ABSTRACT
Central memory (TCM) CD4+ T cells are the principal reservoir of latent HIV-1 infection that persists despite durable, successful antiretroviral therapy (ART). In a study that measured HIV DNA in 17 patients and replication-competent HIV in 4 patients, pools of resting and activated transitional memory (TTM) CD4+ T cells were found to be a reservoir for HIV infection. As defective viruses account for the majority of integrated HIV DNA and do not reflect the actual frequency of latent, replication-competent proviral infection, we assessed the specific contribution of resting TTM cells to latent HIV infection. We measured the frequency of replication-competent HIV in purified resting memory cell subpopulations by a limiting-dilution, quantitative viral outgrowth assay (QVOA). HIV was routinely detected within the resting central memory compartment but was infrequently detected within the resting TTM compartment. These observations suggest that prolonged ART may limit persistent latent infection in the TTM compartment. Our results confirm the importance of latent infection within the TCM compartment and again focus attention on these cells as the most important latent viral reservoir. While proliferation may drive expansion of detectable viral genomes in cells, the frequency of replication-competent HIV must be carefully assessed. Latent infection appears to wane within the transitional memory compartment in patients who have sustained successful viral suppression via ART or were treated very early in infection.
IMPORTANCE Antiretroviral therapy (ART) has led to a significant decrease in morbidity and mortality among HIV-infected patients. However, HIV integrates into the genome of CD4+ T cells, generating pools of long-lived cells that are reservoirs of latent HIV. Two main subsets of CD4+ T cells, central memory and transitional memory cells, were reported to be major reservoirs of HIV infection. However, this study primarily measured the HIV DNA content, which also includes defective proviruses that would not be able to replicate and initiate new rounds of infection. By analyzing the replication-competent virus in both cell subsets, we showed that transitional memory cells may not be a durable reservoir in patients on successful ART.
INTRODUCTION
After HIV infection, there may be several pathways that lead to proviral latency. Most latent infection may be established in activated CD4+ T cells that transition to the memory resting state shortly after infection (1). Mechanisms that maintain latent infection are operable in cells that are in a resting G0 state (2). When the cells are resting, HIV persists but is transcriptionally silent, invisible to immune surveillance, and insensitive to antiretroviral therapy (ART) (3–5). The major cellular reservoir of quiescent but replication-competent viruses that persists despite ART resides in a small pool of resting memory CD4+ T cells (2, 6–10). Previous analysis found that frequencies of HIV DNA within activated cells are dramatically higher than within cells in a resting state, reflecting rapid death of activated cells and suggesting that only a fraction of productively infected activated cells survive to return to the memory resting state (2). Therefore, activated cells do not constitute a stable population in which HIV can persist for years. The definition of subpopulations of resting cells that harbor latent infection that can persist despite durable ART is of critical importance.
A previous study of CD4+ T cells, in which populations were studied irrespective of their activation state, found that central memory (TCM) and transitional memory (TTM) CD4+ T cell subsets were the major reservoirs for HIV infection (11). It was proposed that latent infection was maintained by either antigen-driven proliferation or homeostatic proliferation in TCM or TTM cells, respectively (11). In a more recent study, HIV DNA was also detected in both resting TCM and TTM CD4+ T cells in viremic patients (12). However, the contribution of replication-competent virus within resting TCM and resting TTM cells to the total HIV reservoir in patients with complete suppression of plasma viremia has not been addressed. This is a critical point, given the potential for some cell populations to proliferate and as the majority of HIV DNA detected in resting CD4+ T cells consists of defective, non-replication-competent genomes (2, 13, 14). In addition, the impact of immediate or delayed ART on the distribution of persistent, latent infection in CD4+ T cell subpopulations is not known. Furthermore, inherent differences in biologically distinct reservoirs might influence the design of therapeutic interventions to target TTM CD4+ T or TCM CD4+ T cells.
In the present study, we analyzed the frequency of latent HIV infection in highly purified, resting TCM and TTM CD4+ T cells by a quantitative viral outgrowth assay (QVOA) (15–17) comparing a cohort of patients treated during acute HIV infection (AHI) with patients treated during chronic HIV infection (CHI). We also measured the frequency of HIV infection within naive CD4+ T cells. Our results show that HIV latency is primarily established and maintained in TCM CD4+ T cells and that latency is found in TTM CD4+ T cells in a minority of patients. Our findings suggest that while latent infection may be established within TTM CD4+ T cells, initially supported by homeostatic proliferation, the administration of durable ART allows the decay of infection within this cell population.
MATERIALS AND METHODS
Patients.
All patients provided written informed consent, and studies were approved by the UNC Institutional Review Board. Patients identified in the AHI stage (plasma HIV RNA detected and HIV Western blot negative) were enrolled and initiated ART within 45 days of the estimated date of infection; most patients were treated within 3 weeks of the estimated date of infection. Serial measurements of plasma viremia and CD4+ T cell counts were performed, and when patients were aviremic (<50 HIV RNA copies/ml) on ART for >2 years, cells were obtained by continuous-flow leukapheresis.
Isolation of resting memory CD4+ T cell subpopulations and naive CD4+ T cells.
