Abstract
Over the past decade, rapid advances in epigenomics research have extensively characterized critical roles for chromatin regulatory events during normal periods of eukaryotic cell development and plasticity, as well as part of aberrant processes implicated in human disease. Application of such approaches to studies of the central nervous system (CNS), however, is more recent. Here, we provide a comprehensive overview of currently available tools to analyze neuroepigenomics data, as well as a discussion of pending challenges specific to the field of neuroscience. Integration of numerous unbiased genome-wide and proteomic approaches will be necessary to fully understand the neuroepigenome and the extraordinarily complex nature of the human brain. This will be critical to the development of future diagnostic and therapeutic strategies aimed at alleviating the vast array of heterogeneous and genetically distinct disorders of the CNS.
Introduction
Our current understanding of how the brain adapts and responds over time to a host of environmental challenges, both under normal conditions and in a range of neurological and psychiatric disease states, is incomplete. Although candidate gene approaches have been useful, too little is still known to select the best candidate genes for future investigations. Unbiased approaches are therefore essential to reveal fundamentally new insights into these questions.
Genome-wide studies of expressed RNAs are powerful but not sufficient. This is because many adaptations and maladaptations do not involve alterations in steady state levels of RNAs. Instead, they involve “molecular scars”—chromatin structural alterations at specific genes that alter their inducibility (e.g., priming or desensitization) in response to subsequent challenges1,2. Studies of chromatin are thus required to identify genes affected by this latent form of regulation. Likewise, studies of chromatin endpoints are the primary means of exploring the detailed molecular mechanisms by which the steady state expression or inducibility of genes is affected. Prior to chromatin studies, all efforts to understand mechanisms focused on cell culture, despite the fact that what happens in cultured cells—even cultured neurons—is not always an accurate reflection of what happens within the fully differentiated adult brain. Analogous to the developmental biology and cancer biology fields, where certain epigenomic modifications are seemingly permanent, studies of chromatin in brain have the potential to identify how environmental experiences/challenges lead to life-long changes in neuronal or glial function and in behavior, including disease susceptibility or resilience. Finally, an increasing number of CNS disorders are being shown to be caused by primary abnormalities in chromatin regulatory proteins. Increased knowledge of brain adaptations and disease pathogenesis resulting from explorations of epigenomic mechanisms3-18 has led to the possibility that such information can be mined to generate better diagnostic tests and treatments for a large variety of disabling nervous system disorders (see Table 1 for an update on progress in neuroepigenomics research).
Table 1.
Progress Report of Epigenomic Data from Brain
| Year | Species/Brain region(s) examined | Modification(s)/DNA binding protein(s) examined | Platform(s) | Variable(s) | Key finding(s) | Reference |
|---|---|---|---|---|---|---|
| 2009 | Mouse/embryonic forebrain and midbrain | p300 | ChIP-seq | Basal state | First evidence that in vivo mapping of p300 can be used to accurately identify tissue-specific enhancers and their associated activities |
3 |
| 2010 | Human/prefrontal cortex, neurons vs. non- neuronal cells |
H3K4me3 | ChIP-seq | Age | This study revealed age-correlated neuronal specific epigenomic patterns of reorganizaton demonstrating that H3K4me3 in human prefrontal cortex is highly regulated in a cell type- and subject-specific manner |
4 |
| 2010 | Mouse/adult hippocampus | H4K12ac | ChIP-seq/microarray | Fear conditioned learning |
During periods of learning, aged mice display deregulated H4K12ac and fail to initiate hippocampal gene expression programs associated with memory consolidation |
5 |
| 2011 | Mouse/adult hippocampal dentate granule cells |
5mC and 5hmC | MethylC-seq/BS- seq/microarray |
Electroconvulsive stimulation |
First in vivo genome-wide single base resolution maps of 5mC and 5hmC in adult hippocampal dentate granule cells. These data indicated a functionally important role for stimulus-dependent alterations in the CNS's DNA methylome in the regulation of activity-dependent gene expression |
6 |
| 2011 | Mouse/adult nucleus accumbens | H3K9me3 | ChIP-seq | Chronic cocaine | These data demonstrated that chronic cocaine exposure promotes reduced expression of H3K9me3 within specific heterochromatic loci of the nucleus accumbens epigenome leading to intergenic instability |
7 |
| 2011 | Mouse/early postnatal and adult hippocampus and cerebellum Human/adult cerebellum |
5hmC | Chemical labeling and immunoprecipitation/ ChIP-seq |
Age/Mecp2
overexpression and knockout |
Provided the first genome-wide maps of 5hmC in mouse hippocampus and cerebellum during periods of development and aging. This work further demonstrated alterations in 5hmC genomic content in mouse models of Rett syndrome indicating a critical role for 5hmC in neurodevelopment and human disease |
8 |
| 2011 | Human/adult hippocampus | H3K4me3 | ChIP-seq/RNA-seq | Cocaine and alcohol addiction |
This study identified substance-specific and shared transcriptional and chromatin related changes in human hippocampus of individuals chronically exposed to cocaine and alcohol |
9 |
| 2012 | Rat/adult hippocampus | H3K9me3 | ChIP-seq | Acute restraint stress |
Acute stress was found to result in increased enrichment of heterochromatic H3K9me3 in hippocampus, a finding hypothesized to be important for the repressive maintainence of endogenous retrotransposable elements in brain |
10 |
| 2012 | Mouse/adult cerebellar Purkinje, granule and Bergmann glial cells |
5mC, 5hmC and non-CpG methylation | MeDIP-seq/TRAP- seq |
Cell type, Mecp2 knockout | These data demonstrated that the relationship between 5hmC, genomic distribution of 5mC and gene expression is cell type specific, and that MeCP2 is the major 5hmC-binding protein in brain |
11 |
| 2012 | Human, chimpanzee and macaque/adult prefrontal cortex, neurons vs. non- neuronal cells |
H3K4me3 | ChIP-seq | Species | The findings presented here provided the first insights into human-specific modifications of the neuronal epigenome, with evidence for coordinated epigenetic regulation of sites separated by megabases of interspersed sequence |
12 |
| 2013 | Human and mouse/frontal cortex, neurons vs. non-neuronal cells |
5mC and 5hmC | MethylC-seq/RNA- seq |
Cell type, age | This study reported the genome-wide composition, patterning, cell specificity and dynamics of DNA methylation at single-base resolution in human and mouse cortex throughout lifespan, and identified a novel role for accumulation of non-CpG methylation during brain development |
13 |
| 2013 | Mouse/adult hippocampus | H4K5ac | ChIP-seq/microarray | Fear conditioned learning |
These data provided insights into the mechanisms of gene priming and 'bookmarking' by histone acetlyation following hippocampal memory activation, and proposed that increased H4Kac5 may prime genes for rapid expression following activity |
14 |
| 2013 | Human/adult | H3K4me1, H3K4me3, H3K9me3, H3K27me3, H3K36me3, H3K9ac and H3K27ac |
ChIP-seq/microarray | Cell/tissue types | This study described how global chromatin state transitions relate to chromosomal and nuclear architectures, and hypothesized implications for lineage fidelity, cellular senescence and reprogramming. |
15 |
| 2014 | Mouse/adult nucleus accumbens | H3K4me3, H3K4me1, H3K27me3, H3K9me2, H3K9me3, H3K36me3 and RNA Pol II |
ChIP-seq/RNA-seq | Chronic cocaine | This study demonstrated that repeated cocaine treatment results in an unexpectedly large number of pre-mRNA splicing alterations in nucleus accumbens, and that specific combinations of chromatin changes (i.e., signatures) predict these events |
16 |
| 2014 | Mouse/adult hippocampal dentate granule cells Human brain |
5mC (CpG) and non-CpG methylation (CpH) | BS-seq/ChIP- seq/RNA-seq |
Age/triple Dnmt1, Dnmt3a and Dnmt3b knockout |
This study, using single-base resolution maps of the neuronal DNA methylome, identified high levels of both CpG and CpH methylation, patterns which were shown to be conserved in human brain. Functionally, both mCpGs and mCpHs were shown to repress transcription in vitro and are recognized by MeCP2. Finally, mCpH was demonstrated to be established de novo during neurodevelopment requiring Dnmt3a for active maintenance in neurons |
17 |
| 2014 | Mouse/adult nucleus accumbens | PARP-1 and H3K4me3 | ChIP-seq/RNA-seq | Chronic cocaine | This work demonstrated that chronic cocaine exposure results in increases in PARP-1 expression and activity in nucleus accumbens, which mediates the expression of numerous target genes (e.g., SDK1) to promote cocaine related behaviors |
18 |
Selected list of genome-wide neuroepigenomic analyses carried out in brains of human or other mammalian species since 2009.
A host of genome-wide methods have become available over the past decade leading to increasingly powerful tools for characterizing changes in RNA expression and chromatin modifications, as well as relating the two phenomena. The reader is referred to a companion review of these experimental approaches19. However, application of such methods to the brain, given its distinctive cellular heterogeneity and the need to focus on in vivo models, involves many unique challenges with regards to data analysis. In this review, we provide an overview of such challenges and highlight ways of overcoming them to derive the extraordinary advances promised by epigenomic studies of the nervous system.
RNA Expression Analysis
Genome-wide epigenomic studies typically begin with measures of RNA expression, since ultimately it is the regulation of such expression that serves as the functional readout of epigenomic modifications. Over the past decade, genome-wide RNA expression analysis in brain has served as a powerful tool for identifying transcriptional ‘signatures’ associated with normal neurodevelopment as well as pathological disease states. Historically, such investigations have relied on microarray technology as the primary means of generating transcriptome data in brain; however, since its development, RNA-seq20-22 has proven to be a more powerful tool for assessing transcriptional outputs for a number of reasons. 1) Whereas microarray technology limits researchers to detecting and analyzing transcripts that correspond to existing genomic sequence information, RNA-seq allows for studies of both known and novel transcripts, an approach that is ideal for discovery-based experiments. 2) Since RNA-seq allows for unambiguous mapping of obtained DNA sequences to unique regions of the genome, as opposed to cross-hybridization procedures inherent to microarray technologies, signal to noise ratios are significantly improved. 3) RNA-seq quantifies absolute rather than relative values, thereby allowing for assessments of a large dynamic range of expression levels23-25. Given these considerations, we focus exclusively here on RNA-seq, which provides the most complete and accurate assessment of all expressed RNAs in a given tissue19. Despite the potential power of this approach, the analysis of RNA-seq data is still far from routine and involves numerous bioinformatics challenges, which we review here.
RNA-seq: Initial Methods of Data Processing and Annotation
The raw data produced by RNA-seq (see Figure 1 for initial pipelines of RNA-seq data analysis) is—for each biological sample—tens to hundreds of million short sequences (called reads, typically 50-100 bp) that correspond to random fragments of expressed RNAs present in the original tissue. The first step in analyzing such data is to assess the quality of these reads, which greatly influences downstream bioinformatics outputs. For that purpose, fastqc (http://www.bioinformatics.babraham.ac.uk/projects/fastqc/) can be used. Fastqc is a lightweight, highly efficient and low profile (i.e., requires reduced memory and yields less excessive outputs) program that simply requires raw sequencing reads in fastq format for initial quality control (QC) assessments. Fastqc, however, does not address RNA-seq specific questions, such as exonic vs. intronic alignments, transcript detection rates, etc., making QC determinations for subsequent downstream analyses difficult. To do so, RNA-SeQC26 is often used after fastqc, allowing investigators to examine numerous additional parameters associated with RNA-seq sample quality including yield, alignment and duplication rates, GC bias, contaminating rRNA content, regions of alignment (exon vs. intron vs. intragenic), continuity of coverage, 3′/5′ biases and counts of detectable transcripts, among others. Although RNA-SeQC is a comprehensive program that addresses most QC issues, its usage can be cumbersome requiring a great deal of computational power. In addition, ngs.plot27 can be used in connection with RNA-SeQC or fastqc to generate gene body plots for RNA-seq data, thereby providing an intuitive visualization tool for investigators to examine overall coverage patterns of sequencing reads from transcription start sites (TSSs) to transcription end sites (TESs). This tool allows for the identification of common sequencing abnormalities, such as strong 3’ biases. Although underreported, such QC data are extremely important to interpretations of RNA expression in brain, as differences observed in transcript abundance between control and experimental conditions, although important, are often small in magnitude, owing to a variety of unique challenges encountered when working with neural tissues (Box 1). Since low quality sequencing results in decreased signal to noise, as well as potential biases, such small differences may be inadvertently masked or amplified, thereby leading to high false positive and negative rates.
