Abstract
The physicochemical properties of cellular environments with a high macromolecular content have been systematically characterized to explain differences observed in the diffusion coefficients, kinetics parameters, and thermodynamic properties of proteins inside and outside of cells. However, much less attention has been given to the effects of macromolecular crowding on cell physiology. Here, we review recent findings that shed some light on the role of crowding in various cellular processes, such as reduction of biochemical activities, structural reorganization of the cytoplasm, cytoplasm fluidity, and cellular dormancy. We conclude by presenting some unresolved problems that require the attention of biophysicists, biochemists, and cell physiologists. Although it is still underappreciated, macromolecular crowding plays a critical role in life as we know it.
Main Text
Contrary to the typical in vitro media, the intracellular environment is densely packed with macromolecules, which occupy 5–40% of the total cellular volume (1). This packing is referred to as macromolecular crowding. The environment is described as crowded and not highly concentrated because no single molecular species is necessarily present at a high density. The crowded environment contains different molecules (proteins, nucleic acids, and/or polysaccharides) of various sizes and shapes. Importantly, crowding reduces the volume of solvent that is available for other molecules in the solution, which strongly affects molecules with large molecular weights (2). This excluded-volume effect has important consequences for cellular thermodynamics and is reported to influence several cellular processes (3, 4, 5).
The effects of macromolecular crowding on molecular diffusion inside cells have been thoroughly examined over the past two decades. Experimental, theoretical, and computational studies have shown the existence of a crossover from anomalous to regular diffusion in environments with a high macromolecular content (6, 7, 8, 9, 10, 11). In the diffusion-limited regime, chemical reactions appear to be governed by fractal-like kinetics (12, 13). Theoretical (14, 15, 16) and experimental (17, 18, 19) studies have found that enzyme-catalyzed reactions exhibit fractal-like kinetics in environments with a high macromolecular content (see Fig. 1). Macromolecular crowding can also increase the association rates of proteins (5) and protein-DNA interactions (20). This is consistent with the known effects of crowding in both DNA replication (21) and protein folding (22).
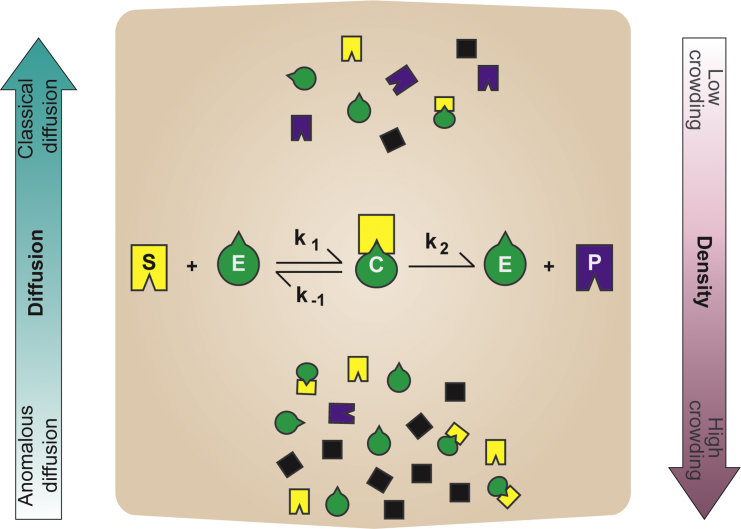
Figure 1.
Macromolecular crowding affects diffusion and the rates of enzyme-catalyzed reactions. In low macromolecular crowding conditions (top), diffusion is classical and differs from the anomalous diffusion observed in more crowded environments (bottom). Under high crowding and diffusion-limited conditions, the forward rate k1 is believed to follow fractal-like kinetics. Association rate coefficients, such as k1, decrease with increasing levels of crowding, but are also observed to increase under certain conditions. The formation of complexes is usually favored by high crowding. In the figure, S, E, C, and P represent the substrate, enzyme, enzyme-substrate complex, and product, respectively. The black squares represent the macromolecular crowding agents.