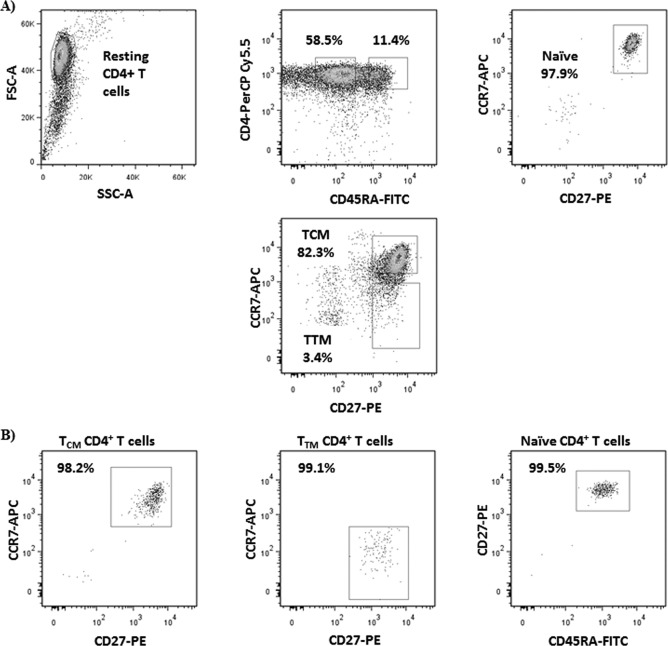
Total resting CD4+ T cells were isolated from the leukapheresis product as previously described (1, 15–17). Following purification, resting CD4+ T cells were incubated for 1 day with the HIV integrase inhibitor L-870812 (1 μM; gift of D. Hazuda, Merck Research Laboratory, West Point, PA) and efavirenz (15 nM) or abacavir (4 μM) as determined by treatment history to ensure the decay of any preintegrated HIV DNA and to avoid any potential de novo infection during cell isolation. Total resting CD4+ T cells were then enriched for memory cells by magnetic separation using a custom cocktail containing CD45RA monoclonal antibody (MAb; Stemcell Technologies, Vancouver, Canada). Afterwards, enriched resting memory CD4+ T cells were incubated in staining buffer with CD45RA-fluorescein isothiocyanate (FITC) and CD4-peridinin chlorophyll protein (PerCP) Cy5.5 (clone HI-100 and clone SK3; BD Pharmingen, San Diego, CA), and CD27-phycoerythrin (PE) and CCR7-allophycocyanin (APC) (clone O323 and clone G043H7; Biolegend, San Diego, CA), for 20 min on ice in the dark, washed twice, resuspended in a phosphate-buffered saline (PBS)-EDTA-HEPES solution, filtered, and isolated by fluorescence-activated cell sorting (FACS) using a Reflection sorter (iCyt, Champagne, IL) or a FACSAria III (BD Biosciences, San Jose, CA). Resting memory CD4+ T cell subsets were defined as CD4+ CD45RA−. TCM CD4+ T cells were gated based on their expression of CD27 and CCR7, while TTM CD4+ T cells were defined as CD27+ CCR7−. Naive CD4+ T cells were CD45RA+ CD27+ CCR7+. Each experiment was validated by performing instrument quality controls and running isotype controls and fluorescence minus one control. Cells were collected in Iscove's modified Dulbecco's medium (IMDM) containing HEPES and glutamine (Gibco, Invitrogen, Carlsbad, CA) and 10% fetal bovine serum (FBS) and 10% penicillin-streptomycin (PenStrep) (both from Sigma-Aldrich, St. Louis, MO). After sorting, an aliquot was used to assess the purity of the sorted T cell subsets, which was >99% (see Fig. 1).
FIG 1.
Representative dot plot examples of presort gating strategy to isolate resting TCM cells (CD4+ CD45RA− CD27+ CCR7+), resting TTM cells (CD4+ CD45RA− CD27+ CCR7−), and naive CD4+ T cells (CD4+ CD45RA+ CD27+ CCR7+) (A) and percentage of postsort purities in these three CD4+ T subsets (B).
Quantitative viral outgrowth assay.
After sorting, resting memory CD4+ T cell subsets and naive cells were cultured in Complete IMDM (cIMDM; containing the supplements HEPES, Gln, 10% FBS, and PenStrep) and activated as previously reported for total resting CD4+ T cells (17). Briefly, 2.5 × 106 to 2.5 × 104 CD4+ T cells of each subset were cultured in limiting dilution in the presence of 1 μg/ml of phytohemagglutinin (PHA), 60 U/ml of interleukin 2 (IL-2), and allogeneic irradiated peripheral blood mononuclear cells (PBMC) for 24 h. Cells were then washed and resuspended in cIMDM with 5 U/ml of IL-2. Allogeneic noninfected PBMC depleted of CD8+ T cells and activated by PHA were used as target cells at a 1:5 ratio (CD4+ T cells/PBMC). Cultures of total resting CD4+ T cells from the same patient were performed in parallel. Medium was replaced every 3 to 4 days. At days 15 and 19 of culture, supernatants were collected and stored at −80°C until further p24 production analysis by a commercially available enzyme-linked immunosorbent assay (ELISA) kit (ABL Inc., Rockville, MD). We scored cultures as positive if p24 was detectable 15 days after cell culture, and rising p24 concentrations were confirmed on day 19.
Total HIV DNA quantification.
Resting memory CD4+ T cell subsets and TCM, TTM, and naive cells were isolated from frozen PBMC, pelleted, and stored at −80°C until DNA extraction was performed. Cellular DNA was extracted using a Qiagen DNA minikit by following the manufacturer's instructions. DNA concentration was measured using a Nanodrop. HIV pol DNA was amplified and droplet digital PCR (ddPCR) performed as previously described (18). Briefly, PCR samples were loaded into the Bio-Rad QX emulsification device and droplets were formed by following the manufacturer's instructions. After 40 cycles of 30 s at 94°C, 60 s at 58°C, and 10 min at 98°C, droplets were analyzed as positive or negative, with the no-template controls serving as the threshold of the limit of quantification, and copy number was calculated by the manufacturer's software (Bio-Rad Quantasoft v.1.2).
