out of AR in experimental animals, as we found no AR immunoreactivity in the brains of iTfm males (Figures 2B,D), while their WT brothers showed AR expression in typical AR positive regions (Figures 2A,C). Circulating T measures revealed that iTfm males have lower adult circulating T levels than their WT counterparts (iTfm: M=4.2 ± 0.23 (SEM) nmol/l; WT: M=29.8 ± 4.9 nmol/l; methods detailed below), further confirming the disruption of AR in these animals since sTfm mice also have lower T levels than WTs (sTfm: M=3.26 ± 0.52 nmol/l; WT: M=19.75 ± 4.39 nmol/l; Zuloaga et al., 2008).
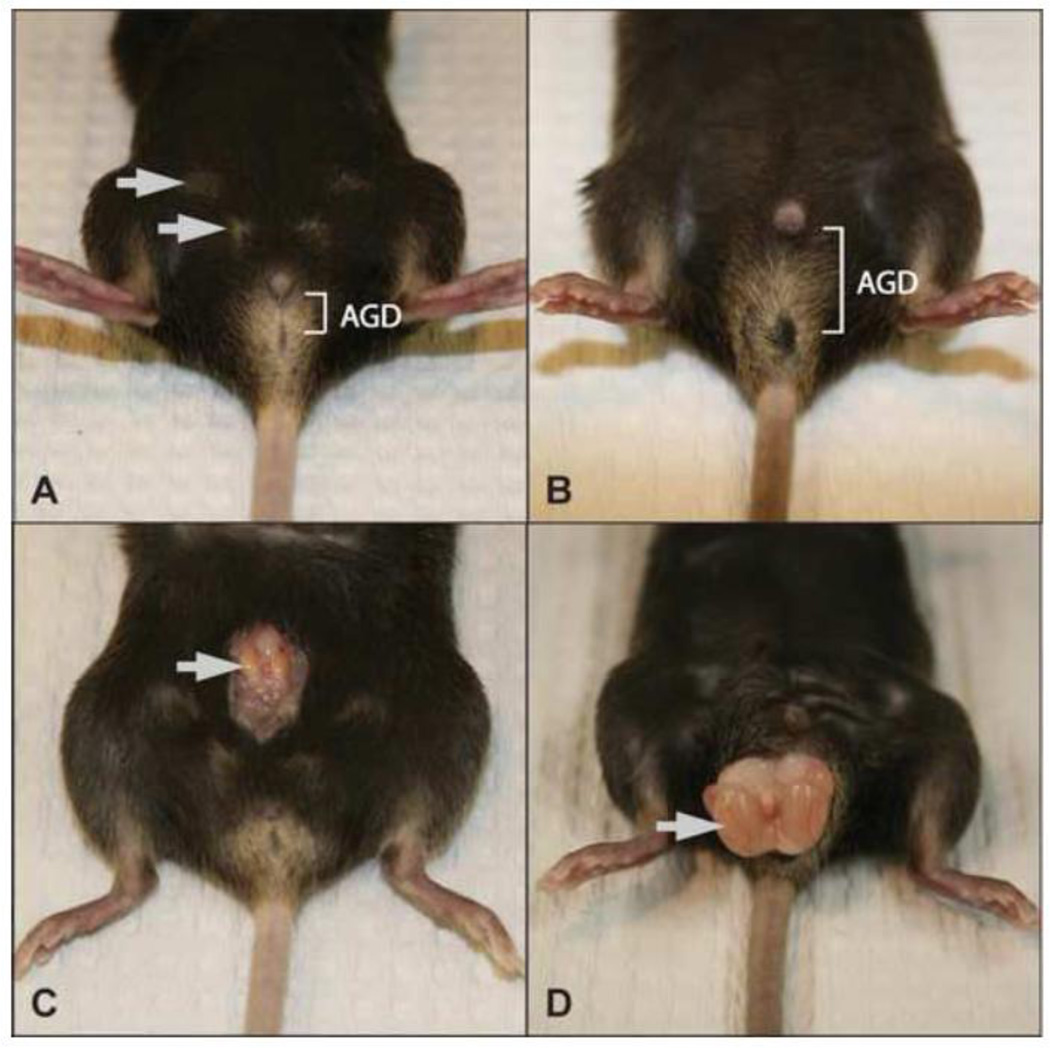
Figure 1.
The external phenotype of iTfm males is feminine. AGD: anogenital distance. Compared to WT males, iTfm males have a visibly shorter anogenital distance (A vs. B) and have much smaller and undescended testis (arrow in C vs. D). iTfm males also have external nipples (arrows in A) typical of WT females but not WT males (B).
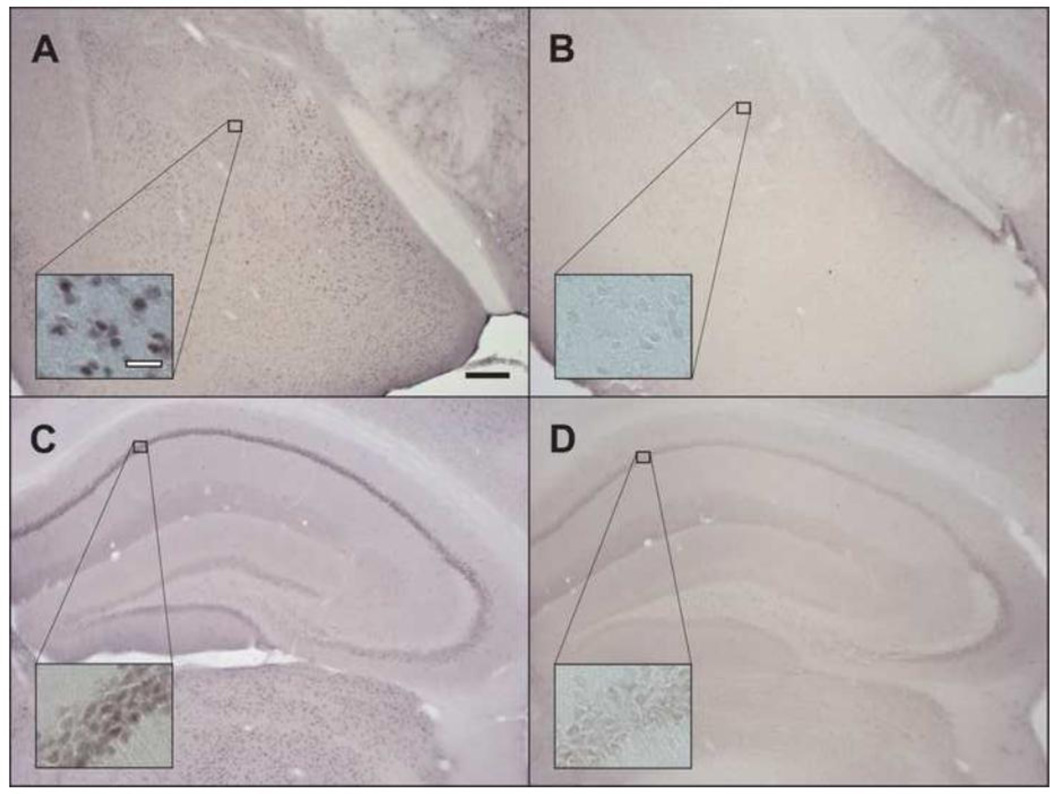
Figure 2.
Brain AR immunoreactivity confirms full AR KO in iTfm animals. Robust nuclear AR immunoreactivity is present in the amygdala (A) and hippocampus (C) of WT males, while absent in the amygdala and hippocampus (B and D, respectively) of iTfm males. Black scale bar: 200µm; white scale bar: 40µm.
