Summary
Protein kinase B (a.k.a. AKT) and the mechanistic target of rapamycin (mTOR) are central regulators of T cell differentiation, proliferation, metabolism and survival. Here, we show that during chronic murine lymphocytic choriomeningitis virus (LCMV) infection, activation of AKT and mTOR are impaired in antiviral cytotoxic T lymphocytes (CTLs), resulting in enhanced activity of the transcription factor FoxO1. Blockade of inhibitory receptor programmed cell death protein 1 (PD-1) in vivo increased mTOR activity in virus-specific CTLs, and its therapeutic effects were abrogated by the mTOR inhibitor rapamycin. FoxO1 functioned as a transcriptional activator of PD-1 that promoted the differentiation of terminally exhausted CTLs. Importantly, FoxO1 null CTLs failed to persist and control chronic viral infection. Collectively, this study identifies that CTLs adapt to persistent infection through a positive feedback pathway (PD-1→FoxO1→PD-1) that functions to both desensitize virus-specific CTLs to antigen and to support their survival during chronic viral infection.
Introduction
Chronic viral infection is a global health concern contributing to millions of deaths annually (Virgin et al., 2009). Viruses that cause chronic infection have evolved strategies to evade immune responses and the persistence of antigen can lead to alterations in cytotoxic T lymphocyte (CTL) proliferation, survival, effector functions and gene expression that lead to the differentiation of dysfunctional or ‘exhausted’ CTLs (Wherry, 2011). Exhausted CTLs that arise during certain chronic infections and cancers are characterized by impaired production of interferon-γ (IFN-γ), tumor necrosis factor-α (TNF-α) and interleukin-2 (IL-2), reduced cytotoxicity, and elevated surface expression of a number of inhibitory receptors, most notably programmed cell death protein 1 (PD-1) (Baitsch et al., 2011; Barber et al., 2006; Wherry, 2011). Upon interaction with its ligands PD-L1 or PD-L2, PD-1 can inhibit proximal T cell antigen receptor (TCR) signaling and suppress CTL function (Chemnitz et al., 2004; Keir et al., 2008; Mueller et al., 2010; Parry et al., 2005; Wei et al., 2013; Yokosuka et al., 2012; Zinselmeyer et al., 2013). Importantly, the induction of PD-1 and CTL exhaustion during chronic viral infection helps to balance the benefits of anti-viral responses and viral control with the costs of immunopathology to the host (Barber et al., 2006; Frebel et al., 2012; Mueller et al., 2010; Zinselmeyer et al., 2013).
Evidence strongly points to a central role for sustained TCR signaling in fine-tuning the expression of PD-1 and many other genes that affect the function and homeostasis of virus-specific CTLs during chronic viral infection (Kao et al., 2011; Keir et al., 2008; Paley et al., 2012; Riley, 2009; Shin et al., 2007; Shin et al., 2009). However, it is unclear how TCR signaling is integrated with transcriptional changes that regulate these processes in CTLs during chronic infection. The activation of phosphoinositide 3-kinase (PI3K), protein kinase B (also known as AKT), and the mechanistic target of rapamycin (mTOR) either as part of mTOR complex 1, mTORC1, or mTORC2 by T cell, cytokine and co-stimulatory receptors are of particular interest because they function in parallel pathways to control many aspects of T cell differentiation, proliferation, function, and survival (Finlay and Cantrell, 2011; Michalek and Rathmell, 2010; Pearce and Pearce, 2013; Powell and Delgoffe, 2010; Rao et al., 2010). Additionally, activation of PI3K, AKT, and mTOR signaling can induce a metabolic switch towards anabolic metabolism and aerobic glycolysis in activated CD8+ T cells that is transcriptionally coordinated in part by c-myc and hypoxia inducible factor-1 (HIF-1) (Doedens et al., 2013; Finlay et al., 2012; Frauwirth et al., 2002; Jacobs et al., 2008; Macintyre et al., 2011; Wang et al., 2011). PI3K, AKT, and mTOR activation can also enhance T-bet transcription factor expression and the expression of several effector molecules including IFN-γ, and granzyme B (Macintyre et al., 2011; Rao et al., 2010; Tomasoni et al., 2011).
Ligation of the inhibitory receptor PD-1 on the surface of activated CTLs results in enhanced expression and/or recruitment of SHP-1, SHP-2 or PTEN phosphatases that dampens proximal TCR signaling and activation of AKT (Patsoukis et al., 2013; Riley, 2009; Yokosuka et al., 2012; Zinselmeyer et al., 2013). Importantly, blockade of PD-1:PD-L1 interactions promotes the expansion of anti-viral CTLs and improves viral control during viral infection (Barber et al., 2006). These findings have made PD-1 a prime therapeutic target for enhancing T cell responses during certain forms of chronic infection and cancer (Speiser et al., 2014). However, the pertinent signaling pathways that underlie the recovery of T cells responses by PD-1:PD-L1 blockade in vivo are not known; such information could offer important insights into the etiology of CTL exhaustion and may also provide a basis for the development of therapies for treating chronic diseases.
While PI3K, AKT, and mTOR signaling enhances effector CTL differentiation, it suppresses the differentiation and maturation of memory CTLs (Araki et al., 2009; Hand et al.; Kim et al., 2012; Pearce et al., 2009; Rao et al., 2010). This occurs, in part, because AKT phosphorylation inhibits the nuclear activity of FoxO transcription factors, namely FoxO1, which positively regulates several genes involved in naïve and memory T cell survival and trafficking including Il7ra, Ccr7, Klf2, Sell (CD62L), Tcf7, Eomes and Bcl2 (Kerdiles et al., 2009; Kerdiles et al., 2010; Kim et al., 2012; Kim et al., 2013; Michelini et al., 2013; Ouyang et al., 2009; Ouyang et al., 2010; Ouyang et al., 2012; Rao et al., 2012; Sullivan et al., 2012a; Sullivan et al., 2012b; Tejera et al., 2013). Moreover, FoxO1 counterbalances effector CTL differentiation via repression of T-bet, IFN-γ and granzyme B expression (Michelini et al., 2013; Ouyang et al., 2012; Rao et al., 2012) However, the precise roles of FoxO1, AKT, or mTOR signaling in controlling functional exhaustion, metabolism and differentiation of CD8+ T cells during chronic infection has not been explored.
In this study, we identified critical roles for PI3K, AKT, and mTOR signaling and FoxO1 transcriptional activity in the homeostasis and differentiation of CD8+ T cells during chronic lymphocytic choriomeningitis virus (LCMV) infection. We found that sustained antigenic signaling from persisting virus led to a reduction, rather than an enhancement, in the ability of virus-specific CD8+ T cells to transduce TCR signals and activate AKT and mTOR. This suppression of mTOR activation was largely due to PD-1:PD-L1 signaling, and rapamycin abolished the restoration of CTL responses and overall therapeutic efficacy of α-PDL1 blockade. Consequently, suppression of AKT led to increased FoxO1 nuclear activity, which was necessary to sustain PD-1 expression and acquisition of terminally exhausted states during chronic infection. However, FoxO1 was also required for Bcl2 expression and the survival of CTLs during chronic infection. These results suggested that increased FoxO1 activity and expression of PD-1 are important adaptations by virus-specific CTLs during persistent infection that support their homeostasis.
Results
TCR activation of PI3K, AKT, and mTOR and markers of anabolic metabolism are poorly sustained in exhausted CD8+ T cells
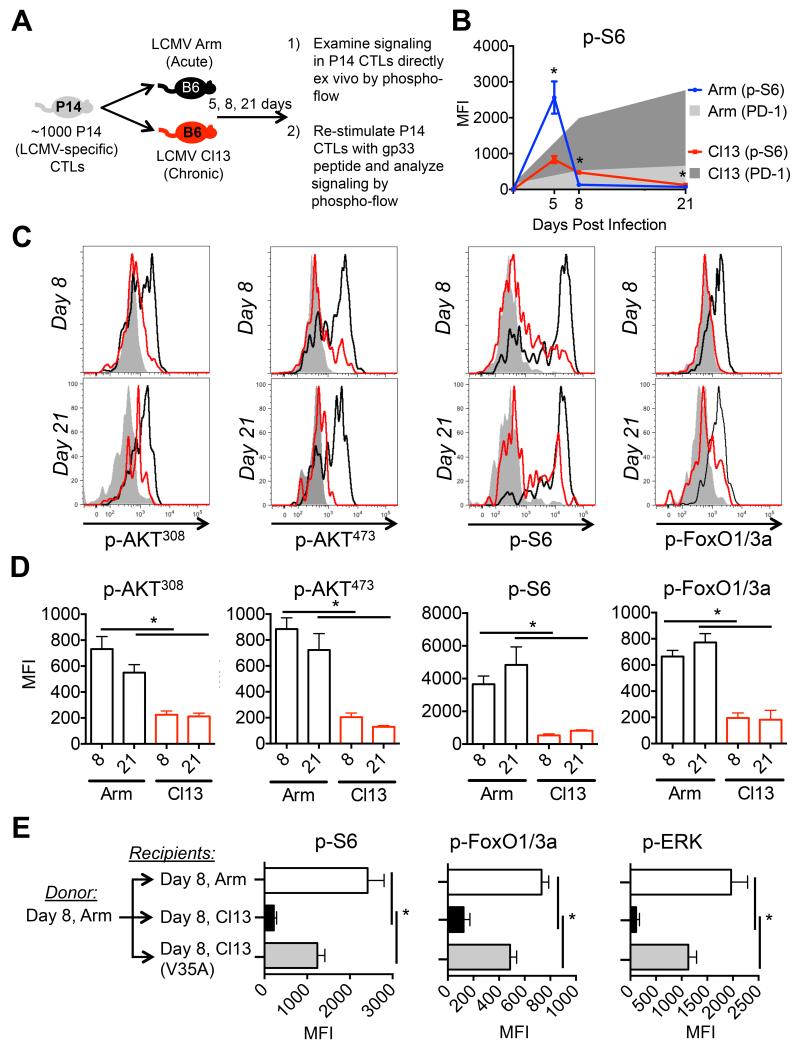
To better understand the regulation of PI3K, AKT, and mTOR signaling in antigen-specific CTLs during acute (LCMV-Arm) and chronic (LCMV-Cl13) viral infection we combined adoptive T cell transfer techniques and phospho-flow cytometry (Figure 1A). While peak phosphorylation of S6, a readout of mTOR activity, (present at day 5 p.i.) was reduced in CTLs in LCMV-Cl13 compared to -Arm infection, p-S6 was not sustained in CTLs during LCMV-Cl13 infection despite the persistence of virus (Figure 1B) (Sullivan et al., 2012b). These data led us to hypothesize that CTLs during chronic infection might be impaired in their ability to sustain the phosphorylation of PI3K, AKT, and mTOR, and that this may contribute to their functional exhaustion.
