TO THE EDITOR: The prospect of reaching a “functional cure” for human immunodeficiency virus (HIV) infection has been raised by recent reports of the “Mississippi baby” and the “Berlin patient.” Both cases are reminiscent of an earlier case described in 1999 involving an HIV-infected patient (also referred to as the “Berlin patient”) in whom viral replication was spontaneously reduced to less than 1000 HIV RNA copies per milliliter despite the absence of antiretroviral treatment (ART).1 At the time of his HIV diagnosis, this patient was immediately treated with ART and hydroxyurea after acute HIV infection, but the patient chose to discontinue treatment shortly thereafter. This surprising level of natural control was attributed to the early treatment, leading to speculation that early intervention might promote HIV-specific cytotoxic T-lymphocyte–mediated control by preserving CD4+ T helper cells.2
A meta-analysis of nearly a dozen studies that were subsequently conducted suggested that in a minority of patients, those who were treated with early ART showed long-term clinical improvement,3 although most changes were observed only transiently. In addition, it was speculated that treatment with hydroxyurea or other immunomodulators might inhibit T-cell activation, which would reduce the pool of target cells and thereby provide an additive benefit. Despite an initial pilot trial with hydroxyurea in patients with chronic HIV infection who had improvement in viral control after treatment interruption,4 this outcome was not confirmed in a larger randomized trial involving patients with primary HIV infection.5
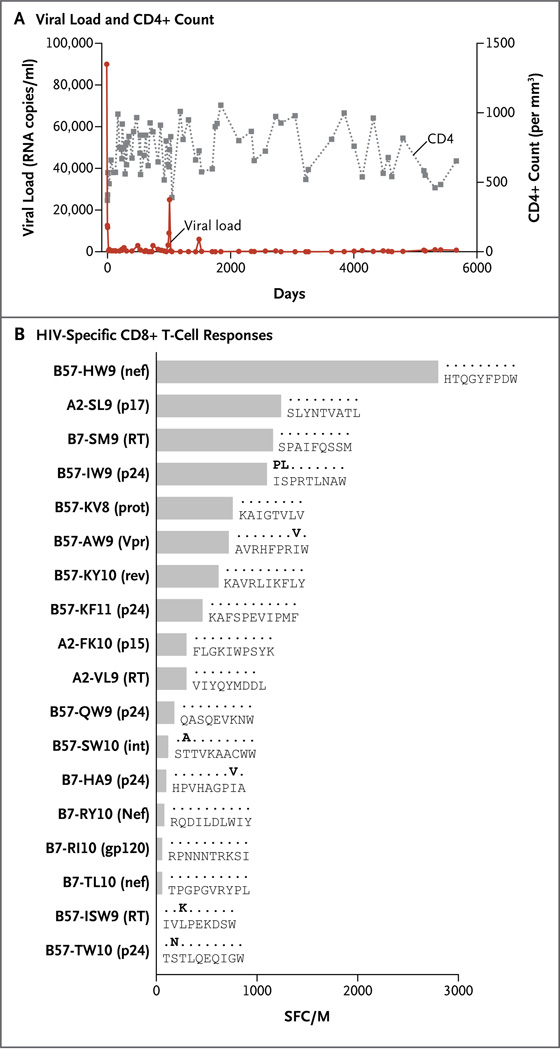
We now present further follow-up on the 1999 Berlin patient, whose identity has remained anonymous (unlike the other Berlin patient, Timothy Ray Brown, whose case was first described in 2008). The study was approved by the local ethics committee at the Charité Medical University and at Massachusetts General Hospital, and the patient provided written informed consent. Viral-load analysis showed that this patient had continual suppression of viral replication in the absence of any ART during the past 15 years, when all treatment was discontinued at the patient’s request. During this period, the mean (±SD) number of HIV RNA copies per milliliter was 2812±11,451 (median, 399; interquartile range, 100 to 923), with one blip to 25,000 copies per milliliter (Fig. 1). Similarly, the patient’s CD4+ T-cell count remained stable, with a mean of approximately 729±167 cells per cubic millimeter. Genotypic analysis revealed that this patient carried the highly protective HLA class I allele HLA-B⋆57. Although no other known genetic protective factors were detected, patients with this allele have on average 0.92 log10 lower viral loads than do all other HIV-infected patients, and HLA-B⋆57 has been shown to be enriched among patients in whom HIV is spontaneously controlled in the absence of ART. Moreover, half the HIV-specific CD8+ T-cell responses that were found in this patient were restricted by HLA-B⋆57, with the most dominant cytotoxic T-lymphocyte–mediated responses directed against a known conserved epitope in Nef, suggesting a dominant role for this response in HIV control. Furthermore, viral sequence analysis of recovered virus revealed typical B⋆57-restricted escape mutations located in two dominant epitopes: B57-ISW9 (RT) and B57-TW10 (p24). Both mutations have been previously suggested to partially impair viral replicative capacity.