Mice born in this iTfm colony were housed in plastic cages (29×18×18cm) at approximately 27°C in a 12:12 LD cycle, and provided ad libitum tap water and rodent chow (Harlan Teklad 8640 Rodent Diet [Madison, WI]). Mice were ear punched for genotyping and weaned at postnatal (PN) day 23 and group housed with other phenotypically similar males or females. For experiments 1 and 3, WT and iTfm males were castrated at postnatal (PN) day 60 and subcutaneously implanted either with testosterone-filled (T) or blank (B) capsules (1.6 mm-inner and 3.2 mm-outer diameter, 1.6 cm effective length). Behavior testing and blood collection took place on PN90–120. All housing conditions and experiments were performed in compliance with guidelines established by the National Institutes of Health Guide for the Care and Use of Laboratory Animals, and were approved by the MSU Institutional Animal Care and Use Committee.
Polymerase chain reaction (PCR) identification of genotype
PCR was run to detect the recombined AR allele and the Sry gene. To discriminate the WT and recombined AR alleles, primers targeted and amplified the sequence that includes the lox sites and exon 2 of the AR gene. The primers used were AGC CTG TAT ACT CAG TTG GGG and AAT GCA TCA CAT TAA GTT GAT ACC. The resulting products were 860bp for the wild type AR, and 400bp for the recombined AR allele. Animals that were positive for both Sry and WT AR were classified as WT males whereas mice positive for both Sry and a recombined AR were classified as iTfm males. The genotype determined by PCR was also verified based on the phenotype of each mouse as described below (experiments 1 and 3), and/or at sacrifice (experiment 2).
AR Immunocytochemistry (ICC)
To facilitate detecting AR, all mice in these studies were injected with 1mg of testosterone propionate in 0.1ml sesame oil sc two hours before being sacrificed. Animals were then injected with an overdose of sodium pentobarbital i.p. and intracardially perfused with 0.9% saline followed by 4% paraformaldehyde in 0.1 M phosphate buffered Saline (PBS, pH 7.4). Brains were collected and post-fixed for 2 hours in 4% paraformaldehyde, at 4°C. Tissues were then transferred into a 20% sucrose solution and kept at 4°C until they sunk to the bottom (approx. 2 days). Once sunk, brains were microtome sectioned in the coronal plane at 35µm. All reactions for the ICC were performed at room temperature and on a rotomixer unless otherwise indicated. Tissues were rinsed in four 5 minute washes in a phosphate-buffered gelatin Triton solution (PBS-GT; 0.1% gelatin, 0.3% Triton X-100, in PBS, pH 7.4), followed by 0.5% sodium borohydride in PBS-GT for 15 minutes, and three 5 minute washes in PBS-GT. Sections were then incubated in 10% NGS in PBS-GT for 1 hour to block non-specific binding of the secondary antibody. Two 5 minute washes in PBS-GT, and later a 10 minute incubation in avidin block followed. This was succeeded by two 5 minute PBS-GT washes and then incubation for 10 minutes in biotin block (Avidin/Biotin Blocking Kit, Vector, cat#SP-2001). Two 5 minute PBS-GT washes took place before the tissue was incubated for 36 hours at 4°C in 1% NGS in PBS-GT with AR primary antisera at 1:5000 concentration (rabbit monoclonal - Abcam, code#ab52615, clone ID EP670Y). Following incubation in the primary antibody, the tissue was rinsed in PBS-GT, and incubated 1 hour in 1% NGS in PBS-GT with biotinylated goat antirabbit secondary antisera at 1:1000 concentration (Jackson Immunoresearch, lot # 88762, code # 711-065-152). Brain sections were rinsed again in PBS-GT, followed by a 1 hour incubation in PBS-GT with an Avidin-Biotin complex solution made 30 minutes before use (1 drop of each solution A and B per 10ml of PBS-GT; ABC Elite Kit [standard], Vector Laboratories, catalogue # PK6100). The tissue was again washed in PBS-GT before being stained with NiCl-enhanced diaminobenzidine (DAB, Sigma, St. Louis, MO) in a 0.05 M Tris Buffer, pH 7.2. Following staining, the tissue was first washed in PBS-GT and then in mounting solution before being mounted and coverslipped after dehydration through graded alcohols and xylene.
Experiment 1: Validation of the iTfm model
To examine whether disabling AR via the cre/lox system produces effects on anxiety-like behavior comparable to that seen in sTfm mice (Zuloaga et al., 2008a), castrated WT and iTfm male mice with T or B capsules were tested for anxiety-related behavior on PN 90–120. Mice were tested on the OF, the NO, the EPM, and the L/D box tests, with 2 days between tests. The order of the last two tests (the EPM and L/D box) was counterbalanced to assess any test-order effect (detailed information below). Mice were taken in their home cages to the behavior testing room to habituate an hour before testing. Mice were tested during their resting phase, 2 hrs after lights on.
Experiment 2: Effects of photoperiodicity on anxiolytic effects of T and AR activity
Additional groups of behaviorally naïve WT and iTfm male mice were tested on the same behavioral assays as above, except the tests were conducted during their active phase, 2 hrs after lights off. Animals were taken to the behavior testing room covered with a dark cloth to minimize exposure to ambient light and remained covered until testing. Mice were only exposed to light during behavioral tests.
Experiment 3: Corticosterone response to mild stress in T-treated WT and iTfm males
Based on findings in experiments 1 and 2, and the relation between anxiety-related behavior and HPA axis activity, experiment 3 was conducted to determine whether disabling AR through recombination alters HPA response to mild stress. Terminal blood samples for corticosterone (CORT) levels were collected on PN90 from behaviorally naïve T-treated WT and iTfm mice at baseline, and at 20, 40 60, and 120 min after a 10-min exposure to the L/D box. Castrated WT males implanted with blank capsules provided an additional control group. Exposure to the L/D box and blood collection took place during the animals’ active phase, 2 hours after lights off, parameters chosen based on the robust group differences seen in Experiment 2 (detailed below).
Castration and Silastic capsule implant for androgen treatment
At PN60 before any testing began, mice in experiments 1 and 3 were anesthetized with isoflurane and their backs shaved for capsule implants. Castrations in WT mice were performed through a 5-mm long incision along the midline of the scrotal sac. Testes were visualized and silk suture was used to tie the vas deferens, blood vessels and associated fat pad before removing the testes. The incision was closed using surgical staples. In iTfm mice, the testes were undescended and small as expected (De Gendt et al., 2004; Figure 1D); thus, an abdominal incision was made through which the testes were visualized on each side of the bladder, tied and removed as described above. The abdominal muscle wall was closed with suture and the overlying skin closed with clips.
During the same period of anesthesia, animals also received subcutaneous implants of Silastic capsules containing either testosterone (T) or left blank (B) (1.6mm inner diameter, 3.2mm outer diameter; 6mm effective release length). Such capsules produce T levels at near-normal circulating levels in mice (Zuloaga et al., 2008a) and were implanted on the dorsal surface just caudal to the interscapular fat pad. The incision over the capsule was closed with surgical staples, and mice were injected with 0.05 ml of Ketofen (100mg/ml, sc) for analgesia.
Anxiety-related behavior testing
All testing chambers were cleaned with 70% EtOH and dried between subjects.
Open field and novel object tests
The OF was a rectangular unlidded Plexiglas box, 40.6 × 40.6 cm with walls 30.5 cm high. Testing took place under fluorescent lights projecting 460 lux throughout the arena. Mice were placed in the testing chamber facing a corner and allowed to move freely for 5 min. Animals were returned to their home cage for 15 min, then placed back into the chamber in which a novel object (a clear cylindrical petri dish with color tape, 5 cm in diameter) had been placed in the center of the field for another 5 min. Locomotor activity was tracked based on the interruption of laser beams and collected by computer software (Versamax). For the OF portion of the test, measures included the latency to enter the middle portion of the arena (a 20.3 × 20.3 cm area located in the center of the field), the number of entries into the center area, the total time spent in the center area, and the number of rearings. For the NO portion of the test, measures included the latency to visit the novel object, the number of visits paid to the object, the total time spent near the object, and the number of rearings performed anywhere in the chamber.