Hoffmann et al. (23) extensively reviewed evidence showing that cell volume regulation is a byproduct of the regulation of cytosolic macromolecular crowding, ion pumps, and channels. Macromolecular crowding plays a role in the signaling of volume perturbation, as discussed by Minton et al. (24) and Burg (25). Burg (25) proposed that cell volume regulation is controlled by a crowding sensor, i.e., a membrane-bound, two-state receptor. One state would exclude volume and therefore be restricted by crowding and trigger osmotic shrinkage. The other state would do exactly the opposite. However, the link between crowding and volume control remains relatively unexplored, and a global control mechanism has not been identified beyond speculation. In this mini-review, we focus our attention on another problem, which remains largely unexplored: the role of crowding in cell physiology and activity. What global, cell-level properties are controlled by macromolecular crowding? What are the physiological consequences of overcrowding in the cell? Here, we address these questions by presenting recent findings that describe the relationship among macromolecular crowding, molecular associations, cellular structure, intracellular signaling, and metabolism regulation in cell physiology.
Macromolecular crowding decreases intracellular signaling and active protein transport
The effects of macromolecular crowding in cell physiology can be investigated by exploiting the phenomenon of osmosis. Cells can be made to experience osmotic compression by adding higher solute concentrations in the extracellular medium. This volume contraction increases the overall concentration of the macromolecular crowding agents in cells. In osmotically stressed bacteria, macromolecular crowding decreases the diffusion coefficients of proteins (10, 11) and increases the association rates of protein-DNA interactions (20). Since it is possible to measure and statistically compare osmotic flux through signaling and metabolic pathways, as well as other cellular functions, volume contraction is widely used to study a variety of cellular processes (26).
In a recent article, Miermont et al. (27) reported a reduction in several biochemical processes, including cell signaling and protein transport in vesicles, as a consequence of a reduction in cell volume. They found that a sudden reduction of yeast cell volume slows down the high-osmolarity glycerol (HOG) pathway required for osmotic adaptation. The strong reduction of cell volume leads to a decrease in the diffusion coefficient of Hog1p, as well as its phosphorylation rate and nuclear import. The inhibition of a volume regulation pathway when volume is strongly perturbed suggests a loss in homeostatic control. It is possible, however, that the reduction of the HOG pathway results from an extreme osmotic stress induced by experimental conditions rather than by the overall increase in the macromolecular crowding concentration. To further investigate this scenario, Miermont et al. (27) investigated the dynamics of other, unrelated biological processes activated through different mechanisms. They found that the dynamics of nuclear translocation of transcription factors (Yap1p, Crz1p, and Mig1p) is similarly reduced under osmotic stress. In addition, vesicular trafficking and endocytosis are also reduced under lower cellular volume. Interestingly, the reduction of these biochemical processes is reversible, at least within a short timescale, through a regulatory volume increase (RVI) of the cells.
Miermont et al. (27) hypothesized that the decrease in protein diffusion and reaction rates by osmotic compression is caused by an increase in the overall concentration of crowding agents. This hypothesis is supported by measurements of protein diffusion in the periplasm of Escherichia coli under osmotic stress (11). After sudden hyperosmotic shock, the cytoplasm loses water as the periplasm gains water. A net gain of water by the periplasm decreases its macromolecular crowding volume fraction. This leads to a 3-fold increase of the diffusion coefficient of a green fluorescent protein in the periplasm. These results are also supported by theoretical simulations that showed a decrease in diffusion coefficients (9, 28) and reaction rates in an increasingly crowded environment (4, 14, 15, 16).
What are the evolutionary consequences of the reversibility of an osmotic-stress-mediated decrease in activity of the aforementioned biological processes? One could argue that single-celled organisms have evolved to use macromolecular agents to survive extreme osmotic stress by entering dormancy. From the physicochemical point of view, the cytoplasm behaves like a colloidal suspension, which can undergo a glass transition by extreme crowding (29).
Macromolecular crowding promotes molecular associations and structural reorganization of the cell
Dormant cells can go through a structural reorganization that makes them physically distinct from active cells. Recently, Petrovska et al. (30) investigated the organizational storage of proteins in dormant cells and found that the glutamine synthetase enzyme (Gln1) forms filaments in yeast cells under low-nutrient conditions. Gln1 catalyzes the ATP-dependent synthesis of glutamine from glutamate and ammonium, but it is inactive in the filament form. In yeast cells, starvation causes the intracellular pH to decrease, which changes the charge state of protein surfaces and promotes protein association. However, the self-assembly of Gln1 into filaments only occurs in the presence of an environment with a high macromolecular crowding content. Interestingly, Petrovska et al. (30) also found other metabolic enzymes assembling into filaments under acidic conditions.