Statistical analyses.
Results are expressed as medians and interquartile ranges (IQ). Nonparametric tests were used for analyses, and P values of >0.05 were considered significant. Differences between categorical groups were compared using the two-tailed Mann-Whitney U test, and correlations between continuous variables were performed by Spearman's test. Estimated frequency of infection in different T cell subsets is expressed as infectious units per million (IUPM) cells, estimated by a maximum likelihood method (15). Statistical analyses were performed using IBM SPSS version 19.0 (Chicago, IL).
RESULTS
Patient characteristics.
Sixteen male patients, nine patients treated during AHI and seven treated during CHI, were included in the present study. The median age was 37 (range, 27 to 51) years. Nine (56%) patients were Caucasian, six (37%) were African-American, and one (6%) patient was Hispanic. Of central importance, patients had been treated for a median of 3.7 (range, 2.6 to 7.8) years and had been suppressed (plasma HIV-1 RNA < 50 copies/ml) for a median of 3.2 (range, 2.2 to 3.8) years; therefore, the virological events measured reflect those that persist despite durably successful ART. The median CD4+ T cell nadir was 371 (range, 166 to 520) cells/mm3, and the median CD4+ T cell count at the time of cell donation for QVOA was 774 (range, 701 to 1,005) cells/mm3. In addition, at the time of the study, clinical comparisons between patients treated during AHI or CHI showed no statistical differences except that the length of time that patients had been on therapy was longer for CHI patients (P = 0.03), but the durations of full suppression were similar (P = 0.26) (Table 1).
TABLE 1.
Comparison of clinical characteristics between patients treated during AHI and CHI
| Infection type | Patient | CD4 nadir (cells/mm3) | VL peak (log10 copies/ml)a | CD4 count (cells/mm3) | Duration of ART (yrs) | Duration of suppression (yrs) |
|---|---|---|---|---|---|---|
| AHI | A.1 | 289 | 5.13 | 674 | 3.61 | 2.91 |
| A.2 | 403 | 5.88 | 730 | 4.45 | 3.75 | |
| A.3 | 371 | 5.19 | 629 | 3.38 | 3.22 | |
| A.4 | 137 | 6.24 | 776 | 4.04 | 3.12 | |
| A.5 | 739 | 4.39 | 1,378 | 3.89 | 3.78 | |
| A.6 | 500 | 6.26 | 723 | 2.78 | 3.59 | |
| A.7 | 606 | 6.06 | 940 | 1.02 | 0.99 | |
| A.8 | 520 | 4.36 | 698 | 0.97 | 0.76 | |
| A.9 | 504 | 5.77 | 1,355 | 2.08 | 1.82 | |
| Median | 137 | 5.76 | 730 | 3.88 | 3.12 | |
| CHI | C.1 | NAb | NA | 711 | 8.48 | 3.31 |
| C.2 | 130 | 5.24 | 903 | 18.90 | 10.62 | |
| C.3 | 81 | 5.58 | 772 | 12.52 | 2.76 | |
| C.4 | 195 | 4.86 | 1,027 | 26.93 | 7.50 | |
| C.5 | 322 | 5.57 | 830 | 3.09 | 2.79 | |
| C.6 | 602 | 5.88 | 1,120 | 5.39 | 5.06 | |
| C.7 | 166 | 5.77 | 474 | 2.54 | 1.99 | |
| Median | 180.5 | 5.57 | 830 | 8.48 | 3.31 | |
| P valuec | 0.059 | 0.79 | 0.03 | 0.26 |
Peak of HIV-1 plasma viral load (VL) in the acute phase of the infection for AHI patients and highest plasma viral load on our records for CHI patients.
NA, not available.
Determined by Mann-Whitney U test.
TCM CD4+ T cells are the major reservoir for HIV infection.
Replication-competent HIV was measured by QVOA within total resting memory CD4+ T cells in all 16 patients and within separated memory CD4+ T cell subpopulations (Table 2). Cell populations were rigorously processed to high purity (Fig. 1) in the presence of antiretrovirals during processing and sorting to prevent spread of infection and the factitious amplification of infection within subpopulations.
TABLE 2.
Frequency of infection within resting memory CD4+ T cells
| Patient | No. of infectious units per million |
No. of DNA copies/106 TTM cellsa | ||
|---|---|---|---|---|
| Total resting CD4+ cells | TCM cells | TTM cells | ||
| A.1 | 2.31 | 4.26 | 2.15 | NA |
| A.7 | 0.90 | 2.07 | 3.74 | NA |
| A.8 | 0.74 | 5.89 | 4.52 | NA |
| A.4 | 7.69 | 3.41 | <2.68 | NA |
| A.3 | <0.10 | 0.16 | <0.16 | NA |
| A.2 | 0.41 | 0.85 | <1.91 | 5.1 |
| A.6 | 0.04 | 0.47 | <4.05 | 12 |
| A.9 | 0.02 | 0.24 | <2.22 | 10.3 |
| A.5 | <0.10 | 0.03 | <0.16 | 26.5 |
| C.7 | 0.44 | 3.67 | <2.23 | 416.7 |
| C.6 | 0.61 | 2.06 | <4.05 | <LOQ |
| C.2 | 0.18 | 0.53 | <1.62 | <LOQ |
| C.1 | 0.02 | <1.39 | <2.23 | <LOQ |
| C.5 | 3.75 | 9.74 | <10.9 | NA |
| C.3 | 2.69 | 4.23 | 2.72 | NA |
| C.4 | 0.11 | 0.77 | 1.82 | NA |
LOQ, limit of quantification; NA, not available.