Figure 1. Laboratory Values and Viral Sequences for the Patient.
Panel A shows the HIV RNA viral load in plasma and CD4+ count in the patient during 15 years of testing. Panel B shows the patient’s HIV-specific CD8+ T-cell responses against previously defined optimal epitopes as determined with the use of an enzyme-linked immunosorbent spot (ELISPOT) assay for high-resolution frequency analysis of interferon-γ–secreting cells. Responses are displayed as spot-forming cells per million peripheral-blood mononuclear cells (SFC/M). Actual viral sequences in samples obtained from the patient are shown on the right. Bold lettering indicates mutations in the targeted epitope.
Although the early initiation of treatment may have long-term benefits for certain patients, a likely explanation for control of viral replication in this patient is genetic background, regardless of intervention. Thus, this case represents a cautionary tale of drawing broad conclusions from a single patient.
Acknowledgments
Supported by grants (R01 AI091450-01 and R01 AI094602-01, to Dr. Streeck) from the National Institute of Allergy and Infectious Diseases.
The views expressed in this letter are those of the authors and do not necessarily represent the positions of the U.S. Army or the Department of Defense.
Footnotes
Disclosure forms provided by the authors are available with the full text of this letter at NEJM.org.
Contributor Information
Heiko Jessen, J2: Private Clinic for Infectious Diseases, Berlin, Germany
Todd M. Allen, Massachusetts General Hospital, Boston, MA
Hendrik Streeck, Email: hstreeck@hivresearch.org, U.S. Military HIV Research Program, Silver Spring, MD.
References
- 1.Lisziewicz J, Rosenberg E, Lieberman J, et al. Control of HIV despite the discontinuation of antiretroviral therapy. N Engl J Med. 1999;340:1683–1684. doi: 10.1056/NEJM199905273402114. [DOI] [PubMed] [Google Scholar]
- 2.Rosenberg ES, Altfeld M, Poon SH, et al. Immune control of HIV-1 after early treatment of acute infection. Nature. 2000;407:523–526. doi: 10.1038/35035103. [DOI] [PubMed] [Google Scholar]
- 3.Hocqueloux L, Prazuck T, Avettand-Fenoel V, et al. Long-term immunovirologic control following antiretroviral therapy interruption in patients treated at the time of primary HIV-1 infection. AIDS. 2010;24:1598–1601. doi: 10.1097/qad.0b013e32833b61ba. [DOI] [PubMed] [Google Scholar]
- 4.García F, Plana M, Arnedo M, et al. A cytostatic drug improves control of HIV-1 replication during structured treatment interruptions: a randomized study. AIDS. 2003;17:43–51. doi: 10.1097/00002030-200301030-00007. [DOI] [PubMed] [Google Scholar]
- 5.Bloch MT, Smith DE, Quan D, et al. The role of hydroxyurea in enhancing the virologic control achieved through structured treatment interruption in primary HIV infection: final results from a randomized clinical trial (Pulse) J Acquir Immune Defic Syndr. 2006;42:192–202. doi: 10.1097/01.qai.0000219779.50668.e6. [DOI] [PubMed] [Google Scholar]