Petrovska et al. (30) proposed that the structural reorganization of proteins into filaments is a mechanism to inactivate and store key metabolic enzymes in states of stress, such as cellular starvation. Sagot et al. (31) reported that under starvation conditions, yeast cells disassemble their cytoskeleton of actin filaments and reorganize it into actin bodies. After the cells are refed with a glucose-rich medium, actin bodies disappear and actin filaments reassemble to form a cytoskeleton. The self-assembly of actin bodies could be driven by macromolecular crowding, as it is well known that crowding increases such molecular association rates and accelerates protein fibrillation (5). Remarkably, macromolecular crowding also plays a role in the self-organization of large molecular complexes that form nuclear structures, such as the nucleolus and other intranuclear bodies (32, 33).
Miermont et al. (27) and Petrovska et al. (30) propose that macromolecular crowding plays a fundamental role in cellular adaption to stressful environmental conditions. Although Petrovska et al. (30) did not show that cell volume decreases with cytoplasmic acidification, there is evidence that Ehrlich ascites tumor cells undergoing a regulatory volume decrease (RVD) have low cytoplasmic pH (34).
Mechanistically, the link between pH and volume regulation has been observed in various cell types, including most mammalian cells and tissues, and involves several different transporter mechanisms (23). One class consists of the Na+/H+ exchangers, which electroneutrally expel cytosolic H+ in response to intracellular acidification (35). This is coupled with Cl−/HCO3− exchange, which jointly imports NaCl (causing RVI) and reduces cytosolic H+ concentration. This would imply that reduced pH, such as in starving conditions, would yield RVI, but ATP depletion inhibits this exchange by limiting PIP-2 binding to the transporter (36). This H+ efflux generally serves to counteract the passive acidifying influx of H+ and HCO3−, but is inhibited during conditions of starvation. Additionally, RVD is induced at low pH due to anion-dependent K+ efflux (37), whereas the overall rate of RVD is decreased in more acidic conditions. Several cation-Cl− cotransporters (CCCs) that have been implicated in RVD are inhibited at pH < 7.5 (KCC1, KCC3, and others), whereas KCC4 is activated. However, KCCCs (and NKCCs) have the ability to import NH4+, probably on the K+-binding site, which leads to cytoplasmic acidification (38).
From this information, we can see that the true link between cytosolic acidification and regulatory volume control is still ambiguous. Specifically, the rate and direction of volume control generally depend on the cell type, the transporter isoform, and changes in ATP conditions in ways that are not completely understood, warranting further investigation.
If a drop in intracellular pH does generally lead to RVD, the increase in crowding agent concentration due to cellular shrinkage may be sufficient to lead to the filament formation reported by Petrovska et al. (30). However, given the inconsistent effects of starvation-induced acidification on volume, we cannot definitively ascertain that RVD is the source of the filament formation in yeast cells. Petrovska et al. (30) offer an alternate link between acidification and the assembly of filaments. They suggest that low cytosolic pH may alter the charge distributions at the interfaces of protein subunits, thereby decreasing intrinsic repulsion, or, alternatively, that the protons allosterically induce structural changes in the subunits. The molecular crowding itself would not be amplified, but the effects of crowding would be exacerbated in interactions with more readily self-assembling structures. Although the details regarding the role of crowding remain unknown, the regulation of cellular volume may serve as an evolutionary adaptation mechanism for cell protection by processes driven by macromolecular crowding. During RVI, the overall macromolecular crowding concentration is low and the cell becomes sensitive to environmental perturbation. During RVD, the overall crowding concentration is high and the cell is protected against environmental perturbations.
A point we have not considered yet is that enzyme inactivation triggered by starvation or lower pH causes cells to enter into a low metabolic state, similar to what is observed in hibernating animals. This effect may be driven by macromolecular crowding, which clusters enzymes into filaments. When clustered into filaments, enzymes are inactive. Is this inactivation a mechanism for a reduction in cellular metabolism? If this is the case, what are the physicochemical and physiological implications of a metabolic reduction?