In two patients treated during AHI (A.3 and A.5), latent infection was not detected in total resting CD4+ T cells, but it was detected within the subpopulation of purified TCM cells as a result of the enrichment of TCM cells, which harbor the majority of the replication-competent HIV. As the majority of patients had preserved CD4 cell counts (Table 1), the frequency of replication-competent HIV in total resting CD4+ T cells did not correlate with CD4+ T cell counts at the time of leukapheresis (P = 0.53). However, in AHI patients the CD4+ T cell nadir was inversely correlated with the infectious units per million (IUPM) total resting CD4+ T cells (P = 0.02; R = −0.748).
The median numbers of TCM CD4+ T cells cultured were 22.5 million (range, 11.6 to 28.5 million) in patients who started ART during AHI and 12.0 million (range, 1.0 to 30.0 million) in patients who started ART once CHI was established. Likely due to the smaller cohort size, there was only a trend toward a lower frequency of latent infection in AHI patients than in CHI patients (P > 0.05), unlike what has been previously reported (1–3). Replication-competent HIV was quantifiable in the TCM CD4+ T cell subset for all patients but one (C.1), in whom infection in the total memory CD4 cell population was rare (IUPM = 0.02 [Table 2]). The frequency of latent infection in TCM CD4+ T cells showed a strong positive correlation with the frequency of infection in total resting CD4+ T cells (P < 0.001; R = 0.928), showing that the TCM CD4+ T cell subset harbored the majority of replication-competent HIV, regardless of the time of ART initiation (P > 0.05).
Replication-competent HIV is infrequently recovered from resting TTM cells.
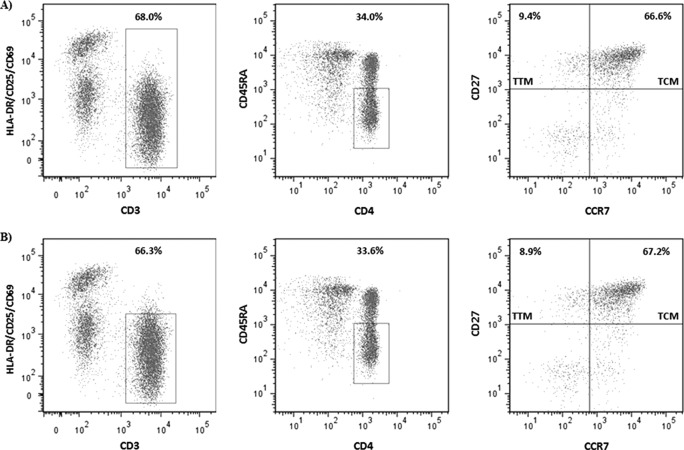
Resting TTM CD4+ T cells were simultaneously purified from the total memory pool, isolated, and cultured in parallel with TCM cells to measure the frequency of latent but replication-competent infection (Table 2). Our protocol for isolating resting cells excludes cells displaying the activation markers HLA-DR and CD25. As these markers may be expressed on a portion of the TTM cells, we analyzed the percentage of these cells that may have been excluded during the procedure for purifying resting memory T cells (Fig. 2). In eight patients, the TTM CD4+ T cells accounted for a mean of 6.46% of the CD3+ CD4+ CD45RA− cell population, but of these cells, only a mean of 0.41% displayed any of the activation markers HLA-DR, CD25, and CD69. In addition, for one AHI patient who had been suppressed for more than 5 years, we performed a QVOA using TTM cells sorted according to HLA-DR expression, and we did not recover any virus either from the total cell pool containing activated cells or from the TTM pool that excluded the activated population (data not shown).
FIG 2.
To illustrate the frequency of activated cells within the memory population, a representative example for one patient is shown within either total CD45RA− CD3+ CD4+ cells without excluding activated cells, defined by a combination of activation markers HLA-DR, CD25, and CD69 (A), or within the nonactivated CD45RA− CD3+ CD4+ compartment (B). In this patient, only 0.5% of the TTM cells display activation markers.
In total, 0.2 to 7.5 million resting TTM CD4+ T cells from nine AHI patients and 0.1 to 5.3 million resting TTM CD4+ T cells from seven CHI patients were used for outgrowth assays. Surprisingly, replication-competent HIV was quantifiable for only 5 of 16 patients, 3 treated during AHI and 2 treated during CHI (Table 2). Interestingly, the three AHI patients in whom HIV was recovered had been suppressed for a mean of 1.58 years, compared to a mean of 3.21 years of suppression in patients with no replication-competent HIV recovered. In addition, it was discovered that the two CHI patients from whom replication-competent virus was recovered had a history of poor therapy adherence with multiple episodes of ART interruption prior to meeting the adherence criteria (uninterrupted successful ART for more than 2 years) that allowed them to participate in this study. Given the lack of recovery of replication-competent HIV within isolated TTM CD4+ T cells, we attempted to measure total HIV DNA in eight patients (four during AHI and four during CHI) in whom replication-competent HIV was not recovered. HIV DNA was detected in the four patients treated during AHI and in only one treated during CHI, while it was below the limit of detection in the other three CHI patients (Table 2). As the number of TTM cells available to perform limiting dilution outgrowth assays was less than that of TCM cells, the ability to apply robust direct statistical comparisons between the two groups was limited. However, overall, we performed 129 cultures of 30 × 106 TTM cells from all patients, and only 9 cultures (6.9%) yielded replication-competent virus, while 132 of 442 (29.8%) were positive in the TCM compartment (Table 3). The numbers of TTM culture replicates were comparable between patients with and without HIV recovery from TTM CD4+ T cells (P = 0.36).
TABLE 3.