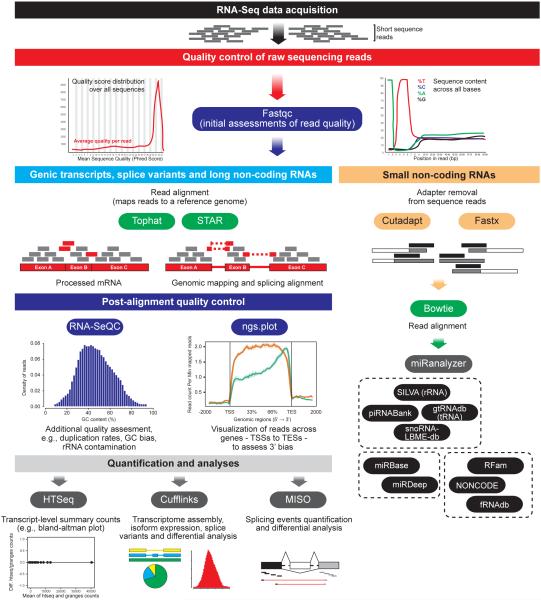
Figure 1. Initial Pipelines of RNA-seq Data Analysis.
Following data acquisition, RNA-seq analyses typically begin with numerous quality control assessments using analytical tools such as fastqc. Next, for analysis of genic transcripts, splice variants and long non-coding RNAs, short sequencing reads can be aligned to a reference genome using programs such as Tophat or STAR. Following alignment, additional quality control assessments can be made with RNA-SeQC and ngs.plot [orange line: RNA-seq plot from a human postmortem brain sample with a high RNA integrity number (RIN = 7.8); green line: RNA-seq plot from a human postmortem brain sample with a low RIN value (RIN = 3) displaying a clear 3’ bias]. ngs.plot image used with permission from27. Finally, to quantify and analyze RNA-seq data, programs such as HTSeq, Cufflinks or MISO are typically used. Depending on experimental purification schemes, researchers may also wish to analyze small ncRNAs from their samples. To do so, following initial quality control analysis, adapters must first be removed from sequence reads using Cutadapt or Fastx, followed by Bowtie alignment to a reference genome and quantitation using a program such as miRanalyzer150. Additional ncRNA specific analyses can similarly be integrated into miRanalyzer’s pipeline, or independent databases (e.g., SILVA, piRNABank, etc.) can be used.
Text Box 1. Unique Challenges in Neuroepigenomics.
Epigenomic studies of the brain are more challenging due to several considerations. In contrast to studies of cultured cells or most peripheral tissues, a discrete brain region of interest must be isolated by microdissection, which adds considerable variability to any study of the brain. Moreover, because many brain regions of interest are very small (~1 mm3 in a mouse), tissue is highly limiting. This necessitates multiple analyses to be performed on different collections of tissue from different cohorts of animals, which further increases the imprecision inherent in brain investigations.
Brain tissue is also highly heterogeneous, with all brain regions containing numerous types of neuronal, glial and vascular cells. The fact that each cell type has a distinct epigenome further increases the “noise” of brain epigenomic data compared to that from simpler systems. In addition, certain diseases might involve shifts in cell type (e.g., loss of neurons in neurodegenerative disorders or invasion of immune cells in neuroinflammatory disorders), which complicates the interpretation of epigenomic data. Epigenomic analysis of individual cell types—the isolation of which or their nuclei is becoming increasingly possible by use of genetic or viral tags141—represents one promising approach for the future. However, it is not yet feasible to obtain the number of isolated cells or nuclei from smaller brain regions that are required for most epigenomic analyses. Refinement of techniques that make ChIP-seq and related procedures possible with a far smaller number of cells or nuclei will advance the field dramatically4,142,143.
It has long been known that regulation of neural phenomena often involves changes in protein levels or activities that are far smaller than those seen in studies of other systems. As just one example, earlier microarray studies of cultured cells, peripheral tissues or tumors might have a 2-fold cutoff, while studies of brain reveal few changes of this magnitude. This is not solely due to the confound of multiple cell types, because similar findings have been obtained from analysis of single cell types. Rather, it is likely that fully differentiated neurons within the context of an intricate circuit do not show the same degree of adaptation displayed by other cell types. This requirement to reliably detect changes of relatively small magnitude (e.g., 20%)—which are demonstrably functionally importante.g., 1,2,15—adds another unique burden on bioinformatics analysis of neuroepigenomic data.
Following initial QC assessments, short sequence reads are mapped to appropriate transcriptomes using splicing-aware alignment tools. Such mapping requires both a reference genome sequence and a description of transcripts (e.g., a format such as GFF) rendering alignment results highly specific to a particular version of the transcriptome, which one can refer to using a database name, as well as a release number (e.g., Ensembl 67). When working with a species in which a comprehensive reference genome is not available, de novo transcriptome assembly can be performed using tools such as Cufflinks28 and Trinity29. A popular choice for RNA-seq data alignment is Tophat30, which wraps around Bowtie as the basic alignment tool but provides additional workflows to accomplish tasks specific to RNA-seq data analysis (e.g., splicing detection). Since spliced alignment is critical to RNA-seq analysis, it has attracted much research effort in recent years. For example, a new alignment program, referred to as STAR31, has been gaining significant market share and attention. In a recent study comparing 11 programs for RNA-seq alignment32, STAR achieved impressive performance across numerous benchmarks including alignment yield, basewise accuracy, mismatch and gap placement, exon junction discovery and suitability for transcript reconstruction. STAR is an ultra-fast, sensitive and precise tool that reduces alignment time from 1-2 days to a few hours for RNA-seq samples containing ~100 million reads, based on 4-core workstation processing capabilities. STAR is limited, however, in that its memory footprint can easily reach 32 GB or more, which is prohibitive for many common servers.
Analysis of Transcripts, Splice Variants and Non-coding RNAs
The next step in data analysis is to infer expression levels of individual transcripts from aligned reads. Simply put, if tissue samples derived from control conditions generate on average 1,000 reads for a given RNA, and samples from an experimental condition generate 2,000 reads, one can conclude a 2-fold induction of that gene’s expression. Such analyses are rarely so simple, because of the expression of multiple transcripts from a single gene and because such transcripts share a majority of their sequences, often differing by only a few or dozens of base pairs. Cufflinks33 solves this problem through the use of a statistical learning approach that assigns a fraction of the total read count to each transcript based on a maximum likelihood principle. In addition, Cufflinks attempts to quantify splicing events by grouping transcripts into TSS groups. A TSS group is a collection of transcripts that share TSSs. Such grouping is based on the rationale that alternative splicing events, such as exon skipping, will only happen between transcripts sharing the same TSS. Two types of splicing events are thereby characterized: 1) alternative splicing, which is defined between different transcripts that are within the same TSS group; and 2) alternative promoter usage, which is defined between transcripts with different TSSs. It should be noted that Cufflinks only reports these events at the level of TSS groups, as well as entire transcripts, and does not provide detailed information regarding which exons demonstrate alternative splicing. In order to obtain more detailed splicing information, Mixture of Isoforms (MISO) model34 can be used. MISO is based on a different design from that of Cufflinks and works on a predefined set of splicing events, such as exon skipping, intron retention, mutual exclusion and alternative 3’ UTR. MISO uses a Bayesian approach, which allows researchers to combine prior knowledge with obtained data for more reliable inferences, to derive relative abundance levels of spliced transcripts. Such detailed information provides an advantage when performing integrative analyses between alternative splicing and other forms of epigenomic regulation, such as the contribution of histone modification states and transcription factor binding events to regulation of alternative splicing. Additional information on alternative transcripts can be obtained from sequencing nuclear RNA as opposed to total cellular RNA. Nuclear RNA contains much larger amounts of sequenced introns, which can provide invaluable information about splicing mechanisms35. RNA-seq analysis of brain tissue has revealed an order of magnitude more alternative transcript production compared to that inferred from older microarray and related technologies16.
RNA-seq also enables the detection and quantification of several types of non-coding RNAs, which are proving to play crucial roles in biological regulation. A subset of long non-coding RNAs (lncRNAs)—similar to protein-coding genes—contain polyadenylated (polyA) tails, which allow for their detection by RNA-seq regardless of the RNA purification procedure used (e.g., ribozero or polyA selection). Identification of non-polyA lncRNAs, however, can only be accomplished through purification procedures preserving total RNA (i.e., polyA+ and polyA−). Since lncRNAs exist in high abundance in mammals, the Ensembl database has recently incorporated a large number of lncRNAs into its gene collection. Thus, predefined lncRNAs can now be analyzed alongside of protein-coding genes in the same sample. If investigators wish to predict a large number of novel, and still un-annotated, lncRNAs, however, this can be accomplished by utilizing histone modification state data to define candidate regions. To do so, ChIP (chromatin immunoprecipitation)-seq data for euchromatic H3K4me3 (trimethylated Lys4 of histone H3) and H3K36me3 need to first be obtained, as described in the next section. Using the intersection of H3K4me3 and H3K36me3 (so called ‘K4-K36 domains,’ as defined through “peak calling”—see below), RNA-seq reads at these domains can be extracted using programs such as bedtools36 to estimate lncRNA abundance levels.
MicroRNA (miRNA) sequencing experiments, which must be run separately from standard RNA-seq experiments, investigate mature miRNAs that are typically 22 nucleotides in length. Since most high-throughput sequencing machines produce reads that are significantly longer than mature miRNAs, the short reads obtained from miR sequencing often contain of portion of adapter sequences. The first step of miRNA analysis is thus to remove adapter sequences using tools such as fastx (http://hannonlab.cshl.edu/fastx_toolkit/). As an alternative, the Cutadapt program37 can be used for these purposes. Cutadapt is useful in that it supports a larger range of sequencing platforms, including color-space reads, which allow basepairs to be encoded in color to reduce sequencing error rates (https://www.biostars.org/p/43855/), in comparison to fastx. Following adapter removal, short reads can be mapped to the reference genome similar to that of data obtained from long RNA sequencing. miRBase38 provides a comprehensive collection of miRNA sequences. Bedtools can then be used to extract read counts for each of the annotated miRs obtained from alignment. If novel prediction of miRNAs from a given sample is desired, the miRDeep39 program can be used. Many additional families of small RNAs (Table 2) can be identified and analyzed with similar approaches40-46,38,47.
Table 2.
Existing Databases for Analysis of ncRNAs
| ncRNAs* | Database | Description | Reference |
|---|---|---|---|
| General purpose | Rfam (http://rfam.xfam.org/) | Sister database of Pfam**, collections of ncRNA families | 45 |
| NONCODE (http://www.noncode.org/) | Database of ncRNA, excluding rRNA and tRNA | 47 | |
| fRNAdb (http://www.ncrna.org/frnadb/) | Database of functional ncRNAs | 43 | |
| miRNA | miRBase (http://www.mirbase.org/) | Database for miRNAs | 38 |
| piRNA | piRNABank (http://pirnabank.ibab.ac.in/) | Database for piRNAs, clustering information provided | 41 |
| snoRNA | snoRNA-LBME-db (https://www-snorna.biotoul.fr/) | Comprehensive database for human snoRNAs | 40 |
| lncRNA | lncRNAdb (http://www.lncrnadb.org/) | Database for lncRNAs | 44 |
| rRNA | SILVA (http://www.arb-silva.de/) | A curated database for rRNAs | 46 |
| tRNA | GtRNAdb (http://gtrnadb.ucsc.edu/) | A genome-wide tRNA database predicted by the program tRNAscan-SE | 42 |
This list is not comprehensive but aims to provide an overview of currently available databases for analyzying ncRNAs from biological samples. The recent identification of novel ncRNAs, such as circRNAs, which may play critical roles in the CNS, are not included, as analysis tools do not currently exist.
PFam: a database widely used in the analysis/annotation of protein sequences that uses hidden Markov model based multiple sequence alignments to provide detailed information about protein families and domains.
Differential Analysis: Approaches, Advantages and Disadvantages
Differential analysis refers to the process of identifying differences in RNA expression levels of individual genes, or of individual splice variants of a single gene, between control and experimental samples. This is not straightforward, as there are numerous tools available, each of which is associated with high rates of false positive or false negative discovery and generates very different lists of regulated genes when applied to the same sequencing data. Generation of ideal differential analytical tools is therefore a focus of great interest in the field.