Macromolecular crowding decreases metabolic processes and cytoplasm fluidity
In a recent article, Parry et al. (39) found that small particles (i.e., proteins) diffuse freely in the bacterial cytoplasm, whereas large particles (i.e., macromolecular complexes) exhibit anomalous diffusion. The diffusion of smaller particles is typical of that found in a liquid phase, and the larger particles display the anomalous diffusion found in heterogeneous glass-like environments. Under 2,4-dinitrophenol-induced starvation, small particles were also restricted in their diffusive motion, but less so than with larger species. The authors discovered this by tracking the movement of fluorescently labeled particles in the bacterium Caulobacter crescentus and E. coli. Parry et al. (39) used volume exclusion (40) to explain this phenomenon, since the anomalous diffusion in heterogeneous glass-like environments is driven by physical crowding. The size dependency of diffusion restriction is compatible with the size dependency of the volume-exclusion effect in that larger particles have less available volume than smaller particles in a similarly crowded environment (3) and thus are more strongly influenced by crowding.
Remarkably, Parry et al. (39) discovered that the diffusion of small particles can become anomalous in dormant bacteria, whereas anomalous diffusion is suppressed by metabolic activity in a particle-size-dependent manner. They found that metabolic activity fluidizes the cytoplasm to enhance molecular motion. How is it possible that a passive physicochemical process can change with the metabolic state of the cell? A multitude of simultaneously occurring cellular processes could play a role in enhancing the diffusion observed in metabolically active cells. Based on the theory of Spitzer (41), Parry et al. (39) hypothesized that metabolic activity continuously changes the structural configuration of the cytoplasm by altering the hydrophobicity and electrostatic interactions of molecules with macromolecular crowding.
We have an alternative theory for the fluidization of the cytoplasm by metabolic activity. In dormant cells, the metabolic activity is suppressed and the cell enters into an ATP-depleted state. Under these conditions, the cells experience a RVD because the active transport pumps responsible for maintaining the homeostatic cell volume are inactive. This will inevitably lead to an overall increase in the macromolecular crowding concentration that hinders diffusion. Once the metabolic activity builds up, the cell will increase its ATP concentration and activate the ATP-dependent transport pumps responsible for volume regulation. The cell will then go through a RVI phase, decreasing the overall macromolecular crowding concentration and increasing molecular diffusion.
In a recent study, Guo et al. (42) provided additional clues regarding the physiological role of macromolecular crowding by investigating the movement of larger intracellular components. They introduced a technique called force spectrum microscopy, which they used to measure the ensemble forces due to the overall activity of the cell. The authors demonstrated that the ensemble forces are due in part to active processes and can be reduced by ATP depletion in the absence of Myosin II activity. Interestingly, the ensemble force reduction scales with the size of the tracer particles; that is, thermal-driven motion is more significant for smaller particles, but not as effective at moving the tracers (100–500 nm), mitochondria, vesicles, or protein complexes, which are transported via active motors. This is consistent with the size selectivity of the slowing effect of crowding on intracellular components. Guo et al. (42) also attribute the motion of individual proteins (Dendra2) to active transport, but, as we pointed out, ATP depletion indirectly halts small-particle transport and the cytoplasm becomes glassy under starvation conditions. This phenomenon was also observed by Parry et al. (39). The most relevant observation made by Guo et al. (42) is that there is a correlation between the overall activity of a cell and the active force fluctuations of protein complexes and organelles in the cell. Transporting and mixing ribosomes may increase the probability that ribosomes will encounter proteins, and the removal of enzyme products for the synthesis sites would increase enzymatic activity. As the ensemble force increases in a cell, there is more mixing and transport of cellular components. As an example, Guo et al. (42) use the human breast cancer cell line MCF-7. This cell line has enhanced metabolic and proliferative rates, and exhibits a higher force spectrum. As cells are considerably confined by macromolecular crowding, there is a decrease in the overall transport and metabolic activity.
Conclusions
Macromolecular crowding is gaining recognition as a major player in intracellular biochemical interactions. Although its effect on diffusion and reaction kinetics has been extensively addressed, very little is known about how macromolecular crowding affects cell physiology. The cytoplasm is densely packed with macromolecules, and the function of many of these macromolecules remains unknown. All cells have evolved to synthesize crowding agents, which are used to regulate diffusion, reaction rates, biochemical processes, and cellular organization. Remarkably, cells have evolved adaptation and survival strategies to enter and exit dormancy by changing the physicochemical properties of the cytoplasm via macromolecular crowding. Macromolecular crowding plays a critical role during the RVD, when the overall concentration of macromolecular crowding is high and cells undergo a glass transition. Based on the findings reviewed in this article, we propose that macromolecular crowding affects cellular physiological processes through cellular volume regulation (see Fig. 2). Cells change between dormant and active states, thereby changing biochemical activity and other processes by RVD and RVI.