Frequency of cultures yielding replication-competent HIV
| Cell type | AHI |
CHI |
||||
|---|---|---|---|---|---|---|
| Total no. of cultures | No. of positive cultures (%) | Total no. of cells cultured | Total no. of cultures | No. of positive cultures (%) | Total no. of cells cultured | |
| TCM | 275 | 78 (28.4) | 139 × 106 | 167 | 54 (32.3) | 114 × 106 |
| TTM | 70 | 6 (8.6) | 17 × 106 | 59 | 3 (5.1) | 13 × 106 |
| Naive | 91 | 3 (3.3) | 38 × 106 | 91 | 18 (19.7) | 25 × 106 |
Frequency of infection in resting TTM cells decays with longer duration of HIV plasma suppression.
The frequency of infection in TTM CD4+ T cells positively correlated with the frequency of infection in TCM CD4+ T cells (R = 0.551; P = 0.027), although no correlation with total resting CD4+ T cells was found (P > 0.05). We found an inverse correlation between the frequency of infection within TTM CD4+ T cells and the duration of ART suppression in patients who initiated treatment during AHI (R = −0.772; P = 0.015) (see Fig. S1 in the supplemental material), which did not achieve significance in patients treated during CHI (P > 0.05). In addition, we also found an inverse correlation between HIV DNA levels within resting TTM cells and the duration the patients had been on therapy (R = −0.805; P = 0.016). These preliminary results suggest that resting cell infection in TTM decays over time, at least in patients treated during AHI.
Latently infected naive CD4+ T cells are found in both AHI and CHI patients.
In 11 of 16 patients, sufficient cells were available to isolate and study latent HIV infection in naive CD4+ T cells. A median of 3.6 million naive cells (range, 2.3 to 9.0 million) were cultured, revealing replication-competent HIV in four patients, two of six treated during AHI (A.4 and A.6) and two of five treated during CHI (C.3 and C.6). It is notable that the two AHI patients had the highest levels of peak of viremia prior to therapy recorded in our cohort, and the two CHI patients had the highest levels of set point viremia prior to ART in this cohort (Table 1), suggesting that latent infection of naive cells may be associated with particularly poor immune control of HIV replication.
DISCUSSION
This is the first study that has exhaustively analyzed the frequency of replication-competent HIV using QVOA within resting memory CD4+ T cell subpopulations. In striking distinction to the uniform recovery of latent HIV from resting TCM cells, we did not recover replication-competent HIV from pools of resting TTM CD4+ T cells in 11 out of 16 patients that had been durable suppressed by ART without interruptions for 2 years or more. This highlights an important distinction between latent infection of TTM and TCM cells and suggests that latent, replication-competent HIV infection does not persist uniformly in the resting TTM population in patients undergoing ART.
TTM CD4+ T cells can harbor high levels of HIV DNA in some patients (Table 2 and reference 11). However, current measurements of replication-competent HIV show that TCM CD4+ T cells are the major latent reservoir after initiation of ART in either the acute or the chronic stage of infection. We detected HIV DNA within resting TTM CD4+ T cells in patients with no replication-competent virus recovery, suggesting an excess of non-replication-competent HIV as demonstrated by Chun et al. (2) and recently reexamined in careful studies by Ericksson et al. (19) and Ho et al. (20). Although other studies have detected HIV DNA in TTM CD4+ T cells (12), these patients were viremic and so latent DNA genomes could not be distinguished from defective genomes or genomes actively expressing viral RNA. Studies that do not exclude activated CD4+ T cells may detect a less stable reservoir (2, 21), which may decay on durable therapy, as suggested by our results. In this study, we used the CD25 marker to exclude activated T cells. However, there is a subset of resting memory CD4+ T cells that express intermediate levels of CD25 (22), so this population merits further analysis.
Homeostatic proliferation of TTM CD4+ T cells has the potential to maintain the presence of replication-competent, integrated HIV DNA in TTM CD4+ T cells (11). We found a strong correlation between longer duration of suppression of viremia and a lower frequency of infection within resting TTM CD4+ T cells in patients treated during AHI, an aspect also supported by the inverse correlation between HIV DNA within TTM cells and the time patients had been on therapy. However, this preliminary observation must be confirmed by including more patients. Our results suggest that while homeostatic proliferation of resting memory TTM CD4+ T cells may contribute to the accumulation and maintenance of latent HIV genomes within TTM CD4+ T cells during the initial months of ART, this influence appears to wane, and replication-competent HIV within resting TTM appears to decay. One recent study supports our results showing expansion of T cell clones containing defective non-replication-competent HIV over time (23). In another study, HIV DNA sequences were significantly increased after long-term suppressive ART (24), consistent with our findings that nondefective viruses may accumulate within the TTM compartment. We were able to quantify HIV DNA in TTM CD4+ T cells in all patients analyzed, but replication-competent virus was infrequently recovered. Moreover, we had the opportunity to analyze the frequency of infection in the resting TTM compartment in patient A.7 1 year following the initial QVOA measurement, finding a decay in the frequency of infection in total resting CD4+ T cells (from 0.902 to 0.496 IUPM cells) and in TCM CD4+ T cells (from 2.070 to 0.747 IUPM cells), with no virus recovered in TTM CD4+ T cells (3.74 declined to <2.23). Our findings are consistent with a model in which latent infection is established within TTM cells but decays on ART, as it does in the first few years of ART (9, 10). Moreover, the only two CHI patients in whom HIV was recovered within TTM cells had a history of poor adherence to ART with several treatment interruptions, suggesting reseeding of the TCM and TTM pools prior to the period of adherence (more than 3 years) that allowed them to be enrolled in this study. Taken together, our results show that TTM CD4+ T cells are not a major reservoir for HIV infection in durably suppressed patients on ART and suggest that while infection of this population can occur, it may not be long-lived. The decline of infection in TTM CD4+ T cells is consistent with this hypothesis. All patients but one included in our study had normal numbers of CD4+ T cells at the time of leukapheresis, due to early or durable and successful ART. In contrast to the previously reported patient population still undergoing CD4 reconstitution (11), the population reported herein had achieved stable CD4+ T cell levels (or never had CD4 depletion), and plasma IL-7 levels were therefore not correlated with the CD4 counts or the frequency of infection in the resting CD4+ T cell populations (data not shown).