Figure 1. Persistent antigen suppresses TCR activation of AKT and mTOR signaling in CTLs during chronic infection.
A) Experimental approach used to study TCR and cytokine signaling in LCMV-specific P14 CTLs during LCMV Armstrong (acute) and LCMV-Clone 13 (chronic) infection by phospho-flow. B) p-S6 (line) and PD-1 (shaded) expression in P14 CTLs directly ex vivo 5, 8, and 21 days after LCMV-Arm (blue, light gray-shaded) or LCMV-Cl13 (red, dark gray-shaded) infection. C) Representative histograms of P14 CTLs from day 8 or day 21 after LCMV-Arm (black line) or -Cl13 (red line) infection were stimulated with gp33 peptide for 60 minutes and p-AKT308, p-AKT473, p-S6 and p-FoxO1/3a were measured by phospho-flow. Shaded histograms are un-stimulated CTLs from LCMV-Cl13 infection. D) Cumulative bar graphs showing the MFI of phospho-flow data from C). E) P14 CTLs from day 8 LCMV-Arm infection were transferred into infection-matched LCMV-Arm (white), -Cl13 (black), or -Cl13(V35A) (gray) recipients. At day 15 (7 days post transfer) P14+ CTLs were stimulated with gp33 peptide for 60 minutes and p-S6, p-FoxO1/3a, and p-ERK were measured by phospho-flow. Data are representative of three independent experiments that included 3-5 mice/group. MFI, mean fluorescence intensity. See also Supplementary Figure 1.
To address this possibility, we directly compared the TCR signaling capacity between two populations of gp33-41-specific TCR transgenic (P14) CTLs isolated from LCMV-Arm or -Cl13 infection. By 8 days post infection (p.i.), CTLs from LCMV-Cl13 infection demonstrated a loss in TCR responsiveness compared to those from LCMV-Arm infection as measured by reduced phosphorylation of both proximal (e.g., Zap-70(319)) and distal signaling molecules, such as ERK(202/204), AKT(308) and AKT(473), m-TOR(2448), and their downstream targets S6(235/236) and FoxO1(24) and Foxo3a(32) (referred to as FoxO1/3a) (Figure 1C-D, Supp. Figure 1A-B; Supp. Figure 1C, includes specificity controls for p-S6 and p-FoxO1/3a by PI3K, AKT, and mTOR). Importantly, the defect in TCR signaling was sustained at later time points (d21 p.i.) and could not be solely explained by decreased expression of the TCR itself or rescued by CD28 co-stimulation (data not shown). However, phorbol ester or phosphatase inhibitor treatment of day 8 CTLs from LCMV-Cl13 infection could at least partially restore phosphorylation of p-Zap70, p-S6, and p-ERK, suggesting active inhibition of TCR signaling (data not shown). Taken together, our data point to a proximal signaling defect in exhausted CTLs that results in dampened phosphorylation of S6, AKT and FoxO1/3a) (Powell et al., 2012).
Next, to determine if the reduction in PI3K, AKT, and mTOR kinase activity in CTLs during chronic infection was a general feature associated with CTL exhaustion, we examined the ability of CTLs from acute and chronic viral infection to respond to cytokine stimulation. Although we found that ability of CTLs from LCMV-Cl13 infected mice to phosphorylate STAT5 and STAT3 transcription factors downstream of IL-2, IL-15, and IL-21, respectively, was not significantly impaired relative to CTLs from LCMV-Arm infected mice, their ability to activate the AKT and mTOR was (Supp. Figures 1D-F). Taken together, these results demonstrate that the progressive loss of effector functions that occurs in CTLs during chronic LCMV infection (Wherry, 2011) correlates with their impaired ability to activate the PI3K, AKT, and mTOR signaling pathways.
Chronic antigen stimulation contributes to many of the phenotypic and molecular changes that define exhausted CTLs during chronic infection (Angelosanto et al., 2012; Brooks et al., 2006; Kao et al., 2011; Paley et al., 2012; Shin et al., 2007). Therefore, to directly test if persistent antigen contributed to TCR desensitization in virus-specific CD8+ T cells during chronic viral infection, we varied the duration of antigen exposure by transferring functional P14 CTLs from acute LCMV-Arm infected animals into recipients infected 8 days previously with either 1) LCMV-Arm, 2) LCMV-Cl13, or 3) LCMV-Cl13-V35A, a mutant strain of LCMV-Cl13 that contains an amino acid substitution in the gp33 epitope and is not recognized by the P14 TCR (Puglielli et al., 2001). Importantly, infection with Cl13-V35A resulted in comparable viremia and elevated PD-1 expression on gp276- and 396-specific CTLs (data not shown) (Shin et al., 2007). Indeed, TCR-dependent phosphorylation of S6, FoxO1/3a, and ERK was elevated in P14 CTLs engrafted into LCMV-Arm or LCMV Cl13-V35A as compared to LCMV Cl13 infected mice (Figure 1E). These findings demonstrate that prolonged contact with antigen underlies the loss in TCR responsiveness and downstream signaling in CTLs during chronic infection.
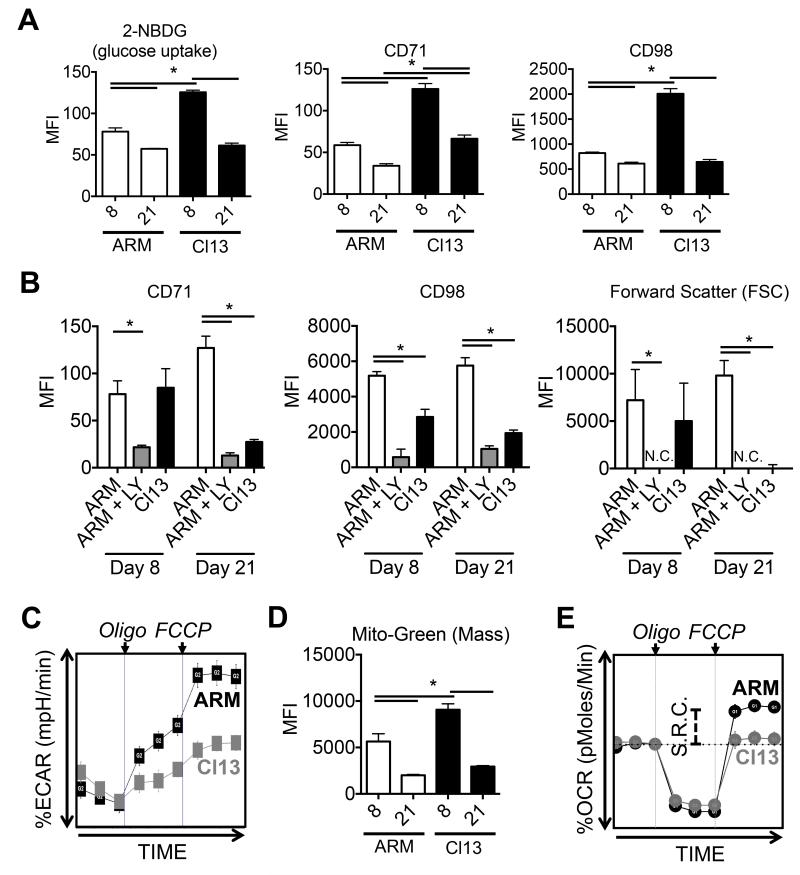
Consistent with their inability to sustain robust mTOR activity (Figure 1B), at day 21 p.i., virus-specific CTLs from chronic LCMV-Cl13 infection were unable to maintain properties of anabolic metabolism such as robust glucose uptake (2-NBDG) and expression of CD71 (transferrin receptor) and CD98 (amino acid transporter) (Figure 2A) (Finlay and Cantrell, 2011; Finlay et al., 2009). Furthermore, while CTLs from acute infection were able to up-regulate CD71, CD98 and initiate blastogenesis in response to TCR re-stimulation in a PI3K, AKT, and mTOR dependent manner, CTLs from LCMV-Cl13 failed to do so (Figure 2B, Supp. Figure 2A). Although glucose uptake was somewhat higher at day 8 in CTLs from chronic relative to acute infection, their baseline extracellular acidification rates (ECAR, a measure of glycolysis) were similar (Supp. Figure 2B). Additionally, CTLs from chronic infection were less efficient in their ability to engage glycolysis when mitochondrial ATP synthesis was blocked by oligomycin (Figure 2C) (van der Windt et al., 2013). Similarly, at day 8 p.i., despite an increase in mitochondrial mass in CTLs from chronic infection (Figure 2D), their baseline oxygen consumption rates (OCR, a measure of mitochondrial oxidative phosphorylation) were comparable to CTLs from acute infection (Supp. Figure 2B). Moreover, when CTLs from chronic infection were treated with the mitochondrial uncoupling reagent, FCCP, their maximal mitochondrial respiration (i.e., spare respiratory capacity, SRC) was reduced compared to those from acute infection (Figure 2E) (van der Windt et al., 2013). Collectively, these data suggest that exhausted CTLs display deficiencies in both glycolytic and oxidative metabolism, which further underscore the decline in TCR-dependent PI3K signaling in virus-specific CTLs during chronic LCMV-Cl13 infection.
Figure 2. An anabolic metabolism is poorly sustained in exhausted CTLs during chronic infection.