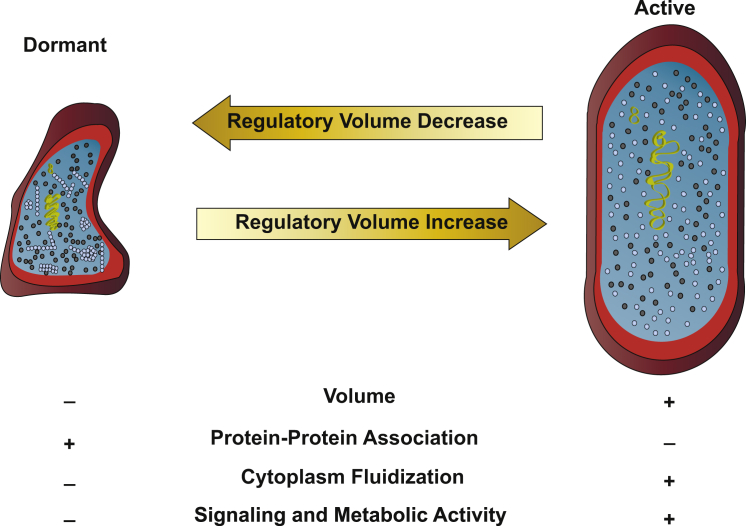
Figure 2.
Modulation of cellular volume to control physiological processes via macromolecular crowding. The active cell and the dormant cell interchange states by undergoing RVD or RVI, respectively. In the dormant cells, the higher concentration of crowding agents (black dots in cytoplasm) leads to the formation of protein complexes. Proteins are indicated by light blue dots, and complexes represent filaments and clusters of proteins. In addition, the chromatin is compressed by higher crowding. Note that the quantity of crowding agents does not necessarily change, but a reduction of volume leads to a higher overall concentration of crowding agents. Upon formation of protein filaments and clusters in response to volume contraction, metabolic activity is slowed, which may correspond to a depletion of nutrients stopping active transport and inhibiting RVI, thus making the cell dormant.
The most remarkable findings recently made by Miermont et al. (27), Petrovska et al. (30), and Parry et al. (39), as discussed in this article, are mostly limited to prokaryotic single-cell organisms. It remains to be investigated whether similar behaviors are found in single-cell organisms lacking cell walls and in multicellular organisms. Many other open questions and challenges concerning this topic exist as well. Recently, it was shown that macromolecular crowding modulates the dynamics of gene expression in artificial cells (43). Theoretical work (44, 45) suggests that macromolecular crowding serves as a modulator of gene expression. The effects of macromolecular crowding as regulators of gene expression remain to be explored systematically in living cells. Can we develop experimental techniques to measure macromolecular crowding inside the cells? A new type of ratiometric fluorescent molecular rotor has been developed to quantify and image intracellular viscosity in live cells (46). If this type of technology can be used to measure the overall concentration of macromolecular crowding, we may be able to resolve several open questions. Is there a certain crowding concentration at which the cytoplasm behaves differently from a simple viscous fluid? What are the mechanisms responsible for the regulation of macromolecular crowding synthesis? How is the regulation of cell volume linked to macromolecular crowding? Is this regulation driven by signaling or metabolism? Lastly, how are signaling and metabolism linked to active pumps and channels to regulate cell volume?
To illustrate our limited understanding of the mechanisms that regulate the effects of macromolecular crowding on cell physiology, we can holistically look at how the recent findings discussed above may tie together. The big picture established by combining these findings is a complicated web of regulatory interactions. This creates a confusing picture and leaves us with a chicken-and-egg type problem about how volume regulation, protein-protein associations, cytoplasm fluidization, and biochemical activity are tied together. We can say that crowding affects metabolic activity through filament storage of enzymes and metabolites, but does metabolism downregulate crowding by inhibiting volume or changing the electrostatic composition of macromolecular crowding directly? We know that volume contraction increases crowding content, but is crowding generally used as a homeostatic volume sensor in all cells? We could have starvation leading to a volume decrease, inducing an overall increase in crowding concentration, and then restricting metabolism. Alternatively, we could have volume contraction due to low ATP availability, leading to an overall high crowding concentration, which in turn would slow the metabolic processes until nutrients became available.