The first step in this process often involves summarizing gene- or gene variant-level read counts using a popular python program called htseq (http://www-huber.embl.de/users/anders/HTSeq/). According to individual needs, bedtools can also be used for gene count summarization. All read counts across genes and samples are then imported into a data matrix so that each row represents a gene, and each column represents a sample. This data matrix serves as the input for downstream differential analyses. Numerous differential analysis tools have been developed in recent years. Two popular choices are DESeq48 and edgeR49, with both methods based on negative binomial testing, which provide an exact test (generalization of the Poisson distribution model) that is ideal for modeling biological variances of read count data. Variance estimates are often problematic for RNA-seq datasets, as sample sizes from animal models are typically small with an N=3 (three biological replicates) used per condition in most experiments. Therefore, numerous statistical methods are used to exploit the relationship between mean- and variance-related information obtained from neighboring genes to stabilize variance estimations. DESeq uses local regression to model mean-variance relationships, while edgeR uses Bayesian methods to “borrow” information from neighboring genes. Both methods generate satisfactory results, and their lists are often consistent. Recently, a new method called voom50 has been developed, which does not depend on negative binomial testing and instead models mean-variance relationships on log-transformed read counts, thereby assigning a precise ‘weight’ for each gene. These weights are then entered into the limma51 empirical Bayes analysis pipeline. This approach provides access to a large collection of analysis tools developed originally for microarrays, which makes “voom” a particularly attractive option. Furthermore, DESeq has been criticized for its extremely conservative outputs, and its authors have acknowledged that their method yields high false negative rates52. Recently, the statistical power of the program has been improved with the introduction of DESeq2 (http://www.bioconductor.org/packages/release/bioc/html/DESeq2.html). Nevertheless, experience with RNA-seq suggests that a larger number of biological replicates are needed to derive the most reliable data53,54.
Cuffdiff, another popular tool for RNA-seq differential analysis, and part of the cufflinks pipeline, is a natural choice for investigators using cufflinks for initial identification of transcripts and splice variants. However, we, along with numerous other groups, have found cuffdiff to generate a high degree of false positives. The authors of cufflinks claim to have improved the reliability of detection in cuffdiff255, but a recent comparison pointed out that cuffdiff2 may be too conservative56. Another reason for avoiding cuffdiff is its restrictive workflow. Cuffdiff only accepts short read alignments and performs read counts, GC content adjustments, 3’ bias corrections and differential analyses in one integrated workflow; based on a standard four-core workstation, it would take 1-2 days to complete analysis for a 20 sample (10 vs. 10) dataset and may crash for datasets with >80 samples. Using htseq for gene counts in connection with limma or DESeq greatly reduces the analysis time to <1 hour.
Performing all of the steps of RNA-seq analysis, including quality assessment, alignment, gene count summarization and differential analysis, can be tedious if investigators have hundreds/thousands of samples (e.g., from studies of clinical populations), as well as dozens of comparisons, to run and examine. Therefore, a new Python-based computational pipeline has been developed SPEctRA (scalable PipEline for RNA-seq Analysis) (https://github.com/shenlab-sinai/complete-seq/) to perform all these tasks with a single command accepting various parameter settings as a configuration file. This pipeline is designed to function within different computational environments. For example, when performing analyses under a portable batch system (PBS) cluster, computer software that allocates computational tasks, SPEctRA will generate batch scripts, automatically submit them and wait for them to finish. A new feature of this pipeline, which will allow it to run in Amazon cloud where both CPU power and memory can be configured on demand for each run, is currently being developed.
Epigenomic Analyses of the Nervous System
ChIP-seq: Initial Methods of Data Processing and Annotation
As with RNA-seq, the raw data obtained from ChIP-seq are tens to hundreds of million short reads per sample that correspond to genomic regions bound to the DNA-binding protein subjected to ChIP (e.g., a modified histone, transcription factor, etc.)19. Quality control and sequence alignment are the first steps in analyzing such ChIP-seq data. Any of several short read aligners, such as bowtie57 or BWA58, can be used to align reads to a reference genome and assess the enrichment of the DNA-binding protein of interest. Alignments are exported as BAM59 files for further analysis. Sequencing quality can then be assessed using fastqc, as described above. If ChIP-seq samples are generated from sonication-based fragmentation methods, then duplicated alignments need to be removed, a procedure easily accomplished with samtools59 or picard (http://picard.sourceforge.net/). In doing so, PCR duplicates are identified as reads aligned to the same genomic location or on the same strand of fragmented DNA. During sonication, genomic DNA is theoretically fragmented at random, thereby rendering it unlikely that two unique reads will align to the same location or on the same strand of DNA. Since micrococcal nuclease (MNase) digestion, as used in “native” ChIP, preferentially digests genomic sequences containing specific nucleotide sequences (i.e., the rate of MNase cleavage 5’ of A or T nucleotides occurs at a 30 times greater rate in comparison to cleavage at G or C nucleotides), the probability of obtaining fragments from the same location or strand is increased. Therefore, removal of these reads with native ChIP samples, unless excessively high (thresholds can be determined using DANPOS60, as described below), is not typically performed. Removal of repeat reads can be problematic when analyzing repeat regions of the genome, which are now known to be important in biological regulation, thus framing a challenge for current bioinformatics innovations. The number of unique reads per sample is an important criterion for the quality of ChIP-seq data. Since antibodies play a key role in the success of any ChIP-seq experiment19, it is vitally important to determine the efficiency and specificity of the antibody being used. For this purpose, the phantomPeak61 tool can be employed, as suggested by the Encyclopedia of DNA Elements (ENCODE) consortium62, to examine the distribution of cross-correlations between represented strands of DNA to determine peak enrichments (see next section).
It is also helpful to visualize ChIP-seq enrichment profiles to ensure quality and diagnose any problems that may exist. Two approaches—localized inspection and global visualization—are used. For local inspection, the IGV genome browser63, which runs on all platforms without uploading data, is popular. With the tools provided by the IGV, BAM file are first converted to TDF files and then loaded onto the browser to display coverage between two chromosomal coordinates. For global visualization, ngs.plot (https://code.google.com/p/ngsplot/)27, which allows for inspection of both average and ‘laid out’ coverages as curves or heatmaps, respectively, at various functional genomic regions, such as TSS, TES, gene body, CpG islands, enhancers, exons and DNase I hypersensitive sites (DHSs). ngs.plot, which is simple to use and supports numerous genomes, can accommodate large alignment files with relatively small memory footprints. A ChIP-seq quality assessment pipeline has been developed (https://github.com/nyshao/chip-seq_preprocess) to perform all the above steps with a single command. This pipeline uses a similar design to the RNA-seq pipeline described previously. When dealing with large sample numbers and multiple comparisons, this pipeline saves significant amounts of time and effort.
Another important quality control consideration during the initial analysis/alignment of ChIP-seq data requires an in depth understanding of the differences between ‘uniquely mapped’ vs. ‘unique reads/tags,’ two terms that are often used interchangeably in the field but are distinctly defined. ‘Uniquely mapped’ reads are identified by all alignment software programs and exclude sequences that align to multiple genomic locations. These excluded sequences likely represent repetitive regions of the genome, or non-repetitive genomic loci that are extremely similar in sequence (although the latter becomes increasingly unlikely with greater read lengths). ‘Unique reads/tags,’ however, refer to reads remaining after PCR de-duplication (i.e., the non-redundant fraction or NRF) using tools such as samtools. PCR de-duplication essentially excludes reads (all copies but one) that align to the same genomic location. These non-unique reads are often PCR duplicates resulting from over-amplification during library processing, most likely due to low starting material or poor antibody efficiency. These reads are thus often removed to avoid PCR amplification bias. However, arguments now exist64,65 to suggest that these duplicative reads may provide increased dynamic range to ChIP signals. For example, if one were to imagine two 100 bp regions of the genome (A and B), where factor X binds strongly to region A and weakly to region B, and if all duplicated reads mapping to these regions were removed (assuming a sequencing read length of 100 bp), the resulting effect would be two reads for each of these regions (one mapping to each strand of DNA), an output that would prevent the investigator from distinguishing the differential binding strengths of factor X to these regions. Therefore, although removing duplicates is a highly conservative measure to prevent PCR amplification bias, it is also likely that this process similarly removes real signals that might be informative for determination of binding interactions. Having said this, without excluding these duplicates, one runs the risk of generating high levels of false positive findings due to increased signal. In our experience, keeping two reads for any identified duplicates (instead of only one) dramatically increases the sensitivity of downstream data analysis and genome browser viewing with IGV, while retaining low false positive discovery rates.
Peak calling—identifying a genomic region that displays a significant level of a DNA-binding protein above background—is an important task in analyzing ChIP-seq data. Currently, dozens of peak calling tools exist, and these methods can generally be separated into two categories: sonication- and MNase-digestion based. For sonication-based peaks, Model-based Analysis of ChIP-Seq (MACS)66 and Hypergeometric Optimization of Motif EnRichment (HOMER)67 are used. In our experience, both methods work very well for punctuated peaks (e.g., H3K4me3), however, if peaks are broad and diffusive (e.g., total histone H3 or H3K9me2), detection can be challenging, and HOMER is generally recommended, as systematic evaluations of broad peak detection are often difficult. For MNase-digested peaks, DANPOS60, which detects not only basal enrichment but also various nucleosomal events (e.g., nucleosomal positioning and occupancy and “fuzziness” between the two), can be used. After peaks are detected, it is useful to determine their location within the genome, such as genes, gene deserts or pericentromeres. A regional analysis tool, which is part of the diffReps68 package—more recently extended as a standalone program (https://github.com/shenlabsinai/region_analysis)—has been developed to perform this task. This program features single command usage and can assign peaks to one of eight distinct categories including proximal promoter (+/− 250 bp of a TSS), promoter 1k (1 kb upstream of a TSS), promoter 3k, gene body, gene desert, pericentromere, subtelomere and other intergenic loci. Regional annotation is a very useful feature, and other alternatives exist to perform this function including the ChIPpeakAnno69 package. After peaks are annotated, enrichment of specific chromatin marks is easily visualized for their genomic distribution using piecharts or plots.
Differential Analysis: Approaches, Advantages and Disadvantages
Differential analysis of ChIP-seq data aims to identify genomic loci or broader regions that display significant changes in enrichment between control and experimental conditions. Although it is natural for investigators to consider differential analyses as an extension of peak calling, in reality the former cannot be measured accurately using standard peak calling methods. Currently, one differential analysis tool70 based on peak calling exists, however, the numerous challenges associated with this approach make it disadvantageous. Differences in antibody efficiency, sequencing biases and manual handling of samples (i.e., sequencing library preparation), can produce very different signal-to-noise ratios between samples, which confounds peak calling. Insufficient sequencing depth (i.e., too few reads per sample) can also result in specific genomic regions having different coverage across different samples and complicate differential analyses. For example, consider the following situation: a 3 kb peak in biological replicate #1 under condition A; two 1 kb peaks with 1 kb gap in biological replicate #2 under condition A; a 2.5 kb peak shifted to the left with lower enrichment in biological replicate 1 under condition B; and a 2 kb peak shifted to the right with higher enrichment in biological replicate 2 under condition B. Such heterogeneity in peak calling, which is common in analyses of brain (see Box 1) makes comparisons difficult.
Further exaggerating this problem is the fact that different peak calling methods, or the same peak calling method with different parameter settings, can yield very different peaks using the same ChIP-seq dataset. Biological replicates are thus essential in neuroepigenomics research to enhance statistical power and increase precision. However, most peak calling methods to date were not designed with biological replicates in mind. To address these challenges, one can use a sliding window-based strategy to ensure that the entire genome is scanned and scored continuously, and that significant regions can be extracted for further analyses. diffReps68, which was developed to address this need, is a Perl-based program that features single command usage and has been applied to several projects. diffReps scans genomes with a predefined window size and performs one of four distinct statistical tests, identifies samples passing pre-defined cutoffs, merges replicates, performs multiple testing corrections and reports results. Using benchmark standards, diffReps is highly sensitive and efficiently controls for false positives (see Figure 2 for examples of ChIP-seq analysis outputs). It is also important to assess the reproducibility among biological replicates as an additional quality control measurement during data processing. To do so, programs such as corrgram71 can be used, which generates Pearson’s correlation coefficients between ChIP-seq signals derived from multiple signals. Alternatively, irreproducible discovery rates (IDRs) can be derived following peak calling to determine the number of ‘bound’ regions observed between replicates, as described72.
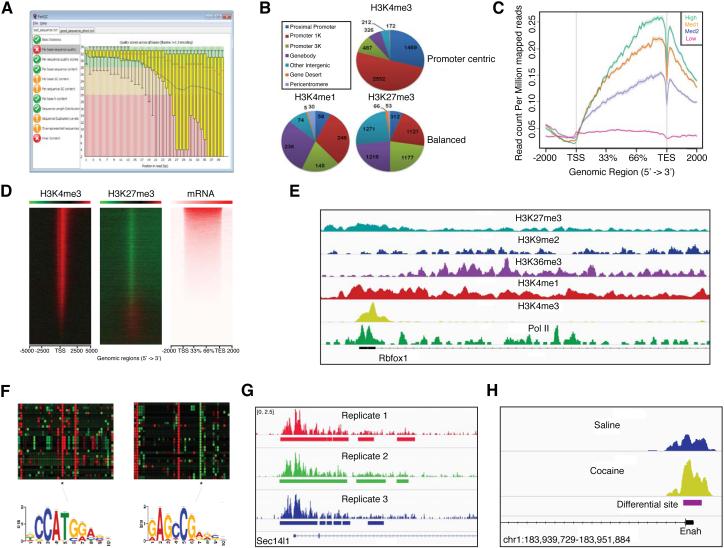
Figure 2. ChIP-seq Analysis of Brain.