A) 2-NDBG staining, and CD71 and CD98 expression in P14 CTLs at day 8 or 21 after LCMV-Arm or -Cl13 infection were measured directly ex vivo using flow cytometry. B) P14 CTLs at day 8 or 21 after from LCMV-Arm or -Cl13 infection were stimulated with gp33 peptide +/− 25nM LY294002 (PI3K inhibitor) for 16-24hrs and CD71 and CD98 expression and forward scatter (FSC) was measured using flow cytometry. C) Seahorse extracellular flux analysis showing the extracellular acidification rate (ECAR) of purified P14 CTLs at day 8 after LCMV-Arm or -Cl13 infection (normalized to baseline) after the addition of oligomycin (ATPase inhibitor) and FCCP (mitochondrial uncoupling agent). D) Mitochondrial green (mass) staining in P14 CTLs using flow cytometry as in A). E) Seahorse extracellular flux analysis showing the oxygen consumption rate (OCR) of purified P14 CTLs at day 8 after LCMV-Arm or -Cl13 infection (normalized to baseline) after the addition of oligomycin (ATPase inhibitor) and FCCP (mitochondrial uncoupling agent). Spare respiratory capacity (S.R.C.) is indicated as the difference between baseline OCR and after the addition of FCCP. Data are representative of three independent experiments that included 3-5 mice/group. MFI, mean fluorescence intensity. See also Supplementary Figure 2.
Therapeutic PD-L1 blockade requires mTOR activation
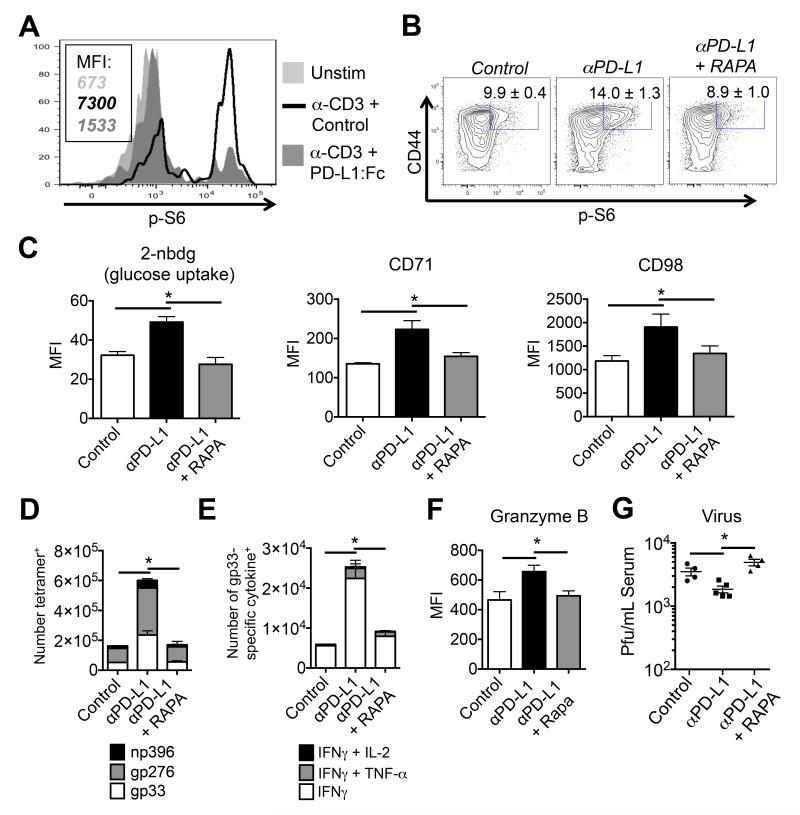
PD-1 is markedly up-regulated on exhausted CTLs and can inhibit proximal TCR signaling and distal AKT, mTOR, S6, and ERK phosphorylation (Francisco et al., 2009; Parry et al., 2005; Sheppard et al., 2004; Yokosuka et al., 2012). Moreover, blocking PD-1:PD-L1 interactions can restore TCR signaling (Fife et al., 2009; Zinselmeyer et al., 2013) and boost antiviral T cell responses and viral control during LCMV-Cl13 infection (Barber et al., 2006). First, we confirmed the ability of PD-1 to suppress mTORC1 activity (based on p-S6 staining) in virus-specific CTLs following TCR stimulation (Figure 3A). Next, to examine if PD-1 suppression of mTOR activity was relevant in antiviral CTLs in vivo, LCMV-Cl13 infected mice were treated with a blocking α-PD-L1 mAb, either with or without the mTORC inhibitor rapamycin. Anti-PD-L1 mAb treatment augmented the amounts of p-S6235/236, CD98, CD71 and glucose uptake in CTLs (Figures 3B-C) (Finlay et al., 2009). The increase in mTOR activity was accompanied by a marked increase in the frequency and number of IFN-γ- and granzyme B (GzmB)-producing virus-specific CTLs and decrease in viral load (Figures 3D-G). Importantly, rapamycin abrogated the beneficial effects of α-PD-L1 blockade on viral control and virus-specific T cell responses, including the aforementioned markers of anabolic metabolism (Figures 3B-F). Taken together, these data demonstrate that PD-1 suppression of the mTOR pathway contributes to CTL exhaustion in vivo and that recovery of mTOR activity is a part of the therapeutic effects of α-PD-L1 during chronic LCMV-Cl13 infection.
Figure 3. PD-1 suppresses mTOR signaling and markers associated with anabolic metabolism in exhausted CTLs during chronic infection.
A) Histograms show amounts of phosphorylated S6 (from day 8 post LCMV-Arm infection) 60 minutes after anti-CD3 (3ug/ml) +/− PD-L1:Fc (red line) or control IgG (20ug/ml) (black line). Shaded histograms are un-stimulated CTLs. MFI’s are indicated. B-F) LCMV-Cl13 infected mice were PBS treated (white-shaded) or treated at day 28 p.i. with α-PD-L1 blocking mAb (200ug/mouse) (black-shaded) alone or in combination with rapamycin (100ug/kg) (red-shaded) for 7 days and then analyzed for: (B) the percentage of p-S6+ CD8+ CD44hi T cells, (C) intracellular staining using 2-NDBG, and surface expression of CD71 and CD98, and forward-scatter (FSC) in gp33 tetramer+ CTLs. D) Stacked bar graphs showing the number of tetramer-positive CTLs (gp33, gp276, and np396), and (E) the number of gp33-specific cytokine producing CTLs. F) The expression of granzyme B in gp33-specific CTLs as in B). G) Viral titers in the serum as determined by plaque assay. Data are representative of three independent experiments that included 3-5 mice/group. MFI, mean fluorescence intensity.
Expression and nuclear retention of the transcription factor, FoxO1, is enhanced in exhausted CD8+ T cells
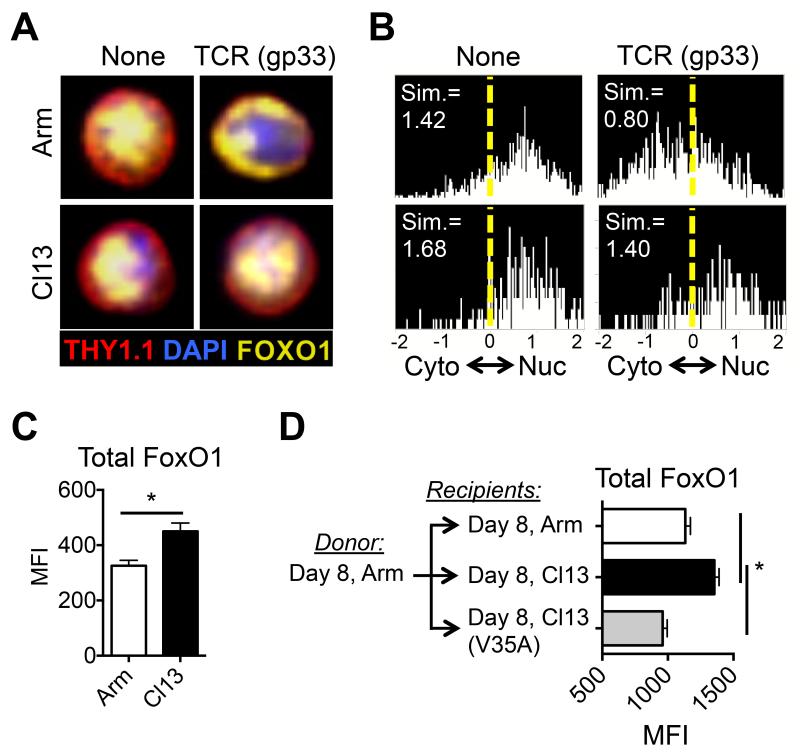
FoxO1 supports the differentiation of regulatory T cells, suppresses effector functions and promotes the differentiation and survival of long-lived memory CD8+ T cells (Hedrick et al., 2012; Kim et al., 2013; Michelini et al., 2013; Ouyang et al., 2012; Tejera et al., 2013). FoxO transcription factors are phosphorylated and inhibited by AKT in response to mTORC2 activation (Biggs et al., 1999; Brunet et al., 1999; Calnan and Brunet, 2008; Rao et al., 2012). Thus, we questioned if the suppression of AKT signaling in CTLs during chronic viral infection results in enhanced FoxO1 activity. Consistent with decreased p-FoxO1/3a following TCR stimulation (Figure 1, Supp. Fig. 1C), CTLs from LCMV-Cl13 infection displayed enhanced nuclear retention of FoxO1 after TCR stimulation (Figures 4A-B) and contained elevated amounts of FoxO1 protein (Figure 4C) compared to those from acute LCMV-Arm infection. Transfer of P14 cells from LCMV-Arm infected animals into recipients infected 8 days previously with LCMV-Arm, -Cl13, or -Cl13-V35A confirmed that FoxO1 expression was up-regulated in an antigen-dependent manner during chronic viral infection (Figure 4D). Furthermore, PD-1 ligation on the surface of effector CTLs (as in Figure 3A) could also effectively suppress the phosphorylation of FoxO1/3a in response to TCR stimulation (data not shown). These data suggest that persistent antigenic stimulation and PD-1 signaling dampens TCR-dependent activation of AKT and mTORC2, leading to enhanced nuclear accumulation of FoxO1 in antiviral CTLs during chronic infection.
Figure 4. Chronic antigen promotes the expression and nuclear retention of FoxO1 in CD8+ T cells during chronic infection.