In short, we have a complex regulatory web of interactions describing fundamental cellular processes, and perhaps the best way to elucidate this is to apply both experimental and theoretical modeling studies. The former would establish evidence of the interactions as demonstrated here, and the latter would provide a means of investigating the nonlinearity and ambiguous feedback interactions mechanistically. In addition, analysis of this complex regulatory network requires the integration of information across spatial, temporal, and functional scales. With the advent of powerful computing platforms and systems biology, the development of multiscale models may enable researchers to comprehensively investigate the effects of macromolecular crowding on cell physiology by testing diverse mechanisms and selecting those that best explain the experimental observations. In the meantime, there is only one certainty: although macromolecular crowding has the potential to radically alter our understanding of the cellular environment, our current scientific knowledge about macromolecular crowding is still in its infancy.
Acknowledgments
We thank Michael Vincent (University of Michigan) for critically reading the manuscript.
MAM was supported by a Mathematical Bioscience Institute postdoctoral fellowship funded through National Science Foundation (Grant No. DMS-0931642). This work was partially supported by the James S. McDonnell Foundation (Grant No. 220020223) under the 21st Century Science Initiative Studying Complex Systems Program and the National Institute of Diabetes and Digestive Diseases (Grant No. R25 DK088752).
Editor: Dennis Bray.
References
- 1.Ellis R.J. Macromolecular crowding: obvious but underappreciated. Trends Biochem. Sci. 2001;26:597–604. doi: 10.1016/s0968-0004(01)01938-7. [DOI] [PubMed] [Google Scholar]
- 2.Hall D., Minton A.P. Macromolecular crowding: qualitative and semiquantitative successes, quantitative challenges. Biochim. Biophys. Acta. 2003;1649:127–139. doi: 10.1016/s1570-9639(03)00167-5. [DOI] [PubMed] [Google Scholar]
- 3.Minton A.P. The influence of macromolecular crowding and macromolecular confinement on biochemical reactions in physiological media. J. Biol. Chem. 2001;276:10577–10580. doi: 10.1074/jbc.R100005200. [DOI] [PubMed] [Google Scholar]
- 4.Schnell S., Turner T.E. Reaction kinetics in intracellular environments with macromolecular crowding: simulations and rate laws. Prog. Biophys. Mol. Biol. 2004;85:235–260. doi: 10.1016/j.pbiomolbio.2004.01.012. [DOI] [PubMed] [Google Scholar]
- 5.Minton A.P. Influence of macromolecular crowding upon the stability and state of association of proteins: predictions and observations. J. Pharm. Sci. 2005;94:1668–1675. doi: 10.1002/jps.20417. [DOI] [PubMed] [Google Scholar]
- 6.Luby-Phelps K. Cytoarchitecture and physical properties of cytoplasm: volume, viscosity, diffusion, intracellular surface area. Int. Rev. Cytol. 2000;192:189–221. doi: 10.1016/s0074-7696(08)60527-6. [DOI] [PubMed] [Google Scholar]
- 7.Kusumi A., Nakada C., Fujiwara T. Paradigm shift of the plasma membrane concept from the two-dimensional continuum fluid to the partitioned fluid: high-speed single-molecule tracking of membrane molecules. Annu. Rev. Biophys. Biomol. Struct. 2005;34:351–378. doi: 10.1146/annurev.biophys.34.040204.144637. [DOI] [PubMed] [Google Scholar]
- 8.Dix J.A., Verkman A.S. Crowding effects on diffusion in solutions and cells. Annu Rev Biophys. 2008;37:247–263. doi: 10.1146/annurev.biophys.37.032807.125824. [DOI] [PubMed] [Google Scholar]
- 9.Vilaseca E., Isvoran A., Mas F. New insights into diffusion in 3D crowded media by Monte Carlo simulations: effect of size, mobility and spatial distribution of obstacles. Phys. Chem. Chem. Phys. 2011;13:7396–7407. doi: 10.1039/c0cp01218a. [DOI] [PubMed] [Google Scholar]
- 10.Mika J.T., van den Bogaart G., Poolman B. Molecular sieving properties of the cytoplasm of Escherichia coli and consequences of osmotic stress. Mol. Microbiol. 2010;77:200–207. doi: 10.1111/j.1365-2958.2010.07201.x. [DOI] [PubMed] [Google Scholar]