A) Assessment of ChIP-seq sample quality, represented here as the sequence quality score vs. bp position using fastqc, is a critical first step in ChIP-seq data analysis and is required for appropriate interpretations of subsequent downstream analyses. The example shows relatively poor quality data, given the high error rate and variability toward the 3’ end of the reads. B) Example pie charts describing the genome-wide distribution patterns of differential histone modification sites for H3K4me3, H3K4me1 and H3K27me3 (as determined using diffReps) in nucleus accumbens (NAc) of saline vs. chronic cocaine treated mice16. C) Example gene body plots for H3K36me3 in NAc of control mice derived by ngs.plot. Lines represent average profiles for four gene groups ordered by mRNA expression levels by RNA-seq, defined as “high,” “med2,” “med1” and “low,” and illustrates increasing levels of this histone mark with increasing gene expression. ngs.plot easily generates these kinds of figures for accessible representations of protein enrichment throughout different functional genomic regions. D) Representative log2 enrichment heat maps generated in ngs.plot for several histone marks vs. DNA input in mouse NAc at TSS ± 5 kb genomic regions. Gene expression levels analyzed by RNA-seq are illustrated by enrichment in the same gene order as ChIP-seq enrichment patterns. Red is high, green is low. E) Integrated genomics viewer (IGV) screenshots for ChIP-seq data of various histone marks in NAc of cocaine treated mice. The genomic region displayed represents the TSS and ~20 kb downstream of the Rbfox1 (A2bp1) gene, a splicing factor which is highly expressed in neurons. F) Chromatin signatures, such as those shown here, can be defined to characterize groups of transcripts displaying similar patterns of chromatin modifications at specific genomic regions following environmental stimuli (e.g., at splicing related regions following cocaine treatment)16. Motif finding can then be used to identify potential transcriptional and splicing associated factors deduced to regulate these signatures. G) IGV genome browser screenshot for ChIP-seq coverage of H3K4me3 from NAc of control mice. Three biological replicates are shown as separate tracks with significant peaks identified by MACS depicted as solid bars beneath the tracks. The genomic region depicted is ~ chr11:116,974,000-116,990,000. These data highlight the difficulty of using peak calling-based approaches to identify differential enrichment patterns across samples. Here, although the three biological replicates appear to be generally similar in size and distribution, MACS identified discordant peaks between the samples due to intrinsic variations in peak location. Unlike MACS, diffReps uses a sliding window approach that allows one to focus on a fixed sized region across all samples (e.g., 1 kb), thus allowing for more unified comparisons across samples. H) ChIP-seq differential analysis using diffReps to compare two groups of samples representing two distinct biological conditions and test for significance. Here, diffReps was used to compare differential H3K4me3 enrichment in NAc between saline vs. cocaine treated mice at the TSS of the Enah gene.
Important questions for ChIP-seq experiments are the read depth required to appropriately make assumptions based on aforementioned analyses, and to determine if experimental sequencing depths are sufficient for the questions being asked. In general, researchers can follow the guidelines established by the ENCODE consortium72, which indicate a minimum of 10 or 20 million uniquely mapped reads for factors displaying punctuated or broad peaks, respectively. One can also perform saturation analyses for each individual factor to assess the sufficiency of sequencing depth. In essence, such analyses repeatedly perform peak calling on a series of samples from the original ChIP-seq library using increasing sampling rates, followed by plotting the number of peaks assigned vs. the sampling rate to identify plateaus indicative of sequencing depth saturation. One caveat for such analyses, however, is that narrow peak binding factors, such as transcription factors, will typically display increased numbers of binding sites/peaks with increasing numbers of reads obtained. This is due to the fact that such factors rarely saturate at the number of reads that are practical for saturation analysis.
Another challenge in analyzing ChIP-seq data involves appropriately annotating chromatin states while examining combinations of chromatin modification patterns in a given sample. To achieve this, an automated computational system, referred to as ChromHMM73, has been developed to integrate ChIP-seq information from multiple histone modifications, chromatin factors, transcription factors, etc. to accurately assess combinatorial and spatial patterns of marks/binding factors in biological samples. Such analyses provide a novel computational tool for ‘learning’ chromatin states, characterizing biological functions and achieving genome-wide visualizations of such annotations. Although numerous methods now exist for analyzing ChIP-seq data, independent biological validation remains an essential step to confirm the accuracy of any data derived from genome-wide approaches.
Overlaying ChIP-seq and RNA-seq Data
In an effort to understand the transcriptional and epigenomic mechanisms underlying RNA expression, a major goal of current research is to merge ChIP-seq and RNA-seq datasets. In fact, if one analyzes a sufficiently large number of epigenomic endpoints (numerous histone modifications, chromatin remodeling factors, transcription factors and other chromatin regulatory proteins) it should in theory be possible to identify “chromatin signatures” that reflect specific modes of transcriptional regulation, such as gene activation or repression as well as gene priming or desensitization.
Accomplishing this task remains challenging given the vast amounts of data (terabytes) involved. One initial approach is to first reduce observed ChIP-seq events to individual genes. This thereby reduces the problem to essentially comparing two gene lists—one from differential analysis of ChIP-seq data and the other from differential analysis of RNA-seq data. GeneOverlap (http://www.bioconductor.org/packages/devel/bioc/html/GeneOverlap.html), a bioconductor package for testing and visualizing gene overlaps, can be used for this purpose. However, such analyses oversimplify the problem. First, a given histone modification can show opposite changes across the span of a given gene. Also, all histone modifications regulate gene expression in a highly cooperative but complex fashion. For example, the histone modification, H3K4me3, is highly enriched at active gene promoters and is often used as an indication of transcriptional initiation. However, H3K4me3 levels at some genes can increase, in concert with other DNA-binding proteins (e.g., RNA polymerase II [Pol 2] serine 5 phosphorylation), in response to a specific stimulus without increasing the expression of its cognate gene, a phenomenon thought to indicate gene “poising.” And in a much smaller number of examples, H3K4me3 has been linked to gene repression when it associates with ING2 (inhibitor of growth family-2), a chromatin regulatory protein74. On the other hand, the histone mark, H3K9me3, which typically enriches within the gene bodies of silenced genes along with numerous other corepressor proteins, may exhibit increased enrichment with distinct binding partners to promote alternative splicing75.
Previously, numerous groups76,77 have attempted to use histone modifications to predict gene expression at baseline states in cultured cells and have achieved good performance. These groups were able to generate predicted expression levels correlating with real expression levels with coefficients as high as 0.8. Their approach worked by separating gene bodies into dozens of bins, where the relative enrichment of histone marks was determined. In this case, enrichment values serve as observed data and corresponding gene expression levels serve as target variables. Such training data were then fed into machine-based learning methods, such as support vector machines or generalized linear models, to learn how the behavior of histone modifications relates to gene expression. This approach, however, has not yet been applied to brain tissue at rest and, in particular, to data examining the relationship between stimulus-induced changes in epigenomic endpoints and gene expression, perhaps the most essential question facing molecular neuroscientists interested in examining the role of epigenomic mechanisms in mediating the impact of environmental manipulations on the brain.
We recently developed a bioinformatics approach that was successfully applied to studying the actions of repeated cocaine exposure in the mouse nucleus accumbens16, a key brain reward region important in addiction. We first correlated ChIP-seq data for six histone modifications and for total Pol 2 binding with changes in gene expression as determined by RNA-seq. While we were able to demonstrate >50 distinct chromatin signatures that correlate with altered RNA expression, the correlations were relatively weak and not deterministic. This suggests that a far larger number of chromatin endpoints are needed to make such predictions; indeed, current research is revealing large numbers of previously unappreciated histone modifications (see below). Additional considerations unique to the analysis of brain tissue are also likely involved (see Box 1).
We used a similar approach to determine whether repeated cocaine-induced changes in alternative splicing in nucleus accumbens correlate with changes in chromatin modifications50. We focused on genomic regions most closely associated with splicing, such as variant exons, alternative donors, alternative acceptors and their neighboring intronic regions, and examined numerous chromatin modifications at these regions. We applied this information extraction procedure to all known transcripts of the mouse genome and calculated the log2 fold changes between repeated cocaine and saline and loaded the values into a data matrix so that each row represents a transcript and each column represents a histone mark-gene region combination (e.g., a H3K4me3 peak at the proximal promoter of that gene). We also used cufflinks to determine the expression changes of these transcripts. K-means clustering was applied on the chromatin modification matrix to identify clusters of transcripts that show similar patterns. These clusters were then correlated with transcripts’ expression changes to determine whether there is any significant overlap. We were able to identify 29 such clusters or chromatin modification signatures. Further analysis of these signatures using motif finding revealed several important splicing factors and transcription factors deduced to be important in their regulation. One of the splicing factors, A2BP1 (Rbfox1/Fox-1), was validated by showing that knocking out A2BP1 in adult nucleus accumbens blocks cocaine’s regulation of several of the genes identified in this genome-wide analysis. Moreover, A2BP1 knockout was shown to attenuate behavioral responses to cocaine16. These findings illustrate how the overlay of ChIP-seq and RNA-seq data can reveal fundamentally new insight into the biological basis of a complex brain disorder.
Overlaying DNA Methylation with Gene Expression Analyses
Methylation of cytosine bases in DNA (5-methylcytosine or 5mC) has historically been viewed as an important mode of gene repression. However, many alternative forms of DNA methylation have been demonstrated in recent years, most importantly, 5-hydroxycytosine (5hmC), which is enriched in brain and associated with gene activation78. Despite the implication of DNA methylation in numerous neuropsychiatric phenomena, virtually all studies to date have focused on individual candidate genes, with genome-wide explorations of brain sorely lacking.
Several methods, described in the companion review19, are used to obtain a genome-wide map of 5mc and 5hmc, but doing so at single nucleotide resolution remains a challenge due to the sequencing costs involved. These methods include genome-wide bisulfite sequencing (BS-seq)—including oxidized BS-seq (oxBS-seq) to distinguish 5mc and 5hmc, RRBS (reduced representation bisulfite sequencing) which focuses on CG rich regions, and meDIP-seq which involves immunoprecipitation of genomic DNA fragments with an antibody directed against 5mC or 5hmC followed by deep sequencing. The human “methylome” can be obtained from a chip-based method, however, available chips do not distinguish between 5mC and 5hmC.
The first step in analyzing BS-seq data is to trim the adaptors and low quality 3’ ends. Trim Galore! (http://www.bioinformatics.babraham.ac.uk/projects/trim_galore/), which wraps around Cutadapt and fastqc to provide extra functionality for RRBS libraries, can be used for this purpose; it reports the sequencing QC by calling fastqc after trimming. Then the reads are aligned to the reference genome. Generally, the aligners need a preprocessing step to tolerate the conversion of C to T. Two strategies of aligners are available. One is a wild-card aligner, such as BSMAP (https://code.google.com/p/bsmap/)79, as all Cs in a reference genome are replaced by a wild-card letter (e.g., “Y”), with both C and T possibly aligned to Y. Another approach is a three-letter aligner, such as Bismark (http://www.bioinformatics.babraham.ac.uk/projects/bismark/)80; here, all Cs in reads and a reference genome are converted to Ts, whereas Gs are converted to As equivalent to C-to-A conversions on the reverse strand, and the aligners process alignments in only three-letter alphabets (A, G and T). Wild-card aligners may reach better genomic coverage while potentially introducing bias to increase DNA methylation levels, compared with three-letter aligners81. After the alignment, the quantification of absolute DNA methylation can be achieved by counting the alignments, referred to as “methylation calling”.
The next step is to detect sites of differential methylated regions (DMRs) in experimental vs. control conditions. Basic analyses like t-tests or Wilcoxon rank tests can be applied; details about more advanced methods have been reviewed recently81. oxbs-sequencing-qc (https://code.google.com/p/oxbs-sequencing-qc) is a pipeline based on Bismark, including trimming of adaptors and low quality, alignment and methylation calling. Users need to implement the detection of DMRs by other analytical tools. MOABS (http://code.google.com/p/moabs/)82 is another pipeline, developed by the authors of BSMAP. It calls and accepts the alignment results from BSMAP or Bismark, and implements the detection of DMRs based on a Beta-Binomial hierarchical model.
As the final step of data analysis, DMR events detected by BS-seq or oxBS-seq are annotated by region_analysis or ChIPpeakAnno, and then integrated with differential expressed genes detected by RNA-seq, or with ChIP-seq characterizations of other epigenomic endpoints, as described in the ChIP-seq section above. Approaches similar to ChIP-seq are used to analyze sequencing data obtained from meDIP-seq experiments. Reads are first evaluated for QC and then aligned to a reference genome using the various tools outlined above, with the number of aligned reads used for methylation calling83. In addition to canonical CpG methylation, highly conserved non-CpG methylation (mCH, where H is either A, T or C) may also play an important role in the central nervous system. It was recently reported that non-CpG methylation accumulates in neurons, but not glia, of the cerebral cortex during development13.