Elevated plus maze test
The EPM consisted of four arms, 25.4 cm in length and 5.7 cm in width, stretched out from a center square at 90 degree intervals. Two of the opposing arms had walls of 13.3 cm in height. The maze rested 43.8 cm from the floor. Testing took place with an overhead lamp that provided 50 lux lighting in the open arms and 25 lux in the closed arms. Animals were placed into the maze facing a closed arm and allowed 10 minutes to move freely. An overhead camera recorded their activity. Measures taken from video recordings included the number of open arm entries (4 paws in an open arm), the number of stretch attends into open arms (2 paws in an open arm), the total percentage of time spent in open arms (time in open arm/[time spent in closed arms + time spent in open arms]), and the number of head dippings while in an open arm.
Light/dark box test
The L/D box consisted of a rectangular Plexiglas arena divided at the center by a wall to yield two areas measuring 24.1×18.4 cm each. The “light” part of the box was open-topped and had 3 transparent walls, while the “dark” part was a fully-enclosed black box. The dividing black wall between the light and dark sides of the box had a 10.2×5.1cm opening, which allowed mice to move freely between the sides. Testing took place with an overhead lamp providing 500 lux in the light area and 2 lux in the dark area. Animals were placed on the light side of the apparatus facing the connecting doorway and allowed 10 minutes to move freely. Behavior coding started after animals first enter the dark side. Measures made from video recordings included number of stretch attends towards the light chamber (2 paws in light side), number of entries into the light chamber (4 paws in light side), total time spent in the light chamber, and number of rearings made during time spent in the light chamber (number of rears/time in light side).
Blood collection for CORT sampling
To study CORT hormone response, we exposed mice to the L/D box two hours into their active phase. Mice were anesthetized with isoflurane and decapitated for blood collection. Blood was collected into heparinized tubes, which were centrifuged at 8°C for 20 minutes at 3000 rcf. Plasma samples were stored at −80°C until analyses. Samples were assayed for corticosterone using Coat-a-Count Corticosterone kits (Diagnostics Products Corporation, Los Angeles, CA. USA) at the Diagnostic Center for Population and Animals Health at MSU. All samples were run in duplicate and the average of the two samples from each mouse was used for analysis. Blood samples for basal T level analysis were taken from intact adult WT and iTfm animals. All procedures were the same as mentioned above, and Coat-a-Count Total Testosterone kits were used for T assays (Diagnostics Products Corporation, Los Angeles, CA. USA).
Statistics
Experiment 1
Analyses for the OF and NO tests consisted of two-way ANOVAs with genotype (WT versus iTfm) and hormone treatment (T versus B) as independent factors. Analysis for the EPM and LD box tests initially consisted of three-way ANOVAs run to determine a potential test order effect with genotype (WT versus iTfm), hormone treatment (T versus B) and test order (EPM/LD versus LD/EPM) as independent factors. As these analyses indicated no effect of test order, subsequent two-way ANOVAs were conducted with genotype and hormone treatment alone. Analyses were followed up by Post-hoc LSD tests.
Experiment 2
Analysis for the OF and NO tests consisted of one-way ANOVAs with genotype (WT versus iTfm) as the independent factor. For the EPM and LD box tests, an initial two-way ANOVA was run with genotype (WT versus iTfm) and test order (EPM/LD versus LD/EPM) as independent factors. As these did not reveal a test order effect, one-way ANOVAs were conducted with genotype as the independent factor.
Experiment 3
Analysis consisted of a two-way ANOVA with group (WT+T, iTfm+T, WT+B) and blood collection time (baseline, 20, 40, 60, 120 minutes after exposure to the L/D box) as independent factors, followed by a post-hoc LSD test. Differences were considered significant when p<0.05. For two-way ANOVAs and pairwise comparisons that showed significant differences, eta squared and Cohen’s d effect sizes were calculated, respectively.
Results
Experiment 1: Validation of the iTfm model in the resting phase
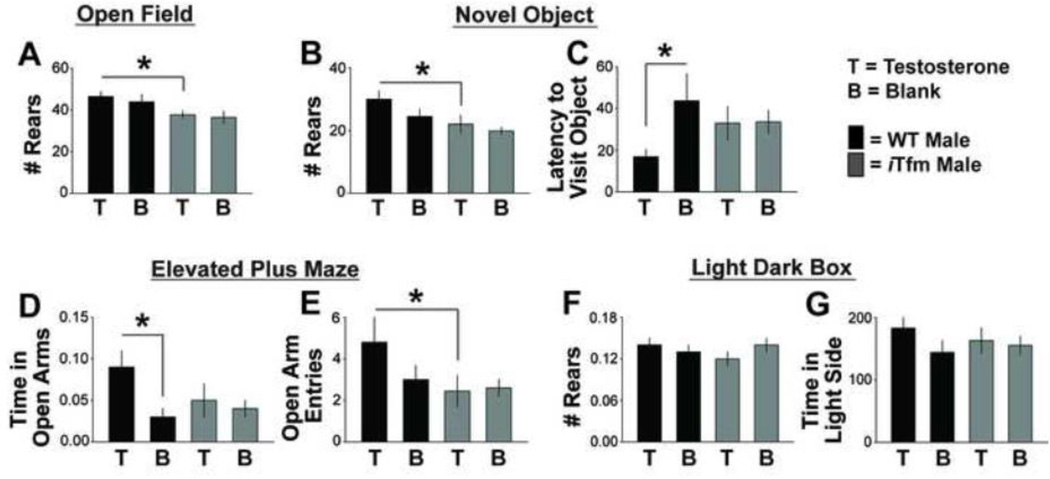
In the resting phase (lights on), iTfm males showed evidence of greater anxiety than wildtype males in several behavioral indices, confirming findings with sTfms (Zuloaga et al., 2008a). For the OF test, there was a significant main effect of genotype on the number of rears (p <.005; d=.806) since WT males reared more than iTfm mice, regardless of hormone treatment. However, there was no main effect of hormone or interaction on number of rears. Post-hoc comparisons confirmed that WT+T mice reared more than either iTfm+T or iTfm+B (all p’s<.05, unless specified; d=1.083 and d=.944, respectively; Figure 3A). There was also a main effect of hormone on the number of center area entries (η2=.009), where animals treated with T entered the area more than those given blank capsules (d=.554), but there was no main effect of genotype or interaction on this measure (data not shown). No main effects or interactions were found for latency to enter or time spent in the center area.
Figure 3.
ARs are necessary to alleviate anxiety-related behavior in mice tested during the resting phase (lights on). Number of rears in the open field (OF) test (A) and the novel object (NO) test (B) both show a main effect of genotype only, with testosterone (T)-treated iTfms showing fewer rears than T-treated WTs. Latency to visit the object in the NO test (C) and total time spent in the open arms of the elevated plus maze (EPM; D) were affected by T treatment only in WT males. WT males given T made more open arm entries than iTfms given T (E). For OF, NO and EPM tests, T treatment has anxiolytic effects only in WT males, not iTfm males, indicating these effects of T are normally mediated through AR. There was no effect of T treatment or genotype in the light dark box (LD) when tested during the resting phase (F, G). These results replicate our previous findings of anxiolytic effects of T in sTfm mice, validating the iTfm model. *p<.05.
For the NO test, there was a significant main effect of genotype on the number of rears (η2=.015) because WT males reared more than iTfm mice (d=.72). There was no main effect of hormone or interaction, paralleling what we found in the OF. Post-hoc comparisons confirmed that WT+T mice reared more than T-treated iTfm males (d=.755; Figure 3B). T treatment also reduced the latency to approach the novel object, but only in WT animals; a post-hoc LSD test showed that WT+T animals approached the object faster than WT+B mice (d=.887; Figure 3C). iTfm males in both groups were intermediate on this measure, not differing from either WT group. No main effect of genotype or hormone, and no interactions were found for number of object visits and total time spent with the object.