In addition, a previous study found that the proviral HIV DNA level was predicted by CD4+ T cell nadir (25). We also found that when ART is initiated during AHI, the frequency of total resting cell infection is correlated with the CD4+ T cell nadir. One possible explanation for the lack of correlation in patients treated during CHI is that while HIV DNA may accumulate over the time of untreated HIV infection, and with higher levels of viremia, the levels of true virologic latency reach an equilibration point and HIV DNA does not accumulate continuously.
Limiting-dilution coculture of resting CD4+ T cells utilizes a protocol whereby cells are exposed to a single round of maximal mitogenic stimulation. Ho et al. recently demonstrated that this protocol may underestimate the frequency of replication-competent HIV (20), due to the stochastic nature of proviral activation even in the face of maximal stimulation, and that virions may be recovered from some cells in some patients with additional rounds of stimulation. In our study, we compared the recovery of latent HIV from resting TCM and TTM populations following a single round of stimulation by the most sensitive assay available (QVOA), using the largest number of cells that could be obtained by apheresis. Assays of HIV DNA very significantly overrepresent frequency of replication-competent proviral infection (2, 19). In addition, HIV DNA/RNA ratio analyses may not accurately reflect the frequency of latent, replication-competent infection due to the potential presence of defective viral DNAs and RNAs (19). Therefore, our assays, performed at the limit of what is currently technically feasible, show that latent infection within TTM populations is less frequent and suggest that TTM infection decays over time on ART.
We also detected replication-competent HIV in naive CD4+ T cells from four patients with poor initial immune control of viremia during AHI, suggesting that innate immune responses in early infection may play a critical role in limiting the establishment of latent infection (26–28). Also, these results imply that other mechanisms such as rapid innate immune responses, more potent HIV-specific CD8 responses, or expression of specific protective alleles, such as HLA-B27/B57 (29), may, at least partially, determine the size of the reservoir of replication-competent HIV. Like for TTM cell populations, the uncommon detection of latent infection in the naive cell population is consistent with latency in these cells being less durable than that in TCM cells, but confirmation of this will require further longitudinal study. However, given the recent description of a population of memory CD4+ T cells with stem cell properties (30) within which latent HIV may persist (31), it should be noted that our method to isolate naive CD4+ T cells (CD45RA+ CD27+ CCR7+) might allow the inclusion of this stem memory subset. Longitudinal study with careful quantitation of replication-competent virus within stem-like CD4+ memory cells will also be required.
In summary, our results demonstrate that replication-competent HIV is primarily found in resting TCM CD4+ T cells and suggest that while homeostatic proliferation of infected resting TTM CD4+ T cells may play a role in the maintenance of latent infection within TTM cells, this effect may be of diminishing importance over years of ART. Therefore, while approaches to deplete persistent infection based on the blockade of homeostatic proliferation in TTM cells (32, 33) may contribute in some way to the eradication of latent HIV infection, approaches that focus on latent virus in TCM CD4+ T cells are of the highest priority.
Supplementary Material
ACKNOWLEDGMENTS
This study was supported by National Institutes of Health grants DA030156M and AI096113 to D.M.M., RR024383 to the UNC TRaCS Institute, and AI50410 to the UNC Center for AIDS Research and an equipment grant from the James B. Pendleton Charitable Trust. N. Soriano-Sarabia was supported by Carlos III Institutes of Health, Spain (postdoctoral contract Sara Borrell CD10/00438).
We are grateful to M. C. Strain for performing digital droplet PCR assays and to C. Gay and J. Eron for expert clinical management and clinical trial support. Finally, we thank R. Bosch and N. Chomont for valuable comments.
Footnotes
Published ahead of print 24 September 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JVI.01900-14.