Reconstruction of Multi-Scale Biological Networks
A high priority for current research is to optimize ways of uniting genome-wide measures of RNA expression and chromatin modifications with human DNA sequence and other clinical data. Several genome-wide methods aimed at assessing individual differences in DNA sequence—including genome-wide association studies (GWAS) or whole exome or genome sequencing—are uncovering large numbers of genetic loci associated with neuropsychiatric disease states such as lzheimer’s disease, Parkinson’s Disease, autism and schizophrenia/bipolar disorder among many others. While these studies are identifying common and rare sequence variations, available data only explain a small portion of the genetic contribution of most of these illnesses84. Also, gene sequence analysis alone is insufficient to uncover causal mechanisms regarding gene/pathway dysregulation that gives rise to the disease. Increasingly available large scale genomic, epigenomic and clinical data informing neuropsychiatric disorders, derived from humans and animal models, is now making it possible for the first time to more comprehensively uncover key mechanisms and regulators of these diseases. For example, the nearly completed Cancer Genome Atlas project (TCGA) aims to provide a comprehensive genomic, epigenomic and pathophysiological characterization of over 30 different types of cancers including glioblastoma85. The ongoing Genotype Tissue Expression project (GTEx) is generating a compendium of genotypic and gene expression data in many human tissues including cerebral cortex and cerebellum86. For such large-scale molecular datasets, several systems/network approaches have been developed to identify and dissect the underlying “interactomes” for the discovery of key mechanisms and causal regulators in normal or pathological biological systems.
Different methods of analyzing correlated gene regulation, such as weighted gene co-expression network analysis (WGCNA)87, aims to capture total interactions among genes in a more comprehensive manner than traditional un-weighted approaches and has been used to identify coexpressed gene modules and key genes associated with glioblastoma88, late-onset lzheimer’s disease (LOAD)89-91 and autism92,93. Given the correlative nature of WGCNA, it can neither predict causal relationships nor identify causal regulators. For instance, to identify master regulators of LOAD, Rhinn et al. developed a differential co-expression analysis (DCA) approach to integrate differential expression and differential correlation to identify APOE ε4 effectors94. While DCA can pinpoint master regulators, DCA by itself does not provide a context of causal networks to further understand the underlying subnetworks and pathways controlled by master regulators.
Recently, a more comprehensive effort was made to build multiscale gene regulatory networks from large scale genetic, genomic and pathophysiological data for identification of key mechanisms and causal regulators in LOAD95. A key component of this multiscale network analysis (MNA) approach is to integrate gene coexpression and causal network analyses so as to make full use of the multiscale biological data. Gene modules that are comprised of highly interacting genes through WGCNA are rank ordered based on their correlations with clinical outcomes and enrichment for differential expression in LOAD when compared with normal controls. The causal relationships among the genes in each module are then determined by causal network inference that integrates gene expression data and prior information derived from expression-associated gene sequence variations such as single nucleotide polymorphisms (SNPs) and quantitative trait loci (QTL). Bayesian networks are then used to identify key causal regulators based on network connectivity.
MNA is in line with the recent finding that no single network inference method is universally best across multiple datasets and the best strategy is to integrate multiple networks constructed from different approaches96. MNA is a natural framework to integrate genomic, epigenomic and clinical data. Gene expression, copy number variations (CNV) and CpG sites can be put together to create comprehensive interaction networks using WGCNA or other approaches to fully capture interactions and coordination among gene expression, DNA methylation and DNA sequence variations. Regulatory relationships identified through the analysis of ChIP-seq data, expression-associated CNVs (eCNVs) and expression-associated DNA methylation sites (eMSs) can be taken as priors for causal network inference97. In addition, such analyses can reveal how DNA sequence variations across individuals help to determine epigenomic modifications (including DNA methylation, histone modifications and many others), both within those regions (“cis”) or at distant interacting genomic regions (“trans”). Genetic and epigenetic markers that are identified in conjunction with altered gene expression reflect molecular features related to phenotypic changes. Several approaches have been developed to formally disentangle the causality among differences in DNA sequence, epigenetic modifications, and gene expression across numerous genes98,99. With increasingly available large scale molecular profiling and clinical data, and the rapid evolution of network inference approaches (see Figure 3), we expect to derive a far more comprehensive picture of molecular pathways and key regulators underlying neuropsychiatric diseases in the next decade.
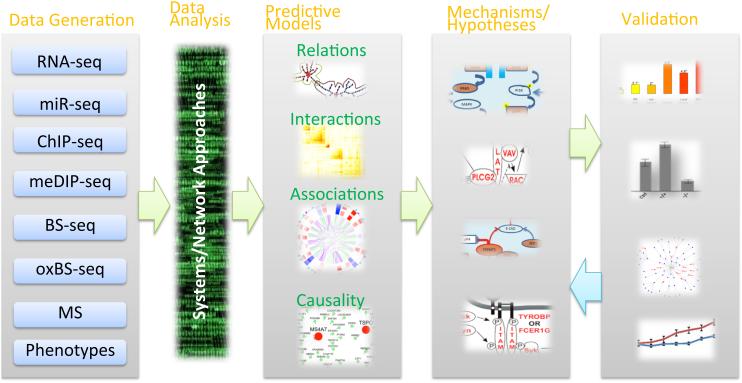
Figure 3. Network Inference Approaches in Neuroscience.
Diagrammatic representation of systems/network approaches to integrate large scale genomic, epigenomic and clinical data to inform investigations of neurological and psychiatric disorders using data derived from humans and animal models. Correlational, interaction and causal analyses can be unified under network level frameworks to generate data-driven hypotheses (i.e., models) concerning the underlying molecular mechanisms or biomarkers resulting in neuronal dysfunction. These models are then further validated experimentally, data that can be fed back into modeling processes to refine biological predictions.
MNA can generate a large number of hypotheses (i.e., models) concerning the underlying molecular mechanisms or biomarkers for complex diseases, such as neuropsychiatric disorders. Validation of MNA-derived models and mechanisms can only be achieved through perturbation (overexpression, knockdown, pharmacological manipulation, etc.) of individual key causal regulators in in vitro and eventually in vivo experiments from which phenotypic changes are examined and compared with predicted outcomes98,88,97,94,95. Full validation of network-based mechanisms for complex diseases, however, is still prohibitive due to high costs, intensive labor efforts and a lack of suitable in vitro and in vivo models for many common nervous system disorders. Recent progress in stem cell research has made it possible to model aspects of neuropsychiatric diseases using induced pluripotent stem cells (iPSCs) derived from reprogrammed somatic cells. Such advances will likely pave a way to systematically investigate genetic and epigenetic endpoints associated with disease states in such in vitro systems, which could then be integrated with data obtained from human postmortem brain tissue and from animal models.
Proteomic Exploration of Epigenomic Mechanisms
Mass Spectrometry in Epigenomics Research
Mass spectrometry (MS) has become a powerful tool for the analyses of DNA methylation and histone modifications100 with some newer applications to small non-coding RNAs101. However, much of this has been at the global chromatin level and not at specific genes102. Identification and quantification of histone PTMs from a variety of cells and tissues have been the focus of several studies, and typically involve examining the protein by Bottom Up, Middle Down or Top Down MS102.
Bottom Up MS refers to digestion of the protein into small (<3 kDa) size peptides, and analysis of those peptide typically by nanoLC-MS/MS. These experiments are fairly high-throughput and easier to perform than Middle or Top Down MS. The Bottom Up MS approach has been coupled with many diverse platforms and quantification schemes (e.g., stable isotope labeling approaches)103 to compare the histone PTM profiles from various cellular sources and states. This Bottom Up approach has been applied to the global analysis of core histone PTMs and variants from whole brain of adult C57BL/6 mice104. This large-scale proteomics analysis identified more than 10,000 peptides creating a dataset that containing 146 modification sites on 1475 peptides in various combinatorial patterns on short peptides. Among these histone peptides, 58 novel sites of modification were discovered. Bottom Up MS in fact has led the way to identify novel marks on histones, such as crotonylation, succinylation, malonylation and 2-hydroxyisobutyrylation of lysine residues105-107 and serine/threonine O-acetylation108.
Middle Down MS involves interrogation of polypeptides over the 3 kDa molecular weight range. These MS experiments allow for long-range spanning PTMs to be identified on the same peptide to determine a long distance combinatorial code, and has mainly been focused on the N-terminal tails of the histone proteins. Currently, it has been determined by this approach that there exists >200 combinatorially modified N-terminal forms of histone H3, with about half as many N-terminal forms existing on histone H4109. These numbers are far lower than those that are theoretically possible, seemingly indicating that much specificity exists for creating multiply modified histone forms. Kelleher and co-workers used this approach to identify histone PTM patterns and variants from whole brain, cerebral cortex, cerebellum and hypothalamus from 7-8 week old Sprague–Dawley rats110. Interesting patterns of modifications were found, including more silencing modifications such as H3K9me3 in the cerebellum.
Analysis of intact histone proteins (Top Down MS) is a challenging task, but allows for the characterization of combinations of modifications across the entire protein sequence. Top Down MS has been used to identify over 700 unique histone forms across all the core histones111 but still remains the most technically challenging MS experiment to perform.
Data Processing for Histone PTM Analyses
As can be inferred from the above overview, the data analysis of histones is very complicated due to the many different types of modifications that can be detected, the distinct combinations of these PTMs that can be found and the low level stoichiometry of many histone PTMs, which can often give rise to false positive assignments. Currently, there are numerous computational approaches for both the qualitative and quantitative analysis of histone proteomics datasets. The general workflow for proteomics data is that peptides are resolved by liquid chromatography, typically electrosprayed ionized into the mass spectrometer and full MS spectra of the precursor ions collected. The precursor peptide ions are then selected and fragmented to obtain tandem mass spectra (MS/MS spectra) by various approaches including high energy C-trap dissociation (HCD), collision induced dissociation (CID) or electron transfer dissociation (ETD). Peptides and associated modifications are identified by searching the MS/MS spectra against an organism specific database.
As histone PTM patterns are challenging, several in-house developed algorithms to deal with these datasets have been developed. (e.g., MILP or PTMap)112-114. However, several other database searching engines are commercially available for analyzing histone proteomics data with the most common being Mascot115 or SEQUEST116 but with many others emerging with different specificities or advantages117-119. Searching for a large number of PTMs in one search drastically increases the number of candidate peptides, slowing computational searching speed, increasing false positive IDs and even identifying less spectra. Therefore, it has been shown with histone data to best search for classes of modifications one at a time to reduce false positive misassignments. Additionally and equally important to these analyses for histone PTM proteomics datasets is the use of a site localization scoring algorithm120. As histone PTMs occur on peptides with multiple acceptor amino acids (e.g., several lysine residues within a single peptide), it is necessary to either manually confirm the fragment ions or use a localization scoring algorithm to confidently determine on which residue the modification site occurs.
Combining Proteomic with Genomic and Epigenomic Analyses
Most histone proteomics data have been generated from global chromatin extractions, and hence the genomic loci involved are usually lost. The strength in being able to combine genomics-epigenomics level information with proteomics data is that an unbiased assessment of histone PTMs could be performed and mapped back to genomic loci. Several approaches based on tagging of proteins or using antibodies to isolate nucleosomes have been performed121-123 and have resulted in a proteomic portrait of the genomic landscape. For example, histone PTMs and other proteins associated with MSL (male-specific lethal) associated proteins in Drosophila S2 cells were recently mapped by quantitative proteomics123. A ChIP-seq and proteomics study also found that Brd (bromodomain) proteins are found on active Hox genes in cultured HEK cells, with distinct patterns of histone H4 acetylation124. However, both of these studies map histone PTMs, not to a specific locus, but rather over a distribution of enriched genes. Experiments that isolate DNA specific sequences have still greater power to establish protein profiles and histone PTM patterns at a distinct gene. One of the first attempts at this was by Kingston and co-workers using PiCh (Proteomics of isolated Chromatin segments) to isolate telomeric repeats125. Other approaches such as ChAP–MS and iChIP have also all been shown to be very useful for isolation of genomic material for mass spectrometry interrogation126,127, however, it remains to be seen if they will be highly adopted.
Human Brain Evolution and the Challenge of Functional Genomics
Cognitive abilities and neurological and psychiatric diseases apparently unique to modern humans may result from genomic features distinguishing our brains from those of other primates. Because protein coding sequences for synaptic and other neuron-specific genes are extremely highly conserved within the Order Primates128, a significant portion of hominid evolution could be due to DNA sequence changes involving regulatory and non-coding DNA129. However, exploring human-specific regulatory DNA is a daunting task considering that the chimpanzee-human genome comparison alone reveals close to 35×106 single bp and 5×106 multi-bp substitutions and insertion/deletion events130, with the majority of DNA in the human genome encoding functionally active transcripts131.