For the EPM, a three-way ANOVA did not indicate a main effect of test order or any interaction of that factor with others. Therefore, data were collapsed across test order for the rest of the analysis. Subsequent two-way ANOVA revealed a significant main effect of genotype on the number of open arm entries (η2=.028), where WT males entered the open arms more often than iTfms (d=.525). No main effect of hormone or interaction was found. Post-hoc LSD revealed that T significantly increased the number of open arm entries (d=.74; Figure 3D), and the time spent in open arms (d=1.203; Figure 3E) only in WT and not in iTfm males. iTfm males, irrespective of hormone treatment condition, were like WTs without T, spending less time in the open arms.
We found no difference in anxiety-like behavior in iTfm males compared to WT males in the LD box during the resting phase (Figure 3F–G). To determine whether circadian time affected these results, we performed follow-up tests on a separate cohort of mice during the active phase (lights off), described next.
Experiment 2: Effects of circadian phase on anxiolytic effects of T and AR activity
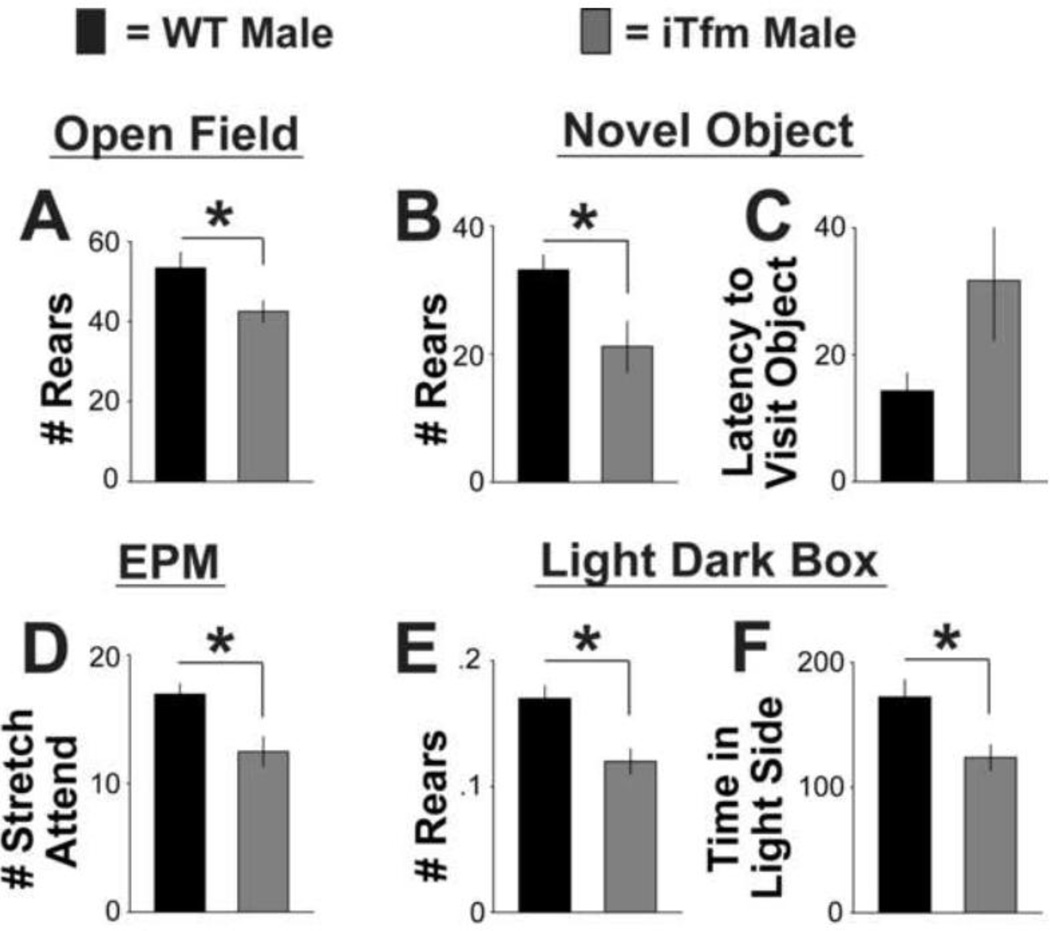
As in the previous tests during the resting phase, we found a difference between WT and iTfm animals in the number of rears in the OF test in the active phase (d=.941; Figure 4A), but no significant differences for time spent in the center, latency to enter the center, or total time spent in the center.
Figure 4.
Anxiety-related behavior is also heightened in intact iTfm male mice compared to intact WT males tested during their active phase (lights off). With one exception (latency to visit object in the novel object test, C), iTfm males show significantly increased levels of anxiety-like behavior compared to WT males based on performance in the open field (A), novel object (B), elevated plus maze (D), and light/dark box (E, F) tests. These results further support the idea that AR plays a role in the modulation of anxiety. Testing during the active phase reveals group differences in the light dark box that were not observed during the resting phase (see Figure 3). *p<.05.
For the NO test, WT mice again reared significantly more than iTfms (d=1.093; Figure 4B), with a trend toward approaching the novel object sooner than iTfm animals (p=.079, two-tailed, Figure 4C). No significant differences were found for latency to visit the novel object, number of object visits, or total time spent with the object.
For the EPM, two-way ANOVAs again indicated no effect of test order. Collapsing across test order, one-way ANOVAs revealed that WT males performed more stretch attends than iTfm mice (d=1.288; Figure 4D), but there were no significant differences for other measures (number of open arm entries, total time spent in open arms, and number of head dips) during the lights off phase.
For the LD box, a two-way ANOVA first confirmed that there was no effect of test order for any of the parameters. Subsequent one-way ANOVAs revealed that WT males reared more often (d=1.418; Figure 4E) and spent more time in the light side (d=1.135; Figure 4F) than did iTfm males when tested in the active phase. There were no significant differences for any other measure in this test.