A) Representative image of P14 CTLs from day 8 after LCMV-Arm or -Cl13 infection were stimulated with gp33 peptide for 60 minutes and nuclear versus cytoplasmic FoxO1 was determined using the Amnis ImagestreamX (Amnis, Seattle, WA). B) Histograms showing the cumulative similarity score (Sim) of FoxO1 and DAPI staining to measure nuclear localization as in A). C) Total FoxO1 expression in gp33-specific CTLs was analyzed by intracellular staining at day 21 after LCMV-Arm and LCMV-Cl13 infection. D) P14 CTLs from day 8 LCMV-Arm infection were transferred into infection-matched LCMV-Arm, -Cl13, or -Cl13(V35A) recipients. At day 15 (7 days post transfer) P14+ CTLs were analyzed for total FoxO1 expression by intracellular staining. Data are representative of three independent experiments that included 3-5 mice/group. MFI, mean fluorescence intensity.
FoxO1 regulates the homeostasis of virus-specific CD8+ T cells during chronic viral infection
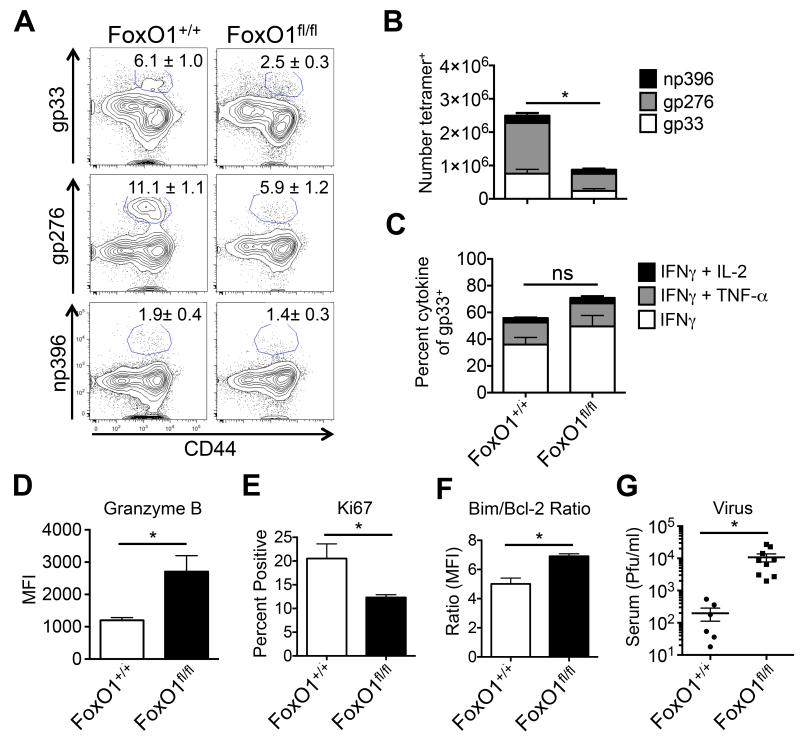
To determine if FoxO1 played a role in CTL exhaustion during chronic infection, we conditionally deleted Foxo1 from virus-specific CD8+ T cells during LCMV-Cl13 infection using Foxo1fl/fl Gzmb-cre+ mice (referred to as FoxO1fl/fl). Deletion of FoxO1 impaired virus-specific CTL responses such that their frequency and number were reduced by nearly 50% at day 21 p.i. compared to wild type (WT) Foxo1+/+ Gzmb-cre+ littermate controls (referred to as FoxO1+/+) (Figures 5A, 5B, Supp. Figure 3A). Cytokine production by FoxO1fl/fl CTLs was only modestly affected, but the expression of Gzmb, a FoxO1-repressed target gene (Macintyre et al., 2011; Michelini et al., 2013; Rao et al., 2012; Tejera et al., 2013), was markedly increased (Figures 5C-D, Supp. Figure 3B). Despite this, viral loads remained significantly higher in FoxO1fl/fl relative to FoxO1+/+ mice, likely owing to a combination of decreased proliferation and survival of LCMV-specific CTLs in the absence of FoxO1 as suggested by Ki67, and the ratio of Bim to Bcl-2 staining, respectively (Figures 5E-G, Supp. Figure 3C) (Kim et al., 2013; Tejera et al., 2013). Taken together, these data propose an intriguing model wherein the suppression of mTOR and AKT, and resulting increase in FoxO1 activity, dampens effector molecule expression, but simultaneously promotes virus-specific CTL survival during chronic infection.
Figure 5. FoxO1 sustains virus-specific CD8+ T cell responses during chronic viral infection.
Foxo1+/+ Gzmb-cre+ (FoxO1+/+) and Foxo1fl/fl Gzmb-cre+ (FoxO1fl/fl) mice were infected with LCMV-Cl13 and at day 21 p.i. the frequency (A) and number (B) of tetramer+ CTLs (gp33, gp276, and np396) was determined in the spleen by flow cytometry. C) FoxO1+/+ and FoxO1fl/fl CTLs as in D) were re-stimulated with gp33 peptide in the presence of brefeldin A and cytokine production was measured by flow cytometry. D-G) FoxO1+/+ and FoxO1fl/fl gp33-tetramer+ CTLs as in A) were examined for expression of (D) granzyme B, (E) Ki67, (F) Bim:Bcl-2 ratio by flow cytometry, and G) serum viral titers were determined by plaque assay. Data are cumulative from four independent experiments (FoxO1+/+, n = 10-13; FoxO1fl/fl, n = 10-17). MFI, mean fluorescence intensity. See also Supplementary Figure 3.
FoxO1 is necessary for the differentiation of PD-1hi Eomeshi terminally exhausted CTLs
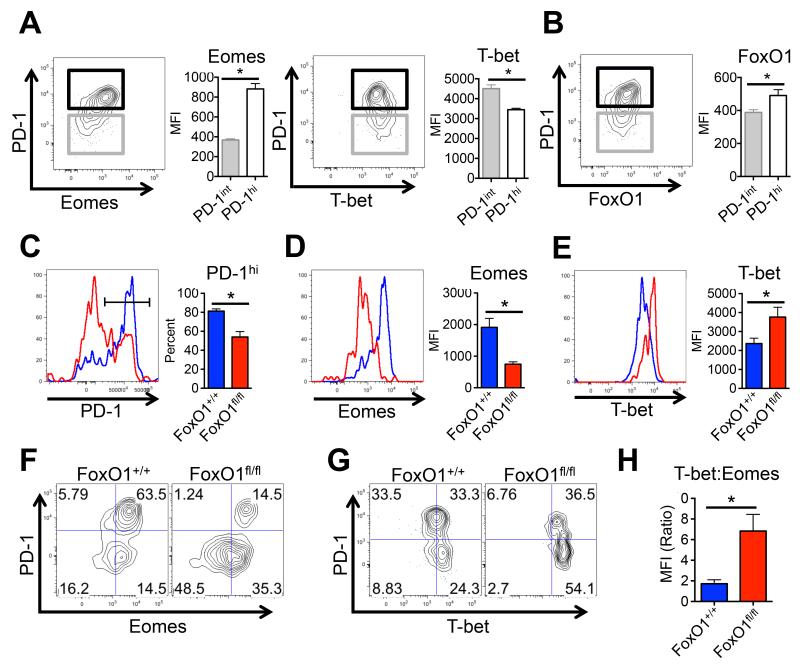
During chronic LCMV infection, the CTL pool is maintained by periodic proliferation and conversion of PD-1int T-bethi → PD-1hi Eomeshi cells (Kao et al., 2011; Paley et al., 2012). Together these subsets cooperate to maintain a durable and partially effective CD8+ T cell response during chronic infection (Paley et al., 2012). Upon further inspection, we found FoxO1 expression was increased in PD1hi CTLs that also expressed higher amounts of Eomes, but lower amounts of T-bet (Figures 6A-B) (Paley et al., 2012). We posited that FoxO1 may regulate the progression and balance between PD-1int T-bethi and PD-1hi Eomeshi CD8+ T cell subsets during chronic infection because it can promote Eomes and suppress T-bet expression in acutely stimulated CD8+ T cells (Michelini et al., 2013; Rao et al., 2012; Rao et al., 2010). Consistent with this model, at day 21 p.i., the expression of PD-1 and Eomes and frequency of PD-1hi Eomeshi CTLs were significantly reduced in the absence of FoxO1, while the proportion of PD-1lo CTL that expressed T-bet were markedly increased (Figure 6C-E, data not shown). Adoptive transfer of FoxO1fl/fl P14 CTL into wild type mice infected with LCMV-Cl13 validated the cell autonomous role for FoxO1 in regulating the formation of PD-1hi Eomeshi CTLs (Supp. Figure 4A). The phenotypes of the FoxO1fl/fl CTLs were more modest at day 8 p.i. compared to day 21 p.i.; for example, Eomes was lower than that of the FoxO1+/+ CTLs, but no significant differences in PD-1 and T-bet expression were observed (Supp. Figure 4B). These data suggest that the dependency on FoxO1 for elevating Eomes, Bcl-2 and PD-1 and repressing T-bet expression increases as chronic infection progresses and likely, the improper regulation of these genes impairs CTL maintenance over time (Figures 6F-H) (Kao et al., 2011; Paley et al., 2012; Rao et al., 2012).
Figure 6. FoxO1 regulates the differentiation of PD-1hiEomeshi and PD-1loTbethi populations.
A) Representative dot plots and, right, cumulative bar graphs showing Eomes, T-bet, and (B) FoxO1 expression in PD-1hi versus PD-1int P14 CTLs at day 21 after LCMV-Cl13 infection as determined by flow cytometry (n = 7 mice/group). C-E) Left, Representative histogram overlays and, right, cumulative bar graphs showing (C) the frequency of PD-1hi CTLs and the MFI of (D) Eomes and (E) T-bet in FoxO1+/+ (blue) and FoxO1fl/fl (red) gp33 tetramer+ CTLs at day 21 after LCMV-Cl13 infection as determined by flow cytometry. F) Representative dot plots of FoxO1+/+ and FoxO1fl/fl gp33-tetramer+ CTLs showing PD-1 versus Eomes, or (G) PD-1 versus T-bet expression at day 21 after LCMV-Cl13 infection. H) The ratio of T-bet to Eomes expression in FoxO1+/+ and FoxO1fl/fl gp33-tetramer+ CTLs at day 21 after LCMV-Cl13 infection is shown. Data are cumulative from four independent experiments (FoxO1+/+, n = 10-13; FoxO1fl/fl, n = 10-17). MFI, mean fluorescence intensity.