Thus, given that transcription is the first step connecting genetic information to phenotype, the initial studies on deep sequencing of human and non-human primate (mostly chimpanzee and macaque) brains are providing a treasure trove of human-specific gene expression signatures, with hundreds or thousands of human-specific transcripts, particularly in the genome’s non-repetitive intergenic regions132, with 16% of the estimated 8,000 adenosine-to-inosine exonic RNA editing sites in the cerebral and cerebellar cortex defined by human-specific substitution patterns133. Deep sequencing of human and non-human primate transcriptomes becomes particularly powerful in conjunction with comparative genome analyses. For example, a recent study demonstrated that gene expression divergences in lipid catabolism pathways in the brain of modern humans are driven by shared ancestry with Neanderthals134. In contrast to the rapidly increasing number of RNA-seq studies in primate brains, the deep sequencing of chromatin, including DNA methylation and histone modification profiles, has barely begun. For example, the recent deep sequencing of H3K4me3 revealed hundreds of regulatory sequences with human-specific epigenomic regulation in prefrontal cortical neurons12.
Future Challenges
Characterizing the epigenome of the brain is a daunting task. The very large number of distinct types of epigenomic regulation—including several different types of DNA methylation and the great diversity and number of histone modifications and chromatin regulatory proteins—highlights the vast amount of ChIP-seq and related data that must be generated to capture the complete epigenome. Moreover, there is not one “brain epigenome” but a distinct epigenome for each neuronal and glia cell type in the brain, which likely is in the thousands. This is based on increasing evidence that patterns of DNA methylation and histone modifications at promoters and enhancers are highly specific for cell type and tissue135,4. A key challenge for the field therefore is to develop a consensus for how many such epigenomes must be generated to drive an improved understanding of the brain and its diseases.
Ultimately, this expanding knowledge of the brain’s epigenomes will shed unique light onto mechanisms underlying transcriptional regulation. Such analyses should incorporate richer RNA-seq datasets that provide not simply a single cross-section of RNA expression levels, but provide detailed time courses of how patterns of RNA expression shift dynamically over time in disease models. As well, work in animal models should be integrated increasingly with studies of postmortem human brain tissue, and even with human gene sequencing data and phenotypic characterizations, to drive translational discoveries. The major bioinformatics challenge is thus to optimize the tools to overlay these different and each vast datasets to derive maximal insight into transcriptional control in health and disease. A crucial step is to ensure that all genome-wide data are placed within the public domain in ways that allow raw datasets to be reanalyzed as advances proceed (Box 2).
Text Box 2. Data Deposition.
The sequence read archive (SRA)144 was established as a joint effort between multiple nations and has become the central depository for next-generation sequencing data. It can be accessed at several different URLs depending on the user’s physical location and preference:
| Provider | URL |
|---|---|
| NCBI | http://www.ncbi.nlm.nih.gov/Traces/sra |
| EBI | http://www.ebi.ac.uk/ena |
| DDBJ | http://www.ddbj.nig.ac.ip/sub/trace_sra-e.html |
For functional genomics studies involving short sequence data, such as transcriptomics, small RNA profiling, ChIP-seq and BS-seq, users can upload their data through Gene Expression Omnibus (GEO) at: http://www.ncbi.nlm.nih.gov/geo/info/seq.html. Although SRA also provides an online submission portal, it is often more convenient to submit data (especially for large studies) using GEO. Creating a GEO submission typically requires compiling all raw data, processed data and a metadata spreadsheet into a folder. These data are then transferred to GEO using FTP. For the metadata spreadsheet, submitters are required to briefly describe their study, list all samples including their properties and associated raw data and processed data files, describe experimental protocols, and elaborate on data processing steps and programs used to analyze their data, as well as parameter settings. After data have been successfully transferred, submitters can send an email to GEO to initiate a review of the submission. A GEO curator will examine uploaded files and provide feedback. A GEO accession number will then be created and provided to the submitter. The submitter can then log into their account and review submissions. At this point, submissions remain private. However, a reviewer link can be created to share submissions with journal reviewers. Once manuscripts associated with submissions have been accepted for publication, submitters can return to their login page and make the data public.
The deposition of all genome-wide data into a single resource worldwide is essential for financial reasons: each study is so expensive and so many studies are needed that it is essential to avoid redundant efforts. Furthermore, such datasets will increasingly be of great use to investigators worldwide as raw data can be reanalyzed with newer, more powerful tools and as interpretation of a new epigenomic mark can be vastly improved by overlays of maps of many available marks.
The particular complexity of the genetic risk for most common brain disorders adds yet another challenge. For example, a recent GWAS of the Psychiatric Genomics Consortium, involving approximately 40,000 subjects, estimated that 8,300 independent SNPs, positioned mostly in intergenic and (protein)-non-coding sequences, contributed to the genetic risk for schizophrenia136. Strikingly, however, there was no significant enrichment for DNAse I hypersensitive sites (which broadly define open chromatin primarily at sites of active promoters, enhancers and expressed genes) identified by the ENCODE project137. This contrasts with the robust enrichment for DNAse I hypersensitive sites of the general GWAS catalog of the National Genome Research Institute138. Because the ENCODE database is exclusively built on epigenomic mappings from peripheral cell lines and tissues, it is clear that similar efforts, focused on brain and some of its surrogates, such as pluripotent stem cell-derived neural cultures139 or cerebral organoids140, are now needed to obtain a deeper understanding of the genetic risk architectures of these complex disorders. Furthermore, extending these analyses to disease-linked alterations in chromatin structure over large genomic regions in brain (Box 3) promises to provide great insights into the molecular causes of these heterogeneous syndromes.
Text Box 3. HiC: A Sequencing Approach to Characterize Interactions Over Large Genomic Regions.
HiC is an unbiased high throughput method to detect chromatin-looping interactions between all loci in the genome145. HiC experiments have shown that genome structure and function are linked. In yeast, looping interaction groups form between highly transcribed genes, co-regulated genes (associated with different motifs) and gene ontology groups146,147. Human HiC studies cluster looping interactions into three groups: self-interacting active gene rich clusters, non-active centromere proximal clusters and non-active centromere distal clusters148. In bacteria, yeast, and humans, chromatin loops place enhancers and promoters in close spatial proximity, which are thought to exist in topological domains bound by insulator proteins.
HiC libraries are created using proximity ligation. Tissue or cells are homogenized and crosslinked using 1% formaldehyde and restricted using an enzyme of choice. The 5’ overhangs are filled in with a biotinylated CTP residue and blunt-end ligation is performed under dilute conditions. The library is sheared for 300-500 bp size selection and biotinylated fragments are immunoprecipitated using streptavidin beads. The library is pair-end sequenced and aligned to the corresponding genome. Reads that map to two locations are scored as interactions and an interaction matrix from all interactions in the experiment is created. An expected interaction matrix is used to calculate the statistical significance of all scored experimental interactions.
Current HiC algorithms model looping interactions probabilistically by taking into account mappability, fragment length and GC percentage (which is correlated with gene density, banding patterns, repetitive content and chromatin marks)148. The best algorithm to date can resolve a human HiC map from cell lines to 5-10 kb using 63% mappable reads (300 million reads mapped from 500 million total reads) using four lanes of Illumina sequencing149. Problems from these algorithms arise from the probabilistic nature of proximity ligation. HiC detects the average chromatin structure within a population of cells. Failure to detect looping interactions does not mean they do not exist, but rather that the current method does not detect them148. In time, the efficiency of algorithms will further increase, and the cost of sequencing will decrease, making HiC a more amenable approach for cell-type specific studies in brain.
To this end, the National Institutes of Health-based initiatives that include epigenomic mappings of human brain, including the Epigenomics Roadmap or, more recently, PsychENCODE, will provide critical starting points to what should be the ultimate goal of modern neuroepigenomics: whole genome, high resolution (single base pair) maps for a large number of epigenomic marks for key anatomically defined brain regions and their important subpopulations of neurons and glia. This type of resource is likely needed to expedite the dramatic, game-changing discoveries from integrated studies of animal models and human patients. Such transformational advances are sorely needed to finally overcome the limited success in diagnostics and drug discovery efforts over the past several decades and to develop truly improved diagnostic tests and treatments for a host of severe neurological and psychiatric disorders.
Acknowledgments
We would like to thank Dr. Alexey Soshnev for help with illustrations.. This work was supported by grants from the National Institute of Mental Health (NIMH): 5R01 MH094698 and P50 MH096890 and the National Institute on Drug Abuse: P01 DA008227.
Footnotes
Competing Financial Interests
The authors declare no competing financial interests
References
- 1.Renthal W, Carle TL, et al. Delta FosB mediates epigenetic desensitization of the c-fos gene after chronic amphetamine exposure. J Neurosci. 2008;28:7344–9. doi: 10.1523/JNEUROSCI.1043-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maze I, Covington HE, 3rd, et al. Essential role of the histone methyltransferase G9a in cocaine-induced plasticity. Science. 2010;327:213–6. doi: 10.1126/science.1179438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Visel A, Blow MJ, et al. ChIP-seq accurately predicts tissue-specific activity of enhancers. Nature. 2009;457:854–8. doi: 10.1038/nature07730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cheung I, Shulha HP, et al. Developmental regulation and individual differences of neuronal H3K4me3 epigenomes in the prefrontal cortex. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:8824–9. doi: 10.1073/pnas.1001702107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peleg S, Sananbenesi F, et al. Altered histone acetylation is associated with age-dependent memory impairment in mice. Science. 2010;328:753–6. doi: 10.1126/science.1186088. [DOI] [PubMed] [Google Scholar]