Experiment 3: Corticosterone response to mild stress in T-treated WT and iTfm males
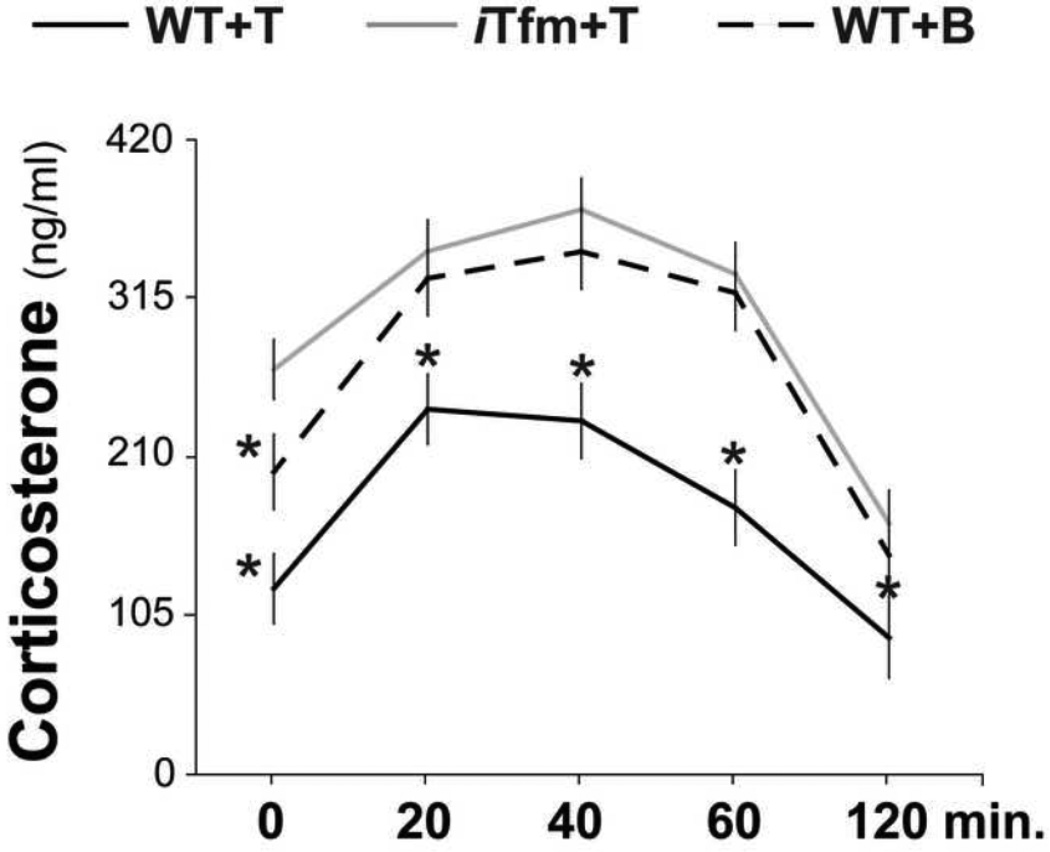
Testosterone had a clear modulatory effect on both the basal levels of corticosterone (CORT) and the time course of recovery after exposure to a mild stressor in WT male mice (Figure 5). Moreover, both baseline and recovery of CORT were affected by disruption of AR. Two-way ANOVA, with treatment group and time of blood collection as factors, indicated significant main effects of group (η2=.036) and time (η2=.055), but no significant interaction. Post-hoc tests revealed that WT+T mice showed consistently lower CORT throughout the HPA response than either iTfm+T (d=1.335) or WT+B (d=1.182) mice. Pairwise comparisons across time also indicated that CORT levels returned to baseline sooner in T-treated WTs compared to the other two groups (iTfm+T and WT+B), since CORT levels at 60 minutes were comparable to baseline levels in WT+T mice (p>0.118) whereas CORT levels remained significantly higher than baseline for both iTfm males and blank-treated WT males at this time point.
Figure 5.
Functional AR is required for T to regulate basal levels of CORT as well as CORT response to an anxiety-provoking stimulus (light dark box). Basal levels of CORT (time point 0) are highest in iTfm males given T and lowest in WT castrates given T, with control-treated WT males intermediate, indicating that T exposure in adulthood normally reduces basal CORT levels by activating ARs. Additionally, despite equivalent T treatment, iTfm males show an even more elevated response and a hastened CORT return to baseline compared to WT males after exposure to an anxiety-provoking situation. *indicates significantly different from iTfm+T males, p<.05.
Discussion
ARs appear to play a role in mediating the anxiolytic effects of testosterone in mice, as measured by anxiety-related behaviors and HPA activity in both the resting and active phases of the circadian cycle. Using cre/lox technology to recapitulate the spontaneous Tfm model (Zuloaga et al., 2008a), we replicated the role of ARs in the anxiolytic effects of T in a so-called “induced” Tfm (iTfm) model and also expanded on these findings. Specifically, T treatment reduced some indices of anxiety only in WT males, not iTfm males. Compared to iTfm+T male mice, WT+T males entered the open arms of the EPM more times, took less time to approach a novel object in the NO test, and showed less exploration in the OF and NO tests, as indicated by the number of rearings. Testing conditions in this first experiment replicated the phenomenon found earlier (Zuloaga et al., 2008a) where sTfm and WT male mice were tested for anxiety in their resting phase (lights on). Both studies indicated an anxiolytic role of AR, although there were some differences between the two Tfm models in terms of which parameters displayed statistically significant differences (Zuloaga et al., 2008a). This may be due to minor differences in how the tests were conducted or scored, as the testing staff was different in the two studies. In sTfms, the natural mutation is caused by a single base deletion in the coding region of the n-terminus, which creates a change in the reading frame, resulting in a truncated AR that lacks both DNA and steroid binding domains (Charest et al., 1991). In iTfms, exon 2 of the AR gene is excised (De Gendt et al., 2004). Exon 2 codes for the 1st zinc finger of the AR DNA-binding domain, and, though this also creates a premature termination of transcription, the resulting transcripts would be different, but as no functional protein is produced in either model, it is difficult to see how the different transcripts would affect behavior.
The role of functional AR was also examined in a different cohort of mice during their active phase (lights off) to assess the possibility that anxiety-related behavior and the apparent role of AR might be affected by circadian phase. To a large extent, we found the same pattern of differences in both phases: WT males reared more in the OF and NO tests, and performed more stretch attends in the EPM, a measure of higher anxiety (McLean et al., 2011). However, differences in anxiety-like behavior between WT and iTfm males in the LD box were revealed only in the active phase, with iTfm males rearing significantly less and spending significantly less time on the light side of the box than WT controls, suggesting an effect of photoperiodicity on the anxiolytic effects of activated AR in this particular test. Two potential mechanisms may underlie this interaction. First, T might work through functional AR in WT mice to dampen the effects of rising CORT levels that occur during the active phase (Halberg et al., 1959; Zuloaga et al., 2011b), which in turn may reduce anxiety-like behavior in the LD box. The effect that we saw of T on CORT levels in WT males in the active phase (Figure 5) is consistent with this view. Second, T and AR may reduce the anxiogenic effects that bright light has on animals during their active phase through a mechanism that is independent of CORT. Since the core of the suprachiasmatic nucleus of the hypothalamus (SCN) responds to photic inputs to the retina, and core cells contain AR (Karatsoreos and Silver, 2007), T may act directly on SCN neurons to regulate their response to light.
Results based on the OF, NO, EPM and LD box tests during the resting and active phases leave questions still unanswered. First, it is not apparent why the anxiolytic effects of AR affect some measures of anxiety-related behavior in these tests and not others, considering that they are all exploration-based anxiety tests. Other studies (Juntti et al., 2010; Raskin et al., 2009) also suggest that T signaling through AR regulates some, but not all, behaviors, including anxiety-related behaviors. Second, since iTfm mice lack AR throughout development and AR is crucial for differentiation of several brain regions and behaviors (Bodo and Rissman, 2007; Dugger et al., 2007; Durazzo et al., 2007; Garcia-Falgueras et al., 2005; Jones and Watson, 2005; Meaney et al., 1983; Morris et al., 2005; Olsen and Whalen, 1981; Rizk et al., 2005; Zuloaga et al., 2008b), it is unclear whether the effects seen are solely due to the adult activational effects of T.
Here, we also demonstrate for the first time that the capacity for T to reduce HPA activity requires a functional AR. WT mice treated with T show lower basal CORT levels than WT+B or iTfm+T males. When exposed to the LD box, all animals showed a CORT increase from their respective baseline levels, which tapers down with time; however, only WT+T males return to baseline levels by 60 minutes after exposure, while WT+B and iTfm+T take longer, indicating a capacity for T to curtail HPA activity through functional AR. Neither the presence of T alone nor functional AR alone was sufficient to curtail HPA activity, as CORT levels in iTfm+T and WT+B mice remained above baseline after 60 minutes. That intact WT male mice have lower baseline CORT compared to mice that lack functional AR has previously been shown in our lab based on sTfm mice (Zuloaga et al., 2008a), but here we additionally demonstrate that T acts through functional AR to lower HPA response and recovery. Since previous work was conducted in the resting phase (Zuloaga et al., 2008a), the present finding also extends the importance of T and AR in regulating HPA activity to the active phase. Our results also agree with Evuarherhe and colleagues’ (2009) finding that T-treated castrated adult rats show lower basal corticosterone levels than castrated controls. In addition, castration increases corticotropin releasing hormone and parvocellular arginine vasopressin mRNA in the paraventricular nucleus of the hypothalamus, a phenomenon that is abolished with T restoration (Evuarherhe et al., 2009; Zhou et al., 1994). It is possible that AR mediates these modulatory effects of T on mRNA levels.