FoxO1 binds to and promotes the expression of PD-1 in CD8+ T cells
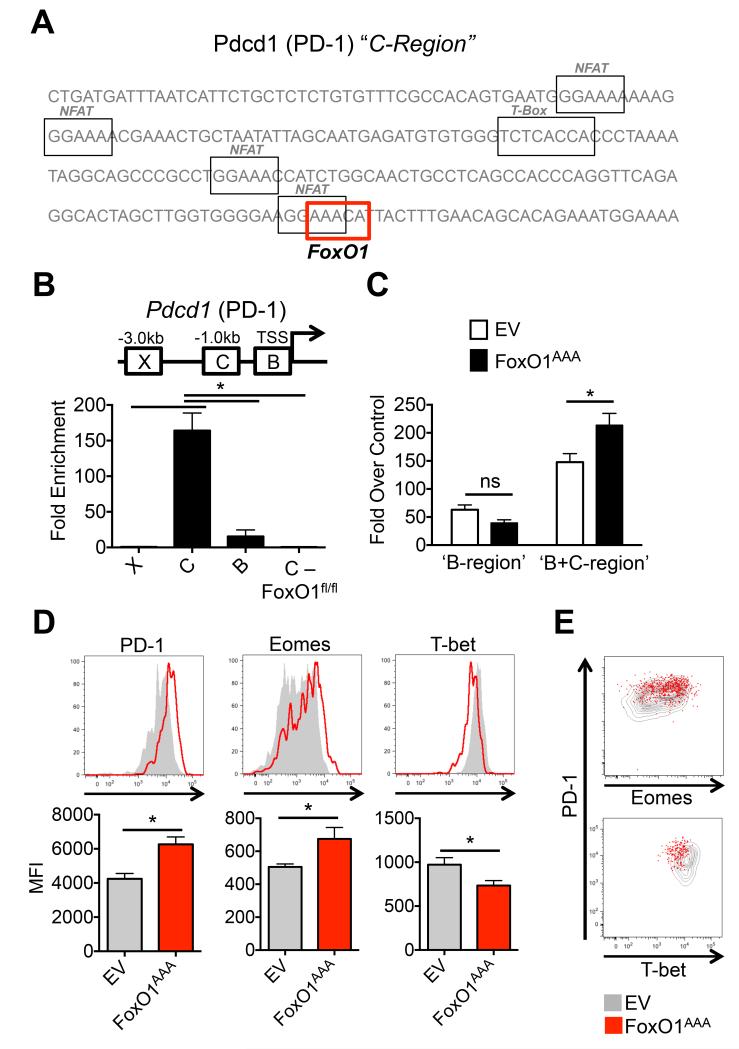
Based on the decreased PD-1 expression in FoxO1fl/fl CD8+ T cells, we hypothesized that, in addition to Eomes (Rao et al., 2012), FoxO1 may also directly control PD-1 (gene symbol Pdcd1) transcription as CTLs transition from PD-1int to PD-1hi states during chronic LCMV infection. Examination of this possibility revealed that the upstream regulatory region of Pdcd1, named the PD-1 ‘C-region’ that is trans-activated by the transcription factor NFATc1 (Oestreich et al., 2008), also contains a putative FoxO1 binding site (Ouyang et al., 2012) (Figure 7A). The ‘C-region’ also contains a T-bet binding site and neighboring Blimp-1 binding site, and overexpression of T-bet or Blimp-1 can suppress PD-1 expression (Kao et al., 2011; Lu et al., 2014). Using chromatin immunoprecipitation (ChIP), we observed FoxO1 binding to the ‘C-region’, but not the neighboring ‘B-region’ in Pdcd1 in in vitro activated CD8+ T cells (Figure 7B). Furthermore, overexpression of FoxO1 was sufficient to enhance the activity of a Pdcd1 promoter luciferase reporter in Jurkat cells when both ‘B-’ and ‘C-regions’ were intact, but not when only the ‘B-region’ was present (Figure 7C). In vitro, PD-1 expression in CD8+ T cells could be enhanced via treatments that increase FoxO1 activity such as inhibition of PI3K, AKT, or mTOR, or through inhibition of glycolysis with 2-deoxy-D-glucose (2-DG) (data not shown) (Chang et al., 2013). To test the role of FoxO1 more directly, we overexpressed an AKT-mediated phosphorylation-resistant mutant of FoxO1, FoxO1AAA, in CTLs in vitro or in vivo during chronic LCMV-Cl13 infection. In vitro, constitutively active FoxO1AAA was able to up-regulate PD-1 expression, an effect that was enhanced further by antigenic re-stimulation (data not shown). In vivo, FoxO1AAA was sufficient to augment the expression of PD-1, Eomes and Bcl-2 while suppressing that of T-bet, effectively shifting the balance towards the differentiation of PD-1hiEomeshi CTLs (Figures 7D-E, data not shown) (Paley et al., 2012). Thus, FoxO1 appears to directly trans-activate the Pdcd1 locus and promote the formation of more terminally exhausted PD-1hi Eomeshi CD8+ T cells during chronic viral infection.
Figure 7. FoxO1 directly regulates PD-1 expression in CD8+ T cells, and promotes the differentiation of PD-1hiEomeshi CD8+ T cells during chronic infection.
A) Outline of putative NFAT, FoxO1, and T-box consensus binding motifs in the ‘C-region’ of the Pdcd1 locus (Oestreich et al., 2008). B) Chromatin immunoprecipitation (ChIP) analysis of FoxO1 binding to the ‘B-’ and ‘C-regions’ (Oestreich et al., 2008) of the Pdcd1 promoter in day 3 in vitro activated P14 CTLs from FoxO1+/+ and FoxO1fl/fl mice. Data are pooled from 3 independent experiments. C) Luciferase reporter assays for Pdcd1 promoter activity in in Jurkat T cells after overnight stimulation with PMA plus ionomycin transfected with MigR1 empty vector (EV) or a constitutively active FoxO1 (FoxO1AAA). Data are pooled from two independent experiments. D) Top, representative histograms and, bottom, cumulative bar graphs showing PD-1, Eomes and T-bet expression in P14+ CTLs retrovirally transduced with EV (gray) or FoxO1AAA (GFP+) (red) and examined at day 15 post LCMV-Cl13 infection. Data are pooled from 3-3 independent experiments (n = 6-9; each group). E) Representative dot plots of P14+ CTLs retrovirally transduced with EV (gray) or FoxO1AAA (red) showing, top, PD-1 versus Eomes or, bottom, PD-1 versus T-bet expression as in D). MFI, mean fluorescence intensity.
Discussion
This study identifies the relevance of fine-tuning of the PI3K, AKT, and mTOR signaling pathways in regulating the differentiation and function of virus-specific CD8+ T cells during chronic viral infection. Our work has characterized the decline in TCR signaling in exhausted CTLs that results in reduced mTORC1 and mTORC2 activity and inability to sustain high rates of aerobic glycolysis and anabolic metabolism. Reactivation of mTOR was necessary to bolster antiviral CTL proliferation and effector functions during PD-L1 blockade therapy, thereby placing mTOR on a central axis controlling CTL exhaustion. Importantly, we identified FoxO1 as a critical rheostat downstream of mTORC2 that controls the expression of PD-1 and Bcl-2, and the balance between Eomeshi and T-bethi CTL populations to maintain the overall homeostasis of virus-specific CTLs during chronic viral infection. These data support the notion that suppression of AKT and mTOR and augmentation of PD-1 expression via increased FoxO1 is both a normal and necessary part of the progression of CTL exhaustion that serves not only to prevent excessive immunopathology, but also to sustain virus-specific CTLs during persistent antigenic stimulation.
Our study, along with that of Sullivan et al. (2012), also brings to light the non-redundant and apparent contrasting roles of FoxO1 and FoxO3a in regulating CTL responses during chronic infection. Although the phospho-flow analysis was unable to distinguish between phosphorylated FoxO1 and FoxO3a, other data using FoxO1-specific antibodies clearly demonstrated the enhanced expression and nuclear retention of FoxO1 protein in PD-1hi CTLs; the specific phosphorylation state and nuclear localization of FoxO3a remains to be investigated. Additionally, whereas FoxO1 was necessary to maintain Bcl-2 expression and CTL survival, FoxO3a promoted Bim expression and CTL apoptosis during chronic LCMV infection (Grayson et al., 2006; Sullivan et al., 2012b). Furthermore, FoxO3a does not appear share the same role as FoxO1 in promoting the differentiation of more terminally exhausted PD-1hi Eomeshi CTLs (Sullivan et al., 2012b). These findings suggest that FoxO1 and FoxO3a can regulate both overlapping and divergent aspects of CD8+ T cell biology, an idea that is further supported by pathway analysis of predicted FoxO1 and FoxO3a target genes in CTLs following acute and chronic viral infection (data not shown). Additionally, this work highlights important dichotomies in the transcriptional targets of FoxO1 in CD8+ T cells between acute and chronic infection. For example, FoxO1 controls expression of Il7r, Ccr7,Sell (CD62L), and Tcf7 in long-lived memory CTLs (Kim et al., 2013; Michelini et al., 2013; Rao et al., 2012; Tejera et al., 2013) that are down-regulated in exhausted CTLs during chronic infection. Similar observations have been made with regard to the transcriptional targets of T-bet and Eomes in CTLs from acute versus chronic LCMV infection (Doering et al., 2012). These data underscore the potential role for distinct epigenetic changes and/or unique transcriptional networks in regulating how these and other transcription factors may have altered functions in the context of a resolving versus a persistent infection. In the future, it will be important to determine the transcriptional program and target genes regulated by FoxO1 and FoxO3a and how and why these may vary in the different settings of acute and chronic infection.