REFERENCES
- 1.Siliciano JD, Kajdas J, Finzi D, Quinn TC, Chadwick K, Margolick JB, Kovacs C, Gange SJ, Siliciano RF. 2003. Long-term follow-up studies confirm the stability of the latent reservoir for HIV-1 in resting CD4+ T cells. Nat. Med. 9:727–728. 10.1038/nm880. [DOI] [PubMed] [Google Scholar]
- 2.Chun TW, Carruth L, Finzi D, Shen X, DiGiuseppe JA, Taylor H, Hermankova M, Chadwick K, Margolick J, Quinn TC, Kuo YH, Brookmeyer R, Zeiger MA, Barditch-Crovo P, Siliciano RF. 1997. Quantification of latent tissue reservoirs and total body viral load in HIV-1 infection. Nature 387:183–188. 10.1038/387183a0. [DOI] [PubMed] [Google Scholar]
- 3.Chun TW, Justement JS, Lempicki RA, Yang J, Dennis G, Jr, Hallahan CW, Sanford C, Pandya P, Liu S, McLaughlin M, Ehler LA, Moir S, Fauci AS. 2003. Gene expression and viral prodution in latently infected, resting CD4+ T cells in viremic versus aviremic HIV-infected individuals. Proc. Natl. Acad. Sci. U. S. A. 100:1908–1913. 10.1073/pnas.0437640100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schröder AR, Shinn P, Chen H, Berry C, Ecker JR, Bushman F. 2002. HIV-1 integration in the human genome favors active genes and local hotspots. Cell 110:521–529. 10.1016/S0092-8674(02)00864-4. [DOI] [PubMed] [Google Scholar]
- 5.Hermankova M, Siliciano JD, Zhou Y, Monie D, Chadwick K, Margolick JB, Quinn TC, Siliciano RF. 2003. Analysis of human immunodeficiency virus type 1 gene expression in latently infected resting CD4+ T lymphocytes in vivo. J. Virol. 77:7383–7392. 10.1128/JVI.77.13.7383-7392.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Finzi D, Blankson J, Siliciano JD, Margolick JB, Chadwick K, Pierson T, Smith K, Lisziewicz J, Lori F, Flexner C, Quinn TC, Chaisson RE, Rosenberg E, Walker B, Gange S, Gallant J, Siliciano RF. 1999. Latent infection of CD4+ T cells provides a mechanism for lifelong persistence of HIV-1, even in patients on effective combination therapy. Nat. Med. 5:512–517. 10.1038/8394. [DOI] [PubMed] [Google Scholar]
- 7.Wong JK, Hezareh M, Günthard HF, Havlir DV, Ignacio CC, Spina CA, Richman DD. 1997. Recovery of replication-competent HIV despite prolonged suppression of plasma viremia. Science 278:1291–1294. 10.1126/science.278.5341.1291. [DOI] [PubMed] [Google Scholar]
- 8.Chun TW, Engel D, Berrey MM, Shea T, Corey L, Fauci AS. 1998. Early establishment of a pool of latently infected, resting CD4(+) T cells during primary HIV-1 infection. Proc. Natl. Acad. Sci. U. S. A. 95:8869–8873. 10.1073/pnas.95.15.8869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Strain MC, Little SJ, Daar ES, Havlir DV, Gunthard HF, Lam RY, Daly OA, Nguyen J, Ignacio CC, Spina CA, Richman DD, Wong JK. 2005. Effect of treatment, during primary infection, on establishment and clearance of cellular reservoirs of HIV-1. J. Infect. Dis. 191:1410–1418. 10.1086/428777. [DOI] [PubMed] [Google Scholar]
- 10.Archin NM, Vaidya NK, Kuruc JD, Liberty AL, Wiegand A, Kearney MF, Cohen MS, Coffin JM, Bosch RJ, Gay CL, Eron JJ, Margolis DM, Perelson AS. 2012. Immediate antiviral therapy appears to restrict resting CD4+ cell HIV-1 infection without accelerating the decay of latent infection. Proc. Natl. Acad. Sci. U. S. A. 109:9523–9528. 10.1073/pnas.1120248109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chomont N, El-Far M, Ancuta P, Trautmann L, Procopio FA, Yassine-Diab B, Boucher G, Boulassel MR, Ghattas G, Brenchley JM, Schacker TW, Hill BJ, Douek DC, Routy JP, Haddad EK, Sékaly RP. 2009. HIV reservoir size and persistence are driven by T cell survival and homeostatic proliferation. Nat. Med. 15:893–900. 10.1038/nm.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Josefsson L, Palmer S, Faria NR, Lemey P, Casazza J, Ambrozak D, Kearney M, Shao W, Kottilil S, Sneller M, Mellors J, Coffin JM, Maldarelli F. 2013. Single cell analysis of lymph node tissue from HIV-1 infected patients reveals that the majority of CD4+ T-cells contain one HIV-1 DNA molecule. PLoS Pathog. 9:e1003432. 10.1371/journal.ppat.1003432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sanchez G, Xu X, Chermann JC, Hirsch I. 1997. Accumulation of defective viral genomes in peripheral blood mononuclear cells of human immunodeficiency virus type 1-infected individuals. J. Virol. 71:2233–2240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kieffer TL, Kwon P, Nettles RE, Han Y, Ray SC, Siliciano RF. 2005. G→A hypermutation in protease and reverse transcriptase regions of human immunodeficiency virus type 1 residing in resting CD4+ T cells in vivo. J. Virol. 79:1975–1980. 10.1128/JVI.79.3.1975-1980.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Macken C. 1999. Design and analysis of serial limiting dilution assays with small sample sizes. J. Immunol. Methods 222:13–29. 10.1016/S0022-1759(98)00133-1. [DOI] [PubMed] [Google Scholar]
- 16.Siliciano JD, Siliciano RF. 2005. Enhanced culture assay for detection and quantification of latently, resting CD4+ T cells carrying replication-competent virus in HIV-1-infected individuals. Methods Mol. Biol. 304:3–15. 10.1385/1-59259-907-9:003. [DOI] [PubMed] [Google Scholar]