- 11.Sochacki K.A., Shkel I.A., Weisshaar J.C. Protein diffusion in the periplasm of E. coli under osmotic stress. Biophys. J. 2011;100:22–31. doi: 10.1016/j.bpj.2010.11.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kopelman R. Rate-processes on fractals: theory, simulations, and experiments. J. Stat. Phys. 1986;42:185–200. [Google Scholar]
- 13.Kopelman R. Fractal reaction kinetics. Science. 1988;241:1620–1626. doi: 10.1126/science.241.4873.1620. [DOI] [PubMed] [Google Scholar]
- 14.Berry H. Monte carlo simulations of enzyme reactions in two dimensions: fractal kinetics and spatial segregation. Biophys. J. 2002;83:1891–1901. doi: 10.1016/S0006-3495(02)73953-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grima R., Schnell S. A systematic investigation of the rate laws valid in intracellular environments. Biophys. Chem. 2006;124:1–10. doi: 10.1016/j.bpc.2006.04.019. [DOI] [PubMed] [Google Scholar]
- 16.Mourão M., Kreitman D., Schnell S. Unravelling the impact of obstacles in diffusion and kinetics of an enzyme catalysed reaction. Phys. Chem. Chem. Phys. 2014;16:4492–4503. doi: 10.1039/c3cp52417e. [DOI] [PubMed] [Google Scholar]
- 17.Lin A.L., Feldman M.S., Kopelman R. Spatially resolved anomalous kinetics of a catalytic reaction: enzymatic glucose oxidation in capillary spaces. J. Phys. Chem. B. 1997;101:7881–7884. [Google Scholar]
- 18.Pastor I., Vilaseca E., Mas F. Effect of crowding by dextrans on the hydrolysis of N-Succinyl-L-phenyl-Ala-p-nitroanilide catalyzed by α-chymotrypsin. J. Phys. Chem. B. 2011;115:1115–1121. doi: 10.1021/jp105296c. [DOI] [PubMed] [Google Scholar]
- 19.Pastor I., Pitulice L., Mas F. Effect of crowding by Dextrans in enzymatic reactions. Biophys. Chem. 2014;185:8–13. doi: 10.1016/j.bpc.2013.10.006. [DOI] [PubMed] [Google Scholar]
- 20.Cayley S., Lewis B.A., Record M.T., Jr. Characterization of the cytoplasm of Escherichia coli K-12 as a function of external osmolarity. Implications for protein-DNA interactions in vivo. J. Mol. Biol. 1991;222:281–300. doi: 10.1016/0022-2836(91)90212-o. [DOI] [PubMed] [Google Scholar]
- 21.Akabayov B., Akabayov S.R., Richardson C.C. Impact of macromolecular crowding on DNA replication. Nat. Commun. 2013;4:1615. doi: 10.1038/ncomms2620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sasahara K., McPhie P., Minton A.P. Effect of dextran on protein stability and conformation attributed to macromolecular crowding. J. Mol. Biol. 2003;326:1227–1237. doi: 10.1016/s0022-2836(02)01443-2. [DOI] [PubMed] [Google Scholar]
- 23.Hoffmann E.K., Lambert I.H., Pedersen S.F. Physiology of cell volume regulation in vertebrates. Physiol. Rev. 2009;89:193–277. doi: 10.1152/physrev.00037.2007. [DOI] [PubMed] [Google Scholar]
- 24.Minton A.P., Colclasure G.C., Parker J.C. Model for the role of macromolecular crowding in regulation of cellular volume. Proc. Natl. Acad. Sci. USA. 1992;89:10504–10506. doi: 10.1073/pnas.89.21.10504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Burg M.B. Macromolecular crowding as a cell volume sensor. Cell. Physiol. Biochem. 2000;10:251–256. doi: 10.1159/000016371. [DOI] [PubMed] [Google Scholar]
- 26.Basser P.J., Schneiderman R., Maroudas A. Mechanical properties of the collagen network in human articular cartilage as measured by osmotic stress technique. Arch. Biochem. Biophys. 1998;351:207–219. doi: 10.1006/abbi.1997.0507. [DOI] [PubMed] [Google Scholar]