Importantly, this study outlines a positive feedback pathway regulating the differentiation and homeostasis of exhausted CTLs during chronic infection, wherein decaying TCR responsiveness, mediated in part by PD-1, lowered PI3K, AKT, and mTOR signaling. HIF-1 is an important downstream target of mTORC1 and thus, our work provides greater explanation for why constitutive HIF-1 activity would sustain aerobic glycolysis and effector functions in CTLs during chronic LCMV infection (Doedens et al., 2013). Further, our work identifies that decreased mTORC2 activity consequently led to elevated FoxO1 activity, which repressed GzmB, but sustained PD-1, Eomes and Bcl2 expression. Thus, FoxO1 aides in the formation and maintenance of more terminally exhausted CD8+ T cells. Identification of FoxO1 as a transcriptional activator of Pdcd1 (PD-1) is an important finding as little is known about the regulation of this locus. Although our data show that FoxO1 can directly bind to a known regulatory element of Pdcd1 and FoxO1AAA can promote the differentiation of PD-1hi CTLs during chronic LCMV infection, it is likely that the drop in PD-1 expression in FoxO1fl/fl CTLs depends in part on increased T-bet expression because T-bet can repress Pdcd1 transcription (Kao et al., 2011; Lu et al., 2014; Michelini et al., 2013; Paley et al., 2012). Although, FoxO1 has not yet been found to bind to the Tbx21 (T-bet) locus directly (Rao et al., 2012), T-bet expression and activity are, nevertheless, markedly increased in the absence of FoxO1 (Michelini et al., 2013; Rao et al., 2012; Tejera et al., 2013). During chronic infection, it is plausible that FoxO1 and T-bet inhibit each others binding in the C region of the Pdcd1 locus thereby imposing divergent effects on PD-1 expression and CTL exhaustion. Alternatively, given the density of NFAT binding sites in the Pdcd1 locus, FoxO1 may cooperate with NFAT to regulate and/or sustain the expression of PD-1 in the presence of persistent antigen during chronic infection (Lu et al., 2014; Oestreich et al., 2008). This idea is similar to the role proposed for NFAT:FoxO1 complexes that coordinate the expression and activity of FoxP3 during CD4+ regulatory T cell differentiation (Samstein et al., 2012). We favor a model in which NFAT activity initiates PD-1 expression, while FoxO1 may help to sustain its expression directly and indirectly through its ability to regulate or compete with T-bet. Together, these data outline a unique transcription factor network that may regulate the expression of PD-1 and the differentiation of exhausted CTLs (Doering et al., 2012).
The FoxO family of transcription factors have a plethora of binding partners and can undergo numerous post-translational modifications (Calnan and Brunet, 2008). In response to nutrient deprivation, phosphorylation of FoxO transcription factors by AMPK on residues distinct from that of AKT redirects its transcriptional activity to different targets (Greer and Brunet, 2005; Greer et al., 2007). Additionally, acetylation of FoxO1 by CBP and its deacetylation by Sirt1 can also regulate its activity and specificity, the latter being suggestive of FoxO1’s role in regulating mitochondrial function and lipid oxidation (Gross et al., 2008). Indeed, future proteomic studies of FoxO1 may help to decipher how its activity may be differentially regulated between acute and chronic infection and the types of co-factors it interacts with in T cells. Additionally, our study has provided proof-of-concept that manipulation of FoxO1, and possibly other members of the PI3K, AKT, and mTOR pathway can regulate the expression and function of PD-1 in exhausted CTLs that could lead to therapeutic options for fighting chronic viral infection or cancer. However, our data would argue that fine-tuning of FoxO1 activities and/or temporal regulation thereof, as opposed to their complete blockade, may serve as more rationale therapeutic design.
Experimental Procedures
Mice and LCMV-Infections
C57BL/6j mice from NCI, and Thy-1.1+ P14 TCR transgenic mice (Pircher et al., 1989) that recognize the H-2Db gp33 epitope were used where indicated. FoxO1-floxed mice (Ouyang et al., 2009) were bred in house onto Gzmb-cre or P14 Gzmb-cre mice (Cui et al., 2011). Mice were intraperitoneally (i.p.) infected with 2×105 plaque forming units (p.f.u.) LCMV Armstrong (Arm) that causes an acute infection or intravenously (i.v.) with 2×106 p.f.u. LCMV Clone 13 (Cl13) that causes a chronic infection. Viral titers were determined using plaque assays as previously described (Cui et al., 2011). The use of all animals was conducted in accordance with Yale University IACUC guidelines.
Flow cytometry, Amnis, and phospho-flow
Cells were surface or intracellular stained using commercially available antibodies and kits from eBioscience or BDBiosciences. Importantly, for all signaling experiments, cells were rested for a period of 2-4 hours at 37°C in 1% RPMI medium to allow for any signals to return to background prior to peptide, α-CD3, or cytokine re-stimulation. Intracellular FoxO1 and phosphorylated (p) p-AKT308, p-AKT473, p-S6235/236, and p-FoxO1/3a24/32, p-Zap70318/p-Syk352, and p-ERK202/204 were detected using paraformaldehyde fixation and methanol permeabilization and antibodies from Cell Signaling. Primary un-conjugated antibodies used in phospho-flow and total FoxO1 staining were detected using secondary staining with anti-rabbit IgG 647 antibody (Molecular Probes). Nuclear FoxO1 localization was performed using Amnis Imagestream and analyzed using Imagestream software. 2-nbdg, mitochondrial green and mitochondrial deep-red staining (Invitrogen) was performed by incubating splenocytes in normal or glucose-free (for 2-nbdg staining) RPMI media at 37°C for 10 minutes, washed, stained for surface markers, and immediately analyzed using using flow cytometry.
Chromatin Immunoprecipitation (ChIP)
Briefly, ~10×106 cells were crosslinked with 1% formaldehyde and quenched with 0.125M glycine as previously described (Ouyang et al., 2012). Nuclei were isolated using 0.1% Triton buffer, and sonicated in SDS lysis buffer using to ~300-500bp size. 5ug of antibody was complexed overnight to anti-rabbit magnetic beads (Invitrogen), and 100ug of chromatin was used per immunoprecipitation (IP) reaction. FoxO1 ChIP antibody (ab39670) was obtained from Abcam. Control rabbit-IgG was obtained from Santa Cruz Biotechnology. Samples were washed with low salt, high salt, LiCl, and TE buffers, eluted with SDS and reverse crosslinked overnight, followed by proteinase K digestion, and DNA purification. Samples were then subjected to quantitative PCR using published primer sets (Oestreich et al., 2008). A region outside of the promoter region ‘X-region’ was used as a negative control (forward, 5′ CAGTATGCAGCTCCTGTCTCC 3′; reverse, 5′ ACACCATGACCAAACCCAAG 3′), and FoxO1 KO P14 CTLs were used to control for antibody-IP specificity. Fold enrichment was calculated over rabbit IgG control.
Retroviral transduction
P14 TCR transgenic mice were superinfected i.v. with 2×106 p.f.u. of LCMV-Arm. Twenty four hours later P14 T cells were spin-transduced with MigR1-EV GFP or MigR1-FoxO1AAA GFP retrovirus (a kind gift from Dr. Terry Unterman, University of Illinois at Chicago) and a small number (1-5×103) cells were adoptively transferred into LCMV-Cl13 infected recipients(Hand et al., 2010; Kao et al., 2011).
Luciferase reporter assays
Briefly, Jurkat T cells were co-transfected with firefly luciferase plasmids containing either the Pdcd1 promoter ‘B-region’ or ‘B+C region’ (a kind gift from Dr. Jeremy Boss, Emory University) and a control Renilla luciferase plasmid using Fugene 6 transfection reagent (Promega). Cells were either left un-stimulated, or stimulated for 18hrs with PMA plus ionomycin to activate PD-1 luciferase reporter activity as previously reported(Oestreich et al., 2008). Luciferase activity was determined using DualGlo Luciferase reagent (Promega). ‘Fold over control’ Pdcd1 promoter luciferase activity was determined by normalizing to Renilla luciferase activity of stimulated over un-stimulated samples.
Seahorse extracelluar flux analysis
Seahorse analysis experiments were performed as previously described (Henao-Mejia et al., 2013). Briefly, day 8 P14 CTLs (Thy1.1+) were purified from LCMV-Arm or -Cl13 infected mice by positive selection using magnetic bead (Stem Cell Technologies) to greater than 90% purity, and plated on a pre-treated poly-D-lysine coated 96-well polystyrene Seahorse plate. Cell were allowed to equilibrate at 37°C for 30 minutes prior to starting the assay. Oligomycin (ATPase inhibitor, 0.5uM) and FCCP (0.2uM) was injected were indicated and extracellular acidification rate (ECAR, mpH/min) oxygen consumption rate (pMoles/min) were measured.
Statistical analysis
Data were analyzed using the Student’s unpaired t-test or with one-way ANOVA analysis with Tukey post-test for multiple comparisons with Prism 6. Error bars are the mean +/− SEM. An asterisk indicating a p value of less than 0.05 (*p < 0.05) is considered significant.
Supplementary Material
Acknowledgements
We thank the Kaech lab and members of the Craft lab for their discussions and critical review of the manuscript, M. Suresh for his thoughtful discussions and suggestions, J. Boss for the PD-1 promoter luciferase plasmids, T. Unterman for the FoxO1-AAA plasmid, and W.K. Ip for Firefly luciferase plasmid and help with luciferase reporter assays. This work was supported by National Institutes of Health (R37AI066232 and R01AI074699 to S.M.K.; R01AI102888 to M.O.L.; F32AI096718 and T325T32AI007019 to M.M.S.; F32AI094791 to H.D.M., and the Shared Instrument Grant, 1-S10-RR-026526-01), and Howard Hughes Medical Institute). I.A.P. was supported by the Australian NHMRC Overseas Biomedical Postdoctoral Fellowship. G.C. was supported by the Yale Trudeau Fellowship.