- 6.Guo JU, Ma DK, et al. Neuronal activity modifies the DNA methylation landscape in the adult brain. Nature Neuroscience. 2011;14:1345–51. doi: 10.1038/nn.2900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maze I, Feng J, et al. Cocaine dynamically regulates heterochromatin and repetitive element unsilencing in nucleus accumbens. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:3035–40. doi: 10.1073/pnas.1015483108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Szulwach KE, Li X, et al. 5-hmC-mediated epigenetic dynamics during postnatal neurodevelopment and aging. Nature Neuroscience. 2011;14:1607–16. doi: 10.1038/nn.2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou Z, Yuan Q, et al. Substance-specific and shared transcription and epigenetic changes in the human hippocampus chronically exposed to cocaine and alcohol. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:6626–31. doi: 10.1073/pnas.1018514108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hunter RG, Murakami G, et al. Acute stress and hippocampal histone H3 lysine 9 trimethylation, a retrotransposon silencing response. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:17657–62. doi: 10.1073/pnas.1215810109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mellen M, Ayata P, et al. MeCP2 binds to 5hmC enriched within active genes and accessible chromatin in the nervous system. Cell. 2012;151:1417–30. doi: 10.1016/j.cell.2012.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shulha HP, Crisci JL, et al. Human-specific histone methylation signatures at transcription start sites in prefrontal neurons. PLoS Biology. 2012;10:e1001427. doi: 10.1371/journal.pbio.1001427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lister R, Mukamel EA, et al. Global epigenomic reconfiguration during mammalian brain development. Science. 2013;341:1237905. doi: 10.1126/science.1237905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Park CS, Rehrauer H, et al. Genome-wide analysis of H4K5 acetylation associated with fear memory in mice. BMC Genomics. 2013;14:539. doi: 10.1186/1471-2164-14-539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhu J, Adli M, et al. Genome-wide chromatin state transitions associated with developmental and environmental cues. Cell. 2013;152:642–54. doi: 10.1016/j.cell.2012.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Feng J, Wilkinson M, et al. Chronic cocaine-regulated epigenomic changes in mouse nucleus accumbens. Genome Biology. 2014;15:R65. doi: 10.1186/gb-2014-15-4-r65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guo JU, Su Y, et al. Distribution, recognition and regulation of non-CpG methylation in the adult mammalian brain. Nature Neuroscience. 2014;17:215–22. doi: 10.1038/nn.3607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scobie KN, Damez-Werno D, et al. Essential role of poly(ADP-ribosyl)ation in cocaine action. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:2005–10. doi: 10.1073/pnas.1319703111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.TBD Other review on epigenetic methods in this special issue. Nature Neuroscience. 2014 [Google Scholar]
- 20.Mortazavi A, Williams BA, et al. Mapping and quantifying mammalian transcriptomes by RNA-Seq. Nature methods. 2008;5:621–8. doi: 10.1038/nmeth.1226. [DOI] [PubMed] [Google Scholar]
- 21.Nagalakshmi U, Wang Z, et al. The transcriptional landscape of the yeast genome defined by RNA sequencing. Science. 2008;320:1344–9. doi: 10.1126/science.1158441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wilhelm BT, Marguerat S, et al. Dynamic repertoire of a eukaryotic transcriptome surveyed at single-nucleotide resolution. Nature. 2008;453:1239–43. doi: 10.1038/nature07002. [DOI] [PubMed] [Google Scholar]
- 23.Marioni JC, Mason CE, et al. RNA-seq: an assessment of technical reproducibility and comparison with gene expression arrays. Genome Research. 2008;18:1509–17. doi: 10.1101/gr.079558.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nookaew I, Papini M, et al. A comprehensive comparison of RNA-Seq-based transcriptome analysis from reads to differential gene expression and cross-comparison with microarrays: a case study in Saccharomyces cerevisiae. Nucleic Acids Research. 2012;40:10084–97. doi: 10.1093/nar/gks804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhao S, Fung-Leung WP, et al. Comparison of RNA-Seq and microarray in transcriptome profiling of activated T cells. PLoS One. 2014;9:e78644. doi: 10.1371/journal.pone.0078644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.DeLuca DS, Levin JZ, et al. RNA-SeQC: RNA-seq metrics for quality control and process optimization. Bioinformatics. 2012;28:1530–1532. doi: 10.1093/bioinformatics/bts196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shen L, Shao N, et al. ngs.plot: Quick mining and visualization of next-generation sequencing data by integrating genomic databases. BMC genomics. 2014;15:284. doi: 10.1186/1471-2164-15-284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Trapnell C, Williams BA, et al. Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nature Biotechnology. 2010;28:511–5. doi: 10.1038/nbt.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grabherr MG, Haas BJ, et al. Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nature Biotechnology. 2011;29:644–52. doi: 10.1038/nbt.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Trapnell C, Pachter L, et al. TopHat: discovering splice junctions with RNA-Seq. Bioinformatics. 2009;25:1105–1111. doi: 10.1093/bioinformatics/btp120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dobin A, Davis CA, et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics. 2013;29:15–21. doi: 10.1093/bioinformatics/bts635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Engstrom PG, Steijger T, et al. Systematic evaluation of spliced alignment programs for RNA-seq data. Nature methods. 2013;10:1185–91. doi: 10.1038/nmeth.2722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Trapnell C, Williams BA, et al. Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat Biotech. 2010;28:511–515. doi: 10.1038/nbt.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Katz Y, Wang ET, et al. Analysis and design of RNA sequencing experiments for identifying isoform regulation. Nature Methods. 2010;7:1009–15. doi: 10.1038/nmeth.1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Deal RB, Henikoff S. A simple method for gene expression and chromatin profiling of individual cell types within a tissue. Developmental Cell. 2010;18:1030–40. doi: 10.1016/j.devcel.2010.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Quinlan AR, Hall IM. BEDTools: a flexible suite of utilities for comparing genomic features. Bioinformatics. 2010;26:841–2. doi: 10.1093/bioinformatics/btq033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Martin M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet.journal. 2011;17:10–12. [Google Scholar]
- 38.Kozomara A, Griffiths-Jones S. miRBase: annotating high confidence microRNAs using deep sequencing data. Nucleic Acids Research. 2014;42:D68–73. doi: 10.1093/nar/gkt1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Friedlander MR, Chen W, et al. Discovering microRNAs from deep sequencing data using miRDeep. Nat Biotech. 2008;26:407–415. doi: 10.1038/nbt1394. [DOI] [PubMed] [Google Scholar]
- 40.Lestrade L, Weber MJ. snoRNA-LBME-db, a comprehensive database of human H/ACA and C/D box snoRNAs. Nucleic Acids Research. 2006;34:D158–62. doi: 10.1093/nar/gkj002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sai Lakshmi S, Agrawal S. piRNABank: a web resource on classified and clustered Piwi-interacting RNAs. Nucleic Acids Research. 2008;36:D173–7. doi: 10.1093/nar/gkm696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chan PP, Lowe TM. GtRNAdb: a database of transfer RNA genes detected in genomic sequence. Nucleic Acids Research. 2009;37:D93–7. doi: 10.1093/nar/gkn787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mituyama T, Yamada K, et al. The Functional RNA Database 3.0: databases to support mining and annotation of functional RNAs. Nucleic Acids Research. 2009;37:D89–92. doi: 10.1093/nar/gkn805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Amaral PP, Clark MB, et al. lncRNAdb: a reference database for long noncoding RNAs. Nucleic Acids Research. 2011;39:D146–51. doi: 10.1093/nar/gkq1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Burge SW, Daub J, et al. Rfam 11.0: 10 years of RNA families. Nucleic Acids Research. 2013;41:D226–32. doi: 10.1093/nar/gks1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Quast C, Pruesse E, et al. The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Research. 2013;41:D590–6. doi: 10.1093/nar/gks1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xie C, Yuan J, et al. NONCODEv4: exploring the world of long non-coding RNA genes. Nucleic Acids Research. 2014;42:D98–103. doi: 10.1093/nar/gkt1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Anders S, Huber W. Differential expression analysis for sequence count data. Genome Biol. 2010;11:R106. doi: 10.1186/gb-2010-11-10-r106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Robinson MD, McCarthy DJ, et al. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics. 2010;26:139–40. doi: 10.1093/bioinformatics/btp616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Law C, Chen Y, et al. voom: precision weights unlock linear model analysis tools for RNA-seq read counts. Genome Biology. 2014;15:R29. doi: 10.1186/gb-2014-15-2-r29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Smyth GK. limma: Linear Models for Microarray Data. In: Gentleman R, Carey V, Huber W, Irizarry R, Dudoit S, editors. Bioinformatics and Computational Biology Solutions Using R and Bioconductor. Springer; New York: 2005. pp. 397–420. [Google Scholar]
- 52.Love MI, Huber W, et al. Moderated estimation of fold change and dispersion for RNA-Seq data with DESeq2. bioRxiv beta. 2014 doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rapaport F, Khanin R, et al. Comprehensive evaluation of differential gene expression analysis methods for RNA-seq data. Genome Biology. 2013;14:R95. doi: 10.1186/gb-2013-14-9-r95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liu Y, Zhou J, et al. RNA-seq differential expression studies: more sequence or more replication? Bioinformatics. 2014;30:301–4. doi: 10.1093/bioinformatics/btt688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Trapnell C, Hendrickson DG, et al. Differential analysis of gene regulation at transcript resolution with RNA-seq. Nat Biotech. 2013;31:46–53. doi: 10.1038/nbt.2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Seyednasrollah F, Laiho A, et al. Comparison of software packages for detecting differential expression in RNA-seq studies. Briefings in Bioinformatics. 2013 doi: 10.1093/bib/bbt086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Langmead B, Trapnell C, et al. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biology. 2009;10:R25. doi: 10.1186/gb-2009-10-3-r25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li H, Durbin R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics. 2009;25:1754–60. doi: 10.1093/bioinformatics/btp324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li H, Handsaker B, et al. The Sequence Alignment/Map format and SAMtools. Bioinformatics. 2009;25:2078–9. doi: 10.1093/bioinformatics/btp352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chen K, Xi Y, et al. DANPOS: dynamic analysis of nucleosome position and occupancy by sequencing. Genome Research. 2013;23:341–51. doi: 10.1101/gr.142067.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kundaje A. Computes quick but highly informative enrichment and quality measures for ChIP-seq/DNase-seq/FAIRE-seq/MNase-seq data. Computer Science Dept., Stanford University, ENCODE Consortium; 2010. [Google Scholar]
- 62.Landt SG, Marinov GK, et al. ChIP-seq guidelines and practices of the ENCODE and modENCODE consortia. Genome Research. 2012;22:1813–1831. doi: 10.1101/gr.136184.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Robinson JT, Thorvaldsdóttir H, et al. Integrative genomics viewer. Nature Biotechnology. 2011;29:24–26. doi: 10.1038/nbt.1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chen Y, Negre N, et al. Systematic evaluation of factors influencing ChIP-seq fidelity. Nature methods. 2012;9:609–14. doi: 10.1038/nmeth.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Carroll TS, Liang Z, et al. Impact of artifact removal on ChIP quality metrics in ChIP-seq and ChIP-exo data. Frontiers in genetics. 2014;5:75. doi: 10.3389/fgene.2014.00075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhang Y, Liu T, et al. Model-based Analysis of ChIP-Seq (MACS) Genome Biology. 2008;9 doi: 10.1186/gb-2008-9-9-r137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Heinz S, Benner C, et al. Simple Combinations of Lineage-Determining Transcription Factors Prime cis-Regulatory Elements Required for Macrophage and B Cell Identities. Molecular Cell. 2010;38:576–589. doi: 10.1016/j.molcel.2010.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shen L, Shao N-Y, et al. diffReps: Detecting Differential Chromatin Modification Sites from ChIP-seq Data with Biological Replicates. PLoS ONE. 2013;8:e65598. doi: 10.1371/journal.pone.0065598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhu LJ, Gazin C, et al. ChIPpeakAnno: a Bioconductor package to annotate ChIP-seq and ChIP-chip data. BMC Bioinformatics. 2010;11:237. doi: 10.1186/1471-2105-11-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Liang K, Keleş S. Detecting differential binding of transcription factors with ChIP-seq. Bioinformatics. 2011 doi: 10.1093/bioinformatics/btr605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wright K. corrgram: Plot a correlogram. R Package version 1.5. 2013 http://CRAN.R-project.org/package=corrgram.