These findings also validate the iTfm mouse model as one suited for testing anxiety mechanisms in mice, which offers possibilities for using the CreLox system to knock out AR selectively, in different regions and/or at different time points. Although it is clear that AR has a role in anxiety-like behavior, the mechanism by which AR activation reduces anxiety is yet to be established. One possibility is that AR stimulation may activate GABAergic drive, known to reduce anxiety (Lydiard, 2003; Rago et al., 1988; Reynolds, 2008). In fact, androgenized female mice show greater GABAergic postsynaptic current frequency and larger hypothalamic cells than control females, an effect blocked by the AR antagonist flutamide (Sullivan and Moenter, 2004), suggesting a capacity of AR to modulate GABA function. Similarly, chronic exposure to anabolic androgenic steroids increases selective GABA(A) receptor subunit mRNAs and GABAergic synaptic current decay in the medial preoptic area in WT male mice, an effect that sTfm mice do not show (Penatti et al., 2009). Since chronic, but not single, exposure to androgens can lower anxiety-related behavior (Fernandez-Guasti and Martinez-Mota, 2005; Penatti et al., 2009) and is blocked by flutamide (Fernandez-Guasti and Martinez-Mota, 2005), it is very likely that prolonged androgen exposure acting on ARs triggers a downstream cascade of events that in time results in altered GABA function, leading to anxiolytic effects.
The present results in mice complement our previous findings in rats, where again Tfm animals show more anxiety-related behaviors than WT males in a variety of tests (Zuloaga et al., 2011b). Thus it seems likely that T also acts through functional AR to reduce anxiety in men. This effect could contribute to sex differences in the prevalence of anxiety disorders. To date, much is known about sex differences in prevalence and course of the disorder and, though little is known about treatment, a few studies show a sex difference in response to treatment (Bekker and van Mens-Verhulst, 2007). Understanding the downstream actions of T and AR might shed light on potential common pathways present in both sexes, potentially leading to treatments for both men and women.
Highlights.
-
-
Testosterone (T) acts through androgen receptors (AR) to produce anxiolytic effects
-
-
Photoperiod interacts with AR’s capacity to modulate some anxiety-related behavior
-
-
T acting through AR lowers baseline corticosterone (CORT) levels
-
-
T acts through AR to hasten HPA return to baseline levels after mild stress
-
-
Cre-lox recombination of AR allele disrupts testosterone’s anxiolytic effects
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aikey JL, Nyby JG, Anmuth DM, James PJ. Testosterone rapidly reduces anxiety in male house mice (Mus musculus) Hormones and behavior. 2002;42:448–460. doi: 10.1006/hbeh.2002.1838. [DOI] [PubMed] [Google Scholar]
- Almeida OP, Waterreus A, Spry N, Flicker L, Martins RN. One year follow-up study of the association between chemical castration, sex hormones, beta-amyloid, memory and depression in men. Psychoneuroendocrino. 2004;29:1071–1081. doi: 10.1016/j.psyneuen.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Amore M, Scarlatti F, Quarta AL, Tagariello P. Partial androgen deficiency, depression and testosterone treatment in aging men. Aging Clin Exp Res. 2009;21:1–8. doi: 10.1007/BF03324891. [DOI] [PubMed] [Google Scholar]
- Bekker MH, van Mens-Verhulst J. Anxiety disorders: sex differences in prevalence, degree, and background, but gender-neutral treatment. Gender medicine. 2007;4(Suppl B):S178–S193. doi: 10.1016/s1550-8579(07)80057-x. [DOI] [PubMed] [Google Scholar]
- Bodo C, Rissman EF. Androgen receptor is essential for sexual differentiation of responses to olfactory cues in mice. Eur J Neurosci. 2007;25:2182–2190. doi: 10.1111/j.1460-9568.2007.05484.x. [DOI] [PubMed] [Google Scholar]
- Charest NJ, Zhou ZX, Lubahn DB, Olsen KL, Wilson EM, French FS. A frameshift mutation destabilizes androgen receptor messenger RNA in the Tfm mouse. Mol Endocrinol. 1991;5:573–581. doi: 10.1210/mend-5-4-573. [DOI] [PubMed] [Google Scholar]
- De Gendt K, Swinnen JV, Saunders PT, Schoonjans L, Dewerchin M, Devos A, Tan K, Atanassova N, Claessens F, Lecureuil C, Heyns W, Carmeliet P, Guillou F, Sharpe RM, Verhoeven G. A Sertoli cell-selective knockout of the androgen receptor causes spermatogenic arrest in meiosis. Proc Natl Acad Sci U S A. 2004;101:1327–1332. doi: 10.1073/pnas.0308114100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean C, Kendell RE. The symptomatology of puerperal illnesses. Br J Psychiatry. 1981;139:128–133. doi: 10.1192/bjp.139.2.128. [DOI] [PubMed] [Google Scholar]
- Decker DA, Pettinga JE, VanderVelde N, Huang RR, Kestin L, Burdakin JH. Estrogen replacement therapy in breast cancer survivors: a matched-controlled series. Menopause. 2003;10:277–285. doi: 10.1097/01.GME.0000061806.76067.E9. [DOI] [PubMed] [Google Scholar]
- Demetrio FN, Renno J, Gianfaldoni A, Goncalves M, Halbe HW, Filho AHGV, Gorenstein C. Effect of estrogen replacement therapy on symptoms of depression and anxiety in non-depressive menopausal women. Arch Women Ment Hlth. 2011;14:479–486. doi: 10.1007/s00737-011-0241-3. [DOI] [PubMed] [Google Scholar]
- Douma SL, Husband C, O'Donnell ME, Barwin BN, Woodend AK. Estrogen-related mood disorders: reproductive life cycle factors. ANS Adv Nurs Sci. 2005;28:364–375. doi: 10.1097/00012272-200510000-00008. [DOI] [PubMed] [Google Scholar]
- Dugger BN, Morris JA, Jordan CL, Breedlove SM. Androgen receptors are required for full masculinization of the ventromedial hypothalamus (VMH) in rats. Hormones and behavior. 2007;51:195–201. doi: 10.1016/j.yhbeh.2006.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durazzo A, Morris JA, Breedlove SM, Jordan CL. Effects of the testicular feminization mutation (tfm) of the androgen receptor gene on BSTMPM volume and morphology in rats. Neurosci Lett. 2007;419:168–171. doi: 10.1016/j.neulet.2007.04.033. [DOI] [PubMed] [Google Scholar]
- Edinger KL, Frye CA. Testosterone's analgesic, anxiolytic, and cognitive-enhancing effects may be due in part to actions of its 5 alpha-reduced metabolites in the hippocampus. Behavioral neuroscience. 2004;118:1352–1364. doi: 10.1037/0735-7044.118.6.1352. [DOI] [PubMed] [Google Scholar]
- Edinger KL, Frye CA. Intrahippocampal administration of an androgen receptor antagonist, flutamide, can increase anxiety-like behavior in intact and DHT-replaced male rats. Hormones and behavior. 2006;50:216–222. doi: 10.1016/j.yhbeh.2006.03.003. [DOI] [PubMed] [Google Scholar]
- Evuarherhe O, Leggett JD, Waite EJ, Kershaw YM, Atkinson HC, Lightman SL. Organizational role for pubertal androgens on adult hypothalamic-pituitary-adrenal sensitivity to testosterone in the male rat. J Physiol-London. 2009;587:2977–2985. doi: 10.1113/jphysiol.2008.168393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Guasti A, Martinez-Mota L. Anxiolytic-like actions of testosterone in the burying behavior test: role of androgen and GABA-benzodiazepine receptors. Psychoneuroendocrino. 2005;30:762–770. doi: 10.1016/j.psyneuen.2005.03.006. [DOI] [PubMed] [Google Scholar]