Footnotes
Author Contributions:
S.M.K and M.M.S. designed all the experiments, analyzed the data, and wrote the manuscript. S.G. assisted with many of the experiments. H.D.M, I.A.P., J.C., and C.P. provided critical reagents for the experiments. G.C. helped with the design of ChIP experiments. M.O.L. provided the FoxO1-floxed mouse strain, expertise with the ChIP experiments, and editing of the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Angelosanto JM, Blackburn SD, Crawford A, Wherry EJ. Progressive loss of memory T cell potential and commitment to exhaustion during chronic viral infection. J Virol. 2012;86:8161–8170. doi: 10.1128/JVI.00889-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araki K, Turner AP, Shaffer VO, Gangappa S, Keller SA, Bachmann MF, Larsen CP, Ahmed R. mTOR regulates memory CD8 T-cell differentiation. Nature. 2009;460:108–112. doi: 10.1038/nature08155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baitsch L, Baumgaertner P, Devevre E, Raghav SK, Legat A, Barba L, Wieckowski S, Bouzourene H, Deplancke B, Romero P, et al. Exhaustion of tumor-specific CD8(+) T cells in metastases from melanoma patients. J Clin Invest. 2011;121:2350–2360. doi: 10.1172/JCI46102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH, Freeman GJ, Ahmed R. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682–687. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- Biggs WH, 3rd, Meisenhelder J, Hunter T, Cavenee WK, Arden KC. Protein kinase B/Akt-mediated phosphorylation promotes nuclear exclusion of the winged helix transcription factor FKHR1. Proc Natl Acad Sci U S A. 1999;96:7421–7426. doi: 10.1073/pnas.96.13.7421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks DG, McGavern DB, Oldstone MB. Reprogramming of antiviral T cells prevents inactivation and restores T cell activity during persistent viral infection. J Clin Invest. 2006;116:1675–1685. doi: 10.1172/JCI26856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, Anderson MJ, Arden KC, Blenis J, Greenberg ME. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999;96:857–868. doi: 10.1016/s0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- Calnan DR, Brunet A. The FoxO code. Oncogene. 2008;27:2276–2288. doi: 10.1038/onc.2008.21. [DOI] [PubMed] [Google Scholar]
- Chang CH, Curtis JD, Maggi LB, Jr., Faubert B, Villarino AV, O’Sullivan D, Huang SC, van der Windt GJ, Blagih J, Qiu J, et al. Posttranscriptional control of T cell effector function by aerobic glycolysis. Cell. 2013;153:1239–1251. doi: 10.1016/j.cell.2013.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chemnitz JM, Parry RV, Nichols KE, June CH, Riley JL. SHP-1 and SHP-2 associate with immunoreceptor tyrosine-based switch motif of programmed death 1 upon primary human T cell stimulation, but only receptor ligation prevents T cell activation. J Immunol. 2004;173:945–954. doi: 10.4049/jimmunol.173.2.945. [DOI] [PubMed] [Google Scholar]
- Cui W, Liu Y, Weinstein JS, Craft J, Kaech SM. An interleukin-21-interleukin-10-STAT3 pathway is critical for functional maturation of memory CD8+ T cells. Immunity. 2011;35:792–805. doi: 10.1016/j.immuni.2011.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doedens AL, Phan AT, Stradner MH, Fujimoto JK, Nguyen JV, Yang E, Johnson RS, Goldrath AW. Hypoxia-inducible factors enhance the effector responses of CD8(+) T cells to persistent antigen. Nat Immunol. 2013;14:1173–1182. doi: 10.1038/ni.2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doering TA, Crawford A, Angelosanto JM, Paley MA, Ziegler CG, Wherry EJ. Network analysis reveals centrally connected genes and pathways involved in CD8+ T cell exhaustion versus memory. Immunity. 2012;37:1130–1144. doi: 10.1016/j.immuni.2012.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fife BT, Pauken KE, Eagar TN, Obu T, Wu J, Tang Q, Azuma M, Krummel MF, Bluestone JA. Interactions between PD-1 and PD-L1 promote tolerance by blocking the TCR-induced stop signal. Nat Immunol. 2009;10:1185–1192. doi: 10.1038/ni.1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlay D, Cantrell DA. Metabolism, migration and memory in cytotoxic T cells. Nat Rev Immunol. 2011;11:109–117. doi: 10.1038/nri2888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlay DK, Rosenzweig E, Sinclair LV, Feijoo-Carnero C, Hukelmann JL, Rolf J, Panteleyev AA, Okkenhaug K, Cantrell DA. PDK1 regulation of mTOR and hypoxia-inducible factor 1 integrate metabolism and migration of CD8+ T cells. J Exp Med. 2012;209:2441–2453. doi: 10.1084/jem.20112607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlay DK, Sinclair LV, Feijoo C, Waugh CM, Hagenbeek TJ, Spits H, Cantrell DA. Phosphoinositide-dependent kinase 1 controls migration and malignant transformation but not cell growth and proliferation in PTEN-null lymphocytes. J Exp Med. 2009;206:2441–2454. doi: 10.1084/jem.20090219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francisco LM, Salinas VH, Brown KE, Vanguri VK, Freeman GJ, Kuchroo VK, Sharpe AH. PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. J Exp Med. 2009;206:3015–3029. doi: 10.1084/jem.20090847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frauwirth KA, Riley JL, Harris MH, Parry RV, Rathmell JC, Plas DR, Elstrom RL, June CH, Thompson CB. The CD28 signaling pathway regulates glucose metabolism. Immunity. 2002;16:769–777. doi: 10.1016/s1074-7613(02)00323-0. [DOI] [PubMed] [Google Scholar]
- Frebel H, Nindl V, Schuepbach RA, Braunschweiler T, Richter K, Vogel J, Wagner CA, Loffing-Cueni D, Kurrer M, Ludewig B, Oxenius A. Programmed death 1 protects from fatal circulatory failure during systemic virus infection of mice. J Exp Med. 2012;209:2485–2499. doi: 10.1084/jem.20121015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grayson JM, Weant AE, Holbrook BC, Hildeman D. Role of Bim in regulating CD8+ T-cell responses during chronic viral infection. J Virol. 2006;80:8627–8638. doi: 10.1128/JVI.00855-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greer EL, Brunet A. FOXO transcription factors at the interface between longevity and tumor suppression. Oncogene. 2005;24:7410–7425. doi: 10.1038/sj.onc.1209086. [DOI] [PubMed] [Google Scholar]
- Greer EL, Oskoui PR, Banko MR, Maniar JM, Gygi MP, Gygi SP, Brunet A. The energy sensor AMP-activated protein kinase directly regulates the mammalian FOXO3 transcription factor. J Biol Chem. 2007;282:30107–30119. doi: 10.1074/jbc.M705325200. [DOI] [PubMed] [Google Scholar]
- Gross DN, van den Heuvel AP, Birnbaum MJ. The role of FoxO in the regulation of metabolism. Oncogene. 2008;27:2320–2336. doi: 10.1038/onc.2008.25. [DOI] [PubMed] [Google Scholar]
- Hand TW, Cui W, Jung YW, Sefik E, Joshi NS, Chandele A, Liu Y, Kaech SM. Differential effects of STAT5 and PI3K/AKT signaling on effector and memory CD8 T-cell survival. Proc Natl Acad Sci U S A. 107:16601–16606. doi: 10.1073/pnas.1003457107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hand TW, Cui W, Jung YW, Sefik E, Joshi NS, Chandele A, Liu Y, Kaech SM. Differential effects of STAT5 and PI3K/AKT signaling on effector and memory CD8 T-cell survival. Proc Natl Acad Sci U S A. 2010;107:16601–16606. doi: 10.1073/pnas.1003457107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedrick SM, Hess Michelini R, Doedens AL, Goldrath AW, Stone EL. FOXO transcription factors throughout T cell biology. Nat Rev Immunol. 2012;12:649–661. doi: 10.1038/nri3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henao-Mejia J, Williams A, Goff LA, Staron M, Licona-Limon P, Kaech SM, Nakayama M, Rinn JL, Flavell RA. The microRNA miR-181 is a critical cellular metabolic rheostat essential for NKT cell ontogenesis and lymphocyte development and homeostasis. Immunity. 2013;38:984–997. doi: 10.1016/j.immuni.2013.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs SR, Herman CE, Maciver NJ, Wofford JA, Wieman HL, Hammen JJ, Rathmell JC. Glucose uptake is limiting in T cell activation and requires CD28-mediated Akt-dependent and independent pathways. J Immunol. 2008;180:4476–4486. doi: 10.4049/jimmunol.180.7.4476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao C, Oestreich KJ, Paley MA, Crawford A, Angelosanto JM, Ali MA, Intlekofer AM, Boss JM, Reiner SL, Weinmann AS, Wherry EJ. Transcription factor T-bet represses expression of the inhibitory receptor PD-1 and sustains virus-specific CD8+ T cell responses during chronic infection. Nat Immunol. 2011;12:663–671. doi: 10.1038/ni.2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerdiles YM, Beisner DR, Tinoco R, Dejean AS, Castrillon DH, DePinho RA, Hedrick SM. Foxo1 links homing and survival of naive T cells by regulating L-selectin, CCR7 and interleukin 7 receptor. Nat Immunol. 2009;10:176–184. doi: 10.1038/ni.1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerdiles YM, Stone EL, Beisner DR, McGargill MA, Ch’en IL, Stockmann C, Katayama CD, Hedrick SM. Foxo transcription factors control regulatory T cell development and function. Immunity. 2010;33:890–904. doi: 10.1016/j.immuni.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim EH, Sullivan JA, Plisch EH, Tejera MM, Jatzek A, Choi KY, Suresh M. Signal integration by Akt regulates CD8 T cell effector and memory differentiation. J Immunol. 2012;188:4305–4314. doi: 10.4049/jimmunol.1103568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MV, Ouyang W, Liao W, Zhang MQ, Li MO. The transcription factor foxo1 controls central-memory CD8(+) T cell responses to infection. Immunity. 2013;39:286–297. doi: 10.1016/j.immuni.2013.