- 72.Landt SG, Marinov GK, et al. ChIP-seq guidelines and practices of the ENCODE and modENCODE consortia. Genome Research. 2012;22:1813–31. doi: 10.1101/gr.136184.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ernst J, Kellis M. ChromHMM: automating chromatin-state discovery and characterization. Nature methods. 2012;9:215–6. doi: 10.1038/nmeth.1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Shi X, Hong T, et al. ING2 PHD domain links histone H3 lysine 4 methylation to active gene repression. Nature. 2006;442:96–9. doi: 10.1038/nature04835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Saint-André V, Batsché E, et al. Histone H3 lysine 9 trimethylation and HP1γ favor inclusion of alternative exons. Nat Struct Mol Biol. 2011;18:337–344. doi: 10.1038/nsmb.1995. [DOI] [PubMed] [Google Scholar]
- 76.Cheng C, Yan KK, et al. A statistical framework for modeling gene expression using chromatin features and application to modENCODE datasets. Genome Biology. 2011;12 doi: 10.1186/gb-2011-12-2-r15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dong X, Greven M, et al. Modeling gene expression using chromatin features in various cellular contexts. Genome Biology. 2012;13:R53. doi: 10.1186/gb-2012-13-9-r53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kriaucionis S, Heintz N. The nuclear DNA base 5-hydroxymethylcytosine is present in Purkinje neurons and the brain. Science. 2009;324:929–30. doi: 10.1126/science.1169786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Xi Y, Li W. BSMAP: whole genome bisulfite sequence MAPping program. BMC bioinformatics. 2009;10:232. doi: 10.1186/1471-2105-10-232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Krueger F, Andrews SR. Bismark: a flexible aligner and methylation caller for Bisulfite-Seq applications. Bioinformatics. 2011;27:1571–2. doi: 10.1093/bioinformatics/btr167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bock C. Analysing and interpreting DNA methylation data. Nature reviews. Genetics. 2012;13:705–19. doi: 10.1038/nrg3273. [DOI] [PubMed] [Google Scholar]
- 82.Sun D, Xi Y, et al. MOABS: model based analysis of bisulfite sequencing data. Genome biology. 2014;15:R38. doi: 10.1186/gb-2014-15-2-r38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Down TA, Rakyan VK, et al. A Bayesian deconvolution strategy for immunoprecipitation-based DNA methylome analysis. Nature Biotechnology. 2008;26:779–85. doi: 10.1038/nbt1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Goldstein DB. Common genetic variation and human traits. N Engl J Med. 2009;360:1696–8. doi: 10.1056/NEJMp0806284. [DOI] [PubMed] [Google Scholar]
- 85.Kandoth C, McLellan MD, et al. Mutational landscape and significance across 12 major cancer types. Nature. 2013;502:333–9. doi: 10.1038/nature12634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Consortium GT. The Genotype-Tissue Expression (GTEx) project. Nat Genet. 2013;45:580–5. doi: 10.1038/ng.2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhang B, Horvath S. A general framework for weighted gene co-expression network analysis. Stat Appl Genet Mol Biol. 2005;4 doi: 10.2202/1544-6115.1128. Article17. [DOI] [PubMed] [Google Scholar]
- 88.Horvath S, Zhang B, et al. Analysis of oncogenic signaling networks in glioblastoma identifies ASPM as a molecular target. Proc Natl Acad Sci U S A. 2006;103:17402–7. doi: 10.1073/pnas.0608396103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Miller JA, Oldham MC, et al. A systems level analysis of transcriptional changes in Alzheimer's disease and normal aging. J Neurosci. 2008;28:1410–20. doi: 10.1523/JNEUROSCI.4098-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Miller JA, Horvath S, et al. Divergence of human and mouse brain transcriptome highlights Alzheimer disease pathways. Proc Natl Acad Sci U S A. 2010;107:12698–703. doi: 10.1073/pnas.0914257107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Miller JA, Woltjer RL, et al. Genes and pathways underlying regional and cell type changes in Alzheimer's disease. Genome Med. 2013;5:48. doi: 10.1186/gm452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Luo R, Sanders SJ, et al. Genome-wide transcriptome profiling reveals the functional impact of rare de novo and recurrent CNVs in autism spectrum disorders. Am J Hum Genet. 2012;91:38–55. doi: 10.1016/j.ajhg.2012.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Parikshak NN, Luo R, et al. Integrative functional genomic analyses implicate specific molecular pathways and circuits in autism. Cell. 2013;155:1008–21. doi: 10.1016/j.cell.2013.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Rhinn H, Fujita R, et al. Integrative genomics identifies APOE epsilon4 effectors in Alzheimer's disease. Nature. 2013;500:45–50. doi: 10.1038/nature12415. [DOI] [PubMed] [Google Scholar]
- 95.Zhang B, Gaiteri C, et al. Integrated Systems Approach Identifies Genetic Nodes and Networks in Late-Onset lzheimer’s Disease. Cell. 2013;153:14. doi: 10.1016/j.cell.2013.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Marbach D, Costello JC, et al. Wisdom of crowds for robust gene network inference. Nat Methods. 2012;9:796–804. doi: 10.1038/nmeth.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhu J, Zhang B, et al. Integrating large-scale functional genomic data to dissect the complexity of yeast regulatory networks. Nat Genet. 2008;40:854–61. doi: 10.1038/ng.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Schadt EE, Lamb J, et al. An integrative genomics approach to infer causal associations between gene expression and disease. Nature Genetics. 2005;37:710–7. doi: 10.1038/ng1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Swarup V, Geschwind DH. Alzheimer's disease: From big data to mechanism. Nature. 2013;500:34–5. doi: 10.1038/nature12457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Evertts AG, Zee BM, et al. Modern approaches for investigating epigenetic signaling pathways. J Appl Physiol (1985) 2010;109:927–33. doi: 10.1152/japplphysiol.00007.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kullolli M, Knouf E, et al. Intact microRNA analysis using high resolution mass spectrometry. J Am Soc Mass Spectrom. 2014;25:80–7. doi: 10.1007/s13361-013-0759-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Karch KR, Denizio JE, et al. Identification and interrogation of combinatorial histone modifications. Front Genet. 2013;4:264. doi: 10.3389/fgene.2013.00264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ong SE, Blagoev B, et al. Stable isotope labeling by amino acids in cell culture, SILAC, as a simple and accurate approach to expression proteomics. Mol Cell Proteomics. 2002;1:376–86. doi: 10.1074/mcp.m200025-mcp200. [DOI] [PubMed] [Google Scholar]
- 104.Tweedie-Cullen RY, Brunner AM, et al. Identification of combinatorial patterns of post-translational modifications on individual histones in the mouse brain. PLoS One. 2012;7:e36980. doi: 10.1371/journal.pone.0036980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Tan M, Luo H, et al. Identification of 67 histone marks and histone lysine crotonylation as a new type of histone modification. Cell. 2011;146:1016–28. doi: 10.1016/j.cell.2011.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Xie Z, Dai J, et al. Lysine succinylation and lysine malonylation in histones. Mol Cell Proteomics. 2012;11:100–7. doi: 10.1074/mcp.M111.015875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Dai L, Peng C, et al. Lysine 2-hydroxyisobutyrylation is a widely distributed active histone mark. Nature chemical biology. 2014 doi: 10.1038/nchembio.1497. [DOI] [PubMed] [Google Scholar]
- 108.Britton LM, Newhart A, et al. Initial characterization of histone H3 serine 10 O-acetylation. Epigenetics. 2013;8:1101–13. doi: 10.4161/epi.26025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Young NL, DiMaggio PA, et al. High throughput characterization of combinatorial histone codes. Mol Cell Proteomics. 2009;8:2266–84. doi: 10.1074/mcp.M900238-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Garcia BA, Siuti N, et al. Characterization of neurohistone variants and post-translational modifications by electron capture dissociation mass spectrometry. International Journal of Mass Spectrometry. 2007;259:184–196. [Google Scholar]
- 111.Tian Z, Tolic N, et al. Enhanced top-down characterization of histone post-translational modifications. Genome Biol. 2012;13:R86. doi: 10.1186/gb-2012-13-10-r86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Frank AM, Pesavento JJ, et al. Interpreting top-down mass spectra using spectral alignment. Anal Chem. 2008;80:2499–505. doi: 10.1021/ac702324u. [DOI] [PubMed] [Google Scholar]
- 113.Chen Y, Chen W, et al. PTMap--a sequence alignment software for unrestricted, accurate, and full-spectrum identification of post-translational modification sites. Proc Natl Acad Sci U S A. 2009;106:761–6. doi: 10.1073/pnas.0811739106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.DiMaggio PA, Jr., Young NL, et al. A mixed integer linear optimization framework for the identification and quantification of targeted post-translational modifications of highly modified proteins using multiplexed electron transfer dissociation tandem mass spectrometry. Mol Cell Proteomics. 2009;8:2527–43. doi: 10.1074/mcp.M900144-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Perkins DN, Pappin DJ, et al. Probability-based protein identification by searching sequence databases using mass spectrometry data. Electrophoresis. 1999;20:3551–67. doi: 10.1002/(SICI)1522-2683(19991201)20:18<3551::AID-ELPS3551>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 116.Eng JK, McCormack AL, et al. An approach to correlate tandem mass spectral data of peptides with amino acid sequences in a protein database. J Am Soc Mass Spectrom. 1994;5:976–89. doi: 10.1016/1044-0305(94)80016-2. [DOI] [PubMed] [Google Scholar]
- 117.Geer LY, Markey SP, et al. Open mass spectrometry search algorithm. J Proteome Res. 2004;3:958–64. doi: 10.1021/pr0499491. [DOI] [PubMed] [Google Scholar]
- 118.Wang LH, Li DQ, et al. pFind 2.0: a software package for peptide and protein identification via tandem mass spectrometry. Rapid Commun Mass Spectrom. 2007;21:2985–91. doi: 10.1002/rcm.3173. [DOI] [PubMed] [Google Scholar]
- 119.Zhang J, Xin L, et al. PEAKS DB: de novo sequencing assisted database search for sensitive and accurate peptide identification. Mol Cell Proteomics. 2012;11 doi: 10.1074/mcp.M111.010587. M111 010587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Beausoleil SA, Villen J, et al. A probability-based approach for high-throughput protein phosphorylation analysis and site localization. Nat Biotechnol. 2006;24:1285–92. doi: 10.1038/nbt1240. [DOI] [PubMed] [Google Scholar]
- 121.Tackett AJ, Dilworth DJ, et al. Proteomic and genomic characterization of chromatin complexes at a boundary. J Cell Biol. 2005;169:35–47. doi: 10.1083/jcb.200502104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Voigt P, LeRoy G, et al. Asymmetrically modified nucleosomes. Cell. 2012;151:181–93. doi: 10.1016/j.cell.2012.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Wang CI, Alekseyenko AA, et al. Chromatin proteins captured by ChIP-mass spectrometry are linked to dosage compensation in Drosophila. Nat Struct Mol Biol. 2013;20:202–9. doi: 10.1038/nsmb.2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Leroy G, Chepelev I, et al. Proteogenomic characterization and mapping of nucleosomes decoded by Brd and HP1 proteins. Genome Biol. 2012;13:R68. doi: 10.1186/gb-2012-13-8-r68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Dejardin J, Kingston RE. Purification of proteins associated with specific genomic Loci. Cell. 2009;136:175–86. doi: 10.1016/j.cell.2008.11.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Hoshino A, Fujii H. Insertional chromatin immunoprecipitation: a method for isolating specific genomic regions. J Biosci Bioeng. 2009;108:446–9. doi: 10.1016/j.jbiosc.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 127.Byrum SD, Taverna SD, et al. Purification of a specific native genomic locus for proteomic analysis. Nucleic Acids Res. 2013;41:e195. doi: 10.1093/nar/gkt822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.King MC, Wilson AC. Evolution at two levels in humans and chimpanzees. Science. 1975;188:107–16. doi: 10.1126/science.1090005. [DOI] [PubMed] [Google Scholar]
- 129.McLean CY, Reno PL, et al. Human-specific loss of regulatory DNA and the evolution of human-specific traits. Nature. 2011;471:216–9. doi: 10.1038/nature09774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Initial sequence of the chimpanzee genome and comparison with the human genome. Nature. 2005;437:69–87. doi: 10.1038/nature04072. [DOI] [PubMed] [Google Scholar]
- 131.Dunham I, Kundaje A, et al. An integrated encyclopedia of DNA elements in the human genome. Nature. 2012;489:57–74. doi: 10.1038/nature11247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Xu AG, He L, et al. Intergenic and repeat transcription in human, chimpanzee and macaque brains measured by RNA-Seq. PLoS Comput Biol. 2010;6:e1000843. doi: 10.1371/journal.pcbi.1000843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Li Z, Bammann H, et al. Evolutionary and ontogenetic changes in RNA editing in human, chimpanzee, and macaque brains. RNA. 2013;19:1693–702. doi: 10.1261/rna.039206.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Khrameeva EE, Bozek K, et al. Neanderthal ancestry drives evolution of lipid catabolism in contemporary Europeans. Nat Commun. 2014;5:3584. doi: 10.1038/ncomms4584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Heintzman ND, Hon GC, et al. Histone modifications at human enhancers reflect global cell-type-specific gene expression. Nature. 2009;459:108–12. doi: 10.1038/nature07829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Ripke S, O'Dushlaine C, et al. Genome-wide association analysis identifies 13 new risk loci for schizophrenia. Nat Genet. 2013;45:1150–9. doi: 10.1038/ng.2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Bernstein BE, Birney E, et al. An integrated encyclopedia of DNA elements in the human genome. Nature. 2012;489:57–74. doi: 10.1038/nature11247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Maurano MT, Humbert R, et al. Systematic localization of common disease-associated variation in regulatory DNA. Science. 2012;337:1190–5. doi: 10.1126/science.1222794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Brennand KJ, Simone A, et al. Modeling psychiatric disorders at the cellular and network levels. Mol Psychiatry. 2012;17:1239–53. doi: 10.1038/mp.2012.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Lancaster MA, Renner M, et al. Cerebral organoids model human brain development and microcephaly. Nature. 2013;501:373–9. doi: 10.1038/nature12517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Jiang Y, Matevossian A, et al. Isolation of neuronal chromatin from brain tissue. BMC neuroscience. 2008;9:42. doi: 10.1186/1471-2202-9-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Evrony GD, Cai X, et al. Single-neuron sequencing analysis of L1 retrotransposition and somatic mutation in the human brain. Cell. 2012;151:483–96. doi: 10.1016/j.cell.2012.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Grindberg RV, Yee-Greenbaum JL, et al. RNA-sequencing from single nuclei. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:19802–7. doi: 10.1073/pnas.1319700110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Shumway M, Cochrane G, et al. Archiving next generation sequencing data. Nucleic Acids Research. 2010;38:D870–D871. doi: 10.1093/nar/gkp1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Lieberman-Aiden E, van Berkum NL, et al. Comprehensive mapping of long-range interactions reveals folding principles of the human genome. Science. 2009;326:289–93. doi: 10.1126/science.1181369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Tanizawa H, Iwasaki O, et al. Mapping of long-range associations throughout the fission yeast genome reveals global genome organization linked to transcriptional regulation. Nucleic acids research. 2010;38:8164–77. doi: 10.1093/nar/gkq955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Gehlen LR, Gruenert G, et al. Chromosome positioning and the clustering of functionally related loci in yeast is driven by chromosomal interactions. Nucleus. 2012;3:370–83. doi: 10.4161/nucl.20971. [DOI] [PubMed] [Google Scholar]
- 148.Yaffe E, Tanay A. Probabilistic modeling of Hi-C contact maps eliminates systematic biases to characterize global chromosomal architecture. Nature genetics. 2011;43:1059–65. doi: 10.1038/ng.947. [DOI] [PubMed] [Google Scholar]
- 149.Jin F, Li Y, et al. A high-resolution map of the three-dimensional chromatin interactome in human cells. Nature. 2013;503:290–4. doi: 10.1038/nature12644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Hackenberg M, Rodriguez-Ezpeleta N, et al. miRanalyzer: an update on the detection and analysis of microRNAs in high-throughput sequencing experiments. Nucleic Acids Research. 2011;39:W132–8. doi: 10.1093/nar/gkr247. [DOI] [PMC free article] [PubMed] [Google Scholar]