- Freeman EW. Treatment of depression associated with the menstrual cycle: premenstrual dysphoria, postpartum depression, and the perimenopause. Dialogues in clinical neuroscience. 2002;4:177–191. doi: 10.31887/DCNS.2002.4.2/efreeman. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye CA. Steroids, reproductive endocrine function, and affect. A review. Minerva ginecologica. 2009;61:541–562. [PubMed] [Google Scholar]
- Frye CA, Edinger K, Sumida K. Androgen administration to aged male mice increases anti-anxiety behavior and enhances cognitive performance. Neuropsychopharmacol. 2008;33:1049–1061. doi: 10.1038/sj.npp.1301498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye CA, Lacey EH. Posttraining androgens' enhancement of cognitive performance is temporally distinct from androgens' increases in affective behavior. Cognitive, affective & behavioral neuroscience. 2001;1:172–182. doi: 10.3758/cabn.1.2.172. [DOI] [PubMed] [Google Scholar]
- Garcia-Falgueras A, Pinos H, Collado P, Pasaro E, Fernandez R, Jordan CL, Segovia S, Guillamon A. The role of the androgen receptor in CNS masculinization. Brain Res. 2005;1035:13–23. doi: 10.1016/j.brainres.2004.11.060. [DOI] [PubMed] [Google Scholar]
- Giltay EJ, Enter D, Zitman FG, Penninx BWJH, van Pelt J, Spinhoven P, Roelofs K. Salivary testosterone: Associations with depression, anxiety disorders, and antidepressant use in a large cohort study. J Psychosom Res. 2012;72:205–213. doi: 10.1016/j.jpsychores.2011.11.014. [DOI] [PubMed] [Google Scholar]
- Greenberg PE, Sisitsky T, Kessler RC, Finkelstein SN, Berndt ER, Davidson JR, Ballenger JC, Fyer AJ. The economic burden of anxiety disorders in the 1990s. J Clin Psychiatry. 1999;60:427–435. doi: 10.4088/jcp.v60n0702. [DOI] [PubMed] [Google Scholar]
- Halberg F, Albrecht PG, Bittner JJ. Corticosterone rhythm of mouse adrenal in relation to serum corticosterone and sampling. The American journal of physiology. 1959;197:1083–1085. doi: 10.1152/ajplegacy.1959.197.5.1083. [DOI] [PubMed] [Google Scholar]
- Handa RJ, Pak TR, Kudwa AE, Lund TD, Hinds L. An alternate pathway for androgen regulation of brain function: Activation of estrogen receptor beta by the metabolite of dihydrotestosterone, 5 alpha-androstane-3 beta,17 beta-diol. Hormones and behavior. 2008;53:741–752. doi: 10.1016/j.yhbeh.2007.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes ZA, Liu F, Platt BJ, Dwyer JM, Pulicicchio CM, Zhang GM, Schechter LE, Rosenzweig-Lipson S, Day M. WAY-200070, a selective agonist of estrogen receptor beta as a potential novel anxiolytic/antidepressant agent. Neuropharmacology. 2008;54:1136–1142. doi: 10.1016/j.neuropharm.2008.03.004. [DOI] [PubMed] [Google Scholar]
- Imwalle DB, Gustafsson JA, Rissman EF. Lack of functional estrogen receptor beta influences anxiety behavior and serotonin content in female mice. Physiology & behavior. 2005;84:157–163. doi: 10.1016/j.physbeh.2004.11.002. [DOI] [PubMed] [Google Scholar]
- Jones BA, Watson NV. Spatial memory performance in androgen insensitive male rats. Physiology & behavior. 2005;85:135–141. doi: 10.1016/j.physbeh.2005.03.023. [DOI] [PubMed] [Google Scholar]
- Juntti SA, Tollkuhn J, Wu MV, Fraser EJ, Soderborg T, Tan S, Honda S, Harada N, Shah NM. The androgen receptor governs the execution, but not programming, of male sexual and territorial behaviors. Neuron. 2010;66:260–272. doi: 10.1016/j.neuron.2010.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karatsoreos IN, Silver R. Minireview: The neuroendocrinology of the Suprachiasmatic nucleus as a conductor of body time in mammals. Endocrinology. 2007;148:5640–5647. doi: 10.1210/en.2007-1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Chiu WT, Demler O, Merikangas KR, Walters EE. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:617–627. doi: 10.1001/archpsyc.62.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiesner J. One woman's low is another woman's high: Paradoxical effects of the menstrual cycle. Psychoneuroendocrino. 2011;36:68–76. doi: 10.1016/j.psyneuen.2010.06.007. [DOI] [PubMed] [Google Scholar]
- Krezel W, Dupont S, Krust A, Chambon P, Chapman PF. Increased anxiety and synaptic plasticity in estrogen receptor beta-deficient mice. P Natl Acad Sci USA. 2001;98:12278–12282. doi: 10.1073/pnas.221451898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llaneza P, Garcia-Portilla MP, Llaneza-Suarez D, Armott B, Perez-Lopez FR. Depressive disorders and the menopause transition. Maturitas. 2012;71:120–130. doi: 10.1016/j.maturitas.2011.11.017. [DOI] [PubMed] [Google Scholar]
- Lund TD, Rovis T, Chung WCJ, Handa RJ. Novel actions of estrogen receptor-beta on anxiety-related behaviors. Endocrinology. 2005;146:797–807. doi: 10.1210/en.2004-1158. [DOI] [PubMed] [Google Scholar]
- Lydiard RB. The role of GABA in anxiety disorders. J Clin Psychiat. 2003;64:21–27. [PubMed] [Google Scholar]
- Marcondes FK, Miguel KJ, Melo LL, Spadari-Bratfisch RC. Estrous cycle influences the response of female rats in the elevated plus-maze test. Physiology & behavior. 2001;74:435–440. doi: 10.1016/s0031-9384(01)00593-5. [DOI] [PubMed] [Google Scholar]
- McLean CP, Asnaani A, Litz BT, Hofmann SG. Gender differences in anxiety disorders: prevalence, course of illness, comorbidity and burden of illness. Journal of psychiatric research. 2011;45:1027–1035. doi: 10.1016/j.jpsychires.2011.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meaney MJ, Stewart J, Poulin P, Mcewen BS. Sexual-Differentiation of Social Play in Rat Pups Is Mediated by the Neonatal Androgen-Receptor System. Neuroendocrinology. 1983;37:85–90. doi: 10.1159/000123524. [DOI] [PubMed] [Google Scholar]
- Meng FT, Ni RJ, Zhang Z, Zhao J, Liu YJ, Zhou JN. Inhibition of oestrogen biosynthesis induces mild anxiety in C57BL/6J ovariectomized female mice. Neurosci Bull. 2011;27:241–250. doi: 10.1007/s12264-011-1014-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menger Y, Bettscheider M, Murgatroyd C, Spengler D. Sex differences in brain epigenetics. Epigenomics. 2010;2:807–821. doi: 10.2217/epi.10.60. [DOI] [PubMed] [Google Scholar]
- Morris JA, Jordan CL, Dugger BN, Breedlove SM. Partial demasculinization of several brain regions in adult male (XY) rats with a dysfunctional androgen receptor gene. J Comp Neurol. 2005;487:217–226. doi: 10.1002/cne.20558. [DOI] [PubMed] [Google Scholar]
- Oliveira AG, Coelho PH, Guedes FD, Mahecha GA, Hess RA, Oliveira CA. 5alpha-Androstane-3beta,17beta-diol (3beta-diol), an estrogenic metabolite of 5alpha-dihydrotestosterone, is a potent modulator of estrogen receptor ERbeta expression in the ventral prostrate of adult rats. Steroids. 2007;72:914–922. doi: 10.1016/j.steroids.2007.08.001. [DOI] [PubMed] [Google Scholar]