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu P, Youngblood BA, Austin JW, Rasheed Mohammed AU, Butler R, Ahmed R, Boss JM. Blimp-1 represses CD8 T cell expression of PD-1 using a feed-forward transcriptional circuit during acute viral infection. J Exp Med. 2014;211:515–527. doi: 10.1084/jem.20130208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macintyre AN, Finlay D, Preston G, Sinclair LV, Waugh CM, Tamas P, Feijoo C, Okkenhaug K, Cantrell DA. Protein kinase B controls transcriptional programs that direct cytotoxic T cell fate but is dispensable for T cell metabolism. Immunity. 2011;34:224–236. doi: 10.1016/j.immuni.2011.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalek RD, Rathmell JC. The metabolic life and times of a T-cell. Immunol Rev. 2010;236:190–202. doi: 10.1111/j.1600-065X.2010.00911.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michelini RH, Doedens AL, Goldrath AW, Hedrick SM. Differentiation of CD8 memory T cells depends on Foxo1. J Exp Med. 2013;210:1189–1200. doi: 10.1084/jem.20130392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller SN, Vanguri VK, Ha SJ, West EE, Keir ME, Glickman JN, Sharpe AH, Ahmed R. PD-L1 has distinct functions in hematopoietic and nonhematopoietic cells in regulating T cell responses during chronic infection in mice. J Clin Invest. 2010;120:2508–2515. doi: 10.1172/JCI40040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oestreich KJ, Yoon H, Ahmed R, Boss JM. NFATc1 regulates PD-1 expression upon T cell activation. J Immunol. 2008;181:4832–4839. doi: 10.4049/jimmunol.181.7.4832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang W, Beckett O, Flavell RA, Li MO. An essential role of the Forkhead-box transcription factor Foxo1 in control of T cell homeostasis and tolerance. Immunity. 2009;30:358–371. doi: 10.1016/j.immuni.2009.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang W, Beckett O, Ma Q, Paik JH, DePinho RA, Li MO. Foxo proteins cooperatively control the differentiation of Foxp3+ regulatory T cells. Nat Immunol. 2010;11:618–627. doi: 10.1038/ni.1884. [DOI] [PubMed] [Google Scholar]
- Ouyang W, Liao W, Luo CT, Yin N, Huse M, Kim MV, Peng M, Chan P, Ma Q, Mo Y, et al. Novel Foxo1-dependent transcriptional programs control T(reg) cell function. Nature. 2012;491:554–559. doi: 10.1038/nature11581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paley MA, Kroy DC, Odorizzi PM, Johnnidis JB, Dolfi DV, Barnett BE, Bikoff EK, Robertson EJ, Lauer GM, Reiner SL, Wherry EJ. Progenitor and terminal subsets of CD8+ T cells cooperate to contain chronic viral infection. Science. 2012;338:1220–1225. doi: 10.1126/science.1229620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parry RV, Chemnitz JM, Frauwirth KA, Lanfranco AR, Braunstein I, Kobayashi SV, Linsley PS, Thompson CB, Riley JL. CTLA-4 and PD-1 receptors inhibit T-cell activation by distinct mechanisms. Mol Cell Biol. 2005;25:9543–9553. doi: 10.1128/MCB.25.21.9543-9553.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patsoukis N, Li L, Sari D, Petkova V, Boussiotis VA. PD-1 increases PTEN phosphatase activity while decreasing PTEN protein stability by inhibiting casein kinase 2. Mol Cell Biol. 2013;33:3091–3098. doi: 10.1128/MCB.00319-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce EL, Pearce EJ. Metabolic pathways in immune cell activation and quiescence. Immunity. 2013;38:633–643. doi: 10.1016/j.immuni.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce EL, Walsh MC, Cejas PJ, Harms GM, Shen H, Wang LS, Jones RG, Choi Y. Enhancing CD8 T-cell memory by modulating fatty acid metabolism. Nature. 2009;460:103–107. doi: 10.1038/nature08097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pircher H, Burki K, Lang R, Hengartner H, Zinkernagel RM. Tolerance induction in double specific T-cell receptor transgenic mice varies with antigen. Nature. 1989;342:559–561. doi: 10.1038/342559a0. [DOI] [PubMed] [Google Scholar]
- Powell JD, Delgoffe GM. The mammalian target of rapamycin: linking T cell differentiation, function, and metabolism. Immunity. 2010;33:301–311. doi: 10.1016/j.immuni.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell JD, Pollizzi KN, Heikamp EB, Horton MR. Regulation of immune responses by mTOR. Annu Rev Immunol. 2012;30:39–68. doi: 10.1146/annurev-immunol-020711-075024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puglielli MT, Zajac AJ, van der Most RG, Dzuris JL, Sette A, Altman JD, Ahmed R. In vivo selection of a lymphocytic choriomeningitis virus variant that affects recognition of the GP33-43 epitope by H-2Db but not H-2Kb. J Virol. 2001;75:5099–5107. doi: 10.1128/JVI.75.11.5099-5107.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao RR, Li Q, Gubbels Bupp MR, Shrikant PA. Transcription factor Foxo1 represses T-bet-mediated effector functions and promotes memory CD8(+) T cell differentiation. Immunity. 2012;36:374–387. doi: 10.1016/j.immuni.2012.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao RR, Li Q, Odunsi K, Shrikant PA. The mTOR kinase determines effector versus memory CD8+ T cell fate by regulating the expression of transcription factors T-bet and Eomesodermin. Immunity. 2010;32:67–78. doi: 10.1016/j.immuni.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley JL. PD-1 signaling in primary T cells. Immunol Rev. 2009;229:114–125. doi: 10.1111/j.1600-065X.2009.00767.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samstein RM, Arvey A, Josefowicz SZ, Peng X, Reynolds A, Sandstrom R, Neph S, Sabo P, Kim JM, Liao W, et al. Foxp3 exploits a pre-existent enhancer landscape for regulatory T cell lineage specification. Cell. 2012;151:153–166. doi: 10.1016/j.cell.2012.06.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheppard KA, Fitz LJ, Lee JM, Benander C, George JA, Wooters J, Qiu Y, Jussif JM, Carter LL, Wood CR, Chaudhary D. PD-1 inhibits T-cell receptor induced phosphorylation of the ZAP70/CD3zeta signalosome and downstream signaling to PKCtheta. FEBS Lett. 2004;574:37–41. doi: 10.1016/j.febslet.2004.07.083. [DOI] [PubMed] [Google Scholar]
- Shin H, Blackburn SD, Blattman JN, Wherry EJ. Viral antigen and extensive division maintain virus-specific CD8 T cells during chronic infection. J Exp Med. 2007;204:941–949. doi: 10.1084/jem.20061937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin H, Blackburn SD, Intlekofer AM, Kao C, Angelosanto JM, Reiner SL, Wherry EJ. A role for the transcriptional repressor Blimp-1 in CD8(+) T cell exhaustion during chronic viral infection. Immunity. 2009;31:309–320. doi: 10.1016/j.immuni.2009.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speiser DE, Utzschneider DT, Oberle SG, Munz C, Romero P, Zehn D. T cell differentiation in chronic infection and cancer: functional adaptation or exhaustion? Nat Rev Immunol. 2014 doi: 10.1038/nri3740. [DOI] [PubMed] [Google Scholar]
- Sullivan JA, Kim EH, Plisch EH, Peng SL, Suresh M. FOXO3 regulates CD8 T cell memory by T cell-intrinsic mechanisms. PLoS Pathog. 2012a;8:e1002533. doi: 10.1371/journal.ppat.1002533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan JA, Kim EH, Plisch EH, Suresh M. FOXO3 regulates the CD8 T cell response to a chronic viral infection. J Virol. 2012b;86:9025–9034. doi: 10.1128/JVI.00942-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tejera MM, Kim EH, Sullivan JA, Plisch EH, Suresh M. FoxO1 controls effector-to-memory transition and maintenance of functional CD8 T cell memory. J Immunol. 2013;191:187–199. doi: 10.4049/jimmunol.1300331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasoni R, Basso V, Pilipow K, Sitia G, Saccani S, Agresti A, Mietton F, Natoli G, Colombetti S, Mondino A. Rapamycin-sensitive signals control TCR/CD28-driven Ifng, Il4 and Foxp3 transcription and promoter region methylation. Eur J Immunol. 2011;41:2086–2096. doi: 10.1002/eji.201041130. [DOI] [PubMed] [Google Scholar]
- van der Windt GJ, O’Sullivan D, Everts B, Huang SC, Buck MD, Curtis JD, Chang CH, Smith AM, Ai T, Faubert B, et al. CD8 memory T cells have a bioenergetic advantage that underlies their rapid recall ability. Proc Natl Acad Sci U S A. 2013;110:14336–14341. doi: 10.1073/pnas.1221740110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virgin HW, Wherry EJ, Ahmed R. Redefining chronic viral infection. Cell. 2009;138:30–50. doi: 10.1016/j.cell.2009.06.036. [DOI] [PubMed] [Google Scholar]
- Wang R, Dillon CP, Shi LZ, Milasta S, Carter R, Finkelstein D, McCormick LL, Fitzgerald P, Chi H, Munger J, Green DR. The transcription factor Myc controls metabolic reprogramming upon T lymphocyte activation. Immunity. 2011;35:871–882. doi: 10.1016/j.immuni.2011.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei F, Zhong S, Ma Z, Kong H, Medvec A, Ahmed R, Freeman GJ, Krogsgaard M, Riley JL. Strength of PD-1 signaling differentially affects T-cell effector functions. Proc Natl Acad Sci U S A. 2013;110:E2480–2489. doi: 10.1073/pnas.1305394110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wherry EJ. T cell exhaustion. Nat Immunol. 2011;12:492–499. doi: 10.1038/ni.2035. [DOI] [PubMed] [Google Scholar]
- Yokosuka T, Takamatsu M, Kobayashi-Imanishi W, Hashimoto-Tane A, Azuma M, Saito T. Programmed cell death 1 forms negative costimulatory microclusters that directly inhibit T cell receptor signaling by recruiting phosphatase SHP2. J Exp Med. 2012;209:1201–1217. doi: 10.1084/jem.20112741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinselmeyer BH, Heydari S, Sacristan C, Nayak D, Cammer M, Herz J, Cheng X, Davis SJ, Dustin ML, McGavern DB. PD-1 promotes immune exhaustion by inducing antiviral T cell motility paralysis. J Exp Med. 2013;210:757–774. doi: 10.1084/jem.20121416. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.