- 27.Miermont A., Waharte F., Hersen P. Severe osmotic compression triggers a slowdown of intracellular signaling, which can be explained by molecular crowding. Proc. Natl. Acad. Sci. USA. 2013;110:5725–5730. doi: 10.1073/pnas.1215367110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saxton M.J. Anomalous diffusion due to obstacles: a Monte Carlo study. Biophys. J. 1994;66:394–401. doi: 10.1016/s0006-3495(94)80789-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhou E.H., Trepat X., Fredberg J.J. Universal behavior of the osmotically compressed cell and its analogy to the colloidal glass transition. Proc. Natl. Acad. Sci. USA. 2009;106:10632–10637. doi: 10.1073/pnas.0901462106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Petrovska I., Nüske E., Alberti S. Filament formation by metabolic enzymes is a specific adaptation to an advanced state of cellular starvation. eLife. 2014;3:e024099. doi: 10.7554/eLife.02409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sagot I., Pinson B., Daignan-Fornier B. Actin bodies in yeast quiescent cells: an immediately available actin reserve? Mol. Biol. Cell. 2006;17:4645–4655. doi: 10.1091/mbc.E06-04-0282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hancock R. A role for macromolecular crowding effects in the assembly and function of compartments in the nucleus. J. Struct. Biol. 2004;146:281–290. doi: 10.1016/j.jsb.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 33.Schnell S., Hancock R. The intranuclear environment. Methods Mol. Biol. 2008;463:3–19. doi: 10.1007/978-1-59745-406-3_1. [DOI] [PubMed] [Google Scholar]
- 34.Levinson C. Regulatory volume increase in Ehrlich ascites tumor cells. Biochim. Biophys. Acta. 1990;1021:1–8. doi: 10.1016/0005-2736(90)90375-x. [DOI] [PubMed] [Google Scholar]
- 35.Orlowski J., Grinstein S. Na+/H+ exchangers of mammalian cells. J. Biol. Chem. 1997;272:22373–22376. doi: 10.1074/jbc.272.36.22373. [DOI] [PubMed] [Google Scholar]
- 36.Aharonovitz O., Zaun H.C., Grinstein S. Intracellular pH regulation by Na(+)/H(+) exchange requires phosphatidylinositol 4,5-bisphosphate. J. Cell Biol. 2000;150:213–224. doi: 10.1083/jcb.150.1.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kramhøft B., Lambert I.H., Jørgensen F. Activation of Cl-dependent K transport in Ehrlich ascites tumor cells. Am. J. Physiol. 1986;251:C369–C379. doi: 10.1152/ajpcell.1986.251.3.C369. [DOI] [PubMed] [Google Scholar]
- 38.Bergeron M.J., Gagnon E., Isenring P. Ammonium transport and pH regulation by K(+)-Cl(−) cotransporters. Am. J. Physiol. Renal Physiol. 2003;285:F68–F78. doi: 10.1152/ajprenal.00032.2003. [DOI] [PubMed] [Google Scholar]
- 39.Parry B.R., Surovtsev I.V., Jacobs-Wagner C. The bacterial cytoplasm has glass-like properties and is fluidized by metabolic activity. Cell. 2014;156:183–194. doi: 10.1016/j.cell.2013.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zimmerman S.B., Trach S.O. Estimation of macromolecule concentrations and excluded volume effects for the cytoplasm of Escherichia coli. J. Mol. Biol. 1991;222:599–620. doi: 10.1016/0022-2836(91)90499-v. [DOI] [PubMed] [Google Scholar]
- 41.Spitzer J. From water and ions to crowded biomacromolecules: in vivo structuring of a prokaryotic cell. Microbiol. Mol. Biol. Rev. 2011;75:491–506. doi: 10.1128/MMBR.00010-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Guo M., Ehrlicher A.J., Weitz D.A. Probing the stochastic, motor-driven properties of the cytoplasm using force spectrum microscopy. Cell. 2014;158:822–832. doi: 10.1016/j.cell.2014.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tan C., Saurabh S., Leduc P. Molecular crowding shapes gene expression in synthetic cellular nanosystems. Nat. Nanotechnol. 2013;8:602–608. doi: 10.1038/nnano.2013.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Klumpp S., Scott M., Hwa T. Molecular crowding limits translation and cell growth. Proc. Natl. Acad. Sci. USA. 2013;110:16754–16759. doi: 10.1073/pnas.1310377110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Matsuda H., Putzel G.G., Szleifer I. Macromolecular crowding as a regulator of gene transcription. Biophys. J. 2014;106:1801–1810. doi: 10.1016/j.bpj.2014.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kuimova M.K., Botchway S.W., Ogilby P.R. Imaging intracellular viscosity of a single cell during photoinduced cell death. Nat. Chem. 2009;1:69–73. doi: 10.1038/nchem.120. [DOI] [PubMed] [Google Scholar]