- Olsen KL, Whalen RE. Hormonal-Control of the Development of Sexual-Behavior in Androgen-Insensitive (Tfm) Rats. Physiology & behavior. 1981;27:883–886. doi: 10.1016/0031-9384(81)90058-5. [DOI] [PubMed] [Google Scholar]
- Penatti CAA, Porter DM, Henderson LP. Chronic Exposure to Anabolic Androgenic Steroids Alters Neuronal Function in the Mammalian Forebrain via Androgen Receptor- and Estrogen Receptor- Mediated Mechanisms. J Neurosci. 2009;29:12484–12496. doi: 10.1523/JNEUROSCI.3108-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rago L, Kiivet RA, Harro J, Pold M. Behavioral-Differences in an Elevated Plus-Maze - Correlation between Anxiety and Decreased Number of Gaba and Benzodiazepine Receptors in Mouse Cerebral-Cortex. N-S Arch Pharmacol. 1988;337:675–678. doi: 10.1007/BF00175795. [DOI] [PubMed] [Google Scholar]
- Raskin K, de Gendt K, Duittoz A, Liere P, Verhoeven G, Tronche F, Mhaouty-Kodja S. Conditional inactivation of androgen receptor gene in the nervous system: effects on male behavioral and neuroendocrine responses. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29:4461–4470. doi: 10.1523/JNEUROSCI.0296-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds DS. The value of genetic and pharmacological approaches to understanding the complexities of GABA(A) receptor subtype functions: The anxiolytic effects of benzodiazepines. Pharmacol Biochem Be. 2008;90:37–42. doi: 10.1016/j.pbb.2008.03.015. [DOI] [PubMed] [Google Scholar]
- Rizk A, Robertson J, Raber J. Behavioral performance of tfm mice supports the beneficial role of androgen receptors in spatial learning and memory. Brain Res. 2005;1034:132–138. doi: 10.1016/j.brainres.2004.12.002. [DOI] [PubMed] [Google Scholar]
- Rocha BA, Fleischer R, Schaeffer JM, Rohrer SP, Hickey GJ. 17 beta-Estradiol-induced antidepressant-like effect in the Forced Swim Test is absent in estrogen receptor-beta knockout (BERKO) mice. Psychopharmacology. 2005;179:637–643. doi: 10.1007/s00213-004-2078-1. [DOI] [PubMed] [Google Scholar]
- Shifren JL, Braunstein GD, Simon JA, Casson PR, Buster JE, Redmond GP, Burki RE, Ginsburg ES, Rosen RC, Leiblum SR, Caramelli KE, Mazer NA. Transdermal testosterone treatment in women with impaired sexual function after oophorectomy. The New England journal of medicine. 2000;343:682–688. doi: 10.1056/NEJM200009073431002. [DOI] [PubMed] [Google Scholar]
- Sternbach H. Age-associated testosterone decline in men: clinical issues for psychiatry. The American journal of psychiatry. 1998;155:1310–1318. doi: 10.1176/ajp.155.10.1310. [DOI] [PubMed] [Google Scholar]
- Sullivan SD, Moenter SM. Prenatal androgens alter GABAergic drive to gonadotropin-releasing hormone neurons: Implications for a common fertility disorder. P Natl Acad Sci USA. 2004;101:7129–7134. doi: 10.1073/pnas.0308058101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tangen T, Mykletun A. Depression and anxiety through the climacteric period: An epidemiological study (HUNT-II) J Psychosom Obst Gyn. 2008;29:125–131. doi: 10.1080/01674820701733945. [DOI] [PubMed] [Google Scholar]
- Thomson J, Oswald I. Effect of oestrogen on the sleep, mood, and anxiety of menopausal women. British medical journal. 1977;2:1317–1319. doi: 10.1136/bmj.2.6098.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walf AA, Frye CA. ERbeta-selective estrogen receptor modulators produce antianxiety behavior when administered systemically to ovariectomized rats. Neuropsychopharmacol. 2005;30:1598–1609. doi: 10.1038/sj.npp.1300713. [DOI] [PubMed] [Google Scholar]
- Walf AA, Frye CA. Estradiol reduces anxiety- and depression-like behavior of aged female mice. Physiology & behavior. 2010;99:169–174. doi: 10.1016/j.physbeh.2009.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walf AA, Koonce C, Manley K, Frye CA. Proestrous compared to diestrous wildtype, but not estrogen receptor beta knockout, mice have better performance in the spontaneous alternation and object recognition tasks and reduced anxiety-like behavior in the elevated plus and mirror maze. Behav Brain Res. 2009;196:254–260. doi: 10.1016/j.bbr.2008.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walf AA, Koonce CJ, Frye CA. Estradiol or Diarylpropionitrile Decrease Anxiety-Like Behavior of Wildtype, but Not Estrogen Receptor Beta Knockout, Mice. Behavioral neuroscience. 2008;122:974–981. doi: 10.1037/a0012749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Alexander G, Berman N, Salehian B, Davidson T, McDonald V, Steiner B, Hull L, Callegari C, Swerdloff RS. Testosterone replacement therapy improves mood in hypogonadal men--a clinical research center study. The Journal of clinical endocrinology and metabolism. 1996;81:3578–3583. doi: 10.1210/jcem.81.10.8855804. [DOI] [PubMed] [Google Scholar]
- Wu MV, Shah NM. Control of masculinization of the brain and behavior. Curr Opin Neurobiol. 2011;21:116–123. doi: 10.1016/j.conb.2010.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yazici K, Pata O, Yazici A, Aktas A, Tot S, Kanik A. [The effects of hormone replacement therapy in menopause on symptoms of anxiety and depression] Turk psikiyatri dergisi = Turkish journal of psychiatry. 2003;14:101–105. [PubMed] [Google Scholar]
- Zahn-Waxler C, Shirtcliff EA, Marceau K. Disorders of childhood and adolescence: gender and psychopathology. Annu Rev Clin Psychol. 2008;4:275–303. doi: 10.1146/annurev.clinpsy.3.022806.091358. [DOI] [PubMed] [Google Scholar]
- Zhou L, Blaustein JD, Devries GJ. Distribution of Androgen Receptor Immunoreactivity in Vasopressin-Immunoreactive and Oxytocin-Immunoreactive Neurons in the Male-Rat Brain. Endocrinology. 1994;134:2622–2627. doi: 10.1210/endo.134.6.8194487. [DOI] [PubMed] [Google Scholar]
- Zuloaga DG, Carbone DL, Hiroi R, Chong DL, Handa RJ. Dexamethasone induces apoptosis in the developing rat amygdala in an age-, region-, and sex-specific manner. Neuroscience. 2011a;199:535–547. doi: 10.1016/j.neuroscience.2011.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuloaga DG, Morris JA, Jordan CL, Breedlove SM. Mice with the testicular feminization mutation demonstrate a role for androgen receptors in the regulation of anxiety-related behaviors and the hypothalamic-pituitary-adrenal axis. Hormones and behavior. 2008a;54:758–766. doi: 10.1016/j.yhbeh.2008.08.004. [DOI] [PubMed] [Google Scholar]
- Zuloaga DG, Poort JE, Jordan CL, Breedlove SM. Male rats with the testicular feminization mutation of the androgen receptor display elevated anxiety-related behavior and corticosterone response to mild stress. Hormones and behavior. 2011b;60:380–388. doi: 10.1016/j.yhbeh.2011.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuloaga DG, Puts DA, Jordan CL, Breedlove SM. The role of androgen receptors in the masculinization of brain and behavior: What we've learned from the testicular feminization mutation. Hormones and behavior. 2008b;53:613–626. doi: 10.1016/j.yhbeh.2008.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]