- 17.Archin NM, Eron JJ, Palmer S, Hartmann-Duff A, Martinson JA, Wiegand A, Bandarenko N, Schmitz JL, Bosch RJ, Landay AL, Coffin JM, Margolis DM. 2008. Valproic acid without intensified antiviral therapy has limited impact on persistent HIV infection of CD4+ T cells. AIDS 22:1131–1135. 10.1097/QAD.0b013e3282fd6df4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Strain MC, Lada SM, Luong T, Rought SE, Gianella S, Terry VH, Spina CA, Woelk CH, Richman DD. 2013. Highly precise measurement of HIV DNA by droplet digital PCR. PLoS One 8:e55943. 10.1371/journal.pone.0055943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eriksson S, Graf EH, Dahl V, Strain MC, Yukl SA, Lysenko ES, Bosch RJ, Lai J, Chioma S, Emad F, Abdel-Mohsen M, Hoh R, Hecht F, Hunt P, Somsouk M, Wong J, Johnston R, Siliciano RF, Richman DD, O'Doherty U, Palmer S, Deeks SG, Siliciano JD. 2013. Comparative analysis of measures of viral reservoirs in HIV-1 eradication studies. PLoS Pathog. 9:e1003174. 10.1371/journal.ppat.1003174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ho YC, Shan L, Hosmane NN, Wang J, Laskey SB, Rosenbloom DI, Lai J, Blankson JN, Siliciano JD, Siliciano RF. 2013. Replication-competent noninduced proviruses in the latent reservoir increase barrier to HIV-1 cure. Cell 155:540–551. 10.1016/j.cell.2013.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pierson TC, Zhou Y, Kieffer TL, Ruff CT, Buck C, Siliciano RF. 2002. Molecular characterization of preintegration latency in human immunodeficiency virus type 1 infection. J. Virol. 76:8518–8531. 10.1128/JVI.76.17.8518-8513.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Triplett TA, Curti BD, Bonafede PR, Miller WL, Walker EB, Weinberg AD. 2012. Defining a functionally distinct subset of human memory CD4+ T cells that are CD25POS and FOXP3NEG. Eur. J. Immunol. 42:1893–1905. 10.1002/eji.201242444. [DOI] [PubMed] [Google Scholar]
- 23.Josefsson L, von Stockenstrom S, Faria NR, Sinclair E, Bacchetti P, Killian M, Epling L, Tan A, Ho T, Lemey P, Shao W, Hunt PW, Somsouk M, Wylie W, Douek DC, Loeb L, Custer J, Hoh R, Poole L, Deeks SG, Hecht F, Palmer S. 2013. The HIV-1 reservoir in eight patients on long-term suppressive antiretroviral therapy is stable with few genetic changes over time. Proc. Natl. Acad. Sci. U. S. A. 110:E4987–E4996. 10.1073/pnas.1308313110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wagner TA, McKernan JL, Tobin NH, Tapia KA, Mullins JI, Frenkel LM. 2013. An increasing proportion of monotypic HIV-1 DNA sequences during antiretroviral treatment suggests proliferation of HIV-infected cells. J. Virol. 87:1770–1778. 10.1128/JVI.01985-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boulassel M-R, Chomont N, Pai NP, Gilmore N, Sékaly RP, Routy JP. 2012. CD4 T cell nadir independently predicts the magnitude of the HIV reservoir after prolonged suppressive antiretroviral therapy. J. Clin. Virol. 53:29–32. 10.1016/j.jcv.2011.09.018. [DOI] [PubMed] [Google Scholar]
- 26.Roederer M, Raju PA, Mitra DK, Herzenberg LA, Herzenberg LA. 1997. HIV does not replicate in naïve CD4 T cells stimulated with CD3/CD28. J. Clin. Invest. 99:1555–1564. 10.1172/JCI119318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang W, Guo J, Yu D, Vorster PJ, Chen W, Wu Y. 2012. A dichotomy in cortical and chemotactic actin activity between human memory and naïve T cells contributes to their differential susceptibility to HIV-1 infection. J. Biol. Chem. 287:35455–35469. 10.1074/jbc.M112.362400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wightman F, Solomon A, Khoury G, Green JA, Gray L, Gorry PR, Ho YS, Saksena NK, Hoy J, Crowe SM, Cameron PU, Lewin SR. 2010. Both CD31(+) and CD31(−) naïve CD4(+) T cells are persistent HIV type 1-infected reservoirs in individuals receiving antiretroviral therapy. J. Infect. Dis. 202:1738–1748. 10.1086/656721. [DOI] [PubMed] [Google Scholar]
- 29.Descours B, Avettand-Fenoel V, Blanc C, Samri A, Mélard A, Supervie V, Theodorou I, Carcelain G, Rouzioux C, Autran B, ALT ANRS CO15 Study Group 2012. Immune responses driven by protective human leukocyte antigen alleles from long-term non progressors are associated with log HIV reservoir in central memory CD4 T cells. Clin. Infect. Dis. 54:1495–1503. 10.1093/cid/cis188. [DOI] [PubMed] [Google Scholar]
- 30.Gattinoni L, Lugli E, Ji Y, Pos Z, Paulos CM, Quigley MF, Almeida JR, Gostick E, Yu Z, Carpenito C, Wang E, Douek DC, Price DA, June CH, Marincola FM, Roederer M, Restifo NP. 2011. A human memory T cell subset with stem cell-like properties. Nat. Med. 17:1290–1297. 10.1038/nm.2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Buzon MJ, Sun H, Li C, Shaw A, Seiss K, Ouyang Z, Martin-Gayo E, Leng J, Henrich TJ, Li JZ, Pereyra F, Zurakowski R, Walker BD, Rosenberg ES, Yu XG, Lichterfeld M. 2014. HIV-1 persistence in CD4+ T cells with stem cell-like properties. Nat. Med. 20:139–142. 10.1038/nm.3445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bosque A, Famiglietti M, Weyrich AS, Goulston C, Planelles V. 2011. Homeostatic proliferation fails to efficiently reactivate HIV-1 latently infected central memory CD4+ T cells. PLoS Pathog. 7:e1002288. 10.1371/journal.ppat.1002288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chomont N, DaFonseca S, Vandergeeten C, Ancuta P, Sékaly RP. 2011. Maintenance of CD4+ T-cell memory and HIV persistence, keeping memory, keeping HIV. Curr. Opin. HIV AIDS 6:30–36. 10.1097/COH.0b013e3283413775. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.